Abstract
Background
Diabetes mellitus is a major risk factor for ischemic stroke. Rising hemoglobin A1c (HbA1c) levels are associated with microvascular diabetes mellitus complication development; however, this relationship has not been established for stroke risk, a macrovascular complication.
Methods and Results
We conducted a systematic review and meta‐analysis of observational cohort and nested case‐control cohort studies assessing the association between rising HbA1c levels and stroke risk in adults (≥18 years old) with and without type 1 or type 2 diabetes mellitus. Random‐effects model meta‐analyses were used to calculate pooled adjusted hazard ratios (HRs) and their precision. The systematic review yielded 36 articles, of which 29 articles (comprising n=532 779 participants) were included in our meta‐analysis. Compared to non–diabetes mellitus range HbA1c (<5.7%), diabetes mellitus range HbA1c (≥6.5%) was associated with an increased risk of first‐ever stroke with average HR (95% confidence interval) of 2.15 (1.76, 2.63), whereas pre–diabetes mellitus range HbA1c (5.7–6.5%) was not (average HR [95% confidence interval], 1.19 [0.87, 1.62]). For every 1% HbA1c increment (or equivalent), the average HR (95% confidence interval) for first‐ever stroke was 1.12 (0.91, 1.39) in non–diabetes mellitus cohorts and 1.17 (1.09, 1.25) in diabetes mellitus cohorts. For every 1% HbA1c increment, both non–diabetes mellitus and diabetes mellitus cohorts had a higher associated risk of first‐ever ischemic stroke with average HR (95% confidence interval) of 1.49 (1.32, 1.69) and 1.24 (1.11, 1.39), respectively.
Conclusions
A rising HbA1c level is associated with increased first‐ever stroke risk in cohorts with a diabetes mellitus diagnosis and increased risk of first‐ever ischemic stroke in non–diabetes mellitus cohorts. These findings suggest that more intensive HbA1c glycemic control targets may be required for optimal ischemic stroke prevention.
Keywords: cerebrovascular disease/stroke, diabetes mellitus, hemoglobin A1c, meta‐analysis, risk
Subject Categories: Cerebrovascular Disease/Stroke; Diabetes, Type 1; Diabetes, Type 2
Clinical Perspective
What Is New?
Using a meta‐analytical approach, we found that higher glycated hemoglobin levels were associated with an increased risk of first‐ever ischemic stroke in both non–diabetes mellitus and diabetes mellitus cohorts.
In people with established diabetes mellitus, higher glycated hemoglobin levels were associated with an increased risk of first‐ever stroke.
What Are the Clinical Implications?
Further interventional studies are needed to examine the effectiveness of more intensive glycemic control targets as part of primary and secondary stroke prevention, in pre–diabetes mellitus and diabetes mellitus cohorts.
Introduction
Strokes represent a heterogeneous group of vascular pathologies that collectively act as a major global burden of mortality and lifelong morbidity. Diabetes mellitus is a major risk factor for the development of stroke, particularly ischemic stroke, with type 2 diabetes mellitus alone known to increase stroke risk 1.5 to 4 fold.1 Macrovascular complications of diabetes mellitus (ischemic heart disease (IHD), stroke, and peripheral vascular disease) represent a major cause of diabetes mellitus related mortality and health‐related expenditure.2, 3
Glycated hemoglobin (HbA1c) is a validated marker of 2 to 3 month glycemic control used within routine diabetes mellitus care. Current American Diabetes Association (ADA) diabetes mellitus management guidelines recommend a base target of HbA1c <7.0% within routine diabetes mellitus care of non‐pregnant adults.4 This target is most validated for microvascular complication risk reduction and has unclear implications for optimal macrovascular risk reductions. Long‐term follow‐up studies of 2 randomized controlled trials (RCTs) have demonstrated beneficial effects on long‐term macrovascular outcomes with more intensive glycemic control, thereby suggesting a metabolic memory effect for hyperglycemia.5, 6
Up to one half of all patients presenting with acute stroke have previously unknown abnormalities of glucose tolerance, with 20% to 40% of patients presenting with hyperglycemia at hospital admission.7 Chronic hyperglycemia has been linked with several stroke risk factors including accelerated atherosclerosis, increased carotid intima media thickness (CIMT), cardiomyocyte dysfunction, atrial fibrillation (AF), and ischemic heart disease.8, 9, 10, 11 In non–diabetes mellitus patients with insulin resistance and recent ischemic stroke or transient ischemic attack, long‐term treatment with pioglitazone reduced the risk of adverse cardiovascular outcomes.12 The outcomes of this trial suggested that in the ischemic stroke subgroup, anti‐hyperglycemic medication may need to start at lower HbA1c thresholds than currently accepted levels.12
No systematic review and meta‐analysis has generated consensus regarding the specific relationship between rising HbA1c level and stroke risk.13, 14 We therefore aimed to fill this knowledge gap by conducting a systematic review and meta‐analysis of observational studies to examine and determine a quantifiable risk relationship for the association between rising HbA1c level and stroke risk, stratified by diabetes mellitus status, stroke temporality, and stroke subtype.
Methods
Study Design and Registration
We systematically reviewed cohort and nested case‐control cohort studies that assessed the association between varying HbA1c level and stroke risk. The protocol implemented as part of this systematic review and meta‐analysis was constructed using the combined recommendations of the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA),15 Meta‐analysis of Observational Studies in Epidemiology (MOOSE) group,16 and the Cochrane Handbook for Systematic Reviews.17 This study is registered with PROSPERO (CRD42017056706). The data, analytic methods, and study materials will be/have been made available to other researchers for purposes of reproducing the results or replicating the procedure. These are available within this manuscript and the associated online data supplement.
Literature Search Strategy
A systematic search of 5 literary databases (MEDLINE, Embase, PubMed, Web of Science, and the Cochrane Library) was performed between February 7, 2017, and March 5, 2017. Medical Subject Headings (MeSH) terms selected were synonymous with the following text words used: glycosylated h(a)emoglobin, HbA 1c , glycated h(a)emoglobin, stroke, cerebral infarction, cerebral h(a)emorrhage, and transient isch(a)emic attack.” MeSH terms were “exploded” to maximise coverage.
Search results were restricted to human (≥18 years old) and English only articles. No publication filters were applied within any database. Search results were managed and duplicate entries removed using EndNote X7.7.1. A manual search of study references was performed for completeness. A complete list of MeSH and text words with Boolean operators applied within MEDLINE are provided in Figure S1.
Inclusion and Exclusion Criteria
Studies were considered for inclusion within meta‐analyses and sensitivity analyses performed if they met the following criteria: (1) presented adjusted hazard ratios (HRs) or risk ratios (RRs, relative risk) for the association between varying HbA1c level and stroke risk, defined by temporality (first‐ever or recurrent) and subtype of event (ischemic, hemorrhagic, other); and (2) involved a minimum follow‐up period of ≥12 months.
We excluded studies that met any of the following criteria: (1) failed the automatic exclusion criteria within the Scottish Intercollegiate Guidelines Network (SIGN)18 quality tool (Data S1); (2) focused on specific subpopulations (including end‐stage kidney disease (ESKD), dialysis, post‐thrombolysis (tPA), post‐myocardial infarction (AMI), and post‐operative cohorts); (3) had insufficient or missing data for extrapolation and quality assessment; and (4) compared diabetes mellitus to non–diabetes mellitus cohorts.
In the source literature, effect sizes (HR or RR) were variably adjusted for covariates, with multiple effect sizes often reported. Consequently, we extracted the most extensively covariate‐adjusted HR or RR data for use in the meta‐analysis. However, if in the original source article adjustment was performed for hypoglycemic medication use, we only included data that were not adjusted for hypoglycemic medication use, as the medication may have biased the association between the exposure and outcome. Data S2 provides a full description of inclusion and exclusion criteria applied during each phase of the search strategy.
Search Protocol Implementation and Data Extraction
Two reviewers (J.P.M., G.P.M.) independently screened all available articles by title and abstract using predefined inclusion and exclusion criteria. Following this, 2 reviewers (J.P.M., V.T.) performed a full‐text review of articles identified through screening using predefined inclusion and exclusion criteria. Objective methodological study quality assessment was performed during full‐text review using 2 critical appraisal checklists for cohort studies.18, 19 Any disagreements were resolved through discussion between reviewers. Narrative synthesis was performed for all studies deemed suitable for inclusion in the meta‐analysis (n=36 studies). Data on key study parameters, including; author(s), year of publication, study location, sample size, participant demography, stroke outcome type, effect size data, and covariate adjustment performed, were extracted in duplicate and are detailed in Tables S1 through S6. Any discrepancies in data extracted were resolved through consultation between authors.
Statistical Analyses and Bias Assessment
The primary outcome measure of interest was the association between rising categorical or 1% increment (or equivalent) HbA1c levels and stroke risk, stratified by diabetes mellitus status, stroke temporality, and stroke subtype. A random‐effects model was used for all meta‐analyses and sensitivity analyses. Meta‐analyses were only performed on strata that contained a minimum of 3 studies (n≥3) to ensure adequate analytical power. RR data were treated as equivalent to HR data in all analyses.
The association between rising categorical HbA1c levels and first‐ever stroke risk was assessed through comparison of stroke risk between American Diabetes Association–defined non–diabetes mellitus range HbA1c (<5.7%) (reference category) to pre–diabetes mellitus range HbA1c (5.7%–6.5%) and diabetes mellitus range HbA1c (≥6.5%) categories. Only studies that used a reference category within non–diabetes mellitus range HbA1c and at least 1 comparator category within pre–diabetes mellitus range or diabetes mellitus range HbA1c were included (Data S3, Figures S2 and S3).
The association between 1% increments (or equivalent) of HbA1c and first‐ever stroke risk was examined. Studies reporting 1 standard deviation (1sd) increment effect sizes were treated as equivalent to 1% HbA1c, as the magnitude of these 1 standard deviation increments ≈ 1% increments (1±0.4%) and the effect sizes quoted approximated the estimated 1% increment equivalents (Tables S1– S6).
Many studies only reported categorical data. A linear regression model was used to estimate natural log‐transformed 1% increment effect sizes and 95% confidence interval (CI) from this pool of categorical data using a method described by Greenland,20 with statistical significance set at P<0.05. This estimated 1% data were then used within separate random‐effects model meta‐analyses performed. A detailed description of this method is provided in Data S4, Figures S4 and S5.
To avoid duplicate data use, whenever 2 studies reported on the same study population, we used the most recent study for the subgroup meta‐analysis performed. Baseline and time‐update mean values of HbA1c were treated as equivalents. Time‐updated HbA1c values were selected in preference to single baseline values in studies that presented both. A random‐effects model was used to generate overall effect sizes from effect size data that had been stratified by variables like sex or ethnicity.
Definitions used to classify stroke and diabetes mellitus status varied greatly within the source literature. Diabetes mellitus status was defined using reported diabetes mellitus status (medical history, clinician or patient reported) and/or glucose or HbA1c measurement at study inclusion. Strokes were classified using International Classification of Diseases,Ninth/Tenth Revision (ICD‐9/10) codes, World Health Organization criteria and/or study‐defined stroke criteria. Stroke event occurrence was identified using hospital admission diagnosis, death certificate details, medical history details, clinician reports, and/or patient‐reported status.
Given the heterogeneity in diabetes mellitus and stroke outcome classification and reporting, the decision was made to assign both diabetes mellitus and stroke outcome status based on each study's reported outcome status. Effect sizes adjusted for diabetes mellitus status were treated as representing non–diabetes mellitus data. Effect sizes adjusted for past stroke history were treated as representing first‐ever stroke data.
Several sensitivity analyses were performed within this study, examining the; (1) effect of combining type 1 and type 2 diabetes mellitus cohorts, (2) importance of ischemic subtype stratification, (3) difference between estimated and quoted 1% HbA1c increment data, and (4) effect of varying levels of covariate adjustment on results obtained. These sensitivity analyses are presented in Figures S6 through S10. The linear‐regression estimated 1% HbA1c meta‐analyses are presented in Figure S11.
Based on the recommendations of the Cochrane Handbook,17 we did not perform formal meta‐regression because of insufficient study number (n<10 studies per subgroup). We assessed for statistical heterogeneity using the I2 statistic. As per Higgins et al,21 we assigned adjectives of “low,” “moderate,” and “high” for I2 statistic values of 25%, 50% and 75%, respectively. I2 statistic values below 25% were assigned the adjective of “low.” The I2 statistic describes the percentage of variation across studies that is attributable to heterogeneity rather than chance.21 Consequently, the categories of I2 statistic magnitude outlined do not refer to the absolute amount of observed heterogeneity.
Publication bias was assessed through construction of funnel plots and performing Egger's test for funnel plot asymmetry for each meta‐analysis performed (Figures S12–S16). Statistical significance for publication bias assessment using Egger's test was set at P<0.05. Stata/IC 14.2 was used for all statistical analyses. Additional sensitivity analyses are presented in Figures S17 through S23.
Results
A total of 5831 articles were identified through the search strategy. Following duplicate removal (n=2279), a total of 3552 articles were screened by title and abstract. Of these, 310 articles were assessed by full‐text review. A total of 56 studies were assessed for inclusion in meta‐analyses performed, of which 20 were excluded for the following reasons: 7 studies did not provide HR or RR data; 3 studies provided only effect sizes adjusted for hypoglycemic medication use; 5 studies provided effect sizes which compared diabetes mellitus to non‐diabetes mellitus participants; 1 study provided HR which compared intensively treated to non‐intensively treated cohorts; 1 study had effect size covariate adjustment and stratification limitations; 1 study used a duplicate study population with incomplete HbA1c strata use in effect size calculation; 1 study used a conditional HR that compared stroke and non‐stroke patient cohorts; and 1 study used a pooled cardiovascular disease outcome without stratifying for a stroke outcome. A detailed overview of the study review process is presented in Figure 1.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41
Figure 1.

PRISMA flowchart outlining search strategy implementation and results at each stage. Results presented outline the number of articles identified during each stage of the search strategy. Duplicate removal was performed using the default duplicate removal function within EndNote X7.7.1. Screening by title and abstract was performed independently by 2 researchers using a defined set of inclusion criteria. Full‐text review, including methodological quality assessment, was subsequently performed using defined inclusion criteria. Following this, articles were assessed for meta‐analytical inclusion through consultation between 2 authors using a separate set of inclusion criteria. Articles deemed suitable for meta‐analyses and sensitivity analyses were stratified based on cohort diabetes mellitus status and stroke outcome. Strata that lacked sufficient article number (n<3 articles) were presented within narrative synthesis only. A total of 29 articles were used in meta‐analyses and sensitivity analyses conducted. The number of studies at each stage (n) is reflected in brackets. AMI indicates acute myocardial infarction; CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL); CVD, cardiovascular disease; ESKD, end‐stage kidney disease; HR, hazard ratio; RR, risk ratio (relative risk); tPA, tissue plasminogen activator.
Of the 36 studies deemed suitable for meta‐analysis, 7 reported recurrent stroke outcome data and were included only in narrative synthesis because of concerns regarding underpowering of meta‐analyses following outcome stratification (Tables S1–S6). A total of 29 studies comprising 532 779 participants were used in meta‐analyses and sensitivity analyses (Figure 1, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41). A narrative summary of baseline participant characteristics within all 36 articles identified for potential inclusion is provided in Tables S1 through S6.
First‐Ever Stroke Risk in American Diabetes Association–Defined HbA1c Ranges
Compared to non–diabetes mellitus range HbA1c (<5.7%), pre–diabetes mellitus range HbA1c (5.7%–6.5%) was not associated with a significant increased risk of first‐ever stroke (average HR [95% CI], 1.19 [0.87, 1.62]). In contrast, diabetes mellitus range HbA1c (≥6.5%) was associated with a significant increased risk of first‐ever stroke when compared to non–diabetes mellitus range HbA1c (average HR [95% CI], 2.15 [1.76, 2.63]).
We identified moderate and low I2 statistic values (I2=61.3% [P=0.051] and I2=0% [P=0.460]) for the pre–diabetes mellitus and diabetes mellitus analyses, respectively. We did not find evidence of significant publication bias for either analysis (Figure S12).
Association Between Study‐Quoted 1% HbA1c Increments and First‐Ever Stroke Risk
Figure 2, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 summarizes the meta‐analyses assessing the association between study‐quoted rising 1% HbA1c increments and first‐ever stroke risk, stratified by diabetes mellitus status and ischemic stroke subtype. For every 1% HbA1c increment (or equivalent), the average HR (95% CI) for first‐ever stroke risk was 1.12 (0.91, 1.39) in non–diabetes mellitus cohorts and 1.17 (1.09, 1.25) in diabetes mellitus cohorts. When restricted to studies only examining first‐ever ischemic stroke, average HRs (95% CI) were 1.49 (1.32, 1.69) and 1.24 (1.11, 1.39) for non–diabetes mellitus and diabetes mellitus cohorts, respectively.
Figure 2.
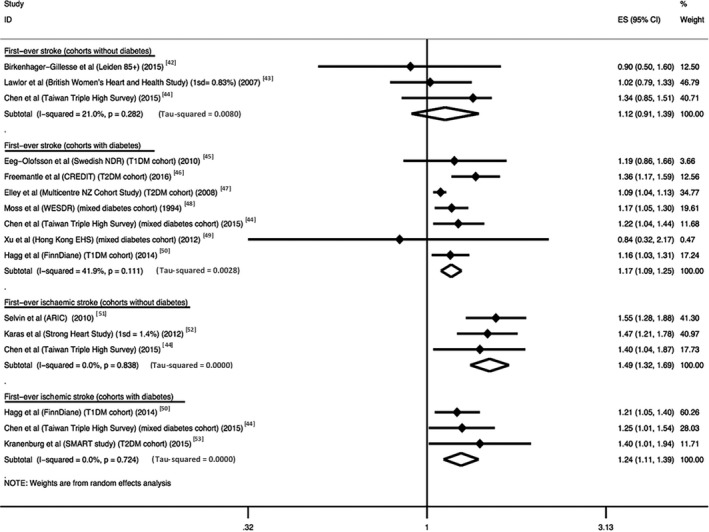
Association between study‐quoted rising 1% HbA1c increments and stratified first‐ever stroke risk. Studies presenting hazard ratio (HR) or risk ratio (RR, relative risk) data assessing the association between rising 1% HbA1c increments and first‐ever stroke risk were identified and used to calculate meta‐analytical effect sizes (ES) (95% CI). RR data were treated as equivalent to HR data. Studies using 1 standard deviation (1 SD) HbA1c increments for effect sizes quoted were treated as equivalent to 1% HbA1c increment data. The corresponding HbA1c increments for each standard deviation are as shown in brackets. Studies were stratified based on the diabetes mellitus status of their cohorts and their restriction of first‐ever stroke to an ischemic stroke subtype. The outcome “first‐ever stroke” reflects any stroke subtype. The outcome “first‐ever ischemic stroke” included only studies that specifically restricted their stroke outcome to first‐ever stroke of ischemic subtype. Diabetes cohorts included studies that measured type 1 diabetes mellitus (T1DM), type 2 diabetes (T2DM) or a combination of both (mixed diabetes mellitus cohort). Non–diabetes mellitus cohorts represented studies that used participants with no diabetes mellitus or whose effect size(s) were adjusted for diabetes mellitus. The I2 statistic values for each subgroup analysis assessing the percentage of variation across studies that is due to heterogeneity, rather than chance, are presented below each subgroup analysis. A random‐effects model using the inverse‐variance method for weighting was used to generate pooled effect sizes for each subgroup. ES=1.0 indicates no statistically significant association.
The I2 statistic value was moderate for the analysis assessing first‐ever stroke risk in patients with diabetes mellitus (I2=59.0%, P=0.012). Sensitivity analysis identified studies with limited covariate adjustment as the likely source of this moderate I2 statistic value (Figure S6). Exclusion of these studies reduced the I2 statistic value from moderate to low (reduction from I2=59.0% [P=0.012] to I2=41.9% [P=0.111]) without inducing significant publication bias or altered pooled effect size significance (average HR [95% CI], 1.14 [1.07, 1.20] prior to exclusion and 1.17 [1.09, 1.25] following exclusion). We did not find evidence of significant publication bias in any subgroup analysis (Figures S13 and S14).
Association Between Linear Regression Estimated 1% HbA1c Increments and First‐Ever Stroke Risk
For every estimated 1% HbA1c increment (or equivalent), the average HR (95% CI) for first‐ever stroke was 1.17 (1.02, 1.34) and 1.17 (1.01, 1.36) for non–diabetes mellitus and diabetes mellitus cohorts, respectively. When restricted to first‐ever ischemic stroke, average HRs (95% CI) were 1.35 (0.91, 2.02) and 1.32 (1.23, 1.42) for non–diabetes mellitus and diabetes mellitus cohorts, respectively (Figure S11). Inclusion of studies with limited covariate adjustment resulted in a high I2 statistic value, as shown in Figure S7. Exclusion of these studies resulted in a reduction of the I2 statistic value from high to moderate (reduction from I2=89.9% [P<0.001] to I2=57.7% [P=0.051]) without inducing statistically significant publication bias (Figures S15 and S16) or altering the significance of the pooled effect sizes (average HR [95% CI], 1.21 [1.05, 1.40] prior to exclusion and 1.17 [1.01, 1.36] following exclusion).
Discussion
We demonstrated a significant association between rising 1% HbA1c increments and first‐ever stroke risk in cohorts with diabetes mellitus. In non–diabetes mellitus cohorts, analysis of estimated 1% HbA1c data revealed a significant relationship with first‐ever stroke, while study‐quoted 1% HbA1c data analyses were significant only for an association with first‐ever ischemic stroke. Analysis of American Diabetes Association diabetes mellitus range HbA1c (≥6.5%) revealed a significant 2.15‐fold increased risk of first‐ever stroke in diabetes mellitus range HbA1c compared to non–diabetes mellitus range HbA1c (<5.7%).
The absence of a clear association between pre–diabetes mellitus range HbA1c and first‐ever stroke identified is in keeping with the results of a previous meta‐analysis by Huang et al.54 This study identified a nonsignificant 5% increased risk of stroke associated with pre–diabetes mellitus range HbA1c following meta‐analysis of only 2 studies.54
Our study expands upon previous meta‐analyses.13, 14 These studies demonstrated a significant association between rising 1% HbA1c increments and stroke risk in patients with type 2 diabetes mellitus (where stroke represented any fatal or nonfatal stroke event) after combining study‐quoted and estimated 1% HbA1c data from a small number of included studies.
Our study addressed these limitations. Our study included 29 studies and studied ischemic stroke specifically. The use of separate meta‐analyses for study‐quoted and linear regression estimated 1% HbA1c data within our meta‐analysis avoids the inherent imputation bias associated with conversion of categorical data into a continuous data set.
Our study suggests the presence of an independent association between chronic hyperglycemia, even in the pre–diabetes mellitus range, and first‐ever stroke risk. The strength of this association was enhanced when restricting stroke outcomes to first‐ever ischemic stroke. This suggests that the inclusion of hemorrhagic and undefined stroke subtypes within the stroke outcome assessed may have blunted the statistical significance of the underlying relationship between hyperglycemia and ischemic stroke. This finding is in keeping with previous research that has suggested that diabetes mellitus is primarily a risk factor for ischemic stroke rather than hemorrhagic stroke,7 and is supported by pathogenic data that links chronic hyperglycemia and ischemic stroke risk factors.8, 9, 10, 11
The DCCT/EDIC (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study) demonstrated a statistically significant risk reduction in macrovascular complication risk in participants managed initially with intensive glycemic control measures following 17 years of follow‐up of a type 1 diabetes mellitus population.5, 55 Similarly, the UKPDS (United Kingdom Prospective Diabetes Study) follow‐up study6 demonstrated that intensive glycemic control in patients with type 2 diabetes mellitus led to fewer macrovascular complications after prolonged follow‐up, suggesting a metabolic memory effect for earlier intensive control.
Despite this, several randomized controlled trials including VADT (Veterans Affairs Diabetes Trial),56 ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation)57 and ACCORD (Action to Control Cardiovascular Risk in Diabetes)58 have refuted the presence of a statistically significant macrovascular complication risk reduction offered by intensified glycemic control, in the context of type 2 diabetes mellitus management. Common limitations within these randomized controlled trials that may explain the incongruence of their results with DCCT/EDIC and UKPDS include; the comparatively short follow‐up intervals used, competing risk confounding associated with the older age of participants used, the inclusion of participants with poorly controlled diabetes mellitus, and, importantly, the inclusion of participants with preexisting cardiovascular disease (may be at heightened risk of hypoglycemia with insulin and sulfonylurea therapy). Newer agents reduce cardiovascular outcomes without major changes in HbA1c or improved glycemic control, but these may act through other pathways and do not deter from the finding that pre–diabetes mellitus, as determined by HbA1c level, appears associated with incident ischemic stroke.59 This is evident within 2 recent glucagon‐like peptide‐1 trials that demonstrated significant reductions in a combined cardiovascular outcome of cardiovascular death, nonfatal acute myocardial infarction, or nonfatal stroke through their use in type 2 diabetes mellitus populations with high cardiovascular disease risk.60, 61
Several measures were implemented within our study with the intention of reducing the magnitude of interstudy heterogeneity. We attempted to reduce heterogeneity by implementing an extensive set of inclusion and exclusion criteria that were designed to reduce common sources of heterogeneity and bias, including but not limited to; variability in study design, variability in effect measures used, insufficient follow‐up time, and insufficient covariate adjustment. Given the implicit heterogeneity of observational study designs, a random‐effects model was used in all analyses performed. The use of subgroup meta‐analyses, stratified for key outcome parameters including stroke subtype (first‐ever ischemic stroke versus first‐ever stroke) and cohort diabetes mellitus status (diabetes mellitus versus non–diabetes mellitus cohorts), further aimed to reduce outcome measure related heterogeneity.
Of the subgroup meta‐analyses focusing on the association between rising 1% HbA1c increments and first‐ever stroke risk, only 2 demonstrated I2 values exceeding 25%, thereby indicating a very good level of heterogeneity control through our study design. Sensitivity analyses performed in Figures S6 and S7 demonstrated reductions in the magnitude of heterogeneity detected from moderate to low and high to moderate, respectively, following exclusion of studies with limited covariate adjustment. Given this result, it is a reasonable assertion that differences in the types and number of covariates adjusted for within individual studies is a likely major contributor to the statistical heterogeneity measured. This is not surprising when considering that many of these variables, including; age, sex, hypertension, smoking status, cholesterol level, and history of cardiovascular disease, are independent risk factors for the development of vascular disease including stroke.
A further potential reason for the statistical heterogeneity measured relates to the unavoidable differences in study definitions for key parameters of diabetes mellitus status and stroke. Decisions made relating to the treatment of studies whose results were statistically adjusted for past history of diabetes mellitus and stroke, and the acceptance of study‐quoted diabetes mellitus and stroke outcome descriptors may also have contributed to the level of statistical heterogeneity detected but were unavoidable given the inherent variability in outcome definitions present within observational study designs.
Our study has limitations. We restricted our studies to those reporting only RR and HR data. The lack of a common, standardized definition for diabetes mellitus across all the studies could result in assessment bias. Likewise, the inclusion of studies with different stroke outcome classification systems is suboptimal given the inherent differences within the classification systems used. The use of a linear regression model to ≈1% HbA1c effect size data from categorical HbA1c data can provide only an approximation of this relationship and could not be combined with quoted data. The variability in covariate adjustment performed (types and number of covariates adjusted for) within the source studies included in our analyses imposed limitations on our ability to examine the independent effects of individual covariate adjustment on the statistical heterogeneity calculated using the I2 statistic. Likewise, the limited number of studies (n<10) within each subgroup meta‐analysis performed precluded the implementation of meta‐regression techniques.
Although a comprehensive search strategy assessing multiple literary databases was performed, we may not have identified all relevant literature on this topic. We also did not include studies addressing this topic in languages other than English. We did not have access to individual patient data that would have permitted more detailed analyses. Our data are, however, in line with an individual patient data meta‐analysis that assessed the risk of a composite outcome of stroke and myocardial infarction with varying levels of HbA1c.62 That study did not report on stroke outcomes separately but was able to identify an increased risk of acute myocardial infarction/stroke in patients with low levels of HbA1c.62 Our methodology was not able to identify such a J‐shaped relationship. The limited number of articles within each subgroup analysis prevented use of formal meta‐regression and analysis of HbA1c as a risk factor for recurrent stroke.
Conclusions
In summary, our study suggests that both continuous and categorical elevations in HbA1c are associated with increased first‐ever ischemic stroke risk, irrespective of diabetes mellitus status. Prevention of macrovascular complications like stroke may need to start at lower HbA1c thresholds. Further interventional studies are needed to explore the effectiveness of more intensive glycemic management within primary and secondary stroke prevention, in pre–diabetes mellitus and diabetes mellitus cohorts.
Sources of Funding
E.I.E. was supported by a Viertel Clinical Investigatorship, RACP Fellowship, and Sir Edward Weary Dunlop Medical Research Foundation research grant and a Stroke Foundation grant.
Disclosures
V.T. reports receiving consulting fees from Medtronic. V.T. is on the steering committee of the DIAGNOSE AF and REACT AF clinical trials sponsored by Medtronic. V.T. reports speaker and consulting fees and travel support by Boehringer Ingelheim, Pfizer/BMS, Daichi‐Sankyo, and Bayer. The remaining authors have no disclosures to report.
Supporting information
Data S1. Scottish Intercollegiate Guidelines Network (SIGN) methodological quality assessment tool [1] automatic exclusion criteria.
Data S2. Inclusion and exclusion criteria for each phase of the search strategy.
Data S3. Association between ADA defined pre–diabetes mellitus and diabetes mellitus range HbA1c and first‐ever stroke risk.
Data S4. Linear regression analysis method for estimating continuous (1% HbA1c increment) effect size data from categorical effect size data
Table S1. The Association Between Rising HbA1c Levels and Stroke Risk in Adults Without Diabetes Mellitus
Table S2. The Association Between Rising HbA1c Levels and Stroke Risk in Adults With Type 1 Diabetes Mellitus
Table S3. The Association Between Rising HbA1c Levels and Stroke Risk in Adults With Type 2 Diabetes Mellitus
Table S4. The Association Between Rising HbA1c Levels and Stroke Risk in Mixed Diabetes Mellitus Cohorts
Table S5. Association Between Rising HbA1c Levels and Ischemic Stroke Risk, in Adults Without Diabetes Mellitus
Table S6. Association Between Rising HbA1c Levels and Ischemic Stroke Risk, in Adults With Diabetes Mellitus
Figure S1. Summary of search terms and Boolean operators used within the search strategy in MEDLINE.
Figure S2. Association between American Diabetes Association–defined pre–diabetes mellitus range HbA1c (5.7–6.5%) and first‐ever stroke risk.
Figure S3. Association between American Diabetes Association–defined diabetes mellitus range HbA1c (≥6.5%) and first‐ever stroke risk.
Figure S4. Linear regression analysis used to confirm linear hypothesis used in estimation of 1% HbA1c data.
Figure S5. 1% HbA1c increment effect size (95% CI) estimation method using the example of Selvin [13].
Figure S6. Sensitivity analysis for inadequate covariate adjustment in study‐quoted 1% HbA1c increment data.
Figure S7. Sensitivity analysis for inadequate covariate adjustment in estimated 1% HbA1c increment data.
Figure S8. Comparison of study‐quoted 1% HbA1c increment first‐ever stroke and first‐ever ischemic stroke effects sizes, in non–diabetes mellitus cohorts.
Figure S9. Comparison of study‐quoted 1% HbA1c increment first‐ever stroke and first‐ever ischemic stroke effects sizes, in diabetes cohorts.
Figure S10. Comparison of study‐quoted and linear regression estimated 1% HbA1c effect size data.
Figure S11. Association between linear regression estimated rising 1% HbA1c increments and stratified first‐ever stroke risk.
Figure S12. Publication bias assessment for intercategorical meta‐analyses within Figures S2 and S3.
Figure S13. Publication bias assessment for subgroup meta‐analyses within Figure 2.
Figure S14. Publication bias assessment for sensitivity analysis within Figure S6.
Figure S15. Publication bias assessment for subgroup meta‐analyses within Figure S11.
Figure S16. Publication bias assessment for sensitivity analysis within Figure S7.
Figure S17. Additional subgroup analysis: association between study‐quoted rising 1% HbA1c increments and first‐ever stroke in non–diabetes mellitus and diabetes mellitus cohorts (as described in Figure 2).
Figure S18. Additional subgroup analysis: association between study‐quoted rising 1% HbA1c increments and first‐ever ischemic stroke in non–diabetes mellitus and diabetes mellitus cohorts (as described in Figure 2).
Figure S19. Additional subgroup analysis: association between study‐quoted rising 1% HbA1c increments and the combined outcome of first‐ever stroke and first‐ever ischemic stroke events, in non–diabetes mellitus cohorts.
Figure S20. Additional subgroup analysis: association between study‐quoted rising 1% HbA1c increments and the combined outcome of first‐ever stroke and first‐ever ischemic stroke events, in diabetes mellitus cohorts.
Figure S21. Additional subgroup analysis: association between study‐quoted rising 1% HbA1c increments and the combined outcome of first‐ever stroke and first‐ever ischemic stroke events, regardless of cohort diabetes mellitus status (combination of Figures S19 and S20).
Figure S22. Additional subgroup analysis: association between first‐ever stroke risk and combined American Diabetes Association–defined pre–diabetes mellitus and diabetes mellitus range HbA1c (≥5.7%), compared to non–diabetes mellitus range HbA1c (<5.7%).
Figure S23. Additional subgroup analysis: comparison of study‐quoted 1% HbA1c increment first‐ever stroke and first‐ever ischemic stroke effect sizes regardless of cohort diabetes mellitus status (combination of Figures S8 and S9).
Acknowledgments
Special thanks to Ms Helen Baxter (Clinical Librarian, Austin Health Services Library, Austin Health) for her advice regarding selection of search terms, literature databases and Boolean operators during search strategy development. The Florey Institute of Neuroscience and Mental Health acknowledges the strong support from the Victorian Government and in particular the funding from the Operational Infrastructure Support Grant.
(J Am Heart Assoc. 2018;7:e007858 DOI: 10.1161/JAHA.117.007858.)29773578
The abstract of this work was presented as an oral presentation at the Stroke Society of Australasia Conference, August 23 to 25, 2017, in Queenstown, New Zealand, and as a poster presentation at the Australian Diabetes Society Conference, August 30 to September 1, 2017, in Perth, Australia.
References
- 1. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82. [Google Scholar]
- 2. Wild SH, Dunn CJ, McKeigue PM, Comte S. Glycemic control and cardiovascular disease in type 2 diabetes: a review. Diabetes Metab Res Rev. 1999;15:197–204. [DOI] [PubMed] [Google Scholar]
- 3. Hogan P, Dall T, Nikolov P; American Diabetes Association . Economic costs of diabetes in the U.S. in 2002. Diabetes Care. 2003;26:917–932. [DOI] [PubMed] [Google Scholar]
- 4. American Diabetes Association . 5. Glycemic targets. Diabetes Care. 2016;39(suppl 1):S39–S46. [DOI] [PubMed] [Google Scholar]
- 5. Nathan DM, Cleary PA, Backlund JYC, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B; DCCT/EDIC Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 7. Baird TA, Parsons MW, Barber PA, Butcher KS, Desmond PM, Tress BM, Colman PG, Jerums G, Chambers BR, Davis SM. The influence of diabetes mellitus and hyperglycaemia on stroke incidence and outcome. J Clin Neurosci. 2002;9:618–626. [DOI] [PubMed] [Google Scholar]
- 8. Pai JK, Cahill LE, Hu FB, Rexrode KM, Manson JE, Rimm EB. Hemoglobin A1c is associated with increased risk of incident coronary heart disease among apparently healthy, nondiabetic men and women. J Am Heart Assoc. 2013;2:e000077. doi: 10.1161/JAHA.112.000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobayashi S, Xu X, Chen K, Liang Q. Suppression of autophagy is protective in high glucose‐induced cardiomyocyte injury. Autophagy. 2012;8:577–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qi W, Zhang N, Korantzopoulos P, Letsas KP, Cheng M, Di F, Tse G, Liu T, Li G. Serum glycated hemoglobin level as a predictor of atrial fibrillation: a systematic review with meta‐analysis and meta‐regression. PLoS One. 2017;12:e0170955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vitelli LL, Shahar E, Heiss G, McGovern PG, Brancati FL, Eckfeldt JH, Folsom AR; Atherosclerosis Risk in Communities (ARIC) Study Investigators . Glycosylated hemoglobin level and carotid intimal‐medial thickening in nondiabetic individuals. The Atherosclerosis Risk in Communities Study. Diabetes Care. 1997;20:1454–1458. [DOI] [PubMed] [Google Scholar]
- 12. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP, Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O'Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR; for the IRIS Trial Investigators . Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Selvin E, Marinopoulos S, Berkenblit G, Rami T, Brancati FL, Powe NR, Golden SH. Meta‐analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141:421–431. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Hu G, Yuan Z, Chen L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta‐analysis. PLoS One. 2012;7:e42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB; for the Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) Group . Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Internet]. Oxford, England: The Cochrane Collaboration; 2011. Available at: http://www.handbook.cochrane.org. Accessed March 21, 2017. [Google Scholar]
- 18. SIGN Methodology Checklist 3: cohort studies. Edinburgh, Scotland: Scottish Intercollegiate Guideline Network; 2012. Available at: http://www.sign.ac.uk/assets/checklist_for_cohort_studies.rtf. Accessed March 21, 2017. [Google Scholar]
- 19. JBI critical appraisal checklist for cohort studies. South Australia: The Joanna Briggs Institute; 2016. Available at: http://joannabriggs.org/assets/docs/critical-appraisal-tools/JBI_Critical_Appraisal-Checklist_for_Cohort_Studies.pdf. Accessed March 21, 2017. Updated tool available from: http://joannabriggs.org/assets/docs/critical-appraisal-tools/JBI_Critical_Appraisal-Checklist_for_Cohort_Studies2017.pdf. [Google Scholar]
- 20. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Law ZK, Nafisah WN, Sahathevan R, Hing JY, Zakaria MF, Shuhari NMM, Ahmad NF, Ting TK, Tan HJ, Azmin S, Remli R, Nawi AM, Ibrahim NM. High prevalence of diabetes in stroke patients and its association with lacunar infarction. Neurol Asia. 2015;20:121–127. [Google Scholar]
- 24. Kuusisto J, Mykkanen L, Pyorala K, Laakso M. Non‐insulin‐dependent diabetes and its metabolic control are important predictors of stroke in elderly subjects. Stroke. 1994;25:1157–1164. [DOI] [PubMed] [Google Scholar]
- 25. Davis TME, Bruce DG, Davis WA. Predictors of first stroke in type 1 diabetes: the Fremantle Diabetes Study. Diabet Med. 2005;22:551–553. [DOI] [PubMed] [Google Scholar]
- 26. Lehto S, Ronnemaa T, Pyorala K, Laakso M. Predictors of stroke in middle‐aged patients with non‐insulin‐dependent diabetes. Stroke. 1996;27:63–68. [DOI] [PubMed] [Google Scholar]
- 27. Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, Holman RR. Risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes: UKPDS 66. Diabetes Care. 2004;27:201–207. [DOI] [PubMed] [Google Scholar]
- 28. Kimura T, Kaneto H, Kanda‐Kimura Y, Shimoda M, Kamei S, Anno T, Kawasaki F, Hashiramoto M, Matsuki M, Mune T, Kaku K. Seven‐year observational study on the association between glycemic control and the new onset of macroangiopathy in Japanese subjects with type 2 diabetes. Intern Med. 2016;55:1419–1424. [DOI] [PubMed] [Google Scholar]
- 29. Eeg‐Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjornsdottir S, Eliasson B. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). J Intern Med. 2010;268:471–482. [DOI] [PubMed] [Google Scholar]
- 30. Gudbjornsdottir S, Eliasson B, Eeg‐Olofsson K, Zethelius B, Cederholm J. Additive effects of glycaemia and dyslipidaemia on risk of cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register. Diabetologia. 2011;54:2544–2551. [DOI] [PubMed] [Google Scholar]
- 31. Vazquez‐Benitez G, Desai JR, Xu S, Goodrich GK, Schroeder EB, Nichols GA, Segal J, Butler MG, Karter AJ, Steiner JF, Newton KM, Morales LS, Pathak RD, Thomas A, Reynolds K, Kirchner HL, Waitzfelder B, Lafata JE, Adibhatla R, Xu Z, O'Connor PJ. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care. 2015;38:905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sunaga K, Miura K, Naruse Y, Sakurai M, Morikawa Y, Kurosawa Y, Nakagawa H. Glycated hemoglobin and risk of stroke, ischemic and hemorrhagic, in Japanese men and women. Cerebrovasc Dis. 2008;26:310–316. [DOI] [PubMed] [Google Scholar]
- 33. Sakurai M, Saitoh S, Miura K, Nakagawa H, Ohnishi H, Akasaka H, Kadota A, Kita Y, Hayakawa T, Ohkubo T, Okayama A, Okamura T, Ueshima H; for the NIPPON DATA90 Research Group . HbA1c and the risks for all‐cause and cardiovascular mortality in the general Japanese population. Diabetes Care. 2013;36:3759–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saliba W, Barnett‐Griness O, Elias M, Rennert G. Glycated hemoglobin and risk of first episode stroke in diabetic patients with atrial fibrillation: a cohort study. Heart Rhythm. 2015;12:886–892. [DOI] [PubMed] [Google Scholar]
- 35. Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereux RB, Kizer JR, Howard BV, Cowan LD, Yeh J, Howard WJ, Wang W, Best L, Lee ET. Incidence and risk factors for stroke in American Indians: the Strong Heart Study. Circulation. 2008;118:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jing J, Pan Y, Zhao X, Zheng H, Jia Q, Li H, Guan L, Liu L, Wang C, Meng X, He Y, Wang Y, Wang Y. Prognosis of ischemic stroke with newly diagnosed diabetes mellitus according to hemoglobin A1c criteria in Chinese population. Stroke. 2016;47:2038–2044. [DOI] [PubMed] [Google Scholar]
- 37. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gosmanov AR, Lu JL, Sumida K, Potukuchi PK, Rhee CM, Kalantar‐Zadeh K, Molnar MZ, Kovesdy CP. Synergistic association of combined glycemic and blood pressure level with risk of complications in US veterans with diabetes. J Hypertens. 2016;34:907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HAW, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia. 2006;49:1761–1769. [DOI] [PubMed] [Google Scholar]
- 40. Yang X, Kong APS, So WY, Ma RCW, Ho CS, Lam CWK, Chow CC, Cockram CS, Tong PCY, Chan JCN. Effects of chronic hyperglycaemia on incident stroke in Hong Kong Chinese patients with type 2 diabetes. Diabetes Metab Res Rev. 2007;23:220–226. [DOI] [PubMed] [Google Scholar]
- 41. Yokoyama H, Matsushima M, Kawai K, Hirao K, Oishi M, Sugimoto H, Takeda H, Minami M, Kobayashi M, Sone H; on behalf of the Japan Diabetes Clinical Data Management Study Group . Low incidence of cardiovascular events in Japanese patients with type 2 diabetes in primary care settings: a prospective cohort study (JDDM 20). Diabet Med. 2011;28:1221–1228. [DOI] [PubMed] [Google Scholar]
- 42. Birkenhager‐Gillesse EG, den Elzen WPJ, Achterberg WP, Mooijaart SP, Gussekloo J, de Craen AJM. Association between glycosylated hemoglobin and cardiovascular events and mortality in older adults without diabetes mellitus in the general population: the Leiden 85‐Plus Study. J Am Geriatr Soc. 2015;63:1059–1066. [DOI] [PubMed] [Google Scholar]
- 43. Lawlor DA, Fraser A, Ebrahim S, Smith GD. Independent associations of fasting insulin, glucose and glycated haemoglobin with stroke and coronary heart disease in older women. PLoS Med. 2007;4:e263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen YY, Lin YJ, Chong E, Chen PC, Chao TF, Chen SA, Chien KL. The impact of diabetes mellitus and corresponding HbA1c levels on the future risks of cardiovascular disease and mortality: a representative cohort study in Taiwan. PLoS One. 2015;10:e0123116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eeg‐Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjornsdottir S, Eliasson B. Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Care. 2010;33:1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Freemantle N, Danchin N, Calvi‐Gries F, Vincent M, Home PD. Relationship of glycaemic control and hypoglycaemic episodes to 4‐year cardiovascular outcomes in people with type 2 diabetes starting insulin. Diabetes Obes Metab. 2016;18:152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elley CR, Kenealy T, Robinson E, Drury PL. Glycated haemoglobin and cardiovascular outcomes in people with type 2 diabetes: a large prospective cohort study. Diabet Med. 2008;25:1295–1301. [DOI] [PubMed] [Google Scholar]
- 48. Moss SE, Klein R, Klein BEK, Meuer SM. The association of glycemia and cause‐specific mortality in a diabetic population. Arch Intern Med. 1994;154:2473–2479. [PubMed] [Google Scholar]
- 49. Xu L, Chan WM, Hui YF, Lam TH. Association between HbA1c and cardiovascular disease mortality in older Hong Kong Chinese with diabetes. Diabet Med. 2012;29:393–398. [DOI] [PubMed] [Google Scholar]
- 50. Hagg S, Thorn LM, Forsblom CM, Gordin D, Saraheimo M, Tolonen N, Waden J, Liebkind R, Putaala J, Tatlisumak T, Groop PH; on behalf of the FinnDiane Study Group . Different risk factor profiles for ischemic and hemorrhagic stroke in type 1 diabetes mellitus. Stroke. 2014;45:2558–2562. [DOI] [PubMed] [Google Scholar]
- 51. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Karas MG, Devereux RB, Wiebers DO, Whisnant JP, Best LG, Lee ET, Howard BV, Roman MJ, Umans JG, Kizer JR. Incremental value of biochemical and echocardiographic measures in prediction of ischemic stroke: the Strong Heart Study. Stroke. 2012;43:720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kranenburg G, van der Graaf Y, van der Leeuw J, Nathoe HMW, de Borst GJ, Kappelle LJ, Visseren FLJ, Westerink J; on behalf of the SMART Study Group . The relation between HbA1c and cardiovascular events in patients with type 2 diabetes with and without vascular disease. Diabetes Care. 2015;38:1930–1936. [DOI] [PubMed] [Google Scholar]
- 54. Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta‐analysis. BMJ. 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. The Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 56. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; for the VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. [DOI] [PubMed] [Google Scholar]
- 57. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 58. Gerstein HC, Miller ME, Ismail‐Beigi F, Largay J, McDonald C, Lochnan HA, Booth GL; for the ACCORD Study Group . Effects of intensive glycaemic control on ischaemic heart disease: analysis of data from the randomised, controlled ACCORD trial. Lancet. 2014;384:1936–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lipska KJ, Krumholz HM. Is hemoglobin A1c the right outcome for studies of diabetes? JAMA. 2017;317:1017–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsboll T; for the SUSTAIN‐6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 61. Marso SP, Daniels GH, Brown‐Frandsen K, Kristensen P, Mann JFE, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; for the LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. The Emerging Risk Factors Collaboration . Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA. 2014;311:1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Scottish Intercollegiate Guidelines Network (SIGN) methodological quality assessment tool [1] automatic exclusion criteria.
Data S2. Inclusion and exclusion criteria for each phase of the search strategy.
Data S3. Association between ADA defined pre–diabetes mellitus and diabetes mellitus range HbA1c and first‐ever stroke risk.
Data S4. Linear regression analysis method for estimating continuous (1% HbA1c increment) effect size data from categorical effect size data
Table S1. The Association Between Rising HbA1c Levels and Stroke Risk in Adults Without Diabetes Mellitus
Table S2. The Association Between Rising HbA1c Levels and Stroke Risk in Adults With Type 1 Diabetes Mellitus
Table S3. The Association Between Rising HbA1c Levels and Stroke Risk in Adults With Type 2 Diabetes Mellitus
Table S4. The Association Between Rising HbA1c Levels and Stroke Risk in Mixed Diabetes Mellitus Cohorts
Table S5. Association Between Rising HbA1c Levels and Ischemic Stroke Risk, in Adults Without Diabetes Mellitus
Table S6. Association Between Rising HbA1c Levels and Ischemic Stroke Risk, in Adults With Diabetes Mellitus
Figure S1. Summary of search terms and Boolean operators used within the search strategy in MEDLINE.
Figure S2. Association between American Diabetes Association–defined pre–diabetes mellitus range HbA1c (5.7–6.5%) and first‐ever stroke risk.
Figure S3. Association between American Diabetes Association–defined diabetes mellitus range HbA1c (≥6.5%) and first‐ever stroke risk.
Figure S4. Linear regression analysis used to confirm linear hypothesis used in estimation of 1% HbA1c data.
Figure S5. 1% HbA1c increment effect size (95% CI) estimation method using the example of Selvin [13].
Figure S6. Sensitivity analysis for inadequate covariate adjustment in study‐quoted 1% HbA1c increment data.
Figure S7. Sensitivity analysis for inadequate covariate adjustment in estimated 1% HbA1c increment data.
Figure S8. Comparison of study‐quoted 1% HbA1c increment first‐ever stroke and first‐ever ischemic stroke effects sizes, in non–diabetes mellitus cohorts.
Figure S9. Comparison of study‐quoted 1% HbA1c increment first‐ever stroke and first‐ever ischemic stroke effects sizes, in diabetes cohorts.
Figure S10. Comparison of study‐quoted and linear regression estimated 1% HbA1c effect size data.
Figure S11. Association between linear regression estimated rising 1% HbA1c increments and stratified first‐ever stroke risk.
Figure S12. Publication bias assessment for intercategorical meta‐analyses within Figures S2 and S3.
Figure S13. Publication bias assessment for subgroup meta‐analyses within Figure 2.
Figure S14. Publication bias assessment for sensitivity analysis within Figure S6.
Figure S15. Publication bias assessment for subgroup meta‐analyses within Figure S11.
Figure S16. Publication bias assessment for sensitivity analysis within Figure S7.
Figure S17. Additional subgroup analysis: association between study‐quoted rising 1% HbA1c increments and first‐ever stroke in non–diabetes mellitus and diabetes mellitus cohorts (as described in Figure 2).
Figure S18. Additional subgroup analysis: association between study‐quoted rising 1% HbA1c increments and first‐ever ischemic stroke in non–diabetes mellitus and diabetes mellitus cohorts (as described in Figure 2).
Figure S19. Additional subgroup analysis: association between study‐quoted rising 1% HbA1c increments and the combined outcome of first‐ever stroke and first‐ever ischemic stroke events, in non–diabetes mellitus cohorts.
Figure S20. Additional subgroup analysis: association between study‐quoted rising 1% HbA1c increments and the combined outcome of first‐ever stroke and first‐ever ischemic stroke events, in diabetes mellitus cohorts.
Figure S21. Additional subgroup analysis: association between study‐quoted rising 1% HbA1c increments and the combined outcome of first‐ever stroke and first‐ever ischemic stroke events, regardless of cohort diabetes mellitus status (combination of Figures S19 and S20).
Figure S22. Additional subgroup analysis: association between first‐ever stroke risk and combined American Diabetes Association–defined pre–diabetes mellitus and diabetes mellitus range HbA1c (≥5.7%), compared to non–diabetes mellitus range HbA1c (<5.7%).
Figure S23. Additional subgroup analysis: comparison of study‐quoted 1% HbA1c increment first‐ever stroke and first‐ever ischemic stroke effect sizes regardless of cohort diabetes mellitus status (combination of Figures S8 and S9).


