Abstract
Background
Higher circulatory corin in patients with cardiac diseases is associated with improved cardiovascular outcomes, and chronic cardiac dysfunction is a well‐known cause of progressive renal dysfunction. This study aimed to determine the role of serum corin in predicting short‐term and long‐term renal outcomes after contrast exposure in patients with suspected coronary artery disease.
Methods and Results
Four hundred one patients who had received coronary angiography were enrolled. Serum corin levels were determined before administration of contrast media. Contrast‐induced nephropathy was defined as a rise in serum creatinine of 0.5 mg/dL or a 25% increase from baseline within 48 hours after the procedure. Progressive renal dysfunction was defined as >50% decrease in estimated glomerular filtration rate after discharge. All patients were followed up for at least 1 year or until the occurrence of death after coronary angiography. Overall, contrast‐induced nephropathy occurred in 23 (5.7%) patients. During a median follow‐up of 529 days, 44 (11.0%) cases had subsequent decline in renal function. After adjustment for demographic characteristics, kidney function, traditional risk factors, and medications, lower corin level was found to be independently associated with higher risk for progressive renal dysfunction (hazard ratio, 0.23; 95% confidence interval, 0.12–0.44) but not for contrast‐induced nephropathy. This inverse correlation remained evident in patients with underlying chronic kidney disease, coronary artery disease, or heart failure.
Conclusions
Lower baseline serum corin was associated with higher risk of renal function decline in patients undergoing coronary angiography. Further studies are needed to verify these results.
Keywords: chronic kidney disease, contrast‐induced nephropathy, corin diagnosis, coronary angiography
Subject Categories: Biomarkers, Percutaneous Coronary Intervention, Coronary Artery Disease
Clinical Perspective
What Is New?
Preprocedural serum corin level predicts long‐term renal outcomes after undergoing coronary angiography.
What Are the Clinical Implications?
Patients with lower serum corin levels require diligent follow‐up of renal function after contrast exposure.
Introduction
It is now clear that cardiac and renal functions are interrelated. Patients with cardiac diseases have an increased risk of progressive renal dysfunction.1, 2 Furthermore, cardiac patients may be further exposed to the risk of contrast‐induced nephropathy (CIN) if they undergo coronary angiography.3, 4 A previous study showed that even mild elevation in serum creatinine after coronary angiography accelerates decline in renal function and increases risk of end‐stage renal disease.5 To identify patients at risk of CIN, several novel biomarkers were studied and a scoring system has been developed.6, 7, 8, 9, 10, 11, 12 However, less is known about a predictive marker for long‐term renal outcomes in patients undergoing coronary angiography.
Altered sensitivity and secretion of natriuretic peptides were observed during the progression of cardiac and renal dysfunction.2 Atrial natriuretic peptide (ANP) is essential for maintaining body fluid homeostasis and exerts antiremodeling effects in the myocardium.13 Pro‐ANP is converted to mature ANP by corin, a transmembrane protein with serine protease activity, which is expressed primarily in cardiomyocytes.14 Various isoforms of corin can be detected in the circulation because of ectodomain shedding, and the level of serum corin is considered a marker of cardiac corin activity.15, 16 Recent studies demonstrated a prognostic role for circulatory corin in relation to various cardiovascular outcomes in patients with acute myocardial infarction, chronic heart failure, and acute stroke.17, 18, 19, 20 However, whether serum corin level could predict renal outcomes in patients with high cardiovascular risks remains uncertain.
Previous studies reported that ANP may protect renal function by increasing glomerular filtration and medullary vasa recta blood flow, and by inhibiting inflammatory reaction.21, 22, 23 Periprocedural ANP infusion has been used to prevent acute kidney injury after angiography or cardiac surgery with various results.24, 25, 26 Therefore, we hypothesized that serum corin levels, which reflect the levels of cardiac corin activation and pro‐ANP processing activity, may predict renal outcomes after contrast exposure in patients with high cardiovascular risks. In this study, we evaluated the relationship between serum corin levels and the incidence of CIN and investigated the predictive role of corin in renal function decline in patients undergoing coronary angiography.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
Initially, a series of 540 consecutive patients who were admitted to a single medical center for coronary angiography between December 2009 and February 2015 were evaluated. First, 107 subjects lost to follow‐up after coronary angiography were excluded. Second, 32 individuals with end‐stage renal disease, which was defined as estimated glomerular filtration rate (eGFR) <15 mL/min per 1.73 m2 or with pre‐existing dialysis, were also excluded. Thus, a total of 401 subjects were enrolled in this study. Before enrollment, the chart of each patient was reviewed in detail to obtain data on medications, smoking status, and risk factors for CIN such as age, pre‐existing renal dysfunction, type 2 diabetes mellitus, and volume depletion. Blood pressure measurements were performed with electronic sphygmomanometers on the day of coronary angiography with standard method. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications. Type 2 diabetes mellitus was defined as fasting plasma glucose ≥126 mg/dL or use of hypoglycemic agents. Chronic kidney disease (CKD) was defined as eGFR <60 mL/min per 1.73 m2. eGFR was calculated using age, sex, and serum levels of blood urea nitrogen, creatinine, and albumin, according to the modified GFR estimating equations for Chinese patients.27 Body mass index was calculated by dividing the weight of the patient in kilograms by the square of the height in meters. Nonionic low‐osmolality contrast medium (iopromide) was used for all patients. The contrast medium was administered intra‐arterially, mainly through transradial catheters. Metformin and nephrotoxic medications such as NSAIDs were discontinued 48 hours before contrast media administration. Before and after contrast media exposure, physiological (0.9%) saline was given intravenously at a rate of 1 mL/kg per hour for 12 hours. In patients with left ventricular dysfunction (ejection fraction <40%) or overt heart failure, the hydration rate was reduced to 0.5 mL/kg per hour. The study protocol was approved by the institutional review board of Taipei Veterans General Hospital. Informed consent was obtained from all participants, and our study complies with the Declaration of Helsinki.
Laboratory Investigations
Blood samples were obtained from each patient after ≥8 hours of fasting, before coronary angiography. Serum levels of uric acid and glucose were measured using a Hitachi 7600 Autoanalyzer (Hitachi Ltd, Tokyo, Japan). Serum creatinine concentration was measured at the time of admission and every day for the following 3 days after contrast media exposure. Urine dipstick analysis was performed by commercial test strip, and proteinuria was defined as a urine protein ≥30 mg per 100 mL in urinalysis. The Mehran risk score,12 a risk stratification system for CIN, was calculated for each patient. Serum concentrations of corin were determined by a commercial enzyme‐linked immunosorbent assay (R&D Systems, Inc, Minneapolis, MN); sensitivity was 7 ng/L. Intra‐ and interassay coefficients were 4.1% and 3.9%, respectively. Patients were classified into 2 groups according to serum corin levels. Subjects with corin concentrations higher than the median were defined as “high corin group”; all others were defined as “low corin group.”
End Points for Clinical Follow‐Up
All patients were evaluated for the occurrence of CIN, which was defined as a rise in serum creatinine concentration of 0.5 mg/dL or a 25% increase from baseline within 48 hours after coronary angiography.28 Patients were advised to visit outpatient clinics regularly after discharge from the hospital. The cohort was followed until January 2016. Patients’ clinical data, including serum creatinine level, were obtained every 3 to 6 months during follow‐up. Progressive renal dysfunction was defined as >50% decrease in eGFR after discharge.
Statistical Analysis
Data were expressed in terms of median (quartiles) for numeric variables and as number (percent) for categorical variables. Clinical and laboratory data were compared using Mann–Whitney U test for continuous variables and Fisher exact test for categorical variables. Logarithmic (log) transformation was performed to achieve normal distribution for skewed variables (eGFR and corin). Incidence of CIN and progressive renal dysfunction were calculated. Survival curves were generated with the Kaplan–Meier method, and survival among groups was compared by log‐rank test. Logistic regression analysis was performed to investigate the relationships of various risk factors to CIN, whereas Cox proportional hazard regression analysis was performed to investigate the risk factors for progressive renal dysfunction. Factors with statistical significance in univariate regression analysis were entered into a final forward stepwise multivariate logistic regression model.
Because of the protective role of corin in cardiac remodeling under stress, we hypothesized that the prognostic values of serum corin level may be more significant in patients with higher cardiovascular risks. We performed a subgroup analysis and stratified the study cohort by the presence of diabetes mellitus, proteinuria, CKD, coronary artery disease (CAD), and heart failure. Data were analyzed using SPSS version 18.0 (SPSS Inc, Chicago, IL). A P<0.05 was regarded as statistically significant.
Results
Baseline Characteristics
The median age of the study population was 71 (interquartile range, 60–81) years old, and 69.3% were male. Table 1 summarizes the baseline characteristics of patients grouped according to serum corin concentrations. There were no differences between patients in the low corin group and those in the high corin group with respect to age, blood pressure, duration of follow‐up, Mehran risk score, smoking status, medical history, medications, serum levels of fasting glucose and uric acid, eGFR, or contrast volume. However, subjects with higher serum corin concentrations were more likely to be male (85.6%, n=172) and to present with higher body mass index (median, 25.9 kg/m2; interquartile range, 23.3–28.7) and hemoglobin (median, 13.3 mg/dL; interquartile range, 12.0–14.1) levels at baseline.
Table 1.
Baseline Characteristics of Patients According to Median Level of Serum Corin
| Characteristic | Total | Corin <1049.9 pg/mL | Corin ≥1049.9 pg/mL | P Valuea |
|---|---|---|---|---|
| n=401 | n=200 | n=201 | ||
| Age, y | 71.0 (60.0–81.0) | 73.0 (61.0–82.0) | 69.0 (59.0–80.0) | 0.068 |
| Sex (male) | 278 (69.3) | 106 (53.0) | 172 (85.6) | <0.001 |
| Follow‐up duration, d | 529.0 (336.0–812.0) | 518.0 (338.0–718.8) | 569.0 (334.0–890.0) | 0.302 |
| Mehran risk score | 4.0 (1.0–7.0) | 4.0 (1.0–8.0) | 4.0 (1.0–7.0) | 0.113 |
| Smoking | 148 (36.9) | 66 (33.0) | 82 (40.8) | 0.106 |
| BMI, kg/m2 | 25.4 (23.2–28.1) | 25.0 (22.9–27.8) | 25.9 (23.3–28.7) | 0.016 |
| Systolic blood pressure, mm Hg | 131.0 (120.0–144.0) | 130.0 (121.0–146.0) | 131.0 (118.5–144.0) | 0.732 |
| Diastolic blood pressure, mm Hg | 75.0 (67.0–83.0) | 74.0 (66.0–83.0) | 75.0 (68.0–82.5) | 0.529 |
| Medical history | ||||
| Hypertension | 284 (70.8) | 137 (68.5) | 147 (73.1) | 0.314 |
| Diabetes mellitus | 133 (33.2) | 64 (32.0) | 69 (34.3) | 0.622 |
| Heart failure | 73 (18.2) | 43 (21.5) | 30 (14.9) | 0.088 |
| Chronic kidney disease | 105 (26.2) | 56 (28.0) | 49 (24.4) | 0.411 |
| Medications | ||||
| ACEi or ARB | 96 (23.9) | 43 (21.5) | 53 (26.3) | 0.254 |
| Diuretics | 46 (11.5) | 26 (13.0) | 20 (10.0) | 0.339 |
| Statin | 109 (27.2) | 49 (24.5) | 60 (29.9) | 0.230 |
| Laboratory data | ||||
| Hemoglobin, g/dL | 12.9 (11.7–14.0) | 12.6 (11.1–13.7) | 13.3 (12.0–14.1) | <0.001 |
| Fasting glucose, mg/dL | 102.0 (92.0–125.0) | 101.0 (91.0–120.0) | 105.0 (93.0–128.0) | 0.336 |
| eGFR, mL/min per 1.73 m2 | 76.1 (58.5–93.6) | 78.0 (55.7–96.1) | 74.8 (60.5–89.0) | 0.408 |
| Uric acid, mg/dL | 6.1 (4.8–7.2) | 6.0 (4.6–7.0) | 6.2 (5.0–7.3) | 0.079 |
| Proteinuria, n (%) | 80 (20.0) | 42 (21.0) | 38 (18.9) | 0.601 |
| Corin, pg/mL | 1049.9 (750.1–1374.8) | 750.1 (544.9–905.1) | 1369.8 (1182.6–1609.8) | <0.001 |
| Contrast volume, mL | 50.0 (50.0–150.0) | 50.0 (50.0–150.0) | 50.0 (50.0–150.0) | 0.174 |
Data are presented as the median (interquartile range) or as total number of patients (%). ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate.
P values were calculated with the use of Mann–Whitney U test for continuous variables and the Fisher exact test for categorical variables.
All patients were successfully followed up for a median duration of 529 days (range, 336–812 days). Of these, 23 (5.7%) developed CIN after coronary angiography. In addition, 44 (11.0%) had progressive renal dysfunction. Patients with higher serum corin concentrations tended to have a lower incidence of progressive renal dysfunction, though this trend was of only borderline significance (8.0% versus 14.0%, P=0.053). However, the incidence of CIN (6.0% versus 5.5%, P=0.840) did not show significant differences between groups. Kaplan–Meier survival analysis was performed to investigate the potential impact of baseline corin levels on adverse event‐free survival. Patients in the high corin group showed significantly lower risk of progressive renal dysfunction than did patients in the low corin group (P=0.017), as illustrated in Figure.
Figure 1.
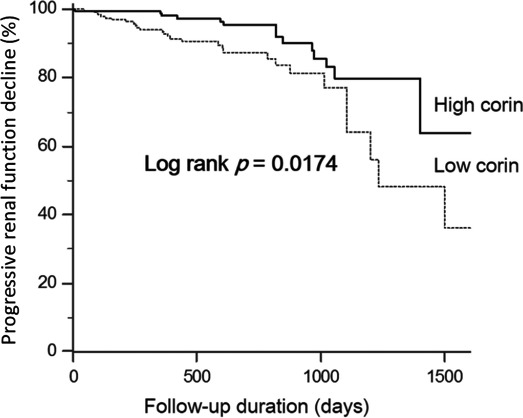
Kaplan–Meier estimate of >50% decline in eGFR in patients with higher or lower serum corin. eGFR indicates estimated glomerular filtration rate.
Independent Correlates of CIN and Predictors of Progressive Renal Dysfunction
In univariate logistic regression analysis, older age (odds ratio [OR], 1.04; 95% confidence interval [CI], 1.00–1.08; P=0.042), higher Mehran risk score (OR, 1.16; 95% CI, 1.06–1.28; P=0.002), history of diabetes mellitus (OR, 3.39; 95% CI, 1.43–8.04; P=0.006), use of angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers (OR, 3.82; 95% CI, 1.63–8.96; P=0.002), and diuretics (OR, 2.98; 95% CI, 1.11–8.00; P=0.030), hemoglobin level (OR, 0.5; 95% CI, 0.38–0.66; P<0.001), and presence of proteinuria (OR, 4.97; 95% CI, 2.11–11.74; P<0.001) were significantly associated with risk of CIN. To identify the independent predictors of CIN, multivariable logistic regression analysis was performed. After adjustment for these significant factors in univariate analysis, hemoglobin level (OR, 0.55; 95% CI, 0.41–0.73; P<0.001), proteinuria (OR, 2.65; 95% CI, 1.04–6.76; P=0.041), and use of angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers (OR, 3.41; 95% CI, 1.36–8.56; P=0.009) remained significantly associated with CIN, as shown in Table 2.
Table 2.
Univariate and Multivariate Analyses of Factors Associated With CIN
| Variable | Univariate Logistic Regression | Multivariate Logistic Regressiona | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age, y | 1.04 | 1.00–1.08 | 0.042 | |||
| Sex (male) | 0.67 | 0.28–1.60 | 0.368 | |||
| Mehran risk score | 1.16 | 1.06–1.28 | 0.002 | |||
| Smoking | 0.91 | 0.38–2.19 | 0.828 | |||
| BMI, kg/m2 | 1.02 | 0.93–1.12 | 0.682 | |||
| Systolic blood pressure, mm Hg | 1.01 | 0.99–1.03 | 0.513 | |||
| Diastolic blood pressure, mm Hg | 0.98 | 0.94–1.01 | 0.191 | |||
| Medical history | ||||||
| Hypertension | 1.54 | 0.56–4.24 | 0.408 | |||
| Diabetes mellitus | 3.39 | 1.43–8.04 | 0.006 | |||
| Heart failure | 2.07 | 0.82–5.23 | 0.124 | |||
| Chronic kidney disease | 0.77 | 0.28–2.14 | 0.618 | |||
| Medications | ||||||
| ACEi or ARB | 3.82 | 1.63–8.96 | 0.002 | 3.41 | 1.36–8.56 | 0.009 |
| Diuretics | 2.98 | 1.11–8.00 | 0.030 | |||
| Statin | 1.79 | 0.75–4.26 | 0.190 | |||
| Laboratory data | ||||||
| Hemoglobin, g/dL | 0.50 | 0.38–0.66 | <0.001 | 0.55 | 0.41–0.73 | <0.001 |
| Fasting glucose, mg/dL | 1.00 | 1.00–1.01 | 0.281 | |||
| eGFRb | 1.01 | 0.99–1.03 | 0.204 | |||
| Uric acid, mg/dL | 0.96 | 0.75–1.23 | 0.761 | |||
| Proteinuria | 4.97 | 2.11–11.74 | <0.001 | 2.65 | 1.04–6.76 | 0.041 |
| Corinb | 0.54 | 0.19–1.57 | 0.260 | |||
| Contrast volume, mL | 1.00 | 1.00–1.01 | 0.731 | |||
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CI, confidence interval; CIN, contrast‐induced nephropathy; eGFR, estimated glomerular filtration rate; OR, odds ratio.
The model consists of age, sex, and variables with P<0.05 in univariate comparison, including Mehran risk score, medical history of diabetes mellitus, medications with ACEi or ARB, diuretics, levels of hemoglobin, and presence of proteinuria.
Log transformation was performed before analysis.
In univariate Cox regression analysis, older age (hazard ratio [HR], 1.04; 95% CI, 1.01–1.07; P=0.007), history of heart failure (HR, 2.28; 95% CI, 1.22–4.26; P=0.009) and CKD (HR, 2.51; 95% CI, 1.37–4.59; P=0.003), presence of proteinuria (HR, 2.66; 95% CI, 1.46–4.87; P=0.001), serum levels of corin (HR, 0.17; 95% CI, 0.09–0.32; P<0.001) and hemoglobin (HR, 0.73; 95% CI; P<0.001), eGFR (HR, 0.07; 95% CI, 0.01–0.34; P=0.001), and presence of CIN (HR, 2.46; 95% CI, 1.03–5.90; P=0.043) were significantly associated with the development of progressive renal dysfunction. After performing multivariable forward‐stepwise Cox regression analysis, circulating corin level (HR, 0.23; 95% CI, 0.12–0.44; P<0.001), hemoglobin level (HR, 0.78; 95% CI, 0.65–0.94; P=0.008), and baseline eGFR (HR, 0.14; 95% CI, 0.03–0.82; P=0.029) remained significantly associated with progressive renal dysfunction, as shown in Table 3. Serum corin level was an independent predictor of progressive renal function decline rather than CIN in patients undergoing coronary angiography.
Table 3.
Univariate and Multivariate Analyses of Factors Associated With Progressive Renal Dysfunctiona
| Variable | Univariate Cox Regression | Multivariate Cox Regressionb | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age, y | 1.04 | 1.01–1.07 | 0.007 | |||
| Sex (male) | 0.86 | 0.46–1.61 | 0.629 | |||
| Smoking | 1.63 | 0.90–2.98 | 0.110 | |||
| BMI, kg/m2 | 0.96 | 0.89–1.04 | 0.341 | |||
| Systolic blood pressure, mm Hg | 1.00 | 0.99–1.02 | 0.786 | |||
| Diastolic blood pressure, mm Hg | 0.98 | 0.96–1.01 | 0.227 | |||
| Medical history | ||||||
| Hypertension | 1.14 | 0.58–2.27 | 0.703 | |||
| Diabetes mellitus | 1.68 | 0.92–3.06 | 0.091 | |||
| Heart failure | 2.28 | 1.22–4.26 | 0.009 | |||
| CKD | 2.51 | 1.37–4.59 | 0.003 | |||
| Medications | ||||||
| ACEi or ARB | 1.36 | 0.72–2.54 | 0.343 | |||
| Diuretics | 1.46 | 0.72–2.94 | 0.294 | |||
| Statin | 0.56 | 0.26–1.21 | 0.138 | |||
| Laboratory data | ||||||
| Hemoglobin, g/dL | 0.73 | 0.62–0.86 | <0.001 | 0.78 | 0.65–0.94 | 0.008 |
| Fasting glucose, mg/dL | 1.00 | 1.00–1.00 | 0.678 | |||
| eGFRc | 0.07 | 0.01–0.34 | 0.001 | 0.14 | 0.03–0.82 | 0.029 |
| Uric acid, mg/dL | 1.16 | 0.98–1.36 | 0.083 | |||
| Proteinuria | 2.66 | 1.46–4.87 | 0.001 | |||
| Corinc | 0.17 | 0.09–0.32 | <0.001 | 0.23 | 0.12–0.44 | <0.001 |
| CIN | 2.46 | 1.03–5.90 | 0.043 | |||
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CI, confidence interval; CIN, contrast‐induced nephropathy; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Progressive renal dysfunction is defined as >50% decrease in eGFR.
The model consists of age, sex, and variables with P<0.05 in univariate comparison, including medical history of heart failure, CKD, levels of hemoglobin, eGFR, corin, presence of proteinuria, and occurrence of CIN.
Log transformation was performed before analysis.
Stratified Analysis of Serum Corin Level in Predicting Progressive Renal Dysfunction
The study cohort was stratified by the presence of diabetes mellitus, proteinuria, CKD, CAD, and heart failure. As shown in Table 4, there were no significant interactions between serum corin level and subgroup with respect to predicting progressive renal dysfunction. However, likely because of lower statistical power, this association did not reach statistical significance in the subsets of patients without CKD, CAD, or heart failure.
Table 4.
Stratified Analysis of Risk of Progressive Renal Dysfunction in Patients Grouped by the Presence of Diabetes Mellitus, Proteinuria, CKD, CAD, and Heart Failure
| Subgroup (Events/Subjects) | Log Corin | Log Corin | P for Interaction | ||
|---|---|---|---|---|---|
| Crude HR (95% CI) | P Value | Adjusted HR (95% CI)a | P Value | ||
| Overall (44/401) | 0.17 (0.09–0.32) | <0.001 | 0.23 (0.12–0.44) | <0.001 | |
| Diabetes mellitus | 0.875 | ||||
| Yes (21/133) | 0.15 (0.04–0.55) | 0.004 | 0.11 (0.03–0.39) | 0.001 | |
| No (23/268) | 0.16 (0.07–0.37) | <0.001 | 0.26 (0.09–0.75) | 0.013 | |
| Proteinuria | 0.842 | ||||
| Yes (21/80) | 0.17 (0.05–0.53) | 0.003 | 0.12 (0.03–0.40) | 0.001 | |
| No (23/321) | 0.18 (0.07–0.45) | <0.001 | 0.29 (0.11–0.77) | 0.013 | |
| CKD | 0.280 | ||||
| Yes (22/105) | 0.19 (0.09–0.41) | <0.001 | 0.23 (0.10–0.51) | <0.001 | |
| No (22/296) | 0.35 (0.06–2.07) | 0.246 | 1.26 (0.11–15.20) | 0.855 | |
| CAD | 0.467 | ||||
| Yes (29/243) | 0.16 (0.08–0.32) | <0.001 | 0.24 (0.12–0.49) | <0.001 | |
| No (15/158) | 0.39 (0.04–3.60) | 0.405 | 0.49 (0.03–9.37) | 0.634 | |
| Heart failure | 0.641 | ||||
| Yes (17/73) | 0.30 (0.14–0.65) | 0.002 | 0.33 (0.15–0.76) | 0.008 | |
| No (27/328) | 0.14 (0.03–0.63) | 0.010 | 0.20 (0.03–1.39) | 0.103 | |
CAD indicates coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Adjusted for hemoglobin, log eGFR.
Discussion
The major findings of the present study are that lower serum corin levels are independently associated with the development of progressive renal dysfunction but not CIN in patients undergoing coronary angiography. The association is attenuated in the relatively robust population without underlying CKD, CAD, or heart failure. To the best of our knowledge, the present study is the first to show an association between serum corin level and decline in renal function, suggesting the predictive role of serum corin levels for long‐term renal outcomes. Our results indicate that lower corin levels may be a novel risk marker for progressive renal dysfunction.
Corin is primarily expressed in ANP‐expressing atrial cardiomyocytes, and acts principally to convert pro‐ANP to ANP.14 Corin protein consists of a transmembrane domain near the N‐terminus, 2 frizzled‐like domains, 8 low‐density lipoprotein receptor repeats, a macrophage scavenger receptor–like domain, and a trypsin‐like protease domain at the C‐terminus. Protease activity is activated by PCSK6, after being cleaved at a conserved site, Arg801‐Ile802.29 Previous studies showed that cardiac corin expression was stimulated in hypertrophic cardiomyocytes, animal models of heart failure, and failing human hearts.16 Corin knock‐out mice had higher blood pressures and enhanced cardiac hypertrophic responses toward increased afterload. Overexpression of cardiac corin in a murine model of dilated cardiomyopathy resulted in improved ejection fraction and survival. In humans, 2 single nucleotide polymorphisms (T555I/Q568P and R539C) of CORIN have been shown to impair zymogen activity.30, 31 Observational studies showed that these variants were associated with higher prevalence of hypertension, more severe cardiac hypertrophic responses, and worse cardiovascular outcomes.32, 33, 34 These results suggest that cardiac corin activity plays a protective role in the process of cardiac remodeling.
Once activated, the extracellular domain of cardiac corin undergoes corin autocleavage and a disintegrin and metalloprotease–mediated proteolysis, which releases various isoforms of corin fragments into the circulation.16 It has been suggested that the shedding of active cardiac corin into the circulation prevents excessive corin activity on cardiomyocytes. Therefore, serum corin level is considered a marker of cardiac corin activity.16 Previous studies demonstrated that serum corin levels were increased in patients with hypertension, atrial fibrillation, and obesity, and were decreased in patients with heart failure and acute stroke.16 Recent published studies also revealed prognostic values of serum corin in patients with cardiovascular diseases.17, 18, 19, 20 Zhou et al showed that reduced serum corin level was an independent predictor of cardiovascular death, hospitalization for heart failure, or recurrent myocardial infarction in patients with acute myocardial infarction.18 The same authors revealed that in patients with heart failure, reduced serum corin level was an independent predictor of cardiovascular death and heart failure readmission.17
In this study, we provided the first evidence that patients with decreased baseline serum corin levels were more likely to encounter progressive renal dysfunction after coronary angiography. One possible explanation is that decreased serum corin level may indicate an early stage of heart failure. It is clear that cardiac dysfunction is a mediator of progressive renal dysfunction.1, 2 Hypoperfusion, venous congestion, excessive production of vasoconstrictive mediators, altered sensitivity, and/or release of endogenous vasodilatory factors, and pharmacotherapies used in the management of heart failure have all been suggested to contribute to the increased risk of CKD in patients with heart failure.2, 35 In our study cohort, patients with a history of heart failure had higher risk of progressive renal dysfunction. However, the association became insignificant when serum corin level was included in the multivariable Cox regression model. A previous study showed that serum corin level was decreased in patients with heart failure.36 In an animal study utilizing a murine model of dilated cardiomyopathy, Tripathi et al demonstrated that cardiac corin expression decreased since the initial stage of heart failure, while the serum levels of ANP and brain natriuretic peptide rose only with terminal heart failure.37 Therefore, patients with early‐stage cardiac dysfunction may have had a decreased serum corin level before heart failure was diagnosed clinically, which in turn increases the risk of progressive renal dysfunction.
Another possible explanation of our findings is that patients with lower serum corin levels may have impaired activation of cardiac corin in response to myocardial stress. It is clear that ANP exerts its cardiac and renal protection effects only after being adequately sliced by corin. Increased levels of unprocessed ANP were found in several pathological conditions, which suggested inadequate corin activation, and were associated with worse cardiac and renal outcomes.38, 39, 40 In our study, decreased serum corin level was associated with the development of progressive renal dysfunction in patient with comorbidities of diabetes mellitus, proteinuria, CKD, heart failure, and CAD. Because the level of serum corin is considered a marker of cardiac corin activity, it is possible that decreased corin compensation in these high‐risk patients causes impaired pro‐ANP activation, which results in an increased risk of progressive renal dysfunction. Further studies are needed to clarify the interaction between serum corin and renal function.
This study had some limitations that should be considered. First, the study population was relatively small, and all participants were of Asian ethnicity and were recruited from a single center. Further studies with a larger number of different participants are required to confirm our findings. Second, the measurements of metabolic acidosis and other relevant biomarkers, such as pro‐ANP and N‐terminal pro‐brain natriuretic peptide, were not available. Thus, we could not provide additional insights into potential mechanisms underlying the association between serum corin levels and progressive renal dysfunction. Finally, our patients had relatively normal renal function at baseline (median eGFR: 76.1 mL/min; CKD: 26.2%), and no end‐stage renal disease was encountered during a median follow‐up of 529 days, which hindered further analysis of the predictive role of corin in the occurrence of end‐stage renal disease. Nevertheless, our study demonstrated that serum corin level is a novel risk marker for renal outcomes in patients with suspected CAD.
In conclusion, although not a predictor for CIN, serum corin is an independent prognostic marker for long‐term renal outcomes in patients undergoing coronary angiography. The current study demonstrated a possible protective role of circulating corin in the pathogenesis of renal dysfunction in patients with suspected CAD. Further multicenter trials are needed to confirm our findings, and studies are warranted to clarify the role of corin in cardiorenal crosstalk.
Author Contributions
Yang and Chou contributed equally in collecting the data, performing the statistical analysis, and drafting the article. P.‐H. Huang, Li, and S.‐S. Huang participated in study design, coordination, and data interpretation.
Sources of Funding
This study was supported, in part, by research grants from the Ministry of Science and Technology of Taiwan (MOST 104‐2314‐B‐075‐047), the Ministry of Science and Technology of Taiwan (MOST 104‐2314‐B‐350‐002), the Novel Bioengineering and Technological Approaches to Solve Two Major Health Problems in Taiwan sponsored by the Taiwan Ministry of Science and Technology Academic Excellence Program (MOST 106‐2633‐B‐009‐001), the Ministry of Health and Welfare (MOHW106‐TDU‐B‐211‐113001), Taipei Veterans General Hospital (V105C‐0207, V106C‐045), and grant from Cheng Hsin General Hospital (CHGH106‐05). These funding agencies had no influence on the study design, data collection or analysis, or decision to publish or preparation of the article.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e008157 DOI: 10.1161/JAHA.117.008157.)29728370
Shang‐Feng Yang and Ruey‐Hsing Chou contributed equally.
Contributor Information
Shao‐Sung Huang, Email: shao_0915@yahoo.com.tw.
Po‐Hsun Huang, Email: huangbsvgh@gmail.com.
References
- 1. Schefold JC, Filippatos G, Hasenfuss G, Anker SD, von Haehling S. Heart failure and kidney dysfunction: epidemiology, mechanisms and management. Nat Rev Nephrol. 2016;7:610–623. [DOI] [PubMed] [Google Scholar]
- 2. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;7:1527–1539. [DOI] [PubMed] [Google Scholar]
- 3. Nash K, Hafeez A, Hou S. Hospital‐acquired renal insufficiency. Am J Kidney Dis. 2002;7:930–936. [DOI] [PubMed] [Google Scholar]
- 4. Eng J, Wilson RF, Subramaniam RM, Zhang A, Suarez‐Cuervo C, Turban S, Choi MJ, Sherrod C, Hutfless S, Iyoha EE, Bass EB. Comparative effect of contrast media type on the incidence of contrast‐induced nephropathy: a systematic review and meta‐analysis. Ann Intern Med. 2016;7:417–424. [DOI] [PubMed] [Google Scholar]
- 5. James MT, Ghali WA, Tonelli M, Faris P, Knudtson ML, Pannu N, Klarenbach SW, Manns BJ, Hemmelgarn BR. Acute kidney injury following coronary angiography is associated with a long‐term decline in kidney function. Kidney Int. 2010;7:803–809. [DOI] [PubMed] [Google Scholar]
- 6. Briguori C, Visconti G, Rivera NV, Focaccio A, Golia B, Giannone R, Castaldo D, De Micco F, Ricciardelli B, Colombo A. Cystatin C and contrast‐induced acute kidney injury. Circulation. 2010;7:2117–2122. [DOI] [PubMed] [Google Scholar]
- 7. Akdeniz D, Celik HT, Kazanci F, Yilmaz H, Yalcin S, Bilgic MA, Ruzgaresen N, Akcay A, Eryonucu B. Is kidney injury molecule 1 a valuable tool for the early diagnosis of contrast‐induced nephropathy? J Investig Med. 2015;7:930–934. [DOI] [PubMed] [Google Scholar]
- 8. Li W, Yu Y, He H, Chen J, Zhang D. Urinary kidney injury molecule‐1 as an early indicator to predict contrast‐induced acute kidney injury in patients with diabetes mellitus undergoing percutaneous coronary intervention. Biomed Rep. 2015;7:509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tong J, Li H, Zhang H, Luo Z, Huang Y, Huang J, He F, Fu J. Neutrophil gelatinase‐associated lipocalin in the prediction of contrast‐induced nephropathy: a systemic review and meta‐analysis. J Cardiovasc Pharmacol. 2015;7:239–245. [DOI] [PubMed] [Google Scholar]
- 10. Torregrosa I, Montoliu C, Urios A, Andres‐Costa MJ, Gimenez‐Garzo C, Juan I, Puchades MJ, Blasco ML, Carratala A, Sanjuan R, Miguel A. Urinary KIM‐1, NGAL and L‐FABP for the diagnosis of AKI in patients with acute coronary syndrome or heart failure undergoing coronary angiography. Heart Vessels. 2015;7:703–711. [DOI] [PubMed] [Google Scholar]
- 11. Chaykovska L, Heunisch F, von Einem G, Alter ML, Hocher CF, Tsuprykov O, Dschietzig T, Kretschmer A, Hocher B. Urinary vitamin D binding protein and KIM‐1 are potent new biomarkers of major adverse renal events in patients undergoing coronary angiography. PLoS One. 2016;7:e0145723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;7:1393–1399. [DOI] [PubMed] [Google Scholar]
- 13. Calvieri C, Rubattu S, Volpe M. Molecular mechanisms underlying cardiac antihypertrophic and antifibrotic effects of natriuretic peptides. J Mol Med (Berl). 2012;7:5–13. [DOI] [PubMed] [Google Scholar]
- 14. Wu Q, Xu‐Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;7:142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong N, Chen S, Wang W, Zhou Y, Wu Q. Corin in clinical laboratory diagnostics. Clin Chim Acta. 2012;7:378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou Y, Wu Q. Corin in natriuretic peptide processing and hypertension. Curr Hypertens Rep. 2014;7:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou X, Chen JC, Liu Y, Yang H, Du K, Kong Y, Xu XH. Plasma corin as a predictor of cardiovascular events in patients with chronic heart failure. JACC Heart Fail. 2016;7:664–669. [DOI] [PubMed] [Google Scholar]
- 18. Zhou X, Chen J, Zhang Q, Shao J, Du K, Xu X, Kong Y. Prognostic value of plasma soluble corin in patients with acute myocardial infarction. J Am Coll Cardiol. 2016;7:2008–2014. [DOI] [PubMed] [Google Scholar]
- 19. Hu W, Chen S, Song Y, Zhu F, Shi J, Han X, Zhou D, Zhi Z, Zhang F, Shen Y, Ma J, Liu CF, Peng H. Serum soluble corin deficiency predicts major disability within 3 months after acute stroke. PLoS One. 2016;7:e0163731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peleg A, Ghanim D, Vered S, Hasin Y. Serum corin is reduced and predicts adverse outcome in non‐ST‐elevation acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2013;7:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marin‐Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre‐glomerular vasodilatation and post‐glomerular vasoconstriction in rat kidney. Nature. 1986;7:473–476. [DOI] [PubMed] [Google Scholar]
- 22. Kiberd BA, Larson TS, Robertson CR, Jamison RL. Effect of atrial natriuretic peptide on vasa recta blood flow in the rat. Am J Physiol. 1987;7:F1112–F1117. [DOI] [PubMed] [Google Scholar]
- 23. Mitaka C, Si MK, Tulafu M, Yu Q, Uchida T, Abe S, Kitagawa M, Ikeda S, Eishi Y, Tomita M. Effects of atrial natriuretic peptide on inter‐organ crosstalk among the kidney, lung, and heart in a rat model of renal ischemia‐reperfusion injury. Intensive Care Med Exp. 2014;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morikawa S, Sone T, Tsuboi H, Mukawa H, Morishima I, Uesugi M, Morita Y, Numaguchi Y, Okumura K, Murohara T. Renal protective effects and the prevention of contrast‐induced nephropathy by atrial natriuretic peptide. J Am Coll Cardiol. 2009;7:1040–1046. [DOI] [PubMed] [Google Scholar]
- 25. Nigwekar SU, Navaneethan SD, Parikh CR, Hix JK. Atrial natriuretic peptide for management of acute kidney injury: a systematic review and meta‐analysis. Clin J Am Soc Nephrol. 2009;7:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitaka C, Ohnuma T, Murayama T, Kunimoto F, Nagashima M, Takei T, Iguchi N, Tomita M; Investigators J . Effects of low‐dose atrial natriuretic peptide infusion on cardiac surgery‐associated acute kidney injury: a multicenter randomized controlled trial. J Crit Care. 2017;7:253–258. [DOI] [PubMed] [Google Scholar]
- 27. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;7:2937–2944. [DOI] [PubMed] [Google Scholar]
- 28. McCullough PA. Contrast‐induced acute kidney injury. J Am Coll Cardiol. 2008;7:1419–1428. [DOI] [PubMed] [Google Scholar]
- 29. Chen S, Cao P, Dong N, Peng J, Zhang C, Wang H, Zhou T, Yang J, Zhang Y, Martelli EE, Naga Prasad SV, Miller RE, Malfait AM, Zhou Y, Wu Q. PCSK6‐mediated corin activation is essential for normal blood pressure. Nat Med. 2015;7:1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;7:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dong N, Fang C, Jiang Y, Zhou T, Liu M, Zhou J, Shen J, Fukuda K, Qin J, Wu Q. Corin mutation R539C from hypertensive patients impairs zymogen activation and generates an inactive alternative ectodomain fragment. J Biol Chem. 2013;7:7867–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, Dries DL. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;7:857–864. [DOI] [PubMed] [Google Scholar]
- 33. Rame JE, Tam SW, McNamara D, Worcel M, Sabolinski ML, Wu AH, Dries DL. Dysfunctional corin I555(P568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ Heart Fail. 2009;7:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;7:2403–2410. [DOI] [PubMed] [Google Scholar]
- 35. Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, Yancy CW, Califf RM, Stevenson LW, Hill JA. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;7:1268–1274. [DOI] [PubMed] [Google Scholar]
- 36. Dong N, Chen S, Yang J, He L, Liu P, Zheng D, Li L, Zhou Y, Ruan C, Plow E, Wu Q. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;7:207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tripathi R, Wang D, Sullivan R, Fan TH, Gladysheva IP, Reed GL. Depressed corin levels indicate early systolic dysfunction before increases of atrial natriuretic peptide/B‐type natriuretic peptide and heart failure development. Hypertension. 2016;7:362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dieplinger B, Mueller T, Kollerits B, Struck J, Ritz E, von Eckardstein A, Haltmayer M, Kronenberg F; Group MS . Pro‐A‐type natriuretic peptide and pro‐adrenomedullin predict progression of chronic kidney disease: the MMKD Study. Kidney Int. 2009;7:408–414. [DOI] [PubMed] [Google Scholar]
- 39. Ogawa N, Komura H, Kuwasako K, Kitamura K, Kato J. Plasma levels of natriuretic peptides and development of chronic kidney disease. BMC Nephrol. 2015;7:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu‐Cai YO, Wu Q. Molecular forms of natriuretic peptides in heart failure and their implications. Heart. 2010;7:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]


