Abstract
Background
Prediction of thrombotic and bleeding risk is important to optimize antithrombotic therapy after percutaneous coronary intervention.
Methods and Results
We developed the prediction rules for thrombotic and bleeding events separately in Japanese patients. Derivation and validation cohorts consisted of 4778 patients from CREDO‐Kyoto (Coronary Revascularization Demonstrating Outcome Study in Kyoto) registry cohort 2 and 4669 patients from RESET (Randomized Evaluation of Sirolimus‐Eluting Versus Everolimus‐Eluting Stent Trial) and NEXT (Nobori Biolimus‐Eluting Versus Xience/Promus Everolimus‐Eluting Stent Trial). Primary thrombotic and bleeding events were a composite of myocardial infarction, definite or probable stent thrombosis or ischemic stroke, and GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) moderate or severe bleeding. The prediction rule for thrombosis assigned 2 points for severe chronic kidney disease, atrial fibrillation, peripheral vascular disease, and anemia and 1 point for age ≥75 years, heart failure, diabetes mellitus, and chronic total occlusion. The prediction rule for bleeding assigned 2 points for thrombocytopenia, severe chronic kidney disease, peripheral vascular disease, and heart failure and 1 point for prior myocardial infarction, malignancy, and atrial fibrillation. In derivation and validation cohorts, area under the curve was 0.68 and 0.64, respectively, for thrombosis and 0.66 and 0.66, respectively, for bleeding. In the validation cohort, a high thrombosis risk score (≥4, n=682) was associated with higher 3‐year incidence of thrombotic events than a score that was intermediate (2–3, n=1178) or low (0–1, n=2809) (7.6%, 3.7%, versus 2.4%, respectively; P<0.0001). A high bleeding risk score (≥3, n=666) was associated with higher incidence of bleeding than scores that were intermediate (1–2, n=1802) or low (0, n=2201) (8.8%, 4.1%, versus 2.3%, respectively; P<0.0001). Among 682 patients at high thrombotic risk, only 39 (5.7%) had low bleeding risk, whereas 401 (58.8%) had high bleeding risk with very high incidence of bleeding (11.6%).
Conclusions
CREDO‐Kyoto thrombotic and bleeding risk scores demonstrated modest accuracy in stratifying thrombotic and bleeding risks; however, a large proportion of patients at high thrombotic risk also had high bleeding risk.
Keywords: bleeding, coronary artery disease, thrombosis
Subject Categories: Percutaneous Coronary Intervention
Clinical Perspective
What Is New?
CREDO‐Kyoto (Coronary Revascularization Demonstrating Outcome Study in Kyoto) thrombotic and bleeding risk scores demonstrated modest accuracy in stratifying thrombotic risk and bleeding risk separately in the derivation and validation cohorts of Japanese patients.
Reflecting the overlap of the risk predictors for thrombosis and bleeding, a large proportion of patients at high thrombotic risk also had high bleeding risk, and their bleeding event rate was very high.
What Are the Clinical Implications?
Our results would provide clinicians determining treatment strategies for antithrombotic therapy with individual risks of thrombotic and bleeding events after percutaneous coronary intervention.
Further studies are warranted to explore optimal antithrombotic therapy in the population at high thrombotic risk for which bleeding risk is also substantial.
Prolonged duration of dual antiplatelet therapy (DAPT) after percutaneous coronary intervention (PCI) was shown to significantly reduce the risk of myocardial infarction (MI) and stent thrombosis (ST) compared with aspirin monotherapy in the DAPT (Dual Antiplatelet Therapy) trial.1 However, prolonged DAPT was also associated with higher risk of bleeding events and marginally higher mortality risk. In meta‐analyses including the DAPT trial, risks of bleeding and mortality were significantly higher in the long DAPT group compared with the short DAPT group.2, 3 These results suggest that predicting the risk of thrombotic and bleeding events is important for determining the intensity of antithrombotic therapy, including the duration of DAPT after PCI in individual patients. The DAPT score was developed to differentiate between ischemic high‐risk patients and bleeding high‐risk patients by using a single scoring system: within the DAPT study, it successfully identified those patients who could benefit from prolonged DAPT without excess bleeding risk.4 The DAPT study, however, excluded those patients who had bleeding events in the first year after PCI; therefore, the DAPT score can be applied only to those bleeding low‐risk patients who could tolerate DAPT for 1 year after PCI. Risk prediction of thrombotic and bleeding events is more important just after PCI than after 1 year. Moreover, patients with high thrombotic risk were also reported to have high bleeding risk.5 It has not been yet adequately addressed whether the use of a single scoring system for evaluating both thrombotic and bleeding risk is superior to the use of scoring systems dedicated to evaluating thrombotic and bleeding risk separately.
We sought to develop the prediction rules for the thrombotic events and the bleeding events separately in a large Japanese observational database of patients undergoing first coronary revascularization and to validate the developed risk scores in another Japanese cohort.
Methods
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
We developed the 2 clinical prediction rules for thrombotic and bleeding events, named the CREDO‐Kyoto (Coronary Revascularization Demonstrating Outcome Study in Kyoto) thrombotic risk score and the CREDO‐Kyoto bleeding risk score.6
We identified the derivation cohort of 4778 patients treated by PCI with exclusive use of sirolimus‐eluting stent from the CREDO‐Kyoto PCI and coronary artery bypass grafting registry cohort 2, which is an investigator‐initiated multicenter registry enrolling consecutive patients who underwent first coronary revascularization procedures among 26 centers in Japan between January 2005 and December 2007 (Figure 1A).7 The validation cohort consisted of 4669 patients treated with PCI with exclusive use of new‐generation drug‐eluting stents (DESs) from the RESET (Randomized Evaluation of Sirolimus‐Eluting Versus Everolimus‐Eluting Stent Trial), and NEXT (Nobori Biolimus‐Eluting Versus Xience/Promus Everolimus‐Eluting Stent Trial) studies (Figure 1B).8, 9 RESET and NEXT are prospective, multicenter, randomized trials in Japan comparing new‐generation everolimus‐eluting stents with first‐generation sirolimus‐eluting stents and comparing new‐generation biolimus‐eluting stents with everolimus‐eluting stents, respectively.8, 9 The relevant review boards at all participating centers for each study approved each research protocol for the 3 studies. Because of retrospective enrollment, the requirement for written informed consent from patients was waived in the CREDO‐Kyoto PCI and coronary artery bypass grafting registry cohort 2; however, we excluded those patients who refused participation in the study when contacted for follow‐up. Written informed consent was obtained from all study patients in RESET and NEXT. We excluded those patients with in‐hospital death, MI, ST, ischemic stroke, and bleeding during the index hospitalization because those in‐hospital events were closely related to the index event and/or the index PCI and thus were not suitable for use in constructing the clinical prediction rules for long‐term thrombotic and bleeding events.
Figure 1.
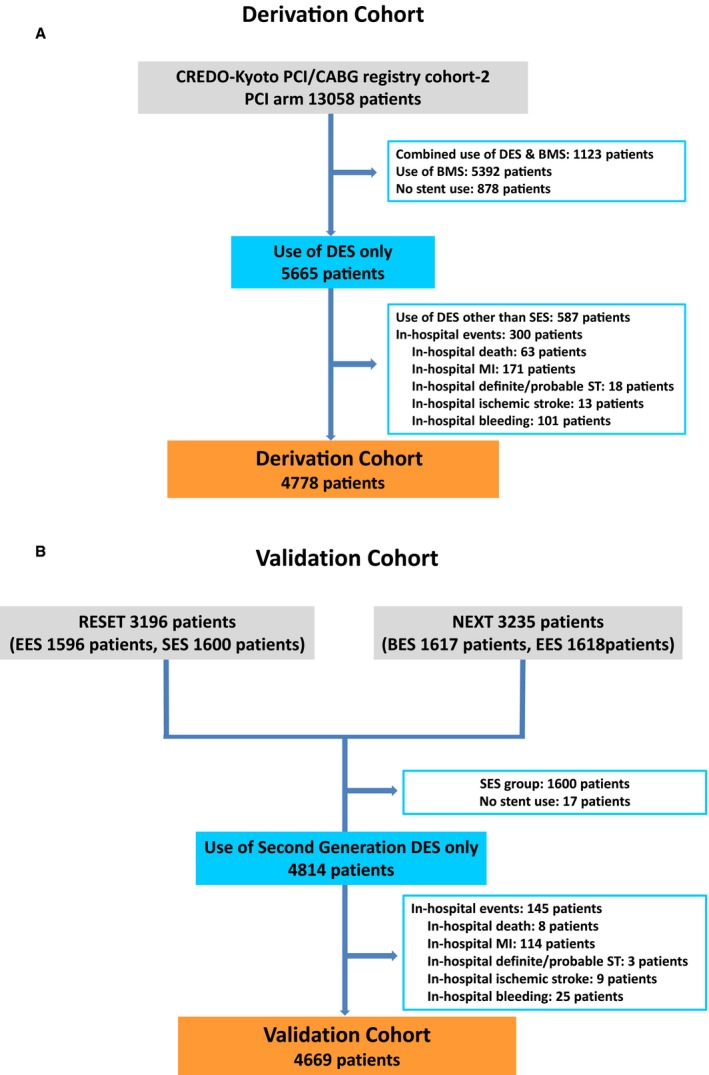
Study flow chart of the derivation cohort (A) and the validation cohort (B). BES indicates biolimus‐eluting stent; BMS, bare metal stent; CABG, coronary artery bypass grafting; CREDO‐Kyoto, Coronary Revascularization Demonstrating Outcome Study in Kyoto; DES, drug‐eluting stent; EES, everolimus‐eluting stent; MI, myocardial infarction; NEXT, Nobori Biolimus‐Eluting Versus Xience/Promus Everolimus‐Eluting Stent Trial; PCI, percutaneous coronary intervention; RESET, Randomized Evaluation of Sirolimus‐Eluting Versus Everolimus‐Eluting Stent Trial; SES, sirolimus‐eluting stent; ST, stent thrombosis.
Definitions
The primary thrombotic event was defined as a composite of MI and definite or probable ST or ischemic stroke, and the primary bleeding event was defined as the GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries) moderate or severe bleeding. MI was adjudicated by the definition of ARTS (Arterial Revascularization Therapies Study) in CREDO‐Kyoto PCI and coronary artery bypass grafting registry cohort 2 and by the definition of ARC (Academic Research Consortium) consensus criteria in RESET and NEXT.10, 11 Both the ARTS and ARC definitions adopted the same criteria for spontaneous MI (biomarker elevation above the upper limit of normal). The present study evaluated postdischarge clinical outcomes after PCI, and the majority of adjudicated MIs were not procedure‐related but rather spontaneous. The definitions for the outcomes other than MI were identical across the 3 studies. ST was defined according to the definition of the ARC.11 Ischemic stroke during follow‐up was defined as stroke requiring hospitalization with symptoms lasting >24 hours. Bleeding was defined according to the GUSTO classification.12 All clinical events were adjudicated by the independent clinical event committees in each study.
Data Collection and Follow‐up
In all 3 studies, demographic, angiographic, and procedural data were collected from hospital charts or databases at each participating center according to the definitions prespecified by the site investigators or by the experienced clinical research coordinators in the study management center (Research Institute for Production Development, Kyoto, Japan). Follow‐up data were obtained from hospital charts or by contacting patients or referring physicians with questions about vital status, subsequent hospitalization, and status of antiplatelet therapy. Persistent DAPT discontinuation was defined as withdrawal of either aspirin or thienopyridine lasting for at least 2 months.13 The follow‐up duration was 5 years in the CREDO‐Kyoto PCI and coronary artery bypass grafting registry cohort 2 and 3 years in RESET and NEXT.8, 9, 14 In the present analysis, follow‐up was truncated at 3 years to standardize follow‐up duration in both the derivation and validation cohorts.
Statistical Analyses
Categorical variables are expressed as number and percentage, and continuous variables are expressed as mean±SD. To compare the characteristics between cohorts, we used the χ2 test for categorical variables and the Student t test or Wilcoxon rank sum test for continuous variables based on the distributions.
To develop clinical prediction rules, we first dichotomized the continuous variables to ensure that the final model did not contain any continuous variables, so clinicians could categorize patients by the presence or absence of a factor without performing any calculations. The cutoff values of age ≥75 years, body mass index <25.0, hemoglobin <11 g/dL, and platelet count <100 000/μL were derived from the previous report.7 Patients with moderate and severe chronic kidney disease (CKD) were defined as those with an estimated glomerular filtration rate (eGFR) ≥30 and <60 mL/min per 1.73 m2 and those with dialysis or eGFR <30 mL/min per 1.73 m2, respectively. Total stent length ≥36 mm and minimum stent size <3.0 mm were determined by the Youden Index on receiver operating characteristic curves. We constructed univariate logistic regression models to assess the strength of the association between the 26 potential predictors and thrombotic events and between the 19 potential predictors and bleeding events in the derivation cohort. Missing values were considered null because the developed clinical prediction rules should allow risk prediction based on the available information for any patient with any missing or uncertain variables. Missing data were found for 85 patients (1.8%) regarding body mass index, for 50 patients (1.0%) regarding eGFR, for 54 patients (1.1%) regarding hemoglobin, and for 22 patients (0.5%) regarding platelet count in the derivation cohort and for 31 patients (0.7%) regarding body mass index, for 25 patients (0.5%) regarding eGFR, for 13 patients (0.3%) regarding hemoglobin, and for 16 patients (0.3%) regarding platelet count in the validation cohort. From clinical context, the missing data of each value were categorized as body mass index <25.0, eGFR ≥60, hemoglobin ≥11.0 g/dL, and platelet count ≥100 000/μL, because physicians have to determine the likelihood of events in the future, even for patients with some missing data, by assuming no risk categories for variables with missing data. Variables found to be associated at P<0.10 in the univariate logistic regression models were included in the multivariate models. We constructed multivariate logistic regression models to select variables associated with the thrombotic and bleeding events separately in the same derivation cohort. Applying the backward model selection procedure to eliminate the variables with higher P values, we finally constructed multivariate logistic regression models using those variables with P<0.05 for each event. The results of the multivariate logistic regression models were then used to develop a clinical prediction model.6 The β coefficient for each variable was divided by the smallest β coefficient and rounded to the nearest integer, which was used as the point for the variable. The risk score for an individual patient was determined by assigning points for each variable present and summing. The discriminatory performances of the models were assessed by receiver operating characteristic curve analysis in the derivation and validation cohorts.15 We calculated the area under the curve (AUC) of each model in the derivation and validation cohorts and compared the corresponding models between derivation and validation cohorts. The resulting continuous distribution of each risk score of all patients in the validation cohort was then stratified into 3 categories of scores according to the level of probability. The intermediate‐risk group was determined so that incidence was similar to that of the entire validation cohort. Those patients with higher and lower risk scores than the those of the intermediate‐risk group were classified as the high‐ and low‐risk groups, respectively.
Cumulative incidence was estimated by the Kaplan–Meier method, and differences were assessed with the log‐rank test for the 3 categorized risk groups. Statistical analyses were conducted by a physician (M.N.) and by a statistician (T.M.) with the use of JMP 10.0 and SAS 9.4 (SAS Institute) software. All statistical analyses were 2‐tailed. P<0.05 was considered statistically significant.
Results
Baseline Characteristics
Because of the differences in the study design, baseline characteristics were significantly different in several aspects between the derivation and validation cohorts. Regarding clinical characteristics, patients in the derivation cohort were younger and had lower body mass indexes than those in the validation cohort. Acute MI, hypertension, current smoking, heart failure, multivessel disease, and atrial fibrillation (AF) were more often found in the derivation cohort, whereas male sex, diabetes mellitus, prior MI, peripheral vascular disease (PVD), and dialysis were more prevalent in the validation cohort. Regarding procedural characteristics, the derivation cohort included more target lesions, longer total stent length, and smaller minimum stent size than the validation cohort. Left anterior descending coronary artery, chronic total occlusion, and bifurcation lesions were more prevalent in the derivation cohort than in the validation cohort. The prevalence of statin use was significantly lower in the derivation cohort than in the validation cohort (Table 1).
Table 1.
Baseline Characteristics of Patients in the Derivation and Validation Cohort
| Derivation Cohort (n=4778) | Validation Cohort (n=4669) | P Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, y | 68.1±10.3 | 69.0±9.8 | <0.0001 |
| Age ≥75 y | 1389 (29) | 1474 (32) | 0.008 |
| Male | 3447 (72) | 3622 (78) | <0.0001 |
| BMI | 23.8±3.4 | 24.2±3.6 | <0.0001 |
| BMI <25.0 | 3083/4693 (66) | 2903/4638 (63) | 0.002 |
| Acute myocardial infarction | 733 (15) | 251 (5.4) | <0.0001 |
| Hypertension | 3965 (83) | 3770 (81) | 0.005 |
| Diabetes mellitus | 1952 (41) | 2136 (46) | <0.0001 |
| On insulin therapy | 499 (10) | 493 (11) | 0.86 |
| Current smoking | 1322 (28) | 900 (19) | <0.0001 |
| Heart failure | 772 (16) | 567 (12) | <0.0001 |
| Multivessel disease | 2762 (58) | 2322 (50) | <0.0001 |
| Prior MI | 641 (13) | 1329 (28) | <0.0001 |
| Prior stroke | 526 (11) | 502 (11) | 0.69 |
| PVD | 371 (7.8) | 465 (10) | 0.0002 |
| Moderate CKD | 1494/4728 (32) | 1546/4644 (33) | 0.08 |
| Severe CKD | 374/4728 (7.9) | 371/4644 (8.0) | 0.89 |
| eGFR <30, not on dialysis | 167/4728 (3.5) | 106/4644 (2.3) | 0.0003 |
| Dialysis | 207 (4.3) | 265 (5.7) | 0.003 |
| AF | 368 (7.7) | 309 (6.6) | 0.04 |
| Anemia (Hb <11 g/dL) | 517/4724 (11) | 558/4656 (12) | 0.11 |
| Platelet count <100 000/μL | 63/4750 (1.3) | 77/4653 (1.7) | 0.19 |
| Cirrhosis | 108 (2.3) | 36 (0.8) | <0.0001 |
| Malignancy | 408 (8.5) | 330 (7.1) | 0.008 |
| Procedural characteristics | |||
| Number of target lesions | 1.46±0.73 | 1.25±0.52 | <0.0001 |
| Target of LAD | 3089 (65) | 2268 (49) | <0.0001 |
| Target of unprotected LMCA | 149 (3.1) | 128 (2.7) | 0.28 |
| Target of CTO | 633 (13) | 347 (7.4) | <0.0001 |
| Target of bifurcation | 1849 (39) | 1087 (23) | <0.0001 |
| Side‐branch stenting | 220 (4.6) | 63 (1.4) | <0.0001 |
| Total number of stents | 1.87±1.18 | 1.55±0.81 | <0.0001 |
| Total stent length, mm | 41.7±29.3 | 32.0±19.9 | <0.0001 |
| Total stent length ≥36 mm | 2277 (48) | 1555 (33) | <0.0001 |
| Minimum stent size, mm | 2.83±0.37 | 2.9±0.39 | <0.0001 |
| Minimum stent size <3.0 mm | 2375 (50) | 2212 (47) | 0.02 |
| Baseline medication | |||
| Medication at hospital discharge | |||
| Antiplatelet therapy | |||
| Thienopyridine | 4765 (99.7) | 4646 (99.5) | 0.08 |
| Ticlopidine | 4240 (89) | 631 (14) | <0.0001 |
| Clopidogrel | 517 (11) | 3981 (86) | <0.0001 |
| Aspirin | 4714 (98.7) | 4655 (99.7) | <0.0001 |
| Cilostazole | 711 (15) | 279 (6.0) | <0.0001 |
| Other medications | |||
| Statins | 2616 (55) | 3569 (76) | <0.0001 |
| Beta‐blockers | 1349 (28) | 1742 (37) | <0.0001 |
| ACE‐I/ARB | 2639 (55) | 2877 (62) | <0.0001 |
| Nitrates | 1776 (37) | 1198 (26) | <0.0001 |
| Calcium channel blockers | 2263 (47) | 2080 (45) | 0.006 |
| Warfarin | 389 (8.1) | 354 (7.6) | 0.31 |
Values are expressed as mean±SD or number (%). Patients with moderate CKD had eGFR ≥30 and <60 mL/min/1.73 m2, and those with severe CKD were on dialysis or had eGFR <30 mL/min/1.73 m2. ACE‐I indicates angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; CKD, chronic kidney disease; CTO, chronic total occlusion; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; LAD, left anterior descending coronary artery; LMCA, left main coronary artery; MI, myocardial infarction; PVD, peripheral vascular disease.
Thrombotic Risk Score
The prediction rule for the thrombotic risk assigned 2 points for severe CKD, AF, PVD, and anemia and 1 point for age ≥75 years, heart failure, diabetes mellitus, and chronic total occlusion (Table 2). The thrombotic risk score ranged from 0 to 12, with peaks at 1 point in the derivation and validation cohorts (Figures 2A and 3). Distribution of the thrombotic risk score categories was comparable in both the derivation and validation cohorts, with the majority of patients in the low thrombotic risk category (Table 3). Patients were classified by thrombotic risk score as high, intermediate, and low: High was ≥4 points (derivation cohort: n=693, 14.5%; validation cohort: n=682, 14.6%), intermediate was 2–3 points (derivation cohort: n=1263, 26.4%; validation cohort: n=1178, 25.2%), and low was 0–1 point (derivation cohort: n=2822 patients, 59.1%; ; validation cohort: n=2809, 60.2%).
Table 2.
Univariate and Multivariate Analysis for Thrombotic and Bleeding Events in the Derivation Cohort
| Univariate | P Value | Multivariate | P Value | Score | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | β Estimate | ||||
| Thrombotic risk score | ||||||
| Age ≥75 y | 1.84 (1.40–2.41) | <0.0001 | 1.71 (1.28–2.28) | 0.536 | 0.0002 | 1 |
| Male | 1.04 (0.78–1.42) | 0.78 | ||||
| BMI <25.0 | 1.11 (0.84–1.47) | 0.47 | ||||
| Acute MI | 1.15 (0.81–1.63) | 0.46 | ||||
| Hypertension | 1.46 (0.99–2.22) | 0.051 | ||||
| Diabetes mellitus | 1.58 (1.21–2.07) | 0.0007 | 1.45 (1.1–1.91) | 0.373 | 0.008 | 1 |
| Current smoking | 1.04 (0.77–1.38) | 0.81 | ||||
| Heart failure | 2.34 (1.71–3.15) | <0.0001 | 1.6 (1.15–2.21) | 0.467 | 0.005 | 1 |
| Prior MI | 1.38 (0.96–1.97) | 0.08 | ||||
| Prior stroke | 1.90 (1.31–2.68) | 0.0009 | ||||
| PVD | 2.08 (1.38–3.1) | 0.0008 | 1.77 (1.18–2.67) | 0.573 | 0.006 | 2 |
| Moderate CKD | 1.80 (1.34–2.41) | <0.0001 | ||||
| Severe CKD | 4.65 (3.12–6.83) | <0.0001 | 2.44 (1.6–3.71) | 0.892 | <0.0001 | 2 |
| AF | 2.13 (1.41–3.12) | 0.0006 | 1.85 (1.22–2.8) | 0.615 | 0.004 | 2 |
| Anemia (Hb <11 g/dL) | 3.17 (2.26–4.38) | <0.0001 | 1.77 (1.21–2.59) | 0.57 | 0.003 | 2 |
| Platelet count <100 000/μL | 0.39 (0.02–1.78) | 0.27 | ||||
| Cirrhosis | 1.63 (0.72–3.2) | 0.22 | ||||
| Malignancy | 1.03 (0.62–1.63) | 0.90 | ||||
| Multivessel disease | 1.44 (1.09–1.91) | 0.009 | ||||
| Target of LAD | 1.22 (0.92–1.63) | 0.17 | ||||
| Target of unprotected LMCA | 1.95 (1.01–3.45) | 0.048 | ||||
| Target of CTO | 1.41 (0.99–2.0) | 0.06 | 1.44 (1.0–2.07) | 0.365 | 0.047 | 1 |
| Target of bifurcation | 0.79 (0.59–1.04) | 0.09 | ||||
| Side‐branch stenting | 0.78 (0.35–1.49) | 0.47 | ||||
| Total stent length ≥36 mm | 1.37 (1.05–1.79) | 0.02 | ||||
| Minimum stent size <3.0 mm | 1.09 (0.83–1.42) | 0.54 | ||||
| Bleeding risk score | ||||||
| Age ≥75 y | 1.49 (1.12–1.96) | 0.007 | ||||
| Male | 1.19 (0.88–1.63) | 0.27 | ||||
| BMI <25.0 | 1.19 (0.9–1.6) | 0.22 | ||||
| Acute MI | 0.88 (0.59–1.27) | 0.49 | ||||
| Hypertension | 1.73 (1.16–2.7) | 0.007 | ||||
| Diabetes mellitus | 1.44 (1.1–1.88) | 0.008 | ||||
| Current smoking | 0.82 (0.60–1.11) | 0.21 | ||||
| Heart failure | 2.75 (2.03–3.69) | <0.0001 | 2.13 (1.54–2.94) | 0.754 | <0.0001 | 2 |
| Prior MI | 2.06 (1.47–2.83) | <0.0001 | 1.68 (1.19–2.37) | 0.519 | 0.003 | 1 |
| Prior stroke | 1.83 (1.26–2.59) | 0.002 | ||||
| PVD | 2.83 (1.95–4.02) | <0.0001 | 2.61 (1.8–3.8) | 0.961 | <0.0001 | 2 |
| Moderate CKD | 1.46 (1.09–1.96) | 0.01 | ||||
| Severe CKD | 3.94 (2.64–5.79) | <0.0001 | 2.65 (1.79–3.9) | 0.973 | <0.0001 | 2 |
| AF | 1.95 (1.27–2.88) | 0.003 | 1.55 (1.01–2.38) | 0.437 | 0.046 | 1 |
| Warfarin | 1.85 (1.22–2.72) | 0.005 | ||||
| Anemia (Hb <11 g/dL) | 2.72 (1.92–3.80) | <0.0001 | ||||
| Platelet count <100 000/μL | 4.80 (2.24–9.37) | 0.0002 | 2.76 (1.31–5.83) | 1.016 | 0.008 | 2 |
| Cirrhosis | 1.84 (0.85–3.51) | 0.11 | ||||
| Malignancy | 1.68 (1.1–2.49) | 0.02 | 1.6 (1.05–2.43) | 0.469 | 0.03 | 1 |
Patients with moderate CKD had eGFR ≥30 and <60 mL/min/1.73 m2, and those with severe CKD were on dialysis or had eGFR <30 mL/min/1.73 m2, respectively. BMI indicates body mass index; CI, confidence interval; CKD, chronic kidney disease; CTO, chronic total occlusion; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; LAD, left anterior descending coronary artery; LMCA, left main coronary artery; MI, myocardial infarction; OR, odds ratio; PVD, peripheral vascular disease.
Figure 2.
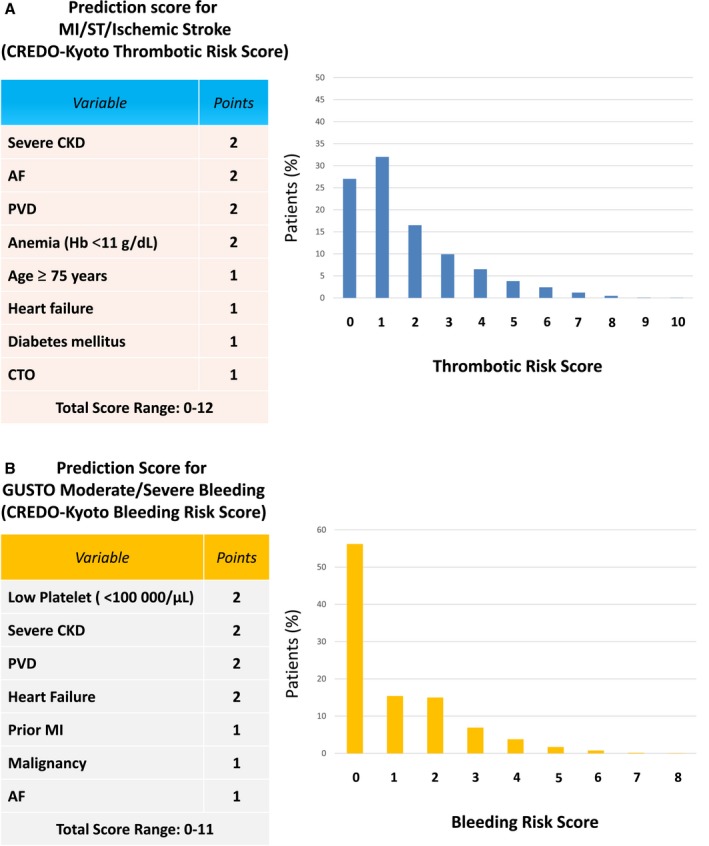
Elements and distribution of the prediction scores. A, Thrombotic risk score in the derivation cohort. B, Bleeding risk score in the derivation cohort. Severe CKD indicates those with dialysis or estimated glomerular filtration rate <30 mL/min per 1.73m2. For the web calculator, see http://www.seiken-j.or.jp/CREDO-Kyoto.risk.score/.26 AF indicates atrial fibrillation; CKD, chronic kidney disease; CREDO‐Kyoto, Coronary Revascularization Demonstrating Outcome Study in Kyoto; CTO, chronic total occlusion; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; Hb, hemoglobin; MI, myocardial infarction; PVD, peripheral vascular disease; ST, definite or probable stent thrombosis.
Figure 3.
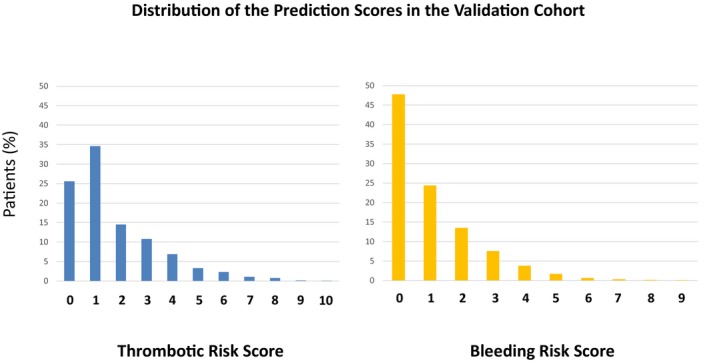
Distribution of the prediction scores in the validation cohort.
Table 3.
Cumulative 3‐Year Incidences of Events According to the Thrombotic and Bleeding Risk Categories in the Derivation and Validation Cohorts
| Thrombotic Risk Score | P Value | Bleeding Risk Score | P Value | |||||
|---|---|---|---|---|---|---|---|---|
| Low | Intermediate | High | Low | Intermediate | High | |||
| Derivation cohort, n | 2822 | 1263 | 693 | 2685 | 1455 | 638 | ||
| MI, ST, or ischemic stroke | 86 (3.1) | 70 (5.8) | 74 (11.8) | <0.0001 | 92 (3.5) | 83 (5.9) | 55 (9.7) | <0.0001 |
| MI | 40 (1.5) | 20 (1.7) | 23 (3.8) | 0.0003 | 40 (1.5) | 22 (1.6) | 21 (3.8) | 0.0007 |
| ST | 23 (0.8) | 10 (0.8) | 11 (1.8) | 0.06 | 22 (0.9) | 12 (0.9) | 10 (1.8) | 0.08 |
| Ischemic stroke | 47 (1.7) | 51 (4.3) | 51 (8.1) | <0.0001 | 51 (2.0) | 64 (4.6) | 34 (6.0) | <0.0001 |
| GUSTO moderate/severe bleeding | 89 (3.2) | 67 (5.6) | 73 (11.6) | <0.0001 | 84 (3.2) | 68 (4.9) | 77 (13.5) | <0.0001 |
| GUSTO severe bleeding | 44 (1.6) | 25 (2.1) | 33 (5.3) | <0.0001 | 42 (1.6) | 26 (1.9) | 34 (6.0) | <0.0001 |
| Death | 91 (3.3) | 100 (8.0) | 179 (26.3) | <0.0001 | 77 (2.9) | 129 (9.0) | 164 (26.2) | <0.0001 |
| Cardiac death | 32 (1.2) | 38 (3.1) | 73 (11.7) | <0.0001 | 22 (0.8) | 55 (3.9) | 66 (11.4) | <0.0001 |
| Noncardiac death | 59 (2.1) | 62 (5.1) | 106 (16.5) | <0.0001 | 55 (2.1) | 74 (5.3) | 98 (16.7) | <0.0001 |
| Persistent DAPT discontinuation | 1386 (50.2) | 599 (49.5) | 331 (53.6) | 0.53 | 1288 (49.1) | 722 (51.9) | 306 (53.1) | 0.24 |
| Validation cohort, n | 2809 | 1178 | 682 | 2201 | 1802 | 666 | ||
| MI, ST, or ischemic stroke | 66 (2.4) | 42 (3.7) | 48 (7.6) | <0.0001 | 48 (2.2) | 65 (3.7) | 43 (7.1) | <0.0001 |
| MI | 30 (1.1) | 18 (1.6) | 24 (3.8) | <0.0001 | 20 (0.9) | 32 (1.8) | 20 (3.3) | 0.0001 |
| ST | 5 (0.2) | 5 (0.5) | 6 (0.9) | 0.01 | 4 (0.2) | 7 (0.4) | 5 (0.8) | 0.06 |
| Ischemic stroke | 36 (1.3) | 26 (2.3) | 24 (3.8) | <0.0001 | 29 (1.4) | 34 (2.0) | 23 (3.8) | 0.0004 |
| GUSTO moderate/severe bleeding | 57 (2.1) | 55 (4.8) | 62 (9.9) | <0.0001 | 49 (2.3) | 72 (4.1) | 54 (8.8) | <0.0001 |
| GUSTO severe bleeding | 37 (1.4) | 40 (3.5) | 41 (6.5) | <0.0001 | 36 (1.7) | 43 (2.5) | 39 (6.3) | <0.0001 |
| Death | 86 (3.1) | 97 (8.4) | 124 (18.4) | <0.0001 | 79 (3.6) | 112 (6.3) | 116 (17.6) | <0.0001 |
| Cardiac death | 25 (0.9) | 29 (2.6) | 54 (8.3) | <0.0001 | 21 (1.0) | 35 (2.0) | 52 (8.2) | <0.0001 |
| Noncardiac death | 61 (2.2) | 68 (6.0) | 70 (11.0) | <0.0001 | 58 (2.7) | 77 (4.4) | 64 (10.2) | <0.0001 |
| Persistent DAPT discontinuation | 1116 (40.8) | 449 (39.9) | 250 (41.0) | 0.9 | 899 (42.0) | 673 (38.7) | 243 (41.0) | 0.1 |
Data reflect patients with the event (cumulative incidence) and are shown as n (%). DAPT indicates dual antiplatelet therapy; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; MI, myocardial infarction; ST, definite or probable stent thrombosis.
The AUC for the thrombotic risk score was 0.68 in the derivation cohort and 0.64 in the validation cohort (Figure 4). The CREDO‐Kyoto thrombotic risk score was validated with modest accuracy in the validation cohort without significant difference in the AUC between the derivation and validation cohorts (P=0.23). Calibration of the model was tested on the entire cohort and proved satisfactory (Figure 5A).
Figure 4.
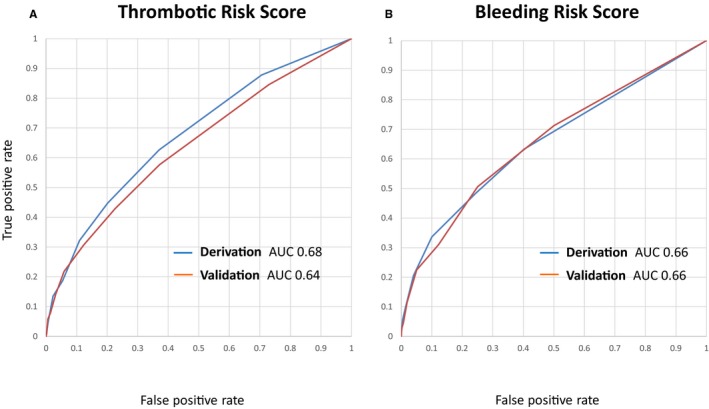
A, AUC for the thrombotic risk score in the derivation and validation cohorts. There was no significant difference between the derivation and validation cohorts (P=0.23). B, AUC for the bleeding risk score in the derivation and the validation cohorts. There was no significant difference between the derivation and validation cohorts (P=0.96). AUC indicates area under the curve; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; MI, myocardial infarction; ST, definite or probable stent thrombosis.
Figure 5.
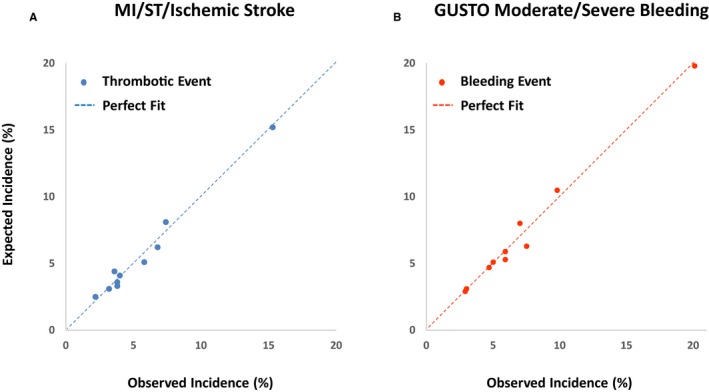
Observed vs predicted incidence of thrombotic and bleeding events. Scatterplot allowing a visual assessment of the linearity of increasing event rates across risk groups. The straight dashed diagonal line represents perfect calibration, and deviations from this line represent over‐ and underprediction of actual risk. A, MI, ST, and ischemic stroke. B, GUSTO moderate/severe bleeding. GUSTO indicates Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; MI, myocardial infarction; ST, definite or probable stent thrombosis.
Bleeding Risk Score
The prediction rule for the bleeding risk assigned 2 points for thrombocytopenia, severe CKD, PVD, and heart failure and 1 point for prior MI, malignancy, and AF (Table 2). The variables incorporated in the bleeding risk score had considerable overlap with those incorporated in the thrombotic risk score (severe CKD, AF, PVD, and heart failure). The bleeding risk score ranged from 0 to 11, with the peak at 0 points in the derivation and validation cohorts (Figures 2B and 3). The distribution of the bleeding risk score categories was comparable in the derivation and validation cohorts, with the majority of patients in the low bleeding risk category (Table 3). Patients were classified by thrombotic risk score as high, intermediate, and low: High was ≥3 points (derivation cohort: n=638, 13.4%; validation cohort: n=666, 14.3%), intermediate was 1–2 points (derivation cohort: n=1455, 30.5%; validation cohort: n=1802, 38.6%), and low was 0 points (derivation cohort: n=2685, 56.2%; validation cohort: n=2201, 47.1%).
The AUC for the bleeding risk score was 0.66 in the derivation cohort and 0.66 in the validation cohort (Figure 4). The CREDO‐Kyoto bleeding risk score was also validated with modest accuracy in the validation cohort without significant difference in AUC between the derivation and validation cohorts (P=0.96). Calibration of the model was tested on the entire cohort and proved satisfactory (Figure 5B).
Clinical Outcomes Over 3 Years in the Derivation and Validation Cohorts
In the derivation cohort, primary thrombotic and bleeding events occurred in 230 patients (5.0%) and in 229 patients (5.0%), respectively, over 3 years. Patients with high thrombotic risk scores in the derivation cohort had higher cumulative 3‐year incidence of primary thrombotic events compared with those with intermediate and low thrombotic risk scores (11.8%, 5.8%, and 3.1%; P<0.0001; Figures 6A and 7A). Patients with high bleeding scores in the derivation cohort had higher cumulative 3‐year incidence of primary bleeding events compared with those with intermediate and low bleeding risk scores (13.5%, 4.9%, and 3.2%; P<0.0001; Figures 6B and 7B). In the validation cohort, primary thrombotic and bleeding events occurred in 156 patients (3.5%) and 175 patients (3.9%), respectively. In the validation cohort, there also was an incremental increase in the cumulative 3‐year incidence of primary thrombotic events with higher thrombotic risk scores (7.6%, 3.7%, and 2.4%, respectively; P<0.0001; Figures 6A and 7C) and an incremental increase in the cumulative 3‐year incidence of primary bleeding events with higher bleeding risk scores (8.8%, 4.1%, and 2.3%, respectively; P<0.0001; Figures 6B and 7D), although the absolute event rates in the validation cohort were lower than those in the derivation cohort (Figure 6).
Figure 6.
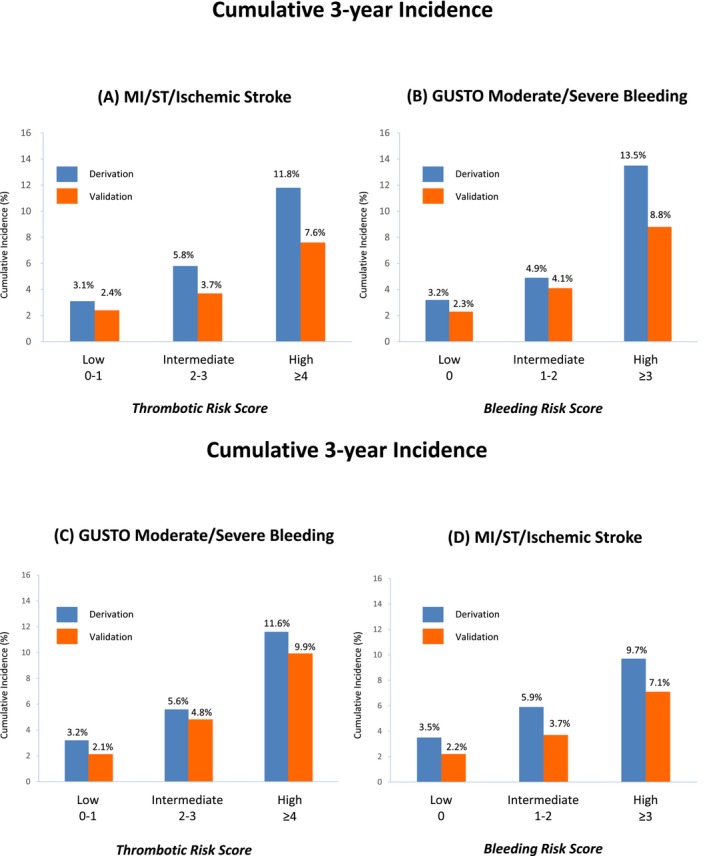
Cumulative 3‐year incidence of thrombotic and bleeding events in the derivation and validation cohorts. A, MI, ST, and ischemic stroke according to the thrombotic risk score categories. B, GUSTO moderate/severe bleeding according to the bleeding risk score categories. C, GUSTO moderate/severe bleeding according to the thrombotic risk score categories. D, MI, ST, and ischemic stroke according to the bleeding risk score categories. GUSTO indicates Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; MI, myocardial infarction; ST, definite or probable stent thrombosis.
Figure 7.
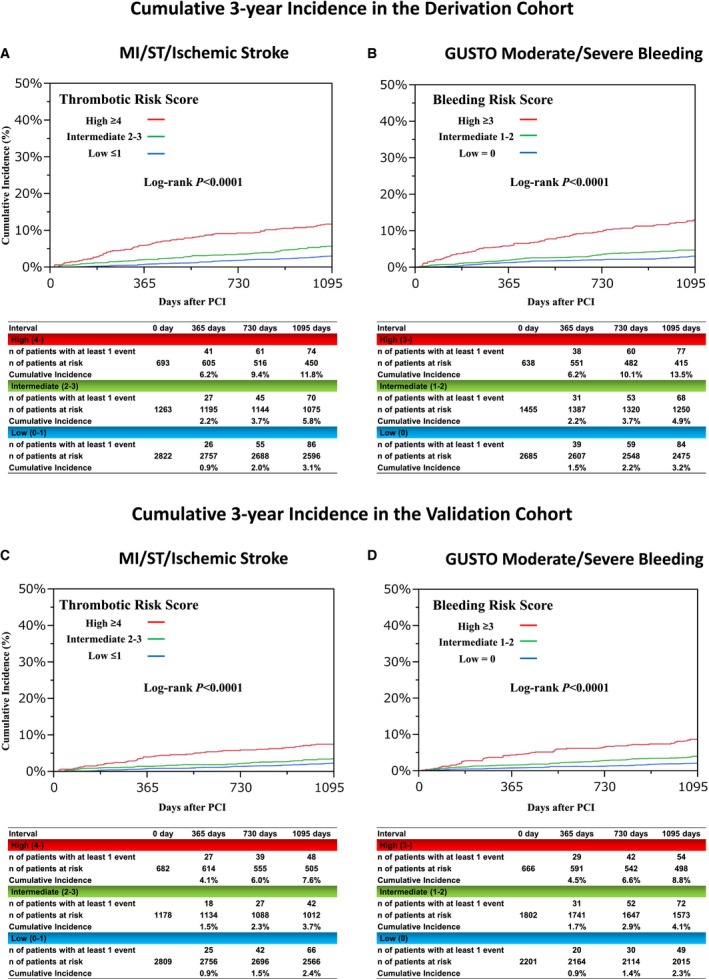
A, Cumulative 3‐year incidence of MI, ST, and ischemic stroke according to the thrombotic risk score categories in the derivation cohort. B, Cumulative 3‐year incidence of GUSTO moderate/severe bleeding according to the bleeding risk score categories in the derivation cohort. C, Cumulative 3‐year incidence of MI, ST, and ischemic stroke according to the thrombotic risk score categories in the validation cohort. D, Cumulative 3‐year incidence of GUSTO moderate/severe bleeding according to the bleeding risk score categories in the validation cohorts. GUSTO indicates Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; MI, myocardial infarction; PCI, percutaneous coronary intervention; ST, definite or probable stent thrombosis.
There was close correlation of thrombotic and bleeding risk. In both the derivation and validation cohorts, the bleeding event rate was also markedly higher in patients with high thrombotic risk scores (Table 3, and Figure 6C). Among 693 and 682 patients with high thrombotic risk scores in the derivation and validation cohorts, respectively, 408 (58.9%) and 401 (58.8%), respectively, also had high bleeding risk scores; both the bleeding and mortality rates for these patients were extremely high (Figure 8, and Table 4). Only 35 patients (5.1%) and 39 patients (5.7%) in the derivation and validation cohorts, respectively, had low bleeding risk scores among those with high thrombotic risk scores (Figure 8). Among those with low thrombotic risk scores, the vast majority had low bleeding risk scores (Figure 8). An incremental increase in the incidence of primary thrombotic and bleeding events were observed with higher bleeding risk scores in patients with high thrombotic risk scores (Figure 9).
Figure 8.
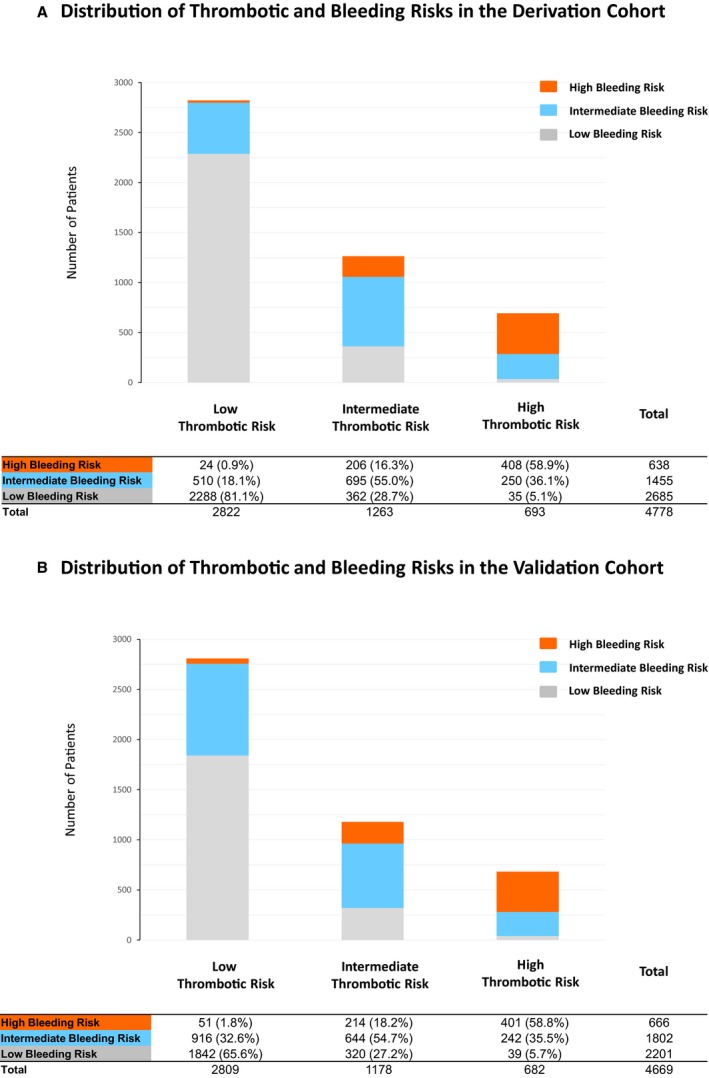
Distribution of the bleeding risk score categories according to the thrombotic risk score categories in the derivation cohort (A) and the validation cohort (B).
Table 4.
Cumulative 3‐Year Incidence of Events Considering Both the Thrombotic and Bleeding Risk Scores in the Derivation and Validation Cohorts
| Bleeding Risk Score (Incidence) | P Value | |||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| Derivation cohort | ||||
| Low thrombotic risk score, n | 2288 | 510 | 24 | |
| MI, ST, or ischemic stroke | 69 (3.1) | 17 (3.4) | 0 (0) | 0.66 |
| GUSTO moderate/severe bleeding | 63 (2.8) | 23 (4.6) | 3 (14.3) | 0.001 |
| GUSTO severe bleeding | 34 (1.5) | 9 (1.8) | 1 (4.2) | 0.44 |
| Death | 52 (2.3) | 34 (6.7) | 5 (21.4) | <0.0001 |
| Intermediate thrombotic risk score, n | 362 | 695 | 206 | |
| MI, ST, or ischemic stroke | 21 (6.0) | 39 (5.9) | 10 (5.2) | 0.93 |
| GUSTO moderate/severe bleeding | 20 (5.7) | 26 (3.9) | 21 (10.9) | 0.0008 |
| GUSTO severe bleeding | 8 (2.3) | 7 (1.1) | 10 (5.2) | 0.002 |
| Death | 19 (5.3) | 51 (7.5) | 30 (14.7) | 0.0003 |
| High thrombotic risk score, n | 35 | 250 | 408 | |
| MI, ST, or ischemic stroke | 2 (5.9) | 27 (11.3) | 45 (12.7) | 0.66 |
| GUSTO moderate/severe bleeding | 1 (3.0) | 19 (8.1) | 53 (14.7) | 0.02 |
| GUSTO severe bleeding | 0 (0) | 10 (4.2) | 23 (6.5) | 0.22 |
| Death | 6 (17.9) | 44 (17.7) | 129 (32.2) | 0.0001 |
| Validation cohort | ||||
| Low thrombotic risk score, n | 1842 | 916 | 51 | |
| MI, ST, or ischemic stroke | 35 (1.9) | 31 (3.5) | 0 (0) | 0.03 |
| GUSTO moderate/severe bleeding | 32 (1.8) | 25 (2.8) | 0 (0) | 0.13 |
| GUSTO severe bleeding | 24 (1.3) | 13 (1.5) | 0 (0) | 0.69 |
| Death | 47 (2.6) | 35 (3.9) | 4 (7.8) | 0.02 |
| Intermediate thrombotic risk score, n | 320 | 644 | 214 | |
| MI, ST, or ischemic stroke | 12 (3.9) | 21 (3.4) | 9 (4.5) | 0.75 |
| GUSTO moderate/severe bleeding | 13 (4.2) | 30 (4.8) | 12 (5.9) | 0.63 |
| GUSTO severe bleeding | 9 (3.0) | 20 (3.2) | 11 (5.5) | 0.24 |
| Death | 26 (8.3) | 44 (6.9) | 27 (12.7) | 0.02 |
| High thrombotic risk score, n | 39 | 242 | 401 | |
| MI, ST, or ischemic stroke | 1 (2.6) | 13 (5.6) | 34 (9.4) | 0.13 |
| GUSTO moderate/severe bleeding | 3 (8.0) | 17 (7.5) | 42 (11.6) | 0.23 |
| GUSTO severe bleeding | 3 (8.0) | 10 (4.5) | 28 (7.7) | 0.24 |
| Death | 6 (16.1) | 33 (13.8) | 85 (21.4) | 0.04 |
Data are shown as n (%). GUSTO indicates Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; MI, myocardial infarction; ST, definite or probable stent thrombosis.
Figure 9.
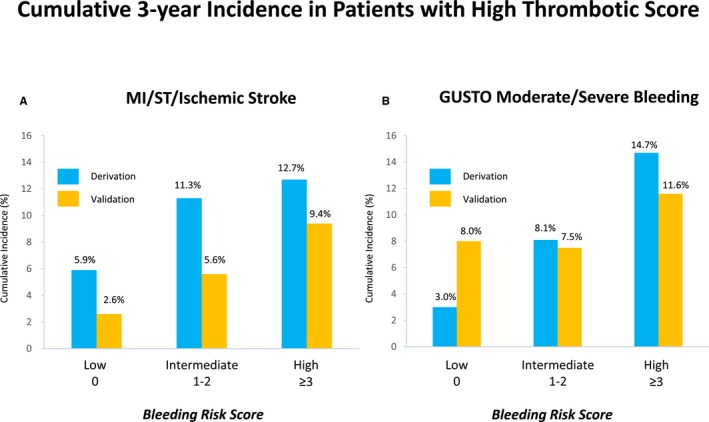
Cumulative 3‐year incidence of thrombotic and bleeding events in patients with high thrombotic scores in the derivation and validation cohorts. A, MI, ST, and ischemic stroke according to the bleeding risk score categories. B, GUSTO moderate/severe bleeding according to the bleeding risk score categories. GUSTO indicates Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; MI, myocardial infarction; ST, definite or probable stent thrombosis.
The cumulative incidence of persistent DAPT discontinuation was not significantly different, regardless of the thrombotic and bleeding risk score categories in both the derivation and validation cohorts (Table 3 and Figure 10).
Figure 10.
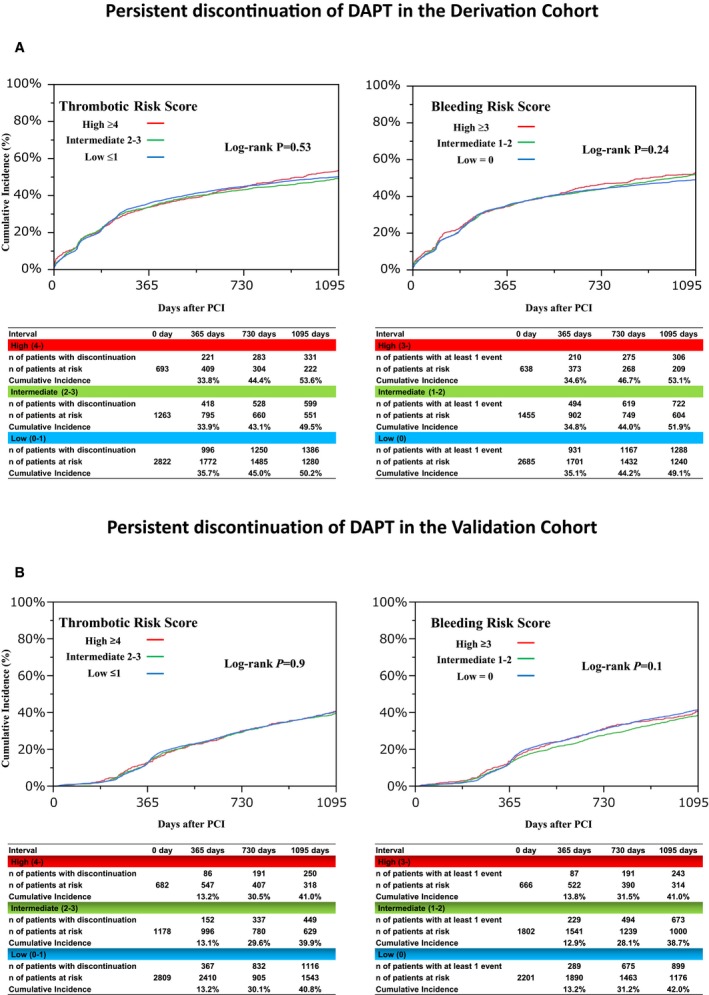
Persistent discontinuation of DAPT according to the risk score categories in the derivation cohort (A) and the validation cohort (B). DAPT indicates dual antiplatelet therapy; PCI, percutaneous coronary intervention.
Discussion
The main findings of this study were as follows. First, the CREDO‐Kyoto thrombotic and bleeding risk scores demonstrated modest accuracy in stratifying thrombotic and bleeding risk separately in the derivation and validation cohorts from Japanese PCI studies. Second, reflecting the overlap of the risk predictors for thrombosis and bleeding, a large proportion of patients with high thrombotic risk also had high bleeding risk, and the bleeding event rate among those patients was very high.
Several thrombotic and bleeding risk scores have been reported previously. A thrombotic risk score was proposed in the DAPT trial, the PARIS (Patterns of Non‐Adherence to Dual Anti‐Platelet Regimen in Stented Patients) registry, and TRA 2°P‐TIMI 50 (Thrombin Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events–Thrombolysis in Myocardial Infarction 50) trial.4, 16, 17 CKD, PVD, age, heart failure, and diabetes mellitus were the common independent predictors for thrombotic events in the previous studies and in the present study.4, 16, 17 In the PARIS registry, the procedural parameters were not included for derivation of the thrombotic score.16 However, in a pooled analysis of 6 randomized trials investigating DAPT durations after PCI, long‐term DAPT compared with short‐term DAPT yielded significant reductions in major adverse cardiac events in the complex PCI group but not in the non–complex PCI group, suggesting that it would be important to include procedural complexity for deriving the thrombotic risk score.18 AF was a characteristic thrombotic risk factor in the current study. Ischemic stroke is included as a thrombotic event in this study and could be the major reason for the emergence of AF as an independent risk factor for thrombotic events. In the DAPT trial and PARIS registry, thrombotic events were defined as the composite of ST or MI, whereas TRA 2°P‐TIMI 50 included ischemic stroke as a thrombotic event.4, 16, 17 Inclusion of ischemic stroke as a component of the thrombotic composite end point would be appropriate because ischemic stroke is clinically as important as MI and is a target of more intensive antithrombotic therapy in patients who underwent PCI.
Bleeding risk score is established in the DAPT study, the PRECISE‐DAPT (Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy) study, the PARIS registry, ADAPT‐DES (Assessment of Dual Antiplatelet Therapy With Drug‐Eluting Stents), HORIZON AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction), and CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA [American College of Cardiology/American Heart Association] Guidelines) trials.4, 5, 16, 19, 20, 21 CKD, PVD, heart failure, and use of anticoagulation (or AF) were the common independent predictors for bleeding events in the previous studies and in the present study.4, 5, 16, 19, 20, 21 In the present study, thrombocytopenia (platelet count <100 000/μL) emerged as an independent risk factor for bleeding events, although it was not evaluated in the previous reports.19, 20, 21 The differences of independent predictors of thrombotic and bleeding events across studies might be due to the differences in race, study design, and patient population, although the risk factors identified in the present study were generally consistent with those in the previous studies. The present prediction rules assessing thrombotic and bleeding risks demonstrated modest accuracy in the derivation and validation cohorts, with AUCs in the range of 0.6 to 0.75, which was regarded as helpful discrimination in the clinical prediction models.22 Similar AUCs were reported in the DAPT and PARIS studies.4, 16 Discussing the superiority of one risk score over another is beyond the scope of this study. Nevertheless, it would be preferable to use risk scores derived in the population with same or similar ethnic and/or geographic characteristics as the patient.
More intensive antithrombotic therapy should be recommended based on the balance between thrombotic risk and bleeding risk in individual patients. The cumulative incidence of persistent DAPT discontinuation in the present study, however, was not different regardless of the thrombotic and bleeding risk score categories, suggesting that appropriate risk stratification was not performed in determining DAPT duration after PCI. Shorter DAPT duration in patients with high bleeding risk might have substantially reduced bleeding events.
There was substantial overlap of the predictors between the thrombotic and bleeding risk scores in this study. CKD, AF, PVD, and heart failure emerged as the common predictors for both thrombotic and bleeding events. Similar results were also seen in the previous studies.4, 16 Reflecting the overlap of the risk predictors, a large proportion of patients with high thrombotic risk also had high bleeding risk, and more intensive antithrombotic therapy would be contraindicated for those patients. Consequently, it would not be sufficient just to stratify thrombotic risk only when considering more intensive antithrombotic therapy. It might be reasonable to evaluate bleeding risk initially to determine the intensity of antithrombotic therapy or the duration of DAPT, as indicated in the 2017 European Society of Cardiology guidelines.23 Further studies are warranted to explore the optimal antithrombotic therapy in the population with high thrombotic risk for whom bleeding risk is also substantial.
Study Limitations
Some limitations to our study should be considered. First, we did not have information on previous history of ST or bleeding events, which could be strongly associated with very high risk for thrombotic or bleeding events, respectively. Second, patients in the validation cohort were derived from randomized controlled trials; therefore, the types of patients included in the validation cohort would be different from those in clinical practice. There is also the chance that some patients might have been included in both the derivation and validation cohorts, because the validation cohort allowed enrollment of those patients with previous PCI. However, the enrollment periods of the 3 studies did not overlap; therefore, there was no possibility of including the same index PCI in different cohorts. Third, the prediction rules were derived from a population treated with early generation DESs, which are no longer used in current clinical practice; however, the prediction rules were validated in a population treated with the currently used new‐generation DESs. Consequently, the differences in the types of DES would not have affected the predictors for thrombosis and bleeding after PCI. Finally, the present study results were based on the patient characteristics and clinical outcomes of Japanese patients. We should be very cautious in extrapolating these results outside Japan. We recently conducted an external validation study for the DAPT score, demonstrating that it could differentiate patients with high ischemic risk from those with high bleeding risk in a Japanese population24; however, the thrombotic event rate was much lower in our validation study in Japanese patients than in the DAPT study. East Asian patients have been reported to have a lower rate of thrombotic events after PCI compared with Western patients.25 The distribution of bleeding risk scores among the patients with high thrombotic risk might be different in a Western population compared with the participants in the present Japanese study and would have important implications for patients selection for intensive antithrombotic therapy after PCI.
Conclusions
The CREDO‐Kyoto thrombotic and bleeding risk scores demonstrated modest accuracy in stratifying thrombotic and bleeding risk separately in the derivation and validation cohorts from Japanese PCI studies. Reflecting the overlap of the risk predictors for thrombosis and bleeding, a large proportion of the patients with high thrombotic risk also had high bleeding risk, and the bleeding event rate for those patients was very high.
Sources of Funding
This work was funded by the Pharmaceuticals and Medical Devices Agency in Japan.
Disclosures
None.
Supporting information
Appendix S1. List of the participating centers and the investigators.
Acknowledgments
We appreciate the support of the coinvestigators participating in the CREDO‐Kyoto (Coronary Revascularization Demonstrating Outcome Study in Kyoto) percutaneous coronary intervention and coronary artery bypass grafting registry cohort 2.
(J Am Heart Assoc. 2018;7:e008708 DOI: 10.1161/JAHA.118.008708.)29789335
References
- 1. Mauri L, Kereiakes DJ, Yeh RW, Driscoll‐Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM. Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palmerini T, Benedetto U, Bacchi‐Reggiani L, Della Riva D, Biondi‐Zoccai G, Feres F, Abizaid A, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Genereux P, Bhatt DL, Orlandi C, De Servi S, Petrou M, Rapezzi C, Stone GW. Mortality in patients treated with extended duration dual antiplatelet therapy after drug‐eluting stent implantation: a pairwise and Bayesian network meta‐analysis of randomised trials. Lancet. 2015;385:2371–2382. [DOI] [PubMed] [Google Scholar]
- 3. Toyota T, Shiomi H, Morimoto T, Natsuaki M, Kimura T. Short versus prolonged dual antiplatelet therapy (DAPT) duration after coronary stent implantation: a comparison between the DAPT study and 9 other trials evaluating DAPT duration. PLoS One. 2017;12:e0174502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Costa F, van Klaveren D, James S, Heg D, Raber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Zanchin T, Palmerini T, Wallentin L, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE‐DAPT) score: a pooled analysis of individual‐patient datasets from clinical trials. Lancet. 2017;389:1025–1034. [DOI] [PubMed] [Google Scholar]
- 6. Tu JV, Naylor CD. Clinical prediction rules. J Clin Epidemiol. 1997;50:743–744. [DOI] [PubMed] [Google Scholar]
- 7. Kimura T, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, Iwabuchi M, Shizuta S, Shiomi H, Tada T, Tazaki J, Kato Y, Hayano M, Abe M, Tamura T, Shirotani M, Miki S, Matsuda M, Takahashi M, Ishii K, Tanaka M, Aoyama T, Doi O, Hattori R, Tatami R, Suwa S, Takizawa A, Takatsu Y, Kato H, Takeda T, Lee JD, Nohara R, Ogawa H, Tei C, Horie M, Kambara H, Fujiwara H, Mitsudo K, Nobuyoshi M, Kita T. Long‐term safety and efficacy of sirolimus‐eluting stents versus bare‐metal stents in real world clinical practice in Japan. Cardiovasc Interv Ther. 2011;26:234–245. [DOI] [PubMed] [Google Scholar]
- 8. Kimura T, Morimoto T, Natsuaki M, Shiomi H, Igarashi K, Kadota K, Tanabe K, Morino Y, Akasaka T, Takatsu Y, Nishikawa H, Yamamoto Y, Nakagawa Y, Hayashi Y, Iwabuchi M, Umeda H, Kawai K, Okada H, Kimura K, Simonton CA, Kozuma K. Comparison of everolimus‐eluting and sirolimus‐eluting coronary stents: 1‐year outcomes from the randomized evaluation of sirolimus‐eluting versus everolimus‐eluting stent trial (RESET). Circulation. 2012;126:1225–1236. [DOI] [PubMed] [Google Scholar]
- 9. Natsuaki M, Kozuma K, Morimoto T, Kadota K, Muramatsu T, Nakagawa Y, Akasaka T, Igarashi K, Tanabe K, Morino Y, Ishikawa T, Nishikawa H, Awata M, Abe M, Okada H, Takatsu Y, Ogata N, Kimura K, Urasawa K, Tarutani Y, Shiode N, Kimura T. Biodegradable polymer biolimus‐eluting stent versus durable polymer everolimus‐eluting stent: a randomized, controlled, noninferiority trial. J Am Coll Cardiol. 2013;62:181–190. [DOI] [PubMed] [Google Scholar]
- 10. Serruys PW, Ong AT, van Herwerden LA, Sousa JE, Jatene A, Bonnier JJ, Schonberger JP, Buller N, Bonser R, Disco C, Backx B, Hugenholtz PG, Firth BG, Unger F. Five‐year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the arterial revascularization therapies study (ARTS) randomized trial. J Am Coll Cardiol. 2005;46:575–581. [DOI] [PubMed] [Google Scholar]
- 11. Mauri L, Hsieh WH, Massaro JM, Ho KK, D'Agostino R, Cutlip DE. Stent thrombosis in randomized clinical trials of drug‐eluting stents. N Engl J Med. 2007;356:1020–1029. [DOI] [PubMed] [Google Scholar]
- 12. The GUSTO investigators . An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. [DOI] [PubMed] [Google Scholar]
- 13. Kimura T, Morimoto T, Nakagawa Y, Tamura T, Kadota K, Yasumoto H, Nishikawa H, Hiasa Y, Muramatsu T, Meguro T, Inoue N, Honda H, Hayashi Y, Miyazaki S, Oshima S, Honda T, Shiode N, Namura M, Sone T, Nobuyoshi M, Kita T, Mitsudo K. Antiplatelet therapy and stent thrombosis after sirolimus‐eluting stent implantation. Circulation. 2009;119:987–995. [DOI] [PubMed] [Google Scholar]
- 14. Natsuaki M, Morimoto T, Furukawa Y, Nakagawa Y, Kadota K, Yamaji K, Ando K, Shizuta S, Shiomi H, Tada T, Tazaki J, Kato Y, Hayano M, Abe M, Tamura T, Shirotani M, Miki S, Matsuda M, Takahashi M, Ishii K, Tanaka M, Aoyama T, Doi O, Hattori R, Kato M, Suwa S, Takizawa A, Takatsu Y, Shinoda E, Eizawa H, Takeda T, Lee JD, Inoko M, Ogawa H, Hamasaki S, Horie M, Nohara R, Kambara H, Fujiwara H, Mitsudo K, Nobuyoshi M, Kita T, Kimura T. Late adverse events after implantation of sirolimus‐eluting stent and bare‐metal stent: long‐term (5‐7 years) follow‐up of the Coronary Revascularization Demonstrating Outcome study‐Kyoto registry Cohort‐2. Circ Cardiovasc Interv. 2014;7:168–179. [DOI] [PubMed] [Google Scholar]
- 15. Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. [DOI] [PubMed] [Google Scholar]
- 16. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzenbichler B, Weisz G, Steg PG, Pocock S. Coronary thrombosis and major bleeding after PCI with drug‐eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67:2224–2234. [DOI] [PubMed] [Google Scholar]
- 17. Bohula EA, Bonaca MP, Braunwald E, Aylward PE, Corbalan R, De Ferrari GM, He P, Lewis BS, Merlini PA, Murphy SA, Sabatine MS, Scirica BM, Morrow DA. Atherothrombotic risk stratification and the efficacy and safety of vorapaxar in patients with stable ischemic heart disease and previous myocardial infarction. Circulation. 2016;134:304–313. [DOI] [PubMed] [Google Scholar]
- 18. Giustino G, Chieffo A, Palmerini T, Valgimigli M, Feres F, Abizaid A, Costa RA, Hong MK, Kim BK, Jang Y, Kim HS, Park KW, Gilard M, Morice MC, Sawaya F, Sardella G, Genereux P, Redfors B, Leon MB, Bhatt DL, Stone GW, Colombo A. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol. 2016;68:1851–1864. [DOI] [PubMed] [Google Scholar]
- 19. Genereux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri E, Yadav M, Francese DP, Palmerini T, Kirtane AJ, Litherland C, Mehran R, Stone GW. Incidence, predictors, and impact of post‐discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66:1036–1045. [DOI] [PubMed] [Google Scholar]
- 20. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556–2566. [DOI] [PubMed] [Google Scholar]
- 21. Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV Jr, Peterson ED, Alexander KP. Baseline risk of major bleeding in non‐ST‐segment‐elevation myocardial infarction: the CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines) bleeding score. Circulation. 2009;119:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alba A, Agoritsas T, Walsh M, Hanna S, Iorio A, Devereaux PJ, McGinn T, Guyatt G. Discrimination and calibration of clinical prediction models. Users’ guides to the medical literature. JAMA. 2017;318:1377–1384. [DOI] [PubMed] [Google Scholar]
- 23. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, Juni P, Kastrati A, Kolh P, Mauri L, Montalescot G, Neumann FJ, Petricevic M, Roffi M, Steg PG, Windecker S, Zamorano JL, Levine GN. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2018;39:213–260. [DOI] [PubMed] [Google Scholar]
- 24. Yoshikawa Y, Shiomi H, Watanabe H, Natsuaki M, Kondo H, Tamura T, Nakagawa Y, Morimoto T, Kimura T. Validating utility of DAPT score in a large pooled cohort from three Japanese PCI studies. Circulation. 2018;137:551–562. [DOI] [PubMed] [Google Scholar]
- 25. Levine GN, Jeong YH, Goto S, Anderson JL, Huo Y, Mega JL, Taubert K, Smith SC Jr. Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI. Nat Rev Cardiol. 2014;11:597–606. [DOI] [PubMed] [Google Scholar]
- 26. http://www.seiken-j.or.jp/CREDO-Kyoto.risk.score/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. List of the participating centers and the investigators.


