Introduction
Getting old can be tough. Cataracts form, joints deteriorate, arteries stiffen, and bone demineralizes. The heart is certainly not immune to senescence. Diastolic function shows perhaps the greatest deterioration: ventricular compliance decreases, and diastolic relaxation becomes prolonged.1, 2 Heart rate and contractility no longer increase as they should when the heart is called on to pump more vigorously.3 When these changes become significant enough, it leads to the clinical syndrome of heart failure (HF) with preserved ejection fraction (HFpEF), the dominant cause of HF in elderly individuals and the quintessential expression of cardiovascular senescence.4, 5
Echocardiography is by far the most commonly used tool to evaluate for diastolic dysfunction (DD). Although certainly not perfect, tissue and Doppler echocardiography enables assessment of ventricular structure, function, and hemodynamics that is helpful diagnostically and prognostically.6, 7, 8, 9 Because DD increases with aging, it has been suggested that we may need to account for this in what we consider to be “normal.” Shah and colleagues have recently proposed new age‐based cutoffs that better predicted incident cardiovascular disease outcomes.10 However, the cutoff values that they proposed were derived from only 401 individuals, and more information is needed.
In this issue of the Journal of the American Heart Association (JAHA), Nayor et al present important new data from the FHS (Framingham Heart Study) to help address this issue.11 The authors examined a healthy reference sample of 2355 participants (mean age, 44 years) without prevalent cardiovascular disease or risk factors. Three echocardiographic variables of diastolic function were analyzed: tissue Doppler lateral mitral annular e′ velocity, the E/e′ ratio, and the E/A ratio. Abnormal values were defined by the outer 10th percentile from the healthy cohort, separated by decade of life and sex. These cutoffs were then applied to the broader FHS sample of 6102 subjects.
Age was the strongest correlate of DD when using a single cut point to define abnormal (not accounting for age or sex), most notably in those with “mild” DD (defined as any 1 of 3 criteria abnormal).11 More than 65% of subjects aged ≥80 years had some element of DD by current single cut point criteria. However, after applying the age‐ and sex‐specific criteria, only 15% to 22% of the sample had mild DD and 10% had moderate or severe DD (defined as 2 or 3 criteria abnormal), and the striking age dependence of DD vanished. In fact, although age was the dominant correlate of DD using the single cut point criteria, it became inversely correlated with DD in the fully adjusted model using age‐ and sex‐specific criteria.
Using age‐ and sex‐specific criteria, mild and moderate‐severe DD were associated with 50% and 65%, respectively, greater hazards of cardiovascular disease events, although this association was no longer significant after adjusting for other cardiovascular risk factors.11 In contrast, using the single cut point criteria, mild DD was no longer predictive, but moderate‐severe DD remained similarly predictive of events. Nayor and colleagues are astute in that they do not conclude from their data that age‐ and sex‐specific reference limits should be used to assess DD, but they rather point out some of the tradeoffs that must be considered by clinicians and investigators, which will require additional study moving forward.11
The authors are to be commended on this important contribution to the literature.11 The large, well‐characterized, community‐based sample, the systematic prospective acquisition of data, and the careful adjudication of events are all major strengths. Like all studies, there are some limitations to consider. For the reference population, the authors were careful to exclude participants with prevalent cardiovascular disease and important risk factors for DD, like obesity, but other factors, such as physical activity and fitness, were not accounted for, and these may strongly affect diastolic function and risk of HFpEF.12, 13 One could make a cogent argument that the reference sample should include individuals who are both active and fit to represent the ideal of “successful” cardiac aging.12, 14 Incident HF events were infrequent, and so a composite cardiovascular outcome that included myocardial infarction, stroke, and claudication was used by the authors.11 The study was not adequately powered to assess for HF‐specific end points.
Despite these limitations, the data from Nayor et al11 provide valuable new insight into cardiac aging while raising questions about how we should define DD across the age spectrum using echocardiography. The velocity of left ventricular diastolic annular motion during early diastole (e′) decreased strikingly with age, by 9% in men and 12% in women per decade. Shah et al also recently observed a precipitous decline in e′ velocity with aging, although the derived cutoff values from their study and the current study to define “abnormal” were different (Table).10, 11 Further study is required to resolve these discrepancies. The E/e′ ratio, a surrogate for left ventricular filling pressures,6, 9 increased with aging, and the slope of this increase appeared to be steeper in women (Table), consistent with published data from other cohorts.1 This is noteworthy when considering how women are more likely to develop HFpEF than men with aging.15 In both studies, the age‐ and sex‐specific cut points for E/e′ did not differ as greatly from the guideline‐based cutoff as e′ velocity did.10, 11
Table 1.
Age, Sex, and Echocardiographic‐Doppler Indexes of Diastolic Function
| Variable | Mean Value in a 40‐Year‐Old Adult Without CVDa | Estimated Change per Decade of Life in an Adult Without CVDb | Relative Change per Decade of Life Without CVD, % | Partition Value for Abnormal in FHS for Age >60 yc | Partition Value for Abnormal in ARIC Study for Age >65 yd |
|---|---|---|---|---|---|
| Lateral E′ velocity, cm/s | |||||
| Women | 13.0 | −1.5 | −12 | <7.7 | <5.1 |
| Men | 13.1 | −1.2 | −9 | <8.1 | <5.4 |
| Lateral E/e′ ratio | |||||
| Women | 4.7 | +0.4 | +9 | >9.0 | >13.3 |
| Men | 5.2 | +0.2 | +4 | >7.4 | >11.5 |
| E/A ratio | |||||
| Women | 1.5 | −0.2 | −13 | <0.8 | NA |
| Men | 1.5 | −0.2 | −13 | <0.7 | NA |
A indicates late diastolic mitral inflow velocity with atrial contraction by pulsed wave Doppler; ARIC, Atherosclerosis Risk in Communities; CVD, cardiovascular disease; E, early diastolic mitral inflow velocity by pulsed wave Doppler; e', early diastolic mitral annular velocity by tissue Doppler; FHS, Framingham Heart Study.
Estimated from Y value at age 40 years in the mean regression lines from Supplemental Figures 2 through 4 from Nayor et al.11
Estimated from the slope of the mean regression lines from Supplemental Figures 2 through 4 from Nayor et al.11
Weighted means based on age distribution from Nayor et al.11
Taken from Shah et al.10
Although age‐specific cutoffs may provide greater insight into prognosis, at least for mild DD,11 one unintended consequence of incorporating these criteria into practice could become a tacit acceptance that any DD is normal. There is no question that the prevalence of DD increases with age, but that does not diminish the fact that DD is harmful. Mild DD in elderly individuals at rest generally does not reflect high filling pressures,16 but what is mild at rest may become profoundly limiting during stress in the setting of HFpEF, where the inability of the ventricle to enhance e′ velocity leads to marked elevation in cardiac filling pressures.17 High filling pressures lead to symptoms of dyspnea and impairments in functional capacity,18, 19 which may perpetuate sedentary behavior and reduce qualify of life. These end points are sometimes more difficult to measure and were not evaluated in the current study, but they can be important to our patients.
The data from Nayor and colleagues show that risk prediction for “hard end points” is improved with age‐specific diastolic indexes,11 but this may incur a cost that we also need to contemplate (Figure). Liberalizing the cutoffs for what is considered normal diastolic function in older adults could worsen the existing problem of underrecognition of HFpEF in people with dyspnea, especially when one considers that the echocardiographic assessments currently in use are poorly sensitive.6 Use of age‐specific criteria may also promote tacit acceptance that DD (when associated with aging) is benign, which is not the case, at least for the symptomatic expression of cardiac insufficiency.5, 17, 19 This could deepen the nihilistic perception that seniors with dyspnea (often attributable to HFpEF) are “just getting old,” rather than experiencing objective limitations in cardiac function.
Figure 1.
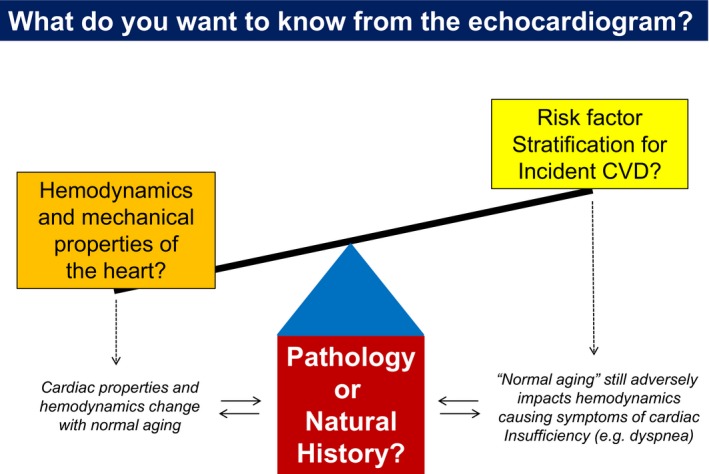
Tradeoffs between considering left ventricular diastolic function in absolute terms as normal or abnormal, or relative to what is seen during normal aging. See text for details. CVD indicates cardiovascular disease.
As shown by Nayor et al in their Figure 1B, the prevalence of DD increases strikingly with age using single cut point criteria.11 This perfectly mirrors what we see plotting the prevalence of HFpEF as a function of age.4 However, using the age‐ and sex‐specific cutoffs, the prevalence of DD remains stable across the lifespan and is much lower in seniors (Figure 1A).11 This is incongruent with what we know about cardiac aging,1 and it would seem inappropriate to label diastolic function as normal when it clearly is not.
It might be helpful to reflect on lessons from the past when considering this dilemma. For years, we thought that “essential” hypertension was an inevitable consequence of aging that was necessary to maintain organ perfusion in older adults, which therefore did not require treatment. Epidemiologic studies including the FHS and then clinical trials eventually proved that is not the case. Why should age‐related DD be any different? Age is typically considered to be an unmodifiable risk factor, but recent studies have shown that interventions, such as exercise training12, 14 or weight loss,20 can reverse or at least mitigate age‐related DD. Even the cellular mechanisms of cardiac aging might one day become treatable.21
There is no question that DD becomes more common as our hearts age.1, 2, 3 That does not mean that we should just accept it or call it normal. When ventricular compliance decreases and relaxation becomes prolonged, the pressure in the left atrium goes up.5 This favors fluid redistribution out of the pulmonary capillaries and into the lung interstitium to cause congestion and dyspnea.19 This sequence of events plays out similarly in people in the third or the ninth decade of life. The lens through which we interpret diastolic data across the lifespan may differ for the epidemiologist, the physiologist, and the clinician. The question we need to ask ourselves is which perspective is best? The answer might be: a little bit of each.
Sources of Funding
Borlaug is supported by R01 HL128526, R01 HL 126638, U01 HL125205, and U10 HL110262, all from the National Institutes of Health. Reddy is supported by NIH T32 HL007111 from the National Institutes of Health.
Disclosures
None.
J Am Heart Assoc. 2018;7:e009462 DOI: 10.1161/JAHA.118.009462.29858373
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, Rodeheffer RJ. Longitudinal changes in left ventricular stiffness: a community‐based study. Circ Heart Fail. 2013;6:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Empel VP, Kaye DM, Borlaug BA. Effects of healthy aging on the cardiopulmonary hemodynamic response to exercise. Am J Cardiol. 2014;114:131–135. [DOI] [PubMed] [Google Scholar]
- 3. Fleg JL, O'Connor F, Gerstenblith G, Becker LC, Clulow J, Schulman SP, Lakatta EG. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78:890–900. [DOI] [PubMed] [Google Scholar]
- 4. Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL; CHS Research Group. Cardiovascular Health Study. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. Am J Cardiol. 2001;87:413–419. [DOI] [PubMed] [Google Scholar]
- 5. Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA. Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart. 2011;97:964–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive‐echocardiographic study. Circulation. 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 8. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 10. Shah AM, Claggett B, Kitzman D, Biering‐Sorensen T, Jensen JS, Cheng S, Matsushita K, Konety S, Folsom AR, Mosley TH, Wright JD, Heiss G, Solomon SD. Contemporary assessment of left ventricular diastolic function in older adults: the Atherosclerosis Risk in Communities Study. Circulation. 2017;135:426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nayor M, Cooper LL, Enserro DM, Xanthakis V, Larson MG, Benjamin EJ, Aragam J, Mitchell GF, Ramachandran R. Left ventricular diastolic dysfunction in the community: impact of diagnostic criteria on the burden, correlates and prognosis. J Am Heart Assoc. 2018;7:e008291 DOI: 10.1161/JAHA.117.008291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arbab‐Zadeh A, Dijk E, Prasad A, Fu Q, Torres P, Zhang R, Thomas JD, Palmer D, Levine BD. Effect of aging and physical activity on left ventricular compliance. Circulation. 2004;110:1799–1805. [DOI] [PubMed] [Google Scholar]
- 13. Pandey A, Cornwell WK III, Willis B, Neeland IJ, Gao A, Leonard D, DeFina L, Berry JD. Body mass index and cardiorespiratory fitness in mid‐life and risk of heart failure hospitalization in older age: findings from the Cooper Center Longitudinal Study. JACC Heart Fail. 2017;5:367–374. [DOI] [PubMed] [Google Scholar]
- 14. Howden EJ, Sarma S, Lawley JS, Opondo M, Cornwell W, Stoller D, Urey MA, Adams‐Huet B, Levine BD. Reversing the cardiac effects of sedentary aging in middle age‐a randomized controlled trial: implications for heart failure prevention. Circulation. 2018;137:1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26:562–568. [DOI] [PubMed] [Google Scholar]
- 16. Prasad A, Okazaki K, Arbab‐Zadeh A, Dijk E, Fu Q, Thomas JD, Levine BD. Abnormalities of Doppler measures of diastolic function in the healthy elderly are not related to alterations of left atrial pressure. Circulation. 2005;111:1499–1503. [DOI] [PubMed] [Google Scholar]
- 17. Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular‐pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reddy YN, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. 2018. Available at: https://doi.org/10.1016/j.jchf.2018.03.003. Accessed May 25, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Obokata M, Olson TP, Reddy YN, Melenovsky V, Kane GC, Borlaug BA. Hemodynamics, dyspnea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018. Available at: https://doi.org/10.1093/eurheartj/ehy268. Accessed May 25, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wohlfahrt P, Redfield MM, Lopez‐Jimenez F, Melenovsky V, Kane GC, Rodeheffer RJ, Borlaug BA. Impact of general and central adiposity on ventricular‐arterial aging in women and men. JACC Heart Fail. 2014;2:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Childs BG, Li H, van Deursen JM. Senescent cells: a therapeutic target for cardiovascular disease. J Clin Invest. 2018;128:1217–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]


