Abstract
Background
Little is known about the association of atrial fibrillation symptom burden with quality of life and outcomes.
Methods and Results
In the Prevention of Thromboembolic Events–European Registry in Atrial Fibrillation (n=6196 patients with atrial fibrillation; mean±SD age, 71.8±10.4 years; 39.7% women), we assessed European Heart Rhythm Association score symptoms and calculated correlations with the standardized health status questionnaire (EQ‐5D‐5L). Patients were followed up for atrial fibrillation therapies and outcomes (stroke/transient ischemic attack/arterial thromboembolism, coronary events, heart failure, and major bleeding) over 1 year. Most individuals (92%) experienced symptoms. Correlations with health status and quality of life were modest. In multivariable‐adjusted regression models, the dichotomized European Heart Rhythm Association score (intermediate/frequent versus never/occasional symptoms) was associated with cardioversions (odds ratio [OR], 1.21; 95% confidence interval [CI], 1.01–1.45) and catheter ablation (OR, 1.97; 95% CI, 1.44–2.69), and inversely related with heart rate control (OR, 0.80; 95% CI, 0.70–0.92) and heart failure incidence (OR, 1.65; 95% CI, 1.16–2.34). Anxiety was inversely related with stroke/transient ischemic attack/arterial thromboembolism (OR, 0.55; 95% CI, 0.32–0.93), whereas chest pain related positively with coronary events (OR, 2.45; 95% CI, 1.42–4.22). Fatigue (OR, 1.84; 95% CI, 1.30–2.60), dyspnea (OR, 2.33; 95% CI, 1.63–3.33), and anxiety (OR, 1.72; 95% CI, 1.16–2.55) were associated with heart failure incidence. Palpitations were positively associated with cardioversion (OR, 1.32; 95% CI, 1.08–1.61) and ablation therapy (OR, 2.02; 95% CI, 1.48–2.76).
Conclusions
A higher symptom burden, in particular palpitations, predicted interventions to restore sinus rhythm. The score itself had limited predictive value, but its individual components were related to different and specific clinical events, and may thus be helpful to target patient management.
Keywords: anticoagulation, atrial fibrillation, atrial fibrillation symptoms, European Heart Rhythm Association score, quality of life
Subject Categories: Quality and Outcomes, Atrial Fibrillation
Clinical Perspective
What Is New?
Most patients with atrial fibrillation are symptomatic (>90%), and the symptom burden slightly decreases during 1 year of follow‐up.
The European Heart Rhythm Association symptoms score and its components are moderately related to quality of life, health care use, and cardiovascular outcomes.
Palpitations are predictive of interventions (cardioversion and ablation therapy) to restore sinus rhythm.
What Are the Clinical Implications?
Compared with other European Heart Rhythm Association symptoms, palpitations appear to be strong triggers of interventions to restore and maintain sinus rhythm.
Atrial fibrillation (AF) is a frequent cardiovascular disease with a high symptom burden compromising daily life.1 The European Heart Rhythm Association (EHRA) score has been suggested to assess symptom quality and the impairment in everyday activities caused by arrhythmia‐related symptoms. The score evaluates 6 symptom dimensions (palpitations, fatigue, dizziness, dyspnea, chest pain, and anxiety) in 4 severities, ranging from none to symptom frequency that leads to a discontinuation of daily activities. Such a score was encouraged as a simple, but relatively specific, quantification of AF‐related symptoms that permits the assessment of functional impairment attributable to AF.2, 3 It may serve as a measure of quality of life (QoL) and of the limitations in everyday activities. It was validated as a useful semiquantitative classification of health status and as an indicator of health utility in a monocentric study in a specialized clinic.4 To date, the EHRA score has not been related to AF‐related interventions and meaningful outcomes in large cohorts. In asymptomatic patients with AF, mortality during 1 year appeared to be higher compared with individuals with symptomatic AF.5 In AF outpatients, EHRA score category ≥2 was related to more frequent hospitalizations and possibly higher bleeding risk.6 Whether AF‐related symptom burden, reflected by the EHRA score, is associated with cardiovascular disease outcomes in AF remains largely unknown. In this context, the Prevention of Thromboembolic Events–European Registry in Atrial Fibrillation7 provided the opportunity to prospectively examine the score and its different dimensions in a contemporary European cohort. We focused on temporal stability of symptoms: symptoms as potential triggers for therapeutic interventions during 1‐year follow‐up. Furthermore, we examined the predictive ability of the score for common adverse events, such as thromboembolism, heart failure, coronary events, and major bleeding, in patients with AF after 1 year.
Methods
We cannot make the individual data available without restrictions attributable to limitations of consent. We will look into possibilities to make access to the original data available on request. Data sets analyzed during this study may be made available on request from Daiichi Sankyo Europe.
Ethics Approval and Consent to Participate
Responsible local Ethics Committees, as required by national regulations in Austria, Germany, Switzerland, Italy, Spain, and the United Kingdom, approved the study before the start of enrollment. In France, no specific approval was needed because of the noninterventional nature of the study.
Study Sample
Over 1 year (2012–2013), 7243 patients aged ≥18 years with AF were recruited from 7 European countries (France, Germany, Austria, Switzerland, Italy, Spain, and the United Kingdom)7 into the Prevention of Thromboembolic Events–European Registry in Atrial Fibrillation and followed up for 12 months, with the last follow‐up in January 2014. Patients were enrolled in 461 centers, mostly cardiology practices and hospitals. Follow‐up information was collected by physician‐administered questionnaire and supplemented from medical records. Because of incomplete longitudinal information, 831 individuals were excluded.
Clinical Variables
Clinical Characteristics
AF was based on a physician's diagnosis and was documented by an ECG or a cardiac device (pacemaker/defibrillator). The enrolling physician provided information on anthropometric data, disease history, current clinical presentation, medications, and cardiovascular interventions. The EHRA score was assessed for palpitations, fatigue, dizziness, dyspnea, chest pain, and anxiety.3 Symptom frequency was classified as never, occasional (less than once per month), intermediate (once per month to almost daily), and frequent (at least daily). The maximum category of any of the 6 individual symptoms resulted in the EHRA score. If information on any of the EHRA symptom dimensions was missing at baseline, patients were excluded, leaving 6196 individuals for the analysis.
We further assessed QoL by validated questionnaires on preference‐based measures of health. The EQ‐5D‐5L provides preference‐based health‐related utility to assess cost‐effectiveness.8 The Perception of Anti‐Coagulant Treatment Questionnaire (PACT‐Q) was specifically designed to quantify expectations and treatment satisfaction with oral anticoagulation.9 Sample items of both questionnaires are provided in Tables S1 and S2.
Interventions and outcomes
We collected information on pharmacological or electrical cardioversion attempts during follow‐up, as well as catheter‐based ablation therapy. As outcomes, we assessed the presence of sinus rhythm on the follow‐up ECG and adequate heart rate control. The latter was defined as a heart rate between 60 and 100 beats per minute during the clinic visit. Disease outcomes were ischemic stroke/transient ischemic attack/arterial embolism, coronary events (acute coronary syndrome/coronary revascularization), heart failure, and major bleeding. Heart failure was defined as a physician‐diagnosed condition or reduced left ventricular ejection fraction. Major bleeding comprised cerebrovascular bleeding and major gastrointestinal or other bleeding events usually requiring blood transfusion.
Statistical Analyses
From 7243 patients, 831 (11.5%) did not have a follow‐up visit. Therefore, the data analysis was performed with complete EHRA score information at baseline and follow‐up in 6196 patients. At baseline, 2.98% of the patients had missing values. To determine the assumption of missing completely at random, we compared the baseline characteristics of the individuals used for the analyses and the patients excluded from the analyses. We did not observe significant differences in the distribution of baseline variables (Table S3).
Variables are presented as mean±SD for continuous variables and number (percentage) for discrete variables. In a multivariable stepwise selection model, we calculated β estimates and F values in relation to the EHRA score.
To understand correlations with other metrics of QoL, Spearman rank correlation coefficients for the EHRA score and its components with EQ‐5D‐5L and PACT‐Q items were calculated.
We examined symptom stability over 1 year comparing the proportion of individuals in each EHRA symptom category at baseline and follow‐up by the McNemar test. We tested for interactions by cardioversion or ablation therapy during follow‐up.
We performed multiple logistic regression models for the EHRA score as a categorical variable and for each symptom dimension separately using ANCOVA across categories in relation to interventions and adverse cardiovascular events. Because of small numbers in the EHRA score categories 1 and 4, we also dichotomized the EHRA score into class 3/4 versus 1/2. Odds ratios (ORs) for the different EHRA classes compared with EHRA score 1 are provided in the Supplemental Material. We chose outcomes on the basis of end points suggested in the literature.1 Interventions included cardioversions (pharmacological and electrical cardioversion) and catheter‐based ablation (pulmonary vein isolation). Major dichotomous adverse cardiovascular outcomes were ischemic stroke/transient ischemic attack/arterial thromboembolic events, coronary events (acute coronary syndrome, including myocardial infarction and unstable angina pectoris, and coronary revascularization), heart failure, and major bleeding. We adjusted for age, sex, and country. In a second model, we additionally adjusted for body mass index, systolic blood pressure/hypertension, diabetes mellitus, current smoking, heart failure, and myocardial infarction. The model examining incident heart failure excluded individuals with heart failure at baseline (N=1723) and patients with missing information about heart failure at baseline (N=165). We chose predictors and potential confounding variables because they have been related to symptoms, disease severity, and outcomes (Data S1).
For adjusted analyses, we used multivariable logistic regression model fitted via the SAS procedure logistic. We calculated P values derived by bootstrapping for the clinical variables to assess the predictive accuracy (Table S4). We also analyzed the area under the curves from resubstitution and from 10‐fold cross validation for the following: (1) the whole model and the EHRA components and (2) the partial model and the EHRA components provided in Tables S5 and S6.
For all the analyses, we used SAS software, version 9.4 (SAS Institute, Cary, NC). A 2‐tailed threshold of 0.05 was chosen to indicate statistical significance. Our analyses are exploratory in nature.
Results
Baseline Characteristics
Baseline characteristics of the sample are provided in Table 1. The mean age was 72 years, and ≈40% were women. Patients showed a substantial cardiovascular risk factor burden and a high prevalence of cardiovascular disease, with ≈10% previous myocardial infarction, ≈30% prevalent heart failure, and 15% previous stroke/transient ischemic attack/other ischemic‐thromboembolic events. Most patients were symptomatic; 92% indicated an EHRA score ≥2. The characteristics of individuals excluded because of missing EHRA score information are shown in Table S7.
Table 1.
Baseline Characteristics of the Study Sample
| Characteristics | Value (N=6196) |
|---|---|
| Age, y | 71.8±10.4 |
| Female sex, N (%) | 2460 (39.7) |
| Body mass index, kg/m2 | 27.9±5.0 |
| Systolic blood pressure, mm Hg | 131.5±16.5 |
| Hypertension, N (%) | 4514 (73.3) |
| Ever smoking, N (%) | 2331 (39.7) |
| Alcohol abuse, N (%) | 157 (2.6) |
| Diabetes mellitus, N (%) | 1368 (22.3) |
| Dyslipidemia, N (%) | 2697 (44.5) |
| History of myocardial infarction, N (%) | 663 (10.7) |
| Heart failure, N (%) | 1723 (28.6) |
| History of ischemic stroke/TIA/other ischemic‐thromboembolic event, N (%) | 924 (15.1) |
| EHRA score ≥2, N (%) | 5695 (91.9) |
| History of cardioversion and/or ablation, N (%) | 2147 (34.7) |
| Interventions during 1 y, N (%) | |
| Cardioversion | 701 (11.3) |
| Catheter‐based ablation | 226 (3.4) |
| Outcomes over 1 y, N (%) | |
| Stroke/TIA/arterial thromboembolic events | 136 (2.2) |
| Coronary events | 140 (2.3) |
| Heart failure | 155 (2.5) |
| Major bleeding | 168 (2.7) |
| Sinus rhythm | 2022 (32.6) |
| Adequate heart rate | 2734 (44.1) |
Data are presented as mean±SD for continuous variables and number (percentage) for discrete variables. In some patients, different variables were missing, as seen from the numbers. EHRA indicates European Heart Rhythm Association; TIA, transient ischemic attack.
Strongest clinical correlates of the EHRA score selected from clinical variables in the baseline table were heart failure and female sex. Furthermore, ever smoking, a history of cardioversion and/or ablation, body mass index, and, with smaller estimates, diabetes mellitus and a history of myocardial infarction were selected (Table 2). Ten‐fold cross‐validation P values showed comparable results (Table S4).
Table 2.
Multivariable Stepwise Selection Model for Clinical Correlates of EHRA Score at Baseline
| Variables | Partial R 2 | Model R 2 | β Value | F Value | P Value |
|---|---|---|---|---|---|
| Female sex | 0.0230 | 0.0590 | 0.361±0.025 | 139.47 | <0.0001 |
| Ever smoking | 0.0104 | 0.0694 | 0.190±0.026 | 62.68 | <0.0001 |
| History of cardioversion and/or ablation | 0.0043 | 0.0737 | 0.124±0.024 | 26.21 | <0.0001 |
| Body mass index, kg/m² | 0.0031 | 0.0769 | 0.011±0.002 | 18.87 | <0.0001 |
| Diabetes mellitus | 0.0008 | 0.0776 | 0.065±0.029 | 4.73 | 0.030 |
| History of myocardial infarction | 0.0008 | 0.0785 | 0.086±0.038 | 5.03 | 0.025 |
| Heart failure | 0.0360 | 0.0360 | 0.387±0.026 | 208.49 | <0.0001 |
All variables from Table 1 were permitted to enter the analysis. Partial R 2 is provided for the variation in EHRA score explained by the clinical variable. β, the regression coefficient, shows estimated change in EHRA score for 1‐unit increment in body mass index or the condition present for categorical variables. EHRA indicates European Heart Rhythm Association.
Correlations With Other Metrics of QoL
Although correlations between EHRA score and its components with EQ‐5D‐5L achieved statistical significance for most bivariate correlations, the strength was moderate (Table 3). The highest Spearman correlation coefficient was observed between EHRA score and the ability to perform usual activities (r s=0.308, P<0.0001). The maximum correlation for components of the EHRA score was seen for dyspnea and usual activities (r s=0.339, P<0.0001).
Table 3.
Spearman Correlation Coefficients for the EHRA Score and Its Components With EQ‐5D‐5L
| EHRA Score | Anxiety | Chest Pain | Palpitations | Dyspnea | Fatigue | Dizziness |
|---|---|---|---|---|---|---|
| Mobility (5 levels) | ||||||
| 0.26876 | 0.14588 | 0.16897 | 0.26876 | 0.30645 | 0.28930 | 0.20188 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Self‐care (5 levels) | ||||||
| 0.19867 | 0.16513 | 0.17959 | 0.11487 | 0.23563 | 0.18963 | 0.19849 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Usual activities (5 levels) | ||||||
| 0.30837 | 0.18304 | 0.21227 | 0.13342 | 0.33949 | 0.31344 | 0.24013 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Pain/discomfort (5 levels) | ||||||
| 0.23616 | 0.18290 | 0.21180 | 0.10504 | 0.26378 | 0.25211 | 0.24013 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Anxiety/depression (5 levels) | ||||||
| 0.27861 | 0.47612 | 0.17020 | 0.10504 | 0.20609 | 0.22386 | 0.18526 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Visual analogue scale (your health today) (numerical) | ||||||
| −0.28372 | −0.21182 | −0.21887 | −0.13869 | −0.30841 | −0.31414 | −0.22712 |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Spearman's r s is provided in the top row, and the corresponding P value is in the bottom row. EHRA indicates European Heart Rhythm Association.
Spearman correlation coefficients of the EHRA score and PACT‐Q items are shown in Table S8. Despite statistical significance, the correlations were weak.
Symptom Development Over Time
In the Figure, we show the proportion of EHRA symptom severity for the 6 dimensions on enrollment and after 1 year. Fatigue was most frequently reported. Only 26.4% of patients at baseline and 32.1% at follow‐up never had symptoms of fatigue. The least frequent symptom of the 6 symptoms of the EHRA score was chest pain, which was never experienced by 70.5% of patients at baseline and 77.6% of patients at follow‐up. Most individuals (92%) experienced at least 1 symptom. Over 1 year, symptom severity appeared to improve for all EHRA dimensions (P<0.001). The largest reduction in symptom frequency was observed for palpitations. The highest symptom stability (ie, patients staying in the same category at baseline and follow‐up) was seen for chest pain (72.8%), dizziness (61.5%), and anxiety (57.7%). These last 3 symptom dimensions did not show significant interactions with cardioversion or ablation therapy. The EHRA score, palpitations, fatigue, and dyspnea showed statistically significant interactions by cardioversion and ablation therapy, with a larger improvement when these interventions were performed successfully, even after adjustment for antiarrhythmic drug use. Results for the unadjusted model are provided in Table S9.
Figure 1.
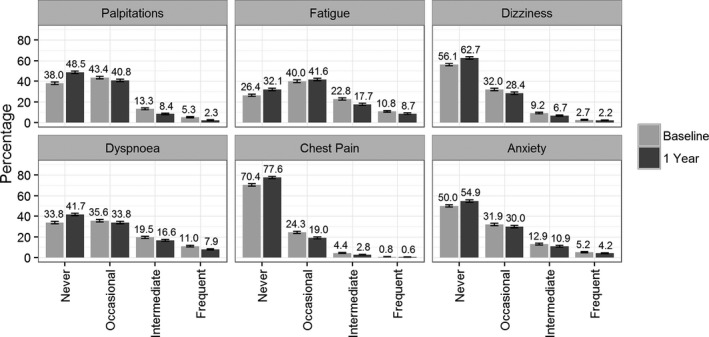
Distribution of European Heart Rhythm Association score categories across the 6 symptom dimensions at baseline and after 1 year. Percentages are provided for each category.
EHRA Score Symptoms and Interventions
In Table 4, we provide risk factor–adjusted logistic regression analyses for EHRA score and EHRA symptom dimensions separately in relation to interventions to restore sinus rhythm for EHRA score class 3/4 (intermediate/frequent) versus 1/2 (never/occasional).
Table 4.
Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Interventions to Restore Sinus Rhythm at 1 Year
| Variable | Odds Ratio for EHRA 3/4 Versus 1/2 | 95% Confidence Interval | P Value | P Value (Adjusted Logistic Regression Across All 4 Categories) |
|---|---|---|---|---|
| Cardioversion, N=701 (11.3%) | ||||
| Palpitations | 1.32 | 1.08 to 1.61 | 0.0073 | <0.0001 |
| Fatigue | 1.16 | 0.97 to 1.39 | 0.11 | 0.18 |
| Dizziness | 1.27 | 0.98 to 1.64 | 0.07 | 0.32 |
| Dyspnea | 1.05 | 0.86 to 1.27 | 0.65 | 0.79 |
| Chest pain | 1.37 | 0.97 to 1.93 | 0.08 | 0.20 |
| Anxiety | 0.79 | 0.63 to 0.99 | 0.042 | 0.08 |
| EHRA score | 1.21 | 1.01 to 1.45 | 0.040 | 0.07 |
| Catheter‐based ablation, N=226 (3.4%) | ||||
| Palpitations | 2.02 | 1.48 to 2.76 | <0.0001 | <0.0001 |
| Fatigue | 1.67 | 1.23 to 2.26 | 0.0009 | 0.0017 |
| Dizziness | 1.11 | 0.70 to 1.75 | 0.65 | 0.90 |
| Dyspnea | 1.19 | 0.84 to 1.68 | 0.26 | 0.008 |
| Chest pain | 1.41 | 0.79 to 2.52 | 0.24 | 0.25 |
| Anxiety | 1.26 | 0.88 to 1.80 | 0.21 | 0.20 |
| EHRA score | 1.97 | 1.44 to 2.69 | <0.0001 | <0.0001 |
| Sinus rhythm at 1 y, N=2022 (32.6%) | ||||
| Palpitations | 1.51 | 1.30 to 1.76 | <0.0001 | <0.0001 |
| Fatigue | 0.98 | 0.86 to 1.12 | 0.76 | 0.93 |
| Dizziness | 0.90 | 0.74 to 1.10 | 0.31 | 0.75 |
| Dyspnea | 0.86 | 0.74 to 0.99 | 0.033 | 0.003 |
| Chest pain | 1.44 | 1.90 to 1.10 | 0.0085 | 0.012 |
| Anxiety | 1.04 | 0.89 to 1.22 | 0.60 | 0.007 |
| EHRA score | 1.06 | 0.93 to 1.20 | 0.40 | 0.048 |
| Adequate heart rate control, N=2734 (44.1%) | ||||
| Palpitations | 0.81 | 0.68 to 0.95 | 0.012 | 0.031 |
| Fatigue | 0.86 | 0.74 to 0.99 | 0.036 | 0.03 |
| Dizziness | 1.004 | 0.82 to 1.23 | 0.97 | 0.23 |
| Dyspnea | 0.88 | 0.76 to 1.02 | 0.10 | 0.16 |
| Chest pain | 0.84 | 0.62 to 1.14 | 0.27 | 0.51 |
| Anxiety | 0.91 | 0.76 to 1.07 | 0.25 | 0.51 |
| EHRA score | 0.80 | 0.70 to 0.92 | 0.0015 | 0.005 |
Odds ratios are provided for EHRA score category 3/4 vs 1/2. P values across all 4 categories are from logistic regression analyses, as implemented in SAS proc logistic. All models are adjusted for age, sex, country, body mass index, systolic blood pressure, hypertension, diabetes mellitus, smoking, heart failure, and history of myocardial infarction. EHRA indicates European Heart Rhythm Association.
EHRA score as a categorical variable was statistically significantly associated with cardioversion therapy (OR, 1.22; 95% confidence interval [CI], 1.01–1.45; P=0.0398) and catheter‐based ablation procedures (OR, 1.97; 95% CI, 1.44–2.69; P<0.0001); and it was inversely associated with adequate heart rate control (OR, 0.80; 95% CI, 0.70–0.92; P=0.0015) at follow‐up. In 10‐fold cross validation, the statistical significance of these interventions remained similar (Table S5). Of the symptoms investigated, a high symptom burden of palpitations was predictive of cardioversion therapy, ablation, and the presence of sinus rhythm at follow‐up, and it was inversely correlated with adequate heart rate control. Statistical significance was more frequently achieved when assessed across all categories. Results for the unadjusted model are provided in Table S10.
Clinical Outcomes
The EHRA score was associated with heart failure incidence during 1 year (OR, 1.65; 95% CI, 1.16–2.34; P=0.0053) (Table 5). Anxiety was inversely related with stroke (OR, 0.55; 95% CI, 0.32–0.93; P=0.0245). Chest pain was associated with coronary events (OR, 2.45; 95% CI, 1.42–4.22; P=0.001). Fatigue (OR, 1.84; 95% CI, 1.30–2.60; P=0.0006), dyspnea (OR, 2.33; 95% CI, 1.63–3.33; P<0.0001), and anxiety (OR, 1.72; 95% CI, 1.16–2.55; P=0.0069) were associated with the incidence of heart failure. None of the EHRA score symptoms were predictive of major bleeding events. P values derived from 10‐fold cross validation are provided in Table S6. Results for the unadjusted model are provided in Table S10. Tables S11 through S15 provide the multivariable adjusted results for EHRA score categories separately, with EHRA class 1 as the reference. Unadjusted models are presented in Tables S14 through S16.
Table 5.
Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
| Variable | Odds Ratio for EHRA 3/4 Versus 1/2 | 95% Confidence Interval | P Value | P Value (Adjusted Logistic Regression Across All 4 Categories) |
|---|---|---|---|---|
| Stroke/TIA/arterial thromboembolic events, N=136 (2.2%) | ||||
| Palpitations | 0.92 | 0.58 to 1.46 | 0.72 | 0.29 |
| Fatigue | 1.04 | 0.72 to 1.51 | 0.82 | 0.92 |
| Dizziness | 1.32 | 0.82 to 2.13 | 0.26 | 0.002 |
| Dyspnea | 1.04 | 0.71 to 1.53 | 0.84 | 0.59 |
| Chest pain | 1.69 | 0.92 to 3.10 | 0.09 | 0.002 |
| Anxiety | 0.55 | 0.32 to 0.93 | 0.025 | 0.08 |
| EHRA score | 1.02 | 0.70 to 1.49 | 0.92 | 0.05 |
| Coronary events, N=140 (2.3%) | ||||
| Palpitations | 0.98 | 0.60 to 1.60 | 0.95 | 0.21 |
| Fatigue | 1.09 | 0.75 to 1.58 | 0.64 | 0.73 |
| Dizziness | 1.16 | 0.70 to 1.91 | 0.57 | 0.72 |
| Dyspnea | 1.32 | 0.90 to 1.93 | 0.16 | 0.02 |
| Chest pain | 2.45 | 1.42 to 4.22 | 0.001 | <0.0001 |
| Anxiety | 1.41 | 0.91 to 2.18 | 0.13 | 0.33 |
| EHRA score | 1.45 | 0.99 to 2.13 | 0.06 | 0.12 |
| Heart failure, N=155 (2.5%) | ||||
| Palpitations | 1.14 | 0.74 to 1.76 | 0.56 | 0.42 |
| Fatigue | 1.84 | 1.30 to 2.60 | 0.0006 | 0.0006 |
| Dizziness | 1.50 | 0.93 to 2.41 | 0.10 | 0.19 |
| Dyspnea | 2.33 | 1.63 to 3.33 | <0.0001 | <0.0001 |
| Chest pain | 1.25 | 0.57 to 2.75 | 0.58 | 0.42 |
| Anxiety | 1.72 | 1.16 to 2.55 | 0.0069 | 0.03 |
| EHRA score | 1.65 | 1.16 to 2.34 | 0.0053 | 0.005 |
| Major bleeding, N=168 (2.7%) | ||||
| Palpitations | 0.88 | 0.55 to 1.41 | 0.59 | 0.78 |
| Fatigue | 1.27 | 0.90 to 1.79 | 0.17 | 0.28 |
| Dizziness | 1.08 | 0.67 to 1.74 | 0.74 | 0.90 |
| Dyspnea | 0.92 | 0.64 to 1.34 | 0.67 | 0.61 |
| Chest pain | 0.91 | 0.42 to 1.98 | 0.81 | 0.74 |
| Anxiety | 1.21 | 0.80 to 1.83 | 0.36 | 0.46 |
| EHRA score | 1.09 | 0.77 to 1.54 | 0.64 | 0.60 |
Odds ratios are provided for EHRA score category 3/4 vs 1/2 in a multivariable‐adjusted model. P values across all 4 categories are from logistic regression analyses, as implemented in SAS proc logistic. Covariates are age, sex, country, body mass index, systolic blood pressure, hypertension, diabetes mellitus, smoking, heart failure, and history of myocardial infarction. Coronary events comprised acute coronary syndrome and coronary revascularization. The model for incident heart failure does not include adjustment for heart failure. EHRA indicates European Heart Rhythm Association; TIA, transient ischemic attack.
Discussion
Main Findings
In a contemporary cohort of patients with AF, the EHRA score and its different symptom dimensions were moderately correlated with commonly used measures of QoL in AF. The score was predictive of interventions to restore sinus rhythm. Among symptoms, only palpitations were consistently related to interventions and rhythm at 1 year. Although the score itself was not strongly related to outcomes, different symptom dimensions were specifically predictive of cardiovascular outcomes (ie, anxiety for stroke; chest pain for coronary events; and fatigue, dyspnea, and anxiety for incident heart failure).
Symptom burden and QoL
The EHRA score has been recommended to specifically quantify AF‐related symptom burden.3 It has been validated with a moderate correlation with the Atrial Fibrillation Effect on Quality‐of‐Life questionnaire.4, 6 Prior studies reported a strong negative correlation between the EHRA class and QoL assessed by components of the EQ‐5D. We can demonstrate a weak correlation of EHRA score or its different symptom dimensions with EQ‐5D‐5L. Generic QoL measures mirror general functioning and well‐being. They may be confounded by general characteristics of patients with AF, such as age, and lack potentially treatable dimensions that are specific to AF.10 No relevant association was shown for PACT‐Q. This finding is plausible, because the PACT‐Q was developed to assess expectations and treatment satisfaction with AF‐related anticoagulation,9 but not necessarily effects of AF on health‐related well‐being.
Symptoms in relation to interventions
In the European AF guidelines, the EHRA score is recommended to tailor treatment for symptoms.11 In our cohort, the EHRA score was a strong predictor of cardioversion and catheter‐based ablation procedures during a 1‐year follow‐up. A higher symptom burden has clearly been associated with ablation therapy.4 The score is most widely used by electrophysiologists who are crucial in the decision process to initiate therapies to restore sinus rhythm.
Among the different symptom dimensions of the EHRA score, palpitations were significantly predictive of interventions to restore sinus rhythm. In addition, they were correlated with the presence of sinus rhythm and, inversely, with heart rate control, at follow‐up. Palpitations are a common symptom and fairly specific in the setting of AF. If a regular rhythm can be achieved, symptoms resolve, which may explain the predictive ability for rhythm control treatment.4 Palpitations thus appear to be a trigger to search for ways to restore and maintain sinus rhythm. In patients with perceived irregular heartbeat, sinus rhythm was more frequently reached rather than heart rate control. The latter was inversely associated with palpitations. The largest reduction in symptom burden over 1 year also was demonstrated for palpitations. Thus, palpitations appear to receive high attention and are targeted as a marker of successful treatment. On the other hand, it needs to be considered that palpitations may be subject to least recall bias and may therefore show stronger associations than other EHRA score components. Other symptom dimensions were not consistently related to interventions and rhythm. Fatigue and dyspnea significantly improved after successful cardioversion and ablation therapies and could be considered as triggers for interventions to improve AF symptom burden.
In our study, the overall symptom burden was high, which may be explained by the specific assessment of all EHRA symptom dimensions required by the protocol. There was a trend towards a lower symptom burden after 1 year, which may indicate the success of regular follow‐up by a cardiologist for the reduction of symptoms, which is a major goal of AF treatment. In particular, we observed significant interactions for cardioversion and ablation therapy that were related to a reduced symptom burden. It is well known that AF‐related therapy helps to improve symptom burden.12, 13, 14 In a prior observational study, QoL in patients with newly diagnosed AF reached normal values under pharmacologic therapy and cardioversion.15 In this context, our data also show that EHRA score symptoms are sensitive to changes related to AF management over 1 year, an important quality of symptom measurement scales.
Symptom burden and outcomes
In our sample, the overall symptom burden measured by the EHRA score showed an association with the incidence of heart failure, but not with other cardiovascular outcomes. Our findings are in line with evidence from the literature. Symptomatic patients (EHRA score ≥2) did not show an increased risk of stroke, myocardial infarction, or mortality in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation.6 Asymptomatic individuals in the AFFIRM (Atrial Fibrillation Follow‐Up Investigation of Rhythm Management) trial had a comparable prognosis after accounting for clinical confounders,16 and asymptomatic subclinical AF carries a significant risk of thromboembolic events.17 In other studies, hospitalizations have been reported to be more frequent in patients with symptoms, and they may have driven associations with combined cardiovascular outcomes.6, 18, 19 Symptoms per se, combined in the EHRA score, do not appear to be strongly predictive of adverse events. Therefore, in line with our data, overall symptom severity does not carry a high prognostic utility.
In contrast, specific symptoms of the EHRA score, which overlap with other cardiovascular disease entities, appear to be more relevant for prognosis. Chest pain was a comparatively uncommon symptom in the Prevention of Thromboembolic Events–European Registry in Atrial Fibrillation, but it was strongly related to coronary events. The inverse association of anxiety with stroke is likely a spurious finding because of the comparatively small number of strokes. Prior cohort studies demonstrated an increased stroke risk in individuals with anxiety.20 In patients with AF, anxiety may be related to better compliance and medication adherence, however we could not demonstrate clear evidence to prove these assumption in our data. Fatigue and dyspnea, classic symptoms of heart failure, were associated with incident disease. Thus, specific symptoms need to be taken into account seriously in clinical practice and may require targeted workup to possibly avoid disease complications.
Limitations
Inherent to registry data from different centers, bias attributable to enrollment decisions, quality of data collection, and follow‐up may have been introduced, despite training of the participating cardiologists and central data management. In addition, the EHRA score calculation in practice often is more subjective, and it shows less measurement accuracy because we used the maximum severity achieved for any of the symptom dimensions, which resulted in a relatively high symptom burden and may slightly impair the comparability with common practice. Compared with a recent study,21 the proportion of interventions (cardioversions or catheter‐based ablation therapies) was small, which may indicate a slightly older and sicker patient sample with more permanent AF and may reduce the generalizability to younger patients.
On the other hand, our large data set provides valuable insights into current symptom‐related health care patterns, treatment decisions, and outcomes.
In conclusion, our prospective data in a contemporary cohort with AF show that the EHRA symptom score and its components are moderately related to QoL and health utility of cardioversion and ablation therapy, and they predict a higher proportion of sinus rhythm after 1 year. Although the EHRA score is not strongly related to outcomes, its specific components should be considered to assess patients’ prognosis. Thus, the EHRA score or slight modifications of it appear to be useful for clinical workup, which needs to be proved in future trials.
Author Contributions
Schnabel designed the analysis, interpreted the data, and wrote the article. Pecen designed the analysis, performed the statistical analysis, and critically reviewed and revised the article. Rzayeva wrote parts of the article, interpreted the data, and critically reviewed the article. Lucerna designed the study, obtained the funding, and critically reviewed the article. Purmah critically reviewed the article. Ojeda interpreted the data, wrote parts of the article, and critically reviewed the article. De Caterina designed the study, obtained the funding, and critically reviewed the article. Kirchhof interpreted the data, designed the study, obtained the funding, and critically reviewed the article.
Sources of Funding
This project has received funding from the European Research Council under the European Union's Horizon 2020 research and innovation program (grant agreement 648131). This work was performed in the context of the Junior Research Alliance symAtrial project funded by the German Ministry of Research and Education (BMBF 01ZX1408A) e:Med—Systems Medicine program. Schnabel is funded by Deutsche Forschungsgemeinschaft (German Research Foundation) Emmy Noether Program Schnabel (SCHN)1149/3‐1. The Prevention of Thromboembolic Events–European Registry in Atrial Fibrillation has been funded by Daiichi Sankyo Europe.
Disclosures
The Prevention of Thromboembolic Events–European Registry in Atrial Fibrillation study sponsor via a contract research organization (SSS International Clinical Research GmbH, Munich, Germany) was Daiichi Sankyo Europe GmbH (Munich, Germany). The study has an independent scientific steering committee. De Caterina reports that his institution received research grant support from Boehringer‐Ingelheim, Bayer, Bristol‐Myers Squibb/Pfizer, Portola and Roche; and honoraria for lectures and/or consulting from Boehringer‐Ingelheim, Bayer, Bristol‐Myers Squibb/Pfizer, Daiichi Sankyo, Lilly, AstraZeneca, Merck, and Novartis. Kirchhof receives research support from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in AF, and has received honoraria from several such companies. Kirchhof is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). The remaining authors have no disclosures to report.
Supporting information
Data S1. Supplementary methods.
Table S1. Selected Items From the EQ‐5D‐5L Questionnaire
Table S2. Selected Items From the Perception of Anticoagulant Treatment Questionnaire (PACT‐Q)14
Table S3. Baseline Characteristics of the Patients Entering the Analyses Compared to Patients Excluded From the Analyses Due to Missing Follow‐Up Information or Missing EHRA Data at Baseline
Table S4. Multivariable Stepwise Selection Model for Variables Correlates of EHRA Score at Baseline
Table S5. Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Interventions to Restore Sinus Rhythm at 1 Year
Table S6. Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S7. Baseline Characteristics of Individuals Excluded Due to Missing Information on Any EHRA Score
Table S8. Spearman Correlation Coefficients for the EHRA Score and its Components With PACT‐Q Items
Table S9. Unadjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Interventions to Restore Sinus Rhythm at 1 Year
Table S10. Unadjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S11. Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S12. Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S13. Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S14. Unadjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S15. Unadjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S16. Unadjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Acknowledgments
We thank all the participants for their time and efforts with the establishment of the registry. The authors had full access to the data and take responsibility for their integrity. All authors read and agreed to the article as written.
(J Am Heart Assoc. 2018;7:e007559 DOI: 10.1161/JAHA.117.007559.)29776959
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wyse DG. Overview of endpoints in atrial fibrillation studies. Heart Rhythm. 2004;1(suppl):B3–B7, discussion B7. [DOI] [PubMed] [Google Scholar]
- 3. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener H‐C, Goette A, Hindricks G, Hohnloser S, Kappenberger L, Kuck K‐H, Lip GYH, Olsson B, Meinertz T, Priori S, Ravens U, Steinbeck G, Svernhage E, Tijssen J, Vincent A, Breithardt G. Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace. 2007;9:1006–1023. [DOI] [PubMed] [Google Scholar]
- 4. Wynn GJ, Todd DM, Webber M, Bonnett L, McShane J, Kirchhof P, Gupta D. The European Heart Rhythm Association symptom classification for atrial fibrillation: validation and improvement through a simple modification. Europace. 2014;16:965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, Sinagra G, Petrescu L, Tavazzi L, Maggioni AP, Lip GYH. Asymptomatic atrial fibrillation: clinical correlates, management, and outcomes in the EORP‐AF Pilot General Registry. Am J Med. 2015;128:509–518.e502. [DOI] [PubMed] [Google Scholar]
- 6. Freeman JV, Simon DN, Go AS, Spertus J, Fonarow GC, Gersh BJ, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Chang P, Peterson ED, Piccini JP. Association between atrial fibrillation symptoms, quality of life, and patient outcomes: results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT‐AF). Circ Cardiovasc Qual Outcomes. 2015;8:393–402. [DOI] [PubMed] [Google Scholar]
- 7. Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey J‐Y, Schilling RJ, Schmitt J, Zamorano JL. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events—European Registry in Atrial Fibrillation (PREFER in AF). Europace. 2014;16:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, Swinburn P, Busschbach J. Measurement properties of the EQ‐5D‐5L compared to the EQ‐5D‐3L across eight patient groups: a multi‐country study. Qual Life Res. 2013;22:1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prins M, Guillemin I, Gilet H, Gabriel S, Essers B, Raskob G, Kahn S. Scoring and psychometric validation of the Perception of Anticoagulant Treatment Questionnaire (PACT‐Q©). Health Qual Life Outcomes. 2009;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabin R, de Charro F. EQ‐5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. [DOI] [PubMed] [Google Scholar]
- 11. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. [DOI] [PubMed] [Google Scholar]
- 12. Kuck KH, Furnkranz A, Chun KR, Metzner A, Ouyang F, Schluter M, Elvan A, Lim HW, Kueffer FJ, Arentz T, Albenque JP, Tondo C, Kuhne M, Sticherling C, Brugada J. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality‐of‐life outcomes in the FIRE AND ICE trial. Eur Heart J. 2016;37:2858–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woźniak‐Skowerska IM, Skowerski MJ, Hoffmann A, Nowak S, Faryan M, Kolasa J, Skowerski T, Szydło K, Wnuk‐Wojnar AM, Mizia‐Stec K. Quality of life in patients with paroxysmal atrial fibrillation after circumferential pulmonary vein ablation. Kardiol Pol. 2016;74:244–250. [DOI] [PubMed] [Google Scholar]
- 14. Nikolaidou T, Channer KS. Chronic atrial fibrillation: a systematic review of medical heart rate control management. Postgrad Med J. 2009;85:303–312. [DOI] [PubMed] [Google Scholar]
- 15. Reynolds MR, Lavelle T, Essebag V, Cohen DJ, Zimetbaum P. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new‐onset atrial fibrillation: the Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152:1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flaker GC, Belew K, Beckman K, Vidaillet H, Kron J, Safford R, Mickel M, Barrell P. Asymptomatic atrial fibrillation: demographic features and prognostic information from the Atrial Fibrillation Follow‐up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149:657–663. [DOI] [PubMed] [Google Scholar]
- 17. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 18. Rienstra M, Vermond RA, Crijns HJGM, Tijssen JGP, Van Gelder IC. Asymptomatic persistent atrial fibrillation and outcome: results of the RACE study. Heart Rhythm. 2014;11:939–945. [DOI] [PubMed] [Google Scholar]
- 19. Vermond RA, Crijns HJGM, Tijssen JGP, Alings AM, Van den Berg MP, Hillege HL, Van Veldhuisen DJ, Van Gelder IC, Rienstra M. Symptom severity is associated with cardiovascular outcome in patients with permanent atrial fibrillation in the RACE II study. Europace. 2014;16:1417–1425. [DOI] [PubMed] [Google Scholar]
- 20. Emdin CA, Odutayo A, Wong CX, Tran J, Hsiao AJ, Hunn BH. Meta‐analysis of anxiety as a risk factor for cardiovascular disease. Am J Cardiol. 2016;118:511–519. [DOI] [PubMed] [Google Scholar]
- 21. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, Dan GA, Kalarus Z, Tavazzi L, Maggioni AP, Lip GY. “Real‐world” management and outcomes of patients with paroxysmal vs. non‐paroxysmal atrial fibrillation in Europe: the EURObservational Research Programme‐Atrial Fibrillation (EORP‐AF) General Pilot Registry. Europace. 2016;18:648–657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary methods.
Table S1. Selected Items From the EQ‐5D‐5L Questionnaire
Table S2. Selected Items From the Perception of Anticoagulant Treatment Questionnaire (PACT‐Q)14
Table S3. Baseline Characteristics of the Patients Entering the Analyses Compared to Patients Excluded From the Analyses Due to Missing Follow‐Up Information or Missing EHRA Data at Baseline
Table S4. Multivariable Stepwise Selection Model for Variables Correlates of EHRA Score at Baseline
Table S5. Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Interventions to Restore Sinus Rhythm at 1 Year
Table S6. Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S7. Baseline Characteristics of Individuals Excluded Due to Missing Information on Any EHRA Score
Table S8. Spearman Correlation Coefficients for the EHRA Score and its Components With PACT‐Q Items
Table S9. Unadjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Interventions to Restore Sinus Rhythm at 1 Year
Table S10. Unadjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S11. Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S12. Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S13. Multivariable‐Adjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S14. Unadjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S15. Unadjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year
Table S16. Unadjusted Logistic Regression Analyses for EHRA Score and EHRA Symptom Dimensions Separately in Relation to Cardiovascular Outcomes at 1 Year


