Abstract
Background
Ideal cardiovascular health metrics (defined by the American Heart Association Life's Simple 7 [LS7]) are suboptimal among blacks, which results in high risk of cardiovascular disease. We examined the association of multiple stressors with LS7 components among blacks.
Methods and Results
Using a community‐based cohort of blacks (N=4383), we examined associations of chronic stress, minor stressors, major life events, and a cumulative stress score with LS7 components (smoking, diet, physical activity, body mass index, blood pressure, total cholesterol, and fasting plasma glucose) and an LS7 composite score. Multivariable logistic regression assessed the odds of achieving intermediate/ideal levels of cardiovascular health adjusted for demographic, socioeconomic, behavioral, and biomedical factors. The LS7 components with the lowest percentages of intermediate/ideal cardiovascular health levels were diet (39%), body mass index (47%), and physical activity (51%). Higher chronic, minor, and cumulative stress scores were associated with decreased odds (odds ratio [OR]) of achieving intermediate/ideal levels for smoking (OR [95% confidence interval], 0.80 [0.73–0.88], 0.84 [0.75–0.94], and 0.81 [0.74–0.90], respectively). Participants with more major life events had decreased odds of achieving intermediate/ideal levels for smoking (OR, 0.84; 95% confidence interval, 0.76–0.92) and fasting plasma glucose (OR, 0.90; 95% confidence interval, 0.82–0.98). Those with higher scores for minor stressors and major life events were less likely to achieve intermediate or ideal LS7 composite scores (OR [95% confidence interval], 0.89 [0.81–0.97] and 0.91 [0.84–0.98], respectively).
Conclusions
Blacks with higher levels of multiple stress measures are less likely to achieve intermediate or ideal levels of overall cardiovascular health (LS7 composite score), specific behaviors (smoking), and biological factors (fasting plasma glucose).
Keywords: blacks, Jackson Heart Study, psychosocial factors, risk factors, stress
Clinical Perspective
What Is New?
Blacks with higher levels of multiple stress measures are less likely to achieve intermediate or ideal levels of cardiovascular health, particularly for global cardiovascular health (Life's Simple 7 composite score) and specific metrics of cardiovascular health behaviors (smoking) and biological factors (fasting plasma glucose).
What Are the Clinical Implications?
To address health disparities in ideal cardiovascular health among blacks compared with whites, attention to alleviating psychosocial stress through culturally sensitive individual interventions, community‐level programs, and policies is warranted.
Introduction
The American Heart Association (AHA) has outlined 7 “simple” targets, termed Life's Simple 7 (LS7), aimed at improving the cardiovascular health (CVH) of Americans by 20% by 2020 and decreasing deaths from cardiovascular disease (CVD) and stroke by 20%.1 To objectively monitor progress toward these goals and to promote CVH, a simplified LS7 metric was devised. This metric includes 4 health behaviors (smoking, diet, physical activity, and body mass index [BMI]) and 3 health factors (blood pressure [BP], total cholesterol, and fasting plasma glucose [FPG]). Each LS7 component is further categorized into 3 categories: ideal, intermediate, and poor.
Recent epidemiologic studies have used this metric to examine the prevalence of CVH in the US population2 and have unmasked striking health disparities in ideal CVH among blacks compared with whites.3, 4 A significantly lower percentage of blacks has ideal levels of the LS7 components than whites, and they have 82% lower odds of meeting at least 5 components of ideal CVH.3 Black women have the lowest ideal CVH among all female racial/ethnic groups (4.2% meeting 5–7 ideal health metrics versus 18.7% for white women).3 A recent analysis of a community‐based cohort of blacks reported an extremely low prevalence of ideal CVH over time,5 with subsequent analyses demonstrating that higher numbers of ideal CVH components are protective for incident cardiovascular events, including fatal and nonfatal myocardial infarction and stroke, in this population.6 Given the persistent disproportionate burden of CVD morbidity and mortality among blacks,7 recent interest has focused on a better understanding of the effects of nontraditional risk factors and specific mediators, including psychosocial factors, on the dismal CVH disparities among blacks.8, 9
A substantial body of evidence has documented the influence of psychosocial factors, such as negative emotional states, acute and chronic stressors, and social support, on CVD risk.10, 11, 12 However, few studies have examined psychosocial factors and CVD risk among blacks, with none of these studies specifically examining the associations of these variables with achievement of ideal CVH, as represented by the AHA LS7.13, 14, 15, 16, 17, 18, 19 Thorough investigation of psychosocial stress in several dimensions and domains is warranted to better determine its effect on CVH outcomes in blacks, given their exposure to greater stressors from life circumstances and discrimination.20
The primary aim of this study was to examine the associations of multidimensional stressors (chronic stress, minor stressors, and major life events [MLEs]) with the LS7 components in blacks. We hypothesized that higher levels of stress would be associated with a lower probability of achieving intermediate or ideal levels of the LS7 components and LS7 composite score after adjusting for traditional sociodemographic factors. Understanding how the stress pathway may mitigate specific LS7 components in a population at risk for CVD is paramount for the development of public health strategies and interventions to reduce the burden of CVD within the black community.
Methods
Data Source
The JHS (Jackson Heart Study) is a population‐based, prospective, cohort study designed to examine CVD risk in black adults (N=5306 participants: 1935 men and 3371 women) aged 21 to 95 years residing in Jackson, MS. Details of the study design, recruitment approach, and measures have been published elsewhere.21, 22, 23 The study enrolled participants from 4 recruitment pools: a random sampling of Jackson residents (17%), the Jackson site of the ARIC (Atherosclerosis Risk in Communities) study (22%), community volunteers from the Jackson area (30%), and family members of other JHS participants (31%). Participants underwent baseline assessments from 2000 to 2004, which included data collection of sociodemographics, medical history, physical examination, laboratory studies, medications, and behavioral risk factors. The data, analytic methods, and study materials can be made available to other researchers for purposes of reproducing the results or replicating the procedure by following the JHS publications' procedures and data use agreements.24 The study was approved by the institutional review boards of University of Mississippi Medical Center, Jackson State University, and Tougaloo College. All participants provided informed consent. The Mayo Clinic Institutional Review Board determined that this secondary data analysis of deidentified data was not human subjects research and thus was exempt from review.
Outcome Ascertainment
In accordance with AHA‐defined criteria, all LS7 components were categorized as poor, intermediate, or ideal CVH for each participant.1 Criteria for each LS7 component are shown in Table 1. For this study, among JHS participants who completed the baseline assessment (2000–2004), we excluded those with missing data on ≥1 LS7 component. We calculated an LS7 composite score, as previously outlined by Thacker and colleagues,25 by assigning 2 points for ideal, 1 point for intermediate, and 0 points for poor for each component and summing all components to calculate a total composite score. The total sum allowed for a continuous measure of global CVH with a range from poor (0 points) to ideal (14 points). The LS7 composite score was further categorized as poor (0–6), intermediate (7–8), or ideal (9–14).
Table 1.
AHA 2020 Strategic Impact Goals: Definition of Poor, Intermediate, and Ideal CVH for Each Goal/Metric for Adults >20 Years
| LS7 Component Goals/Metrics | CVH Definitions | ||
|---|---|---|---|
| Poor | Intermediate | Ideal | |
| Smoking | Current | Former <1 y | Never or former >1 y |
| Healthy diet score (0–5 components)a | 0–1 | 2–3 | 4–5 |
| Physical activity levelb | None |
1–149 min/wk moderate intensity or 1–74 min/wk vigorous intensity or 1–149 min/wk moderate+vigorous intensity |
≥150 min/wk moderate intensity or ≥75 min/wk vigorous intensity or ≥150 min/wk moderate+vigorous intensity |
| Body mass index, kg/m2 | ≥30.0 | 25.0–29.9 | <25.0 |
| Blood pressure, mm Hg |
SBP ≥140 or DBP ≥90 |
SBP 120–139 or DBP 80–89 or treated to goal |
<120/<80 untreated |
| Total cholesterol, mg/dL | ≥240 |
200–239 or treated to goal |
<200 untreated |
| Fasting plasma glucose, mg/dL | ≥126 |
100–125 or treated to goal |
<100 untreated |
Reproduced in part from Shay et al2 with permission. Copyright ©2012, Wolters Kluwer Health, Inc. AHA indicates American Heart Association; CVH, cardiovascular health; DBP, diastolic blood pressure; LS7, Life's Simple 7; SBP, systolic blood pressure.
Healthy diet score components include the following: fruits and vegetables, ≥4.5 cups/day; fish, ≥2 3.5‐ounce servings/week; fiber‐rich whole grains (≥1.1 g fiber per 10 g carbohydrate), ≥3 1‐ounce servings per day; sodium, ≤1500 mg/d; and sugar‐sweetened beverages, <36 fluid‐ounce/week (≤450 kcal/wk). Dietary recommendations are scaled according to a 2000‐kcal/d diet.
Minutes of vigorous activity are equal to 2 times the minutes of moderate activity when moderate and vigorous activities are combined.
Assessment of CVH Behaviors
The 4 AHA‐defined health behaviors were measured at the baseline visit.
Current Smoking: Participants were classified as current, former, or never smokers from self‐reported questionnaires. Poor smoking status was defined as current smokers; intermediate, as former smokers who quit smoking within the past year; and ideal, as never smokers or those who had quit smoking at least 12 months before the baseline visit.
Diet: Specific nutrition components according to the 5 primary dietary goals of the AHA guidelines were assessed by a previously validated, culturally appropriate, short‐form food frequency questionnaire (Delta Nutrition Intervention Research Initiative)26, 27, 28 as follows: at least 4.5 cups/day of fruits and vegetables, ≥2 3.5‐ounce servings/week of fish, ≥3 1‐ounce servings/day of fiber‐rich whole grains (≥1.1 g fiber/10 g carbohydrate), <1500 mg/d of sodium, and <36 fluid‐ounce/week (≤450 kcal/wk) of sugar‐sweetened beverages.
Physical Activity: Participants completed an interviewer‐administered survey that included questions about types, frequency, and duration of specific activities (sports, work/occupational, active living, and home life).29 Three levels of physical activity were defined (in min/wk), according to AHA recommendations1: poor (0 min/wk of physical activity), intermediate (1–149 min/wk of moderate‐intensity activity, 1–74 min/wk of vigorous‐intensity activity, or 1–149 min/wk of moderate+vigorous intensity activity), or ideal (≥150 min/wk of moderate‐intensity activity, ≥75 min/wk of vigorous‐intensity activity, or ≥150 min/wk of moderate+vigorous intensity activity).
BMI: Height (cm) and weight (kg) were measured during the baseline clinical examination and used to calculate BMI (kg/m2). Poor, intermediate, and ideal BMI were defined as BMI >30, 25 to 29.9, and <25 kg/m2, respectively.
Assessment of CVH Biological Factors
The 3 AHA‐defined biological factors were measured at the baseline assessment.
BP: Calculated as the average of 2 sitting BPs after 5 minutes of rest using a random‐zero sphygmomanometer and appropriately sized cuff. BP status was defined as poor (systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg), intermediate (systolic BP 120–139 mm Hg or diastolic BP 80–89 mm Hg if untreated or treated to goal if receiving antihypertensive therapy), or ideal (systolic BP <120 mm Hg and diastolic BP <80 mm Hg, if untreated).
Total Cholesterol: Measured from fasting venous blood samples, as previously described.30 Total cholesterol status was defined as poor (≥240 mg/dL), intermediate (200–239 mg/dL if untreated or treated to goal with lipid‐lowering therapy), or ideal (<200 mg/dL, untreated).
FPG: FPG was measured from fasting venous blood samples with standard glucose oxidase colorimetric methods. FPG status was defined as poor (≥126 mg/dL), intermediate (100–125 mg/dL or treated to goal), or ideal (<100 mg/dL).
Stress Variables
Stress was assessed by 4 measures inclusive of both chronic and acute stress dimensions. Chronic stress was measured using the Global Perceived Stress Scale, which was created for the JHS and adapted from standardized stress scales.22, 31, 32 The 8‐item instrument measures global perceptions of ongoing stressful experiences over the prior 12 months in domains such as employment, legal issues, and racism/discrimination. Participants rated the severity of each domain according to a range of 1 (“not stressful”) to 3 (“very stressful”), with a total ranging from 0 to 24 (Cronbach α, 0.72). Minor stressors (chronic stress dimension) were assessed using the Weekly Stress Inventory, an 87‐item questionnaire measuring recurrent minor irritants during the past week across a broad spectrum of life domains (eg, work tasks, finances, and relationships). Participants were asked to rate the severity of each domain on a 7‐point Likert scale (from 1 [“not stressful”] to 7 [“extremely stressful”]).33, 34 The sum of the ratings ranged from 0 to 493, with high scores reflecting greater weekly stress (Cronbach α, 0.98). Participant MLEs were measured using an 11‐item life events inventory, which assesses short‐term discrete life circumstances. Participants were asked whether they had experienced major negative stressors in the previous 12 months within domains pertaining to personal illness, victim of physical assault, or employment loss.35 The scale ranged from 0 to 11 as the sum of “yes” responses (1 point each) (Cronbach α not calculated because MLE is an index rather than a true scale). Finally, a cumulative stress score was constructed by assigning a separate score of 1 (referent) to 3 to tertiles of chronic stress, minor stressors, and MLEs separately and by summation across all 3 measures (range, 3–9).36 Each stress measure was transformed into SD units in the regression analyses for ease of interpretation.
Covariates
Covariates included baseline age (continuous), sex (men/women), educational attainment, and annual household income. Educational attainment was classified as less than high school, high school graduate/some college, or college graduate or higher. The categories for annual household income were <$25 000, $25 000 to $49 999, and ≥$50 000. Self‐reported medication use for hypertension, hyperlipidemia, and diabetes mellitus was also assessed at baseline.
Statistical Analysis
Participant baseline characteristics were compared by sex using χ2 tests for categorical variables and 2‐sample t tests or Wilcoxon rank sum tests for continuous variables. Multivariable logistic regression models estimated the association of each stress measure (chronic stress, minor stressors, MLEs, and cumulative stress) with achievement of intermediate/ideal levels of CVH using each LS7 component, where odds ratios (ORs) and 95% confidence intervals (CIs) compared intermediate/ideal levels of CVH with poor CVH (referent). For each LS7 component, models were estimated in a sequential manner, including each stress measure in separate models to avoid multicollinearity. Model 1 adjusted for age, sex, and socioeconomic factors (education, income, and insurance status); model 2 adjusted for model 1 covariates plus self‐reported medication use (antihypertensive agents, lipid‐lowering therapies, or diabetic medications) and medical history (hypertension, hyperlipidemia, or diabetes mellitus). An additional model estimated the effect of each stress measure on LS7 composite scores (intermediate/ideal categories). In addition, interaction terms were analyzed to assess whether age and sex modified the associations of interest in the fully adjusted models. For the cumulative stress score, imputation by chained equations was used to address missing individual stress scores before combining into the cumulative score.37 All reported P values reflect a 2‐tailed α=0.05 to establish statistical significance. All data analyses were performed using SAS, 9.3 (SAS Institute, Inc).
Results
Participant Characteristics
Of the 5306 JHS participants who completed the baseline assessment, 923 with missing data on ≥1 LS7 components were excluded. Thus, this analysis includes 4383 participants (64.1% women), and the mean (SD) age was 55.0 (12.8) years. Comparison of the excluded and included participants is shown in Table S1. Participant characteristics stratified by sex are shown in Table 2. Compared with men, women were significantly older (P<0.001) and reported lower annual household income (P<0.001). Women were more likely to hold management/professional or service‐related occupations than men (P<0.001). Men were more likely to report current alcohol use and smoking (both P<0.01). BMI was higher in women than men (P<0.001). In terms of comorbid conditions, a higher proportion of men than women had coronary heart disease (P<0.001), whereas women had higher proportions of diabetes mellitus (P<0.04) and hypertension (P<0.001) than men. Women reported higher scores for chronic stress, MLEs, and cumulative stress than men (all P<0.001).
Table 2.
Baseline Characteristics of JHS Participants, Overall and by Sex
| Characteristic | Total (N=4383) | Men (N=1573) | Women (N=2810) | P Value |
|---|---|---|---|---|
| Age, y | 55.0 (12.8) | 54.1 (12.8) | 55.4 (12.8) | <0.001 |
| Education | 0.42 | |||
| Less than high school | 821 (18.8) | 300 (19.1) | 521 (18.6) | |
| High school graduate/some college | 806 (18.4) | 273 (17.4) | 533 (19.0) | |
| College graduate or greater | 2746 (62.8) | 996 (63.5) | 1750 (62.4) | |
| Annual household income | <0.001 | |||
| <$25 000 | 1390 (31.7) | 371 (23.6) | 1019 (36.3) | |
| $25 000–$49 999 | 1048 (23.9) | 348 (22.1) | 700 (24.9) | |
| ≥$50 000 | 1302 (29.7) | 633 (40.2) | 669 (23.8) | |
| Unknown | 643 (14.7) | 221 (14.0) | 422 (15.0) | |
| Occupation | <0.001 | |||
| Management/professional | 1604 (36.6) | 512 (32.5) | 1092 (38.9) | |
| Service | 1082 (24.7) | 250 (15.9) | 832 (29.6) | |
| Production | 632 (14.4) | 361 (22.9) | 271 (9.6) | |
| Other | 1065 (24.3) | 450 (28.6) | 615 (21.9) | |
| Health insured | 3788 (86.7) | 1366 (87.0) | 2422 (86.5) | 0.64 |
| Current alcohol use | 2025 (46.4) | 946 (60.4) | 1079 (38.6) | <0.001 |
| Current smoker | 551 (12.6) | 281 (17.9) | 270 (9.6) | <0.001 |
| Anthropometrics | ||||
| Body mass index, kg/m2 | 31.7 (7.2) | 29.8 (6.1) | 32.8 (7.5) | <0.001 |
| Waist circumference, cm | 100.5 (16.1) | 101.0 (15.1) | 100.2 (16.7) | 0.12 |
| Comorbid conditions | ||||
| Coronary heart disease | 281 (6.4) | 131 (8.3) | 150 (5.3) | <0.001 |
| Diabetes mellitus | 839 (19.1) | 276 (17.5) | 563 (20.0) | 0.04 |
| Hypertension | 2428 (55.4) | 807 (51.3) | 1621 (57.7) | <0.001 |
| Stress measures | ||||
| Chronic stress score | 5.1 (4.4) | 4.4 (4.1) | 5.5 (4.4) | <0.001 |
| Minor stressors score | 83.2 (81.2) | 79.6 (79.6) | 85.1 (82.0) | 0.08 |
| Major life events score | 1.3 (1.2) | 1.2 (1.1) | 1.4 (1.2) | <0.001 |
| Cumulative stress score | 6.1 (1.7) | 5.9 (1.6) | 6.2 (1.7) | <0.001 |
Data are presented as mean (SD) or number (percentage) of participants. JHS indicates Jackson Heart Study.
Prevalence of CVH Behaviors and Biological Factors
Prevalence estimates for all LS7 components are shown in Figure 1. In terms of ideal CVH behaviors, the highest prevalence rates were reported for smoking status, whereas the lowest prevalence rates were reported for diet. Total cholesterol and FPG were the highest prevalence rates among the ideal CVH biological factors. Overall, the lowest percentages of combined intermediate/ideal CVH among participants were seen for diet (39%), BMI (47%), and physical activity (51%).
Figure 1.
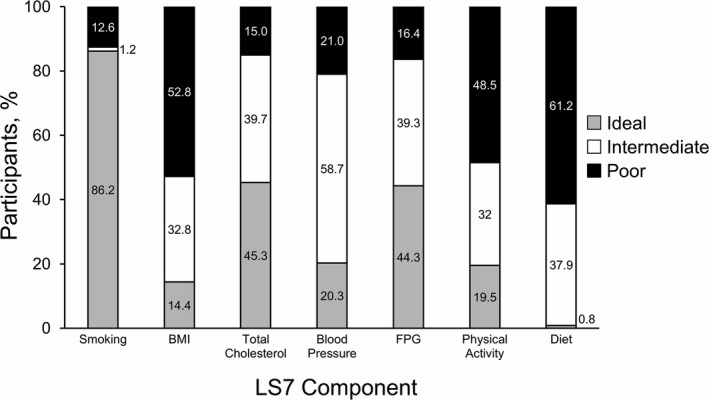
Prevalence of cardiovascular health metrics among Jackson Heart Study participants. Percentage of ideal, intermediate, and poor cardiovascular health behaviors for each component of Life's Simple 7 (LS7). BMI indicates body mass index; FPG, fasting plasma glucose.
Stress Measures and CVH
In the minimally adjusted multivariable model (model 1), higher levels of each stress measure (chronic stress, minor stressors, MLEs, and cumulative stress) were significantly associated with a decreased likelihood of achieving intermediate/ideal levels for smoking (Table S2). The ORs for each LS7 component and LS7 composite score by stress measure for the fully adjusted model (model 2) are shown in Figure 2. Findings were similar for smoking in model 2 as in model 1. The strongest association was seen with chronic stress; a 1‐SD unit increase in chronic stress score was associated with a 20% decreased odds of achieving intermediate/ideal smoking status (OR, 0.80; 95% CI, 0.73–0.88). Higher MLE scores were associated with decreased odds of achieving intermediate/ideal levels for FPG (OR, 0.90; 95% CI, 0.82–0.98). No statistically significant associations were observed between the psychosocial stress measures and other individual LS7 components. Psychosocial stress measures that were associated with a decreased odds of achieving intermediate/ideal LS7 composite score categories were minor stressors (OR, 0.89; 95% CI, 0.81–0.97) and MLEs (OR, 0.91; 95% CI, 0.84–0.98). Associations between stress measures and the LS7 components and composite score did not differ between men and women (ie, there were no significant sex×stress score interactions).
Figure 2.
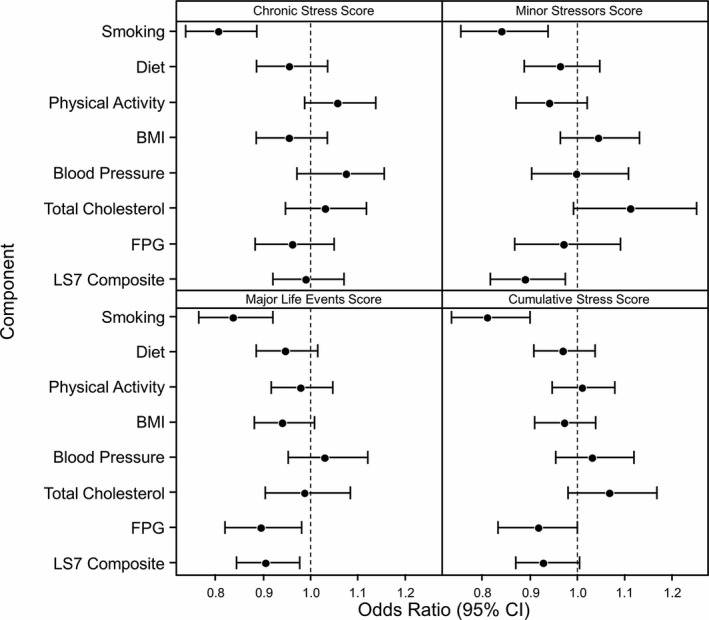
Odds ratios for the association between Life's Simple 7 (LS7) components and stress measures. Model 2: adjusted for age, sex, education, income, insurance status, medication use (antihypertensive agents, lipid‐lowering therapies, or diabetic medications), and medical history (hypertension, hyperlipidemia, or diabetes mellitus). Odds ratios are per 1‐SD unit increase in stress measure. BMI indicates body mass index; CI, confidence interval; FPG, fasting plasma glucose.
The effects of specific stress measures and LS7 components, however, were modified by age (cumulative stress for FPG, P=0.03; MLEs for smoking, P=0.02). In age‐stratified models of FPG and cumulative stress, the odds of achieving intermediate/ideal FPG were decreased among participants ≥55 years (OR, 0.90; 95% CI, 0.80–1.01) but not among participants <55 years (OR, 0.97; 95% CI, 0.83–1.15). For participants with MLEs, the odds of achieving intermediate/ideal smoking status were decreased among those <55 years (OR, 0.79; 95% CI, 0.70–0.89) but not among those ≥55 years (OR, 0.99; 95% CI, 0.84–1.16). There was no statistically significant association between the LS7 composite score and cumulative stress score.
Discussion
The current study examined the association of psychosocial stress with CVH among blacks. The results showed several major novel findings. First, multiple dimensions of psychosocial stress (acute and chronic) were associated with worse global CVH, as measured by the AHA LS7 composite score, and with key individual components, including CVH behavior and biological health factors. Second, the achievement of intermediate/ideal CVH for smoking was lower for blacks with higher levels of multiple dimensions of psychosocial stress. Third, blacks with higher levels of MLEs had decreased odds of being at the intermediate/ideal levels of FPG. To our knowledge, no previous studies have documented these findings in a large sample of blacks.
Prior studies have reported the negative effect of psychological factors, such as depressive symptoms, on CVH. The REGARDS (Reasons for Geographic and Racial Differences in Stroke) study of a nationally representative population‐based cohort, oversampled for blacks (n=6631), found an inverse association between depressive symptoms and ideal CVH for the individual LS7 components and total LS7 score.38 Furthermore, a cross‐sectional analysis of a cohort of French primary care patients enrolled in PPS3 (the Paris Prospective Study) showed a similar association, although it was only observed for CVH behaviors rather than the biological factors.39 Both studies found the strongest association between depressive symptoms and worse CVH for smoking. Sims et al37 examined the association of negative affect (including depressive symptoms) and multidimensional stressors with unhealthy behaviors in black participants in the JHS cohort. Complementary to our findings, negative affect and stress measures (chronic stress, minor stressors, MLEs, and cumulative stress) were associated with greater odds of smoking. Our analyses add to the REGARDS and PPS3 study findings by presenting a comprehensive assessment of multiple stress domains beyond negative emotional states and CVH. These studies together further identify smoking as an adverse behavioral response to psychosocial stress, which is critically important to blacks given their disproportionately higher detrimental tobacco‐related health outcomes, particularly CVD morbidity and mortality.7, 40, 41, 42
Our results suggest that psychosocial stressors are associated with key clinical and behavioral markers of poor CVH within this high‐risk population who also experience multiple inequities (eg, institutional racism, personal discrimination, and classism) that place them at a socioeconomic and health disadvantage. This complex milieu of psychosocial factors influences the disparate and deleterious CVH behaviors, risk factors, and ultimately health outcomes among blacks.8, 9 Blacks report greater cumulative exposure to acute and chronic stressors relative to whites, including stressful living conditions and discrimination.20, 43
There are several potential mechanisms by which psychosocial stress may influence the CVH of blacks. Perceived discrimination, a psychological stressor, has been related to unhealthy behaviors in blacks,19 with an unexpected inverse effect on all‐cause mortality that was partially mediated by perceived stress.44 Studies report the constrained ability of blacks to cope with stress itself through a conceptualized model by Jackson and colleagues45, 46 of “preserving” mental health through unhealthy behaviors as a coping strategy. This theory suggests that when people are chronically exposed to stressful conditions in daily life, they will engage in unhealthy behaviors (eg, smoking or poor eating) to alleviate the symptoms of stress. These unhealthy behaviors may potentially mask the physiological or psychological experiences of poor mental health through the immediate activity of the sympathetic‐adrenal‐medullary system, delayed response of the hypothalamic‐pituitary‐adrenocortical axis, and other environmentally sensitive biological cascades,47, 48 hence the term preserving mental health.
Psychosocial stress exposure, whether acute or chronic, can result in maladaptive changes to the sympathetic nervous system and hypothalamic‐pituitary‐adrenocortical axis, which can lead to atherogenesis and CVD progression through complex multidirectional interactions.49 Recent evidence suggests that chronic everyday stressors are potentially responsible for the rapid progression of CVD, whereas acute lifetime stressors may trigger episodic events of CVD, such as myocardial infarction.50 Our participants showed unfavorable associations of psychosocial stress with selected LS7 components, major CVD risk factors (FPG and smoking), and global CVH (LS7 composite score). This is of major concern for blacks, who are already at a disproportionately high risk for CVD. In addition, evidence suggests that derangements in stress‐elicited responses (allostatic load “wear and tear”) from cumulative stress exposure, particularly extreme, unpredictable, and social threats, may result in hypothalamic‐pituitary‐adrenocortical axis dysregulation. This has been shown to lead to blunted peak cortisol reactivity to acute stress in blacks,51 which over time is a direct biological pathway to insulin resistance.20, 51 This is relevant to our demonstrated association of increased stress from MLEs with poor glycemic control, which could profoundly contribute to the significant disparity in ideal FPG status among blacks compared with whites.3, 52
It is plausible that vulnerability to tobacco use is also closely linked to the stress dysregulation phenomenon across acute and chronic (hence, cumulative) stress dimensions, because these measures were associated with decreased likelihood of reaching intermediate/ideal smoking levels. Complementary to our results, increasing cumulative stress was associated with a consequential increased prevalence of smoking among black women enrolled in a prospective CVD risk study.53 Unfortunately, diminished self‐regulation or limited self‐control while under stressful conditions and environments can trigger smoking and smoking relapses as negative coping behaviors.41 Thus, high stress experienced by blacks may propagate their lower success rates for total smoking cessation than their white counterparts.54 Furthermore, our measure of global CVH as a conglomerate of all LS7 components (behaviors and biological risk factors) was associated with acute and chronic stress dimensions, which supports its utility and sensitivity in capturing a broader range of stress types (such as minor stressors and MLEs) experienced by blacks.
We observed no other significant associations between the psychosocial stress measures and other LS7 components when assessed individually; however, it is possible that correlations may become apparent over a longer exposure time. This postulation is compatible with earlier studies demonstrating that high stress levels over time are associated with increased prevalence of chronic diseases, such as hypertension, hyperlipidemia, and obesity.20, 55 We plan to specifically investigate this hypothesis in future work.
Our findings also suggest potential age‐related influences on the relationship between stress and CVH. Older people experience certain life transitions, such as retirement, which may expose them to unique stressors of daily living (eg, becoming a caregiver or adapting to a fixed income), whereas younger people may continue to navigate the challenges associated with the work environment.56 These personal transitions can have differing effects, ranging from empowering to disruptive (positive to negative), and “off time” or nonanticipation can be associated with high stress. To this end, our results illustrate that older people (who are more likely to be retired) who experience higher negative stressors (inclusive of MLEs and cumulative stress) have worse FPG levels than younger people. This is further compounded by the likelihood that older adults are more susceptible to glucose intolerance than younger adults.57
In terms of tobacco use, blacks tend to have varying smoking patterns across the age continuum. Blacks have a lower prevalence of smoking in adolescence than whites but have higher smoking initiation rates in late adolescence to young adulthood and persistence of smoking into adulthood. This perplexing pattern, referred to as the “crossover” or “convergence” pattern,58, 59 is most likely multifactorial and includes chronic psychosocial stressors that can increase during adulthood (eg, occupational demands, job insecurity, and discrimination) and tobacco industry–targeted marketing to low‐income black younger adults. Consistent with this model, our age‐stratified analyses revealed an even stronger association between stress (MLEs) and smoking among younger people. This is in concordance with a previous prospective study of black adults with high stress exposure, which showed that blacks <55 years were more likely to be current smokers than those of older age.41 Moreover, the relatively younger age of the JHS participants could also explain the lack of associations between the psychosocial stress measures and the other LS7 components because younger people may cope with stress differently than those at middle age and beyond (ie, they may counteract stress with ideal health behaviors: diet and physical activity) and have more ideal biological risk factors (eg, BP and total cholesterol).44, 46
Implications
Blacks are more frequently exposed to stressors linked to socioeconomic position and adverse life circumstances, and it is acknowledged that the eradication of all underlying stress triggers is an insurmountable task given the magnitude of system‐based disadvantages affecting this population.60, 61 Our analyses have both clinical and policy implications. Our findings support, at a minimum, the recognition of and screening for psychosocial stress by clinicians to develop more effective, culturally sensitive lifestyle‐management plans, viewed from the social construct lens of this population, to mitigate CVH disparities.62, 63 To shift the CVH of blacks toward the AHA 2020 goals, interventions endorsing positive psychological well‐being (eg, positive emotions and appraisal, social support, and active coping) are warranted to address modifiable barriers to CVH.10, 64, 65, 66, 67
Practical approaches, including psychosocial interventions incorporating mindfulness, optimism, and resiliency for stress reduction and promotion of healthy behavior in a culturally informed manner, are paramount to producing CVH benefits to at‐risk subgroups.4, 10, 49 Viewed from a contrasting perspective, better ideal CVH is associated with decreased psychological distress and positive mental health outcomes; thus, these approaches seem harmonious for patients and may enhance adherence to recommended therapies.68 Clinical and public health practitioners can have a crucial role in identifying community‐based resources for health promotion, which are not always readily apparent to disadvantaged groups, although doing so is beyond the realm of traditional clinical practice.62, 63, 69 Finally, community‐level programs and policies to counteract or prevent some of the root causes of psychosocial stressors (eg, lack of educational and employment opportunities, unsafe neighborhoods, and institutional racism) are essential to achieving ideal CVH in racial/ethnic minority groups.
Limitations
Although this study advances the current literature on psychosocial influences on CVH disparities, there are several limitations to the current analysis that may affect data interpretation. The cross‐sectional design prevents examining a temporal association between exposure to psychosocial stress and CVH. However, given the prospective nature of the JHS cohort, future studies may investigate this longitudinal association further and uncover the dynamic effects of varying stress dimensions on CVH in blacks. Generalizability to other black populations is limited given our inclusion of participants from a single site in the southern United States with a high proportion of blacks. Several participants had missing data on the LS7 components; however, we integrated a statistical approach to account for these excluded people. Despite these limitations, our study has several notable strengths. First, the JHS is the largest study of CVD in blacks and used standardized procedures for careful adjudication of the LS7 components and validated questionnaires, which facilitated our assessment of multiple dimensions of psychosocial stress measures encompassing an array of domains and social contexts. Second, our simultaneous analysis of individual LS7 components (CVH behaviors and biological factors) and calculation of an LS7 composite score as a global measure of CVH provide an interpretable, relevant, and useful tool for clinicians and patients in healthy lifestyle promotion. Together, our study findings underscore relationships among multiple stressors and CVH, which could account for the persistent CVH disparities in the black population.
Conclusions
The achievement of intermediate or ideal levels of CVH was less likely in blacks with higher levels of multiple measures of stress. This was noted for global CVH (LS7 composite score), as well as specific CVH behaviors (smoking) and biological factors (FPG). These findings further demonstrate that psychosocial factors should be considered in AHA efforts to achieve ideal CVH for all Americans, particularly blacks. Finally, our study provides valuable insight into the importance of alleviating psychosocial stress through lifestyle interventions, strategies, and policies in this high‐risk population to improve CVH.
Sources of Funding
The JHS (Jackson Heart Study) is supported and conducted in collaboration with Jackson State University (HHSN268201300049C and HHSN268201300050C), Tougaloo College (HHSN268201300048C), and the University of Mississippi Medical Center (HHSN268201300046C and HHSN268201300047C) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). Dr Brewer is supported by the Building Interdisciplinary Research Careers in Women's Health Scholars Program (award K12 HD065987‐07) from the National Institutes of Health (NIH) Office of Research on Women's Health and Mayo Clinic Women's Health Research Center and the National Center for Advancing Translational Sciences (NCATS grant KL2 TR002379), a component of the NIH. Dr Sims is supported by the grants P60MD002249 and U54MD008176 from the NIMHD. The views expressed in this article are those of the authors and do not necessarily represent the views of the NHLBI, the NIMHD, the NIH, or the US Department of Health and Human Services. Dr Brewer had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Disclosures
None.
Supporting information
Table S1. Baseline Characteristics of Jackson Heart Study Participants Stratified by Inclusion vs Exclusion Status*
Table S2. Odds Ratios for the Association of LS7 Components and Stress Measures*
Acknowledgments
We thank the JHS (Jackson Heart Study) participants and research team (University of Mississippi Medical Center, Jackson State University, and Tougaloo College) for their long‐term commitment to expanding our knowledge of cardiovascular risk factors toward the eradication of cardiovascular health disparities.
(J Am Heart Assoc. 2018;7:e008855 DOI: 10.1161/JAHA.118.008855.)29871857
Preliminary findings of this study were presented at the American Heart Association Quality of Care and Outcomes Research Scientific Sessions, April 2, 2017, in Arlington, VA.
References
- 1. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task Force, Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 2. Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd‐Jones DM. Status of cardiovascular health in US adults: prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community‐based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011;123:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, Yancy CW; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council . Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 5. Djousse L, Petrone AB, Blackshear C, Griswold M, Harman JL, Clark CR, Talegawkar S, Hickson DA, Gaziano JM, Dubbert PM, Correa A, Tucker KL, Taylor HA. Prevalence and changes over time of ideal cardiovascular health metrics among African‐Americans: the Jackson Heart Study. Prev Med. 2015;74:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ommerborn MJ, Blackshear CT, Hickson DA, Griswold ME, Kwatra J, Djousse L, Clark CR. Ideal cardiovascular health and incident cardiovascular events: the Jackson Heart Study. Am J Prev Med. 2016;51:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz‐Flores S, Davey‐Smith G, Dennison‐Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW; American Heart Association Council on Quality of Care and Outcomes Research, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, Council on Lifestyle and Cardiometabolic Health, and Stroke Council . Social determinants of risk and outcomes for cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 9. Albert MA, Glynn RJ, Buring J, Ridker PM. Impact of traditional and novel risk factors on the relationship between socioeconomic status and incident cardiovascular events. Circulation. 2006;114:2619–2626. [DOI] [PubMed] [Google Scholar]
- 10. Labarthe DR, Kubzansky LD, Boehm JK, Lloyd‐Jones DM, Berry JD, Seligman ME. Positive cardiovascular health: a timely convergence. J Am Coll Cardiol. 2016;68:860–867. [DOI] [PubMed] [Google Scholar]
- 11. Rutledge T, Linke SE, Johnson BD, Bittner V, Krantz DS, Cornell CE, Vaccarino V, Pepine CJ, Handberg EM, Eteiba W, Shaw LJ, Parashar S, Eastwood JA, Vido DA, Merz CN. Relationships between cardiovascular disease risk factors and depressive symptoms as predictors of cardiovascular disease events in women. J Womens Health (Larchmt). 2012;21:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sumner JA, Khodneva Y, Muntner P, Redmond N, Lewis MW, Davidson KW, Edmondson D, Richman J, Safford MM. Effects of concurrent depressive symptoms and perceived stress on cardiovascular risk in low‐ and high‐income participants: findings from the Reasons for Geographical and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2016;5:e003930 DOI: 10.1161/JAHA.116.003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis TT, Everson‐Rose SA, Colvin A, Matthews K, Bromberger JT, Sutton‐Tyrrell K. Interactive effects of race and depressive symptoms on calcification in African American and white women. Psychosom Med. 2009;71:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lewis TT, Guo H, Lunos S, Mendes de Leon CF, Skarupski KA, Evans DA, Everson‐Rose SA. Depressive symptoms and cardiovascular mortality in older black and white adults: evidence for a differential association by race. Circ Cardiovasc Qual Outcomes. 2011;4:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weinstein AA, Abraham P, Diao G, Zeno SA, Deuster PA. Relationship between depressive symptoms and cardiovascular disease risk factors in African American individuals. Depression Res Treat. 2011;2011:836542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Capistrant BD, Gilsanz P, Moon JR, Kosheleva A, Patton KK, Glymour MM. Does the association between depressive symptoms and cardiovascular mortality risk vary by race? Evidence from the health and retirement study. Ethn Dis. 2013;23:155–160. [PMC free article] [PubMed] [Google Scholar]
- 17. Mwendwa DT, Ali MK, Sims RC, Cole AP, Lipscomb MW, Levy SA, Callender CO, Campbell AL. Dispositional depression and hostility are associated with inflammatory markers of cardiovascular disease in African Americans. Brain Behav Immun. 2013;28:72–82. [DOI] [PubMed] [Google Scholar]
- 18. O'Brien EC, Greiner MA, Sims M, Hardy NC, Wang W, Shahar E, Hernandez AF, Curtis LH. Depressive symptoms and risk of cardiovascular events in blacks: findings from the Jackson Heart Study. Circ Cardiovasc Qual Outcomes. 2015;8:552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sims M, Diez‐Roux AV, Gebreab SY, Brenner A, Dubbert P, Wyatt S, Bruce M, Hickson D, Payne T, Taylor H. Perceived discrimination is associated with health behaviours among African‐Americans in the Jackson Heart Study. J Epidemiol Community Health. 2016;70:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gebreab SY, Diez‐Roux AV, Hickson DA, Boykin S, Sims M, Sarpong DF, Taylor HA, Wyatt SB. The contribution of stress to the social patterning of clinical and subclinical CVD risk factors in African Americans: the Jackson Heart Study. Soc Sci Med. 2012;75:1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson heart study. Ethn Dis. 2005;15:S6‐4–S617 [PubMed] [Google Scholar]
- 22. Payne TJ, Wyatt SB, Mosley TH, Dubbert PM, Guiterrez‐Mohammed ML, Calvin RL, Taylor HA Jr, Williams DR. Sociocultural methods in the Jackson Heart Study: conceptual and descriptive overview. Ethn Dis. 2005;15:S6‐38–S6‐48. [PubMed] [Google Scholar]
- 23. Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA Jr. Recruiting African‐American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15:S6‐18–S6‐29. [PubMed] [Google Scholar]
- 24. Jackson Heart Study . Data access [internet]. 2015. Available at: https://www.jacksonheartstudy.org/Research/Study-Data/Data-Access. Accessed May 7, 2018.
- 25. Thacker EL, Gillett SR, Wadley VG, Unverzagt FW, Judd SE, McClure LA, Howard VJ, Cushman M. The American Heart Association Life's Simple 7 and incident cognitive impairment: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2014;3:e000635 DOI: 10.1161/JAHA.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carithers T, Dubbert PM, Crook E, Davy B, Wyatt SB, Bogle ML, Taylor HA Jr, Tucker KL. Dietary assessment in African Americans: methods used in the Jackson Heart Study. Ethn Dis. 2005;15:S6‐49–S6‐55. [PubMed] [Google Scholar]
- 27. Carithers TC, Talegawkar SA, Rowser ML, Henry OR, Dubbert PM, Bogle ML, Taylor HA Jr, Tucker KL. Validity and calibration of food frequency questionnaires used with African‐American adults in the Jackson Heart Study. J Am Diet Assoc. 2009;109:1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Talegawkar SA, Johnson EJ, Carithers TC, Taylor HA Jr, Bogle ML, Tucker KL. Serum carotenoid and tocopherol concentrations vary by dietary pattern among African Americans. J Am Diet Assoc. 2008;108:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dubbert PM, Carithers T, Ainsworth BE, Taylor HA Jr, Wilson G, Wyatt SB. Physical activity assessment methods in the Jackson Heart Study. Ethn Dis. 2005;15:S6‐56–S6‐61. [PubMed] [Google Scholar]
- 30. Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci. 2004;328:131–144. [DOI] [PubMed] [Google Scholar]
- 31. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 32. LePore SJ. Measurement of chronic stressors In: Cohen S, Kessler RC, Gordon LU, eds. Measuring Stress: A Guide for Health and Social Scientists. New York, NY: Oxford University Press; 1997:102–120. [Google Scholar]
- 33. Brantley PJ, Waggoner CD, Jones GN, Rappaport NB. A daily stress inventory: development, reliability, and validity. J Behav Med. 1987;10:61–74. [DOI] [PubMed] [Google Scholar]
- 34. Mosley TH Jr, Payne TJ, Plaud JJ, Johnson CA, Wittrock DA, Seville JL, Penzien DB, Rodriguez G. Psychometric properties of the Weekly Stress Inventory (WSI): extension to a patient sample with coronary heart disease. J Behav Med. 1996;19:273–287. [DOI] [PubMed] [Google Scholar]
- 35. Schulz AJ, Israel BA, Parker EA, Lockett M, Hill Y, Wills R. The east side village health worker partnership: integrating research with action to reduce health disparities. Public Health Rep. 2001;116:548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diez Roux AV, Ranjit N, Powell L, Jackson S, Lewis TT, Shea S, Wu C. Psychosocial factors and coronary calcium in adults without clinical cardiovascular disease. Ann Intern Med. 2006;144:822–831. [DOI] [PubMed] [Google Scholar]
- 37. Sims M, Lipford KJ, Patel N, Ford CD, Min YI, Wyatt SB. Psychosocial factors and behaviors in African Americans: the Jackson Heart Study. Am J Prev Med. 2017;52:S48–S55. [DOI] [PubMed] [Google Scholar]
- 38. Kronish IM, Carson AP, Davidson KW, Muntner P, Safford MM. Depressive symptoms and cardiovascular health by the American Heart Association's definition in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. PLoS One. 2012;7:e52771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gaye B, Prugger C, Perier MC, Thomas F, Plichart M, Guibout C, Lemogne C, Pannier B, Boutouyrie P, Jouven X, Empana JP. High level of depressive symptoms as a barrier to reach an ideal cardiovascular health: the Paris Prospective Study III. Sci Rep. 2016;6:18951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Luger TM, Suls J, Vander Weg MW. How robust is the association between smoking and depression in adults? A meta‐analysis using linear mixed‐effects models. Addict Behav. 2014;39:1418–1429. [DOI] [PubMed] [Google Scholar]
- 41. Slopen N, Dutra LM, Williams DR, Mujahid MS, Lewis TT, Bennett GG, Ryff CD, Albert MA. Psychosocial stressors and cigarette smoking among African American adults in midlife. Nicotine Tob Res. 2012;14:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thorpe RJ Jr, Wilson‐Frederick SM, Bowie JV, Coa K, Clay OJ, LaVeist TA, Whitfield KE. Health behaviors and all‐cause mortality in African American men. Am J Mens Health. 2013;7:8S–18S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sternthal MJ, Slopen N, Williams DR. Racial disparities in health: how much does stress really matter? Du Bois Rev. 2011;8:95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dunlay SM, Lippmann SJ, Greiner MA, O'Brien EC, Chamberlain AM, Mentz RJ, Sims M. Perceived discrimination and cardiovascular outcomes in older African Americans: insights from the Jackson Heart Study. Mayo Clin Proc. 2017;92:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jackson JS, Knight KM. Race and self‐regulatory health behaviors: the role of the stress response and HPA axis in physical and mental health disparities In: Schaie KW, Carstensen LL, eds. Social Structures, Aging, and Self‐Regulation in the Elderly. New York, NY: Springer Pub; 2006:189–208. [Google Scholar]
- 46. Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the hpa axis, and physical and mental health disparities over the life course In: LaVeist TA, Isaac LA, eds. Race, Ethnicity, and Health: A Public Health Reader. San Francisco, CA: Jossey‐Bass; 2013:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hamer M, Malan L. Psychophysiological risk markers of cardiovascular disease. Neurosci Biobehav Rev. 2010;35:76–83. [DOI] [PubMed] [Google Scholar]
- 48. Lucas T, Wegner R, Pierce J, Lumley MA, Laurent HK, Granger DA. Perceived discrimination, racial identity, and multisystem stress response to social evaluative threat among African American men and women. Psychosom Med. 2017;79:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lagraauw HM, Kuiper J, Bot I. Acute and chronic psychological stress as risk factors for cardiovascular disease: insights gained from epidemiological, clinical and experimental studies. Brain Behav Immun. 2015;50:18–30. [DOI] [PubMed] [Google Scholar]
- 50. Okhomina VI, Glover L, Taylor H, Sims M. Dimensions of and responses to perceived discrimination and subclinical disease among African‐Americans in the Jackson Heart Study. J Racial Ethn Health Disparities. 2018. Available at: https://link.springer.com/article/10.1007%2Fs40615-017-0457-7. Accessed May 12, 2018. [DOI] [PubMed] [Google Scholar]
- 51. Obasi EM, Shirtcliff EA, Cavanagh L, Ratliff KL, Pittman DM, Brooks JJ. Hypothalamic‐pituitary‐adrenal reactivity to acute stress: an investigation into the roles of perceived stress and family resources. Prev Sci. 2017;18:923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Effoe VS, Carnethon MR, Echouffo‐Tcheugui JB, Chen H, Joseph JJ, Norwood AF, Bertoni AG. The American Heart Association ideal cardiovascular health and incident type 2 diabetes mellitus among blacks: the Jackson Heart Study. J Am Heart Assoc. 2017;6:e005008 DOI: 10.1161/JAHA.116.005008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Albert MA, Durazo EM, Slopen N, Zaslavsky AM, Buring JE, Silva T, Chasman D, Williams DR. Cumulative psychological stress and cardiovascular disease risk in middle aged and older women: rationale, design, and baseline characteristics. Am Heart J. 2017;192:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kulak JA, Cornelius ME, Fong GT, Giovino GA. Differences in quit attempts and cigarette smoking abstinence between whites and African Americans in the United States: literature review and results from the international tobacco control US survey. Nicotine Tob Res. 2016;18(suppl 1):S79–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salleh MR. Life event, stress and illness. Malays J Med Sci. 2008;15:9–18. [PMC free article] [PubMed] [Google Scholar]
- 56. Wong JD, Shobo Y. The moderating influences of retirement transition, age, and gender on daily stressors and psychological distress. Int J Aging Hum Dev. 2017;85:90–107. [DOI] [PubMed] [Google Scholar]
- 57. Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinol Metab Clin North Am. 2013;42:333–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kandel D, Schaffran C, Hu MC, Thomas Y. Age‐related differences in cigarette smoking among whites and African‐Americans: evidence for the crossover hypothesis. Drug Alcohol Depend. 2011;118:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Roberts ME, Colby SM, Lu B, Ferketich AK. Understanding tobacco use onset among African Americans. Nicotine Tob Res. 2016;18(suppl 1):S49–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Williams DR, Yan Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio‐economic status, stress and discrimination. J Health Psychol. 1997;2:335–351. [DOI] [PubMed] [Google Scholar]
- 61. Ferdinand KC, Nasser SA. Disparate cardiovascular disease rates in African Americans: the role of stress related to self‐reported racial discrimination. Mayo Clin Proc. 2017;92:689–692. [DOI] [PubMed] [Google Scholar]
- 62. Wallen J, Randolph S, Carter‐Pokras O, Feldman R, Kanamori‐Nishimura M. Engaging African Americans in smoking cessation programs. Am J Health Educ. 2014;45:151–157. [Google Scholar]
- 63. Caleyachetty R, Echouffo‐Tcheugui JB, Muennig P, Zhu W, Muntner P, Shimbo D. Association between cumulative social risk and ideal cardiovascular health in US adults: NHANES 1999–2006. Int J Cardiol. 2015;191:296–300. [DOI] [PubMed] [Google Scholar]
- 64. Boehm JK, Kubzansky LD. The heart's content: the association between positive psychological well‐being and cardiovascular health. Psychol Bull. 2012;138:655–691. [DOI] [PubMed] [Google Scholar]
- 65. Kim‐Dorner SJ, Simpson‐McKenzie CO, Poth M, Deuster PA. Psychological and physiological correlates of insulin resistance at fasting and in response to a meal in African Americans and whites. Ethn Dis. 2009;19:104–110. [PubMed] [Google Scholar]
- 66. Fernandez CA, Loucks EB, Arheart KL, Hickson DA, Kohn R, Buka SL, Gjelsvik A. Evaluating the effects of coping style on allostatic load, by sex: the Jackson Heart Study, 2000–2004. Prev Chronic Dis. 2015;12:E165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Watson JM, Logan HL, Tomar SL. The influence of active coping and perceived stress on health disparities in a multi‐ethnic low income sample. BMC Public Health. 2008;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Espana‐Romero V, Artero EG, Lee DC, Sui X, Baruth M, Ruiz JR, Pate RR, Blair SN. A prospective study of ideal cardiovascular health and depressive symptoms. Psychosomatics. 2013;54:525–535. [DOI] [PubMed] [Google Scholar]
- 69. Puckrein GA, Egan BM, Howard G. Social and medical determinants of cardiometabolic health: the big picture. Ethn Dis. 2015;25:521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Jackson Heart Study Participants Stratified by Inclusion vs Exclusion Status*
Table S2. Odds Ratios for the Association of LS7 Components and Stress Measures*


