Abstract
Background
Intensive care unit (ICU) use for initially stable patients presenting with non–ST‐segment–elevation myocardial infarction (NSTEMI) varies widely across hospitals and minimally correlates with severity of illness. We aimed to develop a bedside risk score to assist in identifying high‐risk patients with NSTEMI for ICU admission.
Methods and Results
Using the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry linked to Medicare data, we identified patients with NSTEMI aged ≥65 years without cardiogenic shock or cardiac arrest on presentation. Complications requiring ICU care were defined as subsequent development of cardiac arrest, shock, high‐grade atrioventricular block, respiratory failure, stroke, or death during the index hospitalization. We developed and validated a model and integer risk score (Acute Coronary Treatment and Intervention Outcomes Network (ACTION) ICU risk score) that uses variables present at hospital admission to predict requirement for ICU care. Of 29 973 patients with NSTEMI, 4282 (14%) developed a complication requiring ICU‐level care, yet 12 879 (43%) received care in an ICU. Signs or symptoms of heart failure, initial heart rate, initial systolic blood pressure, initial troponin, initial serum creatinine, prior revascularization, chronic lung disease, ST‐segment depression, and age had statistically significant associations with requirement for ICU care after adjusting for other risk factors. The ACTION ICU risk score had a C‐statistic of 0.72. It identified 11% of patients as having very high risk (>30%) of developing complications requiring ICU care and 49% as having low likelihood (<10%) of requiring an ICU.
Conclusions
The ACTION ICU risk score quantifies the risk of initially stable patients with NSTEMI developing a complication requiring ICU care, and could be used to more effectively allocate limited ICU resources.
Keywords: intensive care unit, model, non–ST‐segment acute coronary syndrome, risk prediction risk score, risk score
Subject Categories: Health Services, Complications, Acute Coronary Syndromes
Clinical Perspective
What Is New?
Of initially stable patients with non–ST‐segment–elevation myocardial infarction, 43% are treated in an intensive care unit (ICU), but only 14% ultimately develop complications requiring ICU care during their hospital stay.
Nine variables available on presentation to the emergency department predict the development of complications requiring ICU care: signs or symptoms of heart failure, initial heart rate, initial systolic blood pressure, initial troponin, initial serum creatinine, prior revascularization, chronic lung disease, ST‐segment depression, and age.
An integer risk score (the Acute Coronary Treatment and Intervention Outcomes Network; ACTION ICU score) based on these variables predicts a patient's risk of developing complications requiring ICU care.
What Are the Clinical Implications?
Current practice patterns suggest that hospitals do not use objective markers of risk when making ICU admission decisions for initially stable patients with non–ST‐segment–elevation myocardial infarction, and no prior risk score existed to predict need for ICU care.
Hospitals and providers can use the ACTION ICU risk score to guide risk‐based location of care decision making for initially stable patients with non–ST‐segment–elevation myocardial infarction by selecting a threshold score and admitting patients with non–ST‐segment–elevation myocardial infarction with scores greater than the threshold to the ICU and scores less than the threshold to a non‐ICU bed.
Considerable interhospital variability in intensive care unit (ICU) use has been observed for patients with non–ST‐segment–elevation myocardial infarction (NSTEMI).1, 2, 3, 4 ICU use is only minimally correlated with severity of illness, with many low‐risk patients treated in the ICU and many high‐risk patients not treated in the ICU.4 In one study, 41% of patients with NSTEMI with a predicted risk of in‐hospital mortality <1% were treated in the ICU, and more than half of patients with initial serum troponin elevations >10 times the institution's upper limit of normal were not treated in an ICU.4 Given non–risk‐driven use patterns, it is perhaps unsurprising that higher hospital‐level ICU use is not associated with lower patient mortality.1, 2, 3, 4
ICU care is considerably more expensive than care in a general or step‐down hospital unit, but 1 in 8 US hospitals routinely treat hemodynamically stable patients with NSTEMI in the ICU.4, 5 Among initially stable patients with NSTEMI, only a minority deteriorate clinically while hospitalized and require ICU‐level care for stabilization of critical illness. Rational resource use decision making requires an early risk stratification tool that can be practically implemented to guide selective risk‐based ICU use.6 Although several mortality risk scores have been developed for patients with NSTEMI,7, 8, 9, 10 the performance of these risk scores has not been evaluated for the purpose of predicting need for ICU level of care among initially stable patients with NSTEMI, nor has a risk score been developed for this purpose.
The Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry collects detailed clinical data for consecutive patients with MI treated in routine practice in the United States.11, 12 Using this registry, linked to Centers for Medicare and Medicaid Services data, we aimed to derive and validate a multivariable model and bedside risk score (the ACTION ICU risk score) that uses clinical information available at the time of hospital admission to predict the in‐hospital development of a complication requiring ICU care, including cardiac arrest, shock, heart block requiring pacemaker placement, stroke, respiratory failure, or death.
Methods
The data, analytic methods, and study materials cannot be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patient Population
The ACTION Registry is a quality improvement registry that captures consecutive patients presenting to participating hospitals with ischemic symptoms and diagnosed with STEMI or NSTEMI.11, 12 Patients ≥65 years old in the ACTION Registry have previously been linked to their Centers for Medicare and Medicaid Services claims data using 5 indirect identifiers (date of birth, sex, hospital identifier, date of admission, and date of discharge).13, 14, 15 Of 44 915 NSTEMI admissions in the linked database between April 1, 2011, and December 31, 2012, we excluded 12 433 patients who initially presented to hospitals that do not participate in the ACTION Registry, and were transferred from those hospitals into ACTION Registry hospitals, because triage decisions for these patients may involve uncaptured patient characteristics (such as hospital course up to the time of transfer) and hospital transfer policies. We excluded patients with cardiac arrest (n=688) or cardiogenic shock (n=473) on admission, because these patients have a clear requirement for ICU care. For patients with multiple MI admissions during the study period (n=1348), follow‐up began at the start of the first admission. The prediction model and ACTION ICU risk score were therefore constructed using data for 29 973 unique patients presenting to 499 hospitals (Figure 1).
Figure 1.
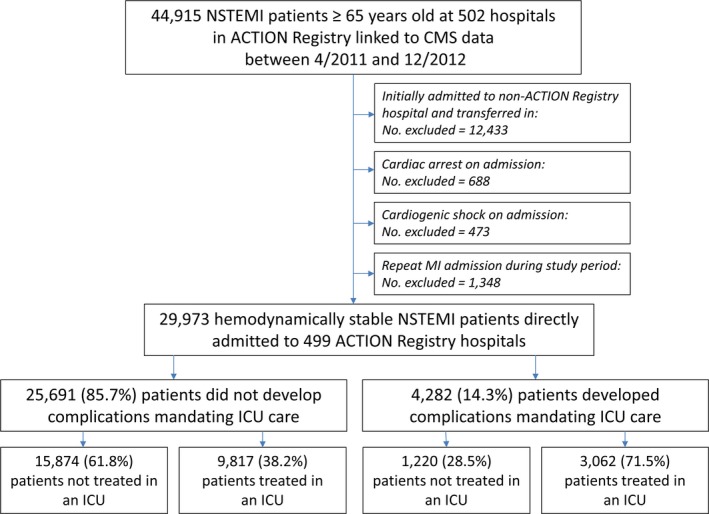
Study flow. ACTION indicates Acute Coronary Treatment and Intervention Outcomes Network; CMS, Centers for Medicare and Medicaid Services; ICU, intensive care unit; NSTEMI, non–ST‐segment–elevation myocardial infarction.
Data Definitions
This risk model was designed to predict, at the time of admission, the subsequent development of in‐hospital complications requiring ICU care. We defined complications requiring ICU care as cardiac arrest, shock, heart block requiring pacemaker placement, respiratory failure, stroke, or death occurring any time during the hospitalization. These complications were chosen on the basis of clinical judgment and reflect events that frequently require ICU interventions, such as use of vasopressors, mechanical hemodynamic support devices, mechanical ventilation, central venous access, and invasive hemodynamic monitoring. Complications were identified both by the ACTION Registry data collection form and Centers for Medicare and Medicaid Services claims data (Table S1). Candidate variables for the model were identified from a list of all ACTION Registry variables and medical history variables from Centers for Medicare and Medicaid Services claims data that would be known to a provider determining location of care at the time of hospital admission (Data S1).16, 17, 18
Statistical Analysis
Baseline patient characteristics and laboratory results were stratified by requiring ICU care status. Categorical variables were reported as frequencies with percentages, and continuous variables were reported as medians with 25th and 75th percentiles. χ2 and Wilcoxon rank‐sum tests were used to compare categorical and continuous variables, respectively.
Before building a model, continuous variables were investigated for nonlinearity, and plots of continuous variables versus outcomes were reviewed to create linear splines. We created a multivariable logistic regression model using generalized estimating equations and backward selection of candidate variables (α to stay=0.05) for the primary outcome of complications requiring ICU care.19 Generalized estimating equations were used to account for clustering of outcomes by hospital. A more parsimonious model was derived by removing the least important factors, on the basis of χ2, stopping when the linear predictor for the reduced model maintained 98% of the predictive power of the full model.20 The predictive performance of the final multivariable model was assessed for discrimination by the C‐statistic and calibration by plotting observed versus predicted probabilities for each decile of predicted risk. The model was validated by bootstrapping, and this same procedure was used to generate confidence intervals for the C‐statistic. A point score for predicting requirement for ICU care (ACTION ICU risk score) was derived by categorizing continuous variables and assigning point values to each predictor according to its strength of association with the primary end point. Points were assigned on the natural log scale, and the estimated coefficients were converted to the nearest whole number that best preserved the relative magnitude. The accuracy of this approximation was checked by calculating the R2 for the point score as a predictor of the continuous X*Beta20 and the R2 for event rates associated with the point score as a predictor of the continuous model event probabilities across patients. We validated the multivariable model and ACTION ICU risk score by deriving an optimism‐corrected C‐statistic.21 We calculated the in‐hospital mortality rate for patients in different ACTION ICU risk score strata, and we identified the number of patients in each stratum who died without being treated in the ICU. For comparison, we recalibrated the Global Registry of Acute Coronary Events (GRACE) mortality risk score8 in our derivation cohort and calculated a C‐statistic to predict complications requiring ICU care.
For categorical variables, missing values were imputed by the most frequent group. Rates of missing values were <1% for all candidate variables. All statistical analyses were performed at the Duke Clinical Research Institute using SAS software, version 9.4 (SAS Institute). This project was supported by a grant from the Agency for Healthcare Research and Quality (U19H2O21092). The Duke University Medical Center Institutional Review Board approved this study and granted a waiver of informed consent and authorization.
Results
Among 29 973 patients with NSTEMI ≥65 years old without cardiac arrest or cardiogenic shock on presentation who were admitted to 499 hospitals across the United States, the median age was 78 years; 46.7% were women, and 37.2% of these patients had at least 1 inpatient admission in the prior year (Table 1). Overall, 4282 patients (14.3%) developed a complication requiring ICU care: 720 (2.4%) developed cardiac arrest, 1228 (4.1%) developed shock, 411 (1.4%) had heart block requiring pacemaker placement, 2774 (9.3%) had respiratory failure or were mechanically ventilated, 185 (0.6%) developed a stroke or intracranial hemorrhage, and 1196 (4.0%) died. Among patients who developed at least 1 complication requiring ICU care, 1420 (33.2%) had >1 complication. Among patients who died, 935 (78.2%) had at least 1 other complication.
Table 1.
Patient Characteristics
| Characteristics | Overall (N=29 973) | ICU‐Requiring Complication (n=4282) | No Complication (n=25 691) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 77 (71–84) | 80 (73–86) | 77 (70–84) | <0.001 |
| Female sex | 13 996 (46.7) | 2159 (50.4) | 11 837 (46.1) | <0.001 |
| Nonwhite race | 4364 (14.6) | 625 (14.6) | 3739 (14.6) | 0.94 |
| Medical history | ||||
| Diabetes mellitus | 11 989 (40.0) | 1892 (44.2) | 10 097 (39.3) | <0.001 |
| Hypertension | 25 943 (86.6) | 3738 (87.3) | 22 205 (86.4) | 0.13 |
| Prior MI | 9249 (30.9) | 1335 (31.2) | 7914 (30.8) | 0.63 |
| Prior HF | 7158 (23.9) | 1516 (35.4) | 5642 (22.0) | <0.001 |
| Prior PCI | 8615 (28.7) | 989 (23.1) | 7626 (29.7) | 0.001 |
| Prior CABG | 7343 (24.5) | 966 (22.6) | 6377 (24.8) | <0.001 |
| Prior atrial fibrillation | 4482 (15.0) | 768 (17.9) | 3714 (14.5) | <0.001 |
| Prior stroke | 4032 (13.5) | 726 (17.0) | 3306 (12.9) | <0.001 |
| Peripheral arterial disease | 4844 (16.2) | 814 (19.0) | 4030 (15.7) | <0.001 |
| Current/recent smoker | 3851 (12.8) | 587 (13.7) | 3264 (12.7) | 0.07 |
| Chronic lung disease | 6294 (21.0) | 1269 (29.6) | 5025 (19.6) | <0.001 |
| Current dialysis | 1192 (4.0) | 254 (5.9) | 938 (3.7) | <0.001 |
| Alcohol abusea | 120 (0.4) | 22 (0.5) | 98 (0.4) | 0.20 |
| Dementiaa | 630 (2.1) | 135 (3.2) | 495 (1.9) | <0.001 |
| Liver diseasea | 184 (0.6) | 33 (0.8) | 151 (0.6) | 0.16 |
| Hypothyroidisma | 2187 (7.3) | 402 (9.4) | 1785 (6.9) | <0.001 |
| Drug abusea | 54 (0.2) | 9 (0.2) | 45 (0.2) | 0.62 |
| Any hospital admissiona | 11 159 (37.2) | 1951 (45.6) | 9208 (35.8) | <0.001 |
| Signs and symptoms at presentation | ||||
| Weight, kg | 77 (65–91) | 74 (63–88) | 78 (66–91) | <0.001 |
| Height, cm | 168 (160–177) | 168 (160–175) | 168 (160–178) | <0.001 |
| HF | 7566 (25.2) | 2085 (48.7) | 5481 (21.3) | <0.001 |
| Heart rate, beats/min | 85 (72–101) | 94 (77–110) | 84 (71–100) | <0.001 |
| Systolic blood pressure, mm Hg | 146 (125–169) | 137 (115–160) | 148 (128–170) | <0.001 |
| New or presumed new ST‐segment depression | 6118 (20.4) | 1037 (24.2) | 5081 (19.8) | <0.001 |
| Initial laboratory values | ||||
| Troponin ratio, ×ULN | 2.4 (0.7–12.0) | 4.4 (1.0–26.3) | 2.3 (0.6–10.7) | <0.001 |
| Serum creatinine, mg/dLb | 1.1 (0.9–1.5) | 1.3 (1.0–1.8) | 1.1 (0.9–1.4) | <0.001 |
| Hemoglobin, g/dL | 12.9 (11.4–14.2) | 12.3 (10.7–13.7) | 13.0 (11.5–14.3) | <0.001 |
Categorical variables are presented as frequency (percentage); continuous variables are presented as median (25th–75th percentile). CABG indicates coronary artery bypass graft surgery; HF, heart failure; ICU, intensive care unit; MI, myocardial infarction; PCI, percutaneous coronary intervention; ULN, upper limit of normal.
Within the past 12 months from the index admission (not including the index admission).
Among nondialysis patients.
Although 4282 patients with NSTEMI (14.3%) developed a complication requiring ICU‐level care, 12 879 (43.0%) received care in an ICU. Of the patients who were treated in an ICU, only 3062 (23.8%) had a complication requiring ICU care. Of the 25 691 patients without any ICU‐requiring complications, 9817 (38.2%) were treated in an ICU (Figure 1).
Univariable associations between baseline characteristics and requirement for ICU care are shown in Table S2. The final multivariable prediction model is shown in Table 2; the equation is provided in Data S2. Nine variables were included in the final multivariable prediction model and ACTION ICU risk score: signs or symptoms of heart failure on first medical contact, initial heart rate, initial systolic blood pressure, initial troponin level, initial serum creatinine level, prior coronary revascularization, chronic lung disease, ST‐segment depression on the ECG, and age. The C‐statistic (95% confidence interval) for the 9‐variable model was 0.73 (0.72–0.74), and the calibration curve is shown in Figure 2. Figure S1 shows calibration curves in sex and age subgroups. Overall, and in all subsets, differences between observed and expected probabilities were small relative to the spread of predicted probabilities, indicating good calibration. The integer‐based ACTION ICU risk score (Table 3) had similar discrimination: C‐statistic, 0.72 (95% confidence interval, 0.71–0.73). The R2 for the point score as a predictor of the continuous X*Beta was 0.95. The R2 for event rates associated with the point score as a predictor of the continuous model event probabilities (across patients) was 0.94, indicating that the approximation of the multivariable model with an integer‐based risk score was successful and little information was lost. Internal validation indicated low optimism in observed C‐statistics, <0.001, and the optimism‐corrected C‐statistic did not change. For comparison, the correlation between the recalibrated GRACE risk score and the likelihood of a patient developing complications requiring ICU care (Figure S2) was less linear, with a C‐statistic of 0.69.
Table 2.
Multivariable Model of In‐Hospital Development of Complications Requiring ICU Care
| Variables | Odds Ratio | 95% Confidence Interval | χ2 |
|---|---|---|---|
| Signs or symptoms of heart failure | 2.64 | 2.42–2.87 | 506 |
| Heart rate | |||
| Per 5 bpm ↑ and <75 bpm | 0.90 | 0.87–0.94 | 218 |
| Per 5 bpm ↑ and ≥75 bpm | 1.12 | 1.10–1.14 | |
| Initial serum creatinine (per 1 mg/dL ↑) | 1.49 | 1.41–1.59 | 167 |
| Systolic blood pressure (per 10 mm Hg ↑) | 0.90 | 0.89–0.92 | 164 |
| Initial troponin ratio (per 5× ULN ↑) | 1.07 | 1.05–1.08 | 128 |
| Chronic lung disease | 1.42 | 1.31–1.55 | 65 |
| Prior revascularization | 0.76 | 0.70–0.82 | 52 |
| New or presumed new ST‐segment depressiona | 1.24 | 1.14–1.35 | 24 |
| Age (per 5 year ↑) | 1.07 | 1.04–1.10 | 20 |
Bpm indicates beats per minute; ICU, intensive care unit; ULN, upper limit of normal.
vs T‐wave inversion, transient ST‐segment elevation lasting <20 minutes, or none.
Figure 2.

Calibration curve of the multivariable model. Line graph shows observed vs predicted probability of developing a complication requiring intensive care unit (ICU) care by decile of predicted risk.
Table 3.
ACTION ICU Risk Score
| Age, y | Points | Serum Creatinine, mg/dL | Points | Heart Rate, beats/min | Points | Systolic Blood Pressure, mm Hg | Points | Initial Troponin, ×ULN | Points |
|---|---|---|---|---|---|---|---|---|---|
| <70 | 0 | <1.1 | 0 | <85 | 0 | <125 | 3 | <12 | 0 |
| ≥70 | 1 | ≥1.1 | 1 | 85–100 | 1 | 125–145 | 1 | ≥12 | 2 |
| ≥100 | 3 | ≥145 | 0 |
| Signs or Symptoms of HF | Points | ST Depression | Points | Prior Revascularization | Points | Chronic Lung Disease | Points |
|---|---|---|---|---|---|---|---|
| No | 0 | No | 0 | No | 1 | No | 0 |
| Yes | 5 | Yes | 1 | Yes | 0 | Yes | 2 |
To calculate a patient's risk score, add up the point values for each variable. For example, a patient who is 66 years old gets 0 points, and a patient who is 72 years old gets 1 point; a patient with signs or symptoms of heart failure on presentation gets 5 points, and a patient without heart failure signs or symptoms gets 0 points. Scores can range from 0 to 19 points. ACTION indicates Acute Coronary Treatment and Intervention Outcomes Network; HF, heart failure; ICU, intensive care unit; ULN, upper limit of normal.
Patient Risk Prediction
Figure 3 shows the proportion of patients with each ACTION ICU risk score developing a complication requiring ICU care; the likelihood of developing a complication requiring ICU care increased >10‐fold from the lowest to the highest scores, from 3.4% to 39.3%. Overall, 14.6% of patients had an ACTION ICU risk score ≤2, corresponding to a very low likelihood (<5%) of clinical deterioration requiring ICU care; 48.5% of patients had a score ≤5, corresponding to a <10% risk of requiring ICU care, and 11.3% of patients had an ACTION ICU risk score ≥12, corresponding to a >30% risk of requiring ICU care. The sensitivity, specificity, positive predictive value, and negative predictive value of each selected ACTION ICU risk score threshold are shown in Table S3.
Figure 3.
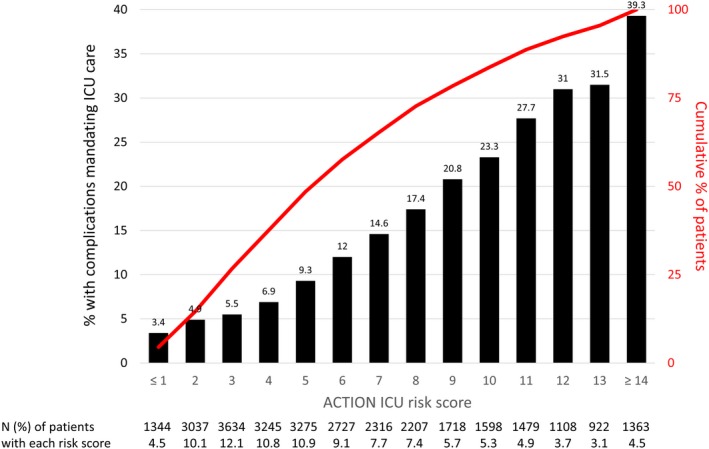
Proportion of patients requiring intensive care unit (ICU) care by Acute Coronary Treatment and Intervention Outcomes Network (ACTION) ICU risk score. Bar graph shows the proportion of patients developing complications requiring ICU care for each ACTION ICU score, and overlaid line graph shows the cumulative proportion of patients with a score less than or equal to each score.
Of the 4282 patients who developed a complication requiring ICU care, 478 died (11.2%) and were never cared for in the ICU. Table S4 shows the proportion of patients who died and those who died without receiving ICU care, stratified by ACTION ICU risk score. Among 4381 patients who had ACTION ICU risk score ≤2, 17 (0.4%) died without being treated in the ICU. In contrast, 150 of 3393 patients (4.4%) with ACTION ICU risk score ≥12 died without ever being treated in the ICU.
Discussion
The ACTION ICU risk score uses 9 variables present at the time of admission to predict the likelihood that a hemodynamically stable patient presenting with NSTEMI will develop an in‐hospital complication that requires ICU care. This risk score is of clinical importance given that 43% of patients with NSTEMI without cardiogenic shock or cardiac arrest are treated in the ICU in contemporary practice, yet limited and expensive ICU resources may only be needed in a smaller proportion of these patients.4 Use of the risk score could help hospitals identify patients at highest risk of clinical deterioration requiring ICU care for direct admission to the ICU, while safely admitting lower‐risk patients to a non‐ICU setting.
Risk Prediction in Patients With NSTEMI
Goldman et al developed a risk score to predict need for intensive care in patients presenting with acute chest pain, but this model was derived using data from the 1980s when early invasive management was not routinely used.22 The GRACE Freedom‐From‐Events score was developed to predict in‐hospital adverse events, including recurrent MI, arrhythmia, heart failure, shock, bleeding, stroke, and death.23 Many of these adverse events (eg, atrial fibrillation) do not necessitate ICU‐level care unless patients are hemodynamically unstable. Several mortality risk scores have been derived from registry, clinical trial, and administrative databases,7, 8, 9, 10, 24, 25 but none of these have been evaluated for the purpose of predicting fatal or nonfatal complications requiring ICU care. The ACTION ICU risk score is thus the first to predict the need for ICU care in a contemporary population with NSTEMI.
Our risk score overlaps with mortality risk scores in variables such as age, ST‐segment depression, blood pressure, and cardiac biomarker elevation7, 8, 24; these are clinical factors associated with infarct size that correlate with the likelihood of developing ICU‐requiring cardiac complications. Unlike MI mortality risk scores, the ACTION ICU score includes chronic lung disease, likely because of an association between chronic lung disease and development of respiratory failure requiring ICU care.26 Although prior coronary artery disease is typically a marker of higher risk in mortality risk models,7 prior revascularization was associated with lower likelihood of developing in‐hospital complications requiring ICU care. Patients with prior revascularization may present earlier after symptom onset,27, 28 with earlier treatment associated with lower likelihood of complications.
The ACTION ICU risk score demonstrated good predictive ability compared with risk scores commonly applied in the population with acute coronary syndromes, such as the Thrombolysis in Myocardial Infarction mortality risk score (C‐statistic, 0.65) or the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress Adverse outcomes with Early implementation of the American College of Cardiology/American Heart Association guidelines) bleeding score (C‐statistic, 0.71).7, 29 The risk of complications requiring ICU care ranged from 3.4% for patients with ACTION ICU risk scores ≤1 to 39.3% for patients with ACTION ICU risk scores ≥14. The ACTION ICU score performed better than the GRACE risk score in predicting in‐hospital complications requiring ICU care, which was expected, because the GRACE risk score was not developed to predict this outcome. More important, despite the GRACE risk score's long track record as a mortality prediction score, its performance for the prediction of ICU‐requiring complications has not been previously evaluated.
Clinical Implications of Risk‐Based ICU Admission
Nationally, the total number of critical care beds has increased by 6.5% even as the total number of hospital beds has decreased by 4.2%.30 A total of 1 in 8 hospitals currently treat >70% of their patients with NSTEMI in the ICU, and several recent reports have identified considerable interhospital variability in ICU use for these patients both in the United States and internationally, with current ICU triaging decisions based largely on local practices or provider preferences.1, 2, 3, 4 In the United States, ≈40% of low‐risk patients with NSTEMI are treated in the ICU; this approach likely expends unnecessary ICU resources on the large number of patients with NSTEMI who are low risk and can be safely monitored and treated in a non‐ICU setting.4 Hospital charges for care in the ICU are >$3500 per day greater than for care in a non‐ICU ward.5 With increasing prevalence of bundled payments that provide fixed per‐patient reimbursement for patients with MI in the United States, hospitals are likely to scrutinize ICU resource use patterns to reduce unnecessary ICU use.31
Although overuse of ICUs for patients at low risk of deterioration may lead to economic consequences, underuse of ICUs for high‐risk patients may affect patient safety. Despite advances in the medical, interventional, and surgical management of NSTEMI, a proportion of initially stable patients still deteriorate clinically after admission. In fact, a third of initially stable patients who developed a complication had multiple indications for ICU care. As shown in patients with heart failure, high‐risk patients have better outcomes if they are admitted early to an ICU rather than transferred there on clinical deterioration.32, 33, 34, 35 The scenario clinicians would like to avoid is the patient dying before their ICU‐requiring condition could be managed in the ICU; our study showed that 11% of patients with NSTEMI who developed a condition requiring ICU level of care died without ever being treated in the ICU. Although a few of these deaths may have been expected (with do not resuscitate orders), with patients and caregivers electing to remain outside of the ICU, some of these deaths may have been “preventable” if the patient was triaged early to the ICU. Furthermore, urgent care of a deteriorating patient outside of the ICU may divert resources away from other patients in the same ward, potentially worsening outcomes for these patients.36 Selective admission of patients with NSTEMI at high risk of clinical deterioration directly to the ICU would maximize patient safety, while allowing limited ICU beds to be used by patients with truly critical illnesses. Using the ACTION ICU risk score, patients with low predicted risk (ACTION ICU score, ≤2) have a low rate of mortality (0.8%) and low likelihood of death outside of the ICU (0.4%), but these risks increase 10‐fold with ACTION risk score ≥12 (mortality rate, 11.5%, with death outside of the ICU in 4.4%).
Hospitals need a practical evidence‐based tool that can guide risk‐based location of care decision making for patients with NSTEMI who are hemodynamically stable at the time of presentation. Individual hospitals will likely select different risk score thresholds on the basis of patient case mix, ICU size, and resource availability outside of the ICU at these hospitals. A hospital with limited telemetry‐equipped beds or high patient/nurse ratios in the non‐ICU setting may select a lower threshold. For example, using a threshold of 5 would triage 50% of patients with NSTEMI to the ICU for closer monitoring, and non–ICU‐treated patients would have a <10% predicted likelihood of developing a complication requiring ICU care. In contrast, a hospital with limited ICU bed availability may elect to use an ACTION ICU risk score threshold of 12, which would triage only 10% of very high‐risk patients with NSTEMI to the ICU. Hospitals that are currently high ICU users could potentially use the ACTION ICU score to reduce ICU use. For example, our institution previously treated all patients with NSTEMI in the ICU for the first 12 to 24 hours and frequently had ICU bed shortages. We recently implemented the ACTION ICU score with a threshold of 5 to reserve ICU beds for higher‐risk patients. For hospitals electing to maintain their current level of ICU use, the ACTION ICU risk score could help select patients at greatest likelihood of needing the ICU.
In contemporary practice, risk scores can be feasibly calculated automatically at the point of care to guide location of care decisions without the need for providers to memorize the score. At our institution, the ACTION ICU risk score has been integrated into our electronic health record and is automatically calculated when patients with NSTEMI are evaluated in the emergency department. Ongoing prospective research (http://www.clinicaltrials.gov. Unique identifier: NCT03390270) will assess whether automatic calculation and use of the ACTION ICU risk score, alongside clinical judgment, in ICU admission decisions for initially stable patients with NSTEMI results in more cost‐effective care while improving patient safety when compared against clinical judgment alone.
Limitations
Our study population consisted of Medicare patients ≥65 years old; therefore, this risk model may not perform as well in younger patients. The ACTION Registry does not capture all clinical features that may be used to make ICU decisions, such as ongoing chest discomfort, dynamic ECG changes, electrical instability short of cardiac arrest, or oxygen saturation levels, and it is unclear how these factors may relate to need for ICU care. Conditions mandating ICU care were selected on the basis of clinical judgment and experience to reflect conditions that require delivery of a critical care intervention or 1:1 nurse/patient monitoring, but different resources at individual hospitals may necessitate different practice patterns with regard to ICU use. In addition, clinical deterioration occurring later in the hospital course may or may not be relevant to initial admission decisions. Despite these limitations, the ACTION ICU score is the only risk score designed to predict requirement for ICU care in patients with NSTEMI. Given non–risk‐based patterns of ICU use, use of even a limited risk score is likely to improve on the status quo.
Conclusion
The ACTION ICU risk score uses 9 variables on admission to predict the likelihood that an initially stable patients with NSTEMI will develop a complication requiring ICU‐level care. Bedside application of this risk model has practice‐changing potential, because current practice suggests hospitals do not use objective markers of risk when making ICU triage decisions for initially stable patients with NSTEMI. The use of this objective risk stratification tool may help hospitals effectively use limited ICU resources while ensuring that high‐risk patients are cared for safely.
Sources of Funding
This study was funded by the Agency for Healthcare Research and Quality, grant U19H2021092 (Wang).
Disclosures
Fanaroff reports grants from the National Institutes of Health (5T32HL069749‐13) and American Heart Association (17FTF33661087) during the conduct of the study, and Gilead Sciences outside the submitted work. Garratt reports receiving consulting fees/honoraria from Abbott Vascular, CeloNova, and Jarvk Heart, holding equity in LifeCuff, Inc, and GDS, and performing legal reviews for Swensen, Perer & Kontos all outside of the submitted work. Peterson reports receiving consulting fees from AstraZeneca, Merck, Janssen, Boehringer Ingelheim, and Bayer, and research grants from Janssen and Eli Lilly, all outside of the submitted work. His disclosures can be found at https://www.dcri.org/about-us/conflict-of-interest. Newby reports receiving consulting fees from Roche Diagnostics and Philips Healthcare, and research grants from Roche Diagnostics and Abbott, all outside the submitted work. Her disclosures can be found at https://www.dcri.org/about-us/conflict-of-interest. de Lemos reports consulting fees from Roche Diagnostics, Abbott Diagnostics, and Siemen's Health Care, and research grants from Roche Diagnostics and Abbott Diagnostics, all outside the submitted work. Kosiborod reports receiving consulting fees from AstraZeneca, Eli Lilly, Amgen, Regeneron, Takeda, Edwards Lifesciences, Gilead Sciences, Roche, and Genentech, and research grants from the American Heart Association, Gilead Sciences, Genentech, Sanofi, and Eisai, all outside of the submitted work. Wang reports receiving honoraria from AstraZeneca, Eli Lilly, and Merch, and research grants from Gilead Sciences, Eli Lilly, Daiichi Sanyo, AstraZeneca, Boston Scientific, Bristol‐Meyers‐Squibb, Regeneron, and GlaxoSmithKline, all outside of the submitted work. The remaining authors have no disclosures to report.
Supporting information
Data S1. Candidate variables for the multivariable model.
Data S2. Prediction equation for requiring ICU care model.
Table S1. Definition of Outcomes Mandating ICU Care
Table S2. Univariable Associations Between Baseline Predictors and Requirement for ICU Care
Table S3. Test Characteristics of the ACTION ICU Score in the Development Cohort at Selected Thresholds
Table S4. Proportion of Patients Who Died and Died Without Treatment in the ICU by Predicted ACTION ICU Model Risk
Figure S1. Calibration curve of the multivariable model in male patients (A), female patient (B), patients <75 years old (C), patients 75 to 84 years old (D), and patients ≥85 years old (E).
Figure S2. Proportion of patients requiring ICU care by GRACE risk score.
(J Am Heart Assoc. 2018;7:e008894 DOI: 10.1161/JAHA.118.008894.)29802146
References
- 1. Chen R, Strait KM, Dharmarajan K, Li S‐X, Ranasinghe I, Martin J, Fazel R, Masoudi FA, Cooke CR, Nallamothu BK. Hospital variation in admission to intensive care units for patients with acute myocardial infarction. Am Heart J. 2015;170:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Diepen S, Lin M, Bakal JA, McAlister FA, Kaul P, Katz JN, Fordyce CB, Southern DA, Graham MM, Wilton SB, Newby LK, Granger CB, Ezekowitz JA. Do stable non‐ST segment elevation acute coronary syndromes require admission to coronary care units? Am Heart J. 2016;175:184–192. [DOI] [PubMed] [Google Scholar]
- 3. Insam C, Paccaud F, Marques‐Vidal P. The region makes the difference: disparities in management of acute myocardial infarction within Switzerland. Eur J Prev Cardiol. 2014;21:541–548. [DOI] [PubMed] [Google Scholar]
- 4. Fanaroff AC, Peterson ED, Chen AY, Thomas L, Doll JA, Fordyce CB, Newby LK, Amsterdam EA, Kosiborod MN, de Lemos JA, Wang TY. Intensive care unit utilization and mortality among Medicare patients hospitalized with non–ST‐segment elevation myocardial infarction. JAMA Cardiol. 2017;2:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halpern NA, Pastores SM. Critical care medicine in the United States 2000–2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. [DOI] [PubMed] [Google Scholar]
- 6. Katz JN. Who belongs in the cardiac intensive care unit? JAMA Cardiol. 2017;2:45–46. [DOI] [PubMed] [Google Scholar]
- 7. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non‐ST elevation MI: a method for prognostication and therapeutic decision making. J Am Med Assoc. 2000;284:835–842. [DOI] [PubMed] [Google Scholar]
- 8. Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA. Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Arch Intern Med. 2003;163:2345–2353. [DOI] [PubMed] [Google Scholar]
- 9. Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, Akkerhuis KM, Harrington RA, Deckers JW, Armstrong PW, Lincoff AM, Califf RM, Topol EJ, Simoons ML; The PURSUIT Investigators . Predictors of outcome in patients with acute coronary syndromes without persistent ST‐segment elevation: results from an international trial of 9461 patients. Circulation. 2000;101:2557–2567. [DOI] [PubMed] [Google Scholar]
- 10. Chin CT, Chen AY, Wang TY, Alexander KP, Mathews R, Rumsfeld JS, Cannon CP, Fonarow GC, Peterson ED, Roe MT. Risk adjustment for in‐hospital mortality of contemporary patients with acute myocardial infarction: the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) Registry®–Get With The Guidelines (GWTG)™ acute myocardial infarction mortality model and risk score. Am Heart J. 2011;161:113–122.e112. [DOI] [PubMed] [Google Scholar]
- 11. Peterson ED, Roe MT, Chen AY, Fonarow GC, Lytle BL, Cannon CP, Rumsfeld JS. The NCDR ACTION Registry–GWTG: transforming contemporary acute myocardial infarction clinical care. Heart. 2010;96:1798–1802. [DOI] [PubMed] [Google Scholar]
- 12. Peterson ED, Roe MT, Rumsfeld JS, Shaw RE, Brindis RG, Fonarow GC, Cannon CP. A call to ACTION (Acute Coronary Treatment and Intervention Outcomes Network) a national effort to promote timely clinical feedback and support continuous quality improvement for acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2009;2:491–499. [DOI] [PubMed] [Google Scholar]
- 13. Shah RU, de Lemos JA, Wang TY, Chen AY, Thomas L, Sutton NR, Fang JC, Scirica BM, Henry TD, Granger CB. Post‐hospital outcomes of patients with acute myocardial infarction with cardiogenic shock: findings from the NCDR. J Am Coll Cardiol. 2016;67:739–747. [DOI] [PubMed] [Google Scholar]
- 14. Pokorney SD, Miller AL, Chen AY, Thomas L, Fonarow GC, de Lemos JA, Al‐Khatib SM, Peterson ED, Wang TY. Implantable cardioverter‐defibrillator use among Medicare patients with low ejection fraction after acute myocardial infarction. J Am Med Assoc. 2015;313:2433–2440. [DOI] [PubMed] [Google Scholar]
- 15. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cowen ME, Strawderman RL, Czerwinski JL, Smith MJ, Halasyamani LK. Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013;8:229–235. [DOI] [PubMed] [Google Scholar]
- 17. Johnston JA, Wagner DP, Timmons S, Welsh D, Tsevat J, Render ML. Impact of different measures of comorbid disease on predicted mortality of intensive care unit patients. Med Care. 2002;40:929–940. [DOI] [PubMed] [Google Scholar]
- 18. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J‐C, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 19. Liang K‐Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 20. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York, NY: Springer; 2015. [Google Scholar]
- 21. Steyerberg EW, Harrell FE, Borsboom GJ, Eijkemans M, Vergouwe Y, Habbema JDF. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–781. [DOI] [PubMed] [Google Scholar]
- 22. Goldman L, Cook EF, Johnson PA, Brand DA, Rouan GW, Lee TH. Prediction of the need for intensive care in patients who come to the emergency departments with acute chest pain. N Engl J Med. 1996;334:1498–1504. [DOI] [PubMed] [Google Scholar]
- 23. Brieger D, Fox KA, FitzGerald G, Eagle KA, Budaj A, Avezum A, Granger CB, Costa B, Anderson FA, Steg PG. Predicting freedom from clinical events in non‐ST‐elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95:888–894. [DOI] [PubMed] [Google Scholar]
- 24. McNamara RL, Kennedy KF, Cohen DJ, Diercks DB, Moscucci M, Ramee S, Wang TY, Connolly T, Spertus JA. Predicting in‐hospital mortality in patients with acute myocardial infarction. J Am Coll Cardiol. 2016;68:626–635. [DOI] [PubMed] [Google Scholar]
- 25. Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand S‐LT. An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–1692. [DOI] [PubMed] [Google Scholar]
- 26. Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A. The APACHE III prognostic system: risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. [DOI] [PubMed] [Google Scholar]
- 27. Goldberg RJ, Steg PG, Sadiq I, Granger CB, Jackson EA, Budaj A, Brieger D, Avezum A, Goodman S. Extent of, and factors associated with, delay to hospital presentation in patients with acute coronary disease (the GRACE registry). Am J Cardiol. 2002;89:791–796. [DOI] [PubMed] [Google Scholar]
- 28. Goldberg RJ, Spencer FA, Fox KA, Brieger D, Steg PG, Gurfinkel E, Dedrick R, Gore JM. Prehospital delay in patients with acute coronary syndromes (from the Global Registry of Acute Coronary Events [GRACE]). Am J Cardiol. 2009;103:598–603. [DOI] [PubMed] [Google Scholar]
- 29. Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT. Baseline risk of major bleeding in non–ST‐segment–elevation myocardial infarction. Circulation. 2009;119:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morrow DA, Fang JC, Fintel DJ, Granger CB, Katz JN, Kushner FG, Kuvin JT, Lopez‐Sendon J, McAreavey D, Nallamothu B. Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models. Circulation. 2012;126:1408–1428. [DOI] [PubMed] [Google Scholar]
- 31. Centers for Medicare & Medicaid Services . Notice of proposed rulemaking for bundled payment models for high‐quality, coordinated cardiac and hip fracture care. 2016. Available at: https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2016-Fact-sheets-items/2016-07-25.html. Accessed February 9, 2018.
- 32. Molina JAD, Seow E, Heng BH, Chong WF, Ho B. Outcomes of direct and indirect medical intensive care unit admissions from the emergency department of an acute care hospital: a retrospective cohort study. BMJ Open. 2014;4:e005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chioncel O, Ambrosy AP, Filipescu D, Bubenek S, Vinereanu D, Petris A, Collins SP, Macarie C, Gheorghiade M. Patterns of intensive care unit admissions in patients hospitalized for heart failure: insights from the RO‐AHFS registry. J Cardiovasc Med. 2015;16:331–340. [DOI] [PubMed] [Google Scholar]
- 34. Liu V, Kipnis P, Rizk NW, Escobar GJ. Adverse outcomes associated with delayed intensive care unit transfers in an integrated healthcare system. J Hosp Med. 2012;7:224–230. [DOI] [PubMed] [Google Scholar]
- 35. Young MP, Gooder VJ, Bride K, James B, Fisher ES. Inpatient transfers to the intensive care unit. J Gen Intern Med. 2003;18:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Volchenboum SL, Mayampurath A, Goksu‐Gursoy G, Edelson DP, Howell MD, Churpek MM. Association between in‐hospital critical illness events and outcomes in patients on the same ward. J Am Med Assoc. 2016;316:2674–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Candidate variables for the multivariable model.
Data S2. Prediction equation for requiring ICU care model.
Table S1. Definition of Outcomes Mandating ICU Care
Table S2. Univariable Associations Between Baseline Predictors and Requirement for ICU Care
Table S3. Test Characteristics of the ACTION ICU Score in the Development Cohort at Selected Thresholds
Table S4. Proportion of Patients Who Died and Died Without Treatment in the ICU by Predicted ACTION ICU Model Risk
Figure S1. Calibration curve of the multivariable model in male patients (A), female patient (B), patients <75 years old (C), patients 75 to 84 years old (D), and patients ≥85 years old (E).
Figure S2. Proportion of patients requiring ICU care by GRACE risk score.


