Initially restricted to high‐risk and inoperable patients with symptomatic severe aortic stenosis (AS), transcatheter aortic valve replacement (TAVR) is increasingly being offered to younger and lower‐risk patients with fewer comorbidities who are otherwise good operative candidates and until now would have undergone surgical aortic valve replacement (SAVR). This evolution in clinical practice is underpinned by robust evidence, but do the data support the universal use of TAVR in all patients with AS, or is there still a role for surgery? In this review, we will review the data of TAVR in operable patients, examine potential limitations of TAVR in younger patients, and highlight the areas in which more research is required.
Concepts of Risk Assessment
First, it is imperative to recognize that the widely used risk classification scheme for patients undergoing TAVR (extreme, high, intermediate, or low risk) is an artificial construct, important for the conduct of clinical trials, but not necessarily applicable to everyday clinical practice. The commonly used risk scores (Society of Thoracic Surgeons [STS], EuroSCORE, and EuroSCORE II) are designed to assess the risk of surgery and not TAVR. Although dedicated TAVR risk scores have been proposed, including the US STS/American College of Cardiology Transcatheter Valve Therapies,1 these are not widely used in clinical practice. Surgical risk scores typically overestimate the risk of TAVR. Arbitrarily selected STS thresholds to define risk categories for the pivotal TAVR trials were modified over time and varied between trials. Furthermore, the SAVR patient population in the STS database has changed in recent years, with most high‐risk patients now undergoing TAVR. As a result, individual patients' STS scores have fallen, meaning there is overlap between clinical trial risk‐defined patient populations.2 Second, “low‐risk” does not necessarily mean “young.” For example, it is theoretically possible for a 90‐year‐old man with severe AS and hypertension, but no other comorbidity, to have an STS score <3%, even though most Heart Teams would probably consider his surgical risk to be substantial. Although this example is extreme, it highlights that score‐based risk assessment is just 1 factor to consider when weighing the relative advantages and disadvantages of TAVR or SAVR. In this review, we will focus on those patients who are deemed “operable” by the multidisciplinary Heart Team, analogous to the low‐ and intermediate‐risk cohorts from the pivotal trials.
Clinical Trials in Operable Patients
Figure 1 illustrates the regulatory timeline of TAVR in the United States. TAVR has been proven to be an effective treatment for patients with symptomatic severe AS and high or extreme surgical risk.3, 4, 5 Observational studies in Europe were the first to suggest that TAVR is safe in low‐ and intermediate‐risk patients, with low rates of procedural complications and short‐term mortality,6, 7, 8, 9 although a recent meta‐analysis suggested increased intermediate‐term mortality with TAVR compared with SAVR (relative risk 1.45, 95% confidence interval, 1.11–1.89, P=0.006) with median follow‐up of 2 years.10 More robust data were provided by the NOTION (Nordic Aortic Valve Intervention Trial), which randomized primarily low‐risk patients to SAVR versus TAVR with the self‐expanding CoreValve THV (Medtronic, Minneapolis, MN).11 There were no differences between groups in 2‐year mortality or composite outcome of all‐cause mortality, stroke, or myocardial infarction (Tables 1 and 2). TAVR patients required a more permanent pacemaker (PPM), but had lower life‐threatening bleeding, acute kidney injury, and new‐onset atrial fibrillation.
Figure 1.

Regulatory timeline of TAVR in the US. ESRD indicates end‐stage renal disease; EU, European Union; FDA, US Food and Drug Administration; TAVR, transcatheter aortic valve replacement.
Table 1.
Summary of Key Findings From TAVR Cohorts of Clinical Trials in Low‐ and Intermediate‐Risk Patients
| Type of Transcatheter Heart Valve | PARTNER 212 | SURTAVI14 | NOTION11 | SAPIEN 3 IR13 |
|---|---|---|---|---|
| Edwards Sapien XT | Medtronic CoreValve or Evolut R | Medtronic CoreValve | Edwards Sapien 3 | |
| Time to end point | 30 d | 30 d | 30 d | 30 d |
| All‐cause mortality | 3.9% | 2.2% | 2.1% | 1.1% |
| Disabling stroke | 3.2% | 1.2% | 1.4% | 1.0% |
| Paravalvular leak (≥ moderate) | 3.7% | 3.5%a | 15.3%b | 3.8% |
| Major vascular complications | 7.9% | 6.0% | 5.6%a | 6.1% |
| Major and life‐threatening bleeding | 10.4% | 12.2% | 11.3%a | 4.6% |
| Acute kidney injury (stage 2 or 3) | 1.3% | 1.7% | 0.7%a | 0.5% |
| New permanent pacemaker implantation | 8.5% | 25.9% | 34.1% | 10.2% |
| Time to end point | 2 y | 2 y | 2 y | 1 y |
| All‐cause mortality | 16.7% | 11.4% | 8.0% | 7.4% |
| Disabling stroke | 6.2% | 2.6% | 3.6% | 2.3% |
| Paravalvular leak (≥ moderate) | 5.5% | 5.7% | 15.7% | 1.5% |
| New permanent pacemaker implantation | 11.8% | 25.6% | 41.3% | 12.4% |
NOTION indicates Nordic Aortic Valve Intervention Trial; PARTNER 2, Placement of Aortic Transcatheter Valves; SURTAVI, Surgical Replacement and Transcatheter Aortic Valve Implantation; TAVR, transcatheter aortic valve replacement.
End point at hospital discharge.
End point at 3 months.
Table 2.
Comparison of 30‐Day Outcomes With TAVR Versus SAVR in Clinical Trials of Low‐ and Intermediate‐Risk Patients
| PPM Implantation | Stroke | Moderate or Severe PVL | New Atrial Fibrillation | |||||
|---|---|---|---|---|---|---|---|---|
| TAVR | SAVR | TAVR | SAVR | TAVR | SAVR | TAVR | SAVR | |
| PARTNER 212 | 8.5% | 6.9% | 3.2% | 4.3% | 3.7%a | 0.6%a | 9.1%a | 26.4%a |
| SURTAVI14 | 25.9%a | 6.6%a | 1.2% | 2.5% | 3.5%a | 0.7%a | 12.9%a | 43.4a |
| NOTION11 | 34.1%a | 1.6%a | 1.4% | 3.0% | 15.3%a | 1.8%a | 16.9%a | 57.8%a |
| SAPIEN 3 IR13 | 10.2% | 7.3% | 1.0%a | 4.4%a | 3.8%a | 0.6%a | 3.2%a | 28.5%a |
NOTION indicates Nordic Aortic Valve Intervention Trial; PARTNER 2, Placement of Aortic Transcatheter Valves; PPM, permanent pacemaker; PVL, paravalvular leakage; SAVR, surgical aortic valve replacement; SURTAVI, Surgical Replacement and Transcatheter Aortic Valve Implantation; TAVR, transcatheter aortic valve replacement.
Statistically significant difference.
Results from the first pivotal randomized clinical trial of TAVR in intermediate‐risk patients using the balloon‐expandable Sapien XT transcatheter heart valve (THV) (Edwards Lifesciences, Irvine, CA) were published in 2016.12 TAVR was noninferior to surgery with regard to death and disabling stroke, but there was a higher rate of moderate/severe paravalvular leakage (PVL) with TAVR versus SAVR. Similar to NOTION, TAVR was associated with lower rates of severe kidney injury, severe bleeding, and new‐onset atrial fibrillation. Use of the newer‐generation Sapien 3 THV in intermediate‐risk patients may be associated with even better results. Data from the registry arm of the PARTNER 2 (Placement of Aortic Transcatheter Valves) study comparing TAVR with the Sapien 3 THV with a propensity‐matched surgical cohort demonstrated both noninferiority and superiority of TAVR versus SAVR for the composite outcome of death, stroke, and moderate/severe aortic regurgitation, with overall low complication rates using this newer THV (Table 1).13 Comparable findings were revealed in the SURTAVI (Surgical Replacement and Transcatheter Aortic Valve Implantation) trial using the self‐expanding CoreValve or Evolut R THV in intermediate‐risk patients.14 At 2 years, there was no difference in the composite outcome of all‐cause death or disabling stroke. As with the balloon‐expandable valves, the PPM implantation rate was higher with TAVR, but new‐onset atrial fibrillation, significant bleeding, and acute kidney injury rates were lower (Table 1).
In addition, it is important to appreciate that most subjects were elderly in the clinical trials of intermediate‐risk patients, with mean age 79 to 82 years. Therefore, the results cannot necessarily be extrapolated to younger patients.
Potential Limitations of TAVR in Operable Patients
Because TAVR was initially reserved for extreme‐ and high‐risk patients with advanced age and multiple comorbidities, very few patients lived long enough to test the lifespan of their bioprosthetic THV. So far, 5‐year THV hemodynamic data appear comparable to surgical bioprostheses with no evidence of increased early structural valve deterioration.15, 16, 17 Nonetheless, long‐term THV durability remains unknown, which tempers widespread adoption of TAVR in younger patients. Mechanisms to explain the recent observation that THV appear more susceptible to subclinical leaflet thrombosis compared with surgical bioprostheses are still unclear.18, 19 The first comprehensive data on durability will likely come from long‐term follow‐up of patients from the intermediate‐risk PARTNER 2 and SURTAVI trials. Additionally, the randomized US clinical trials in low‐risk patients will follow subjects for 10 years with yearly echocardiography to assess for structural valve deterioration, and all include a subgroup of patients undergoing 4‐dimensional contrast‐enhanced cardiac computed tomography to assess for leaflet thrombosis and restricted leaflet motion. In the meantime, as more patients undergo TAVR, THV failure will become more common. TAVR‐in‐TAVR has been shown to be safe, with comparable short‐ and midterm clinical and hemodynamic outcomes to valve‐in‐valve TAVR for failed surgical bioprostheses.20, 21
Over the past decade, the morbidity associated with the first‐generation TAVR valves has dramatically improved. The increased risk of periprocedural stroke with TAVR compared with SAVR was a concern in high‐risk patients. However, with device and procedural improvements, the rate of disabling stroke was consistently lower with TAVR versus SAVR in intermediate‐risk patients (Table 2). Additionally, the rate of new‐onset atrial fibrillation was significantly lower with TAVR versus SAVR in all of the clinical trials (Table 2). In the TAVR cohort of the NOTION study using the first‐generation CoreValve self‐expanding THV, the rate of clinically relevant PVL at 30 days, defined as moderate or severe by echocardiography, was 15.3%. The PARTNER 2 and SAPIEN 3 IR studies using balloon‐expandable THV (Edwards Sapien XT or Sapien 3) demonstrated much lower rates of significant PVL under 4%, as did the SURTAVI trial using self‐expanding THV (CoreValve or Evolut R) (Table 1). The Sapien 3 valve features a sealing skirt, and the latest‐generation CoreValve Evolut PRO also incorporates a pericardial tissue wrap specifically designed to reduce PVL. However, the need for new PPM implantation remains the Achilles’ heel of TAVR, with 30‐day PPM rates of ≈10% for balloon‐expandable THV13 and ≈25% for self‐expanding THV14 (Table 1). In the SURTAVI trial, there was no difference in PPM rate between the CoreValve and Evolut R THV (25.5% versus 26.7%, respectively), although only 16% of patients in the study received the newer Evolut R THV. This compares with PPM implantation rates after SAVR of 1.6% to 7.3% in these same studies. Strategies for high implantation, accurate computed tomography–based sizing, and newer TAVR devices may reduce the rates of PPM implantation in the future. The long‐term consequences of the need for a PPM following either TAVR or SAVR are unknown.
Do Clinical Trials of TAVR in Low‐Risk Patients Herald the End of Isolated SAVR?
So long as TAVR was restricted for use only in high‐ and extreme‐risk patients, there remained a clear role for surgery for the many more patients with symptomatic severe AS who were operable. However, data from PARTNER 2 and SURTAVI have convincingly demonstrated that TAVR is noninferior to SAVR in intermediate‐risk patients and, if performed via transfemoral access, is associated with improved early health status improvements compared with SAVR.22 Thus, in 2018, it is reasonable to favor TAVR in all operable patients with symptomatic severe AS and increased surgical risk.
Table 3 summarizes the study design of currently enrolling randomized clinical trials of TAVR in low‐risk patients.23, 24, 25, 26 The first results from these studies are expected in 2018. Assuming these results demonstrate noninferiority of TAVR versus SAVR, in the future it will likely be reasonable to consider TAVR in all patients with symptomatic severe AS, regardless of operative risk (Figure 2). If this is the case, then we wholeheartedly support continued Heart Team collaboration between interventional cardiologists and cardiothoracic surgeons, although the requirement for 2 surgeons to evaluate each patient for operative risk before TAVR would become obsolete. Availability of TAVR for all, regardless of operative risk, would also allow more opportunity for patient preference to direct decision‐making. Indeed, many low‐risk patients may prefer TAVR over SAVR for personal reasons that current guidelines and indications cannot encompass.
Table 3.
Ongoing Clinical Trials in Low‐Risk Patients
| Name | Unique Identifier | Population | Study Design | Primary End Point | THV in TAVR Arm | Sample Size |
|---|---|---|---|---|---|---|
| LRT23 | NCT02628899 |
No age restriction STS ≤3% |
Feasibility study Prospective TAVR arm with historical SAVR controls |
All‐cause mortality at 30 d |
Transfemoral SAPIEN 3 or Evolut R/PRO |
200 TAVR in main arm Up to 100 TAVR in bicuspid arm |
| PARTNER 324 | NCT02675114 |
Age ≥65 y STS <4% |
Noninferiority Randomized TAVR vs SAVR |
All‐cause mortality, all stroke, and rehospitalization at 1 y |
Transfemoral SAPIEN 3 |
614 TAVR 614 SAVR |
| Medtronic TAVR in low risk patients25 | NCT02701283 |
No age restriction STS <3% |
Noninferiority Randomized TAVR vs SAVR |
All‐cause mortality or disabling stroke at 2 y | Transfemoral or subclavian Evolut R |
625 TAVR 625 SAVR |
| NOTION 226 | NCT02825134 |
Age 18 to 75 y STS <4% |
Noninferiority Randomized TAVR vs SAVR |
Composite rate of all‐cause mortality, myocardial infarction and stroke at 1 y |
Transfemoral Any CE‐approved THV |
496 TAVR 496 SAVR |
CE indicates Conformité Européene; NOTION, Nordic Aortic Valve Intervention Trial; PARTNER 2, Placement of Aortic Transcatheter Valves; SAVR, surgical aortic valve replacement; STS, Society of Thoracic Surgeons; TAVR, transcatheter aortic valve replacement; THV, transcatheter heart valve.
Figure 2.
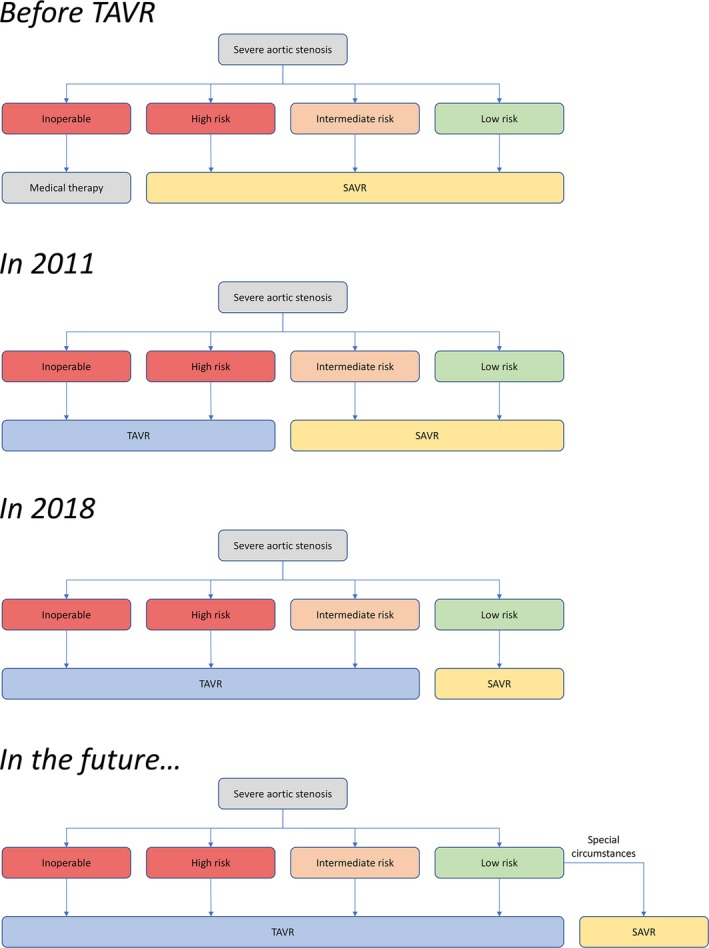
Evolution of the treatment algorithm for patients with aortic stenosis in the United States. SAVR indicates surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
However, risk assessment is but 1 element of the decision‐making process. Special circumstances may still favor SAVR, namely, patient life expectancy, patients' preference for a mechanical valve, aortic valve and root anatomy, available vascular access, and comorbidities (Table 4).
Table 4.
Indications for SAVR in Operable Patients
| Indications |
|---|
| 1. Young patient requiring a mechanical valve |
| 2. Bicuspid aortic stenosis with dilation of the ascending aorta |
| 3. Very large aortic annulus |
| 4. Patients ineligible for transfemoral access |
| 5. Aortic stenosis with multivessel coronary artery disease |
SAVR indicates surgical aortic valve replacement.
Young Patients Requiring a Mechanical Valve
The 2017 American College of Cardiology/American Heart Association focused guideline update on the management of patients with valvular heart disease lowered the age cutoff above which a bioprosthetic valve is reasonable to 50 years with a Class IIa recommendation.27 In patients under the age of 50 years, the guideline still recommends a mechanical valve unless the patient has a clear contraindication to anticoagulation. There is nonetheless a trend toward increased use of bioprosthetic surgical valves even in patients under the age of 50 years,28 often driven by patient preference and desire to avoid long‐term anticoagulation. Availability of newer mechanical valves that can safely be maintained with lower‐dose warfarin (target INR 1.5–2.0) and low‐dose aspirin29 may convince more patients to select a mechanical prosthesis. Conversely, patients and physicians may feel more comfortable selecting a bioprosthesis because valve‐in‐valve TAVR is now an option if a surgical bioprosthesis fails in the future.21 Irrespective of whether a mechanical valve or bioprosthesis is selected, aortic root enlargement surgery should be considered in patients with a small aortic annulus to prevent patient–prosthesis mismatch and facilitate TAVR valve‐in‐valve for the future.
Bicuspid Aortic Stenosis
The prevalence of a bicuspid aortic valve in the general population is 1% to 2%, with a 2:1 male‐to‐female ratio.30, 31 The specific genetic locus and protein abnormality in patients with a bicuspid aortic valve have not yet been identified; however, the tissue abnormality is not confined to the valve leaflets and these patients are at increased risk of aortic aneurysm and dissection. Although most cases of bicuspid aortic valve are sporadic, familial clusters have been identified. The high incidence of familial clustering is suggestive of autosomal‐dominant inheritance with reduced penetrance.32
Nearly all patients with a bicuspid aortic valve will require valve surgery during their lifetime. A study of excised aortic valves revealed bicuspid morphology in 62% of patients aged 50 to 70 years undergoing isolated SAVR before the advent of TAVR.33 Bicuspid aortic valves are more prone to calcific degeneration leading to stenosis at a young age, and therefore, patients with bicuspid AS are likely to fall into low‐ and intermediate‐risk categories. Computed tomography analysis of aortic annulus and valve morphology demonstrates more eccentric annular calcification in bicuspid versus tricuspid valves (68% versus 32%).34 Consequently, TAVR could theoretically be associated with increased risk in patients with bicuspid versus tricuspid valves. First, the rate of moderate or severe PVL could be higher. Observational studies have reported PVL rates in bicuspid valves to range from 9.6% to 28.4%.35, 36 However, careful valve sizing using cardiac computed tomography angiography can reduce the incidence of PVL substantially.36 Use of newer‐generation valves that incorporate features to reduce PVL may be particularly advantageous in the setting of bicuspid AS. Second, the rate of valve embolization could be higher, although the same observational studies suggest that embolization with conversion to open chest surgery occurs rarely in bicuspid AS (2.2–4.0%).36, 37 Third, the rate of prosthesis–patient mismatch could be higher because the abnormal aortic valve morphology could prevent full expansion of the transcatheter valve.38 Fourth, the rate of PPM implantation could be higher, although a German TAVR registry analysis actually reported lower rates in patients with bicuspid versus tricuspid valves (17% versus 35%).39
All of the pivotal randomized clinical trials comparing TAVR and SAVR excluded patients with bicuspid aortic valves. However, observational studies suggest that TAVR may be safe and effective in this setting. A multicenter registry of 108 patients reported 30‐day and 1‐year mortality rates of 8.3% and 16.9%, respectively.35 An analysis of 139 low‐ and intermediate‐risk patients (mean STS score 4.9±3.4%) in 12 European centers corroborated these findings with 1‐year mortality of 17.5%.36 Another multicenter observational study comparing TAVR in patients with bicuspid versus tricuspid aortic valves did not demonstrate any difference in 30‐day mortality.40 A comparison of outcomes in high‐risk patients with bicuspid versus tricuspid aortic valves from the German national TAVR registry also demonstrated no difference in 1‐year mortality.39 A recent multicenter observational study of 51 patients in Canada and Europe with bicuspid aortic valve undergoing TAVR with the latest‐generation Edwards Sapien 3 valve reported promising results with low 30‐day mortality (3.9%) and no clinically significant PVL. These results suggest that the latest‐generation balloon‐expandable THV may achieve superior hemodynamic results compared with earlier‐generation devices. However, the rate of new PPM implantation was high at 23.5%.41 Further data on the safety and feasibility of TAVR in low‐risk patients with bicuspid AS will be provided by the LRT (Low Risk TAVR) study, which includes a separate bicuspid registry arm.23
Regardless of these promising results, up to 80% of adult patients with bicuspid AS have concomitant dilation of the ascending aorta, and half of these patients meet criteria for surgical repair.42, 43 According to current guidelines, patients with ascending aorta diameter ≥4.5 cm should undergo surgical repair at the time of aortic valve replacement for bicuspid aortic valve pathology,44 and therefore TAVR is probably inappropriate. However, in patients with ascending aorta <4.5 cm in diameter, the best strategy is to review historical imaging of the aorta to assess the rate of dilation. Rapid progression may push toward SAVR and ascending aortic repair, whereas slowing or absence of progression over preceding years may reassure that TAVR is a reasonable strategy.
Very Large Aortic Annulus
Commercially available THV are indicated for aortic annuli measuring up to ≈30 mm in diameter (maximum area 683 mm2 for the 29‐mm Sapien 3; maximum perimeter 94.2 mm for the 34 mm Evolut R). Case reports and a small series support the safety and feasibility of overexpansion of the 29‐mm Sapien 3 THV up to a maximum annulus area of 800 mm2, which roughly corresponds to an annulus diameter of 32 mm.45, 46 However, the effect of overexpansion on THV leaflet function and long‐term durability is not known. The data for CoreValve THV in large annuli are less favorable with high rates of implantation of a second valve.47 Therefore, at present, SAVR should still be considered in patients with a very large annulus.
Patients Ineligible for Transfemoral Access
In the PARTNER 1 study in high‐risk patients, transthoracic access was an independent predictor of 2‐year all‐cause mortality with hazard ratio 1.52 (95% confidence interval, 1.12–2.07), P=0.008.48 Similar findings were observed in the PARTNER 2 study in intermediate‐risk patients, with hazard ratio 1.55 (95% confidence interval, 1.23–1.96), P<0.001.12 A much smaller prospective randomized trial of transapical TAVR versus SAVR in operable patients (the STACCATO trial) was stopped early because of a higher complication rate in the transapical TAVR arm, specifically death and stroke.49 Furthermore, health status improvements using the Kansas City Cardiomyopathy Questionnaire at 30 days in the PARTNER 2 study were greater with TAVR compared with SAVR, but only in patients who underwent transfemoral TAVR.22 Those who underwent transthoracic TAVR (transapical or transaortic) did not show any early health status improvement benefit over surgery. Transfemoral TAVR has been shown to be more cost effective than transthoracic TAVR in high‐risk patients50 and was recently demonstrated to be more cost effective than SAVR in intermediate‐risk patients (Cohen DJ, Meeting Presentation, Transcatheter Cardiovascular Therapeutics, 2017). Combined, these data suggest that there is likely to be little benefit of TAVR over SAVR in operable patients who are ineligible for transfemoral access because of small or diseased iliofemoral arteries. However, newer options for percutaneous or surgical minimally invasive alternate access, such as transcaval,51 subclavian,52 transaxillary,53 or carotid,54 have been shown to be safe and appear to avoid the morbidity of transthoracic access, although none have been compared with transthoracic access in a randomized trial. Some can be performed with patients under conscious sedation, allowing rapid ambulation after TAVR and shorter hospital length of stay. Further studies are needed to determine whether TAVR via these newer alternate access approaches confers benefit over SAVR in operable patients. Indeed, all of the ongoing clinical trials in low‐risk patients (Table 3) mandate transfemoral access exclusively and therefore will not provide any new information on alternate access in low‐risk patients.
Multivessel Coronary Artery Disease
Patients with unrevascularized multivessel coronary artery disease were excluded from all of the pivotal TAVR trials, including from ongoing trials in low‐risk patients. The Interventional Section Leadership Council of the American College of Cardiology recommends that patients undergo limited percutaneous coronary intervention (PCI) to proximal coronary stenoses before TAVR55 even though this indication is not consistent with current PCI guidelines. It is important to recognize that this recommendation is based on expert consensus in the absence of randomized clinical trial data. However, a number of studies are under way to address this paucity of data. The ACTIVATION (percutaneous coronary intervention prior to transcatheter aortic valve implantation) study is a randomized trial of PCI versus no PCI before TAVR.56 The FAITAVI (Functional assessment in TAVI) study, a randomized trial of fractional flow reserve versus angiography‐guided PCI before TAVR, aims to explore the role of invasive physiological assessment of coronary stenoses to guide revascularization in patients with AS.57
Conclusions
In summary, the use of TAVR in intermediate‐risk patients with both balloon‐expandable and self‐expanding THV is supported by robust data from multiple randomized clinical trials. Comparable data in low‐risk patients are not yet available because the pivotal trials are ongoing. Nonetheless, the balance of evidence in operable patients is certainly leaning toward the use of TAVR as the preferred strategy for most patients with symptomatic severe AS. Choice of strategy should continue to be personalized based on individual patient demographics, aortic valve and root anatomy, available vascular access, and relevant comorbidities.
Disclosures
Rogers reports consulting for Medtronic. Thourani reports consulting for Abbott Vascular, Boston Scientific, Claret Medical, Edwards Lifesciences, JenaValve, and Gore Medical. Waksman reports consulting for Abbott Vascular, Biosensors International, Biotronik, Boston Scientific, Medtronic Vascular, Symetis, Lifetech; Speakers Bureau: AstraZeneca, Boston Scientific, Biotronik, Abbott Vascular; grant support from Biosensors International, Biotronik, Boston Scientific, Edwards Lifesciences, and Abbott Vascular.
J Am Heart Assoc. 2018;7:e007147 DOI: 10.1161/JAHA.117.007147.29754127
References
- 1. Edwards FH, Cohen DJ, O'Brien SM, Peterson ED, Mack MJ, Shahian DM, Grover FL, Tuzcu EM, Thourani VH, Carroll J, Brennan JM, Brindis RG, Rumsfeld J, Holmes DR Jr; Steering Committee of the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy R . Development and validation of a risk prediction model for in‐hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016;1:46–52. [DOI] [PubMed] [Google Scholar]
- 2. Rogers T, Koifman E, Patel N, Gai J, Torguson R, Corso P, Waksman R. Society of Thoracic Surgeons score variance results in risk reclassification of patients undergoing transcatheter aortic valve replacement. JAMA Cardiol. 2017;2:455–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; Investigators PT . Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 4. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ; Investigators PT . Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med. 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 5. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK; Investigators USCC . Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med. 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 6. Lange R, Bleiziffer S, Mazzitelli D, Elhmidi Y, Opitz A, Krane M, Deutsch MA, Ruge H, Brockmann G, Voss B, Schreiber C, Tassani P, Piazza N. Improvements in transcatheter aortic valve implantation outcomes in lower surgical risk patients: a glimpse into the future. J Am Coll Cardiol. 2012;59:280–287. [DOI] [PubMed] [Google Scholar]
- 7. Wenaweser P, Stortecky S, Schwander S, Heg D, Huber C, Pilgrim T, Gloekler S, O'Sullivan CJ, Meier B, Juni P, Carrel T, Windecker S. Clinical outcomes of patients with estimated low or intermediate surgical risk undergoing transcatheter aortic valve implantation. Eur Heart J. 2013;34:1894–1905. [DOI] [PubMed] [Google Scholar]
- 8. Tamburino C, Barbanti M, D'Errigo P, Ranucci M, Onorati F, Covello RD, Santini F, Rosato S, Santoro G, Fusco D, Grossi C, Seccareccia F; Group OR . 1‐year outcomes after transfemoral transcatheter or surgical aortic valve replacement: results from the Italian OBSERVANT Study. J Am Coll Cardiol. 2015;66:804–812. [DOI] [PubMed] [Google Scholar]
- 9. Schymik G, Heimeshoff M, Bramlage P, Herbinger T, Wurth A, Pilz L, Schymik JS, Wondraschek R, Suselbeck T, Gerhardus J, Luik A, Gonska BD, Tzamalis P, Posival H, Schmitt C, Schrofel H. A comparison of transcatheter aortic valve implantation and surgical aortic valve replacement in 1,141 patients with severe symptomatic aortic stenosis and less than high risk. Catheter Cardiovasc Interv. 2015;86:738–744. [DOI] [PubMed] [Google Scholar]
- 10. Witberg G, Lador A, Yahav D, Kornowski R. Transcatheter versus surgical aortic valve replacement in patients at low surgical risk: a meta‐analysis of randomized trials and propensity score matched observational studies. Catheter Cardiovasc Interv. 2018. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1002/ccd.27518. Accessed May 7, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Thyregod HG, Steinbruchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrom T, Clemmensen P, Hansen PB, Andersen LW, Olsen PS, Sondergaard L. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1‐year results from the all‐comers NOTION randomized clinical trial. J Am Coll Cardiol. 2015;65:2184–2194. [DOI] [PubMed] [Google Scholar]
- 12. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; Investigators P . Transcatheter or surgical aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 13. Thourani VH, Kodali S, Makkar RR, Herrmann HC, Williams M, Babaliaros V, Smalling R, Lim S, Malaisrie SC, Kapadia S, Szeto WY, Greason KL, Kereiakes D, Ailawadi G, Whisenant BK, Devireddy C, Leipsic J, Hahn RT, Pibarot P, Weissman NJ, Jaber WA, Cohen DJ, Suri R, Tuzcu EM, Svensson LG, Webb JG, Moses JW, Mack MJ, Miller DC, Smith CR, Alu MC, Parvataneni R, D'Agostino RB Jr, Leon MB. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate‐risk patients: a propensity score analysis. Lancet. 2016;387:2218–2225. [DOI] [PubMed] [Google Scholar]
- 14. Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP; Investigators S . Surgical or transcatheter aortic‐valve replacement in intermediate‐risk patients. N Engl J Med. 2017;376:1321–1331. [DOI] [PubMed] [Google Scholar]
- 15. Mack MJ, Leon MB, Smith CR, Miller DC, Moses JW, Tuzcu EM, Webb JG, Douglas PS, Anderson WN, Blackstone EH, Kodali SK, Makkar RR, Fontana GP, Kapadia S, Bavaria J, Hahn RT, Thourani VH, Babaliaros V, Pichard A, Herrmann HC, Brown DL, Williams M, Akin J, Davidson MJ, Svensson LG; PARTNER 1 trial investigators . 5‐year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2477–2484. [DOI] [PubMed] [Google Scholar]
- 16. Barbanti M, Petronio AS, Ettori F, Latib A, Bedogni F, De Marco F, Poli A, Boschetti C, De Carlo M, Fiorina C, Colombo A, Brambilla N, Bruschi G, Martina P, Pandolfi C, Giannini C, Curello S, Sgroi C, Gulino S, Patane M, Ohno Y, Tamburino C, Attizzani GF, Imme S, Gentili A, Tamburino C. 5‐year outcomes after transcatheter aortic valve implantation with corevalve prosthesis. JACC Cardiovasc Interv. 2015;8:1084–1091. [DOI] [PubMed] [Google Scholar]
- 17. Daubert MA, Weissman NJ, Hahn RT, Pibarot P, Parvataneni R, Mack MJ, Svensson LG, Gopal D, Kapadia S, Siegel RJ, Kodali SK, Szeto WY, Makkar R, Leon MB, Douglas PS. Long‐term valve performance of TAVR and SAVR: a report from the PARTNER I trial. JACC Cardiovasc Imaging. 2017;10:15–25. [DOI] [PubMed] [Google Scholar]
- 18. Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, De Backer O, Asch FM, Ruiz CE, Olsen NT, Trento A, Friedman J, Berman D, Cheng W, Kashif M, Jelnin V, Kliger CA, Guo H, Pichard AD, Weissman NJ, Kapadia S, Manasse E, Bhatt DL, Leon MB, Sondergaard L. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. 2015;373:2015–2024. [DOI] [PubMed] [Google Scholar]
- 19. Chakravarty T, Sondergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, Jilaihawi H, Shiota T, Abramowitz Y, Jorgensen TH, Rami T, Israr S, Fontana G, de Knegt M, Fuchs A, Lyden P, Trento A, Bhatt DL, Leon MB, Makkar RR; RESOLVE; SAVORY Investigators . Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. 2017;389:2383–2392. [DOI] [PubMed] [Google Scholar]
- 20. Barbanti M, Webb JG, Tamburino C, Van Mieghem NM, Makkar RR, Piazza N, Latib A, Sinning JM, Won‐Keun K, Bleiziffer S, Bedogni F, Kapadia S, Tchetche D, Rodes‐Cabau J, Fiorina C, Nombela‐Franco L, De Marco F, de Jaegere PP, Chakravarty T, Vaquerizo B, Colombo A, Svensson L, Lange R, Nickenig G, Mollmann H, Walther T, Della Rosa F, Elhmidi Y, Dvir D, Brambilla N, Imme S, Sgroi C, Gulino S, Todaro D, Pilato G, Petronio AS, Tamburino C. Outcomes of redo transcatheter aortic valve replacement for the treatment of postprocedural and late occurrence of paravalvular regurgitation and transcatheter valve failure. Circ Cardiovasc Interv. 2016;9:e003930. [DOI] [PubMed] [Google Scholar]
- 21. Dvir D, Webb JG, Bleiziffer S, Pasic M, Waksman R, Kodali S, Barbanti M, Latib A, Schaefer U, Rodes‐Cabau J, Treede H, Piazza N, Hildick‐Smith D, Himbert D, Walther T, Hengstenberg C, Nissen H, Bekeredjian R, Presbitero P, Ferrari E, Segev A, de Weger A, Windecker S, Moat NE, Napodano M, Wilbring M, Cerillo AG, Brecker S, Tchetche D, Lefevre T, De Marco F, Fiorina C, Petronio AS, Teles RC, Testa L, Laborde JC, Leon MB, Kornowski R; Valve‐in‐Valve International Data Registry I . Transcatheter aortic valve implantation in failed bioprosthetic surgical valves. JAMA. 2014;312:162–170. [DOI] [PubMed] [Google Scholar]
- 22. Baron SJ, Arnold SV, Wang K, Magnuson EA, Chinnakondepali K, Makkar R, Herrmann HC, Kodali S, Thourani VH, Kapadia S, Svensson L, Brown DL, Mack MJ, Smith CR, Leon MB, Cohen DJ; PARTNER 2 Investigators . Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rogers T, Torguson R, Bastian R, Corso P, Waksman R. Feasibility of transcatheter aortic valve replacement in low‐risk patients with symptomatic severe aortic stenosis: rationale and design of the Low Risk TAVR (LRT) study. Am Heart J. 2017;189:103–109. [DOI] [PubMed] [Google Scholar]
- 24. The safety and effectiveness of the SAPIEN 3 transcatheter heart valve in low risk patients with aortic stenosis (PARTNER 3). https://clinicaltrials.Gov/ct2/show/nct02675114. Accessed October 10, 2016.
- 25. Medtronic transcatheter aortic valve replacement in low risk patients. https://clinicaltrials.Gov/ct2/show/nct02701283. Accessed October 10, 2016.
- 26. Comparison of transcatheter versus surgical aortic valve replacement in younger low surgical risk patients with severe aortic stenosis (NOTION‐2). https://clinicaltrials.Gov/ct2/show/nct02825134. Accessed October 10, 2016.
- 27. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM III, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 28. Head SJ, Celik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017;38:2183–2191. [DOI] [PubMed] [Google Scholar]
- 29. Puskas J, Gerdisch M, Nichols D, Quinn R, Anderson C, Rhenman B, Fermin L, McGrath M, Kong B, Hughes C, Sethi G, Wait M, Martin T, Graeve A; PROACT Investigators . Reduced anticoagulation after mechanical aortic valve replacement: interim results from the prospective randomized on‐X valve anticoagulation clinical trial randomized Food and Drug Administration investigational device exemption trial. J Thorac Cardiovasc Surg. 2014;147:1202–1210; discussion 1210‐1201 [DOI] [PubMed] [Google Scholar]
- 30. Ward C. Clinical significance of the bicuspid aortic valve. Heart. 2000;83:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Williams DS. Bicuspid aortic valve. J Insur Med. 2006;38:72–74. [PubMed] [Google Scholar]
- 32. Clementi M, Notari L, Borghi A, Tenconi R. Familial congenital bicuspid aortic valve: a disorder of uncertain inheritance. Am J Med Genet. 1996;62:336–338. [DOI] [PubMed] [Google Scholar]
- 33. Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation. 2005;111:920–925. [DOI] [PubMed] [Google Scholar]
- 34. Philip F, Faza NN, Schoenhagen P, Desai MY, Tuzcu EM, Svensson LG, Kapadia SR. Aortic annulus and root characteristics in severe aortic stenosis due to bicuspid aortic valve and tricuspid aortic valves: implications for transcatheter aortic valve therapies. Catheter Cardiovasc Interv. 2015;86:E88–E98. [DOI] [PubMed] [Google Scholar]
- 35. Yousef A, Simard T, Webb J, Rodes‐Cabau J, Costopoulos C, Kochman J, Hernandez‐Garcia JM, Chiam PT, Welsh RC, Wijeysundera HC, Garcia E, Ribeiro HB, Latib A, Huczek Z, Shanks M, Testa L, Farkouh ME, Dvir D, Velianou JL, Lam BK, Pourdjabbar A, Glover C, Hibbert B, Labinaz M. Transcatheter aortic valve implantation in patients with bicuspid aortic valve: a patient level multi‐center analysis. Int J Cardiol. 2015;189:282–288. [DOI] [PubMed] [Google Scholar]
- 36. Mylotte D, Lefevre T, Sondergaard L, Watanabe Y, Modine T, Dvir D, Bosmans J, Tchetche D, Kornowski R, Sinning JM, Theriault‐Lauzier P, O'Sullivan CJ, Barbanti M, Debry N, Buithieu J, Codner P, Dorfmeister M, Martucci G, Nickenig G, Wenaweser P, Tamburino C, Grube E, Webb JG, Windecker S, Lange R, Piazza N. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol. 2014;64:2330–2339. [DOI] [PubMed] [Google Scholar]
- 37. Kochman J, Huczek Z, Scislo P, Dabrowski M, Chmielak Z, Szymanski P, Witkowski A, Parma R, Ochala A, Chodor P, Wilczek K, Reczuch KW, Kubler P, Rymuza B, Koltowski L, Scibisz A, Wilimski R, Grube E, Opolski G. Comparison of one‐ and 12‐month outcomes of transcatheter aortic valve replacement in patients with severely stenotic bicuspid versus tricuspid aortic valves (results from a multicenter registry). Am J Cardiol. 2014;114:757–762. [DOI] [PubMed] [Google Scholar]
- 38. Hamdan A, Kornowski R. Transcatheter aortic valve implantation for bicuspid aortic valve stenosis. Catheter Cardiovasc Interv. 2015;86:331–333. [DOI] [PubMed] [Google Scholar]
- 39. Bauer T, Linke A, Sievert H, Kahlert P, Hambrecht R, Nickenig G, Hauptmann KE, Sack S, Gerckens U, Schneider S, Zeymer U, Zahn R. Comparison of the effectiveness of transcatheter aortic valve implantation in patients with stenotic bicuspid versus tricuspid aortic valves (from the German TAVI Registry). Am J Cardiol. 2014;113:518–521. [DOI] [PubMed] [Google Scholar]
- 40. Phan K, Wong S, Phan S, Ha H, Qian P, Yan TD. Transcatheter aortic valve implantation (TAVI) in patients with bicuspid aortic valve stenosis—systematic review and meta‐analysis. Heart Lung Circ. 2015;24:649–659. [DOI] [PubMed] [Google Scholar]
- 41. Perlman GY, Blanke P, Dvir D, Pache G, Modine T, Barbanti M, Holy EW, Treede H, Ruile P, Neumann FJ, Gandolfo C, Saia F, Tamburino C, Mak G, Thompson C, Wood D, Leipsic J, Webb JG. Bicuspid aortic valve stenosis: favorable early outcomes with a next‐generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv. 2016;9:817–824. [DOI] [PubMed] [Google Scholar]
- 42. Della Corte A, Bancone C, Quarto C, Dialetto G, Covino FE, Scardone M, Caianiello G, Cotrufo M. Predictors of ascending aortic dilatation with bicuspid aortic valve: a wide spectrum of disease expression. Eur J Cardiothorac Surg. 2007;31:397–404; discussion 404‐395 [DOI] [PubMed] [Google Scholar]
- 43. Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–1929. [DOI] [PubMed] [Google Scholar]
- 44. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD; ACC/AHA Task Force Members . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:e521–e643. [DOI] [PubMed] [Google Scholar]
- 45. Mathur M, McCabe JM, Aldea G, Pal J, Don CW. Overexpansion of the 29 mm SAPIEN 3 transcatheter heart valve in patients with large aortic annuli (area > 683 mm(2)): a case series. Catheter Cardiovasc Interv. 2017. Available at: https://onlinelibrary.wiley.com/doi/10.1002/ccd.27190. Accessed May 7, 2018. [DOI] [PubMed] [Google Scholar]
- 46. Barr P, Ormiston J, Stewart J, Nand P, Ramanathan T, Webster M. Transcatheter aortic valve implantation in patients with a large aortic annulus. Heart Lung Circ. 2018;27:e11–e14. [DOI] [PubMed] [Google Scholar]
- 47. Attizzani GF, Ohno Y, Latib A, Petronio AS, Giannini C, Ettori F, Curello S, Bedogni F, Todaro D, Brambilla N, Bruschi G, Colombo P, Presbitero P, Fiorilli R, Poli A, Martina P, Colombo A, Barbanti M, Tamburino C. Acute and long‐term (2‐years) clinical outcomes of the CoreValve 31 mm in large aortic annuli: a multicenter study. Int J Cardiol. 2017;227:543–549. [DOI] [PubMed] [Google Scholar]
- 48. Elmariah S, Fearon WF, Inglessis I, Vlahakes GJ, Lindman BR, Alu MC, Crowley A, Kodali S, Leon MB, Svensson L, Pibarot P, Hahn RT, Thourani VH, Palacios IF, Miller DC, Douglas PS, Passeri JJ; PARTNER Trial Investigators and PARTNER Publications Office . Transapical transcatheter aortic valve replacement is associated with increased cardiac mortality in patients with left ventricular dysfunction: insights from the PARTNER I trial. JACC Cardiovasc Interv. 2017;10:2414–2422. [DOI] [PubMed] [Google Scholar]
- 49. Nielsen HH, Klaaborg KE, Nissen H, Terp K, Mortensen PE, Kjeldsen BJ, Jakobsen CJ, Andersen HR, Egeblad H, Krusell LR, Thuesen L, Hjortdal VE. A prospective, randomised trial of transapical transcatheter aortic valve implantation vs. surgical aortic valve replacement in operable elderly patients with aortic stenosis: the STACCATO trial. EuroIntervention. 2012;8:383–389. [DOI] [PubMed] [Google Scholar]
- 50. Reynolds MR, Magnuson EA, Lei Y, Wang K, Vilain K, Li H, Walczak J, Pinto DS, Thourani VH, Svensson LG, Mack MJ, Miller DC, Satler LE, Bavaria J, Smith CR, Leon MB, Cohen DJ; PARTNER Investigators . Cost‐effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high‐risk patients with severe aortic stenosis: results of the PARTNER (placement of aortic transcatheter valves) trial (Cohort A). J Am Coll Cardiol. 2012;60:2683–2692. [DOI] [PubMed] [Google Scholar]
- 51. Greenbaum AB, Babaliaros VC, Chen MY, Stine AM, Rogers T, O'Neill WW, Paone G, Thourani VH, Muhammad KI, Leonardi RA, Ramee S, Troendle JF, Lederman RJ. Transcaval access and closure for transcatheter aortic valve replacement: a prospective investigation. J Am Coll Cardiol. 2017;69:511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frohlich GM, Baxter PD, Malkin CJ, Scott DJ, Moat NE, Hildick‐Smith D, Cunningham D, MacCarthy PA, Trivedi U, de Belder MA, Ludman PF, Blackman DJ; National Institute for Cardiovascular Outcomes Research . Comparative survival after transapical, direct aortic, and subclavian transcatheter aortic valve implantation (data from the UK TAVI Registry). Am J Cardiol. 2015;116:1555–1559. [DOI] [PubMed] [Google Scholar]
- 53. Mathur M, Hira RS, Smith BM, Lombardi WL, McCabe JM. Fully percutaneous technique for transaxillary implantation of the impella CP. JACC Cardiovasc Interv. 2016;9:1196–1198. [DOI] [PubMed] [Google Scholar]
- 54. Mylotte D, Sudre A, Teiger E, Obadia JF, Lee M, Spence M, Khamis H, Al Nooryani A, Delhaye C, Amr G, Koussa M, Debry N, Piazza N, Modine T. Transcarotid transcatheter aortic valve replacement: feasibility and safety. JACC Cardiovasc Interv. 2016;9:472–480. [DOI] [PubMed] [Google Scholar]
- 55. Ramee S, Anwaruddin S, Kumar G, Piana RN, Babaliaros V, Rab T, Klein LW; Aortic Stenosis AUCWG, Interventional Section of the Leadership Council of the American College of Cardiology . The rationale for performance of coronary angiography and stenting before transcatheter aortic valve replacement: from the Interventional Section Leadership Council of the American College of Cardiology. JACC Cardiovasc Interv. 2016;9:2371–2375. [DOI] [PubMed] [Google Scholar]
- 56. Khawaja MZ, Wang D, Pocock S, Redwood SR, Thomas MR. The percutaneous coronary intervention prior to transcatheter aortic valve implantation (ACTIVATION) trial: study protocol for a randomized controlled trial. Trials. 2014;15:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Functional assessment in TAVI: FAITAVI (FAITAVI). https://clinicaltrials.Gov/ct2/show/nct03360591. Accessed February 19, 2018.


