Abstract
Background
Midregional proadrenomedullin (MR‐proADM) has demonstrated prognostic potential after myocardial infarction (MI). Yet, the prognostic value of MR‐proADM at admission has not been examined in patients with ST‐segment–elevation MI (STEMI).
Methods and Results
The aim of this substudy, DANAMI‐3 (The Danish Study of Optimal Acute Treatment of Patients with ST‐segment–elevation myocardial infarction), was to examine the associations of admission concentrations of MR‐proADM with short‐ and long‐term mortality and hospital admission for heart failure in patients with ST‐segment–elevation myocardial infarction. Outcomes were assessed using Cox proportional hazard models and area under the curve using receiver operating characteristics. In total, 1122 patients were included. The median concentration of MR‐proADM was 0.64 nmol/L (25th–75th percentiles, 0.53–0.79). Within 30 days 23 patients (2.0%) died and during a 3‐year follow‐up 80 (7.1%) died and 38 (3.4%) were admitted for heart failure. A doubling of MR‐proADM was, in adjusted models, associated with an increased risk of 30‐day mortality (hazard ratio, 2.67; 95% confidence interval, 1.01–7.11; P=0.049), long‐term mortality (hazard ratio, 3.23; 95% confidence interval, 1.97–5.29; P<0.0001), and heart failure (hazard ratio, 2.71; 95% confidence interval, 1.32–5.58; P=0.007). For 30‐day and 3‐year mortality, the area under the curve for MR‐proADM was 0.77 and 0.78, respectively. For 3‐year mortality, area under the curve (0.84) of the adjusted model marginally changed (0.85; P=0.02) after addition of MR‐proADM.
Conclusions
Elevation of admission MR‐proADM was associated with long‐term mortality and heart failure, whereas the association with short‐term mortality was borderline significant. MR‐proADM may be a marker of prognosis after ST‐segment–elevation myocardial infarction but does not seem to add substantial prognostic information to established clinical models.
Clinical Trial Registration
URL: http://www.ClinicalTrials.gov/. Unique identifiers: NCT01435408 and NCT01960933.
Keywords: biomarker, midregional proadrenomedullin, myocardial infarction, prognosis, ST‐segment–elevation myocardial infarction
Subject Categories: Myocardial Infarction, Prognosis, Biomarkers
Clinical Perspective
What Is New?
This study demonstrates that elevation of midregional proadrenomedullin from blood samples taken immediately at presentation to the hospital is independently associated with increased risk of short‐ and long‐term all‐cause mortality, short‐ and long‐term cardiovascular mortality, and hospital admission for heart failure in patients with ST‐segment–elevation myocardial infarction.
This indicates that the adrenomedullin system is activated after ST‐segment–elevation myocardial infarction and that midregional proadrenomedullin measured in the early phase after an ST‐segment–elevation myocardial infarction may be a useful marker of prognosis.
What Are the Clinical Implications?
A single prognostic biomarker might be easier for physicians to take into account for early risk stratification instead of several clinical markers.
However, midregional proadrenomedullin only minimally changed the discriminatory ability of a multivariable clinical model for long‐term all‐cause mortality, whereas the discriminatory ability for short‐term mortality was not significantly changed.
Future randomized clinical studies are needed to evaluate if an early risk strategy with admission concentrations of midregional proadrenomedullin, for example with a cut‐off concentration of 0.79 nmol/L, can improve clinical outcomes.
Myocardial infarction remains a major cause of mortality and morbidity worldwide.1 The current preventive strategies and recommendations of early reperfusion with primary percutaneous coronary intervention (PCI) have improved the outcomes in patients with ST‐segment–elevation myocardial infarction (STEMI).2, 3 However, mortality remains substantial and cardiovascular causes, including heart failure, remain the leading causes of mortality during the first month after STEMI.4, 5 Therefore, early assessment of risk is important for effective and alert management of patients, after reperfusion is achieved.
Adrenomedullin (ADM) is a 52‐amino‐acid peptide derived from a larger precursor termed pre‐proadrenomedullin (185 amino‐acid residues). It was originally isolated from human pheochromocytoma tissue and has subsequently been detected in several human tissues including the adrenal medulla, heart, lung, kidney, gastrointestinal organs, brain, vascular endothelium, and vascular smooth muscle cells.6, 7, 8 ADM causes potent vasodilation and hypotension, increases cardiac output, and induces diuresis and natriuresis;6, 9 elevated levels of ADM in myocardial infarction and in heart failure10, 11, 12, 13 have been shown to be associated with adverse outcomes.14, 15 Despite this, it has been technically difficult to introduce ADM as a biomarker in plasma because of instability in vivo.16, 17 This has been solved by developing an assay for measurement of the stable peptide fragment midregional proadrenomedullin (MR‐proADM). It is generated from post‐translational processing of pre‐proadrenomedullin and is secreted in equimolar amounts to ADM.18
The prognostic value of MR‐proADM concentrations at admission has not been examined in a large STEMI cohort prior to PCI. We examined the associations of MR‐proADM concentrations at admission with short‐ and long‐term mortality and hospital admission for heart failure in patients with STEMI undergoing PCI.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. All data are stored electronically at the Clinical Trial Unit at Rigshospitalet and can be available for other researchers by contacting members (Thomas Engstrøm or Lars Køber) of the DANAMI‐3 (The Danish Study of Optimal Acute Treatment of Patients with ST‐segment–elevation myocardial infarction) steering committee affiliated to Rigshospitalet.
Study Design and Patients
This study was a post hoc substudy of the DANAMI‐3 trial program. The rationale and design of the DANAMI‐3 trial program has previously been reported.19 In short, the program comprised three randomized controlled multicenter trials investigating the effect of: (1) ischemic post‐conditioning induced by repetitive interruptions of blood flow immediately after reopening versus conventional primary PCI, DANAMI‐3‐iPOST;20 (2) deferred stent implantation in the infarct‐related lesion vs conventional primary PCI with immediate stent implantation, DANAMI‐3‐DEFER;21 and (3) fractional flow reserve‐guided complete revascularization versus treatment of the culprit lesion only in patients with multivessel disease, DANAMI‐3‐PRIMULTI.22 The program included patients (aged >18 years) admitted to the hospital with chest pain of <12 hours duration and ST‐segment–elevation greater than 0.1 mV in at least 2 contiguous leads or documented newly developed left bundle‐branch block. Major exclusion criteria were the inability to provide consent because of unconsciousness or cardiogenic shock, stent thrombosis, or need of acute coronary artery bypass surgery. All patients included in this single center substudy were referred to Rigshospitalet, Copenhagen, (1 of the 4 primary PCI centers in DANAMI‐3) and had blood samples obtained at admission for MR‐proADM measurement.
Ethics
The Regional Ethics Committee in Copenhagen approved the study.19 The DANAMI‐3 trial program was conducted in compliance with the Helsinki II Declaration. Written informed consent was obtained from all participants.
Blood Sampling
All blood samples were obtained immediately upon arrival in the catheterization laboratory before PCI was initiated. In a subset of patients additionally 2 samples were collected with a median time of 5.7 hours (25th–75th percentiles, 5.2–6.6) and of 12.8 hours (25th–75th percentiles, 11.6–15.7) after presentation. Blood samples were collected into tubes containing EDTA. After centrifugation, plasma was stored at −80°C until later biochemical analyses.
Biochemical Analysis
MR‐proADM was measured using a chemiluminescence immunoassay (BRAHMS MR‐proADM KRYPTOR) on an automated platform (BRAHMS KRYPTOR compact PLUS, Thermo Fisher Scientific, Hennigsdorf, Germany).23 The lower limit of detection was 0.05 nmol/L. The functional assay sensitivity, defined as the MR‐proADM concentration with an interassay coefficient of variation of <20%, was 0.25 nmol/L. Intra‐assay and interassay coefficient of variation at different concentrations of MR‐proADM were as follows; between 0.2 and 0.5 nmol/L, ≤10% and ≤20%, respectively; and >6 nmol/L, <3.5% and ≤6%, respectively.
We measured high‐sensitivity cardiac troponin T (hs‐cTnT) using the Elecsys high Troponin T high‐sensitive assay (Cobas). The limit of detection was 5 ng/L and the 10% CV precision was 13 ng/L. The 99th percentile concentration in a healthy population is reported to be 14 ng/L.24 We measured creatinine and hemoglobin by routine methods. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.25
Outcomes
The primary outcome was long‐term all‐cause mortality. All‐cause mortality and cardiovascular mortality within 30 days, long‐term cardiovascular mortality, and hospital admission for heart failure were evaluated as secondary outcomes. All study outcomes were identified using the National Danish Heart Registry and were subsequently validated by an independent clinical event committee using hospital records. Deaths were classified as cardiovascular unless another cause was obvious, as determined by the clinical event committee. Hospital admission for heart failure was defined as development of heart failure after primary PCI, new hospital admission to an acute care facility of at least 6 hours with a worsening of existing heart failure requiring treatment, or extended index hospital admission because of worsening heart failure.
Statistical Analysis
Patients were grouped according to quartiles of MR‐proADM concentrations. Baseline characteristics are presented as numbers (percentages) for categorical variables and as medians with 25th to 75th percentiles for continuous variables. Baseline characteristics were compared between groups by use of the Kruskal–Wallis rank‐sum test for continuous variables and the Cochran‐Armitage, chi squared, or Fisher's exact test as appropriate for categorical variables.
Kaplan–Meier curves and log‐rank tests were used to compare unadjusted differences across baseline levels of MR‐proADM divided into quartiles. Hazard ratios (HR) were assessed by use of univariate and multivariable Cox proportional hazards models. Patients lost to follow‐up because of emigration were censored at date of emigration. Levels of MR‐proADM were transformed using a base 2‐logarithmic transformation, and therefore, the presented HRs refer to a doubling in level of MR‐proADM. Secondly, the two highest quartiles of MR‐proADM were compared individually with quartile 1 and 2 as a combined reference.
For each outcome event, two multivariable models were applied. Multivariable model 1 included adjustment for age and sex, whereas multivariable model 2 was adjusted for age, sex, time since onset of symptoms, left ventricular ejection fraction, heart rate, eGFR, angiographic thrombolysis in myocardial infarction (TIMI) flow before primary PCI, anterior myocardial infarction, log2‐transformed peak concentrations of hs‐cTnT, and medical history of following variables: diabetes mellitus, hypertension, history of smoking, previous myocardial infarction, previous stroke, and congestive heart failure.
Assumptions underlying the proportional hazard models (proportional hazards and linearity of continuous variables) were tested and found valid. We tested for interactions with MR‐proADM and age, sex, time since onset of symptoms, diabetes mellitus, heart rate, left ventricular ejection fraction, and hs‐cTnT on all outcomes and were reported if significant after adjustment for multiple testing. To assess whether MR‐proADM was influenced by treatment allocation, we tested for interactions with MR‐proADM and randomization in iPOST, DEFER, and PRIMULTI on all outcomes.
To assess the predictive effects of MR‐proADM on 30‐days and 3‐year all‐cause mortality, we calculated the area under the curve (AUC) of receiver operating characteristics and evaluated the effects of MR‐proADM in addition to peak concentrations of hs‐cTnT and to models with well‐established risk factors and clinical variables (same variables as in the multivariable Cox model 2) using the approach of DeLong et al.26 By use of the Youden index, cut‐off values for MR‐proADM were calculated for short‐ and long‐term mortality, respectively. Comparison between serial samples of MR‐proADM was performed with repeated measures analysis of variance.
All tests were 2‐sided, and a P value of <0.05 was considered to indicate statistical significance. All analyses were performed with SAS statistical software, version 9.4 (SAS Institute, Chapel Hill, North Carolina, USA).
Results
Baseline Characteristics
Between March 21, 2011, and February 2, 2014, a total number of 2239 patients were enrolled in the DANAMI‐3 trial at 4 different primary PCI centers. Blood samples for MR‐proADM measurements were collected between November 10, 2011, and February 2, 2014, at Rigshospitalet. In this period, a total of 1292 consecutive patients were enrolled in the DANAMI‐3 trial at Rigshospitalet and MR‐proADM was measured in 1122 patients (86.8%) at admission (Figure 1).
Figure 1.
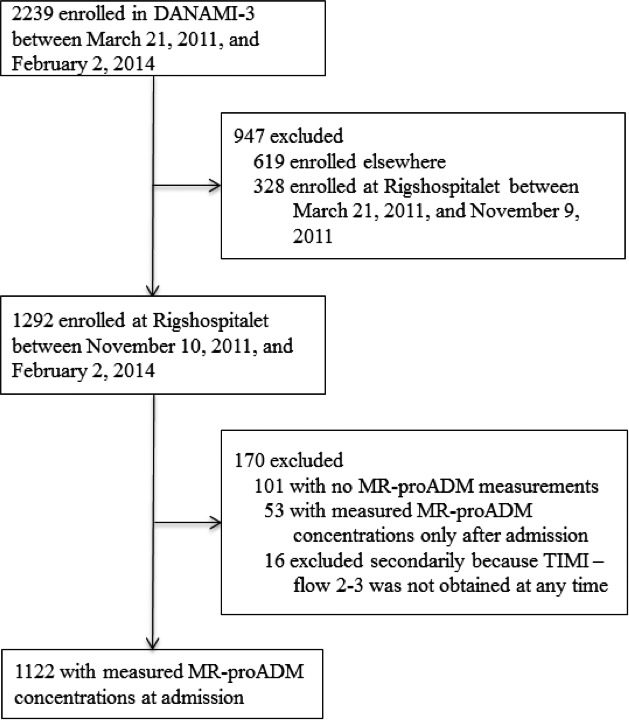
CONSORT study flow diagram. MR‐proADM indicates midregional proadrenomedullin; TIMI‐flow, angiographic thrombolysis in myocardial infarction flow.
The median interval from onset of symptoms until blood sampling was 2.8 hours (25th–75th percentiles, 2.1–4.4). MR‐proADM concentrations at admission did not differ considerably according to duration of symptoms (Figure S1). There were some differences in baseline characteristics between patients in this substudy and the remaining patients in DANAMI‐3: systolic and diastolic blood pressure, eGFR, TIMI‐flow before PCI, the prevalence of hyperlipidemia, and the prevalence of multivessel disease were significantly higher among patients with measured MR‐proADM, whereas left ventricular ejection fraction was significantly lower among these patients (Table S1).
Baseline characteristics of patients in this substudy, according to quartiles of MR‐proADM concentrations at admission, are shown in Table 1. Median age was 62 years (25th–75th percentiles, 53–70) and plasma concentrations of MR‐proADM ranged from 0.14 to 4.76 nmol/L with a median of 0.64 nmol/L. All quartiles contained more men than women, but the proportion of women increased with higher quartile level. The proportion of patients with diabetes mellitus, heart failure, and hypertension increased with higher quartile level, as did age, body‐mass index, and baseline and peak hs‐cTnT. In contrast, higher quartile level was associated with lower levels of left ventricular ejection fraction, hemoglobin, diastolic blood pressure, and eGFR.
Table 1.
Baseline Characteristics of the 1122 Patients With STEMI According to Quartiles of MR‐proADM at Admission
| All (N=1122) | MR‐pro‐ADM Quartiles (nmol/L) | P Value | ||||
|---|---|---|---|---|---|---|
| 1st ≤0.52 (n=279) | 2nd 0.53 to 0.64 (n=282) | 3rd 0.65 to 0.78 (n=281) | 4th ≥0.0.79 (n=280) | |||
| Male sex, n (%) | 848 (75.6) | 233 (83.5) | 219 (77.7) | 209 (74.4) | 187 (66.8) | <0.0001 |
| Age, y | 62 (53–70) | 54 (47–62) | 58 (52–66) | 65 (55–71) | 71 (62–80) | <0.0001 |
| Medical history | ||||||
| Previous myocardial infarction, n (%) | 73 (6.5) | 14 (5.0) | 15 (15.3) | 22 (7.8) | 22 (7.9) | 0.09 |
| Congestive heart failure, n (%) | 47 (4.2) | 7 (2.5) | 5 (1.8) | 13 (4.6) | 22 (7.9) | 0.0004 |
| Previous stroke, n (%) | 49 (4.4) | 9 (3.2) | 9 (3.2) | 15 (5.3) | 16 (5.7) | 0.08 |
| Diabetes mellitus, n (%) | 110 (9.8) | 22 (7.9) | 24 (8.5) | 25 (8.9) | 39 (13.9) | 0.02 |
| Hypertension, n (%) | 450 (40.1) | 82 (29.4) | 100 (35.6) | 115 (40.9) | 153 (54.6) | <0.0001 |
| Hyperlipidemia, n (%) | 393 (35.0) | 94 (33.7) | 107 (37.9) | 93 (33.1) | 99 (35.4) | 1.0 |
| History of smoking, n (%) | 897 (80.0) | 215 (77.1) | 226 (80.1) | 230 (81.9) | 226 (80.7) | 0.24 |
| Body‐mass index, kg/m2 | 26.6 (24.2–29.6) | 26.5 (24.2–28.7) | 26.0 (23.8–29.2) | 26.6 (24.5–30.4) | 27.1 (24.4–30.2) | 0.03 |
| Multivessel disease, n (%) | 428 (38.2) | 105 (37.6) | 111 (39.4) | 97 (34.5) | 115 (41.1) | 0.67 |
| TIMI‐flow before PCI, n (%) | 0.50 | |||||
| 0 | 572 (51.0) | 145 (52.0) | 131 (46.5) | 147 (52.3) | 149 (53.2) | |
| 1 | 95 (8.5) | 24 (8.6) | 31 (11.0) | 20 (7.1) | 20 (7.1) | |
| 2 | 199 (17.7) | 49 (17.6) | 45 (16.0) | 55 (19.6) | 50 (17.9) | |
| 3 | 256 (22.8) | 61 (21.9) | 75 (26.6) | 59 (21.0) | 61 (21.8) | |
| Left ventricular ejection fraction, % | 50 (40–55) | 50 (45–55) | 50 (40–55) | 50 (40–55) | 45 (35–50) | <0.0001 |
| Clinical data at admission | ||||||
| Heart rate, bpm | 73 (61–87) | 70 (60–84) | 72.5 (61–87) | 74 (64–84) | 75 (59–92) | 0.06 |
| Systolic BP, mm Hg | 133 (118–149) | 133 (122–149) | 131 (116–149) | 134 (119–148) | 131 (115–151) | 0.29 |
| Diastolic BP, mm Hg | 82 (72–93) | 84 (76–94) | 82 (72–97) | 84 (72–93.5) | 80 (70–91) | 0.003 |
| Infarct location | 0.07 | |||||
| Anterior, n (%) | 478 (42.6) | 105 (37.6) | 139 (49.3) | 123 (43.8) | 111 (39.6) | |
| Inferior, n (%) | 595 (26.7) | 162 (58.1) | 130 (46.1) | 142 (50.5) | 161 (57.5) | |
| Posterior, n (%) | 47 (4.2) | 12 (4.3) | 12 (4.3) | 16 (5.7) | 7 (2.5) | |
| Left bundle branch block, n (%) | 2 (<1.0) | 0 (0) | 1 (<1.0) | 0 (0) | 1 (<1.0) | |
| Laboratory values | ||||||
| Baseline hs‐cTnT, ng/L | 71 (27–190) | 55 (23–146) | 68 (25–186) | 72 (29–155) | 100 (34–353) | <0.0001 |
| Peak hs‐cTnT, ng/L | 2945 (1160–6270) | 2625 (1130–5120) | 2730 (1030–6070) | 3340 (1155–6970) | 3485 (1330–7490) | 0.02 |
| eGFR, mL/min per 1.73 m2 | 91 (76–100) | 100 (93–108) | 95 (85–103) | 89 (77–97) | 69 (53–86) | <0.0001 |
| Hemoglobin, mmol/L | 8.7 (8.1–9.2) | 8.8 (8.3–9.3) | 8.8 (8.2–9.2) | 8.7 (8.2–9.2) | 8.5 (7.7–9.0) | <0.0001 |
Data are presented as median values (25th–75th percentiles) for continuous variables and as numbers (percentages) for categorical variables. Unless indicated otherwise, the laboratory values are from blood samples obtained at admission. BP indicates blood pressure; bpm, beats per minute; eGFR, estimated glomerular filtration rate; hs‐cTnT, high‐sensitivity cardiac troponin T; MR‐proADM, midregional proadrenomedullin; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; TIMI‐flow, angiographic thrombolysis in myocardial infarction flow.
Survival Analysis
Median follow‐up was 1105 days (25th–75th percentiles, 917–1281) among survivors. Five patients emigrated 65 to 510 days after inclusion. Within 30 days 23 (2.1%) patients died, all from cardiovascular causes. During follow‐up, 80 (7.1%) deaths occurred of which 45 (4.0%) were from cardiovascular causes and 38 (3.4%) patients were admitted with heart failure. Of the 35 noncardiovascular deaths, 24 patients died from cancer, and 11 patients died from other causes. Kaplan–Meier survival analysis showed that the overall differences between quartiles of MR‐proADM concentrations were significant (P<0.0001, Figure 2). However, no significant difference was found between quartile 1 and 2 (P=0.27). The number of deaths from all causes increased with higher quartile level; 4 (1.4%) in the first quartile, 8 (2.8%) in the second quartile, 17 (6.1%) in the third quartile and 51 (18.2%) in the fourth quartile, as did the number of cardiovascular deaths; 4 (1.4%) in the first quartile, 4 (1.4%) in the second quartile, 8 (2.9%) in the third quartile and 29 (10.4%) in the fourth quartile.
Figure 2.
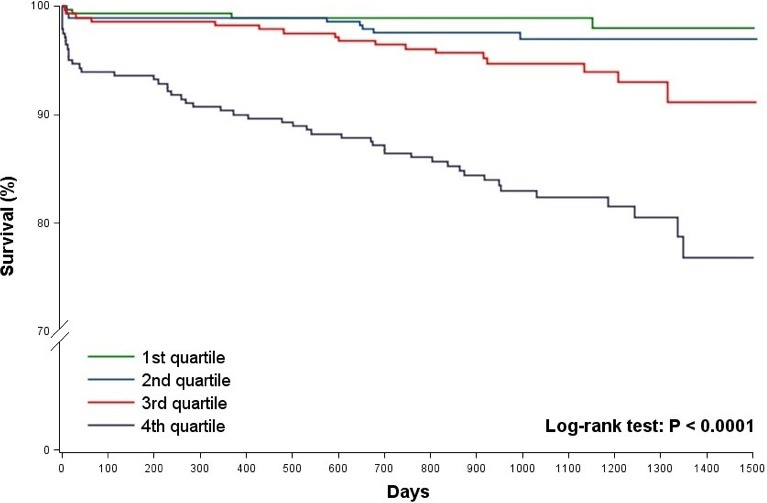
Overall survival among patients with STEMI, according to quartiles of admission MR‐proADM. The MR‐proADM levels were as follows: first quartile ≤0.52 nmol/L, second quartile 0.53 to 0.64 nmol/L, third quartile 0.65 to 0.78 nmol/L and fourth quartile ≥0.79 nmol/L. MR‐proADM indicates midregional proadrenomedullin; STEMI, ST‐segment–elevation myocardial infarction.
In univariate analysis, 30‐day mortality increased with higher plasma concentrations of MR‐proADM (HR, 3.93 per doubling; 95% confidence interval [CI], 2.51–6.14; P<0.0001) (Table 2). This association remained significant after adjusting for age and sex (HR, 3.14; 95% CI, 1.86–5.33; P<0.0001), and after additional adjustment in the multivariable model 2 (HR, 2.67; 95% CI, 1.01–7.11; P=0.049). These results were identical for 30‐day cardiovascular mortality, since all deaths within 30 days were from cardiovascular cause.
Table 2.
Associations Between Admission MR‐proADM and Prognosis
| Outcome | Univariate Model | Multivariable Model 1 | Multivariable Model 2 | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| 30‐day all‐cause mortality | 3.93 (2.51–6.14) | <0.0001 | 3.14 (1.86–5.33) | <0.0001 | 2.67 (1.01–7.11) | 0.049 |
| 30‐day cardiovascular mortality | 3.93 (2.51–6.14) | <0.0001 | 3.14 (1.86–5.33) | <0.0001 | 2.67 (1.01–7.11) | 0.049 |
| Long‐term all‐cause mortality | 3.43 (2.67–4.41) | <0.0001 | 2.59 (1.91–3.52) | <0.0001 | 3.23 (1.97–5.29) | <0.0001 |
| Long‐term cardiovascular mortality | 3.64 (2.63–5.04) | <0.0001 | 3.06 (2.10–4.45) | <0.0001 | 3.17 (1.56–6.42) | 0.002 |
| Hospital admission for heart failure | 3.45 (2.40–5.00) | <0.0001 | 3.17 (2.12–4.73) | <0.0001 | 2.71 (1.32–5.58) | 0.007 |
Model 1 was adjusted for age and sex. Model 2 was adjusted additionally for age, sex, time since onset of symptoms, left ventricular ejection fraction, heart rate, estimated glomerular filtration rate, TIMI‐flow before primary PCI, anterior myocardial infarction, log2‐transformed peak concentrations of hs‐cTnT, and medical history of the following variables: diabetes mellitus, hypertension, history of smoking, previous myocardial infarction, previous stroke, and congestive heart failure. CI indicates confidence interval; HR, hazard ratio; hs‐cTnT, high‐sensitivity cardiac troponin T; MR‐proADM, midregional proadrenomedullin; PCI, primary percutaneous coronary intervention; TIMI‐flow, angiographic thrombolysis in myocardial infarction flow.
For long‐term all‐cause mortality, univariate analysis showed that a doubling of MR‐proADM concentrations was associated with a 3.43 increase in HR (95% CI, 2.67–4.41; P<0.0001). After adjusting for age and sex, MR‐proADM was still associated with long‐term all‐cause mortality (HR, 2.59; 95% CI, 1.91–3.52; P<0.0001) and remained significant after additional multivariable adjustment (HR, 3.23; 95% CI, 1.97–5.29; P<0.0001). Besides elevated MR‐proADM concentrations, older age (HR, 2.44 per 10 year increase; 95% CI, 1.82–3.25), lower left ventricular ejection fraction (HR, 0.97 per 1% increase; 95% CI, 0.94–1.00), diabetes mellitus (HR, 2.28; 95% CI, 1.20–4.34), history of smoking (HR, 2.43; 95% CI, 1.21–4.90) and higher heart rate (HR, 1.18 per 10 beats per minute; 95% CI, 1.05–1.33) were significantly associated with higher risk of death in multivariable analysis.
In univariate Cox analysis with MR‐proADM as a categorical variable the HRs in quartile 3 and 4 were; 2.87 (95% CI, 1.37–6.01; P=0.005) and 9.16 (95% CI, 4.88–17.18; P<0.0001; see Figure 3) with the two first quartiles as a combined reference. This association remained significant in quartile 4 when adjusted for age and sex (HR, 4.70; 95% CI, 2.37–9.33; P<0.0001) and after additional multivariable adjustment (HR, 4.25; 95% CI, 1.98–9.15; P=0.0002).
Figure 3.
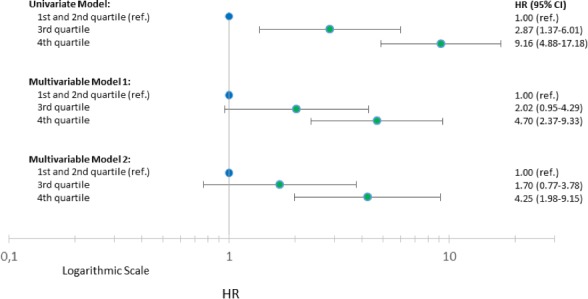
Univariate and multivariable cox analysis for all‐cause mortality according to quartiles of MR‐proADM among patients with STEMI. Model 1 was adjusted for age and sex. Model 2 was adjusted additionally for age, sex, time since onset of symptoms, left ventricular ejection fraction, heart rate, estimated glomerular filtration rate, TIMI‐flow before primary PCI, anterior myocardial infarction, log2‐transformed peak concentrations of hs‐cTnT, and medical history of following variables: diabetes mellitus, hypertension, history of smoking, previous myocardial infarction, previous stroke, and congestive heart failure. CI indicates confidence interval; HR, hazard ratio; hs‐cTnT, high‐sensitivity cardiac troponin T; MR‐proADM, midregional proadrenomedullin; PCI, primary percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; TIMI‐flow, angiographic thrombolysis in myocardial infarction flow.
Interaction analysis did not reveal any important interaction with randomization in DANAMI‐3‐DEFER, DANAMI‐3‐PRIMULTI or DANAMI‐3‐iPOST (data not shown).
For long‐term cardiovascular mortality and hospital admission for heart failure, analysis of elevated MR‐proADM concentrations in multivariable model 2 revealed HRs of 3.17 (95% CI, 1.56–6.42; P=0.002) and 2.71 (95% CI, 1.32–5.58; P=0.007), respectively (see Table 2).
Receiver Operating Characteristics Analysis
Receiver operating characteristics analysis of MR‐proADM on 30‐day mortality yielded an AUC of 0.77 (95% CI, 0.65–0.88; Table 3). AUC of peak concentrations of hs‐cTnT was 0.68 (95% CI 0.55–0.81) and improved significantly to 0.83 (95% CI, 0.73–0.92; P=0.03) after the addition of MR‐proADM, whereas hs‐cTnT did not significantly improve the discriminatory ability of MR‐proADM (P=0.07). For a multivariable model including the same variables as in the multivariable Cox model 2, the AUC was 0.91 (95% CI, 0.85–0.97) and did not change significantly with the inclusion of MR‐proADM (AUC 0.92, 95% CI, 0.86–0.98; P=0.23). For long‐term mortality, the AUC of MR‐proADM was 0.78 (95% CI, 0.73–0.84; Table 3), whereas the AUC of the multivariable model was 0.84 (95% CI, 0.78–0.90) and increased minimally but significantly to 0.85 (95% CI, 0.80–0.91; P=0.02) after the addition of MR‐proADM. Receiver operating characteristics analysis provided a cut‐off value of 0.87 nmol/L (65.2% sensitivity, 84.1% specificity) for short‐term mortality and a cut‐off value of 0.80 nmol/L (65.3% sensitivity, 78.4% specificity) for long‐term mortality.
Table 3.
Area Under the Curve of Receiver Operating Characteristics for 30‐Day and 3‐Year All‐Cause Mortality
| Outcome | Model | AUC | 95% CI | P Value for Testa |
|---|---|---|---|---|
| 30‐day mortality | MR‐proADM | 0.77 | 0.65 to 0.88 | ··· |
| Peak hs‐cTnT | 0.68 | 0.55 to 0.81 | ··· | |
| Peak hs‐cTnT+MR‐proADM | 0.83 | 0.73 to 0.92 | 0.03 | |
| Multivariable model | 0.91 | 0.85 to 0.97 | ··· | |
| Multivariable model+MR‐proADM | 0.92 | 0.86 to 0.98 | 0.23 | |
| 3‐year mortality | MR‐proADM | 0.78 | 0.73 to 0.84 | ··· |
| Peak hs‐cTnT | 0.59 | 0.51 to 0.66 | ··· | |
| Peak hs‐cTnT+MR‐proADM | 0.78 | 0.72 to 0.84 | <0.0001 | |
| Multivariable model | 0.84 | 0.78 to 0.90 | ··· | |
| Multivariable model+MR‐proADM | 0.85 | 0.80 to 0.91 | 0.02 |
AUC indicates area under the curve; CI, confidence interval; hs‐cTnT, high‐sensitivity cardiac troponin T; MR‐proADM, midregional proadrenomedullin.
Test for the effect of MR‐proADM in addition to peak hs‐cTnT and the multivariable models (same variables as in the multivariable Cox regression model 2).
Serial Measurements of MR‐proADM
Serial measurements with 3 samples of MR‐proADM were performed in 448 patients. Repeated measures analysis of variance showed that MR‐proADM concentrations increased significantly (P<0.0001) in this subgroup of patients with the following median levels for sample 1, 2, and 3; 0.64 nmol/L (25th–75th percentiles, 0.54–0.78), 0.73 nmol/L (0.62–0.92), and 0.76 nmol/L (0.63–0.94).
Discussion
Our study shows that MR‐proADM elevation at presentation to the hospital in patients with STEMI prior to PCI is independently associated with long‐term all‐cause mortality, long‐term cardiovascular mortality, and hospital admission for heart failure, whereas the association with short‐term all‐cause mortality and short‐term cardiovascular mortality was borderline significant. Furthermore, MR‐proADM concentrations seem relatively unchanged when compared with duration of symptoms after an STEMI, which contrast other biomarkers such as troponins and copeptin.27, 28, 29 This is the first study on MR‐proADM measurement at admission in a large STEMI cohort prior to PCI. Thus, these results add evidence to available information about the value of MR‐proADM as a possible marker of risk in patients presenting with acute chest pain,30, 31 acute dyspnea,32 stable angina pectoris,33 non‐ST‐segment–elevation myocardial infarction (NSTEMI)34 and mixed populations of acute coronary syndrome.33, 35, 36
Patients with plasma concentrations of 0.79 nmol/L or higher (upper quartile) were of particularly high risk with early separation of mortality. This threshold effect for MR‐proADM is supported by previous findings from studies on the prognostic value of ADM and MR‐proADM after acute myocardial infarction. Katayama et al reported that only a high level of plasma ADM in STEMI patients was independently associated with 30‐day mortality when measured 24 hours after onset of chest pain.15 The LAMP (Leicester Acute Myocardial Infarction Peptide) study showed that MR‐proADM was most informative with concentrations above the fourth quartile (1.04 nmol/L).35 The LAMP study included 983 patients with acute myocardial infarction, either STEMI or NSTEMI, with a median follow‐up of 342 days and with MR‐proADM concentrations measured at discharge (3–5 days after the event). They concluded that discharge concentrations of MR‐proADM added predictive information to N‐terminal pro‐B type natriuretic peptide (NT‐pro‐BNP). Our study differs from the LAMP study, as we examined admission concentrations of MR‐proADM in blood samples obtained immediately before primary PCI was performed in patients with STEMI.
Our reported AUC of 0.78 on long‐term mortality is consistent with that reported in the LAMP study (0.77) for the combined end point of death and heart failure.35 In a long‐term follow‐up study (median 3.6 years) of a subgroup of patients with acute coronary syndrome, Wild et al found an AUC of 0.64 on a combined end point of cardiovascular death and non‐fatal myocardial infarction after 2 years and reported a cut‐off value of 0.70 nmol/L.33 Tzikas et al identified patients at high risk in the upper tertile (0.71 nmol/L) of patients with acute chest pain.31 These cut‐off values are relatively similar to our cut‐off value for long‐term mortality (0.80 nmol/L) and for the upper quartile (≥0.79 nmol/L). Plasma concentrations in healthy individuals have been reported to be ≈0.4 nmol/L.37, 38
In this study, admission concentrations of MR‐proADM showed superior prognostic value for both short‐ and long‐term mortality compared with peak concentrations of hs‐cTnT and the discriminatory ability of hs‐cTnT improved with the addition of MR‐proADM. Investigators of the LAMP II study showed that admission MR‐proADM was a stronger predictor than discharge concentrations, and that it was the only independent predictor (superior to NT‐proBNP and the Global Registry of Acute Coronary Events [GRACE] risk score) of early mortality in a large cohort of patients with NSTEMI.34
The LAMP II study reported a considerably higher AUC (0.90) and a higher optimal cut‐off value (1.11 nmol/L) for short‐term mortality compared with our study (0.87 nmol/L). Possible reasons for this discrepancy are that we included younger patients, fewer women, and patients with better renal function, and that we excluded patients at high risk because of unconsciousness or cardiogenic shock, stent thrombosis, or need of urgent surgery. This, along with the fact that only a limited number of deaths occurred, may explain why we only found a borderline significant association between elevated concentrations of MR‐proADM and short‐term all‐cause mortality after multivariable adjustment.
In the LAMP II study, preliminary data suggested that MR‐proADM concentrations above the median (>0.81 nmol/L) could help to identify patients who would benefit from revascularization. We were not able to replicate such data as all patients had STEMI. However, this would be relevant to investigate in future studies.
As might be expected, cardiovascular death was the predominant cause of death within the first month (23 of 23 deaths).5 In full follow‐up, non‐cardiovascular causes of death were almost as frequent as cardiovascular causes of death (35 of 80 deaths). This suggests that the risk of cardiovascular events decreases over time, and therefore, risk assessment is particularly indicated in the early phase after an STEMI. However, non‐cardiovascular death is likely to be influenced by cardiovascular disease. ADM is secreted in response to multiple conditions including shear stress, increased cardiac work load, ischemia, hypoxia, acidosis, catecholamines, angiotensin II, vasopressin, and inflammatory damage by cytokines.9 Through vasodilator, diuretic, natriuretic, and inotropic properties, ADM may try to regulate blood pressure and cardiac contractility, and maintain water and electrolyte homeostasis.6, 9 Thus, increments of ADM in STEMI patients most likely reflect a counterbalanced response to ischemic burden and hemodynamic stress. Moreover, the level of ADM is increased in relation to the severity of heart failure.39
Plasma ADM concentrations seem to peak between 24 to 48 hours after onset of symptoms;10, 11 nevertheless, we showed that elevation of MR‐proADM in STEMI patients prior to PCI were independently associated with poor outcome. In our study, MR‐proADM at admission appeared stable according to duration of symptoms and was relatively stable in later serial measurements, though we observed a doubtful clinically relevant but significant increase of MR‐proADM; this contrasts levels of troponins which have delayed elevations of circulating levels27, 28 and levels of copeptin which seem to peak in the early phase after an acute myocardial infarction with a subsequently rapid decline.29
Study Limitations
This study was a single center, post hoc substudy. We observed minor differences in systolic and diastolic blood pressure, eGFR, TIMI‐flow before PCI and multivessel disease between patients in our substudy and the remaining DANAMI‐3 trial participants. The frozen samples were stored for up to 5 years before analysis of MR‐proADM, potentially leading to biomarker degradation, although this marker has previously been shown to be stable under these conditions.23
Conclusions
Elevated plasma concentrations of MR‐proADM at admission were associated with an increased risk of short‐ and long‐term all‐cause mortality, short‐ and long‐term cardiovascular mortality, and hospital admission for heart failure, in patients with STEMI, independent of established risk factors. In addition, patients with MR‐proADM concentrations of 0.79 nmol/L or higher were of particularly high risk, and MR‐proADM improved the discriminatory ability of peak hs‐cTnT for both short‐term and long‐term all‐cause mortality. These findings indicate that MR‐proADM, measured at admission, may be a marker of prognosis after STEMI. However, the association between elevated MR‐proADM and short‐term mortality only reached borderline significance after multivariable adjustment, and MR‐proADM did not add clinical relevant prognostic information to multivariable receiver operating characteristics analysis on either short‐ or long‐term all‐cause mortality.
Sources of Funding
This research is supported by the Danish Agency for Science, Technology and Innovation and by the Danish Council for Strategic Research (EDITORS: Eastern Denmark Initiative to Improve Revascularization Strategies, grant 09‐066994).
Disclosures
Dr Engstrøm has received speaker fees from St. Jude Medical and Boston Scientific; and has served on the advisory board of AstraZeneca and Bayer A/S. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Characteristics of Included and Excluded Patients
Figure S1. Levels of MR‐proADM according to duration of symptoms until baseline blood sampling.
Acknowledgments
Members of the DANAMI‐3 steering committee: Henning Kelbæk, Thomas Engstrøm, Lars Køber, Steffen Helqvist, Lene Holmvang, Dan E. Høfsten, and Lene Kløvgaard, Department of Cardiology, Rigshospitalet, University of Copenhagen, Denmark. Hans‐Henrik Tilsted, Department of Cardiology, Rigshospitalet, University of Copenhagen, Denmark, and Department of Cardiology, Aalborg University Hospital, Aalborg, Denmark. Hans Erik Bøtker, Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark. Lissette Okels Jensen, Department of Cardiology, Odense University Hospital, Odense, Denmark.
(J Am Heart Assoc. 2018;7:e008123 DOI: 10.1161/JAHA.117.008123.)29776961
The findings of this work were presented at the American College of Cardiology Annual Scientific Session & Expo, March 17 to 19, 2017, in Washington DC.
References
- 1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Rev Esp Cardiol (Engl ed). 2017;70:1082. [DOI] [PubMed] [Google Scholar]
- 2. Boersma E. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in‐hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J. 2006;27:779–788. [DOI] [PubMed] [Google Scholar]
- 3. Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. [DOI] [PubMed] [Google Scholar]
- 4. Kristensen SD, Laut KG, Fajadet J, Kaifoszova Z, Kala P, Di Mario C, Wijns W, Clemmensen P, Agladze V, Antoniades L, Alhabib KF, De Boer MJ, Claeys MJ, Deleanu D, Dudek D, Erglis A, Gilard M, Goktekin O, Guagliumi G, Gudnason T, Hansen KW, Huber K, James S, Janota T, Jennings S, Kajander O, Kanakakis J, Karamfiloff KK, Kedev S, Kornowski R, Ludman PF, Merkely B, Milicic D, Najafov R, Nicolini FA, Noc M, Ostojic M, Pereira H, Radovanovic D, Sabate M, Sobhy M, Sokolov M, Studencan M, Terzic I, Wahler S, Widimsky P. Reperfusion therapy for ST elevation acute myocardial infarction 2010/2011: current status in 37 ESC countries. Eur Heart J. 2014;35:1957–1970. [DOI] [PubMed] [Google Scholar]
- 5. Pedersen F, Butrymovich V, Kelbaek H, Wachtell K, Helqvist S, Kastrup J, Holmvang L, Clemmensen P, Engstrom T, Grande P, Saunamaki K, Jorgensen E. Short‐ and long‐term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol. 2014;64:2101–2108. [DOI] [PubMed] [Google Scholar]
- 6. Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. 1993. Biochem Biophys Res Commun. 2012;425:548–555. [DOI] [PubMed] [Google Scholar]
- 7. Ichiki Y, Kitamura K, Kangawa K, Kawamoto M, Matsuo H, Eto T. Distribution and characterization of immunoreactive adrenomedullin in human tissue and plasma. FEBS Lett. 1994;338:6–10. [DOI] [PubMed] [Google Scholar]
- 8. Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, Eto T, Matsuo H. Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994;201:1160–1166. [DOI] [PubMed] [Google Scholar]
- 9. Kitamura K, Kangawa K, Eto T. Adrenomedullin and PAMP: discovery, structures, and cardiovascular functions. Microsc Res Tech. 2002;57:3–13. [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi K, Kitamura K, Hirayama N, Date H, Kashiwagi T, Ikushima I, Hanada Y, Nagatomo Y, Takenaga M, Ishikawa T, Imamura T, Koiwaya Y, Eto T. Increased plasma adrenomedullin in acute myocardial infarction. Am Heart J. 1996;131:676–680. [DOI] [PubMed] [Google Scholar]
- 11. Miyao Y, Nishikimi T, Goto Y, Miyazaki S, Daikoku S, Morii I, Matsumoto T, Takishita S, Miyata A, Matsuo H, Kangawa K, Nonogi H. Increased plasma adrenomedullin levels in patients with acute myocardial infarction in proportion to the clinical severity. Heart. 1998;79:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kato J, Kobayashi K, Etoh T, Tanaka M, Kitamura K, Imamura T, Koiwaya Y, Kangawa K, Eto T. Plasma adrenomedullin concentration in patients with heart failure. J Clin Endocrinol Metab. 1996;81:180–183. [DOI] [PubMed] [Google Scholar]
- 13. Jougasaki M, Rodeheffer RJ, Redfield MM, Yamamoto K, Wei CM, McKinley LJ, Burnett JC Jr. Cardiac secretion of adrenomedullin in human heart failure. J Clin Invest. 1996;97:2370–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagaya N, Goto Y, Satoh T, Sumida H, Kojima S, Miyatake K, Kangawa K. Intravenous adrenomedullin in myocardial function and energy metabolism in patients after myocardial infarction. J Cardiovasc Pharmacol. 2002;39:754–760. [DOI] [PubMed] [Google Scholar]
- 15. Katayama T, Nakashima H, Furudono S, Honda Y, Suzuki S, Yano K. Evaluation of neurohumoral activation (adrenomedullin, BNP, catecholamines, etc.) in patients with acute myocardial infarction. Intern Med. 2004;43:1015–1022. [DOI] [PubMed] [Google Scholar]
- 16. Pio R, Martinez A, Unsworth EJ, Kowalak JA, Bengoechea JA, Zipfel PF, Elsasser TH, Cuttitta F. Complement factor H is a serum‐binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J Biol Chem. 2001;276:12292–12300. [DOI] [PubMed] [Google Scholar]
- 17. Lewis LK, Smith MW, Yandle TG, Richards AM, Nicholls MG. Adrenomedullin(1‐52) measured in human plasma by radioimmunoassay: plasma concentration, adsorption, and storage. Clin Chem. 1998;44:571–577. [PubMed] [Google Scholar]
- 18. Struck J, Tao C, Morgenthaler NG, Bergmann A. Identification of an adrenomedullin precursor fragment in plasma of sepsis patients. Peptides. 2004;25:1369–1372. [DOI] [PubMed] [Google Scholar]
- 19. Hofsten DE, Kelbaek H, Helqvist S, Klovgaard L, Holmvang L, Clemmensen P, Torp‐Pedersen C, Tilsted HH, Botker HE, Jensen LO, Kober L, Engstrom T. The Third DANish Study of Optimal Acute Treatment of Patients with ST‐segment Elevation Myocardial Infarction: ischemic postconditioning or deferred stent implantation versus conventional primary angioplasty and complete revascularization versus treatment of culprit lesion only: rationale and design of the DANAMI 3 trial program. Am Heart J. 2015;169:613–621. [DOI] [PubMed] [Google Scholar]
- 20. Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Clemmensen P, Holmvang L, Jorgensen E, Pedersen F, Saunamaki K, Ravkilde J, Tilsted HH, Villadsen A, Aaroe J, Jensen SE, Raungaard B, Botker HE, Terkelsen CJ, Maeng M, Kaltoft A, Krusell LR, Jensen LO, Veien KT, Kofoed KF, Torp‐Pedersen C, Kyhl K, Nepper‐Christensen L, Treiman M, Vejlstrup N, Ahtarovski K, Lonborg J, Kober L. Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST‐segment elevation myocardial infarction: a randomized clinical trial. JAMA Cardiol. 2017;2:490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kelbaek H, Hofsten DE, Kober L, Helqvist S, Klovgaard L, Holmvang L, Jorgensen E, Pedersen F, Saunamaki K, De Backer O, Bang LE, Kofoed KF, Lonborg J, Ahtarovski K, Vejlstrup N, Botker HE, Terkelsen CJ, Christiansen EH, Ravkilde J, Tilsted HH, Villadsen AB, Aaroe J, Jensen SE, Raungaard B, Jensen LO, Clemmensen P, Grande P, Madsen JK, Torp‐Pedersen C, Engstrom T. Deferred versus conventional stent implantation in patients with ST‐segment elevation myocardial infarction (DANAMI 3‐DEFER): an open‐label, randomised controlled trial. Lancet. 2016;387:2199–2206. [DOI] [PubMed] [Google Scholar]
- 22. Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Holmvang L, Jorgensen E, Pedersen F, Saunamaki K, Clemmensen P, De Backer O, Ravkilde J, Tilsted HH, Villadsen AB, Aaroe J, Jensen SE, Raungaard B, Kober L. Complete revascularisation versus treatment of the culprit lesion only in patients with ST‐segment elevation myocardial infarction and multivessel disease (DANAMI‐3‐PRIMULTI): an open‐label, randomised controlled trial. Lancet. 2015;386:665–671. [DOI] [PubMed] [Google Scholar]
- 23. Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51:1823–1829. [DOI] [PubMed] [Google Scholar]
- 24. Zhelev Z, Hyde C, Youngman E, Rogers M, Fleming S, Slade T, Coelho H, Jones‐Hughes T, Nikolaou V. Diagnostic accuracy of single baseline measurement of Elecsys Troponin T high‐sensitive assay for diagnosis of acute myocardial infarction in emergency department: systematic review and meta‐analysis. BMJ. 2015;350:h15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 27. Giannitsis E, Becker M, Kurz K, Hess G, Zdunek D, Katus HA. High‐sensitivity cardiac troponin T for early prediction of evolving non‐ST‐segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem. 2010;56:642–650. [DOI] [PubMed] [Google Scholar]
- 28. Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, Biedert S, Schaub N, Buerge C, Potocki M, Noveanu M, Breidthardt T, Twerenbold R, Winkler K, Bingisser R, Mueller C. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009;361:858–867. [DOI] [PubMed] [Google Scholar]
- 29. Reichlin T, Hochholzer W, Stelzig C, Laule K, Freidank H, Morgenthaler NG, Bergmann A, Potocki M, Noveanu M, Breidthardt T, Christ A, Boldanova T, Merki R, Schaub N, Bingisser R, Christ M, Mueller C. Incremental value of copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol. 2009;54:60–68. [DOI] [PubMed] [Google Scholar]
- 30. Haaf P, Twerenbold R, Reichlin T, Faoro J, Reiter M, Meune C, Steuer S, Bassetti S, Ziller R, Balmelli C, Campodarve I, Zellweger C, Kilchenmann A, Irfan A, Papassotiriou J, Drexler B, Mueller C. Mid‐regional pro‐adrenomedullin in the early evaluation of acute chest pain patients. Int J Cardiol. 2013;168:1048–1055. [DOI] [PubMed] [Google Scholar]
- 31. Tzikas S, Keller T, Ojeda FM, Zeller T, Wild PS, Lubos E, Kunde J, Baldus S, Bickel C, Lackner KJ, Munzel TF, Blankenberg S. MR‐proANP and MR‐proADM for risk stratification of patients with acute chest pain. Heart. 2013;99:388–395. [DOI] [PubMed] [Google Scholar]
- 32. Maisel A, Mueller C, Nowak R, Peacock WF, Landsberg JW, Ponikowski P, Mockel M, Hogan C, Wu AH, Richards M, Clopton P, Filippatos GS, Di Somma S, Anand I, Ng L, Daniels LB, Neath SX, Christenson R, Potocki M, McCord J, Terracciano G, Kremastinos D, Hartmann O, von Haehling S, Bergmann A, Morgenthaler NG, Anker SD. Mid‐region pro‐hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2010;55:2062–2076. [DOI] [PubMed] [Google Scholar]
- 33. Wild PS, Schnabel RB, Lubos E, Zeller T, Sinning CR, Keller T, Tzikas S, Lackner KJ, Peetz D, Rupprecht HJ, Bickel C, Morgenthaler NG, Papassotiriou J, Tiret L, Munzel T, Blankenberg S. Midregional proadrenomedullin for prediction of cardiovascular events in coronary artery disease: results from the AtheroGene study. Clin Chem. 2012;58:226–236. [DOI] [PubMed] [Google Scholar]
- 34. Dhillon OS, Khan SQ, Narayan HK, Ng KH, Struck J, Quinn PA, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL. Prognostic value of mid‐regional pro‐adrenomedullin levels taken on admission and discharge in non‐ST‐elevation myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) II study. J Am Coll Cardiol. 2010;56:125–133. [DOI] [PubMed] [Google Scholar]
- 35. Khan SQ, O'Brien RJ, Struck J, Quinn P, Morgenthaler N, Squire I, Davies J, Bergmann A, Ng LL. Prognostic value of midregional pro‐adrenomedullin in patients with acute myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol. 2007;49:1525–1532. [DOI] [PubMed] [Google Scholar]
- 36. Klip IT, Voors AA, Anker SD, Hillege HL, Struck J, Squire I, van Veldhuisen DJ, Dickstein K. Prognostic value of mid‐regional pro‐adrenomedullin in patients with heart failure after an acute myocardial infarction. Heart. 2011;97:892–898. [DOI] [PubMed] [Google Scholar]
- 37. Neumann JT, Tzikas S, Funke‐Kaiser A, Wilde S, Appelbaum S, Keller T, Ojeda‐Echevarria F, Zeller T, Zwiener I, Sinning CR, Jagodzinski A, Schnabel RB, Lackner KJ, Munzel T, Blankenberg S, Wild PS, Sydow K. Association of MR‐proadrenomedullin with cardiovascular risk factors and subclinical cardiovascular disease. Atherosclerosis. 2013;228:451–459. [DOI] [PubMed] [Google Scholar]
- 38. Melander O, Newton‐Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, Persson M, Smith JG, Magnusson M, Christensson A, Struck J, Morgenthaler NG, Bergmann A, Pencina MJ, Wang TJ. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jougasaki M, Wei CM, McKinley LJ, Burnett JC Jr. Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation. 1995;92:286–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Included and Excluded Patients
Figure S1. Levels of MR‐proADM according to duration of symptoms until baseline blood sampling.


