Abstract
Background
Cardiovascular diseases (CVDs) and stroke are the highest and third highest causes of death, respectively, in the whole United States. It is well established that both long‐ and short‐term exposure to particulate air pollution (particulate matter with diameters <2.5 μm [PM 2.5]) increases the risks of both CVD and stroke mortality.
Methods and Results
We combined county‐level data for CVD and stroke mortality, and prevalence of hypertension and obesity, with spatial patterns of PM 2.5 and ozone in a cross‐sectional ecological study. We found significant positive associations between both CVD (β=15.4, P<0.001) and stroke (β=2.7, P<0.001) mortality with PM 2.5. Ozone had significant link with just CVD (β=1372.1, P<0.001). Once poverty, ethnicity, and education were taken into account, there were still significant positive associations between PM 2.5 and both CVD (β=1.2, P<0.001) and stroke (β=1.1, P<0.001) mortality. Moreover, the association between CVD and ozone remained after adjustment for these factors (β=21.8, P<0.001). PM 2.5 and ozone were independent risk factors. The impact of PM 2.5 on CVD and stroke mortality was strongly dependent on the prevalence of obesity. Hypertension partially mediated the associations of PM 2.5 and mortality from CVD and stroke.
Conclusions
There was a spatial association between PM 2.5 exposure and the leading causes of death and disability in United States. The effect of PM 2.5 was considerably greater in areas where obesity is more prevalent. Hypertension is a possible mediator of the association of PM 2.5 and both CVD and stroke.
Keywords: cardiovascular diseases, education, obesity, particulate matter, PM2.5, stroke
Subject Categories: Cerebrovascular Disease/Stroke, Epidemiology, Lifestyle, Obesity, Cardiovascular Disease
Clinical Perspective
What Is New?
It has been suggested that particulate air pollution (particulate matter with diameters <2.5 μm) increases the risk of cardiovascular disease and stroke. However, the evidence is mixed and the possible interaction with other factors, such as ozone levels and obesity, are unclear.
We explored spatial variation in particulate matter with diameters <2.5 μm, ozone, hypertension, obesity, and the mortality from cardiovascular disease and stroke, across the mainland United States.
We found independent associations between particulate matter with diameters <2.5 μm and ozone on cardiovascular disease and stroke mortality that were mediated, at least in part, via hypertension.
Effects of particulate matter with diameters <2.5 μm were greater in areas where there were more individuals with obesity.
What Are the Clinical Implications?
If these associations are causal, then avoiding high levels of air pollution may reduce the risk of cardiovascular disease and stroke, particularly in individuals with obesity.
Introduction
According to the latest report of American Heart Association, each year ≈17.3 million people die globally of cardiovascular disease (CVD), accounting for 30% of global mortality.1 Stroke is the second most common cause of death, and third most important cause of disability worldwide.2 In the United States in 2015, CVD was the leading cause of death, accounting for 864 000 deaths.3 Apart from genetic and lifestyle risk factors, exposure to air pollution, specifically fine particulate matter (PM; particle size <2.5 μm [PM2.5]), has been suggested to be linked with CVD and stroke mortality.4, 5
Although considerable effort has been directed at controlling and monitoring ambient concentrations of PM for the past 30 years, and these efforts have been largely successful, levels of air pollution in larger US cities still occasionally exceed by an order of magnitude the recommended safe levels of PM2.5 pollution.6 Worldwide, it has been suggested that particulate air pollution is responsible for >3 million deaths every year.5, 7 PM2.5 can be deposited in the upper and lower airways, and because of its small size, it can reach more deeply into the lungs and circulation. Therefore, it has been implicated in deleterious outcomes, such as CVD, stroke, and endothelial dysfunction.5, 8 In the case of endothelial dysfunction, a previous study suggested that PM2.5 can rapidly exert its harmful impacts on blood pressure (BP) within just 2 hours of exposure.9 Moreover, in 36 US metropolitan areas, among postmenopausal women, long‐term exposure to PM was linked with the incidence of CVD and death.10
The American Heart Association already concluded in 2004 that PM contributes to cardiovascular morbidity and mortality, and this opinion was reinforced by a comprehensive review, meta‐analysis, and updated statement in 2009.5 Nevertheless, since that time, there have been several large studies that have failed to find any association between PM2.5 and the risk of CVD.11, 12, 13, 14, 15 For example, in the multicenter ESCAPE (European Study of Cohorts for Air Pollution Effects), which involved 367 383 participants, they reported that in a joint analysis of data from 22 European cohorts, most hazard ratios for the association of long‐term exposure to air pollutants with mortality from overall CVD and with specific CVDs were ≈113 (ie, there was no elevated risk).
The data about links of stroke to air pollution levels are also inconsistent. In detail, some studies have reported a positive association between PM2.5 and the risk of stroke.16, 17, 18, 19, 20, 21 For instance, across 204 US counties, a 10‐μg/m3 increase in short‐term PM2.5 concentration was associated with a 0.8% increased chance of hospital admission for stroke.21 However, other studies failed to find such an association.22, 23, 24, 25 For example, a time‐stratified case‐crossover was used to assess the associations between short‐term outdoor PM2.5 and emergency department admissions for stroke among elderly people (≥65 years of age) in Canada, and no association between outdoor measures of air pollution and all stroke visits was detected.22 Results from French National Program on Air Pollution Health Effects, which was conducted between 1998 and 2003, also found no association between short‐term PM2.5 exposure and stroke.25 In case of the heterogeneity and controversy between current studies, previous meta‐analyses have also reported that the direction of the association between PM2.5 levels and the risk of different types of stroke is not consistent across studies.26, 27, 28 These meta‐analyses have suggested this high heterogeneity may be attributable to differences in the definition of outcomes, different techniques for measuring the PM2.5, using different confounders in the models for evaluation, and applying different databases with varying levels of socioeconomic factors (income, education, and ethnicity) status.28
In their comprehensive review of the association between particulate air pollution and CVD, Brook and colleagues5 highlighted several key areas in need of future study. First, they noted that levels of PM2.5 might covary with other levels of air pollutions and, hence, the causal factor driving the CVD effects might not be PM2.5 but these covariates. This could explain why more recent large cohorts have failed to detect associations (previously reviewed). One potential covariate is ozone. A recent meta‐analysis and systematic review drew attention to the paucity of studies about the association between long‐term exposure to ozone and mortality.29 Brook et al5 also concluded that there was indicative evidence that obese individuals may be a susceptible population at greater risk for cardiovascular events due to PM2.5 exposure, and that “This is a tremendously important public health issue to corroborate because of the enormous and growing prevalence of obesity worldwide”.5 A cross‐sectional study of Chinese adults suggested that being overweight or obese may enhance the effect of air pollution on prevalence of CVD and stroke.30
With respect to the unclear mechanism of any impact of PM2.5 on CVD and stroke pathological characteristics, an important question is, if it is the causal agent, whether PM2.5 induces deleterious effects directly, or via impacts on other factors (such as hypertension). These complexities in the associations can be clarified using mediation analysis31; in particular, use of the counterfactual framework (or Neyman‐Rubin model) in mediation analysis allows unbiased estimates of direct and indirect effects to be obtained.31, 32 In the present article, in the light of recent reports of no significant association, we aimed to determine whether there is an association between the long‐term spatial variation in both PM2.5 and ozone levels across the mainland United States and the spatial patterns of mortality from CVD and stroke, and the prevalence of hypertension. Second, we evaluated the impact of varying levels of obesity prevalence on the relationship between long‐term PM2.5 exposure and mortality from CVD and stroke. Third, we aimed to assess whether hypertension mediates any association between long‐term PM2.5 and mortality from CVD and stroke. We accomplished this by matching data on the disease mortalities and prevalence from the US Centers for Disease Control and Prevention (CDC; between 2011 and 2013) with data on the spatial patterns of air pollution (PM2.5; 2003–2011) from the US National Aeronautics and Space Administration.
Methods
Mortality and Prevalence
The data, analytic methods, and study materials will be/have been made available to other researchers for purposes of reproducing the results or replicating the procedure.33 We used data on mortality rate (per 100 000 individuals) for CVD and stroke (between 2011 and 2013, aged >35 years) and prevalence of obesity from the publicly available databases compiled by the CDC (http://www.cdc.gov). Annual CVD mortality was defined as the number of deaths per 100 000 person‐years attributable to circulatory causes (International Statistical Classification of Diseases, Tenth Revision, codes I00–I99). Codes I60 to I69 were considered for cerebrovascular diseases. We obtained data on prevalence of hypertension (hypertension was defined as systolic BP of at least 140 mm Hg, self‐reported use of antihypertensive treatment, or both) from county health rankings and roadmaps organization (http://www.countyhealthrankings.org). These data were estimated on the basis of the National Health and Nutrition Examination Survey in five 2‐year waves from 1999 to 2008, including 26 349 adults aged ≥30 years, and the Behavioral Risk Factor Surveillance System from 1997 to 2009, including 1 283 722 adults aged ≥30 years. This is a collaboration of the Robert Wood Johnson Foundation and the University of Wisconsin Population Health Institute. The organization compiles data at the county level from several national government sources, including health behaviors, clinical care, social and economic factors, and aspects of the physical environment; preparation, definition, download, and sorting of data on prevalence of obesity, poverty, and ethnicity have been explained elsewhere.34 Briefly, the prevalence of obesity in this database was estimated using data from the CDC Behavioral Risk Factor Surveillance System, which is a monthly state‐based telephone survey of a nationally representative sample of adults aged >20 years old. Because it is telephone based, it excludes individuals living in care homes or those without a telephone. More than 400 000 individuals are contacted annually to take part in the survey, which has been running since 1984. In the telephone interview, individuals self‐report their height and weight in response to the questions “About how much do you weigh without shoes?” and “About how tall are you without shoes?,” which are then converted, if necessary, to kilograms and meters, respectively, before calculating the body mass index (BMI; kilograms per meter squared). A BMI >30 kg/m2 is then classed as obese using the World Health Organization standard for whites.35 This is applied independent of actual ethnicity. Individuals normally overestimate their own height and underestimate their own weight in a self‐report setting36, 37 and, hence, these estimates are likely to be conservative. Despite the small bias of 0.7 to 1.3 kg/m2, validation studies confirm a strong correlation between self‐reported and actual BMI.38, 39 Obesity prevalence data in 2012 were available for 3143 counties or county equivalents from the continental United States, reflecting a population of ≈170 million adults. A previous variogram analysis34 showed that the county‐level data are a suitable spatial scale at which to seek disease associations for obesity prevalence. Previous studies have used the same database to study factors influencing obesity prevalence,40 links of obesity to restaurant densities,41 and links of obesity and diabetic patients to PM2.5.42 Institutional review board approval was obtained by CDC, and each subject signed informed consent. All the data are already in the public domain from sites identified in the main text.33
Particulate Air Pollution
Estimates of PM2.5 for each county were obtained for 2003 to 2011, as documented on CDC WONDER (http://wonder.cdc.gov), specifically file D80a. Reported measures are the average daily estimates of PM2.5 over the 7‐year interval in micrograms per cubic meter (μg/m3). The values were interpolated to the county level, from 10‐km square spatial area grids covering the 48 contiguous United States (not including Alaska and Hawaii). Two sources of environmental data were used as input to the surfacing algorithm, US Environmental Protection Agency Air Quality System PM2.5 in situ data and US National Aeronautics and Space Administration Moderate Resolution Imaging Spectroradiometer aerosol optical depth remotely sensed data. Data on ozone were obtained from http://www.epa.gov, which is the formal website for the US Environmental Protection Agency. Ozone levels were only available for 801 of the 3111 counties.
Covariates
Data of county‐level poverty (percentage in poverty and average income), education, and ethnicity were obtained from the US Census, 2010 census data (http://www.census.gov/2010census), specifically files PVY01, PVY02, INC01, INCO2, INCO3, IPE01, and RHI02. The distributions of ethnicity data showed heteroscedasticity in the variance. We tried various transformations to remove this, but none was completely successful. The outcome of the final analysis was robust to the type of data transformation used.
Statistical Analysis
We used univariate linear regression to investigate the associations between mortality (CVD and stroke) and prevalence (hypertension) data (dependent variables) with the PM2.5 levels (independent variable) for each county (identified by the federal information processing standard code, which is a 5‐digit code that allows counties and county equivalent units to be uniquely identified). To assess if the relationships were nonlinear, we included higher‐order polynomial terms into the regression model and tested if they were significant. In the absence of significant higher‐order polynomials, we assumed the relationships were linear. We then sought associations between these dependent variables and the records of county‐level poverty (percentage in poverty), ethnicity (percentage black), and education (percentage with bachelor's degree or higher). All data were checked for normality (Kolmogorov‐Smirnov tests) before analysis and, where necessary, were transformed (with the use of log or arcsine transformation). These data allowed us to adjust mortality (CVD and stroke) and prevalence (hypertension) data at the county level for these potential confounders (poverty, education, and ethnicity), and then examine the associations of the adjusted levels to PM2.5. For a subsample of the data (n=801 counties), we estimated the association between ozone (independent) and mortality from CVD and stroke and prevalence of the hypertension in both the crude and adjusted model (ethnicity, poverty, and education). We have also applied a multiple linear regression to evaluate if ozone and PM2.5 were independent predictors of the mortality from CVD and stroke and prevalence of the hypertension in both crude and adjusted models (ethnicity, poverty, and education). Multicollinearity for the multiple linear regressions was assessed with variance inflation factors at each step.43 Multicollinearity was considered high when the variance inflation factor was >10.43
We also quantified the impact of obesity on the link between PM2.5 with CVD and stroke mortality by applying the moderation model using the SPSS Macro developed by Preacher and Hayes44; by applying this Macro, we could simultaneously test the moderator impact of the variable of interest, obesity, adjusting for the confounding factors. Moreover, this approach allowed visualization of the impact of each SD change in the potential moderators on the relationship between independent and dependent variables. We tested for the presence of an effect of obesity in both crude and adjusted models (adjusted for ethnicity, poverty, and education).
We assessed the total, direct, and indirect effects of PM2.5 on mortality from CVD and stroke, with hypertension as a mediator, by using the counterfactual framework.31, 32 In this approach, the total effect can be decomposed into direct effects (not mediated by hypertension) and indirect effects (mediated via hypertension). The SPSS Macro developed by Preacher and Hayes44 was used to evaluate the direct effect and the indirect effect of PM2.5 on mortality from CVD and stroke, with hypertension as mediator. A product‐of‐coefficients test was used because it has the potential to detect significant mediation effects in the absence of a significant intervention effect.45, 46 Using single‐mediator models, the SPSS Macro was used to calculate all regression coefficients that were adjusted for baseline values. In brief, the Macro generates output that includes the following steps. First, the total effect (γ coefficient) of the intervention on the outcome variable (eg, mortality from CVD and stroke) is estimated by regression. Second, the action theory test is then used to examine the effect of the intervention (PM2.5) on the hypothesized mediators (α coefficient and hypertension). Third, the conceptual theory test examines the association between changes in the hypothesized mediators and changes in the dependent variables (ie, mortality from CVD and stroke; β coefficient). The program also estimates the direct (γ′ coefficient) and indirect (α×β product of coefficients) effects. Sobel's test was used to test the significance of the indirect effect. The proportion of the mediation effect was calculated using the following equation: (α×β)/(α×β+γ).47 Full or complete mediation is present when the total effect (the γ path) is significant, the direct effect (the γ′ path) is nonsignificant, and α×β is significant, whereas partial or incomplete mediation is present when both the direct and indirect effects were significant. Inconsistent mediation is present when neither the total nor the direct effect is significant, but α×β is significant.47 SPSS software, version 11.5 (SPSS Inc, Chicago, IL) was used for statistical analysis. P<0.05 was considered statistically significant.
Results
Mean and SD for distribution of all of dependent and independent variables and number of counties that each variable covered are shown in Table 1. There was a positive relationship between both crude level of CVD and stroke mortality with the annual average level of PM2.5 (Table 2). The β values that increase in the levels of PM2.5 air pollution by 5 μg/m3 would be associated with an increase in the mortality from CVD by 77 people per 100 000 population (21.2% increase on mean levels) and from stroke by 13.5 people per 100 000 population (16.9% increase on mean levels). Also, prevalence of hypertension (percentage) had a strong positive relationship with PM2.5. The β values suggest that an increase in PM2.5 air pollution by 5 μg/m3 would be associated with a 4.3% increase in hypertension. In all 3 cases, the relationships were not improved by the addition of higher‐order polynomial predictors, indicating the relationships were not significantly different from linear.
Table 1.
Distribution of Dependent and Independent Variables
| Variables | Value, Mean±SD | No. of Counties |
|---|---|---|
| PM2.5, μg/m3 | 11.9±1.7 | 3111 |
| Ozone, ppm | 0.06±0.01 | 801 |
| CVD mortality, per 100 000 population | 362.9±82.8 | 3134 |
| Stroke mortality, per 100 000 population | 79.9±16.2 | 3119 |
| Obesity by body mass index, kg/m2 | 30.8±4.5 | 3143 |
| Poverty, % | 16.8±6.4 | 3159 |
| Education, % | 20.1±8.9 | 3160 |
CVD indicates cardiovascular disease; and PM2.5, particulate matter with diameters <2.5 μm.
Table 2.
Details of the Association Between PM2.5 and Ozone With Mortality From CVD and Stroke and Prevalence of Hypertension in Both Crude and Adjusted Models
| Outcomes | PM2.5 | Ozone | |||||
|---|---|---|---|---|---|---|---|
| β | 95% CI | P Value | β | 95% CI | P Value | ||
| Crude model | CVD mortality | 15.4 | 13.7 to 17.0 | <0.001 | 1372.1 | 859.1 to 1888.9 | <0.001 |
| Stroke mortality | 2.7 | 2.41 to 3.05 | <0.001 | 88.3 | −17.3 to 194.0 | 0.101 | |
| Hypertension prevalence | 0.0086 | 0.0079 to 0.0092 | <0.001 | 0.25 | 0.19 to 0.49 | 0.034 | |
| Adjusted model | CVD mortality | 1.2 | 1.0 to 1.4 | <0.001 | 21.8 | 15.7 to 28.0 | <0.001 |
| Stroke mortality | 1.1 | 0.77 to 1.3 | <0.001 | 4.90 | −1.6 to 11.4 | 0.143 | |
| Hypertension prevalence | 0.18 | 0.16 to 0.20 | <0.001 | 5.09 | −2.4 to 12.4 | 0.183 | |
Simple and multivariate linear regression applied. Adjusted model: adjusted for poverty, ethnicity, and education. CVD and stroke mortality (per 100 000 population), ozone concentration (ppm), PM2.5 concentration (μg/m3). CI indicates confidence interval; CVD, cardiovascular disease; and PM2.5, particulate matter with diameters <2.5 μm.
Overall mortality rates from CVD and stroke were greater where there was more poverty (β=6.73 [95% confidence interval {CI}, 6.35–7.11] and β=1.08 [95% CI, 1.02–1.16], respectively; both P<0.001), a higher proportion of blacks (β=14.3 [95% CI, 13.0–15.7] and β=2.85 [95% CI, 2.58–3.11], respectively; both P<0.001), and a less educated population (β=−4.88 [95% CI, −5.16 to −4.60] and β=−0.71 [95% CI, −0.77 to −0.65], respectively; both P<0.001). With respect to hypertension, we found that counties with higher levels of poverty (β=0.4; 95% CI, 0.38–0.41; P<0.001), a greater proportion of blacks (β=1.3; 95% CI, 1.11–1.4; P<0.001), and less educated populations (β=−0.23; 95% CI, −0.25 to −0.22; P<0.001) had a greater prevalence of hypertension.
Once we adjusted the models for these 3 confounding factors (poverty, ethnicity, and education), there were still positive and significant associations between adjusted mortality attributable to CVD (β=1.2; 95% CI, 1.0–1.4; P<0.001; Table 2) and stroke (β=1.1; 95% CI, 0.77–1.3; P<0.001; Table 2) and the PM2.5 level. Finding suggested that increases in the levels of PM2.5 air pollution by 5 μg/m3 were associated with increases in the mortality from CVD by only 6 people per 100 000 population (0.12%) and from stroke by 5.5 people per 100 000 population (6.8%). Although these mortalities appear small when multiplied by the adult population of the United States that they refer to, they amount to 1125 and 1375 deaths per annum from CVD and stroke, respectively. In addition, the association between prevalence of hypertension and PM2.5 remained significant after we took account of these confounding factors (β=0.18; 95% CI, 0.16–0.20; P<0.001; Table 2). The β values suggest that an increase in PM2.5 air pollution by 5 μg/m3 was associated with a 0.9% increase in the prevalence of hypertension.
In the crude model, there was a positive association between ozone (mean, 0.073 ppm) levels and CVD mortality (Table 2). The β values suggested that increases in the levels of ozone air pollution by 0.01 ppm (a 13% increase relative to mean levels) would be associated with increases in the mortality from CVD by 13.7 people per 100 000 population (3.7% increase on mean levels). For hypertension prevalence, the β values indicate that an increase in ozone air pollution by 0.01 ppm was associated with a 0.25% increase in hypertension. After adjustment for poverty, ethnicity, and education, the association between CVD mortality and ozone remained (β=21.8; 95% CI, 15.7–28.0; P<0.001; Table 2). However, the link between hypertension prevalence and ozone was no longer significant (β=5.09; 95% CI, −2.4 to 12.4; P=0.183; Table 2). No significant association between ozone concentration and stroke mortality in either the crude or adjusted model was observed (P>0.101, Table 2). To determine the joint impact of PM2.5 and ozone, we included both of them as independent predictors in a multiple linear regression (collinearity checked). For CVD mortality (adjusted for poverty, ethnicity, and education), both ozone and PM2.5 were significant independent factors with no significant interaction (pooled R 2=0.111; for PM2.5: β=0.12; 95% CI, 0.085–0.156; P<0.001; for ozone: β=13.3; 95% CI, 6.7–19.4; P<0.001; P=0.117 for interaction; Table 3).
Table 3.
Details of the Analysis for Multiple Regression Models
| Models | Equation | t Value | P Value | Collinearity Test (VIF) | R 2 | |||
|---|---|---|---|---|---|---|---|---|
| 1 | Crude | CVD mortality vs ozone and PM2.5 | Ozone | y=404X 1+13.7X 2+143.0 | 1.1 | 0.240 | 0.87 | 0.143 |
| PM2.5 | 9.0 | <0.001 | 0.87 | |||||
| Interaction | −3.7 | <0.001 | 0.98 | |||||
| 2 | Stroke mortality vs ozone and PM2.5 | Ozone | y=−92.3X 1+2.29X 2+46.1 | −2.0 | 0.045 | 0.87 | 0.131 | |
| PM2.5 | 9.5 | <0.001 | 0.87 | |||||
| Interaction | −3.2 | <0.001 | 0.98 | |||||
| 3 | Hypertension prevalence vs ozone and PM2.5 | Ozone | y=−0.25X 1+0.007X 2+0.314 | −2.4 | 0.016 | 0.87 | 0.153 | |
| PM2.5 | 10.4 | <0.001 | 0.87 | |||||
| Interaction | −4.3 | <0.001 | 0.98 | |||||
| 4 | Adjusted | CVD mortality vs ozone and PM2.5 | Ozone | y=13.7X 1+0.123X 2−2.43 | 3.9 | <0.001 | 0.87 | 0.111 |
| PM2.5 | 6.6 | <0.001 | 0.87 | |||||
| Interaction | −1.5 | 0.117 | 0.98 | |||||
| 5 | Stroke mortality vs ozone and PM2.5 | Ozone | y=−2.44X 1+0.135X 2−1.57 | −0.8 | 0.379 | 0.87 | 0.071 | |
| PM2.5 | 6.9 | <0.001 | 0.87 | |||||
| Interaction | −2.2 | 0.026 | 0.98 | |||||
| 6 | Hypertension prevalence vs ozone and PM2.5 | Ozone | y=−6.85X 1+0.172X 2−1.73 | −1.9 | 0.054 | 0.87 | 0.084 | |
| PM2.5 | 7.7 | <0.001 | 0.87 | |||||
| Interaction | −3.3 | 0.018 | 0.98 | |||||
Multiple linear regressions conducted. Adjusted model is corrected for poverty, ethnicity, and education. X 1=ozone concentration (ppm), X 2=PM2.5 concentration (μg/m3), CVD, and stroke mortality (per 100 000 population). CVD indicates cardiovascular disease; PM2.5, particulate matter with diameters <2.5 μm; and VIF, variance inflation factor.
We also determined the relationships between mortality from CVD and stroke at different prevalences of obesity (Figure 1A through 1D). In the crude model for CVD mortality, when the concentration of PM2.5 changed from low (mean−SD, 10.2 μg/m3) to high (mean+SD, 13.6 μg/m3), the rate of mortality in the low obesity prevalence areas (prevalence, 26.2%) changed from 309.7 to 325.8 deaths per 100 000 individuals per annum (an increase of 16.1). However, at the high prevalence of obesity (35.3%), it changed from 383.7 to 430.4 deaths per 100 000 individuals per year (an increase of 46.7 deaths per annum), suggesting obesity strongly modulates the impact of PM2.5 on CVD mortality (Figure 1A). This impact was robust even after adjustment for poverty, ethnicity, and education. As the concentration of PM2.5 changed from low to high, in low obesity prevalence regions, CVD mortality changed from 332.1 to 353.3 deaths (per 100 000 individuals per annum; 21.1 increase), and it changed from 369.0 to 401.1 deaths (per 100 000 individuals per annum; 32.1 increase) for counties with a high obesity rate (Figure 1B). In the same analysis for stroke mortality, when the concentration of PM2.5 changed from low (10.2 μg/m3) to high (13.6 μg/m3), rate of the mortality in the low obesity category (26.2%) changed from 70.9 to 74.6 deaths per 100 000 individuals per annum (an increase of 3.7); however, at the high prevalence of obesity (35.3%), it increased from 82.3 to 90.8 deaths per 100 000 individuals per annum (an increase of 8.5). Hence, obesity prevalence also had a strong modulating effect on the impact of PM2.5 on stroke mortality (Figure 1C). Once we adjusted the model for poverty, ethnicity, and education, we found for stroke mortality, when the concentration of PM2.5 changed from low to high rate, the mortality in the low obesity category changed from 74.7 to 78.8 deaths per 100 000 individuals per annum (an increase of 4.1); however, at the high prevalence of obesity, it increased from 80.4 to 85.7 deaths per 100 000 individuals per annum (an increase of 5.3). In Figure 2A and 2B, 3‐dimensional plots illustrating the impact of obesity on the link between CVD and stroke mortality with PM2.5 are presented.
Figure 1.
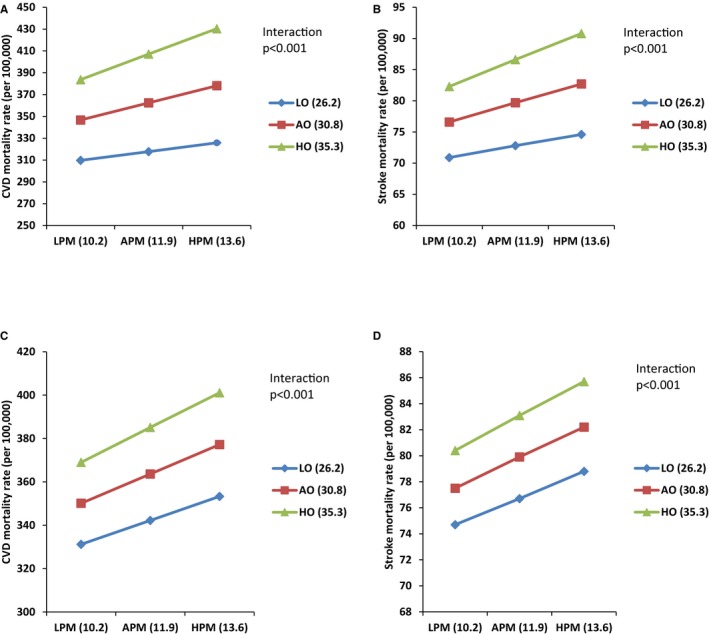
Impact of the variable level of obesity prevalence on the link between particulate matter with diameters <2.5 μm (PM 2.5) and cardiovascular disease (CVD) and stroke mortality in crude and adjusted models. A, Crude model of impact of different level of obesity on the association between CVD mortality and PM 2.5 level (μg/m3). B, Adjusted model (ethnicity, poverty, and education) of impact of different level of obesity on the association between CVD mortality and PM 2.5 level. C, Crude model of impact of different level of obesity on the association between stroke mortality and PM 2.5 level. D, Adjusted model (ethnicity, poverty, and education) of impact of different level of obesity on the association between stroke mortality and PM 2.5 level. AO indicates average obesity; APM, average PM 2.5; HO, high obesity; HPM, high PM 2.5; LO, low obesity; and LPM, low PM 2.5.
Figure 2.
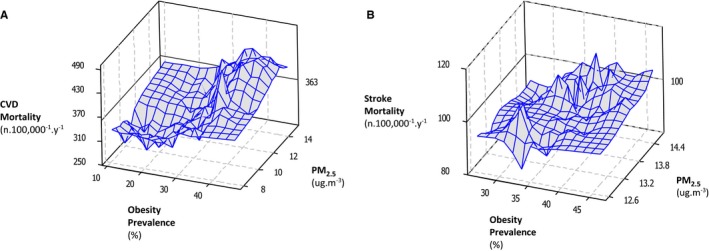
Plots (3 dimensional) of the association between particulate matter with diameters <2.5 μm (PM2.5) and obesity with either cardiovascular disease (CVD) or stroke mortality. A, Association between PM 2.5 and obesity with CVD mortality. B, Association between PM 2.5 and obesity with stroke mortality.
In the mediation analysis, we used 2 separate models for mortality from CVD and stroke (adjusted for poverty, education, and ethnicity). This analysis suggested that hypertension partially mediates the association between PM2.5 and CVD mortality (action theory, 0.0029; conceptual theory, 1204.4; direct effect, 5.24; total effect, 8.7; proportion of mediation, 40.1%; all P<0.001) and stroke mortality (action theory, 0.0029; conceptual theory, 195.9; direct effect, 0.92; total effect, 1.4; proportion of mediation, 38.2%; all P<0.001).
Discussion
In the current study, we aimed to evaluate the spatial association between several disease outcomes, specifically mortality from CVD and stroke, and the prevalence of hypertension, and the long‐term exposure levels of PM2.5 and ozone. Using this large nationwide survey population (>3100 US counties representing >170 million individuals), we found that the counties with higher levels of air pollution (PM2.5) had greater risk of mortality from CVD and stroke; also, individuals living in these areas were more likely to have hypertension. These associations were much reduced in effect size, but remained significant after adjustment for potential confounding factors, including poverty, ethnicity, and education. We have also found the link between ozone and CVD mortality in both the crude and adjusted model; however, no association remained between hypertension prevalence and ozone levels, once we adjusted the model for poverty, ethnicity, and education. In a multiple pollutant model, PM2.5 and ozone were independent risk factors for CVD mortality. Moreover, in areas with higher rates of obesity, PM2.5 had a more deleterious impact on both CVD and stroke mortality. This effect was robust after adjustment for potential confounders. Using counterfactual mediation analysis, we showed that the association between PM2.5 and both CVD and stroke mortality was partially mediated by the association between PM2.5 and hypertension. These data confirm the 2009 scientific statement of the American Heart Association on PM and CVD.5
Cardiovascular Disease
The present results were in line with other studies that have suggested the level of long‐term exposure to PM2.5 is associated with higher risk of CVD.5, 13, 20, 21, 48, 49, 50, 51, 52, 53 For example, using a much smaller sample of 106 US counties, a positive and significant association was observed between long‐term county‐specific estimates of PM2.5 and cardiovascular hospitalizations.49 Another study using data from 6 US cities (n=8111 adults) concluded that long‐term air pollution contributes to excess mortality.54 Several time series studies, specifically the National Morbidity, Mortality and Air Pollution Study (long‐term) and the Air Pollution and Health: A European Approach (short‐term), have shown that temporal variations in PM2.5 were significantly associated with daily all‐cause and CVD death.55, 56
In contrast, a large cohort study (22 European cohort studies; total population, 367 383 participants, with 9994 deaths from CVD) failed to find any association between long‐term PM2.5 exposure and CVD death.13 The authors reported that this negative finding could be attributable to relying on data from mortality registries; there may be coding differences in death certificates among countries and among ESCAPE areas. Moreover, they applied land‐use regression models used for exposure assessment, which were based on air pollution measurements from 2008 to 2011, whereas the cohort studies included in ESCAPE started much earlier (1985–2007, with most studies starting in the mid‐1990s).13 In another study (combining 22 European cohort studies), the total study population consisted of 367 251 participants who contributed 5 118 039 person‐years at risk (average follow‐up, 13.9 years), of whom 29 076 died from natural causes during follow‐up.15 Despite this large sample, they failed to find any association between long‐term PM2.5 exposure and CVD death.15 Again, a negative result in this study could be because of the discrepancy in timing between the data collection for disease risk and pollution measures. Data collection for some of these large European cohorts began in 1985 before air pollution monitoring networks were widely established; air pollution was measured between 2008 and 2011. The authors extrapolated exposures for each cohort back to the start of the study period and, therefore, exposure misclassification might have occurred. Pooled individual patient data were not available in this analysis, which limited the power of the study to assess heterogeneity and susceptibility across different risk groups and regions.15
Some plausible pathophysiological mechanisms have been suggested for the harmful effects of PM2.5 on health. Both animal and human experiments have reported that exposure to PM2.5 may cause inflammation of the respiratory system; in particular, exposure to PM2.5 may result in elevated systemic inflammation and an increased risk of cardiovascular stress.57, 58, 59, 60 It has been suggested that, in humans, exposure to air pollution can lead to increased levels of interleukin 6 and can increase the production of C‐reactive protein, which is known as an independent risk factor for CVD.61 Each 100‐μg/m3 increase in PM2.5 was suggested to increase the C‐reactive protein by 8.1 mg/L.62 Human studies have also reported microRNAs, which are involved in the processes of systemic inflammation, endothelial dysfunction, and atherosclerosis, might be stimulated by PM2.5.63 Exposing mice to PM2.5 (143.8 μg/m3) for 6 hours showed that there was elevated gene expression of inflammation‐related genes, such as tumor necrosis factor‐α, tumor necrosis factor‐β, interleukin 6, and interleukin 8.64 It is well established that increased levels of proinflammatory cytokines and proinflammatory mediators (tumor necrosis factor‐α, tumor necrosis factor‐β, interleukin 6, and interleukin 8) are closely related to increased blood coagulability, leading to endothelial dysfunction.59, 65
We found there was a strong modulating effect of the level of obesity on the relationship between CVD and PM2.5. Similarly, a cross‐sectional study of Chinese adults (n=24 845, aged 18–74 years) reported that being overweight and obese may enhance the effects of air pollution on the prevalence of CVDs and stroke in northeastern metropolitan China.30 Furthermore, in another study between adults in eastern Massachusetts, it has been reported that there are greater autonomic cardiac responses to PM2.5 in obese workers, supporting the hypothesis that obesity may impart greater susceptibility to acute cardiovascular effects of fine particles.66 A prevalence of obesity 1 SD above the average resulted in the impact of PM2.5 being 3× greater than in areas where the prevalence of obesity was 1 SD below average. In the adjusted models, 1 SD higher than the mean prevalence of obesity resulted in higher rate of CVD mortality rate (in same level of PM2.5). The mechanism for this association remains uncertain at present, but because obesity is a low‐grade inflammatory state, this may interact with the inflammatory effects of PM2.5 previously detailed above (see more detailed discussion later under stroke). More physiological studies on the different responses of obese and lean individuals exposed to different levels of PM2.5 are required to better understand this effect. Consistent with Brook et al,5 we found no evidence of any nonlinearity in the response, suggesting no lower safe limit for long‐term exposure.
Stroke
In accord with several previous studies, people living in the areas with higher levels of the PM2.5 had elevated risk of stroke mortality. Previous studies reported that a 10‐μg/m3 increase in short‐term PM2.5 concentration may be associated with a 1% to 5% increase in the likelihood of stroke.21, 23, 67 In review articles, it has been proposed that increased inflammation, vascular reactivity, and endothelial dysfunction might be reasons for the underlying impact of PM2.5 on stroke.64, 68 The Dijon Stroke Register in France reported that long‐term PM2.5 exposure was most strongly associated with strokes caused by cardiometabolic risk factors, which indicated an indirect impact of the PM2.5 on stroke incidence.24
An earlier study has suggested that even in developed countries, where annual mean air pollution concentrations meet current international standards, small (5‐μg/m3) increases in long‐term PM2.5 concentration were linked with a 19% increase in the risk of stroke.69 Our results on long‐term PM2.5 exposure for comparison suggested that each 5‐μg/m3 increase in PM2.5 leads to ≈52% increase in likelihood of stroke (adjusted for poverty, education, and ethnicity) and, therefore, support these previous data. In another study, it has been suggested that short‐term increases of 3.0 μg/m3 in PM2.5 levels are associated with changes in cerebrovascular hemodynamics, including increased cerebrovascular resistance and reduced cerebral blood flow.70
We found there was a strong modulating effect of the level of obesity on the relationship between stroke and PM2.5. A prevalence of obesity 1 SD above the average resulted in the impact of PM2.5 being 3× greater than in areas where the prevalence of obesity was 1 SD below average. In the adjusted models, 1 SD higher than means for obesity resulted in higher rate of stroke mortality rate (in same level of PM2.5). A handful of previous studies have addressed the impact of obesity on the association between PM2.5 and CVD and stroke. For example, 2 cohort (long‐term exposure) studies in women have shown that a greater BMI enhances the susceptibility for PM‐induced CVD mortality10, 71; however, they have indicated that their findings need to be confirmed by other studies.10, 71 Another study concerned the impact of short‐term air pollution exposure on C‐reactive protein, as an independent risk factor for CVD; the authors have reported that the associations between air pollution exposure and C‐reactive protein were strongest and most consistent for the 14 individuals with obesity.72 Another study aimed to evaluate whether and to what extent obesity can affect relationships between long‐term PM2.5 exposure and BP. The authors have reported positive associations between PM2.5 exposure and BP; however, they suggested that it was more visible and consistent among those who were obese.73 Possibly, obese subjects have higher level of oxidative stress and reactive oxygen species production,74 which may lead to CVD and stroke mortality.75 Another possible pathway includes the association of obesity with established CVD risk factors (eg, metabolic syndrome, enhanced insulin resistance, and hyperglycemia) that are cumulatively damaging to the endothelium,76 and risk factors as potential modulators of the endothelial phenotype in obesity, including oxidative stress74 and chronic inflammation.77, 78 In this case, our findings suggested that in areas with higher prevalence of obesity, the population may be at increased likelihood of CVD and stroke morbidity associated with ambient air pollution.
Hypertension and Mediation of the CVD and Stroke Effects
We found that there was an association between higher concentrations of the PM2.5 and greater risk of hypertension. There are relatively few previous studies in the United States,79, 80, 81, 82, 83, 84, 85, 86, 87 and in these studies, there was consensus that BP increased after exposure (both long‐ and short‐term) to PM2.5.79, 80, 81, 82, 83, 84, 85, 86, 87 However, most of these studies have low sample size (n<500),79, 80, 81, 82, 83, 84, 86, 87 with just 1 study on a population of >5000 individuals with short‐term exposure.85 In another study, it was reported that short‐term PM2.5 exposure leads to no changes in BP.88 However, it has been suggested that the lack of statistical significance of the BP elevation in this study was most probably explained by an inadequate sample size and, hence, low power.88
In an incidence study of 3236 black women in Los Angeles, CA, it was reported that when long‐term PM2.5 exposure was analyzed separately, the incidence rate ratio for hypertension for a 10‐μg/m3 increase was 1.48 (95% CI, 0.95–2.31).89 Results from the MESA (Multi‐Ethnic Study of Atherosclerosis) showed that systolic BP was positively associated with short‐term PM2.5 exposure. A 10‐mg/m3 increase in PM2.5 for 30 days was associated with 1.12–mm Hg higher pulse pressure and 0.99–mm Hg higher systolic BP.85 A recent meta‐analysis that included 22 studies suggested that BP was positively related to PM2.5 exposure, with an elevation of 1.39 mm Hg (95% CI, 0.874–1.912 mm Hg) and 0.89 mm Hg (95% CI, 0.49–1.299 mm Hg) per 10‐μg/m3 increase for systolic BP and diastolic BP, respectively.90 A small study of normotensive, nonsmoking, healthy adults (n=23) suggested that short‐term exposure to PM2.5 significantly increased the diastolic BP.91
In the current study, the link between CVD and stroke mortality and long‐term PM2.5 level was affected by hypertension. In this regard, 2 links in the pathway are important. First, there is an association between hypertension and mortality from CVD and stroke, which is well known.92, 93 Second, there is an association between PM2.5 concentration and hypertension, which was previously reviewed. Several possible biological mechanisms could be responsible for the association between airborne PM and BP. Studies revealed that PM2.5 may lead to elevated production of reactive oxygen species, resulting in oxidative stress and systemic inflammation, which can result in damage to NO‐dependent vascular dilation and increasing vasoconstrictor in ex vivo and in vivo experiments.5, 94, 95, 96 As another possible biological mechanism, there is positive association between endothelin‐1, a putative potent endogenous vasoconstrictor that causes vascular endothelial dysfunction, and PM2.5.97, 98, 99 However, it is suggested that the main and most prominent route for impact of PM2.5 on vascular function is via downregulation of NO production.100 Exposure to PM2.5 can prevent endogenous NO synthesis.100 Decreased bioavailability of NO not only can lead to elevated BP but also increases the instability of BP, which itself is considered a risk factor for CVD.100 Systemic inflammation and vascular dysfunction might explain the longer cumulative PM2.5 effects on BP. Moreover, the autonomic nervous system is involved in the regulation of BP, and the activation of the sympathetic nervous system plays an important role in the upregulation of BP.
Impact of Ozone
In line with some previous studies, we found that ozone is a likely risk factor for CVD. For example, a short‐term increase in ozone was associated with acute coronary events in middle‐aged adults with no preexisting CVD.101 Moreover, in another study, short‐term exposure to ozone was associated with out‐of‐hospital cardiac arrest.102 In the case of stroke, some studies reported an increased risk of stroke after a short‐term increase in ozone,103 whereas some investigations did not find any association,104 which is in line with the long‐term (absence of) effects in our study. Moreover, a recent meta‐analysis and systematic review reported no evidence of associations between long‐term annual ozone concentrations and the risk of death from CVD.29 In another study that used data from the study cohort of the American Cancer Society CPS II (Cancer Prevention Study II), it was reported that ozone and PM2.5 single‐pollutant models were associated with CVD, whereas in contrast with our findings, where significant independent effects persisted, once PM2.5 and ozone were included in a multiple regression model, the correlation with ozone disappeared.105 However, the authors reported high collinearity between the concentrations of ozone and PM2.5, and they stated that the effects of ozone could, therefore, be confounded by the presence of PM2.5 because of this collinearity.105 In another study, short‐term ozone exposure was linked to BP.106 Moreover, 2 previous studies found that ozone exposure was negatively (beneficially) or not associated with BP,80, 107 but a recent study has reported a positive association between ozone and both systolic and diastolic BP measurements at slightly higher exposure concentrations.108
Limitations and Strengths
The findings of the present study should be considered in the context of some clear limitations. First, the results were based on a cross‐sectional ecological study, and although it is nationally representative, and well powered, this type of design cannot demonstrate causality in the associations. We took account of what we considered were likely to be the most important confounding factors. However, there may be other factors that are also important that we did not account for, such as physical activity levels, which may in theory be depressed at high levels of air pollution. However, a study performed on Han Chinese, living in Beijing at high levels of air pollution, found no link between PM2.5 levels and objectively measured physical activity levels,109 perhaps indicating physical activity is not normally depressed by air pollution, particularly at the much lower levels involved in the current study. Another limitation is that we had no data on other sources of air pollution, such as PM with diameters <10 μm, NO, and sulfur dioxide levels, which may covary with PM2.5 levels.5 Hence, any one of these additional sources of pollution may be the ultimate causal factor. Although the sources of data that we applied for current study were all independent government institutions, some of the data are potentially prone to error (eg, the measures of obesity [BMI] were based on self‐reported heights and weights). To end this, we recommend in the future study to take account of sex and age for analysis.
Although our analysis was not based on individual data, we had a large sample, which included individuals selected by “disproportionate stratified sampling,” and, therefore, the results obtained from these nationally representative samples can be extrapolated to the general population. Moreover, the Behavioral Risk Factor Surveillance System is largest study of its kind in United States and covers a comprehensive geographic area. Furthermore, 1 of the reported weaknesses of previous studies was the criteria and procedures used to measure the PM2.5. However, in the current study, the data on PM2.5 originated from US National Aeronautics and Space Administration, which has highly validated and accurate database.42 The data sources we used as covariates were all ultimately derived from well‐established and respected US government departments, including the US Census.
Conclusion
Spatial variation in long‐term exposure to PM2.5 across the United States was associated with increased risk of both CVD and stroke mortality. Ozone was an independent risk factor for CVD. The effect of PM2.5 on CVD and stroke was substantially modulated by the level of obesity, being 3× higher for CVD and 2× higher for stroke, in areas with high compared with low obesity levels. Moreover, areas with increased PM2.5 concentrations had populations with a markedly higher level of hypertension. Our analysis suggested that hypertension has an indirect effect on the link between CVD and stroke mortality with PM2.5.
Author Contributions
Mazidi: Collection, analysis, and interpretation of data and cowriting of article. Speakman: Study conception and design, cowriting of article, and interpretation of data.
Sources of Funding
During this work, Speakman was supported by a 1000 Talents Professorship of the Chinese Government and a Wolfson Merit Professorship by the Royal Society. Mazidi was supported by The Third World Academy of Sciences Presidential Studentship and Chinese Academy of Sciences. These funders took no part in the design, implementation, analysis, and interpretation of the data.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e008006 DOI: 10.1161/JAHA.117.008006.)29848499
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker‐Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez‐Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez‐Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui‐Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. US EPA . Integrated Science Assessment (ISA) for Particulate Matter (Final Report). Washington, DC: DUSEPA; 2009. [Google Scholar]
- 5. Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez‐Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 6. Forman HJ, Finch CE. A critical review of assays for hazardous components of air pollution. Free Radic Biol Med. 2018;117:202–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker‐Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan‐Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez‐Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez‐Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stockl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens. 2009;3:332–350. [DOI] [PubMed] [Google Scholar]
- 9. Byrd JB, Morishita M, Bard RL, Das R, Wang L, Sun Z, Spino C, Harkema J, Dvonch JT, Rajagopalan S, Brook RD. Acute increase in blood pressure during inhalation of coarse particulate matter air pollution from an urban location. J Am Soc Hypertens. 2016;10:133–139.e134. [DOI] [PubMed] [Google Scholar]
- 10. Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long‐term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. [DOI] [PubMed] [Google Scholar]
- 11. Barclay JL, Miller BG, Dick S, Dennekamp M, Ford I, Hillis GS, Ayres JG, Seaton A. A panel study of air pollution in subjects with heart failure: negative results in treated patients. Occup Environ Med. 2009;66:325–334. [DOI] [PubMed] [Google Scholar]
- 12. O'Donnell MJ, Fang J, Mittleman MA, Kapral MK, Wellenius GA. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology. 2011;22:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beelen R, Stafoggia M, Raaschou‐Nielsen O, Andersen ZJ, Xun WW, Katsouyanni K, Dimakopoulou K, Brunekreef B, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Houthuijs D, Nieuwenhuijsen M, Oudin A, Forsberg B, Olsson D, Salomaa V, Lanki T, Yli‐Tuomi T, Oftedal B, Aamodt G, Nafstad P, De Faire U, Pedersen NL, Ostenson CG, Fratiglioni L, Penell J, Korek M, Pyko A, Eriksen KT, Tjonneland A, Becker T, Eeftens M, Bots M, Meliefste K, Wang M, Bueno‐de‐Mesquita B, Sugiri D, Kramer U, Heinrich J, de Hoogh K, Key T, Peters A, Cyrys J, Concin H, Nagel G, Ineichen A, Schaffner E, Probst‐Hensch N, Dratva J, Ducret‐Stich R, Vilier A, Clavel‐Chapelon F, Stempfelet M, Grioni S, Krogh V, Tsai MY, Marcon A, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Katsoulis M, Trichopoulou A, Vineis P, Hoek G. Long‐term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts. Epidemiology. 2014;25:368–378. [DOI] [PubMed] [Google Scholar]
- 14. Willocks LJ, Bhaskar A, Ramsay CN, Lee D, Brewster DH, Fischbacher CM, Chalmers J, Morris G, Scott EM. Cardiovascular disease and air pollution in Scotland: no association or insufficient data and study design? BMC Public Health. 2012;12:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beelen R, Raaschou‐Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer P, Nieuwenhuijsen M, Vineis P, Xun WW, Katsouyanni K, Dimakopoulou K, Oudin A, Forsberg B, Modig L, Havulinna AS, Lanki T, Turunen A, Oftedal B, Nystad W, Nafstad P, De Faire U, Pedersen NL, Ostenson CG, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Overvad K, Ellermann T, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno‐de‐Mesquita B, Sugiri D, Kramer U, Heinrich J, de Hoogh K, Key T, Peters A, Hampel R, Concin H, Nagel G, Ineichen A, Schaffner E, Probst‐Hensch N, Kunzli N, Schindler C, Schikowski T, Adam M, Phuleria H, Vilier A, Clavel‐Chapelon F, Declercq C, Grioni S, Krogh V, Tsai MY, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Katsoulis M, Trichopoulou A, Brunekreef B, Hoek G. Effects of long‐term exposure to air pollution on natural‐cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383:785–795. [DOI] [PubMed] [Google Scholar]
- 16. Andersen ZJ, Olsen TS, Andersen KK, Loft S, Ketzel M, Raaschou‐Nielsen O. Association between short‐term exposure to ultrafine particles and hospital admissions for stroke in Copenhagen, Denmark. Eur Heart J. 2010;31:2034–2040. [DOI] [PubMed] [Google Scholar]
- 17. Mateen FJ, Brook RD. Air pollution as an emerging global risk factor for stroke. JAMA. 2011;305:1240–1241. [DOI] [PubMed] [Google Scholar]
- 18. Oudin A, Stromberg U, Jakobsson K, Stroh E, Bjork J. Estimation of short‐term effects of air pollution on stroke hospital admissions in southern Sweden. Neuroepidemiology. 2010;34:131–142. [DOI] [PubMed] [Google Scholar]
- 19. Hong YC, Lee JT, Kim H, Kwon HJ. Air pollution: a new risk factor in ischemic stroke mortality. Stroke. 2002;33:2165–2169. [DOI] [PubMed] [Google Scholar]
- 20. Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among Medicare beneficiaries. Stroke. 2005;36:2549–2553. [DOI] [PubMed] [Google Scholar]
- 21. Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Villeneuve PJ, Chen L, Stieb D, Rowe BH. Associations between outdoor air pollution and emergency department visits for stroke in Edmonton, Canada. Eur J Epidemiol. 2006;21:689–700. [DOI] [PubMed] [Google Scholar]
- 23. Lisabeth LD, Escobar JD, Dvonch JT, Sanchez BN, Majersik JJ, Brown DL, Smith MA, Morgenstern LB. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol. 2008;64:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henrotin JB, Besancenot JP, Bejot Y, Giroud M. Short‐term effects of ozone air pollution on ischaemic stroke occurrence: a case‐crossover analysis from a 10‐year population‐based study in Dijon, France. Occup Environ Med. 2007;64:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larrieu S, Jusot JF, Blanchard M, Prouvost H, Declercq C, Fabre P, Pascal L, Tertre AL, Wagner V, Riviere S, Chardon B, Borrelli D, Cassadou S, Eilstein D, Lefranc A. Short term effects of air pollution on hospitalizations for cardiovascular diseases in eight French cities: the PSAS program. Sci Total Environ. 2007;387:105–112. [DOI] [PubMed] [Google Scholar]
- 26. Shin HH, Fann N, Burnett RT, Cohen A, Hubbell BJ. Outdoor fine particles and nonfatal strokes: systematic review and meta‐analysis. Epidemiology. 2014;25:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Eliot MN, Wellenius GA. Short‐term changes in ambient particulate matter and risk of stroke: a systematic review and meta‐analysis. J Am Heart Assoc. 2014;3:e000983 DOI: 10.1161/JAHA.114.000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shah AS, Lee KK, McAllister DA, Hunter A, Nair H, Whiteley W, Langrish JP, Newby DE, Mills NL. Short term exposure to air pollution and stroke: systematic review and meta‐analysis. BMJ. 2015;350:h1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Atkinson RW, Butland BK, Dimitroulopoulou C, Heal MR, Stedman JR, Carslaw N, Jarvis D, Heaviside C, Vardoulakis S, Walton H, Anderson HR. Long‐term exposure to ambient ozone and mortality: a quantitative systematic review and meta‐analysis of evidence from cohort studies. BMJ Open. 2016;6:e009493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Qin XD, Qian Z, Vaughn MG, Trevathan E, Emo B, Paul G, Ren WH, Hao YT, Dong GH. Gender‐specific differences of interaction between obesity and air pollution on stroke and cardiovascular diseases in Chinese adults from a high pollution range area: a large population based cross sectional study. Sci Total Environ. 2015;529:243–248. [DOI] [PubMed] [Google Scholar]
- 31. Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42:1511–1519. [DOI] [PubMed] [Google Scholar]
- 32. Robins JM, Greenland S. Identifiability and exchangeability for direct and indirect effects. Epidemiology. 1992;3:143–155. [DOI] [PubMed] [Google Scholar]
- 33. Mazidi M, Speakman JR. Higher densities of fast‐food and full‐service restaurants are not associated with obesity prevalence. Am J Clin Nutr. 2017;106:603–613. [DOI] [PubMed] [Google Scholar]
- 34. Speakman JR, Heidari‐Bakavoli S. Type 2 diabetes, but not obesity, prevalence is positively associated with ambient temperature. Sci Rep. 2016;6:30409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. World Health Organization . Obesity: Preventing and Managing a Global Epidemic. Geneva, Switzerland: WHO; 1997. [Google Scholar]
- 36. Rowland ML. Self‐reported weight and height. Am J Clin Nutr. 1990;52:1125–1133. [DOI] [PubMed] [Google Scholar]
- 37. Villanueva EV. The validity of self‐reported weight in US adults: a population based cross‐sectional study. BMC Public Health. 2001;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self‐reported height and weight in 4808 EPIC‐Oxford participants. Public Health Nutr. 2002;5:561–565. [DOI] [PubMed] [Google Scholar]
- 39. Dekkers JC, van Wier MF, Hendriksen IJ, Twisk JW, van Mechelen W. Accuracy of self‐reported body weight, height and waist circumference in a Dutch overweight working population. BMC Med Res Methodol. 2008;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michimi A, Wimberly MC. Associations of supermarket accessibility with obesity and fruit and vegetable consumption in the conterminous United States. Int J Health Geogr. 2010;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mazidi M, Speakman JR. Higher densities of fast‐food and full‐service restaurants are not associated with obesity prevalence. Am J Clin Nutr. 2017;106:603–613. [DOI] [PubMed] [Google Scholar]
- 42. Mazidi M, Speakman JR. Ambient particulate air pollution (PM2.5) is associated with the ratio of type 2 diabetes to obesity. Sci Rep. 2017;7:9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Slinker BK, Glantz SA. Multiple regression for physiological data analysis: the problem of multicollinearity. Am J Physiol. 1985;249:R1–R12. [DOI] [PubMed] [Google Scholar]
- 44. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. [DOI] [PubMed] [Google Scholar]
- 45. VanderWeele TJ. Mediation and mechanism. Eur J Epidemiol. 2009;24:217–224. [DOI] [PubMed] [Google Scholar]
- 46. VanderWeele TJ. A three‐way decomposition of a total effect into direct, indirect, and interactive effects. Epidemiology. 2013;24:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lippmann M, Ito K, Hwang JS, Maciejczyk P, Chen LC. Cardiovascular effects of nickel in ambient air. Environ Health Perspect. 2006;114:1662–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bell ML, Ebisu K, Peng RD, Samet JM, Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med. 2009;179:1115–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dominici F, Peng RD, Barr CD, Bell ML. Protecting human health from air pollution: shifting from a single‐pollutant to a multipollutant approach. Epidemiology. 2010;21:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Johnston FH, Hanigan IC, Henderson SB, Morgan GG. Evaluation of interventions to reduce air pollution from biomass smoke on mortality in Launceston, Australia: retrospective analysis of daily mortality, 1994–2007. BMJ. 2013;346:e8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kunzli N, Jerrett M, Garcia‐Esteban R, Basagana X, Beckermann B, Gilliland F, Medina M, Peters J, Hodis HN, Mack WJ. Ambient air pollution and the progression of atherosclerosis in adults. PLoS One. 2010;5:e9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hoffmann B, Moebus S, Mohlenkamp S, Stang A, Lehmann N, Dragano N, Schmermund A, Memmesheimer M, Mann K, Erbel R, Jockel KH. Residential exposure to traffic is associated with coronary atherosclerosis. Circulation. 2007;116:489–496. [DOI] [PubMed] [Google Scholar]
- 54. Dockery DW, Pope CA III, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–1759. [DOI] [PubMed] [Google Scholar]
- 55. Stylianou M, Nicolich MJ. Cumulative effects and threshold levels in air pollution mortality: data analysis of nine large US cities using the NMMAPS dataset. Environ Pollut. 2009;157:2216–2223. [DOI] [PubMed] [Google Scholar]
- 56. Samoli E, Analitis A, Touloumi G, Schwartz J, Anderson HR, Sunyer J, Bisanti L, Zmirou D, Vonk JM, Pekkanen J, Goodman P, Paldy A, Schindler C, Katsouyanni K. Estimating the exposure‐response relationships between particulate matter and mortality within the APHEA multicity project. Environ Health Perspect. 2005;113:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Quay JL, Reed W, Samet J, Devlin RB. Air pollution particles induce IL‐6 gene expression in human airway epithelial cells via NF‐kappaB activation. Am J Respir Cell Mol Biol. 1998;19:98–106. [DOI] [PubMed] [Google Scholar]
- 58. Veronesi B, Oortgiesen M, Carter JD, Devlin RB. Particulate matter initiates inflammatory cytokine release by activation of capsaicin and acid receptors in a human bronchial epithelial cell line. Toxicol Appl Pharmacol. 1999;154:106–115. [DOI] [PubMed] [Google Scholar]
- 59. van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med. 2001;164:826–830. [DOI] [PubMed] [Google Scholar]
- 60. Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T, Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. [DOI] [PubMed] [Google Scholar]
- 61. Ruckerl R, Greven S, Ljungman P, Aalto P, Antoniades C, Bellander T, Berglind N, Chrysohoou C, Forastiere F, Jacquemin B, von Klot S, Koenig W, Kuchenhoff H, Lanki T, Pekkanen J, Perucci CA, Schneider A, Sunyer J, Peters A. Air pollution and inflammation (interleukin‐6, C‐reactive protein, fibrinogen) in myocardial infarction survivors. Environ Health Perspect. 2007;115:1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pope CA III, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, Eatough DJ. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fossati S, Baccarelli A, Zanobetti A, Hoxha M, Vokonas PS, Wright RO, Schwartz J. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology. 2014;25:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martinelli N, Olivieri O, Girelli D. Air particulate matter and cardiovascular disease: a narrative review. Eur J Intern Med. 2013;24:295–302. [DOI] [PubMed] [Google Scholar]
- 65. Meier R, Cascio WE, Ghio AJ, Wild P, Danuser B, Riediker M. Associations of short‐term particle and noise exposures with markers of cardiovascular and respiratory health among highway maintenance workers. Environ Health Perspect. 2014;122:726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen JC, Cavallari JM, Stone PH, Christiani DC. Obesity is a modifier of autonomic cardiac responses to fine metal particulates. Environ Health Perspect. 2007;115:1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chan CC, Chuang KJ, Chien LC, Chen WJ, Chang WT. Urban air pollution and emergency admissions for cerebrovascular diseases in Taipei, Taiwan. Eur Heart J. 2006;27:1238–1244. [DOI] [PubMed] [Google Scholar]
- 68. O'Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton ES, Schwartz J. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med. 2007;64:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stafoggia M, Cesaroni G, Peters A, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, Cyrys J, de Faire U, de Hoogh K, Eriksen KT, Fratiglioni L, Galassi C, Gigante B, Havulinna AS, Hennig F, Hilding A, Hoek G, Hoffmann B, Houthuijs D, Korek M, Lanki T, Leander K, Magnusson PK, Meisinger C, Migliore E, Overvad K, Ostenson CG, Pedersen NL, Pekkanen J, Penell J, Pershagen G, Pundt N, Pyko A, Raaschou‐Nielsen O, Ranzi A, Ricceri F, Sacerdote C, Swart WJ, Turunen AW, Vineis P, Weimar C, Weinmayr G, Wolf K, Brunekreef B, Forastiere F. Long‐term exposure to ambient air pollution and incidence of cerebrovascular events: results from 11 European cohorts within the ESCAPE project. Environ Health Perspect. 2014;122:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wellenius GA, Boyle LD, Wilker EH, Sorond FA, Coull BA, Koutrakis P, Mittleman MA, Lipsitz LA. Ambient fine particulate matter alters cerebral hemodynamics in the elderly. Stroke. 2013;44:1532–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Puett RC, Schwartz J, Hart JE, Yanosky JD, Speizer FE, Suh H, Paciorek CJ, Neas LM, Laden F. Chronic particulate exposure, mortality, and coronary heart disease in the Nurses' Health Study. Am J Epidemiol. 2008;168:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kannan S, Dvonch JT, Schulz AJ, Israel BA, Mentz G, House J, Max P, Reyes AG. Exposure to fine particulate matter and acute effects on blood pressure: effect modification by measures of obesity and location. J Epidemiol Community Health. 2010;64:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, Chade AR, Lerman LO, Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol. 2007;292:H904–H911. [DOI] [PubMed] [Google Scholar]
- 75. Sugamura K, Keaney JF Jr. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Meyers MR, Gokce N. Endothelial dysfunction in obesity: etiological role in atherosclerosis. Curr Opin Endocrinol Diabetes Obes. 2007;14:365–369. [DOI] [PubMed] [Google Scholar]
- 77. Ferrante AW Jr. Obesity‐induced inflammation: a metabolic dialogue in the language of inflammation. J Intern Med. 2007;262:408–414. [DOI] [PubMed] [Google Scholar]
- 78. Yatsuya H. Pathophysiologic mechanisms of obesity and related metabolic disorders: an epidemiologic study using questionnaire and serologic biomarkers. J Epidemiol. 2007;17:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rich DQ, Zareba W, Beckett W, Hopke PK, Oakes D, Frampton MW, Bisognano J, Chalupa D, Bausch J, O'Shea K, Wang Y, Utell MJ. Are ambient ultrafine, accumulation mode, and fine particles associated with adverse cardiac responses in patients undergoing cardiac rehabilitation? Environ Health Perspect. 2012;120:1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hoffmann B, Luttmann‐Gibson H, Cohen A, Zanobetti A, de Souza C, Foley C, Suh HH, Coull BA, Schwartz J, Mittleman M, Stone P, Horton E, Gold DR. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ Health Perspect. 2012;120:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brook RD, Bard RL, Burnett RT, Shin HH, Vette A, Croghan C, Phillips M, Rodes C, Thornburg J, Williams R. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med. 2011;68:224–230. [DOI] [PubMed] [Google Scholar]
- 82. Mordukhovich I, Wilker E, Suh H, Wright R, Sparrow D, Vokonas PS, Schwartz J. Black carbon exposure, oxidative stress genes, and blood pressure in a repeated‐measures study. Environ Health Perspect. 2009;117:1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Delfino RJ, Tjoa T, Gillen DL, Staimer N, Polidori A, Arhami M, Jamner L, Sioutas C, Longhurst J. Traffic‐related air pollution and blood pressure in elderly subjects with coronary artery disease. Epidemiology. 2010;21:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mar TF, Koenig JQ, Jansen K, Sullivan J, Kaufman J, Trenga CA, Siahpush SH, Liu LJ, Neas L. Fine particulate air pollution and cardiorespiratory effects in the elderly. Epidemiology. 2005;16:681–687. [DOI] [PubMed] [Google Scholar]
- 85. Auchincloss AH, Diez Roux AV, Dvonch JT, Brown PL, Barr RG, Daviglus ML, Goff DC, Kaufman JD, O'Neill MS. Associations between recent exposure to ambient fine particulate matter and blood pressure in the Multi‐Ethnic Study of Atherosclerosis (MESA). Environ Health Perspect. 2008;116:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dvonch JT, Kannan S, Schulz AJ, Keeler GJ, Mentz G, House J, Benjamin A, Max P, Bard RL, Brook RD. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;53:853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zanobetti A, Canner MJ, Stone PH, Schwartz J, Sher D, Eagan‐Bengston E, Gates KA, Hartley LH, Suh H, Gold DR. Ambient pollution and blood pressure in cardiac rehabilitation patients. Circulation. 2004;110:2184–2189. [DOI] [PubMed] [Google Scholar]
- 88. Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. [DOI] [PubMed] [Google Scholar]
- 89. Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in Los Angeles. Circulation. 2012;125:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liang R, Zhang B, Zhao X, Ruan Y, Lian H, Fan Z. Effect of exposure to PM2.5 on blood pressure: a systematic review and meta‐analysis. J Hypertens. 2014;32:2130–2140; discussion 2141. [DOI] [PubMed] [Google Scholar]
- 91. Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environ Health Perspect. 2005;113:1052–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Franklin SS, Wong ND. Hypertension and cardiovascular disease: contributions of the Framingham Heart Study. Glob Heart. 2013;8:49–57. [DOI] [PubMed] [Google Scholar]
- 93. Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–1059. [DOI] [PubMed] [Google Scholar]
- 94. Courtois A, Prouillac C, Baudrimont I, Ohayon‐Courtes C, Freund‐Michel V, Dubois M, Lisbonne‐Autissier M, Marthan R, Savineau JP, Muller B. Characterization of the components of urban particulate matter mediating impairment of nitric oxide‐dependent relaxation in intrapulmonary arteries. J Appl Toxicol. 2014;34:667–674. [DOI] [PubMed] [Google Scholar]
- 95. Bouthillier L, Vincent R, Goegan P, Adamson IY, Bjarnason S, Stewart M, Guenette J, Potvin M, Kumarathasan P. Acute effects of inhaled urban particles and ozone: lung morphology, macrophage activity, and plasma endothelin‐1. Am J Pathol. 1998;153:1873–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Heagerty AM, Heerkens EH, Izzard AS. Small artery structure and function in hypertension. J Cell Mol Med. 2010;14:1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Calderon‐Garciduenas L, Villarreal‐Calderon R, Valencia‐Salazar G, Henriquez‐Roldan C, Gutierrez‐Castrellon P, Torres‐Jardon R, Osnaya‐Brizuela N, Romero L, Torres‐Jardon R, Solt A, Reed W. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal Toxicol. 2008;20:499–506. [DOI] [PubMed] [Google Scholar]
- 98. Brook RD. Cardiovascular effects of air pollution. Clin Sci (Lond). 2008;115:175–187. [DOI] [PubMed] [Google Scholar]
- 99. Haynes WG, Ferro CJ, O'Kane KP, Somerville D, Lomax CC, Webb DJ. Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation. 1996;93:1860–1870. [DOI] [PubMed] [Google Scholar]
- 100. Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. [DOI] [PubMed] [Google Scholar]
- 101. Ruidavets JB, Cournot M, Cassadou S, Giroux M, Meybeck M, Ferrieres J. Ozone air pollution is associated with acute myocardial infarction. Circulation. 2005;111:563–569. [DOI] [PubMed] [Google Scholar]
- 102. Raza A, Bellander T, Bero‐Bedada G, Dahlquist M, Hollenberg J, Jonsson M, Lind T, Rosenqvist M, Svensson L, Ljungman PL. Short‐term effects of air pollution on out‐of‐hospital cardiac arrest in Stockholm. Eur Heart J. 2014;35:861–868. [DOI] [PubMed] [Google Scholar]
- 103. Henrotin JB, Zeller M, Lorgis L, Cottin Y, Giroud M, Bejot Y. Evidence of the role of short‐term exposure to ozone on ischaemic cerebral and cardiac events: the Dijon Vascular Project (DIVA). Heart. 2010;96:1990–1996. [DOI] [PubMed] [Google Scholar]
- 104. Yorifuji T, Suzuki E, Kashima S. Cardiovascular emergency hospital visits and hourly changes in air pollution. Stroke. 2014;45:1264–1268. [DOI] [PubMed] [Google Scholar]
- 105. Jerrett M, Burnett RT, Pope CA III, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long‐term ozone exposure and mortality. N Engl J Med. 2009;360:1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Day DB, Xiang J, Mo J, Li F, Chung M, Gong J, Weschler CJ, Ohman‐Strickland PA, Sundell J, Weng W, Zhang Y, Zhang JJ. Association of ozone exposure with cardiorespiratory pathophysiologic mechanisms in healthy adults. JAMA Intern Med. 2017;177:1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold DR, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Weichenthal S, Hatzopoulou M, Goldberg MS. Exposure to traffic‐related air pollution during physical activity and acute changes in blood pressure, autonomic and micro‐vascular function in women: a cross‐over study. Part Fibre Toxicol. 2014;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang G, Li B, Zhang X, Niu C, Li J, Li L, Speakman JR. No seasonal variation in physical activity of Han Chinese living in Beijing. Int J Behav Nutr Phys Act. 2017;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]


