Abstract
Background
Recent studies suggest that circulating concentrations of specific ceramide species may be associated with coronary risk and mortality. We sought to determine the relations between the most abundant plasma ceramide species of differing acyl chain lengths and the risk of coronary heart disease (CHD) and mortality in community‐based samples.
Methods and Results
We developed a liquid chromatography/mass spectrometry assay to quantify plasma C24:0, C22:0, and C16:0 ceramides and ratios of these very–long‐chain/long‐chain ceramides in 2642 FHS (Framingham Heart Study) participants and in 3134 SHIP (Study of Health in Pomerania) participants. Over a mean follow‐up of 6 years in FHS, there were 88 CHD and 90 heart failure (HF) events and 239 deaths. Over a median follow‐up time in SHIP of 5.75 years for CHD and HF and 8.24 years for mortality, there were 209 CHD and 146 HF events and 377 deaths. In meta‐analysis of the 2 cohorts and adjusting for standard CHD risk factors, C24:0/C16:0 ceramide ratios were inversely associated with incident CHD (hazard ratio per average SD increment, 0.79; 95% confidence interval, 0.71–0.89; P<0.0001) and inversely associated with incident HF (hazard ratio, 0.78; 95% confidence interval, 0.61–1.00; P=0.046). Moreover, the C24:0/C16:0 and C22:0/C16:0 ceramide ratios were inversely associated with all‐cause mortality (C24:0/C16:0: hazard ratio, 0.60; 95% confidence interval, 0.56–0.65; P<0.0001; C22:0/C16:0: hazard ratio, 0.65; 95% confidence interval, 0.60–0.70; P<0.0001).
Conclusions
The ratio of C24:0/C16:0 ceramides in blood may be a valuable new biomarker of CHD risk, HF risk, and all‐cause mortality in the community.
Keywords: cardiovascular disease risk factors, ceramides, mortality
Subject Categories: Epidemiology
Clinical Perspective
What Is New?
We developed a high‐throughput Food and Drug Administration–compliant liquid chromatography/mass spectrometry assay to quantify ratios of very–long‐chain/long‐chain ceramides in the plasma.
Applying this assay to the FHS (Framingham Heart Study) and the SHIP (Study of Health in Pomerania), we observed that higher plasma C24:0/C16:0 ceramide ratios are associated with lower rates of incident coronary heart disease and all‐cause mortality over a mean follow‐up of 6 years.
The C24:0/C16:0 ceramide ratio improves risk prediction for all‐cause mortality.
What Are the Clinical Implications?
The C24:0/C16:0 ceramide ratio provides predictive information about coronary heart disease and mortality in the general population years before the actual onset of disease.
Specific ceramide molecular species likely reflect distinct pathophysiological processes. Approaches to risk prediction that consider this molecular diversity of sphingolipids are likely to be most effective.
Introduction
Ceramides are a large class of bioactive sphingolipids that contain a sphingoid base linked to a fatty acyl chain. In mammals, the de novo synthesis pathway includes a family of 6 ceramide synthase enzymes that direct acylation of sphingoid bases with distinct, but overlapping, tissue distributions and acyl chain specificities.1 The resultant ceramides are highly compartmentalized within cells and enriched in specific tissues.
High levels of total tissue ceramides have been implicated in metabolic and cardiovascular diseases (CVDs) in animal models. Genetic and pharmacological interventions that decrease total plasma ceramides prevent diet‐ and glucocorticoid‐induced insulin resistance, atherosclerosis, and metabolic cardiomyopathy in mice.2, 3, 4 These studies provided early evidence that total ceramides might serve as a useful diagnostic tool or therapeutic target.
Recent studies raise the possibility that the deleterious cardiometabolic effects of ceramides may relate more to remodeling of the ceramide acyl chain species, rather than total ceramide levels. In mice with altered expression of ceramide synthase 2 and ceramide synthase 6, increases in long‐chain ceramide species (eg, C16:0 ceramide) and decreases in very–long‐chain species (eg, C22:0 and C24:0 ceramides) in metabolic tissues are associated with diet‐induced glucose intolerance, nonalcoholic steatohepatitis, and insulin resistance.5, 6 In retrospective case‐control studies of patients with coronary heart disease (CHD), high plasma C16:0 ceramide, low C24:0 ceramide, and low C24:0/C16:0 ceramide ratios were directly related to cardiovascular mortality over 3 years.7, 8 To test the hypothesis that higher ratios of plasma very–long‐chain ceramides/long‐chain ceramides are inversely associated with risk of CHD and mortality in the general population, we related ratios of 3 abundant plasma ceramides9 to the risk of CHD and all‐cause mortality in 2 large community‐based samples.
Methods
Quantification of Ceramides
We developed a fully validated liquid chromatography/tandem mass spectrometry assay to quantify C24:0, C22:0, and C16:0 ceramides in frozen fasting plasma samples (see Data S1).
Healthy Volunteers
To assess performance of the ceramide assay, we recruited healthy nonsmoking men (n=12) and women (n=12), aged 40 to 60 years (mean age, 41 years), at Washington University (St Louis, MO). Subjects were free of hypertension, diabetes mellitus (normal glucose tolerance test result), CVD (normal stress echocardiogram result), and other major systemic illness. Morning fasting plasma was obtained from venous blood draws 2 weeks apart to determine range and mean values at each time. Absolute difference and percentage change were calculated per subject, and Student's 1‐sample t‐test was used to determine if mean percentage change differed from zero. The Washington University Institutional Review Board approved this study protocol.
FHS Samples
We evaluated participants from the FHS (Framingham Heart Study) Offspring Cohort who attended the eighth examination cycle (2005–2008) when fasting plasma samples were obtained. The Boston University Medical Center (Boston, MA) and Washington University Institutional Review Boards approved this study protocol.
From a total of 2812 participants at the eighth examination cycle, we used 4 different participant samples (Figure S1). Sample 1, used to examine clinical correlates of ceramides, excluded individuals who were missing plasma samples (n=140) or covariates (n=30), giving a final sample size of 2642. From this, additional samples to examine outcomes of interest excluded those with the prevalent disease of interest or missing follow‐up time. Sample 2 (n=2336) examined incident CHD, which includes myocardial infarction, coronary insufficiency (history of prolonged ischemic chest pain, accompanied by transient ischemic S‐T segment or T‐wave changes in the ECG, but not by development of Q‐wave abnormality or by serum enzyme changes characteristic of myocardial necrosis), and angina pectoris. Sample 3 (n=2542) examined incident heart failure (HF). Sample 4 (n=2633) examined mortality. Criteria for these events, adjudication process, and criteria for covariates have been previously published.10 All events were adjudicated during the follow‐up from baseline through 2012.
SHIP Samples
We used data and plasma samples from the first cohort of the SHIP (Study of Health in Pomerania), a northern European community‐based study.11 The study was approved by the Ethics Committee of the University Medicine Greifswald and the Washington University Institutional Review Board. CHD and HF events were evaluated between the SHIP‐1 (2002–2006) and SHIP‐2 (2008–2012) examination cycle. Mortality was tracked from SHIP‐1 through March 2016. From 3300 participants who attended SHIP‐1 and had fasting plasma samples, 88 were excluded for missing ceramide values and 78 were removed for missing covariates, yielding sample A (n=3134, Figure S2) that was used to assess clinical correlates. Samples B (examined CHD, n=1848) and C (examined HF, n=1935) were created from the 2333 participants who also attended SHIP‐2, when CHD and HF status was reassessed. Sample D, used to assess mortality, is the same as sample A but with 1 additional individual removed because of a missing death date (n=3133). These samples mirror samples 2, 3, and 4 in our FHS analysis (exclusions for prevalent disease of interest, unknown disease status at SHIP‐1 or SHIP‐2 examinations, unknown follow‐up time, missing ceramide values, or missing covariates). Definitions for events and criteria for covariates were aligned to those used in FHS.
Analysis of FHS and SHIP Samples
Using data from FHS sample 1 participants, we fit multiple linear regression models to assess correlates of the C24:0/C16:0 and C22:0/C16:0 ceramide ratios and C24:0, C22:0, and C16:0 ceramide levels (separate models for each ratio or lipid). Ceramide levels served as the dependent variable, whereas age, sex, body mass index, systolic blood pressure (SBP), antihypertensive medications, smoking status, diabetes mellitus, ratio of total/high‐density lipoprotein (HDL) cholesterol, triglycerides, lipid‐lowering medication, and prevalent CVD served as independent variables. After confirming that the proportional hazards assumption was satisfied, we used data from participants in samples 2, 3, and 4 to perform Cox regression, evaluating the association of ceramide ratios or ceramide levels with CHD, HF, all‐cause mortality, CVD mortality, and non‐CVD mortality (separate models for each event and for each ceramide ratio or species), adjusting for age, sex, body mass index, SBP, diabetes mellitus, smoking status, antihypertensive medications, the ratio of total/HDL cholesterol, triglycerides, and lipid‐lowering medication. All‐cause mortality models were additionally adjusted for prevalent CVD. We created cumulative incidence plots to assess incidence of events by tertiles of ceramide ratio.
Analyses were repeated using SHIP samples A through D. Cox proportional hazards regression models were used to examine the association between ceramides and mortality, because exact death dates were known. Because exact dates of cardiovascular events are not available in SHIP, when modeling the association between ceramides and CHD or HF, we used a constant hazard model with a Poisson distribution and an offset equal to the log follow‐up time, rather than Cox proportional hazards regression.12 We used the midpoint between an individual's SHIP‐1 and SHIP‐2 examination dates as the event date for those who developed CHD or HF between these examinations (see Data S1). All models were adjusted for the same covariates used in FHS analyses.
Meta‐analyses were performed using FHS and SHIP samples, using an increment for ceramide ratio or ceramide level that reflected the average SD for the ratio or level between FHS and SHIP (eg, an increase of 3.0 for the C24:0/C16:0 ratio). Maximum likelihood random effect models were used to account for the moderate heterogeneity indicated by the values of I2. Statistical significance was assessed using P<0.05. FHS and meta‐analyses were performed using SAS, version 9.3. (SAS Institute Inc, Cary, NC); SHIP analyses were performed using Stata, version 14.2 (Stata Corp, 2015).
The incremental effect of ceramide ratios over standard CVD risk factors was assessed in FHS and SHIP by examining the change in C‐statistics between models without versus with ceramide ratios.
This study complies with the Declaration of Helsinki. All human subjects provided informed written consent. FHS data are available through dbGAP (accession pending). SHIP data are publicly available for scientific and quality control purposes (apply at http://www.community-medicine.de).
Results
High‐Throughput Assay for Quantification of Ceramides
We modified a validated liquid chromatography/tandem mass spectrometry assay to simultaneously quantify C16:0, C22:0, and C24:0 ceramides, which are the most abundant long‐ and very–long‐chain ceramide species in human plasma.9, 13 Linear dynamic ranges for C16:0, C22:0, and C24:0 ceramides in this triplex assay were 0.01 to 2, 0.04 to 8, and 0.1 to 20 μg/mL, respectively, which encompass values reported for each of these species in human plasma.9 The intra‐assay and interassay precisions were within 7.8%, 7.6%, and 6.9% coefficient of variation for C16:0, C22:0, and C24:0 ceramides, respectively. The intra‐assay and interassay accuracy values were within ±3.2%, ±4.5%, and ±4.9% deviation of the nominal concentration values for C16:0, C22:0, and C24:0 ceramides, respectively. Stability of the ceramides in human plasma was determined to be acceptable after 5 freeze‐thaw cycles (difference, <5% for each). These data indicate that the triplex assay is accurate, precise, and rugged.
We initially used this assay to quantify the ratios of C24:0/C16:0 and C22:0/C16:0 ceramides in fasting plasma samples obtained 2 weeks apart from 24 healthy nonsmoking volunteers who were free of diabetes mellitus, hypertension, and obstructive CHD. Values for C16:0, C22:0, and C24:0 ceramides were within the linear range for each analyte (Table S1). Values for the C24:0/C16:0 ceramide ratio were greater than for the C22:0/C16:0 ceramide ratio, reflecting greater abundance of the C24:0 species. The difference and percentage change were calculated per subject and then aggregated and summarized by the mean difference and mean percentage change, respectively. Between samples drawn 2 weeks apart, the ceramide ratios were not significantly different.
Associations Between Ceramide Ratios and Standard Risk Factors in FHS and SHIP
We then used the triplex assay to quantify C24:0/C16:0 and C22:0/C16:0 ratios in plasma from the Offspring Cohort participants of FHS, and results were validated in SHIP and meta‐analyses. Overall, FHS and SHIP participants were middle‐aged to older individuals, and more than half the participants were women (Table 1). Values for ceramide ratios were normally distributed in both FHS and SHIP in both women and men (Figure 1), as were values for each of the individual species (Figure S3). C24:0 ceramide was nearly 4‐fold more abundant than C22:0 ceramide, and 12‐fold more abundant than C16:0 ceramide.
Table 1.
Descriptive Characteristics of Largest Study Samples in FHS (Sample 1) and SHIP (Sample A) at Baseline
| Characteristics | FHS (n=2642) | SHIP (n=3134) |
|---|---|---|
| Age, y | 66.2±9.0 | 54.0±15.1 |
| Men | 1208 (45.7) | 1508 (48.1) |
| Body mass index, kg/m2 | 28.3±5.4 | 27.9±4.9 |
| Systolic blood pressure, mm Hg | 128.4±17.2 | 132.2±19.4 |
| Diastolic blood pressure, mm Hg | 73.4±10.1 | 81.4±10.5 |
| Total cholesterol, mg/dL | 186.1±37.2 | 214.4±45.1 |
| HDL cholesterol, mg/dL | 57.4±18.2 | 45.6±16.3 |
| Triglycerides, mg/dL | 117.8±67.8 | 162.0±152.6 |
| Plasma C16:0 ceramide, μg/mL | 0.2±0.04 | 0.2±0.05 |
| Plasma C22:0 ceramide, μg/mL | 0.6±0.2 | 0.7±0.2 |
| Plasma C24:0 ceramide, μg/mL | 2.3±0.6 | 2.5±0.7 |
| Plasma C22:0/C16:0 ceramide | 3.8±0.8 | 3.1±0.6 |
| Plasma C24:0/C16:0 ceramide | 14.0±3.4 | 11.8±2.6 |
| Hypertension | 1540 (58.3) | 1983 (63.3) |
| Antihypertensive medication use | 1280 (48.5) | 1292 (41.2) |
| Lipid‐lowering medication use | 1128 (42.7) | 457 (14.6) |
| Diabetes mellitus | 364 (13.8) | 455 (14.5) |
| Smokers | 236 (8.9) | 820 (26.2) |
Values are mean±SD for continuous variables and number (percentage) for categorical variables. FHS indicates Framingham Heart Study; HDL, high‐density lipoprotein; SHIP, Study of Health in Pomerania.
Figure 1.
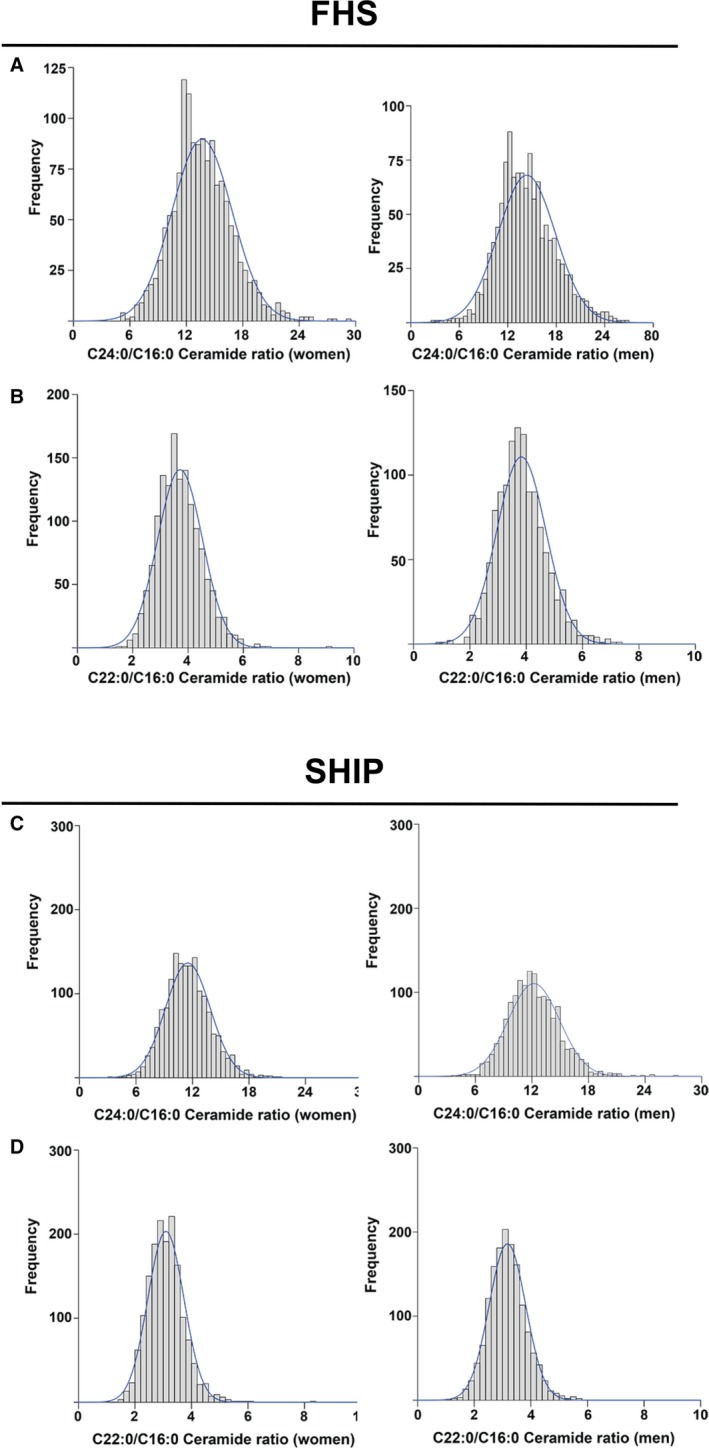
Distributions of plasma ceramide ratios in FHS (Framingham Heart Study) and SHIP (Study of Health in Pomerania). Plots display distribution of values for C24:0/C16:0 (A and C) and C22:0/C16:0 (B and D) ceramide ratios in female and male FHS participants at examination 8 (A and B) and in SHIP participants at SHIP‐1 examination (C and D).
In multiple linear regression models in both FHS and SHIP, age, use of antihypertensive medication, smoking status, and prior CVD were inversely associated with plasma C24:0/C16:0 ceramide ratio, whereas male sex and SBP were directly associated with the ratio (all P<0.03, Table 2). Diabetes mellitus status was not associated with the C24:0/C16:0 ratio in either FHS or SHIP. In FHS and in SHIP, age was inversely associated with the C22:0/C16:0 ceramide ratio, whereas body mass index, total/HDL cholesterol, and triglycerides were directly associated with the C22:0/C16:0 ceramide ratio (Table S2). We did not observe any statistically significant association between male sex and C22:0/C16:0 in either cohort. In FHS only, antihypertensive medication, smoking status, and prevalent CVD were inversely associated with C22:0/C16:0. Although diabetes mellitus was directly associated with the C22:0/C16:0 ratio in FHS, this finding was not replicated in SHIP. In both studies, male sex, use of antihypertensive medication, and use of lipid‐lowering medication were inversely associated with each ceramide species individually, whereas total/HDL cholesterol and triglycerides were directly associated with each ceramide species individually (Table S3). There were no strong associations between ceramides and testosterone levels that replicated across both studies (data not shown).
Table 2.
Clinical Correlates of Plasma C24:0/C16:0 Ceramide Ratios in FHS and SHIP
| Variable | FHS | SHIP | ||
|---|---|---|---|---|
| β Estimate | P Value | β Estimate | P Value | |
| Age | −0.085 | <0.0001 | −0.031 | <0.001 |
| Male sex | 0.813 | <0.0001 | 0.554 | <0.001 |
| Body mass index | −0.009 | 0.48 | −0.013 | 0.20 |
| Systolic blood pressure | 0.012 | 0.0023 | 0.013 | <0.001 |
| Antihypertensive medication | −0.485 | 0.0007 | −0.276 | 0.018 |
| Smoking status | −0.567 | 0.0114 | −0.238 | 0.026 |
| Diabetes mellitus status | 0.205 | 0.30 | −0.017 | 0.90 |
| Total/HDL cholesterol | −0.253 | 0.0021 | 0.064 | 0.015 |
| Triglycerides | 0.008 | <0.0001 | 0.0005 | 0.17 |
| Lipid‐lowering medication | 0.103 | 0.47 | 0.322 | 0.024 |
| Prevalent CVD | −0.707 | 0.0001 | −0.305 | 0.007 |
Multiple linear regression models were used, in which the ceramide ratio served as the dependent variable and clinical correlates served as independent variables. β Estimates represent the increase in plasma ceramide levels per‐unit increase in continuous variables and for the presence (vs absence) of dichotomous variables. CVD indicates cardiovascular disease; FHS, Framingham Heart Study; HDL, high‐density lipoprotein; SHIP, Study of Health in Pomerania.
Association Between Ceramide Ratios and Incidence of CHD, HF, and All‐Cause Mortality
In FHS, there were 88 CHD and 90 HF events, as well as 239 deaths during a mean follow‐up of 6 years. In SHIP, there were 209 CHD and 146 HF events over a median follow‐up of 5.75 years and 377 deaths over a median follow‐up of 8.24 years. Cumulative incidence of CHD, HF, and all‐cause mortality decreased across ceramide 24:0/16:0 tertiles in FHS, with the highest incidence in the lowest ceramide ratio tertile (Figure 2). The increase in median ceramide 24:0/16:0 ratio across tertiles was large (81% increase from tertile 1–2, 62% increase from tertile 2–3) compared with the differences observed in repeated measures of the ratio determined at 2‐week intervals (6%). Thus, intraindividual variability in the ceramide ratio was unlikely to have altered an individual's tertile rank. Similarly, cumulative incidence of HF and all‐cause mortality, but not CHD, decreased across ceramide 22:0/16:0 tertiles (Figure S4). Together, these findings indicate that in FHS, individuals with the lowest ratios of very–long‐chain/long‐chain ceramides were at greatest risk for CHD, HF, and all‐cause mortality.
Figure 2.
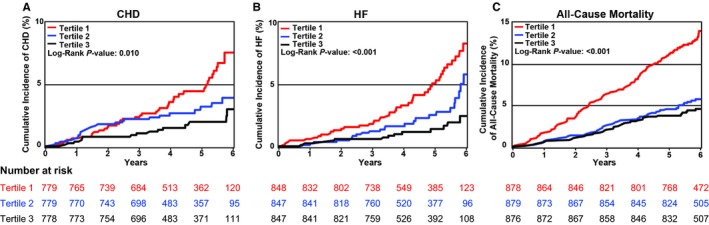
Cumulative incidence of coronary heart disease (CHD), heart failure (HF), and all‐cause mortality in FHS (Framingham Heart Study) by tertiles of plasma C24:0/C16:0 ceramide ratio. Cumulative incidence of CHD (A), HF (B), and all‐cause mortality (C) are reported for tertiles of C24:0/C16:0 ceramide ratio. Tertile 1 includes participants with ceramide levels at the 33rd percentile or lower (2.8–12.3 μg/mL); tertile 2 includes participants with ceramide levels between >33rd and <66th percentile (12.3–15.1 μg/mL); tertile 3 includes participants with ceramide levels ≥66th percentile (15.1–29.2 μg/mL).
Multivariable‐adjusted risk of CHD, HF, and mortality was estimated separately in FHS and SHIP through use of the survival models and then combined through meta‐analysis. In the meta‐analysis, we found a significant inverse association between C24:0/C16:0 ceramide ratio and incident CHD, with little variability in effect size because of the between‐study variation (Figure 3). This inverse association was significant in the SHIP study, but borderline significant in FHS (P=0.08). The C24:0/C16:0 ceramide ratio was inversely associated with incident HF in meta‐analysis and in FHS, but not in SHIP. We observed similar, but weaker, trends for association between C22:0/C16:0 ceramide ratio and incident CHD and HF (Figure S5). Most striking in our meta‐analysis was an inverse association between the C24:0/C16:0 ceramide ratio and all‐cause mortality (Figure 3). This relationship was also observed in each study analyzed individually and reflected inverse associations with both CVD mortality and non‐CVD mortality (Figure 4). Associations between the C22:0/C16:0 ratio and mortality (all‐cause, CVD, and non‐CVD mortality) were similar in our meta‐analysis. Multivariable‐adjusted analyses for individual ceramide species suggest that our findings for the C24:0/C16:0 ceramide ratio were driven by significant inverse associations between C24:0 ceramide and incident CHD and all‐cause mortality and by significant direct association between C16:0 ceramide and all‐cause mortality (Figure S6 and S7).
Figure 3.
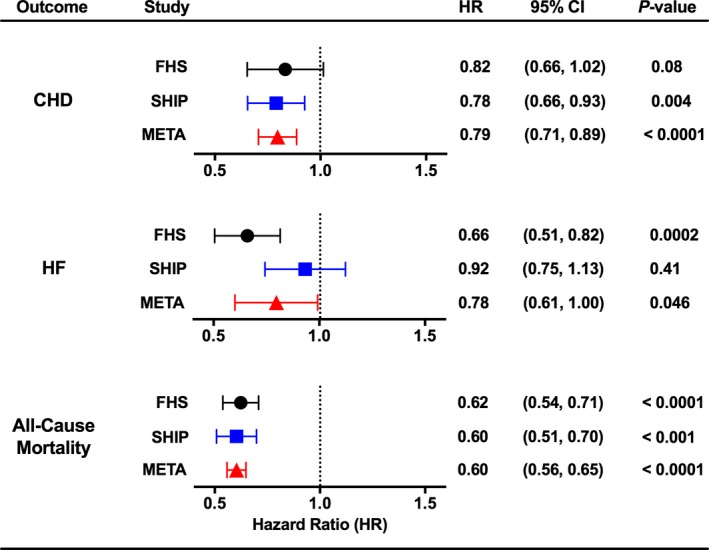
Risk of coronary heart disease (CHD), heart failure (HF), and all‐cause mortality by C24:0/C16:0 ceramide ratio. Hazard ratios (HRs) for CHD, HF, and all‐cause mortality are reported with 95% confidence intervals (CIs) for a 3‐unit increase in the C24:0/C16:0 ceramide ratio (average of SDs between FHS [Framingham Heart Study] and SHIP [Study of Health in Pomerania]), adjusting for all other variables in the model. Data are shown from analysis of subjects in FHS, SHIP, and the combined meta‐analysis (META). I2=0 for CHD and for all‐cause mortality; I2=0.81 for HF.
Figure 4.
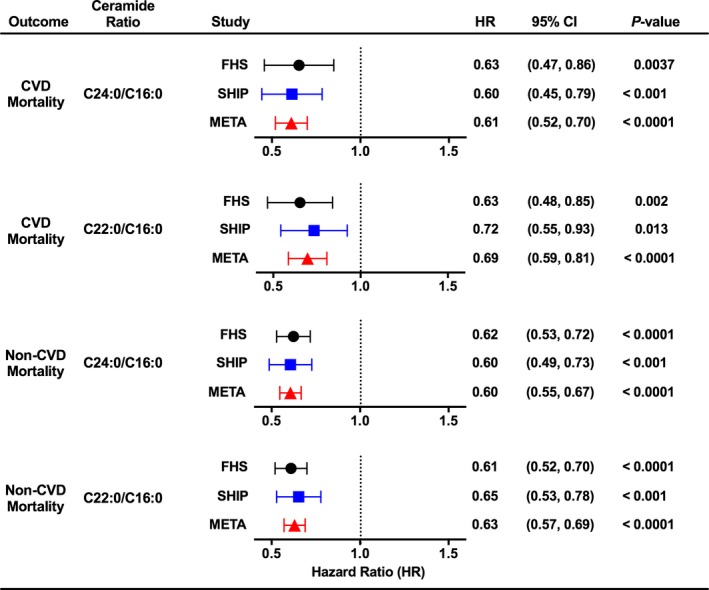
Risk of cardiovascular disease (CVD) mortality and non‐CVD mortality by ceramide ratios. Hazard ratios (HRs) for CVD mortality and non‐CVD mortality are reported with 95% confidence intervals (CIs) for a 3‐unit increase in C24:0/C16:0 ceramide ratio and for a 0.7‐unit increase in C22:0/C16:0 ceramide ratio (for each ratio, average of SDs between FHS [Framingham Heart Study] and SHIP [Study of Health in Pomerania]), adjusting for all other variables in the model. Data are shown from analysis of subjects in FHS, SHIP, and the combined meta‐analysis (META). I2=0 for CVD mortality and for non‐CVD mortality.
To assess the predictive value of the C24:0/C16:0 and C22:0/C16:0 ceramide ratios, we added the ceramide ratios from FHS and SHIP to base models including standard coronary risk factors of age, sex, body mass index, total/HDL cholesterol, triglycerides, lipid‐lowering medications, SBP, antihypertensive therapy, diabetes mellitus, and smoking. Addition of the C24:0/C16:0 ceramide ratio did not affect the C‐statistic for CHD, HF, or CVD mortality (Table 3). However, addition of the C24:0/C16:0 ceramide ratio improved the C‐statistic for all‐cause mortality from 0.756 to 0.776 in FHS and from 0.8706 to 0.8768 in SHIP. In FHS, addition of the C24:0/C16:0 ceramide ratio also improved the C‐statistic for non‐CVD mortality. The 95% confidence intervals for these changes in C‐statistic excluded 0. Overall, similar improvements in the C‐statistic were observed for the C22:0/C16:0 ceramide ratio in FHS, but not in SHIP. In FHS, the effect on all‐cause mortality of adding the C24:0/C16:0 ceramide ratio to the model was similar to the effect of adding high‐sensitivity C‐reactive protein (Table S4). In SHIP, however, the 95% confidence interval for the change in C‐statistic with addition of C‐reactive protein to the base model did not exclude 0. In SHIP, the effects on all‐cause mortality of adding N‐terminal brain natriuretic peptide were similar to the effects of adding the C24:0/C16:0 ratio, and N‐terminal brain natriuretic peptide also improved the C‐statistic for CVD mortality. However, N‐terminal brain natriuretic peptide was not quantified at examination 8 in FHS.
Table 3.
Incremental Effect of Incorporating Ceramide Ratios on Model Discrimination
| Predictors in Model | Incident CHD | Incident HF | All‐Cause Mortalitya | CVD Mortalitya | Non‐CVD Mortalitya |
|---|---|---|---|---|---|
| FHS samples | |||||
| SRFsb | 0.703 | 0.841 | 0.756 | 0.834 | 0.743 |
| SRF+C22:0/C16:0 ceramide ratio | 0.716 | 0.844 | 0.780c | 0.837 | 0.771c |
| SRF+C24:0/C16:0 ceramide ratio | 0.714 | 0.844 | 0.776c | 0.832 | 0.768c |
| SHIP samples | |||||
| SRFsb | 0.7163 | 0.6691 | 0.8706 | 0.9181 | 0.8537 |
| SRF+C22:0/C16:0 ceramide ratio | 0.7165 | 0.6697 | 0.8754 | 0.9205 | 0.8590 |
| SRF+C24:0/C16:0 ceramide ratio | 0.7216 | 0.6710 | 0.8768c | 0.9224 | 0.8607 |
Data are given as C‐statistics. CHD indicates coronary heart disease; CVD, cardiovascular disease; FHS, Framingham Heart Study; HF, heart failure; SHIP, Study of Health in Pomerania; SRF, standard risk factor.
Prevalent CVD added to SRFs.
Standard risk factors: age, sex, body mass index, systolic blood pressure, antihypertensive medication, current smoking status, diabetes mellitus, total/high‐density lipoprotein cholesterol, triglycerides, and lipid‐lowering medication.
Confidence interval for change in C‐statistic excludes 0.
Discussion
In 2 large community‐based observational studies, we observed higher plasma C24:0/C16:0 ceramide ratios are associated with lower rates of incident CHD and all‐cause mortality over a mean follow‐up of ≈6 years. Meta‐analyses estimated that for every 3‐unit increase in plasma C24:0/C16:0 ratio, there was a 21% lower hazard of developing clinical CHD, a 22% lower hazard of developing clinical HF, and a 40% lower hazard of all‐cause mortality in multivariable adjusted models. We found a similar inverse association for C22:0/C16:0 ceramide ratio and all‐cause mortality. Consistent with these observations, we noted that the C24:0/C16:0 ceramide ratio correlated inversely with several known risk factors for CVD and with prevalent CVD in both FHS and SHIP. Recent lipidomic analyses in case‐control studies demonstrated that the C24:0/C16:0 ratio in individuals with CHD is inversely related to prevalent CHD and CVD death.7, 8 Our investigation provides the first demonstration that the ratio of very‐long‐chain/long‐chain ceramide molecular species in plasma is an independent predictor of CHD and all‐cause mortality risk in the general population.
Our findings were unanticipated, because prior observations that total plasma ceramides are directly associated with CVD risk factors suggested that plasma ceramides reflect changes in lipid metabolism that promote CHD and mortality.14, 15 In addition, recent analysis of subjects in the PREDIMED (Prevention with Mediterranean Diet) trial, who were at high cardiovascular risk, indicated that C24:0, C22:0, and C16:0 ceramides were positively associated with prevalent and incident CVD.16 By contrast, we find the C24:0/C16:0 ceramide ratio is inversely associated with the coronary risk factors of age and smoking status and also inversely associated with prevalent CVD in both FHS and SHIP. Moreover, the C24:0/C16:0 ceramide ratio and C24:0 ceramide are inversely associated with incident CHD and incident HF in our analyses. Differences between our findings and the prior study may relate to differences in mass spectrometry method for quantification of ceramide species. The prior analysis used a broad liquid chromatography/mass spectrometry survey of plasma lipids that has not been validated for the specific C24:0, C22:0, and C16:0 ceramide species and does not provide absolute quantification. Herein, we developed and applied a targeted Food and Drug Administration–compliant assay, which provides absolute quantification of 3 ceramides (including authentic deuterated internal standards for each) with high accuracy and precision and with minimal interbatch variability. These attributes strengthen the conclusions of our analyses over nontargeted analyses. We cannot rule out that inherent differences between study populations (individuals at high cardiovascular risk in the PREDIMED trial versus community‐based sampling in our investigation) could have also contributed.
Our meta‐analysis revealed a strong inverse association of the C24:0/C16:0 and C22:0/C16:0 ratios with all‐cause mortality, even after adjusting for established coronary risk factors, that reflected significant inverse associations in both FHS and SHIP. Inverse association of the ratios with CVD mortality is consistent with inverse associations with coronary risk factors and prevalent CVD. However, reasons for strong inverse associations of the ceramide ratios with non‐CVD mortality will require future investigation. Ceramide ratios may be related to common pathological mechanisms in many disease processes and thus may be markers of overall health. In FHS and in SHIP, both ceramide ratios favorably affected the C‐statistic for all‐cause mortality, providing incremental information after adjustment for standard CVD risk factors. Previous case‐control studies showed that the C24:0/C16:0 ceramide ratio is inversely related to CVD mortality among patients with CHD.7, 8 Our results demonstrate that quantification of plasma C24:0/C16:0 or C22:0/C16:0 ratios provides prognostic information in the general population that includes both men and women free of prevalent CVD and over a broad age range. Furthermore, our observations indicate that lower circulating levels of these very–long‐chain ceramides may antedate the clinical CVD events by several years.
Traditional lipid‐based risk factors for CHD are important pillars of risk prediction, but lack sensitivity, particularly among those at intermediate risk. Addition of the C24:0/C16:0 ceramide ratio to the model fit for mortality has a modest effect (≈3% in FHS) on area under the receiver‐operator characteristic curve, as quantified by the C‐statistic. However, even accepted measures in cardiovascular risk prediction that are widely used in clinical practice confer small changes in the C‐statistic when considered individually, reflecting the relative insensitivity of the C‐statistic as a metric for risk prediction in prospective cohorts of healthy individuals.17, 18 Adding the ceramide ratio as a predictor of mortality in the FHS and SHIP populations compares favorably with the effects of adding high‐sensitivity C‐reactive protein or N‐terminal brain natriuretic peptide to predictive models, both of which are broadly used in clinical settings.19 Moreover, high‐sensitivity C‐reactive protein has been important in motivating new approaches for targeting systemic inflammation to decrease cardiovascular risk.20 Likewise, remodeling of ceramide molecular species has the potential to inform about biological features not captured in traditional risk factors and, thus, potential for identification of novel pathways for targeting treatment.
Changes in the relative distribution of acyl chains among the most abundant plasma ceramides suggest remodeling of the plasma lipidome. The opposite associations for C24:0 and C16:0 ceramides with all‐cause mortality suggest that ceramides of differing acyl chain lengths have distinct biological effects. Overall, the stronger association for the C24:0/C16:0 ratio compared with C22:0/C16:0 ratio suggests greater biological significance of the more abundant very–long‐chain ceramide species. Differences in plasma ceramides likely reflect perturbations in the composition of plasma lipoproteins and could contribute to differential risk for atherosclerosis. They may also reflect changes in the composition and function of cellular membranes within tissues, because in addition to the generation of plasma ceramides by secreted sphingomyelinases, ceramides are also secreted by parenchymal tissues.21, 22 Given observations that ceramides accumulate in the myocardium in the setting of metabolic cardiomyopathy and HF, and given that accumulation of ceramides promotes cardiomyocyte oxidative stress and mitophagy, it is possible that enhanced ability to export very–long‐chain ceramides from tissues underlies the inverse relationships between plasma ratios of C24:0/C16:0 and CHD and mortality.23, 24 Future studies will be required to elucidate the relation of plasma ratios to ceramide content in specific tissues and how this affects the pathogenesis of vascular disease and related outcomes.
Study Limitations
Several limitations of the present study merit consideration. First, although the inverse association of the C24:0/C16:0 ceramide ratio with incident CHD was significant in meta‐analyses and in analysis of SHIP alone (after adjusting for established coronary risk factors), the association was borderline significant in FHS alone. Inverse association with incident HF was observed in FHS but not in SHIP. This association was statistically significant in our meta‐analyses, despite high heterogeneity. Although the definitions of CHD and HF in our analyses of SHIP mirrored those used in FHS, one potential reason for the differences in associations for CHD and HF between the studies may be differences in follow‐up methods (continuous surveillance in FHS versus ascertainment at time of follow‐up examinations in SHIP). Extension of our findings to other cohorts with continuous surveillance could strengthen our conclusions. Second, although ceramide ratios did not change in our control subjects over 2 weeks, this time is brief relative to the period of follow‐up in FHS and SHIP. The stability of ceramide ratios over longer periods remains to be determined, an area for future investigation. Third, future studies will be needed to determine how generalizable our findings in 2 largely white cohorts may be to other races and ethnic groups. Application of our ceramide ratio assay to larger numbers of multiethnic individuals and to samples followed up for longer periods will further elucidate the potential utility of this biomarker for stratification of mortality risk. Finally, our observations indicate that higher plasma C24:0/C16:0 ceramide ratios are associated with decreased risk of several outcomes. Discovery of interventions that increase this ratio will enable determination of the utility of the ceramide ratio as a treatment target for affecting disease risk.
Conclusions
Our robust method to simultaneously quantify the most abundant circulating very–long‐chain and long‐chain ceramides provides predictive information about CHD, HF, and mortality in the general population years before the actual onset of disease. Our findings add to a growing body of evidence that specific ceramide molecular species have disparate biological functions and reflect distinct pathophysiological processes. Approaches that take into account this molecular diversity of sphingolipids are likely to be most effective for risk prediction.
Sources of Funding
This work was supported by the National Institutes of Health (P20 HL113444 and P30 DK020579 to Schaffer; T32 GM074905 to Duncan; and N01‐HL25195 and HHSN268201500001I to Vasan).
Disclosures
A patent application for use of the ceramide biomarkers is pending (Schaffer, Ory, Peterson, Vasan, Xanthakis, Jiang, and Duncan).
Supporting information
Data S1. Supplemental Methods.
Table S1. C16:0, C22:0 and C24:0 Ceramides and C24:0/C16:0 and C22:0/C16:0 Ceramide Ratios in Healthy Volunteers
Table S2. Clinical Correlates of Plasma C22:0/C16:0 Ceramide Ratios in FHS and SHIP
Table S3. Clinical Correlates of Individual Plasma Ceramide Species in FHS and SHIP
Table S4. Incremental Effect of Incorporating hsCRP and ntBNP on Model Discrimination
Figure S1. Generation of FHS samples. From 2812 participants in the Offspring Cohort who attended their eighth examination cycle, 4 participant samples were created based on availability of plasma samples and covariate data. For Sample 1, individuals were excluded if they were missing ceramide values or covariates. For Samples 2 and 3, individuals with prevalent coronary heart disease (CHD, sample 2) and heart failure (HF, sample 3) were excluded. For Sample 4, individuals with missing follow‐up time were excluded.
Figure S2. Generation of SHIP samples. From 3300 participants who attended SHIP‐1, 4 participant samples were created based on availability of plasma samples and covariate data. For Sample A, individuals were excluded if they were missing ceramide values or covariate data. Samples B and C included those who had ceramide and covariate data and also attended SHIP‐2 when CHD and HF were assessed (Sample B excluded those with CHD at SHIP‐1 and Sample C excluded those with HF at SHIP‐1. Samples B and C excluded individuals with uncertain event status at SHIP‐2). Sample D excluded those with missing ceramides or covariates or with unknown death date.
Figure S3. Distributions of plasma ceramides in FHS and SHIP. Plots display distribution of values for C24:0 (A, D), C22:0 (B, E), and C16:0 (C, F) ceramides in women and men FHS participants at examination 8 (A, B, C) and in SHIP participants at SHIP‐1 examination (D, E, F).
Figure S4. Cumulative incidence of coronary heart disease, heart failure, and all‐cause mortality in FHS by tertiles of plasma C22:0/C16:0 ceramide ratio. Cumulative incidence of coronary heart disease (CHD, A), heart failure (HF, B), and all‐cause mortality (C) are reported for tertiles of C22:0/C16:0 ceramide ratio. Tertile 1 includes participants with ceramide levels ≤ the 33rd percentile [1.0, 3.4]; tertile 2 includes participants with ceramide levels between the 33rd and 66th percentile [3.4, 4.1]; tertile 3 includes participants with ceramide levels ≥ the 66th percentile [4.1, 10.5].
Figure S5. Risk of coronary heart disease, heart failure, and all‐cause mortality by C22:0/C16:0 ceramide ratio. Hazard ratios (HR) for coronary heart disease (CHD), heart failure (HF), and all‐cause mortality are reported with 95% confidence intervals (CI) for a 0.7‐unit increase in C22:0/C16:0 ceramide ratio (average of standard deviations between FHS and SHIP), adjusting for all other variables in the model. Data is shown from analysis of subjects in FHS, SHIP and the combined meta‐analysis. I2=0 for CHD; I2=0.81 for HF; I2=0.13 for allcause mortality.
Figure S6. Risk of coronary heart disease, heart failure, and all‐cause mortality by C24:0 ceramide level. Hazard ratios (HR) for coronary heart disease (CHD), heart failure (HF), and all‐cause mortality are reported with 95% confidence intervals (CI) for a 0.65 μg/mL increase in C24:0 ceramide level (average of standard deviations between FHS and SHIP), adjusting for all other variables in the model. Data is shown from analysis of subjects in FHS, SHIP and the combined meta‐analysis. I2<0.0001 for CHD; I2=0.71 for HF; I2=0 for all‐cause mortality.
Figure S7. Risk of coronary heart disease, heart failure, and all‐cause mortality by C16:0 ceramide level. Hazard ratios for coronary heart disease (CHD), heart failure (HF), and all‐cause mortality are reported with 95% confidence intervals (CI) for a 0.045 μg/mL increase in C16:0 ceramide level (average of standard deviations between FHS and SHIP), adjusting for all other variables in the model. Data is shown from analysis of subjects in FHS, SHIP and the combined meta‐analysis. I2=0 for CHD and for HF. I2=0.26 for all‐cause mortality.
Acknowledgments
We thank Eric Novak, MS, for assistance with statistical analyses.
(J Am Heart Assoc. 2018;7:e007931 DOI: 10.1161/JAHA.117.007931.)29728014
Contributor Information
Ramachandran S. Vasan, Email: vasan@bu.edu.
Jean E. Schaffer, Email: jschaff@wustl.edu.
References
- 1. Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta. 2014;1841:671–681. [DOI] [PubMed] [Google Scholar]
- 2. Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid‐, saturated‐fat‐, and obesity‐induced insulin resistance. Cell Metab. 2007;5:167–179. [DOI] [PubMed] [Google Scholar]
- 3. Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chakraborty M, Lou C, Huan C, Kuo MS, Park TS, Cao G, Jiang XC. Myeloid cell‐specific serine palmitoyltransferase subunit 2 haploinsufficiency reduces murine atherosclerosis. J Clin Invest. 2013;123:1784–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Ohman MK, Takeda K, Sugii S, Pewzner‐Jung Y, Futerman AH, Summers SA. CerS2 haploinsufficiency inhibits beta‐oxidation and confers susceptibility to diet‐induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. [DOI] [PubMed] [Google Scholar]
- 6. Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Bronneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Bluher M, Kronke M, Bruning JC. Obesity‐induced CerS6‐dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. [DOI] [PubMed] [Google Scholar]
- 7. Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvanne T, Hurme R, Gouni‐Berthold I, Berthold HK, Kleber ME, Laaksonen R, Marz W. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab. 2014;99:E45–E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laaksonen R, Ekroos K, Sysi‐Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, Marz W, Scharnagl H, Stojakovic T, Vlachopoulou E, Lokki ML, Nieminen MS, Klingenberg R, Matter CM, Hornemann T, Juni P, Rodondi N, Raber L, Windecker S, Gencer B, Pedersen ER, Tell GS, Nygard O, Mach F, Sinisalo J, Luscher TF. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL‐cholesterol. Eur Heart J. 2016;37:1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kannel WB, Wolf PA, Garrison RJ. Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements: Framingham heart study, 30 year follow‐up. Natl Inst Health Pub. 1987. Available at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwjElpvjm-_YAhVHslMKHVHUBm4QFggnMAA&url=https%3A%2F%2Fbiolincc.nhlbi.nih.gov%2Fstatic%2Fstudies%2Fframcohort%2F30-Year_Followup_(Section_34).pdf%3Flink_time%3D2017-11-30_11%3A45%3A55.638362&usg=AOvVaw1_Kk0y_T9fj7bJ1YVFEs5F. Accessed April 4, 2018. [Google Scholar]
- 11. Dorr M, Wolff B, Robinson DM, John U, Ludemann J, Meng W, Felix SB, Volzke H. The association of thyroid function with cardiac mass and left ventricular hypertrophy. J Clin Endocrinol Metab. 2005;90:673–677. [DOI] [PubMed] [Google Scholar]
- 12. Royston P, Lambert PC. Fexible Parametric Survival Analysis Using Stata: Beyond the Cox Model. College Station, TX, USA: Stata Press; 2011. [Google Scholar]
- 13. Jiang H, Hsu FF, Farmer MS, Peterson LR, Schaffer JE, Ory DS, Jiang X. Development and validation of LC‐MS/MS method for determination of very long acyl chain (c22:0 and c24:0) ceramides in human plasma. Anal Bioanal Chem. 2013;405:7357–7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Mello VD, Lankinen M, Schwab U, Kolehmainen M, Lehto S, Seppanen‐Laakso T, Oresic M, Pulkkinen L, Uusitupa M, Erkkila AT. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL‐6 concentration in patients with coronary heart disease. Diabetologia. 2009;52:2612–2615. [DOI] [PubMed] [Google Scholar]
- 16. Wang DD, Toledo E, Hruby A, Rosner BA, Willett WC, Sun Q, Razquin C, Zheng Y, Ruiz‐Canela M, Guasch‐Ferre M, Corella D, Gomez‐Gracia E, Fiol M, Estruch R, Ros E, Lapetra J, Fito M, Aros F, Serra‐Majem L, Lee CH, Clish CB, Liang L, Salas‐Salvado J, Martinez‐Gonzalez MA, Hu FB. Plasma ceramides, Mediterranean diet, and incident cardiovascular disease in the PREDIMED trial (Prevencion con Dieta Mediterranea). Circulation. 2017;135:2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. [DOI] [PubMed] [Google Scholar]
- 18. Ridker PM. C‐reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49:2129–2138. [DOI] [PubMed] [Google Scholar]
- 19. Melander O, Newton‐Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ridker PM. Moving beyond JUPITER: will inhibiting inflammation reduce vascular event rates? Curr Atheroscler Rep. 2013;15:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merrill AH Jr, Lingrell S, Wang E, Nikolova‐Karakashian M, Vales TR, Vance DE. Sphingolipid biosynthesis de novo by rat hepatocytes in culture: ceramide and sphingomyelin are associated with, but not required for, very low density lipoprotein secretion. J Biol Chem. 1995;270:13834–13841. [DOI] [PubMed] [Google Scholar]
- 22. Schissel SL, Schuchman EH, Williams KJ, Tabas I. Zn2+‐stimulated sphingomyelinase is secreted by many cell types and is a product of the acid sphingomyelinase gene. J Biol Chem. 1996;271:18431–18436. [DOI] [PubMed] [Google Scholar]
- 23. Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knoll R, Milting H, Chung CS, Jorde U, Naka Y, Mancini DM, Goldberg IJ, Schulze PC. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Law BA, Liao X, Moore KS, Southard A, Roddy P, Ji R, Szulc Z, Bielawska A, Schulze PC, Cowart LA. Lipotoxic very‐long‐chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 2018;32:1403–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. C16:0, C22:0 and C24:0 Ceramides and C24:0/C16:0 and C22:0/C16:0 Ceramide Ratios in Healthy Volunteers
Table S2. Clinical Correlates of Plasma C22:0/C16:0 Ceramide Ratios in FHS and SHIP
Table S3. Clinical Correlates of Individual Plasma Ceramide Species in FHS and SHIP
Table S4. Incremental Effect of Incorporating hsCRP and ntBNP on Model Discrimination
Figure S1. Generation of FHS samples. From 2812 participants in the Offspring Cohort who attended their eighth examination cycle, 4 participant samples were created based on availability of plasma samples and covariate data. For Sample 1, individuals were excluded if they were missing ceramide values or covariates. For Samples 2 and 3, individuals with prevalent coronary heart disease (CHD, sample 2) and heart failure (HF, sample 3) were excluded. For Sample 4, individuals with missing follow‐up time were excluded.
Figure S2. Generation of SHIP samples. From 3300 participants who attended SHIP‐1, 4 participant samples were created based on availability of plasma samples and covariate data. For Sample A, individuals were excluded if they were missing ceramide values or covariate data. Samples B and C included those who had ceramide and covariate data and also attended SHIP‐2 when CHD and HF were assessed (Sample B excluded those with CHD at SHIP‐1 and Sample C excluded those with HF at SHIP‐1. Samples B and C excluded individuals with uncertain event status at SHIP‐2). Sample D excluded those with missing ceramides or covariates or with unknown death date.
Figure S3. Distributions of plasma ceramides in FHS and SHIP. Plots display distribution of values for C24:0 (A, D), C22:0 (B, E), and C16:0 (C, F) ceramides in women and men FHS participants at examination 8 (A, B, C) and in SHIP participants at SHIP‐1 examination (D, E, F).
Figure S4. Cumulative incidence of coronary heart disease, heart failure, and all‐cause mortality in FHS by tertiles of plasma C22:0/C16:0 ceramide ratio. Cumulative incidence of coronary heart disease (CHD, A), heart failure (HF, B), and all‐cause mortality (C) are reported for tertiles of C22:0/C16:0 ceramide ratio. Tertile 1 includes participants with ceramide levels ≤ the 33rd percentile [1.0, 3.4]; tertile 2 includes participants with ceramide levels between the 33rd and 66th percentile [3.4, 4.1]; tertile 3 includes participants with ceramide levels ≥ the 66th percentile [4.1, 10.5].
Figure S5. Risk of coronary heart disease, heart failure, and all‐cause mortality by C22:0/C16:0 ceramide ratio. Hazard ratios (HR) for coronary heart disease (CHD), heart failure (HF), and all‐cause mortality are reported with 95% confidence intervals (CI) for a 0.7‐unit increase in C22:0/C16:0 ceramide ratio (average of standard deviations between FHS and SHIP), adjusting for all other variables in the model. Data is shown from analysis of subjects in FHS, SHIP and the combined meta‐analysis. I2=0 for CHD; I2=0.81 for HF; I2=0.13 for allcause mortality.
Figure S6. Risk of coronary heart disease, heart failure, and all‐cause mortality by C24:0 ceramide level. Hazard ratios (HR) for coronary heart disease (CHD), heart failure (HF), and all‐cause mortality are reported with 95% confidence intervals (CI) for a 0.65 μg/mL increase in C24:0 ceramide level (average of standard deviations between FHS and SHIP), adjusting for all other variables in the model. Data is shown from analysis of subjects in FHS, SHIP and the combined meta‐analysis. I2<0.0001 for CHD; I2=0.71 for HF; I2=0 for all‐cause mortality.
Figure S7. Risk of coronary heart disease, heart failure, and all‐cause mortality by C16:0 ceramide level. Hazard ratios for coronary heart disease (CHD), heart failure (HF), and all‐cause mortality are reported with 95% confidence intervals (CI) for a 0.045 μg/mL increase in C16:0 ceramide level (average of standard deviations between FHS and SHIP), adjusting for all other variables in the model. Data is shown from analysis of subjects in FHS, SHIP and the combined meta‐analysis. I2=0 for CHD and for HF. I2=0.26 for all‐cause mortality.


