Abstract
Background
Cerebral microbleeds (CMBs) are hypothesized downstream markers of brain damage caused by vascular and amyloid pathologic mechanisms. The aim of this study was to determine whether CMB count and location are associated with an increased risk for mild cognitive impairment (MCI) in patients with essential hypertension without a history of transient ischemic attack or stroke.
Methods and Results
In this cross‐sectional study, patients were prospectively enrolled from consecutive outpatients with essential hypertension 50 years and older at 3 centers in northern China. Generalized linear Poisson models were used to determine the association between the number and location of CMBs and MCI in patients with hypertension. The association of microbleeds with different cognitive domains was estimated using linear mixed models. The presence, number, and distribution of CMBs were greater in patients with hypertension who had MCI (P<0.001). The presence of any CMBs, strictly lobar CMBs, and deep or infratentorial CMBs were all related to MCI after adjusting for age, sex, education, cardiovascular risk factors, body mass index, intima‐media thickness, the presence of silent lacunar infarctions, white matter lesion grade, and brain atrophy. Furthermore, the presence of multiple microbleeds (≥5) was associated with lower Montreal Cognitive Assessment total scores and worse performance on specific domains of cognitive tests, such as global cognitive function, information processing speed, and motor speed.
Conclusions
This study suggests that the presence of and a greater number of cerebral CMBs independently correlate with MCI in patients with essential hypertension without a history of transient ischemic attack or stroke.
Keywords: cerebral microbleed, cerebral small vessel disease, risk factor, hypertension, mild cognitive impairment
Subject Categories: Hypertension, Cognitive Impairment, Vascular Disease, Clinical Studies, Magnetic Resonance Imaging (MRI)
Clinical Perspective
What Is New?
This study suggests that the presence of any cerebral microbleeds, strictly lobar cerebral microbleeds, and deep or infratentorial cerebral microbleeds were all related to mild cognitive impairment in patients with essential hypertension without a history of transient ischemic attack or stroke after adjusting for conventional risk factors.
Furthermore, the presence of multiple microbleeds (≥5) was associated with lower Montreal Cognitive Assessment total scores and worse performance on specific domains of cognitive tests, such as global cognitive function, information processing speed, and motor speed.
What Are the Clinical Implications?
Our findings suggest that the presence and greater number of cerebral microbleeds independently correlate with mild cognitive impairment in patients with essential hypertension without a history of transient ischemic attack or stroke.
Introduction
Mild cognitive impairment (MCI) is widely regarded as the intermediate stage of cognitive impairment between the changes seen in normal cognitive aging and those associated with dementia. Elderly patients with MCI constitute a high‐risk population for developing dementia, particularly Alzheimer's disease (AD).1 MCI has received considerable research attention for its potential importance in early identification and intervention for people with a risk for dementia.2, 3
Vascular pathology plays a prominent role in cognitive decline and dementia. Cerebral microbleeds (CMBs), as a form of cerebral small vessel disease, are reported to be highly prevalent in memory clinic patients and in patients with AD.4, 5 Moreover, Some studies have previously shown that the presence of numerous microbleeds, especially in a strictly lobar location, is associated with worse performance on tests that measure cognitive function.6 Growing evidence suggests that hypertension is the most powerful modifiable risk factor for cerebral vessel dysfunction and may contribute to consequent cognitive decline.7, 8 Few studies have examined the relationship between CMBs and MCI in patients with hypertension. In the present study, we aimed to evaluate whether CMBs and, more specifically, CMB count and location are associated with an increased risk for MCI in patients with essential hypertension without a history of transient ischemic attack (TIA) or stroke.
Materials and Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
Participants were prospectively enrolled from consecutive outpatients with essential hypertension 50 years and older who visited the Department of Neurology between December 2015 and December 2016 at 3 centers in northern China. All patients were entered in a prospective electronic database and underwent a standardized interview and physical examination. Hypertension was defined as blood pressure (BP) ≥140/90 mm Hg at 3 different times or the use of antihypertensive medications. Carotid intima‐media thickness (IMT) was measured to reflect the severity of atherosclerosis, and magnetic resonance imaging (MRI) was performed to evaluate suspicious neurological symptoms (ie, headache or dizziness, vertigo, numbness, syncope, or subjective memory impairment). When neither neurological symptoms nor a history of stroke or TIA were identified, the patient was considered eligible for the study.
During the study period, 1254 patients with essential hypertension were identified as candidates (Figure). We then excluded 22 patients whose MRI examinations were not completed. Patients with a history of stroke or TIA (n=169), dementia (n=36), brain tumor (n=1), or depressive disorder (n=33) or those undergoing hemodialysis (n=3) or brain surgery (n=7) were excluded to eliminate any effects of clinically evident diseases on CMBs.
Figure 1.
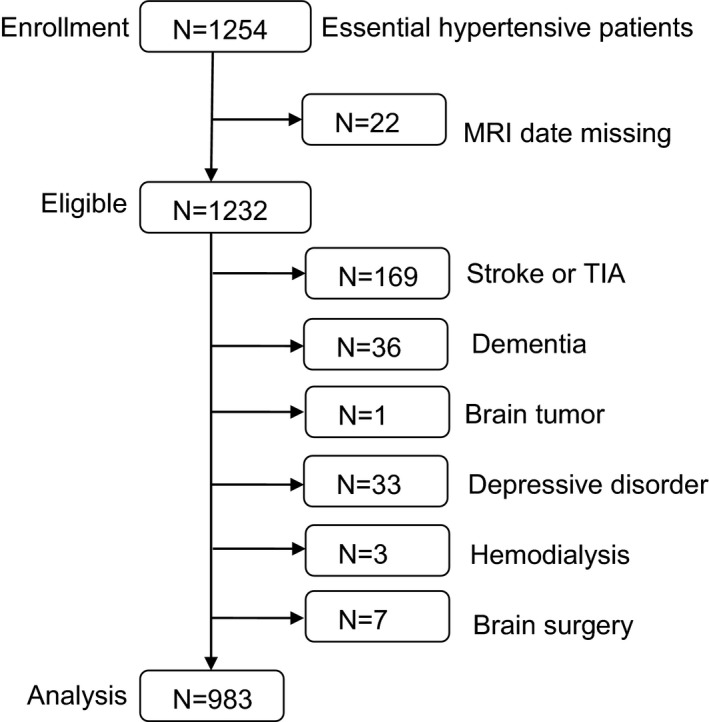
Description of the study population. MRI indicates magnetic resonance imaging; TIA, transient ischemic attack.
The study followed the Declaration of Helsinki and was approved by the ethics committee of Weihai Municipal Hospital, and all patients provided written informed consent before inclusion.
Diagnosis of MCI
The clinical diagnosis of MCI was made according to the established Petersen criteria,1 including subjective complaints of memory deficits, abnormal memory functioning for age, the absence of dementia according to a diagnostic examination (Clinical Dementia Rating <0.5), and normal everyday functioning on the activities of daily living scale. Patients with MCI with depressive disorder were excluded.9 After enrollment, cognitive function was evaluated by a Chinese‐version questionnaire of the Montreal Cognitive Assessment10 the Letter Digit Substitution Task, Word Fluency Test, Purdue Pegboard Test, 15‐Word Verbal Learning Test, and Stroop test.11 Z scores (individual test score minus the mean test score divided by the SD) were generated for each cognitive test, except for the Montreal Cognitive Assessment. We computed compound scores for global cognition (the mean Z score of the Letter Digit Substitution Task, Stroop interference subtask, Word Fluency Test, delayed recall of the 15‐Word Verbal Learning Test, and Purdue Pegboard Test), memory (the mean Z score of immediate and delayed recall of the 15‐Word Verbal Learning Test), information processing speed (the mean Z score of the Stroop reading and color naming subtasks and Letter Digit Substitution Task), executive functioning (the mean Z score of the Letter Digit Substitution Task, Stroop interference subtask, and Word Fluency Test), and motor speed (the mean Z score of the Purdue Pegboard Test). The neuropsychological tests were administered in a quiet, well‐lit room and under standard circumstances by trained examiners.
Brain MRI Data
All 983 participants with hypertension underwent MRI examinations with susceptibility‐weighted imaging. In addition, all patients received conventional T1‐ and T2‐weighted, diffusion‐weighted imaging scans and fluid‐attenuated inversion recovery sequence imaging. MRI examinations were performed with a Magnetom Trio whole‐body 3.0‐T MRI scanner (Siemens) with a 40‐mT/m gradient. A receiver‐only 8‐channel phased‐array head coil was used in all acquisitions with an integrated parallel acquisition technique. Susceptibility‐weighted imaging was acquired as a fully velocity compensated, 3‐dimensional gradient echo sequence using the following parameters: repetition time, 27 ms; time of echo, 20 ms; flip angle, 15°; matrix, 350×445; field of view, 192×220 mm; and slice thickness, 1.2 mm. Minimal intensity projection images were reconstructed on a console by overlaying the 6 adjacent slices from the original susceptibility‐weighted imaging, with a slice thickness of 7.2 mm.12, 13 CMBs were defined as focal areas with very low signal intensity, <10 mm.14 Hypointense lesions were excluded if they appeared to be vascular flow voids (based on sulcal location or linear shape), basal ganglia mineralization, or artifacts from an adjacent bone or sinus. The presence and number of CMBs on susceptibility‐weighted imaging were independently interpreted by 2 experienced neuroradiologists and determined by consensus. CMBs were classified manually as lobar CMBs with or without cerebellar CMBs (suggestive of cerebral amyloid angiopathy) and deep or infratentorial CMBs with or without lobar CMBs (suggestive of hypertensive arteriopathy).15
The interrater reliability for the whole group for the presence of CMBs was κ=0.824 and the intraclass correlation coefficient for the number of CMBs was κ=0.871, which indicates good agreement.
A white matter lesion (WMH) was defined as at least 1 focal lesion in the cerebral white matter with corresponding hyperintensity on fluid‐attenuated inversion recovery images. The Fazekas scale was used to score the white matter lesions. Scores of 0 to 2 were given for 3 periventricular hyperintensities (PVH; range: 0–6) and scores of 0 to 6 were given for a deep WMH (DWMH) in the temporal, frontal, parietal, or occipital lobe (DWMH; range: 0–24).16
Silent lacunar infarctions (SLIs) were defined as focal lesions >3 mm and <15 mm, with a hyperintense rim on fluid‐attenuated inversion recovery images, corresponding hyperintensity on T2‐weighted images, and corresponding hypointensity on T1‐weighted images.
Brain atrophy was defined as total brain tissue volume expressed as percentage of intracranial volume. Total brain volume and intracranial volume were calculated on the axial T1‐weighted inversion recovery and FLAIR images of all participants using the k‐nearest neighbor‐based probabilistic segmentation.17
Other Measurements
The diagnosis of diabetes mellitus was based on repeated pathologic blood tests indicating fasting values ≥126 mg/dL or value loads ≥200 mg/dL 2 hours after oral glucose administration or the use of antidiabetic treatment. Hyperlipidemia was determined when total cholesterol was ≥200 mg/dL or when low‐density lipoprotein cholesterol was ≥130 mg/dL. Alcohol was accepted as a risk factor if current consumption reached 300 g/wk. A history of smoking was coded if the patient smoked during the 3 months before the most recent stroke event. Total protein, serum albumin, total cholesterol, triglycerides, low‐density lipoprotein cholesterol, serum urea nitrogen, and hemoglobin levels were measured by standard laboratory methods. Body mass index (BMI) was also calculated.
Color Doppler ultrasound was used to locate the thickest site of the carotid artery intima‐media and to measure the thickness at this site, 2 upstream sites, and 1 site that was 1 cm downstream, each of which were measured 6 times to obtain average values.
Statistical Analysis
SPSS version 13.0 statistical software (SPSS Inc) was used for the statistical analysis. Tests for the homogeneity of variances were performed. The Kolmogorov–Smirnov test was also performed to ascertain the normality of the distribution of continuous variables. The normally distributed measurement data were represented as the mean±SD (x̅±SD) and the results of the 2 groups were compared using a t test. The counting data were represented as a ratio and the results of the 2 groups were compared using χ2 test. Generalized linear Poisson models were used to investigate the distributional patterns of CMBs relative to MCI in patients with hypertension without a history of TIA or stroke. Linear mixed models with added random effects were used to determine the relationship between CMB count per topographic distribution in the brain and cognitive decline in specific domains. Differences with a P<0.05 were considered statistically significant.
Results
Characteristics of the Sample
Among 983 patients with hypertension, 164 patients had a clinical diagnosis of MCI. CMBs were detected in 215 patients with hypertension (21.9%). Of the 1062 identified CMBs, 285 (26.8%) were located in the basal ganglia, 154 (14.5%) in the thalamus, 151 (14.2%) in the brainstem (mostly in the pons), 120 (11.3%) in the cerebellum, 236 (22.2%) in the subcortical white matter or periventricular white matter, and 116 (10.9%) in the cortex. In the univariate analysis, age, the degrees of PVH and DWMH, and the prevalence of SLIs were higher in patients with hypertension with MCI. The total brain volume/intracranial volume ratio was lower in patients with hypertension with MCI than in those without MCI (P<0.032). Furthermore, the presence, number, and distribution of CMBs were greater in patients with hypertension and MCI (P<0.001) (Table 1).
Table 1.
Demographic and Clinical Characteristics of Enrolled Patients With Hypertension Without and With MCI
| Characteristics | All Patients (N=983) | No MCI (n=819) | MCI (n=164) | P Value |
|---|---|---|---|---|
| Age, y | 64.38+8.26 | 64.38±8.26 | 65.52±8.85 | 0.13 |
| Men, No. (%) | 539 (54.8) | 452 (55.2) | 87 (53.0) | 0.34 |
| DM, No. (%) | 168 (17.1) | 138 (16.8) | 30 (18.3) | 0.36 |
| Education, mean±SD, y | 7.6±4.3 | 7.9±4.7 | 7.0±4.1 | 0.11 |
| Hyperlipidemia, No. (%) | 543 (55.2) | 451 (55.1) | 92 (56.1) | 0.44 |
| Current smoker, No. (%) | 162 (16.5) | 129 (15.8) | 33 (20.1) | 0.11 |
| Current drinker, No. (%) | 221 (22.5) | 186 (22.7) | 35 (21.3) | 0.39 |
| Duration of hypertension, mean±SD, mo | 61.7±17.3 | 59.9±16.8 | 63.2±18.4 | 0.27 |
| SBP, mm Hg | 139±18 | 138±16 | 144±21 | 0.12 |
| DBP, mm Hg | 82±13 | 80±12 | 84±14 | 0.21 |
| Pulse pressure, mm Hg | 61±6 | 60±5 | 63±8 | 0.14 |
| Use of antihypertensive drugs, No. (%) | 535 (54.4) | 451 (52.9) | 84 (51.2) | 0.21 |
| Calcium channel blockers, No. (%) | 319 (32.5) | 272 (33.2) | 47 (28.7) | 0.15 |
| ACEI/ARB, No. (%) | 419 (42.6) | 351 (42.9) | 68 (41.5) | 0.41 |
| β‐Blockers, No. (%) | 183 (18.6) | 159 (19.4) | 24 (14.6) | 0.09 |
| Diuretics, No. (%) | 137 (13.9) | 116 (14.2) | 21 (12.8) | 0.38 |
| Use of antithrombotic drugs, No. (%) | 202 (20.5) | 165 (20.2) | 37 (22.6) | 0.27 |
| Use of lipid‐lowering drugs, No. (%) | 332 (33.8) | 281 (34.3) | 51 (31.1) | 0.24 |
| FBG, mg/dL | 115.16±21.78 | 111.45±20.38 | 117.73±22.91 | 0.39 |
| TC, mg/dL | 175.61±27.18 | 171.32±25.61 | 179.24±29.17 | 0.34 |
| LDL, mg/dL | 129.83±31.42 | 126.58±28.17 | 131.18±32.72 | 0.27 |
| Hemoglobin, g/L | 139.27±10.18 | 142.34±11.11 | 136.55±9.87 | 0.35 |
| BMI, kg/m2 | 24.78±5.14 | 23.98±5.52 | 25.29±6.71 | 0.23 |
| IMT, mm | 1.13±0.24 | 1.10±0.19 | 1.15±0.25 | 0.14 |
| BUN, mg/dL | 58.62 ±28.13 | 53.15±25.26 | 62.15±29.54 | 0.21 |
| SLI, No. (%) | 459 (46.7) | 365 (44.6) | 94 (57.3) | 0.002 |
| PVH, median (IQR) | 2 (1–5) | 2 (1–3) | 3 (2–6) | 0.007 |
| DWMH, median (IQR) | 5 (2–10) | 4 (1–8) | 7 (3–11) | 0.003 |
| Brain atrophy, mean±SD | 0.820±0.025 | 0.827±0.027 | 0.814±0.022 | 0.032 |
| Presence of CMBs, No. (%) | 215 (21.9) | 106 (12.9) | 109 (66.5) | <0.001 |
| No. of CMBs, median (IQR) | 0 (0–0) | 0 (0–0) | 9 (0–19) | <0.001 |
| Distribution of CMBs, No. (%) | ||||
| Lobar | 67 (6.8) | 35 (4.3) | 32 (19.5) | <0.001 |
| Deep or infratentorial | 148 (15.1) | 71 (8.7) | 77 (47.0) | |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BUN, serum urea nitrogen; CMBs, cerebral microbleeds; DBP, diastolic blood pressure; DM, diabetes mellitus; DWMH, deep white matter hyperintensities; FBG, fasting blood glucose; IMT, intima‐media thickness; IQR, interquartile range; LDL, low‐density lipoprotein cholesterol; MCI, mild cognitive impairment; PVH, periventricular hyperintensities; SBP, systolic blood pressure; SLI, silent lacunar infarction; TC, total cholesterol.
We assessed the use of medications in all enrolled patients. The results indicate no significant differences in medication use between the groups (Table 1).
Adjusted Relationship Between the Number and Location of CMBs and MCI in Patients With Hypertension
Generalized linear Poisson models were used to determine the association between the number and location of CMBs and MCI in patients with hypertension, as shown in Table 2. The total number of CMBs and presence of ≥5 CMBs were all related to MCI (odds ratio [OR], 1.66 [P=0.019]; OR, 2.45 [P=0.017], respectively) after adjusting for age, sex, education, diabetes mellitus, hyperlipidemia, current smoker, current drinker, BP levels, BMI, IMT, presence of SLI, WMH (PVH, DWMH) grade, and brain atrophy (model 2). No significant correlations were found between the presence of 1 and 2 to 4 CMBs and MCI after adjusting for confounders. For CMB location, the presence of any CMBs, lobar CMBs, and deep or infratentorial CMBs were all related to MCI (OR, 2.18 [P=0.024]; OR, 1.55 [P=0.028]; OR, 2.74 [P=0.019], respectively) after adjusting for age, sex, education, diabetes mellitus, hyperlipidemia, current smoker, current drinker, BP levels, BMI, IMT, presence of SLI, WMH (PVH, DWMH) grade, and brain atrophy (model 2). Generalized linear Poisson models also found the presence of cerebellar CMBs was related to MCI (OR, 1.41 [P=0.043]) after adjusting for these confounders.
Table 2.
CMBs Distributional Pattern Relevant to MCI in Patients With Hypertension
| Model 0 | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| CMB No. | ||||||
| Total No. | 2.81 (1.73–3.91) | 0.001 | 2.67 (1.35–3.75) | 0.005 | 1.66 (1.15–2.78) | 0.019 |
| 1 vs none | 1.30 (0.81–2.14) | 0.109 | 1.24 (0.75–2.08) | 0.142 | 1.09 (0.65–1.82) | 0.288 |
| 2 to 4 vs none | 1.61 (0.91–3.32) | 0.037 | 1.38 (0.71–2.94) | 0.071 | 1.14 (0.74–2.91) | 0.181 |
| ≥5 vs none | 5.47 (2.06–11.42) | <0.001 | 4.53 (1.85–11.31) | 0.004 | 2.45 (1.35–9.54) | 0.017 |
| CMB location | ||||||
| Any CMBs | 9.91 (3.14–15.71) | <0.001 | 7.17 (2.73–12.32) | 0.001 | 2.18 (1.33–4.86) | 0.024 |
| Lobar | 4.39 (2.15–9.43) | <0.001 | 3.59 (1.24–6.23) | 0.011 | 1.55 (0.81–3.22) | 0.028 |
| Deep or infratentorial | 8.12 (4.62–13.27) | <0.001 | 5.33 (2.57–10.69) | 0.006 | 2.74 (1.24–4.78) | 0.019 |
Model 0: unadjusted. Model 1: adjusted for age, sex. Model 2: adjusted for age, sex, education, diabetes mellitus, hyperlipidemia, current smoker, current drinker, blood pressure, body mass index, intima‐media thickness, presence of silent lacunar infarction, white matter hyperintensities (periventricular hyperintensities, deep white matter hyperintensities) grade, and brain atrophy. CI indicates confidence interval; CMB, cerebral microbleed; OR, odds ratio; MCI, mild cognitive impairment.
Association Between Deep or Infratentorial CMBs and Performance on Cognitive Tests in Patients With Hypertension
Table 3 shows the association of deep or infratentorial CMBs with performance on cognitive tests in patients with hypertension and MCI. The number of deep or infratentorial CMBs per SD increase was significantly related to lower MoCA total scores and worse performance on tests of motor speed. Compared with no microbleeds, the presence of numerous (≥5) CMBs was significantly associated with lower MoCA total scores and with worse performance on tests of global cognitive function, information processing speed, executive function, and motor speed, but not with memory performance (model 0 in Table 3). Additional adjustments for age and sex did not change these results (model 1 in Table 3). Statistical significance remained for the association between (numerous) microbleeds and lower MoCA total scores and worse performance on global cognitive function, information processing speed, and motor speed upon adjusting for age, sex, education, diabetes mellitus, hyperlipidemia, current smoker, current drinker, BP levels, BMI, IMT, presence of SLI, WMH (PVH, DWMH) grade, and brain atrophy (model 2 in Table 3).
Table 3.
Association Between Deep or Infratentorial CMBs and Performance on Cognitive Tests in Patients With Hypertension
| Difference in Test Scores (95% CI) | ||||||
|---|---|---|---|---|---|---|
| MoCA Scores | Z Score Global Cognitive Function | Z Score Memory | Z Score Information Processing Speed | Z Score Executive Function | Z Score Motor Speed | |
| CMB No., per SD increase, Model 0 | −0.12 (−0.34 to 0.14)† | −0.04 (−0.06 to 0.03) | −0.03 (−0.06 to 0.03) | −0.01 (−0.06 to 0.05) | −0.03 (−0.11 to 0.12) | −0.10 (−0.35 to 0.11)† |
| 1 vs none, model 0 | −0.03 (−0.12 to 0.12) | 0.04 (−0.04 to 0.14) | 0.05 (−0.10 to 0.23) | 0.06 (−0.09 to 0.13) | 0.03 (−0.08 to 0.13) | 0.12 (−0.06 to 0.23) |
| 2 to 4 vs none, model 0 | −0.14 (−0.41 to 0.07) | −0.04 (−0.13 to 0.12) | −0.01 (−0.16 to 0.19) | −0.04 (−0.12 to 0.10) | −0.06 (−0.17 to 0.10) | −0.19 (−0.94 to 0.15) |
| ≥5 vs none, model 0 | −0.69 (−1.61 to −0.13)* | −0.41 (−1.45 to −0.09)* | −0.15 (−0.34 to 0.11) | −0.34 (−0.55 to −0.11)† | −0.28 (−0.46 to 0.02)† | −0.71 (−1.66 to −0.13)* |
| CMB No., per SD increase, model 1 | −0.07 (−0.29 to 0.12)† | −0.02 (−0.05 to 0.03) | −0.04 (−0.05 to 0.04) | −0.02 (−0.07 to 0.03) | −0.04 (−0.15 to 0.11) | −0.10 (−0.31 to 0.11)† |
| 1 vs none, model 1 | −0.02 (−0.12 to 0.11) | 0.06 (−0.07 to 0.13) | 0.06 (−0.11 to 0.28) | 0.09 (−0.10 to 0.14) | 0.04 (−0.07 to 0.16) | 0.11 (−0.06 to 0.22) |
| 2 to 4 vs none, model 1 | −0.14 (−0.40 to 0.08) | −0.04 (−0.10 to 0.13) | −0.03 (−0.19 to 0.20) | −0.03 (−0.12 to 0.10) | −0.04 (−0.19 to 0.17) | −0.21 (−0.89 to 0.13) |
| ≥5 vs none, model 1 | −0.67 (−1.65 to −0.12)* | −0.41 (−1.42 to −0.08)* | −0.19 (−0.33 to 0.11) | −0.35 (−0.57 to −0.13)† | −0.26 (−0.41 to 0.06)† | −0.68 (−1.63 to −0.13)* |
| CMB No., per SD increase, model 2 | −0.04 (−0.14 to 0.07) | −0.02 (−0.06 to 0.03) | −0.01 (−0.06 to 0.04) | 0.02 (−0.05 to 0.10) | −0.02 (−0.14 to 0.12) | −0.06 (−0.20 to 0.11) |
| 1 vs none, model 2 | −0.00 (−0.08 to 0.09) | 0.07 (−0.05 to 0.10) | 0.07 (−0.09 to 0.28) | 0.08 (−0.07 to 0.21) | 0.03 (−0.07 to 0.13) | 0.12 (−0.07 to 0.26) |
| 2 to 4 vs none, model 2 | −0.06 (−0.31 to 0.07) | −0.01 (−0.08 to 0.17) | 0.04 (−0.16 to 0.23) | −0.01 (−0.11 to 0.14) | −0.03 (−0.16 to 0.20) | −0.13 (−0.39 to 0.09) |
| ≥5 vs none, model 2 | −0.32 (−1.14 to 0.06)† | −0.23 (−1.07 to 0.05)† | −0.07 (−0.25 to 0.21) | −0.23 (−0.45 to −0.06)† | −0.07 (−0.22 to 0.25) | −0.43 (−1.31 to −0.08)† |
Model 0: unadjusted. Model 1: adjusted for age, sex. Model 2: adjusted for age, sex, education, diabetes mellitus, hyperlipidemia, current smoker, current drinker, blood pressure, body mass index, intima‐media thickness, presence of silent lacunar infarction, white matter hyperintensities (periventricular hyperintensities, deep white matter hyperintensities) grade, and brain atrophy. CI indicates confidence interval; CMB, cerebral microbleed; MoCA, Montreal Cognitive Assessment.
*P<0.01; † P<0.05.
Association Between Lobar CMBs and Performance on Cognitive Tests in Patients With Hypertension
Table 4 shows the association of strictly lobar CMBs with performance on cognitive tests in patients with hypertension with MCI. Per SD increase, a higher CMB number was significantly associated with lower MoCA total scores and worse performance on tests of information processing speed. When analyzed per category, the presence of numerous (≥5) CMBs was significantly associated with lower MoCA total scores and with worse performance on tests of global cognitive function, memory, information processing speed, executive function, and motor speed (model 0 in Table 4). Additional adjustments for age and sex did not change these results (Model 1 in Table 4). When additionally adjusting for confounders, including age, sex, education, diabetes mellitus, hyperlipidemia, current smoker, current drinker, BP levels, BMI, IMT, presence of SLI, WMH (PVH, DWMH) grade, and brain atrophy (model 2 in Table 4), the presence of ≥5 lobar CMBs remained associated with lower MoCA total scores and with worse performance on global cognitive function and information processing speed (model 2 in Table 4). No significant correlations were found between the presence of 1 or 2 to 4 lobar CMBs and MoCA total scores or compound scores of neuropsychological tests of specific cognitive domains in patients with hypertension.
Table 4.
Association Between Lobar CMBs and Performance on Cognitive Tests in Patients With Hypertension
| Difference in Test Scores (95% CI) | ||||||
|---|---|---|---|---|---|---|
| MoCA Scores | Z Score Global Cognitive Function | Z Score Memory | Z Score Information Processing Speed | Z Score Executive Function | Z Score Motor Speed | |
| CMB No., per SD increase, model 0 | −0.09 (−0.17 to 0.05)† | −0.03 (−0.11 to 0.04) | −0.03 (−0.14 to 0.09) | −0.07 (−0.14 to 0.02)† | −0.03 (−0.06 to 0.05) | −0.03 (−0.15 to 0.04) |
| 1 vs none, model 0 | −0.02 (−0.16 to 0.14) | 0.00 (−0.12 to 0.09) | 0.00 (−0.08 to 0.08) | 0.02 (−0.07 to 0.12) | −0.01 (−0.10 to 0.09) | 0.03 (−0.08 to 0.10) |
| 2 to 4 vs none, model 0 | −0.16 (−0.47 to 0.09) | −0.03 (−0.16 to 0.11) | −0.02 (−0.12 to 0.10) | −0.12 (−0.33 to 0.13) | −0.14 (−0.37 to 0.14) | −0.06 (−0.18 to 0.14) |
| ≥5 vs none, model 0 | −0.60 (−1.52 to −0.12)* | −0.37 (−0.79 to 0.02)† | −0.36 (−0.77 to 0.06)† | −0.73 (−1.67 to −0.11)* | −0.34 (−0.65 to −0.08)† | −0.29 (−0.51 to −0.08)† |
| CMB No., per SD increase, model 1 | −0.08 (−0.12 to 0.09)† | −0.03 (−0.09 to 0.03) | −0.01 (−0.13 to 0.07) | −0.08 (−0.14 to 0.03)† | −0.03 (−0.09 to 0.06) | −0.04 (−0.14 to 0.05) |
| 1 vs none, model 1 | −0.04 (−0.17 to 0.13) | 0.00 (−0.08 to 0.07) | 0.00 (−0.08 to 0.10) | 0.01 (−0.06 to 0.13) | −0.01 (−0.07 to 0.08) | 0.03 (−0.09 to 0.16) |
| 2 to 4 vs none, model 1 | −0.15 (−0.43 to 0.09) | −0.03 (−0.16 to 0.11) | −0.02 (−0.14 to 0.11) | −0.11 (−0.26 to 0.11) | −0.12 (−0.44 to 0.14) | −0.06 (−0.19 to 0.13) |
| ≥5 vs none, model 1 | −0.51 (−1.47 to −0.06)† | −0.35 (−0.81 to 0.02)† | −0.33 (−0.85 to 0.03)† | −0.73 (−1.65 to −0.11)* | −0.33 (−0.58 to −0.09)† | −0.27 (−0.44 to −0.09)† |
| CMB No., per SD increase, model 2 | −0.03 (−0.07 to 0.04) | −0.01 (−0.04 to 0.02) | −0.01 (−0.12 to 0.09) | −0.06 (−0.13 to 0.09)† | −0.01 (−0.06 to 0.04) | −0.01 (−0.11 to 0.06) |
| 1 vs none, model 2 | −0.04 (−0.15 to 0.12) | 0.00 (−0.05 to 0.07) | 0.00 (−0.07 to 0.09) | 0.03 (−0.05 to 0.11) | 0.00 (−0.07 to 0.08) | 0.03 (−0.06 to 0.17) |
| 2 to 4 vs none, model 2 | −0.12 (−0.44 to 0.10) | −0.02 (−0.11 to 0.07) | −0.01 (−0.11 to 0.09) | −0.09 (−0.21 to 0.08) | −0.06 (−0.26 to 0.05) | −0.04 (−0.15 to 0.13) |
| ≥5 vs none, model 2 | −0.36 (−1.19 to 0.05)† | −0.23 (−0.42 to −0.06)† | −0.17 (−0.47 to 0.07) | −0.43 (−1.36 to −0.09)† | −0.13 (−0.35 to −0.02) | −0.11 (−0.33 to −0.05) |
Model 0: unadjusted. Model 1: adjusted for age, sex. Model 2: adjusted for age, sex, education, diabetes mellitus, hyperlipidemia, current smoker, current drinker, blood pressure, body mass index, intima‐media thickness, presence of silent lacunar infarction, white matter hyperintensities (periventricular hyperintensities, deep white matter hyperintensities) grade, and brain atrophy. CI indicates confidence interval; CMB, cerebral microbleed; MoCA, Montreal Cognitive Assessment
*P<0.01; † P<0.05.
Discussion
In the present study, we investigated the association between CMBs and MCI in 983 patients with hypertension at 3 centers in northern China. Based on the results from this study, the rate of CMBs in patients with hypertension was 21.9%. Our results are consistent with those of previous studies.18 The presence, number, and distribution of CMBs were greater in patients with hypertension with MCI than in those without MCI. Generalized linear Poisson models showed that the total number of CMBs and the presence of ≥5 CMBs were all related to MCI after adjusting for age, sex, education, diabetes mellitus, hyperlipidemia, current smoker, current drinker, BP levels, BMI, IMT, presence of SLI, WMH (PVH, DWMH) grade, and brain atrophy. The presence of any CMBs, strictly lobar CMBs, and deep or infratentorial CMBs were all related to MCI after adjusting for confounders. Furthermore, the presence of multiple microbleeds (≥5) is associated with lower MoCA total scores and with worse performance on specific domains of cognitive tests, such as global cognitive function, information processing speed, and motor speed. To the best of our knowledge, no previous studies have investigated the association between CMBs and MCI in patients with essential hypertension without a history of TIA or stroke.
Previous cross‐sectional and longitudinal studies6, 19, 20 in healthy adults also demonstrated that CMBs, especially in large numbers, are related to lower Mini‐Mental State Examination scores, worse information processing, and worse executive functioning. Other studies also found that CMBs were associated with cognitive decline in patients with ischemic stroke or TIA and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy.21, 22, 23
The pathological differences in CMBs according to distribution is now well‐known, with CMBs in deep or infratentorial regions considered to be associated with hypertensive microangiopathy, whereas strictly lobar CMBs are considered attributable to neurodegenerative pathology and cerebral amyloid angiopathy.24 Recent studies in experimental models have shown that hypertension promotes β‐secretase activity and increases cerebral vascular amyloid deposits and that hypertension‐mediated impairment of vascular repair mechanisms may contribute to the faster development of neuropathology and cognitive deficits in experimental models of AD.25, 26 CMBs were postulated as the potential “missing link” in the interaction between cerebral amyloid angiopathy and hypertensive arteriolosclerosis in the pathogenesis of AD.27 The robust associations we assessed between the presence and number of cerebral CMBs and cognitive function further support this notion.
Mechanisms by which CMBs influence cognitive function remain speculative and may be causal or noncausal.28 CMBs may represent a proxy measure of cerebral vascular disease overall, and their presence may influence cognition indirectly. This hypothesis is supported by our findings because we found associations with multiple CMBs in widespread areas rather than with single or multiple CMBs clustered in a specific brain region. However, histopathologic studies have shown that the presence of CMBs indicates widespread damage in arterioles by hypertension, amyloid deposition and surrounding gliosis, infarction, or even necrosis, resulting in microstructural damage of the surrounding white matter.29, 30 Therefore, CMBs may disrupt white matter tracts involved in cognitive function, leading to damage to the neural networks superimposed by the effects of frequently co‐occurring white matter lesions and lacunar infarctions. This tissue damage, which is not visible on conventional MRI, can be assessed in future studies with new techniques such as diffusion tensor imaging or resting state MRI. We found that CMBs were related to cognitive performance independent of age, sex, education, major cardiovascular factors, WMHs, and SLI. This finding, together with results from pathological studies showing that CMBs are frequently characterized by surrounding microstructural damage,30 suggests that CMBs have a direct effect on cognitive performance rather than simply reflecting the presence of other markers of cerebral small vessel disease because our findings were independent of the presence of WMHs and SLI.
We observed an association between lobar and deep or infratentorial CMBs and decline in information processing speed, but not in declarative memory. Information processing speed tasks may be the most sensitive to vascular pathology.31, 32 Furthermore, accelerated decline in information processing function may be a harbinger for future decline in cognition related to AD. Notably, among cognitively normal adults older than 60 years, fibrillar forms of β‐amyloid were related to reductions in measures of processing speed, but not declarative memory, a hallmark feature of AD.33 In our study, we found an association between lobar (suggestive of cerebral amyloid angiopathy) and deep or infratentorial CMBs (suggestive of hypertensive arteriopathy) and performance reductions in speed tasks in patients with essential hypertension. Our study emphasizes the role of vascular disease in the pathogenesis of cognitive impairment. Accumulating evidence suggests that vascular damage may be particularly important in the initiation of neurodegenerative disease.34, 35
Study Limitations
Our study has some limitations. First, the present investigation was a cross‐sectional study. We need to conduct a longitudinal study to validate the causal relationship between CMBs and MCI. Second, our results are limited to the cohort of elderly individuals with hypertension; therefore, they cannot be generalized to the general population. Third, we were unable to characterize the precise cause of cognitive impairment using molecular ligands (eg, β‐amyloid) or pathological confirmation.
Conclusions
We found that the presence and greater number of cerebral CMBs independently correlate with MCI in patients with essential hypertension without a history of TIA or stroke, and that this relationship is driven not only by strictly lobar CMBs but also by deep or infratentorial CMBs. These results suggest that CMBs are clinically not as silent as they have been considered to be, which may help us understand the role of vascular disease in cognitive decline. Studies with longitudinal follow‐up should identify whether the presence at baseline and/or increases in CMBs over time can predict the future development of dementia.
Sources of Funding
The present study was supported by the Natural Science Foundation of Shandong Province (ZR2017 MH011), the Development Plan of Medical Sciences of Shandong Province (2016 WS0636), and the Development Plan of Sciences of Weihai City (2016GNS029‐1).
Disclosures
None.
(J Am Heart Assoc. 2018;7:e008453 DOI: 10.1161/JAHA.117.008453.)29858365
References
- 1. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. [DOI] [PubMed] [Google Scholar]
- 2. Knopman DS, Petersen RC. Mild cognitive impairment and mild dementia: a clinical perspective. Mayo Clin Proc. 2014;89:1452–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cordonnier C, van der Flier WM, Sluimer JD, Leys D, Barkhof F, Scheltens P. Prevalence and severity of microbleeds in a memory clinic setting. Neurology. 2006;66:1356–1360. [DOI] [PubMed] [Google Scholar]
- 5. Hanyu H, Tanaka Y, Shimizu S, Takasaki M, Abe K. Cerebral microbleeds in Alzheimer's disease. J Neurol. 2003;250:1496–1497. [DOI] [PubMed] [Google Scholar]
- 6. Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78:326–333. [DOI] [PubMed] [Google Scholar]
- 7. Jellinger KA, Attems J. Prevalence of dementia disorders in the oldest‐old: an autopsy study. Acta Neuropathol. 2010;119:421–433. [DOI] [PubMed] [Google Scholar]
- 8. Nelson PT, Head E, Schmitt FA, Davis PR, Neltner JH, Jicha GA, Abner EL, Smith CD, Van Eldik LJ, Kryscio RJ, Scheff SW. Alzheimer's disease is not “brain aging”: neuropathological, genetic, and epidemiological human studies. Acta Neuropathol. 2011;121:571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmer K, Di Iulio F, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Spalletta G. Neuropsychiatric predictors of progression from amnestic‐mild cognitive impairment to Alzheimer's disease: the role of depression and apathy. J Alzheimers Dis. 2010;20:175–183. [DOI] [PubMed] [Google Scholar]
- 10. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 11. Hoogendam YY, Hofman A, van der Geest JN, van der Lugt A, Ikram MA. Patterns of cognitive function in aging: the Rotterdam Study. Eur J Epidemiol. 2014;29:133–140. [DOI] [PubMed] [Google Scholar]
- 12. Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, Greenberg SM, Dickerson BC. MR imaging detection of cerebral microbleeds: effect of susceptibility‐weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009;30:338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004;52:612–618. [DOI] [PubMed] [Google Scholar]
- 14. Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–1420. [DOI] [PubMed] [Google Scholar]
- 15. Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. [DOI] [PubMed] [Google Scholar]
- 16. Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, Steinling M, Valk J. A semiquantitative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. [DOI] [PubMed] [Google Scholar]
- 17. de Bresser J, Portegies MP, Leemans A, Biessels GJ, Kappelle LJ, Viergever MA. A comparison of MR based segmentation methods for measuring brain atrophy progression. NeuroImage. 2011;54:760–768. [DOI] [PubMed] [Google Scholar]
- 18. Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al‐Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM; Microbleed Study Group . Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takashima Y, Mori T, Hashimoto M, Kinukawa N, Uchino A, Yuzuriha T, Yao H. Clinical correlating factors and cognitive function in community‐dwelling healthy subjects with cerebral microbleeds. J Stroke Cerebrovasc Dis. 2011;20:105–110. [DOI] [PubMed] [Google Scholar]
- 20. Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, Koudstaal PJ, Ikram MA, Vernooij MW. Association of Cerebral Microbleeds With Cognitive Decline and Dementia. JAMA Neurol. 2016;73:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gregoire SM, Smith K, Jäger HR, Benjamin M, Kallis C, Brown MM, Cipolotti L, Werring DJ. Cerebral microbleeds and long‐term cognitive outcome: longitudinal cohort study of stroke clinic patients. Cerebrovasc Dis. 2012;33:430–435. [DOI] [PubMed] [Google Scholar]
- 22. Wang Z, Wong A, Liu W, Yang J, Chu WC, Au L, Lau A, Chan A, Xiong Y, Soo Y, Leung T, Wong LK, Mok VC. Cerebral microbleeds and cognitive function in ischemic stroke or transient ischemic attack patients. Dement Geriatr Cogn Disord. 2015;40:130–136. [DOI] [PubMed] [Google Scholar]
- 23. Liem MK, Lesnik Oberstein SA, Haan J, van der Neut IL, Ferrari MD, van Buchem MA, Middelkoop HA, van der Grond J. MRI correlates of cognitive decline in CADASIL: a 7‐year follow‐up study. Neurology. 2009;72:143–148. [DOI] [PubMed] [Google Scholar]
- 24. Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. 2010;41:S103–S106. [DOI] [PubMed] [Google Scholar]
- 25. Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, Iadecola C. Hypertension enhances Aβ‐induced neurovascular dysfunction, promotes β‐secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab. 2016;36:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cifuentes D, Poittevin M, Dere E, Broquères‐You D, Bonnin P, Benessiano J, Pocard M, Mariani J, Kubis N, Merkulova‐Rainon T, Lévy BI. Hypertension accelerates the progression of Alzheimer‐like pathology in a mouse model of the disease. Hypertension. 2015;65:218–224. [DOI] [PubMed] [Google Scholar]
- 27. Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain. 2011;134:335–344. [DOI] [PubMed] [Google Scholar]
- 28. Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci. 2010;299:131–135. [DOI] [PubMed] [Google Scholar]
- 29. Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, Hartung HP. Histopathologic analysis of foci of signal loss on gradient‐echo T2*‐ weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy‐related microbleeds. Am J Neuroradiol. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 30. Schrag M, McAuley G, Pomakian J, Jiffry A, Tung S, Mueller C, Vinters HV, Haacke EM, Holshouser B, Kido D, Kirsch WM. Correlation of hypointensities in susceptibility‐weighted images to tissue histology in dementia patients with cerebral amyloid angiopathy; a postmortem MRI study. Acta Neuropathol. 2010;119:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz L, Looi JC, Wen W, Zagami AS. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912–919. [DOI] [PubMed] [Google Scholar]
- 32. Case NF, Charlton A, Zwiers A, Batool S, McCreary CR, Hogan DB, Ismail Z, Zerna C, Coutts SB, Frayne R, Goodyear B, Haffenden A, Smith EE. Cerebral amyloid angiopathy is associated with executive dysfunction and mild cognitive impairment. Stroke. 2016;47:2010–2016. [DOI] [PubMed] [Google Scholar]
- 33. Rodrigue KM, Kennedy KM, Devous MD Sr, Rieck JR, Hebrank AC, Diaz‐Arrastia R, Mathews D, Park DC. β‐Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drachman DA. The amyloid hypothesis, time to move on: amyloid is the downstream result, not cause, of Alzheimer's disease. Alzheimers Dement. 2014;10:372–380. [DOI] [PubMed] [Google Scholar]
- 35. Marchesi VT. Alzheimer's dementia begins as a disease of small blood vessels, damaged by oxidative‐induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011;25:5–13. [DOI] [PubMed] [Google Scholar]


