Abstract
Background
We sought to identify patient and surgical factors associated with time to hospital discharge in patients undergoing complete repair for tetralogy of Fallot.
Methods and Results
We performed a prospective cohort study of patients with tetralogy of Fallot admitted for complete repair between May 1, 2012 and June 2, 2017 at Children's Hospital of Philadelphia with detailed demographic, clinical, and operative characteristics. The primary outcome was time to hospital discharge. Cox proportional hazards models were used to identify patient and operative predictors of time to hospital discharge. We enrolled 151 subjects, 62.8% male, 65.6% non‐Hispanic white, and 9.9% non‐Hispanic black. The median time to hospital discharge was 7 days (interquartile range 4, 12). Five patients died in the hospital, all of whom underwent tetralogy of Fallot repair beyond the neonatal period. Greater birth weight was associated with higher rate of hospital discharge (hazard ratio [HR]=1.35, 95% confidence interval (CI) =1.11, 1.64), while absent pulmonary valve versus pulmonary stenosis (HR=0.27, 95% CI=0.08, 0.91), pulmonary valve atresia versus pulmonary stenosis (HR=0.57, 95% CI=0.33, 0.97), presence of aortopulmonary collaterals (HR=0.44, 95% CI=0.24, 0.84), complete repair performed in the neonatal period (<30 days of life) (HR=0.45, 95% CI=0.27, 0.75), more than 1 cardiopulmonary bypass run (HR=0.33, 95% CI=0.18, 0.61), and longer aortic cross‐clamp time (HR [per 10 minutes]=0.88, 95% CI=0.79, 0.97) were associated with lower rate of hospital discharge.
Conclusions
Postoperative hospital stay after complete repair of tetralogy of Fallot is in part determined by patient and operative factors. Some (eg, surgical strategy for the symptomatic neonate) may be modifiable. These results may impact patient counseling, choice of surgical approach, and postoperative care.
Keywords: hospital stay, outcome, surgery, tetralogy of Fallot
Subject Categories: Clinical Studies, Pediatrics, Mortality/Survival
Clinical Perspective
What Is New?
In a relatively large prospective cohort study we identified patient and operative factors associated with time to hospital discharge after complete surgical repair for tetralogy of Fallot. Prospective data collection allowed for data quality and completeness.
What Are the Clinical Implications?
The study found that patient and operative factors are associated with longer time to hospital discharge after initial surgical repair for tetralogy of Fallot.
These factors included smaller birth weight, pulmonary valve atresia or absent leaflets, presence of aortopulmonary collaterals, surgical repair in the neonatal period, repeated cardiopulmonary bypass runs, and longer aortic cross‐clamp time.
Some of these factors might be modifiable and could impact on family counseling and management strategies.
These findings might generate hypotheses for studies examining the long‐term outcomes in this patient population.
Introduction
In an era of low mortality following complete repair for tetralogy of Fallot (TOF), postoperative morbidity is an important surgical outcome. Postoperative length of hospital stay (LOHS) is one of the most important outcome measures for pediatric congenital heart surgery.1, 2, 3 Not only is LOHS a metric of early surgical outcome, it is also associated with long‐term neurodevelopmental outcomes in children with congenital heart defects.4, 5 In TOF, the postoperative hospital course can vary by length of mechanical ventilation, postoperative complications, and LOHS.6, 7 Some cited risk factors for prolonged hospital stay include aortic cross‐clamp time, delayed sternal closure, length of intubation, extubation failure, and worse technical performance scores.7, 8 However, most studies examining predictors of LOHS are retrospective without detailed research‐grade data collection, use clinical databases without granular data points, and focus on a particular feature of TOF, such as timing of repair, genotype, or subtypes of TOF (for example, pulmonary atresia and association with atrioventricular canal defects).9, 10 We sought to identify patient and surgical factors associated with postoperative LOHS with prospective ascertainment of data. Identifying risk factors for perioperative outcomes after complete repair for TOF might identify opportunities to modify early as well as late outcomes.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Ethical Approval of the Study Protocol
The study protocol was approved by the Institutional Review Board for the Protection of Human Subjects at Children's Hospital of Philadelphia, and parents of subjects gave informed consent to participating in the study.
Study Population
We conducted a prospective cohort study of patients who underwent complete repair for TOF at Children's Hospital of Philadelphia. The study sample for this analysis consisted of patients who underwent complete surgical repair for TOF in a single operation between May 1, 2012 and June 2, 2017, whether or not it was preceded by placement of an aortopulmonary (AP) shunt or other palliative procedure (eg, stent in the right ventricular [RV] outflow tract or in the ductus arteriosus). We excluded patients who received a staged repair with unifocalization of the pulmonary arteries and conduit placement followed by later closure of the ventricular septal defect. We also excluded patients who died following a palliative procedure or had completion of repair after 2 years of age.
Patient Screening and Recruitment
Research coordinators screened all patients on the surgical previsit schedules, surgical schedules, and new admissions to the cardiac intensive care unit for eligibility. Parents of patients who appeared to meet the inclusion criteria were approached in the surgical previsit clinic or in the cardiac intensive care unit (preoperatively when possible, or in the immediate postoperative period), and informed consent was obtained.
Data Collection and Variables
Hospital medical records were reviewed to abstract the relevant clinical variables from the index hospitalization, including demographics and birth history. The preoperative history included feeding status and oxygen saturation on admission, use of prostaglandin preoperatively, prior readmissions, and from where the patient had been admitted (eg, home, another hospital).
Cardiac diagnosis was based on the review of preoperative echocardiograms and review of reports from other imaging (computerized tomography or magnetic resonance imaging) or procedures (catheterizations). Anatomy was divided according to the pulmonary valve morphology: stenosis, atresia, absent leaflets, and TOF with complete common atrioventricular canal defect. Presence of AP collateral vessels was recorded. Extracardiac findings and genetic syndromes were recorded.
Complete repair was categorized as (1) closure of ventricular septal defect (VSD) without need for relief of RV outflow tract obstruction; (2) VSD closure and relief of RV outflow tract obstruction with placement of a patch without crossing the pulmonary valve annulus (nontransannular patch); (3) VSD closure and relief of RV outflow tract obstruction with placement of a patch crossing the pulmonary valve annulus (transannular patch); and (4) VSD closure and placement of an RV‐to–pulmonary artery conduit.
Perioperative data collection started at the time of surgery and occurred daily. From the operative procedure, we collected the following times: operating room, cardiopulmonary bypass (CPB), aortic cross clamp, and deep hypothermic circulatory arrest when used. The number of bypass runs was recorded. The lowest temperature on CPB and use of modified ultrafiltration and steroids were recorded.
The operative intervention itself was detailed in terms of type of approach, status of the atrial septum, VSD closure, and outflow tract/pulmonary artery interventions.
Daily summary records were created, and information on respiratory support, feeding, medications, and complications was recorded. Noncardiac events, such as number of consulting services, infections requiring antibiotic treatment, postoperative seizures confirmed by electroencephalogram, pleural effusions requiring drainage, and tracheostomy, were recorded, as were cardiac events such as pericardial effusions requiring pericardiocentesis, arrhythmias requiring treatment, postoperative cardiac catheterizations, reoperations, and cardiac arrest requiring resuscitation.
Discharge data included destination (home or chronic care facility), feeding status, and medications. Daily chart review was discontinued at 30 days if the patient was still hospitalized, and data such as oxygen saturation, medications, mode of feeding, and disposition were collected from the discharge summary.
The primary outcome of interest was time to hospital discharge (THD). THD was defined as the time in days between the day of surgery and the day of discharge from the hospital, which was considered the “event” in the time‐to‐event analysis. Discharge was either to home or to a chronic care facility. Death before discharge was considered as a prolonged THD and was imputed as 800 days (longer than the longest THD) and then censored with no “event” (hospital discharge).
Only patient and operative predictors, as opposed to postoperative variables, were considered in the multivariable models in order to focus on causal and potentially modifiable features that might change the treatment approach. For example, a requirement for longer mechanical ventilation after surgery or more days of intravenous inotropes was expected to be strongly predictive of THD discharge, but such findings would not be particularly helpful in changing the preoperative or operative approach to the patient (and, being in the causal pathway, could mask the clinical determinants of outcomes themselves).
Statistical Analysis
Descriptive statistics were calculated as frequency counts and percentages for categorical variables and mean and standard deviation or median with interquartile range (IQR) for continuous variables. The primary outcome of interest was THD. For the purposes of presenting the cohort, we created 3 groups based on quartiles of THD. Two‐sample t tests or Wilcoxon rank‐sum tests for continuous variables and chi‐squared tests of independence or Fisher exact tests for categorical variables were used to compare the subjects with THD in the first and fourth quartile.
We used univariable and multivariable Cox proportional hazards models to assess the associations between clinical risk factors and THD. Cox proportional hazards are semiparametric because they make specific assumptions about the probability distribution of event times, although they are based on parametric regression models. We used purposeful selection of covariates in building the multivariate models. Patient and operative variables examined in univariable models were considered in the multivariable model if P<0.20. Covariates for the multivariable model were also selected a priori based on clinical plausibility and data availability and retained for clinical or statistical significance. We assessed the collinearity among the predictors. We forced “surgeon” and use of deep hypothermic circulatory arrest into the multivariable models. Possible interactions were explored, such as between pulmonary valve atresia and neonatal repair and pulmonary valve atresia and AP collaterals. Results from the Cox proportional hazards models are reported as hazard ratios (HRs) with 95% confidence intervals (95% CIs) and P values. In this analysis, a HR <1 indicated a lower rate of discharge and therefore longer THD, and HR >1 indicated a higher rate of discharge (shorter THD).
Proportional‐hazards assumptions were examined by including products of variables with log‐time in the model; overall model fit was assessed using Cox‐Snell residuals.11 Sensitivity analyses were performed to detect outlier/influential‐point effects via graphical analysis of the deviance residuals and the difference between the regression coefficient calculated for all of the data and the regression coefficient calculated with the observation deleted; all diagnostic evaluations gave satisfactory results. Sensitivity analysis was performed after excluding patients with absent pulmonary valve leaflets. P<0.05 was considered statistically significant. All analyses were performed using Stata 14.2 (Stata Corporation, College Station, TX).
Results
Informed consent was sought from the parents of 181 children and was obtained for 151 children (83%), who were enrolled. Sixty‐two percent were male children, 65.6% were non‐Hispanic white, and 9.9% were non‐Hispanic black. Most were diagnosed prenatally, born at term, and were discharged home after birth. A genetic diagnosis was identified in 38 patients (25.2%). Median oxygen saturation before surgery was 93% (IQR 86, 97). Prostaglandin was used immediately before surgery in a minority of subjects (9.9%). The vast majority of subjects had pulmonary valve stenosis (80.1%). Aortopulmonary collaterals were present in 15 subjects (7 with pulmonary valve stenosis and 8 with pulmonary valve atresia). Palliative procedures were performed in 33.8% and included placement of an AP shunt, pulmonary valve balloon dilation, and placement of a stent in the right ventricular outflow tract or in the ductus arteriosus (Tables 1, 2 through 3).
Table 1.
Summary Statistics of the Total Cohort and by Quartile of Time to Hospital Discharge (N=151)
| Total | 1 to 4 Days | 5 to 11 Days | ≥12 Days | P Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age at admission, mo | 3.6 (2.1, 5.2) | 3.2 (2.1, 4.4) | 4.1 (2.8, 5.5) | 2.6 (0.0, 5.3) | 0.37 |
| Sex male, n (%) | 94 (62.3) | 24 (61.5) | 46 (63.9) | 24 (60.0) | 0.87 |
| Ethnicity, n (%) | |||||
| Hispanic/Latino | 26 (17.2) | 4 (10.3) | 16 (22.2) | 6 (15.0) | |
| Not Hispanic/Latino | 125 (82.8) | 35 (89.7) | 56 (77.8) | 34 (85.0) | 0.53 |
| Race | |||||
| White | 121 (80.1) | 32 (82.1) | 56 (77.8) | 33 (82.5) | |
| Black | 15 (9.9) | 2 (5.1) | 9 (12.5) | 4 (10.0) | |
| Asian | 5 (3.3) | 2 (5.1) | 2 (2.8) | 1 (2.5) | |
| More than 1 race | 10 (6.6) | 3 (7.7) | 5 (6.9) | 2 (5.0) | 0.84 |
| Birth history | |||||
| Birth weight, kg | 3.0 (2.5, 3.5) | 3.3 (2.9, 3.6) | 3.0 (2.5, 3.5) | 2.8 (2.2, 3.1) | <0.001 |
| Gestational age, wk | 38.4 (37.0, 39.0) | 39.0 (39.0, 39.3) | 38.2 (36.5, 39.0) | 37.6 (35.0, 39.0) | <0.001 |
| Prenatal diagnosis | 109 (72.2) | 22 (56.4) | 55 (76.4) | 32 (80.0) | 0.049 |
| Gestational age | |||||
| <37 wks | 36 (24.0) | 3 (7.9) | 21 (29.2) | 12 (30.0) | |
| ≥37 wks | 114 (76.0) | 35 (92.1) | 51 (70.8) | 28 (70.0) | 0.02 |
| Discharged home after birth | 110 (72.8) | 35 (89.7) | 56 (77.8) | 19 (47.5) | <0.001 |
| Preoperative history | |||||
| Oxygen saturation before surgery (%) | 93.0 (86.0, 97.0) | 95.5 (90.0, 99.0) | 93.0 (86.0, 97.0) | 87.0 (84.0, 95.0) | <0.001 |
| Feeding status at admission | |||||
| Oral | 118 (78.1) | 39 (100) | 59 (81.9) | 20 (50.0) | |
| Oral/nasogastric | 9 (6.0) | 0 (0.0) | 7 (9.7) | 2 (5.0) | |
| Nasogastric | 14 (9.3) | 0 (0.0) | 5 (6.9) | 9 (22.5) | |
| NPO | 10 (6.6) | 0 (0.0) | 1 (1.4) | 9 (22.5) | <0.001 |
| Prostaglandin use at admission | 15 (9.9) | 1 (2.6) | 2 (2.8) | 12 (30.0) | 0.001 |
| Oxygen use on admission | 22 (14.6) | 4 (10.3) | 7 (9.7) | 11 (27.5) | 0.08 |
| Prior hospitalization | 26 (17.2) | 3 (7.7) | 15 (20.8) | 8 (20.0) | 0.19 |
| Patient admitted from | |||||
| Home | 116 (76.8) | 34 (87.2) | 60 (83.3) | 22 (55.0) | |
| Another unit | 22 (14.6) | 3 (7.7) | 7 (9.7) | 12 (30.0) | |
| Outside hospital | 13 (8.6) | 2 (5.1) | 5 (6.9) | 6 (15.0) | 0.008 |
Continuous variables are presented as median (IQR). Counts are presented as n (%). P values represent comparisons between 1‐4 days and ≥12 days in the hospital. NPO stands for nil per os, indicating that the patient was not receiving oral or gastric feeds; IQR, interquartile range.
Table 2.
Cardiac Diagnoses and Extracardiac Findings
| Total | 1 to 4 Days | 5 to 11 Days | ≥12 Days | P Value | |
|---|---|---|---|---|---|
| Cardiac diagnosis based on initial echocardiogram | |||||
| Height, cm | 51.0 (47.0, 57.0) | 53.0 (49.0, 58.0) | 51.5 (48.0, 59.0) | 47.5 (45.0, 51.0) | <0.001 |
| Weight, kg | 3.7 (2.9, 5.0) | 4.0 (3.4, 5.1) | 3.8 (2.8, 5.6) | 3.0 (2.4, 3.7) | <0.001 |
| Body surface area, m2 | 0.2 (0.2, 0.3) | 0.3 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.2) | <0.001 |
| Pulmonary valve anatomy | |||||
| Stenosis | 121 (80.1) | 38 (97.4) | 59 (81.9) | 24 (60.0) | |
| Atresia | 26 (17.2) | 1 (2.6) | 12 (16.7) | 13 (32.5) | |
| Absent | 4 (2.6) | 0 (0.0) | 1 (1.4) | 3 (7.5) | <0.001 |
| Atresia with pulmonary artery segment | 16 (61.5) | 1 (100) | 7 (58.3) | 8 (61.5) | 0.99 |
| Aortopulmonary collaterals | 15 (9.9) | 0 (0.0) | 8 (11.1) | 7 (17.5) | 0.01 |
| Continuous pulmonary arteries | 141 (93.4) | 38 (97.4) | 66 (91.7) | 37 (92.5) | 0.62 |
| Atrial septum | |||||
| Intact | 23 (15.2) | 13 (33.3) | 9 (12.5) | 1 (2.5) | |
| Patent foramen ovale | 115 (76.2) | 25 (64.1) | 58 (80.6) | 32 (80.0) | |
| Secundum atrial septal defect | 11 (7.3) | 1 (2.6) | 4 (5.6) | 6 (15.0) | |
| Other | 2 (1.3) | 0 (0.0) | 1 (1.4) | 1 (2.5) | <0.001 |
| Ventricular septal defect | |||||
| Single | 147 (97.4) | 38 (97.4) | 69 (95.8) | 40 (100) | |
| Multiple | 4 (2.6) | 1 (2.6) | 3 (4.2) | 0 (0.0) | 0.49 |
| Ventricular septal defect type | |||||
| Malalignment | 141 (93.4) | 36 (92.3) | 67 (93.1) | 38 (95.0) | |
| Conal septal hypoplasia with malalignment | 10 (6.6) | 3 (7.7) | 5 (6.9) | 2 (5.0) | 0.68 |
| Endocardial cushion effect | 6 (4.5) | 0 (0.0) | 2 (3.2) | 4 (11.8) | 0.045 |
| Aortic arch side | |||||
| Unknown | 2 (1.3) | 1 (2.6) | 1 (1.4) | 0 (0.0) | |
| Left | 104 (68.9) | 29 (74.4) | 52 (72.2) | 23 (57.5) | |
| Right | 45 (29.8) | 9 (23.1) | 19 (26.4) | 17 (42.5) | 0.096 |
| Aortic arch branching | |||||
| Unknown | 6 (4.1) | 2 (5.4) | 2 (2.9) | 2 (5.0) | |
| Left aortic arch, normal branching | 87 (59.2) | 26 (70.3) | 40 (57.1) | 21 (52.5) | |
| Right aortic arch | 34 (23.1) | 6 (16.2) | 16 (22.9) | 12 (30.0) | |
| Common brachiocephalic trunk | 7 (4.8) | 3 (8.1) | 3 (4.3) | 1 (2.5) | |
| Aberrant right subclavian artery | 8 (5.4) | 0 (0.0) | 7 (10.0) | 1 (2.5) | |
| Aberrant left subclavian artery | 5 (3.4) | 0 (0.0) | 2 (2.9) | 3 (7.5) | 0.093 |
| Pulmonary valve annulus, if measurable | 5.9 (4.9, 7.1) | 6.0 (5.0, 7.5) | 6.2 (5.4, 7.3) | 5.0 (3.8, 6.0) | 0.003 |
| Pulmonary valve annulus z‐score | −2.0 (−2.6, −1.5) | −1.9 (−2.6, −1.5) | −2.0 (−2.4, −1.2) | −2.3 (−2.9, −2.0) | 0.066 |
| Right pulmonary artery diameter, mm | 4.3 (3.6, 5.2) | 4.5 (4.0, 5.4) | 4.4 (3.8, 5.1) | 3.9 (3.0, 5.1) | 0.012 |
| Right pulmonary artery z‐score | −1.2 (−1.9, −0.6) | −1.2 (−1.8, −0.5) | −1.1 (−1.9, −0.6) | −1.3 (−2.1, −0.6) | 0.62 |
| Left pulmonary artery diameter, mm | 4.0 (3.3, 5.0) | 4.0 (3.4, 5.0) | 4.1 (3.5, 5.5) | 3.7 (2.8, 4.0) | 0.044 |
| Left pulmonary artery z‐score | −1.3 (−2.0, −0.6) | −1.6 (−2.1, −0.3) | −1.1 (−1.8, −0.3) | −1.6 (−2.1, −0.9) | 0.59 |
| Aortic valve annulus, mm | 9.3 (8.1, 11.2) | 10.1 (9.0, 11.5) | 9.0 (8.5, 10.8) | 8.7 (7.4, 10.2) | 0.014 |
| Aortic valve annulus z‐score | 2.5 (1.3, 3.8) | 2.8 (1.4, 3.8) | 2.1 (1.3, 3.7) | 2.5 (0.9, 3.8) | 0.667 |
| Aortic root annulus, mm | 12.0 (10.8, 13.9) | 12.5 (11.6, 14.5) | 11.9 (11.0, 13.9) | 11.7 (10.0, 13.0) | 0.027 |
| Aortic root annulus z‐score | 1.9 (1.3, 2.6) | 1.9 (1.1, 2.7) | 1.9 (1.3, 2.7) | 2.0 (1.2, 2.4) | 0.80 |
| Aortic sinotubular junction, mm | 9.9 (8.6, 11.3) | 10.7 (9.0, 11.5) | 9.6 (8.6, 11.3) | 9.3 (8.0, 11.0) | 0.021 |
| Aortic sinotubular junction z‐score | 1.6 (0.8, 2.9) | 1.6 (1.1, 3.0) | 1.8 (0.8, 2.9) | 1.6 (0.8, 2.5) | 0.70 |
| Preoperative interventions | |||||
| No preoperative intervention | 122 (80.8) | 36 (92.3) | 54 (75.0) | 32 (80.0) | 0.19 |
| Pulmonary valve balloon dilation | 3 (2.0) | 0 (0.0) | 1 (1.4) | 2 (5.0) | 0.49 |
| Right ventricular outflow tract stent | 2 (1.3) | 0 (0.0) | 2 (2.8) | 0 (0.0) | ··· |
| Ductus arteriosus stent | 3 (2.0) | 0 (0.0) | 3 (4.2) | 0 (0.0) | ··· |
| Blalock‐Taussig shunt | 16 (10.6) | 3 (7.7) | 9 (12.5) | 4 (10.0) | 0.99 |
| Central shunt | 5 (3.3) | 0 (0.0) | 3 (4.2) | 2 (5.0) | 0.49 |
| Mee procedure | 1 (0.7) | 0 (0.0) | 1 (1.4) | 0 (0.0) | ··· |
| Pulmonary arterioplasty | 5 (3.3) | 1 (2.6) | 3 (4.2) | 1 (2.5) | 0.99 |
| Other | 16 (10.6) | 0 (0.0) | 12 (16.7) | 4 (10.0) | 0.12 |
| Extracardiac findings | |||||
| Genetic consultation obtained | 102 (67.5) | 27 (69.2) | 44 (61.1) | 31 (77.5) | 0.406 |
| Extracardiac malformations | 64 (42.4) | 17 (43.6) | 27 (37.5) | 20 (50.0) | 0.57 |
| Facial dysmorphia | 48 (31.8) | 10 (25.6) | 17 (23.6) | 21 (52.5) | 0.015 |
| Genetic diagnosis identified | 38 (25.2) | 3 (7.7) | 19 (26.4) | 16 (40.0) | 0.001 |
| Genetic diagnosis type | |||||
| Normal | 113 (74.8) | 36 (92.3) | 53 (73.6) | 24 (60.0) | |
| CHARGE | 1 (0.7) | 0 (0.0) | 0 (0.0) | 1 (2.5) | |
| DiGeorge syndrome | 13 (8.6) | 2 (5.1) | 7 (9.7) | 4 (10.0) | |
| Trisomy 21 | 7 (4.6) | 0 (0.0) | 4 (5.6) | 3 (7.5) | |
| VACTERL | 5 (3.3) | 0 (0.0) | 2 (2.8) | 3 (7.5) | |
| Syndromic | 2 (1.3) | 0 (0.0) | 1 (1.4) | 1 (2.5) | |
| Other | 10 (6.6) | 1 (2.6) | 5 (6.9) | 4 (10.0) | 0.019 |
| 22q11.2 deletion status | |||||
| Deleted | 13 (9.7) | 2 (5.9) | 7 (10.9) | 4 (11.1) | |
| Nondeleted | 121 (90.3) | 32 (94.1) | 57 (89.1) | 32 (88.9) | 0.67 |
Continuous variables are presented as median (25th to 75th percentiles). Counts are presented as n (%). P values represent comparisons between 1‐4 days and ≥12 days in the hospital. CHARGE indicates the association of coloboma, heart defect, choanal atresia, growth retardation, and genital and ear abnormalities; VACTERL, association of vertebral defects, anal atresia, cardiac defects, tracheoesophageal fistula, renal and limb anomalies.
Table 3.
Operative, Admission, and Discharge Characteristics
| Total | 1 to 4 Days | 5 to 11 Days | ≥12 Days | P Value | |
|---|---|---|---|---|---|
| Operative characteristics | |||||
| Age at surgery, mo | 3.6 (2.2, 5.2) | 3.3 (2.2, 4.4) | 4.1 (2.8, 5.5) | 3.0 (0.4, 5.3) | 0.58 |
| Neonatal repair | 23 (15.2) | 2 (5.1) | 6 (8.3) | 15 (37.5) | 0.001 |
| Height at surgery, cm | 58.5 (54, 62.7) | 58.0 (56.0, 63.0) | 59.1 (55.0, 63.6) | 55.0 (47.0, 60.5) | 0.007 |
| Weight at surgery, kg | 5.3 (4.1, 6.2) | 5.3 (4.6, 6.0) | 5.7 (4.4, 6.5) | 4.9 (2.9, 5.6) | 0.043 |
| Hours in the operating room | 3.7 (3.0, 4.6) | 2.8 (2.5, 3.4) | 3.8 (3.2, 4.6) | 4.1 (3.4, 5.1) | <0.001 |
| Number of CPB runs | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.0) | 1.0 (1.0, 1.5) | 0.001 |
| Total CPB time, min | 63.0 (37.0, 86.0) | 32.0 (26.0, 53.0) | 66.0 (45.0, 90.5) | 76.5 (62.5, 112.0) | <0.001 |
| Aortic cross‐clamp time, min | 40.0 (25.0, 63.0) | 25.0 (20.0, 43.0) | 48.0 (30.0, 64.5) | 52.0 (29.5, 75.5) | <0.001 |
| Use of DHCA | 12 (7.9) | 0 (0.0) | 6 (8.3) | 6 (15.0) | 0.026 |
| DHCA time, min | 25.5 (16.0, 35.5) | ··· | 23.5 (16.0, 41.0) | 25.5 (16.0, 28.0) | ··· |
| Lowest pH on CPB | 7.3 (7.3, 7.4) | 7.3 (7.3, 7.4) | 7.3 (7.3, 7.4) | 7.3 (7.2, 7.4) | 0.097 |
| Lowest esophageal temperature, °C | 35.2 (33.8, 36.0) | 36.0 (35.1, 36.1) | 35.0 (33.9, 36.0) | 34.0 (25.9, 36.0) | <0.001 |
| Lowest hematocrit on CPB | 37.0 (33.0, 37.0) | 37.0 (37.0, 37.0) | 37.0 (34.0, 37.0) | 33.0 (27.5, 37.0) | <0.001 |
| Used modified ultrafiltration | 151 (100) | 39 (100) | 72 (100) | 40 (100) | |
| Steroid use | 30 (20.0) | 4 (10.3) | 13 (18.1) | 13 (33.3) | 0.014 |
| Operative procedure | |||||
| Repair | |||||
| Staged | 2 (1.3) | 0 (0.0) | 0 (0.0) | 2 (5.0) | |
| Complete | 148 (98.7) | 38 (100) | 72 (100) | 38 (95.0) | 0.49 |
| Foramen ovale | |||||
| Closed | 37 (24.7) | 15 (38.5) | 15 (20.8) | 7 (17.9) | |
| Left open | 100 (66.7) | 20 (51.3) | 51 (70.8) | 29 (74.4) | |
| Created | 13 (8.7) | 4 (10.3) | 6 (8.3) | 3 (7.7) | 0.078 |
| Access | |||||
| Transatrial | 53 (35.3) | 23 (59.0) | 24 (33.3) | 6 (15.4) | |
| Ventriculotomy | 26 (17.3) | 2 (5.1) | 15 (20.8) | 9 (23.1) | |
| Transatrial and ventriculotomy | 71 (47.3) | 14 (35.9) | 33 (45.8) | 24 (61.5) | <0.001 |
| Operative procedure for the VSD | |||||
| Closed | 147 (98.0) | 39 (100) | 70 (97.2) | 38 (97.4) | |
| Fenestrated | 2 (1.3) | 0 (0.0) | 2 (2.8) | 0 (0.0) | |
| Unrepaired | 1 (0.7) | 0 (0.0) | 0 (0.0) | 1 (2.6) | 0.99 |
| Outflow tract repair | |||||
| None | 23 (15.2) | 11 (28.2) | 8 (11.1) | 4 (10.0) | |
| Transannular patch | 87 (57.6) | 19 (48.7) | 41 (56.9) | 27 (67.5) | |
| Nontransannular patch | 7 (4.6) | 1 (2.6) | 5 (6.9) | 1 (2.5) | |
| RV‐PA conduit | 19 (12.6) | 0 (0.0) | 12 (16.7) | 7 (17.5) | |
| Pulmonary valvotomy | 13 (8.6) | 7 (17.9) | 6 (8.3) | 0 (0.0) | |
| TAP and pulmonary valvotomy | 1 (0.7) | 1 (2.6) | 0 (0.0) | 0 (0.0) | |
| Non‐TAP and pulmonary valvotomy | 1 (0.7) | 0 (0.0) | 0 (0.0) | 1 (2.5) | <0.001 |
| Intervention in the pulmonary arteries | |||||
| None | 85 (56.3) | 28 (71.8) | 40 (55.6) | 17 (42.5) | |
| PA plasty | 17 (11.3) | 3 (7.7) | 9 (12.5) | 5 (12.5) | |
| PA plication | 3 (2.0) | 0 (0.0) | 1 (1.4) | 2 (5.0) | |
| Unifocalization | 4 (2.6) | 0 (0.0) | 2 (2.8) | 2 (5.0) | |
| Discontinuous PA plasty | 1 (0.7) | 0 (0.0) | 1 (1.4) | 0 (0.0) | |
| Patch extended to LPA | 37 (24.5) | 8 (20.5) | 17 (23.6) | 12 (30.0) | |
| PA plasty and patch extended to LPA | 3 (2.0) | 0 (0.0) | 2 (2.8) | 1 (2.5) | |
| Unifocalization and discontinuous PA plasty | 1 (0.7) | 0 (0.0) | 0 (0.0) | 1 (2.5) | 0.079 |
| Closure of atrial septal defect | 4 (2.6) | 0 (0.0) | 2 (2.8) | 2 (5.0) | 0.49 |
| Atrioventricular canal repair | 6 (4.0) | 0 (0.0) | 2 (2.8) | 4 (10.0) | 0.12 |
| BT shunt take down | 17 (11.3) | 3 (7.7) | 9 (12.5) | 5 (12.5) | 0.71 |
| Collateral ligation | 1 (0.7) | 0 (0.0) | 1 (1.4) | 0 (0.0) | ··· |
| Division of vascular ring | 2 (1.3) | 0 (0.0) | 2 (2.8) | 0 (0.0) | ··· |
| Hospital discharge characteristics | |||||
| Hospital stay from surgery, d | 7.0 (4.0, 11.0) | 4.0 (4.0, 4.0) | 7.0 (5.0, 8.0) | 34.0 (14.0, 65.0) | <0.001 |
| Prolonged stay >30 d | |||||
| No | 132 (87.4) | 39 (100) | 72 (100) | 21 (52.5) | |
| Yes | 19 (12.6) | 0 (0.0) | 0 (0.0) | 19 (47.5) | <0.001 |
| Discharge status | |||||
| Death | 5 (3.3) | 0 (0.0) | 0 (0.0) | 5 (12.5) | |
| Home | 142 (94.0) | 39 (100) | 70 (97.2) | 33 (82.5) | |
| Other hospital/chronic care facility | 4 (2.6) | 0 (0.0) | 2 (2.8) | 2 (5.0) | 0.012 |
| O2 saturation (%) | 96.0 (92.0, 98.0) | 97.0 (95.0, 100.0) | 95.0 (91.0, 97.0) | 97.0 (91.0, 98.0) | 0.089 |
| Number of medications at discharge | 2.0 (1.0, 3.0) | 1.0 (1.0, 2.0) | 2.0 (1.0, 3.0) | 2.5 (1.0, 5.0) | 0.015 |
| Feeding status | |||||
| Oral | 99 (67.8) | 39 (100) | 53 (73.6) | 7 (20.0) | |
| Oral/nasogastric/gastric | 29 (19.9) | 0 (0.0) | 14 (19.4) | 15 (42.9) | |
| Nasogastric/gastric | 18 (12.3) | 0 (0.0) | 5 (6.9) | 13 (37.1) | <0.001 |
| Use of oxygen on discharge, n (%) | 3 (2.1) | 0 (0.0) | 1 (1.4) | 2 (5.7) | 0.22 |
Continuous variables are presented as median (25th to 75th percentiles), counts are presented as n (%). P values represent comparisons between 1‐4 days and ≥12 days in the hospital. BT shunt indicates Blalock‐Taussig shunt; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; LPA, left pulmonary artery; RV‐PA, right ventricle to pulmonary artery; TAP, transannular patch; VSD, ventricular septal defect.
Demographic characteristics were comparable among quartiles. Compared to patients in the first quartile of THD, those in the fourth quartile of THD had lower birth weight, lower gestational age, greater prevalence of prenatal diagnosis, greater prevalence of prematurity, lower preoperative oxygen saturation, and were less likely to be discharged home after birth (Table 1). Moreover, they were more likely to undergo complete repair in the neonatal period (Table 3).
With respect to the cardiac anatomy, those in the fourth quartile of THD had higher prevalence of pulmonary valve atresia/absent pulmonary valve leaflets, higher prevalence of AP collaterals, and higher prevalence of endocardial cushion defects as compared to those in the first quartile. Although measurements for the pulmonary valve annulus, pulmonary artery diameters, and aortic dimensions were, overall, smaller for those in the fourth THD quartile, the Z scores were comparable to those in the first THD quartile. There was no difference by THD quartile in terms of preoperative interventions, extracardiac malformations, and prevalence of 22q11.2 deletion syndrome. Those in the fourth THD had greater prevalence of genetic syndromes (Table 2).
The median age at surgery was 3.6 months (IQR 2.2, 5.2); 23 subjects (15%) underwent complete repair in the newborn period. Eighty‐eight subjects (58%) received a transannular patch at the time of complete repair. The median duration of CPB was 63 minutes (IQR 37, 86), and aortic cross‐clamp time was 40 minutes (IQR 25, 63).
Postoperative events are detailed in Table 4. Seventeen (11.3%) patients had a cardiac event, 26 patients (17.2%) had a postoperative cardiac catheterization, and 11 patients required extracorporeal membrane oxygenation postoperatively (7.3%). The THD was 7 days (IQR 4.0, 11).
Table 4.
Summary Statistics of Events by Length of Hospital Stay Quartiles (N=151)
| Total | 1 to 4 Days | 5 to 11 Days | ≥12 Days | P Value | |
|---|---|---|---|---|---|
| Number of patients with ≥1 event (cardiac+noncardiac) | 48 (31.8) | 3 (7.7) | 20 (27.8) | 25 (62.5) | <0.001 |
| Number of patients with ≥1 cardiac event | 17 (11.3) | 0 (0.0) | 2 (2.8) | 15 (37.5) | <0.001 |
| Number of patients with ≥1 noncardiac event | 31 (20.5) | 3 (7.7) | 14 (19.4) | 14 (35.0) | 0.005 |
| Number of patients with ≥1 catheterization | 26 (17.2) | 1 (2.6) | 8 (11.1) | 17 (42.5) | <0.001 |
| Number of patients who had postoperative ECMO | 11 (7.3) | 0 (0.0) | 0 (0.0) | 11 (27.5) | <0.001 |
| Number of patients with ≥1 consultation to a subspecialty | 50 (33.1) | 11 (28.2) | 22 (30.6) | 17 (42.5) | 0.241 |
Frequencies are expressed as n (%). P‐values compare frequencies between 1‐4 days and ≥12 days in the hospital. ECMO indicates extracorporeal membrane oxygenation.
Predictors of THD
Univariable analysis showed that greater birth weight was associated with higher risk of hospital discharge (shorter THD), whereas earlier gestational age, smaller pulmonary valve annulus Z score, pulmonary valve atresia and absent pulmonary valve leaflets (as opposed to pulmonary valve stenosis), presence of AP collaterals, presence of a genetic diagnosis, greater than 1 CPB run, longer CPB and aortic cross‐clamp time, use of deep hypothermic circulatory arrest, and lower hematocrit while on CPB were statistically significantly associated with lower risk of hospital discharge (longer THD). Results from Cox regression models evaluating the association of the factors with THD are shown in Table 5.
Table 5.
Relationship Between Time to Discharge and Predictors
| Predictor | Univariable Analysis | |
|---|---|---|
| Hazard Ratio (95% CI) | P Value | |
| Race, white vs other | 1.00 (0.67, 1.51) | 0.990 |
| Birth weight, kg | 1.36 (1.10, 1.67) | 0.005 |
| Gestational age, wk | 1.08 (1.01, 1.15) | 0.029 |
| Genetic diagnosis present | 0.65 (0.44, 0.95) | 0.025 |
| PVA, atresia vs stenosis | 0.50 (0.32, 0.79) | 0.003 |
| PVA, absent vs stenosis | 0.26 (0.08, 0.82) | 0.021 |
| Pulmonary valve annulus z‐score | 1.30 (1.04, 1.62) | 0.023 |
| Left pulmonary artery z‐score | 0.98 (0.91, 1.07) | 0.691 |
| Right pulmonary artery z‐score | 0.96 (0.89, 1.04) | 0.346 |
| Aortopulmonary collaterals | 0.49 (0.28, 0.87) | 0.016 |
| Age at surgery, mo | 1.03 (0.97, 1.09) | 0.356 |
| Age at surgery, each 10 d | 1.01 (0.99, 1.03) | 0.356 |
| Weight at surgery, kg | 1.10 (1.00, 1.21) | 0.041 |
| Number of CPB runs, 2 to 3 to 5 vs 1 | 0.42 (0.24, 0.74) | 0.003 |
| Total CPB time (10‐min increase) | 0.86 (0.82, 0.91) | <0.001 |
| Total aortic cross‐clamp time (10‐min increase) | 0.89 (0.84, 0.95) | 0.001 |
| DHCA used | 0.56 (0.30, 1.03) | 0.063 |
| Lowest hematocrit on CPB | 1.06 (1.02, 1.10) | 0.002 |
| Feeding status at admission, NPO vs oral/nasogastric feeds | 0.43 (0.23, 0.83) | 0.012 |
| Neonatal vs nonneonatal repair | 0.54 (0.35, 0.85) | 0.008 |
CI indicates confidence interval; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; NPO, nothing by mouth; PVA, pulmonary valve anatomy.
Birth weight and gestational age were highly correlated, but due to missing gestational age for 12 subjects, birth weight was retained in the multivariable model. Aortic cross‐clamp time was collinear with CPB time; therefore, CPB was removed from the multivariable model.
On multivariable Cox regression, birth weight, pulmonary valve absent/atresia, AP collaterals, number of CPB runs (≥2 versus 1), total aortic cross‐clamp time, and complete repair during neonatal versus nonneonatal period were significantly associated with THD. These results remained significant after adjusting for surgeon. Aortic cross‐clamp time was associated with THD independently of number of CPB runs (Table 6).
Table 6.
Multivariable Cox Proportional Hazards Model
| Predictor | Univariable Model | Multivariable Model | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Race, white vs other | 1.00 (0.67, 1.51) | 0.990 | ··· | ··· |
| Birth weight, kg | 1.36 (1.10, 1.67) | 0.005 | 1.36 (1.06, 1.60) | 0.011 |
| Pulmonary valve anatomy | ||||
| Atresia vs stenosis | 0.50 (0.32, 0.79) | 0.003 | 0.53 (0.39, 1.04) | 0.036 |
| Absent vs stenosis | 0.26 (0.08, 0.82) | 0.021 | 0.28 (0.08, 0.97) | 0.044 |
| Aortopulmonary collaterals | 0.49 (0.28, 0.87) | 0.016 | 0.44 (0.24, 0.84) | 0.018 |
| Number of CPB runs, >1 vs 1 | 0.42 (0.24, 0.74) | 0.003 | 0.34 (0.19, 0.63) | 0.001 |
| Total aortic cross‐clamp time (10‐min increase) | 0.89 (0.84, 0.95) | 0.001 | 0.88 (0.79, 0.97) | 0.009 |
| Use of DHCA | 0.56 (0.30, 1.03) | 0.063 | 0.74 (0.31, 1.75) | 0.491 |
| Lowest hematocrit on CPB | 1.06 (1.02, 1.10) | 0.002 | 1.05 (0.99, 1.10) | 0.097 |
| Neonatal vs nonneonatal repair | 0.54 (0.35, 0.85) | 0.008 | 0.52 (0.31, 0.85) | 0.010 |
Results from the Cox proportional hazards models adjusting for surgeon are reported as hazard ratios with 95% confidence intervals. These covariates explain 41% of the variability in time to hospital discharge. Postoperative variables were excluded, as they are collinear with the outcome. CI indicates confidence interval; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; HR, hazard ratio.
As the birth weight increased by 1 kg, the rate of discharge increased by 35%. (HR [95% CI]: 1.35 [1.11, 1.65], P=0.003). The rate of discharge decreased by 67% for patients who had more than 1 CPB run (HR [95% CI]: 0.33 [0.18, 0.61], P=0.0004). For each 10‐minute increase in total aortic cross‐clamp time, the risk of discharge decreased by 12% (HR [95% CI]: 0.88 [0.79, 0.97], P=0.009). The risk of discharge decreased by 55% for patients who had complete repair during the neonatal period compared to those who had complete repair after the neonatal period (HR [95% CI]: 0.45 [0.27, 0.75]). Patients born with pulmonary valve atresia had a lower rate of discharge compared to those with pulmonary valve stenosis (HR [95% CI]: 0.57 [0.33, 0.97], P=0.038). Presence of AP collaterals was associated with 66% lower rate of discharge (HR [95% CI]: 0.44 [0.24, 0.84], P=0.0118).
Survival functions from the multivariate Cox regression models illustrate the relationship of birth weight, total aortic cross‐clamp time, and complete repair during neonatal period versus nonneonatal period with time to discharge while holding other covariates constant at their mean values (Figure A through D).
Figure 1.
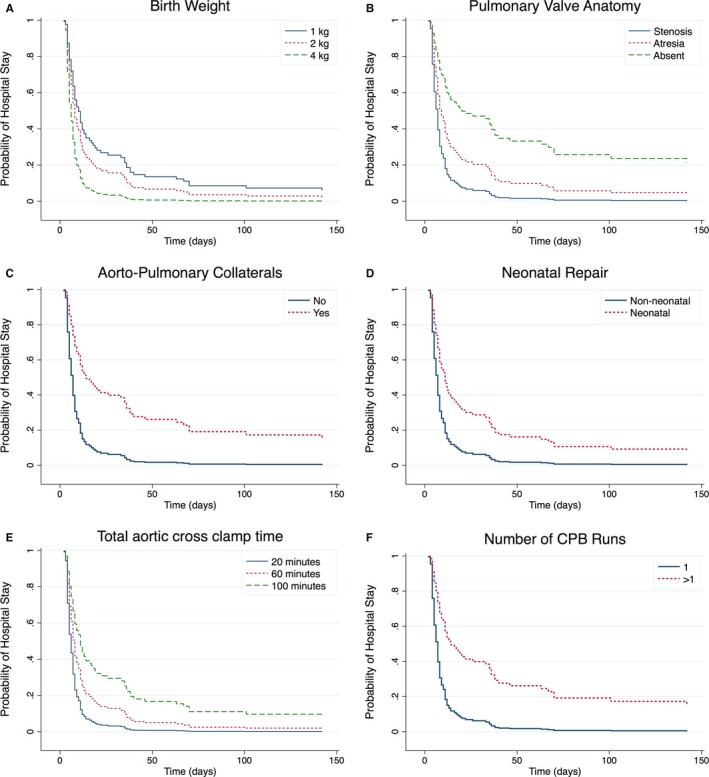
Figures A through F depict the survival function of being in the hospital postoperatively, adjusted by surgeon, and stratified by: A) Birth weight, B) Pulmonary valve anatomy, C) Presence of aorto‐pulmonary collateral vessels, D) Timing of complete TOF repair, E) Aortic cross clamp time, and F) Number of cardiopulmonary bypass runs. CPB indicates cardiopulmonary bypass; TOF, tetralogy of Fallot.
Discussion
In this prospective, single‐center cohort study, we identified patient and operative risk factors for THD after complete surgical repair for TOF. On adjusted analysis, we found that lower birth weight, pulmonary valve anatomy (atresia or absent), neonatal complete repair, more than 1 CPB run, and longer aortic cross‐clamp time were associated with reduced risk of longer time to discharge from the hospital. These factors accounted for 41% of the variance in the THD.
Length of hospital stay is an important outcome variable for the assessment of operative outcomes when postoperative mortality is low. Even 1 additional day in the hospital is associated with significant morbidity and excess costs in TOF.12 Therefore, even modest reductions in THD might lead not only to improved outcomes but also to substantial health cost savings.
We have identified potentially modifiable patient and operative risk factors for THD. Lower birth weight (but not weight at surgery) was associated with longer time to postoperative discharge. Birth weight has been described as a predictor of outcome after congenital heart surgery, in particular when the weight is less than 2.5 kg.13 In TOF, studies have shown that low birth weight is associated with length of hospital stay.14, 15, 16 Lower birth weight may reflect fetal health, placental heath, or a genetic basis.17, 18, 19 Our findings indicate that subjects should not be electively delivered early, and future study is needed to seek the basis of low birth weight in TOF.
Compared to pulmonary valve stenosis, absent pulmonary valve, pulmonary valve atresia, and presence of AP collaterals were associated with increased THD, independent of other covariates. Previous studies of these less common subtypes of TOF have been inconsistent, particularly with respect to associations with reoperations, reinterventions, and genetic syndromes.20, 21, 22, 23 Our findings suggest greater disease severity in patients with absent leaflets or with pulmonary valve atresia and in those with AP collaterals and highlight the impact of anatomy on outcome, even when pulmonary atresia is present without the combination of AP collaterals or the presence of AP collaterals alone, regardless of pulmonary valve anatomy. Contrary to other studies, there was no association between pulmonary artery size and THD, possibly because the median Z score for the right and left pulmonary arteries were within normal limits despite the fact that both had negative median Z scores.24, 25, 26
Whether neonatal repair is preferable to a neonatal palliative procedure preceding complete repair at an older age has been the subject of intense debate.27, 28 In this study, neonatal repair was independently associated with longer THD. Other studies have shown that neonatal repair for TOF is associated with increased circulatory support time, length of mechanical ventilation, greater use of postoperative inotropes, longer LOHS, and subsequent reoperations.15, 22, 27, 29, 30 The neonatal heart might be less “mature” and more vulnerable in the symptomatic neonate with TOF and thus less able to tolerate exposures such as cardiopulmonary bypass and ventriculotomy during surgical repair. Neurologic vulnerability of the neonate as compared to infant may also be an important variable in this decision, but a variable that has not been fully addressed. In this study we were not able to identify patients who would benefit from early repair or candidates for early repair with favorable outcomes. This might be possible in a large, multicenter study with longitudinal follow‐up data.
We also found that repeated CPB runs (independently of CPB or aortic cross‐clamp duration) were associated with THD. Repeated CPB runs suggest technical issues with the surgical repair, such as residual lesions or inability to separate from CPB, and have been directly associated with mortality in congenial heart surgery.31 If a patient had more than 1 CPB run, it is expected for that patient to have a more protracted postoperative recovery and longer hospital stay.
Finally, we found that longer duration of aortic cross clamp was associated with longer THD. Aortic cross‐clamp time was associated with adverse mortality in neonates undergoing congenital heart surgery.32 Aortic cross‐clamp time can also impact longer‐term outcome, as shown by an association with greater right ventricular fibrosis burden 10 years after complete repair for TOF.33
Extracardiac malformations and 22q11.2 deletion were not associated with THD. This finding was consistent with our prior report but contrary to other studies that reported increased THD in TOF patients with 22q11.2 deletion or in patients with congenital heart defects and genetic syndromes.9, 34, 35 The lack of significance in our study could be ascribed in part to the fact that 22q11.2 deletion was not assessed in the entire cohort. Future additional genetic analyses are required in this cohort.
We acknowledge certain limitations to our study. First, this was a single‐center study, and some of the findings may reflect a preferred surgical approach and techniques, which may not be generalizable to other centers. Second, this study examined predictors of THD, and due to low mortality, risk factors could not be analyzed. Third, we examined the data up to hospital discharge, and therefore, the long‐term impact of these factors remains to be identified. Finally, this study did not allow us to identify a group of patients who would benefit from early TOF repair, as we could not make inferences in terms of deciding on the operative approach. Nonetheless, we were able to study a large number of patients with TOF operated on at a single institution who were prospectively enrolled, allowing for optimal data collection and observation. In addition, because we only included subjects with a complete repair with or without a palliative procedure, we were able to study a relatively homogeneous population in terms of cardiac phenotype and surgery in the current era. Given the relative rarity of patients who currently require more complex surgical management, these results apply to the majority of TOF patients.
In summary, our findings indicate that patient (birth weight, pulmonary valve anatomy) and operative factors (number of CPB runs and duration of aortic cross‐clamp time) are associated with THD. Taken together, these factors explain a substantial amount of the variability in THD, independently of each another and irrespective of surgeon. Most of these factors are potentially modifiable: birth weight, with improved prenatal care and counseling; repeated CPB runs/cross‐clamp time (modifiable by patient selection, operative technique), neonatal repair (modifiable by patient selection, staged repairs). Examining the continued impact of these factors on outcome is warranted.
Sources of Funding
Dr Mercer‐Rosa receives research support from the NIH National Heart, Lung, and Blood Institute, grant K01 HL125521, and by Pulmonary Hypertension Association Supplement to HL125521; Dr Kawut receives support from the NIH National Heart, Lung, and Blood Institute, K24HL10384.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e008719 DOI: 10.1161/JAHA.118.008719.)29769202
References
- 1. Liu M, Druschel CM, Hannan EL. Risk‐adjusted prolonged length of stay as an alternative outcome measure for pediatric congenital cardiac surgery. Ann Thorac Surg. 2014;97:2154–2159. [DOI] [PubMed] [Google Scholar]
- 2. Welke KF, Karamlou T, Ungerleider RM, Diggs BS. Mortality rate is not a valid indicator of quality differences between pediatric cardiac surgical programs. Ann Thorac Surg. 2010;89:139–144; discussion 145‐146. [DOI] [PubMed] [Google Scholar]
- 3. Jacobs JP, Jacobs ML, Austin EH III, Mavroudis C, Pasquali SK, Lacour‐Gayet FG, Tchervenkov CI, Walters H III, Bacha EA, Nido PJ, Fraser CD, Gaynor JW, Hirsch JC, Morales DL, Pourmoghadam KK, Tweddell JS, Prager RL, Mayer JE. Quality measures for congenital and pediatric cardiac surgery. World J Pediatr Congenit Heart Surg. 2012;3:32–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Limperopoulos C, Majnemer A, Shevell MI, Rohlicek C, Rosenblatt B, Tchervenkov C, Darwish HZ. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J Pediatr. 2002;141:51–58. [DOI] [PubMed] [Google Scholar]
- 5. Newburger JW, Wypij D, Bellinger DC, du Plessis AJ, Kuban KC, Rappaport LA, Almirall D, Wessel DL, Jonas RA, Wernovsky G. Length of stay after infant heart surgery is related to cognitive outcome at age 8 years. J Pediatr. 2003;143:67–73. [DOI] [PubMed] [Google Scholar]
- 6. Chiu SN, Wang JK, Chen HC, Lin MT, Wu ET, Chen CA, Huang SC, Chang CI, Chen YS, Chiu IS, Chen CL, Wu MH. Long‐term survival and unnatural deaths of patients with repaired tetralogy of Fallot in an Asian cohort. Circ Cardiovasc Qual Outcomes. 2012;5:120–125. [DOI] [PubMed] [Google Scholar]
- 7. Lodin D, Mavrothalassitis O, Haberer K, Sunderji S, Quek RGW, Peyvandi S, Moon‐Grady A, Karamlou T. Revisiting the utility of technical performance scores following tetralogy of Fallot repair. J Thorac Cardiovasc Surg. 2017;154:585–595.e3. [DOI] [PubMed] [Google Scholar]
- 8. Dodgen AL, Dodgen AC, Swearingen CJ, Gossett JM, Dasgupta R, Butt W, Deshpande JK, Gupta P. Characteristics and hemodynamic effects of extubation failure in children undergoing complete repair for tetralogy of Fallot. Pediatr Cardiol. 2013;34:1455–1462. [DOI] [PubMed] [Google Scholar]
- 9. Mercer‐Rosa L, Pinto N, Yang W, Tanel R, Goldmuntz E. 22q11.2 deletion syndrome is associated with perioperative outcome in tetralogy of Fallot. J Thorac Cardiovasc Surg. 2013;146:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauser‐Heaton H, Borquez A, Han B, Ladd M, Asija R, Downey L, Koth A, Algaze CA, Wise‐Faberowski L, Perry SB, Shin A, Peng LF, Hanley FL, McElhinney DB. Programmatic approach to management of tetralogy of Fallot with major aortopulmonary collateral arteries: a 15‐year experience with 458 patients. Circ Cardiovasc Interv. 2017;10:e004952. [DOI] [PubMed] [Google Scholar]
- 11. Cox DSE. A general definition of residuals. J R Stat Soc Series B Stat Methodol. 1968;30:248–275. [Google Scholar]
- 12. Pasquali SK, He X, Jacobs ML, Shah SS, Peterson ED, Gaies MG, Hall M, Gaynor JW, Hill KD, Mayer JE, Li JS, Jacobs JP. Excess costs associated with complications and prolonged length of stay after congenital heart surgery. Ann Thorac Surg. 2014;98:1660–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gaynor JW, Wernovsky G, Jarvik GP, Bernbaum J, Gerdes M, Zackai E, Nord AS, Clancy RR, Nicolson SC, Spray TL. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2007;133:1344–1353, 1353.e1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peer SM, Zurakowski D, Jonas RA, Sinha P. Early primary repair of tetralogy of Fallot does not lead to increased postoperative resource utilization. Ann Thorac Surg. 2014;98:2173–2179; discussion 2179‐2180. [DOI] [PubMed] [Google Scholar]
- 15. Kirsch RE, Glatz AC, Gaynor JW, Nicolson SC, Spray TL, Wernovsky G, Bird GL. Results of elective repair at 6 months or younger in 277 patients with tetralogy of Fallot: a 14‐year experience at a single center. J Thorac Cardiovasc Surg. 2014;147:713–717. [DOI] [PubMed] [Google Scholar]
- 16. Egbe AC, Uppu SC, Mittnacht AJ, Joashi U, Ho D, Nguyen K, Srivastava S. Primary tetralogy of Fallot repair: predictors of intensive care unit morbidity. Asian Cardiovasc Thorac Ann. 2014;22:794–799. [DOI] [PubMed] [Google Scholar]
- 17. Rosenthal GL. Patterns of prenatal growth among infants with cardiovascular malformations: possible fetal hemodynamic effects. Am J Epidemiol. 1996;143:505–513. [DOI] [PubMed] [Google Scholar]
- 18. Llurba E, Sanchez O, Ferrer Q, Nicolaides KH, Ruiz A, Dominguez C, Sanchez‐de‐Toledo J, Garcia‐Garcia B, Soro G, Arevalo S, Goya M, Suy A, Perez‐Hoyos S, Alijotas‐Reig J, Carreras E, Cabero L. Maternal and foetal angiogenic imbalance in congenital heart defects. Eur Heart J. 2014;35:701–707. [DOI] [PubMed] [Google Scholar]
- 19. Matthiesen NB, Henriksen TB, Agergaard P, Gaynor JW, Bach CC, Hjortdal VE, Ostergaard JR. Congenital heart defects and indices of placental and fetal growth in a nationwide study of 924 422 liveborn infants. Circulation. 2016;134:1546–1556. [DOI] [PubMed] [Google Scholar]
- 20. Carotti A, Marino B, Di Donato RM. Influence of chromosome 22q11.2 microdeletion on surgical outcome after treatment of tetralogy of Fallot with pulmonary atresia. J Thorac Cardiovasc Surg. 2003;126:1666–1667. [DOI] [PubMed] [Google Scholar]
- 21. Mahle WT, Crisalli J, Coleman K, Campbell RM, Tam VK, Vincent RN, Kanter KR. Deletion of chromosome 22q11.2 and outcome in patients with pulmonary atresia and ventricular septal defect. Ann Thorac Surg. 2003;76:567–571. [DOI] [PubMed] [Google Scholar]
- 22. Pigula FA, Khalil PN, Mayer JE, del Nido PJ, Jonas RA. Repair of tetralogy of Fallot in neonates and young infants. Circulation. 1999;100:II157–II161. [DOI] [PubMed] [Google Scholar]
- 23. Sandoval JP, Chaturvedi RR, Benson L, Morgan G, Van Arsdell G, Honjo O, Caldarone C, Lee KJ. Right ventricular outflow tract stenting in tetralogy of Fallot infants with risk factors for early primary repair. Circ Cardiovasc Interv. 2016;9:e003979. [DOI] [PubMed] [Google Scholar]
- 24. van Dongen EI, Glansdorp AG, Mildner RJ, McCrindle BW, Sakopoulos AG, VanArsdell G, Williams WG, Bohn D. The influence of perioperative factors on outcomes in children aged less than 18 months after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2003;126:703–710. [DOI] [PubMed] [Google Scholar]
- 25. Li S, Zhang Y, Li S, Wang X, Zhang R, Lu Z, Yan J. Risk factors associated with prolonged mechanical ventilation after corrective surgery for tetralogy of Fallot. Congenit Heart Dis. 2015;10:254–262. [DOI] [PubMed] [Google Scholar]
- 26. Groh MA, Meliones JN, Bove EL, Kirklin JW, Blackstone EH, Lupinetti FM, Snider AR, Rosenthal A. Repair of tetralogy of Fallot in infancy. Effect of pulmonary artery size on outcome. Circulation. 1991;84:III206–III212. [PubMed] [Google Scholar]
- 27. Loomba RS, Buelow MW, Woods RK. Complete repair of tetralogy of Fallot in the neonatal versus non‐neonatal period: a meta‐analysis. Pediatr Cardiol. 2017;38:893–901. [DOI] [PubMed] [Google Scholar]
- 28. Steiner MB, Tang X, Gossett JM, Malik S, Prodhan P. Timing of complete repair of non‐ductal‐dependent tetralogy of Fallot and short‐term postoperative outcomes, a multicenter analysis. J Thorac Cardiovasc Surg. 2014;147:1299–1305. [DOI] [PubMed] [Google Scholar]
- 29. Kanter KR, Kogon BE, Kirshbom PM, Carlock PR. Symptomatic neonatal tetralogy of Fallot: repair or shunt? Ann Thorac Surg. 2010;89:858–863. [DOI] [PubMed] [Google Scholar]
- 30. Wilder TJ, Van Arsdell GS, Benson L, Pham‐Hung E, Gritti M, Page A, Caldarone CA, Hickey EJ. Young infants with severe tetralogy of Fallot: early primary surgery versus transcatheter palliation. J Thorac Cardiovasc Surg. 2017;154:1692–1700.e2. [DOI] [PubMed] [Google Scholar]
- 31. Terada Y, Tachibana K, Takeuchi M, Kinouchi K. [Outcome of the pediatric patients who required repeated cardiopulmonary bypass during the repair of congenital heart disease]. Masui. 2015;64:139–144. [PubMed] [Google Scholar]
- 32. Kansy A, Tobota Z, Maruszewski P, Maruszewski B. Analysis of 14,843 neonatal congenital heart surgical procedures in the European Association for Cardiothoracic Surgery Congenital Database. Ann Thorac Surg. 2010;89:1255–1259. [DOI] [PubMed] [Google Scholar]
- 33. Yim D, Riesenkampff E, Caro‐Dominguez P, Yoo SJ, Seed M, Grosse‐Wortmann L. Assessment of diffuse ventricular myocardial fibrosis using native T1 in children with repaired tetralogy of Fallot. Circ Cardiovasc Imaging. 2017;10:e005695. [DOI] [PubMed] [Google Scholar]
- 34. McDonald R, Dodgen A, Goyal S, Gossett JM, Shinkawa T, Uppu SC, Blanco C, Garcia X, Bhutta AT, Imamura M, Gupta P. Impact of 22q11.2 deletion on the postoperative course of children after cardiac surgery. Pediatr Cardiol. 2013;34:341–347. [DOI] [PubMed] [Google Scholar]
- 35. Simsic JM, Coleman K, Maher KO, Cuadrado A, Kirshbom PM. Do neonates with genetic abnormalities have an increased morbidity and mortality following cardiac surgery? Congenit Heart Dis. 2009;4:160–165. [DOI] [PubMed] [Google Scholar]


