Abstract
Background
Frailty is associated with greater mortality; however, whether frail patients primarily die of cardiovascular disease (CVD) or non‐CVD causes is unknown.
Methods and Results
We assessed the cause of death in relation to frailty status, measured at baseline, among 3135 community‐dwelling older men in the MrOS Sleep (Outcomes of Sleep Disorders in Older Men) study. Absolute probability and risk of CVD mortality associated with frailty status were estimated with traditional methods that used censoring and newer methods that considered non‐CVD mortality as a competing risk. Of the 3135 men (mean age: 76.4±5.6 years), 475 (15.2%) were frail. During an average follow‐up of 9.2 years, 1275 (40.7%) men died, including 445 (34.9%) from CVD and 828 (64.9%) from non‐CVD causes (2 deaths unadjudicated). Both CVD and non‐CVD mortality risk increased with frailty. Cumulative absolute probability of CVD death at 10 years among frail men was 23.8% (20.2–27.6%) using the competing risk method versus 32.5% (27.3–37.8%) using the traditional Kaplan–Meier method (41.5% [95% confidence interval, 36.9–45.9%] and 48.6% [95% confidence interval, 43.6–53.4%], respectively, for non‐CVD mortality). The multivariable‐adjusted risk of CVD death among frail versus robust men was 1.38 (95% confidence interval, 0.99–1.92) using the competing risk method versus 1.84 (95% confidence interval, 1.35–2.51) using the traditional Cox proportional hazards method.
Conclusions
Among community‐dwelling older men, ≈35% of the deaths were due to CVD. Frail men were at increased risk of CVD death, but ignoring the competing risk of non‐CVD mortality overestimated their long‐term probability and relative risk of CVD death.
Keywords: cardiovascular disease, cardiovascular disease risk factors, functional capacity impairment, mortality
Subject Categories: Aging, Cardiovascular Disease, Risk Factors, Epidemiology, Mortality/Survival
Clinical Perspective
What Is New?
Among community‐dwelling older men, frailty is associated with increases in both cardiovascular disease (CVD) and non‐CVD mortality.
Among these patients, ≈35% of the deaths were due to CVD and 65% were due to non‐CVD causes.
Ignoring the competing risk of non‐CVD mortality overestimates the long‐term probability and relative risk of CVD death.
What Are the Clinical Implications?
Frailty status and the competing risk of non‐CVD mortality need to be taken into account when assessing the benefit of CVD interventions regarding mortality.
Studies assessing the association of frailty or its individual components with risk of CVD death should account for the competing risk of non‐CVD death.
Introduction
With the rapid expansion of the number of elderly patients seeking cardiovascular care, frailty status has been suggested as an important factor to consider in clinical decision‐making.1, 2 Frailty, defined as a biological syndrome that reflects a state of decreased physical reserve and increased vulnerability to stressors, is associated with prevalent cardiovascular disease (CVD). Prospective studies of older adults have reported independent associations of frailty with higher risks of comorbidity, disability, all‐cause mortality, and hospitalization.1, 3, 4, 5, 6, 7, 8, 9 Frailty has also been associated with worse outcomes after acute CVD events and a higher likelihood of adverse consequences after CVD interventions.1, 10, 11, 12, 13
Emergence of new interventions, such as transcatheter aortic valve replacement, and improvement of existing interventions have increased the feasibility of treating CVD in later stages of life.14, 15, 16 Devices such as implantable cardioverter–defibrillators are being used prophylactically to prevent sudden cardiac death in patients of all ages, including older adults.17, 18, 19, 20 Shown to prolong life in younger, more robust patients who have participated in randomized clinical trials, these CVD interventions are applied to older, more frail patients for whom the competing risk of non‐CVD death is higher.21, 22 However, because of more advanced disease, greater burden of comorbidity, and higher incidence of adverse consequences, frail patients may accrue less survival benefit from CVD therapies than younger, more robust patients. To assess the likelihood of an improvement in survival associated with a CVD intervention among frail patients, it is necessary to estimate the probability of cause‐specific (CVD versus non‐CVD) mortality first.
The primary objective of this study was to estimate the probability of CVD mortality by frailty status, with and without consideration of non‐CVD mortality as a competing risk. We hypothesized that frailty would be associated with non‐CVD mortality and that traditional analyses that did not consider the competing risk of non‐CVD death would result in overestimation of the risk of CVD death among frail older men.
Methods
MrOS (Osteoporotic Fractures in Men) data, analytic methods, and study materials have been made available to other researchers for purposes of reproducing the results or replicating the procedure.23
Participants
The MrOS study (n=5994) enrolled community‐dwelling men aged 65 years and older between 2000 and 2002, from 6 geographic regions in the United States: Birmingham, Alabama; Minneapolis, Minnesota; Palo Alto, California; the Monongahela Valley near Pittsburgh, Pennsylvania; Portland, Oregon; and San Diego, California.24, 25 Men with a history of bilateral hip replacement or those who were unable to walk without the assistance of another person were not eligible for the study. A subset of MrOS participants (n=3135) were enrolled in the MrOS Sleep (Outcomes of Sleep Disorders in Older Men) ancillary study between 2003 and 2005. These men had both a clinic examination with assessment of frailty and adjudication of vital status, including cause of death, and are included in the present analysis. The research protocols were approved by the institutional review board at each participating institution, and all participants gave written informed consent.
Frailty Assessment
Frailty status was defined at the initial MrOS Sleep examination using criteria similar to those proposed by Fried et al,26 using data collected in the Cardiovascular Health Study:
Shrinking, identified by unintentional weight loss of ≥5% between the MrOS baseline and sleep examination (mean±SD years between examinations: 3.38±0.48);
Weakness, identified by grip strength at the sleep examination in the lowest quintile stratified by body mass index (quartiles);
Poor energy, identified by an answer of “no” to the question, “Do you feel full of energy?” from the Geriatric Depression Scale,27 administered at the sleep examination;
Slowness, identified by a walk speed at the sleep examination in the lowest quintile stratified by standing height (median); and
Low physical activity level at the sleep examination, identified by a Physical Activity Scale for the Elderly28 score in the lowest quintile.
Men with none of these components were categorized as robust, those with 1 or 2 components were categorized as intermediate stage, and those with ≥3 components were categorized as frail.
Adjudication of Mortality
Men were contacted every 4 months by postcard and/or phone to ascertain vital status. A total of 135 individuals terminated study participation before the end of follow‐up. Among the remaining 3000 participants, the follow‐up for vital status was 99% complete. Deaths were centrally adjudicated as being due to CVD versus a non‐CVD cause by 2 experts at the MrOS Coordinating Center using death certificates, medical records, and a validated, prespecified adjudication protocol. Cause of death was categorized according to the International Classification of Diseases, Ninth Revision (ICD‐9) codes for underlying disease as CVD (394.9, 396.9–442, 443.9, 459.7, 459.9, 785.51, and 966.71).29 The follow‐up for mortality in this analysis ended in February 2016. Mean duration of follow‐up after the sleep examination for mortality was 9.2±3.0 years.
Definition of CVD and Other Measurements
Prevalent CVD was defined as having a history of coronary heart disease (including myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, or angina), peripheral vascular disease, valvular heart disease, or congestive heart failure.
At the sleep examination, participants completed a questionnaire and were interviewed regarding smoking status and history of physician‐diagnosed selected medical conditions including stroke, diabetes mellitus, hypertension, coronary heart disease, peripheral vascular disease, valvular heart disease, congestive heart failure, and chronic obstructive pulmonary disease.
Statistical Analysis
Characteristics of the 3135 participants in the MrOS Sleep examination were compared across the 3 categories of frailty status using ANOVA (or nonparametric equivalent Kruskal–Wallis) tests for continuous variables and χ2 tests for categorical variables. Among men who died during follow‐up, we compared baseline characteristics, including frailty status, for those who experienced a CVD death versus a non‐CVD death.
To estimate the absolute probability of CVD mortality during follow‐up by frailty status category, we used 2 approaches: (1) a traditional Kaplan–Meier survival method that treats non‐CVD death as a censored observation and (2) a cumulative incidence function that considers non‐CVD mortality as a competing risk.30 Similarly, we used 2 approaches (with and without accounting for the competing risk of non‐CVD death) to determine adjusted associations of frailty status category with risk of CVD mortality. Men whose cause of death was uncertain were censored in both analyses. Taking robust individuals as the referent group, we used conventional Cox proportional hazards regression models that treat non‐CVD mortality as an uninformative censoring event and subdistribution hazards models proposed by Fine and Gray21, 31 that consider non‐CVD death as a competing risk. In subdistribution models, men who died of non‐CVD mortality are treated as an informative event and remain in the risk set until the end of study follow‐up.30 This permits estimation of the hazard ratio of CVD mortality, which takes competing events into consideration. If the incidence rate of the competing risk is moderate to high, subdistribution hazards ratios tend to be smaller in magnitude than those from traditional Cox proportional hazards regression because those with the competing event are usually more similar to those with the outcome of interest than those who are event‐free.
In multivariable analysis the associations were first adjusted for age, race, and site (base models). Multivariable models were further adjusted for selected risk factors for CVD death including smoking status, stroke, diabetes mellitus, hypertension, coronary heart disease, peripheral vascular disease, valvular heart disease, congestive heart failure, and chronic obstructive lung disease.
In secondary analyses, we repeated the above analyses among the subgroup of men with prevalent CVD at baseline. Among the overall cohort, we also estimated the absolute probability and adjusted risk of non‐CVD mortality according to frailty status in men, using statistical approaches with and without consideration of CVD mortality as a competing risk. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
The mean age of the men who participated in the study was 76.4±5.6 years. Of the 3135 men, 475 (15.2%) were categorized as frail, 1717 (54.8%) were intermediate stage, and 943 (30.1%) were robust (Table 1). Greater frailty status was associated with older age and higher prevalence of comorbid medical conditions (Table 1). Among the subset of men with prevalent CVD at baseline (n=1103, 35.2% of the entire cohort), 22.1% were frail and 20.1% were robust.
Table 1.
Characteristics of 3135 Men at MrOS Baseline Sleep Visit According to Frailty Status
| Characteristic | Frail (n=475) | Intermediate Stage (n=1717) | Robust (n=943) | P Value |
|---|---|---|---|---|
| Age, y, mean (SD) | 80.1 (5.9) | 76.5 (5.4) | 74.5 (4.7) | <0.001 |
| White race, n (%) | 425 (89.5) | 1545 (90.0) | 846 (89.7) | 0.94 |
| Smoking status | 0.28 | |||
| Never | 168 (35.4) | 676 (39.4) | 391 (41.5) | |
| Former | 295 (62.2) | 1005 (58.5) | 534 (56.7) | |
| Current | 11 (2.3) | 36 (2.1) | 17 (1.8) | |
| Selected medical conditions, n (%) | ||||
| Stroke | 37 (7.8) | 63 (3.7) | 17 (1.8) | <0.001 |
| Diabetes mellitus | 106 (22.4) | 228 (13.3) | 83 (8.8) | <0.001 |
| Hypertension | 345 (72.8) | 1054 (61.4) | 448 (47.5) | <0.001 |
| Coronary heart disease | 199 (41.9) | 544 (31.7) | 180 (19.1) | <0.001 |
| Peripheral vascular disease | 74 (15.6) | 132 (7.7) | 46 (4.9) | <0.001 |
| Valvular heart disease | 18 (3.8) | 42 (2.4) | 7 (0.7) | <0.001 |
| Congestive heart failure | 60 (12.7) | 102 (5.9) | 31 (3.3) | <0.001 |
| COPD | 38 (8.0) | 95 (5.5) | 31 (3.3) | 0.001 |
| Cancer | 147 (30.9) | 506 (29.5) | 222 (23.5) | 0.001 |
| CKDa | 220 (48.8) | 490 (29.3) | 165 (17.9) | <0.0001 |
CKD indicates chronic kidney disease (estimated glomerular filtration rate <60 mL/min); COPD, chronic obstructive pulmonary disease; MrOS, Osteoporotic Fractures in Men.
Number missing: 101.
During an average follow‐up of 9.2±3.0 years, 1275 (40.7%) men died (445 [34.9%] CVD deaths, 828 [64.9%] non‐CVD deaths and 2 [0.2%] unadjudicated). The distribution of the frailty status was not significantly different between men who died of CVD versus non‐CVD causes (27.4% and 23.9%, respectively, were frail; 18.2% and 19.1%, respectively, were robust). Men with CVD death compared with those with non‐CVD death had a higher prevalence of hypertension, coronary heart disease, and congestive heart failure (Table S1).
Incidence and Risk of CVD Mortality by Traditional and Competing‐Risk Methods
The cumulative incidence of CVD mortality increased with greater frailty status using the traditional Kaplan–Meier method and the competing‐risk method (Table 2). However, estimates were lower using the competing‐risk method compared with the traditional approach, and the magnitude of the difference in probabilities estimated by the 2 methods was greater with increasing duration of follow‐up (Figure 1). Cumulative absolute probability of CVD mortality among frail men, for example, was 13.2% (95% confidence interval [CI], 10.9–15.7%) at 5 years and 32.5% (95% CI, 27.3–37.8%) at 10 years using the traditional Kaplan–Meier method versus 11.7% (95% CI, 9.8–13.9%) at 5 years and 23.8% (95% CI, 20.2–27.6%) at 10 years using the competing‐risk method (Table 2).
Table 2.
Cumulative Incidence of CVD and Non‐CVD Mortality in Relation to Frailty Status Using Kaplan–Meier versus Competing‐Risk Method
| Frailty Status | Cumulative Incidence, % (95% CI) | |||
|---|---|---|---|---|
| Kaplan–Meier Method | Competing Risk Method | |||
| 5 y | 10 y | 5 y | 10 y | |
| CVD Mortality | ||||
| Robust | 2.4 (2.2–2.6) | 8.0 (7.0–9.0) | 2.3 (2.1–2.6) | 7.4 (6.6–8.4) |
| Intermediate | 5.3 (4.9–5.8) | 15.3 (13.9–16.8) | 5.0 (4.6–5.5) | 13.1 (12.0–14.3) |
| Frail | 13.2 (10.9–15.7) | 32.5 (27.3–37.8) | 11.7 (9.8–13.9) | 23.8 (20.2–27.6) |
| Non‐CVD mortality | ||||
| Robust | 4.6 (4.1–5.2) | 15.7 (13.8–17.6) | 4.6 (4.1–5.1) | 15.0 (13.3–16.8) |
| Intermediate | 9.9 (9.0–10.8) | 27.4 (25.3–29.6) | 9.6 (8.8–10.5) | 25.4 (23.5–27.4) |
| Frail | 22.9 (19.4–26.7) | 48.6 (43.6–53.4) | 21.4 (18.1–24.8) | 41.5 (36.9–45.9) |
CI indicates confidence interval; CVD, cardiovascular disease.
Figure 1.
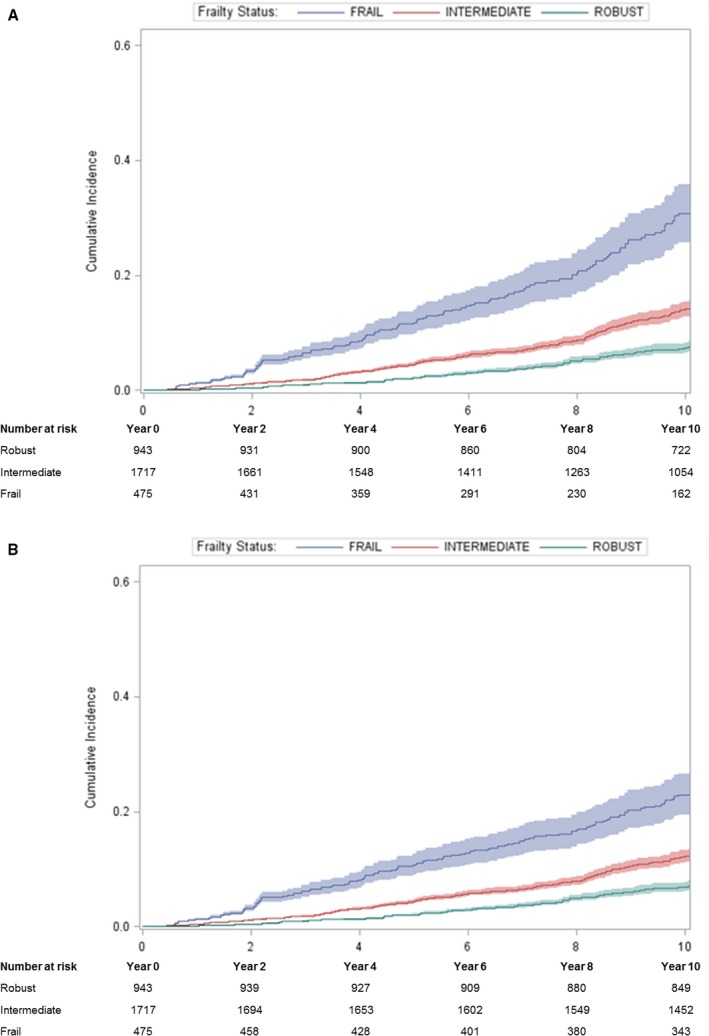
Cumulative absolute probabilities of CVD mortality in relation to frailty status using (A) the Kaplan–Meier method and (B) the competing risk method. CVD indicates cardiovascular disease.
Those who were frail or intermediate stage had a higher risk of CVD mortality than those who were robust, using both the traditional Cox proportional hazards model and the subdistribution (competing‐risk) model, but point estimates of the association were lower using the competing‐risk approach (Table 3). Compared with robust men, the age‐ and race‐adjusted risk of CVD mortality among frail men was 2.73‐fold higher (95% CI, 2.03–3.68) using the traditional Cox model versus 1.98‐fold higher (95% CI, 1.45–2.71) using the competing‐risk model (Table 3). After further adjustment for smoking and comorbid medical conditions (stroke, diabetes mellitus, hypertension, coronary heart disease, peripheral vascular disease, valvular heart disease, congestive heart failure, and chronic obstructive lung disease), the adjusted risk of CVD mortality among frail men was 1.84‐fold higher (95% CI, 1.35–2.51) using the traditional Cox model versus 1.38‐fold higher (95% CI, 0.99–1.92) using the using the competing‐risk model.
Table 3.
Traditional Cox Proportional Hazards Models and Subdistribution Competing‐Risk Models for Association of Frailty Status With CVD and Non‐CVD Mortality
| Frailty Status | Hazard Ratio (95% CI)a | |||||
|---|---|---|---|---|---|---|
| CVD Mortality | Non‐CVD Mortality | |||||
| Events, n | Cox Proportional Hazards Model | Subdistribution Model | Events, n | Cox Proportional Hazards Model | Subdistribution Model | |
| Robust | 81 | 1.00 (referent) | 1.00 (referent) | 158 | 1.00 (referent) | 1.00 (referent) |
| Intermediate stage | 242 | 1.55 (1.20–1.99) | 1.38 (1.07–1.78) | 472 | 1.62 (1.35–1.94) | 1.56 (1.30–1.87) |
| Frail | 122 | 2.73 (2.03–3.68) | 1.98 (1.45–2.71) | 198 | 2.57 (2.06–3.21) | 2.16 (1.73–2.70) |
CI indicates confidence interval; CVD, cardiovascular disease.
Adjusted for site, age, and race.
Among the subgroup of 1103 men with prevalent CVD, the cumulative incidence of CVD mortality was higher than the whole cohort, but the association of frailty status with CVD mortality and the attenuation of estimates with competing‐risk analyses were similar to those among the overall cohort (results not shown).
Incidence and Risk of Non‐CVD Mortality by Traditional and Competing‐Risk Methods
The cumulative incidence of non‐CVD mortality among frail men, although higher than CVD mortality, also increased with greater frailty status. Estimates of the absolute probability of non‐CVD death were lower using the competing‐risk method compared with the traditional approach (Figure 2). However, the attenuation in estimates of absolute probabilities of non‐CVD mortality using the competing‐risk versus traditional approach was smaller in magnitude than that observed for the outcome of CVD death: 22.9% (95% CI, 19.4–26.7%) at 5 years and 48.6% (95% CI, 43.6–53.4%) at 10 years using the traditional Kaplan–Meier method versus 21.4% (95% CI, 18.1–24.8%) at 5 years and 41.5% (95% CI, 36.9–45.9%) at 10 years using the competing‐risk method (Table 2). Similarly, age‐ and race‐adjusted risk of non‐CVD mortality among frail men was 2.57‐fold higher (95% CI, 2.06–3.21) using the traditional Cox model versus 2.16‐fold higher (95% CI, 1.73–2.70) using the competing‐risk model (Table 3).
Figure 2.
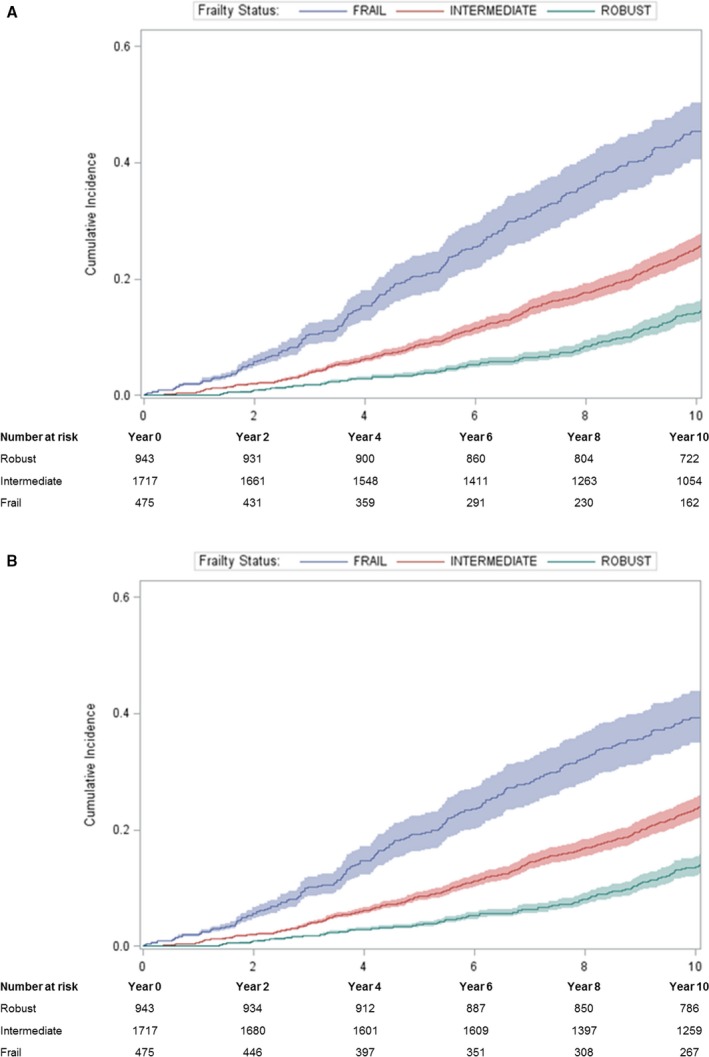
Cumulative absolute probabilities of non‐CVD mortality in relation to frailty status using (A) the Kaplan–Meier method and (B) the competing risk method. CVD indicates cardiovascular disease.
Discussion
Among this cohort of 3135 community‐dwelling older men, 45% died during an average follow‐up of 9.2 years with ≈35% of deaths adjudicated as due to CVD causes and 65% of deaths due to non‐CVD causes. Frail men compared with robust men had an increased risk of CVD mortality and non‐CVD mortality. Traditional analytic methods that ignored the competing risk of non‐CVD death among frail men substantially overestimated their long‐term absolute probability and relative risk of CVD death. Findings regarding comparison of traditional and competing‐risk approaches were similar when the analyses were restricted to men with prevalent CVD at baseline. These results suggest that the benefit of cardiovascular therapeutic interventions in reducing CVD mortality among frail older adults should be weighed against the high risk of competing non‐CVD mortality.
Frailty or its individual components, such as slowness, have been associated with higher odds of clinical and subclinical CVD and a higher risk of CVD mortality.1 In the Three‐City study, slow gait speed was associated with an increased risk of CVD death (hazard ratio: 2.9) but not mortality from cancer or other causes (hazard ratio: 1.0).32 In the EPESE (Established Populations for Epidemiologic Studies of the Elderly) study, impaired mobility was associated with a higher risk of CVD death.33 Among patients with peripheral arterial disease, frail men had a higher risk of CVD mortality.34, 35, 36 In our study, the multivariable‐adjusted risk of CVD death was 1.84 times higher among frail compared with robust men; this risk was similar in magnitude to that reported in previous studies. Nevertheless, in the analyses that considered non‐CVD death as a competing risk, the association between frailty and CVD death was reduced by 25% to 1.38. Considering the sizable burden of non‐CVD mortality in frail patients, studies assessing the association of frailty or its individual components with risk of CVD death should account for the competing risk of non‐CVD death.
Previous studies have shown that frailty is a factor in the decision of whether or not to implement a therapeutic cardiovascular intervention in frail patients with CVD. Compared with robust patients, frail patients with CVD are less likely to receive therapeutic CVD medications or be referred for invasive cardiovascular procedures.1, 18 The decision regarding whether or not to initiate a treatment or refer a patient to a cardiovascular intervention is complex and involves the clinician's personal intuition.37 Although frailty is associated with a higher risk of CVD mortality despite taking into account the competing risk of non‐CVD death, frail patients also have a higher risk of adverse outcomes after therapeutic CVD interventions.1 Previous studies have reported that preoperative frailty or gait speed was associated with higher postoperative mortality and morbidity.38, 39, 40, 41 A post hoc analysis from SCD‐HeFT (Sudden Cardiac Death in Heart Failure Trial) showed that patients who were unable to cover 288 m in a 6‐minute walk test were unlikely to have mortality reduction from implantable cardioverter–defibrillator implantation.42 Although the present analysis indicated that the likelihood of both CVD death and death from non‐CVD causes was highest among frail men, the latter was nearly twice as likely to be the cause of death (≈65% versus 35% of deaths) in this community‐dwelling cohort of older men. Our results suggest that the benefit of cardiovascular therapeutic interventions in reducing CVD mortality among frail older adults should be weighed against the high risk of competing non‐CVD mortality with corresponding greater uncertainty of benefit. Furthermore, reporting for clinical trials evaluating efficacy of cardiovascular interventions in older adults should include absolute risks and risk ratios for non‐CVD mortality in addition to total mortality to better ascertain study relevance to older frail adults and to evaluate possible non‐CVD benefits of those interventions. Inclusion of these results would better inform clinical decision‐making in the aged population.
More than 20 measures of frailty have been used in previous published reports, but no consensus exists about the best method to incorporate into clinical practice.1 Most tools measure ≥1 of the 5 core domains that define frailty: slowness, weakness, low physical activity, exhaustion, and shrinking.1 The method developed by Fried et al26 from the Cardiovascular Health Study has been one of the most frequently cited frailty tools and has been associated with mortality and disability in large cohorts of community‐dwelling older adults.1 This method, however, is not easy to incorporate into a clinical setting, and the distribution of some of its measures such as weakness, slowness, and physical activity requires knowledge of the quartiles in each patient population. Consequently, simpler methods for assessment of frailty status, such as the Study of Osteopathic Fractures scale and the Short Physical Performance Battery, have been developed and validated.4, 43, 44 In contrast to these multi‐item frailty scales, 5‐m gait speed has been advocated as a single component of frailty that has been associated with poor outcomes after CVD interventions.32, 45, 46 Recently, sarcopenia, assessed by psoas muscle area or pectoralis muscle volume, has been associated with poor outcomes after cardiac procedures.47, 48, 49, 50 Future research needs to evaluate the validity and clinical utility of simple frailty assessment tools that fit in the time constraints and competing demands of busy clinical practice.
Strengths and Limitations
A strength of this study cohort is the inclusion of community‐dwelling older men from 6 geographic locations in the United States, suggesting that the results can be generalized to other communities; however, the inclusion of only men (mostly white) is a limitation. Although the adjudication of the cause of death was performed independently using a prespecified protocol, determining the cause of death in older individuals may be inherently prone to error. Furthermore, although the greatest impact of cardiovascular therapeutic interventions is expected to be on CVD mortality, it is possible that these interventions could affect (reduce or increase) the risk of non‐CVD mortality. An intervention that prevents myocardial infarction, for example, could also reduce the risk of deconditioning and falls by way of maintaining mobility. Conversely, such an intervention (eg, coronary bypass surgery) could increase the risk of falls in the postoperative period by causing deconditioning.51
Conclusions
Among community‐dwelling older men, ≈35% of the deaths are due to CVD mortality and 65% are due to non‐CVD causes. Frailty is associated with a higher risk of both CVD and non‐CVD mortality in these men; therefore, CVD interventions may improve the survival of these patients. Nevertheless, non‐CVD death is a major competing event to a CVD death among frail men. Not taking into account this competing risk among frail men overestimates their long‐term absolute probability of CVD death and adjusted CVD mortality risk. The results of this study suggest that a competing‐risk approach should be used among frail patients, including those with prevalent CVD, to better inform CVD‐death risk assessment and clinical decision‐making.
Sources of Funding
The MrOS (Osteoporotic Fractures in Men) study is supported by National Institutes of Health funding. The National Institute on Aging, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Center for Advancing Translational Sciences, and NIH Roadmap for Medical Research provide support under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute provides funding for the MrOS Sleep (Outcomes of Sleep Disorders in Older Men) ancillary study under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. This article is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
Disclosures
None.
Supporting information
Table S1. Characteristics of Men Who Died From CVD vs Non‐CVD Causes
(J Am Heart Assoc. 2018;7:e008974 DOI: 10.1161/JAHA.118.008974.)29728373
References
- 1. Afilalo J, Alexander KP, Mack MJ, Maurer MS, Green P, Allen LA, Popma JJ, Ferrucci L, Forman DE. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;7:747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bell SP, Orr NM, Dodson JA, Rich MW, Wenger NK, Blum K, Harold JG, Tinetti ME, Maurer MS, Forman DE. What to expect from the evolving field of geriatric cardiology. J Am Coll Cardiol. 2015;7:1286–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett‐Connor E, Orwoll ES. Frailty in older men: prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;7:1216–1223. [DOI] [PubMed] [Google Scholar]
- 4. Ensrud KE, Ewing SK, Taylor BC, Fink HA, Cawthon PM, Stone KL, Hillier TA, Cauley JA, Hochberg MC, Rodondi N, Tracy JK, Cummings SR. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Intern Med. 2008;7:382–389. [DOI] [PubMed] [Google Scholar]
- 5. Ensrud KE, Ewing SK, Cawthon PM, Fink HA, Taylor BC, Cauley JA, Dam TT, Marshall LM, Orwoll ES, Cummings SR. A comparison of frailty indexes for the prediction of falls, disability, fractures, and mortality in older men. J Am Geriatr Soc. 2009;7:492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klein BE, Klein R, Knudtson MD, Lee KE. Frailty, morbidity and survival. Arch Gerontol Geriatr. 2005;7:141–149. [DOI] [PubMed] [Google Scholar]
- 7. McNallan SM, Chamberlain AM, Gerber Y, Singh M, Kane RL, Weston SA, Dunlay SM, Jiang R, Roger VL. Measuring frailty in heart failure: a community perspective. Am Heart J. 2013;7:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newman AB, Gottdiener JS, Mcburnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;7:M158–M166. [DOI] [PubMed] [Google Scholar]
- 9. Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;7:1321–1330. [DOI] [PubMed] [Google Scholar]
- 10. Ekerstad N, Swahn E, Janzon M, Alfredsson J, Lofmark R, Lindenberger M, Carlsson P. Frailty is independently associated with short‐term outcomes for elderly patients with non‐ST‐segment elevation myocardial infarction. Circulation. 2011;7:2397–2404. [DOI] [PubMed] [Google Scholar]
- 11. Murali‐Krishnan R, Iqbal J, Rowe R, Hatem E, Parviz Y, Richardson J, Sultan A, Gunn J. Impact of frailty on outcomes after percutaneous coronary intervention: a prospective cohort study. Open Heart. 2015;7:e000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shimura T, Yamamoto M, Kano S, Kagase A, Kodama A, Koyama Y, Tsuchikane E, Suzuki T, Otsuka T, Kohsaka S, Tada N, Yamanaka F, Naganuma T, Araki M, Shirai S, Watanabe Y, Hayashida K. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation. 2017;7:2013–2024. [DOI] [PubMed] [Google Scholar]
- 13. Singh M, Rihal CS, Lennon RJ, Spertus JA, Nair KS, Roger VL. Influence of frailty and health status on outcomes in patients with coronary disease undergoing percutaneous revascularization. Circ Cardiovasc Qual Outcomes. 2011;7:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Afilalo J, Lauck S, Kim DH, Lefevre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, Arora RC, Noiseux N, Rassi A, Palacios IF, Genereux P, Lindman BR, Asgar AW, Kim CA, Trnkus A, Morais JA, Langlois Y, Rudski LG, Morin JF, Popma JJ, Webb JG, Perrault LP. Frailty in older adults undergoing aortic valve replacement: the FRAILTY‐AVR study. J Am Coll Cardiol. 2017;7:689–700. [DOI] [PubMed] [Google Scholar]
- 15. Raza SS, Li JM, John R, Chen LY, Tholakanahalli VN, Mbai M, Adabag AS. Long‐term mortality and pacing outcomes of patients with permanent pacemaker implantation after cardiac surgery. Pacing Clin Electrophysiol. 2011;7:331–338. [DOI] [PubMed] [Google Scholar]
- 16. Vakil K, Roukoz H, Sarraf M, Krishnan B, Reisman M, Levy WC, Adabag S. Safety and efficacy of the MitraClip(R) system for severe mitral regurgitation: a systematic review. Catheter Cardiovasc Interv. 2014;7:129–136. [DOI] [PubMed] [Google Scholar]
- 17. Adabag S, Patton KK, Buxton AE, Rector TS, Ensrud KE, Vakil K, Levy WC, Poole JE. Association of implantable cardioverter defibrillators with survival in patients with and without improved ejection fraction: secondary analysis of the Sudden Cardiac Death in Heart Failure Trial. JAMA Cardiol. 2017;7:767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bibas L, Levi M, Touchette J, Mardigyan V, Bernier M, Essebag V, Afilalo J. Implications of frailty in elderly patients with electrophysiological conditions. JACC: Clin Electrophysiol. 2016;7:288–294. [DOI] [PubMed] [Google Scholar]
- 19. Hess PL, Al‐Khatib SM, Han JY, Edwards R, Bardy GH, Bigger JT, Buxton A, Cappato R, Dorian P, Hallstrom A, Kadish AH, Kudenchuk PJ, Lee KL, Mark DB, Moss AJ, Steinman R, Inoue LY, Sanders G. Survival benefit of the primary prevention implantable cardioverter‐defibrillator among older patients: does age matter? An analysis of pooled data from 5 clinical trials. Circ Cardiovasc Qual Outcomes. 2015;7:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naksuk N, Saab A, Li JM, Florea V, Akkaya M, Anand IS, Benditt DG, Adabag S. Incidence of appropriate shock in implantable cardioverter‐defibrillator patients with improved ejection fraction. J Card Fail. 2013;7:426–430. [DOI] [PubMed] [Google Scholar]
- 21. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;7:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ensrud KE, Harrison SL, Cauley JA, Langsetmo L, Schousboe JT, Kado DM, Gourlay ML, Lyons JG, Fredman L, Napoli N, Crandall CJ, Lewis CE, Orwoll ES, Stefanick ML, Cawthon PM. Impact of competing risk of mortality on association of weight loss with risk of central body fractures in older men: a prospective cohort study. J Bone Miner Res. 2017;7:624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. San Francisco Coordinating Center, University of California San Francisco and California Pacific Medical Center Research Institute. MrOS Online. Available at: https://mrosdata.sfcc-cpmc.net. Accessed January 4, 2018.
- 24. Blank JB, Cawthon PM, Carrion‐Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;7:557–568. [DOI] [PubMed] [Google Scholar]
- 25. Orwoll E, Blank JB, Barrett‐Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study—a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;7:569–585. [DOI] [PubMed] [Google Scholar]
- 26. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, Mcburnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;7:M146–M156. [DOI] [PubMed] [Google Scholar]
- 27. Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;7:165–173. [Google Scholar]
- 28. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;7:153–162. [DOI] [PubMed] [Google Scholar]
- 29. Koo BB, Blackwell T, Ancoli‐Israel S, Stone KL, Stefanick ML, Redline S; Osteoporotic Fractures in Men (MrOS) Study Group . Association of incident cardiovascular disease with periodic limb movements during sleep in older men: Outcomes of Sleep Disorders in Older Men (MrOS) Study. Circulation. 2011;7:1223–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J. 2004;7:103–112. [Google Scholar]
- 31. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;7:496–509. [Google Scholar]
- 32. Dumurgier J, Elbaz A, Ducimetiere P, Tavernier B, Alperovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. BMJ. 2009;7:b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Corti MC, Salive ME, Guralnik JM. Serum albumin and physical function as predictors of coronary heart disease mortality and incidence in older persons. J Clin Epidemiol. 1996;7:519–526. [DOI] [PubMed] [Google Scholar]
- 34. Garcia S, Marston N, Sandoval Y, Pierpont G, Adabag S, Brenes J, Santilli S, McFalls EO. Prognostic value of 12‐lead electrocardiogram and peak troponin I level after vascular surgery. J Vasc Surg. 2013;7:166–172. [DOI] [PubMed] [Google Scholar]
- 35. McDermott MM, Guralnik JM, Tian L, Ferrucci L, Liu K, Liao Y, Criqui MH. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;7:974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh S, Bailey KR, Noheria A, Kullo IJ. Frailty across the spectrum of ankle‐brachial index. Angiology. 2012;7:229–236. [DOI] [PubMed] [Google Scholar]
- 37. Jain R, Duval S, Adabag S. How accurate is the eyeball test?: a comparison of physician's subjective assessment versus statistical methods in estimating mortality risk after cardiac surgery. Circ Cardiovasc Qual Outcomes. 2014;7:151–156. [DOI] [PubMed] [Google Scholar]
- 38. Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;7:973–978. [DOI] [PubMed] [Google Scholar]
- 39. Sundermann S, Dademasch A, Praetorius J, Kempfert J, Dewey T, Falk V, Mohr FW, Walther T. Comprehensive assessment of frailty for elderly high‐risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;7:33–37. [DOI] [PubMed] [Google Scholar]
- 40. Sundermann S, Dademasch A, Rastan A, Praetorius J, Rodriguez H, Walther T, Mohr FW, Falk V. One‐year follow‐up of patients undergoing elective cardiac surgery assessed with the Comprehensive Assessment of Frailty test and its simplified form. Interact Cardiovasc Thorac Surg. 2011;7:119–123. [DOI] [PubMed] [Google Scholar]
- 41. Afilalo J, Eisenberg MJ, Morin JF, Bergman H, Monette J, Noiseux N, Perrault LP, Alexander KP, Langlois Y, Dendukuri N, Chamoun P, Kasparian G, Robichaud S, Gharacholou SM, Boivin JF. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;7:1668–1676. [DOI] [PubMed] [Google Scholar]
- 42. Fishbein DP, Hellkamp AS, Mark DB, Walsh MN, Poole JE, Anderson J, Johnson G, Lee KL, Bardy GH. Use of the 6‐min walk distance to identify variations in treatment benefits from implantable cardioverter‐defibrillator and amiodarone: results from the SCD‐HeFT (Sudden Cardiac Death in Heart Failure Trial). J Am Coll Cardiol. 2014;7:2560–2568. [DOI] [PubMed] [Google Scholar]
- 43. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;7:M85–M94. [DOI] [PubMed] [Google Scholar]
- 44. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;7:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Abellan KG, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette GS, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community‐dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;7:881–889. [DOI] [PubMed] [Google Scholar]
- 46. Ling CH, Taekema D, de Craen AJ, Gussekloo J, Westendorp RG, Maier AB. Handgrip strength and mortality in the oldest old population: the Leiden 85‐plus study. CMAJ. 2010;7:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Drudi LM, Phung K, Ades M, Zuckerman J, Mullie L, Steinmetz OK, Obrand DI, Afilalo J. Psoas muscle area predicts all‐cause mortality after endovascular and open aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2016;7:764–769. [DOI] [PubMed] [Google Scholar]
- 48. Garg L, Agrawal S, Pew T, Hanzel GS, Abbas AE, Gallagher MJ, Shannon FL, Hanson ID. Psoas muscle area as a predictor of outcomes in transcatheter aortic valve implantation. Am J Cardiol. 2017;7:457–460. [DOI] [PubMed] [Google Scholar]
- 49. Zuckerman J, Ades M, Mullie L, Trnkus A, Morin JF, Langlois Y, Ma F, Levental M, Morais JA, Afilalo J. Psoas muscle area and length of stay in older adults undergoing cardiac operations. Ann Thorac Surg. 2017;7:1498–1504. [DOI] [PubMed] [Google Scholar]
- 50. Teigen LM, John R, Kuchnia AJ, Nagel EM, Earthman CP, Kealhofer J, Martin C, Cogswell R. Preoperative pectoralis muscle quantity and attenuation by computed tomography are novel and powerful predictors of mortality after left ventricular assist device implantation. Circ Heart Fail 2017;7:772–776. [DOI] [PubMed] [Google Scholar]
- 51. Garcia S, Ko B, Adabag S. Contrast‐induced nephropathy and risk of acute kidney injury and mortality after cardiac operations. Ann Thorac Surg. 2012;94:772–776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of Men Who Died From CVD vs Non‐CVD Causes


