Abstract
Background
Young women (aged ≤55 years) with acute myocardial infarction (AMI) have poorer health status outcomes than similarly aged men. Low omega‐3 fatty acids (FAs) have been implicated as risk factors for cardiovascular outcomes in AMI patients, but it is not clear whether young women have similar or different post‐AMI omega‐3 FA profiles compared with young men.
Methods and Results
We assessed the sex differences in post‐AMI omega‐3 FAs and the associations of these biomarkers with patient‐reported outcomes (symptom, functioning status, and quality of life) at 12‐month follow‐up, using data from 2985 US adults with AMI aged 18 to 55 years enrolled in the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients) study. Biomarkers including eicosapentaenoic acid, docosahexaenoic acid, arachidonic acid (AA), eicosapentaenoic acid/AA ratio, omega‐3/omega‐6 ratio, and omega‐3 index were measured 1 month after AMI. Overall, the omega‐3 FAs and AA were similar in young men and women with AMI. In both unadjusted and adjusted analysis (controlling for age, sex, race, smoking, hypertension, diabetes mellitus, body mass index, and health status score at 1 month), omega‐3 FAs and AA were not significantly associated with 12‐month health status scores using the Bonferroni corrected statistical threshold.
Conclusions
We found no evidence of sex differences in omega‐3 FAs and AA in young men and women 1 month after AMI. Omega‐3 FAs and AA at 1‐month after AMI were generally not associated with 12‐month patient‐reported health status after adjusting for patient demographic, clinical characteristics, and the corresponding 1‐month health status score.
Keywords: acute myocardial infarction, fatty acid, health status, omega‐3 fatty acids, women
Subject Categories: Quality and Outcomes, Cardiovascular Disease, Secondary Prevention, Women
Clinical Perspective
What Is New?
There was no evidence of sex differences in omega‐3 fatty acids (FAs) and arachidonic acid levels in young men and women 1 month after acute myocardial infarction (AMI).
Omega‐3 FAs and arachidonic acid levels at 1‐month after AMI were generally not associated with 12‐month patient‐reported health status after adjusting for patient demographic, clinical characteristics, and the corresponding 1‐month health status score.
There was no evidence of sex difference in the association between 1‐month omega‐3 FAs levels and 12‐month patient‐reported health status.
What Are the Clinical Implications?
Our finding of no evidence of sex differences in omega‐3 FAs and arachidonic acid levels does not support the hypothesis that low omega‐3 FAs and arachidonic acid explain why young women with AMI have poorer health status outcomes than men.
In younger AMI patients where the recurrent event rate is low, our study suggests that improving omega‐3 and ‐6 FA levels may have little gain in improving patient's recovery.
Our findings of no independent association between omega‐3 FAs levels and patient‐reported health outcomes call into question treatment with omega‐3 FA supplementation in young patients with AMI, which is recommended by the current guidelines.
More studies are needed to assess omega‐3 and omega‐6 FA supplementation, especially in younger patients with AMI.
Introduction
Young women (aged ≤55 years) with acute myocardial infarction (AMI) represent an extreme phenotype and have higher risk for mortality and health status outcomes compared with similarly aged men.1, 2 While these sex differences in outcomes are getting increasing attentions, important questions remain as to factors that contribute to the sex differences in outcomes. Low omega‐3 and ‐6 fatty acids (FAs) have been implicated as risk factors for cardiovascular outcomes in patients with coronary artery diseases.3, 4 The incorporation of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) into the myocardium has been shown to alter the dynamics of sodium and calcium channel function.5 Therefore, differences in levels of omega‐3 and ‐6 FAs may be associated with differences in outcomes. However, it is not clear whether there are important sex differences in the levels of omega‐3 and ‐6 FA biomarkers or in the relationships between these biomarkers and outcomes among young patients with AMI. Understanding sex differences in the prevalence of omega‐3 and ‐6 FA biomarkers and their potential association with sex differences in outcomes in this population may not only inform opportunities for secondary prevention, but also ways to reduce sex differences in health outcomes after AMI.1
Prior studies conducted in the general population have shown significant sex differences in blood levels of omega‐3 FAs. Women in the general population have higher circulating DHA concentrations, but lower circulating EPA and DPA concentrations compared with men and this difference is independent of dietary intake.6, 7, 8, 9 However, data on young patients with AMI are limited, as omega‐3 and ‐6 FAs are not routinely measured in post‐AMI care. Moreover, previous clinical trials that assessed the benefits of omega‐3 FA supplementations on secondary prevention of cardiovascular diseases have focused on clinical outcomes.10, 11, 12 Few studies have assessed the potential role of omega‐3 and ‐6 FAs in influencing patient‐reported outcomes pertaining to cardiovascular health. These patient‐reported health outcomes are particularly important for younger AMI patients because their mortality rate is low and a principle goal of AMI care is to improve patients’ health status (symptom, functioning status, and quality of life).
Accordingly, we used data from the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients)13 study to assess sex differences in omega‐3 FAs and omega‐6 arachidonic acid at 1‐month post‐AMI and the associations of these biomarkers with patient‐reported health outcomes (symptom, functioning status, and quality of life) 12 months after AMI. This study was a prespecified analysis of VIRGO.
Methods
Participants and Study Design
The data that support the findings of this study are available from the corresponding author upon reasonable request and funding for de‐identification of protected health information in the study. Details about the design of the VIRGO study have been previously described.13 In brief, VIRGO was a prospective, observational study designed to investigate the demographic, clinical, psychosocial, biological, and behavioral factors associated with worse outcomes in young women with AMI.14 Between August 2008 and May 2012, participants aged 18 to 55 years were recruited into VIRGO from 103 US, 24 Spanish, and 3 Australian hospitals. Participants were recruited using a 2:1 female to male ratio to increase the proportion of young women in the sample. Of the 6538 patients screened at the participating sites, 3572 were eligible and enrolled. For this analysis, we focused on patients from the United States only (n=2985, 84% of the total sample) as omega‐3 and ‐6 FA levels were measured only in US participants.
Participants were considered eligible for the VIRGO study if they had increased cardiac biomarkers indicative of myocardial necrosis (with at least 1 cardiac biomarker above the 99th percentile of the upper reference limit) within 24 hours of admission. We also required evidence of acute myocardial ischemia, including at least one of the following: symptoms of ischemia, ECG changes indicative of new ischemia, or imaging evidence of infarction. Participants were excluded if they: were previously enrolled in VIRGO; did not speak English or Spanish; were unable to provide informed consent; had developed elevated cardiac markers because of elective coronary revascularization; or had AMI because of physical trauma. Participants who did not have omega‐3 and ‐6 FA laboratory measurements were also excluded for this study (see Figure 1 for details about sample selection flowchart). Each participating institution obtained institutional review board approval, and informed consent was obtained from each participant.
Figure 1.
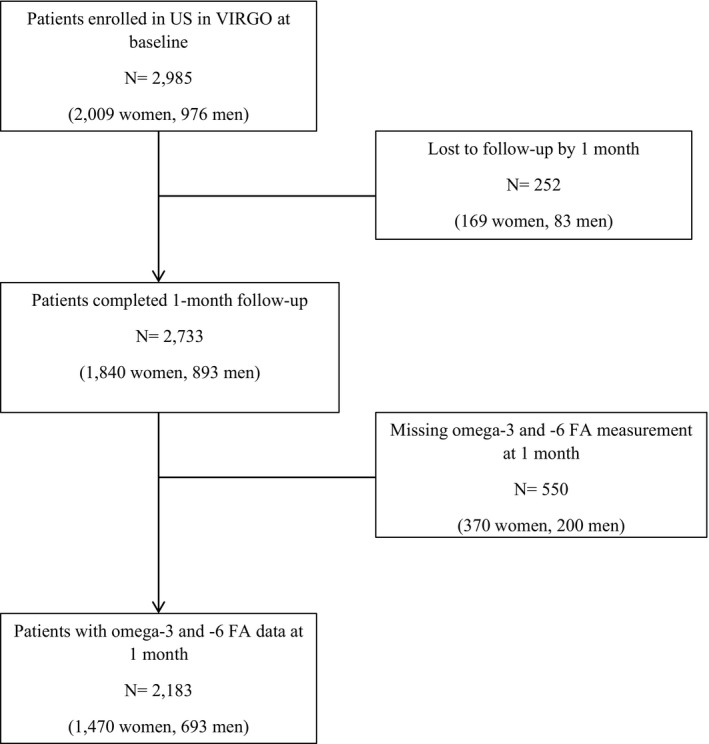
Flowchart of sample selection in the analysis. VIRGO indicates Variation in Recovery: Role of Gender on Outcomes of Young Acute Myocardial Infarction Patients. FA indicates fatty acids.
Omega‐3 and ‐6 Fatty Acids Assessment
To identify a time of clinical stability, we collected blood samples from the US participants at 1‐month post‐AMI discharge for assessment of omega‐3 and ‐6 FAs including EPA, DHA, arachidonic acid (AA), EPA/AA ratio, omega‐3/omega‐6 ratio, and omega‐3 index. Liquid chromatography‐tandem mass (LC‐MS) spectrometry was used to measure EPA, DHA and AA from plasma phospholipids. Measurements were reported as a percentage of total phospholipid fatty acids and the technique sensitively quantifies any individual fatty acid of >0.1%. The total number of fatty acids measured in LC‐MS assay was 19 (Table S1). EPA/AA ratio was quantified as the ratio of EPA divided by AA. The omega‐3/omega‐6 ratio was expressed as the sum of omega‐3 FAs divided by the sum of omega‐6 FAs. The omega‐3 index was calculated as the sum of EPA and DHA divided by the total phospholipid fatty acids. The omega‐3 FA and AA measurements were performed at Quest Diagnostics, San Juan Capistrano, CA. The laboratory was certified by the National Heart, Lung, and Blood Institute and Centers for Disease Control and Prevention lipid standardization program.
Data Collection and Variables
Baseline information, including patient's sociodemographics, clinical presentation, cardiac risk factors, non‐cardiac comorbidities, and AMI treatment, were obtained both from medical records and from standardized in‐person interviews during the index AMI admission. Specifically, we assessed baseline sociodemographics with marital status, education levels, health insurance, employment and financial status (whether or not the patient had enough money to make ends meet by the end of the month).15 Clinical severity of AMI was assessed by AMI diagnosis (ST‐elevation AMI), left ventricular ejection fraction <40%, Global Registry of Acute Coronary Events (GRACE) risk score, and time to presentation after symptom onset. Additionally, we assessed cardiac risk factors, comorbidities (eg, prior heart disease, history of hypertension, diabetes mellitus, and dyslipidemia), obesity, height‐waist circumference, smoking, physical activity levels, and depression on each participant. Physical activity was measured with the Behavioral Risk Factor Surveillance Survey physical activity instrument, an instrument that has been validated and shown to have good reliability in young adults.16 Reperfusion during hospitalization, as well as discharge medications (statin, aspirin, beta‐blocker, and angiotensin‐converting enzyme inhibitor/angiotensin II receptor blockers) were also ascertained.
Information on angina‐specific and overall health status for each patient was assessed at 1 and 12 months after discharge. Specifically, angina frequency, angina‐related physical limitations, and angina‐related quality of life were measured using the Seattle Angina Questionnaire.17, 18, 19 General health status was measured using the 12‐item Short‐Form Survey (SF‐12) physical component summary and mental component summary scores.20 Health‐related quality of life was measured using the EuroQol (EQ‐5D) utility index and visual analog scale.21 The EQ‐5D utility index scores between 0 and 1, where 1 means the best possible health and 0 means death. All other health status scores range from 0 to 100, and the higher the score, the better symptoms, functioning or quality of life.
Statistical Analyses
Patient characteristics at baseline were assessed for the overall sample and compared by sex. We calculated medians with interquartile ranges (IQRs) for continuous variables and frequencies for categorical variables. For continuous variables that have a normal/near normal distribution, student's t‐tests were used to compare baseline characteristics between men and women. For continuous variables that did not have a normal distribution, Wilcoxon rank sum tests were used. For categorical variables that had at least 5 patients in each cell, chi‐squared tests were used to test the differences by sex, and Fisher's exact tests were used for those variables that had <5 patients in ≥1 cells. The same statistical testing methods were used to compare omega‐3 FAs and AA values by sex.
We performed subgroup analyses by types of AMI (ST‐elevation AMI [STEMI] or non–ST‐elevation AMI [NSTEMI]) to determine whether sex differences in omega‐3 FAs and AA vary by AMI type. We also compared the distributions of omega‐3 FAs and AA at 1 month in VIRGO study with those in a healthy population obtained through a large national laboratory in the United States.22, 23 Omega‐3 FAs and AA in this healthy population were measured using the same methods as those in the VIRGO study. The omega‐3 index (the sum of EPA and DHA divided by the total phospholipid fatty acids) was evaluated as a continuous variable in the models.
We performed unadjusted and adjusted analyses to assess the associations between 1‐month omega‐3/‐6 FAs (omega‐3 index, DHA, EPA, AA) and 12‐month health status scores for men and women with AMI, using linear regression models. We considered 12‐month health status scores (Seattle Angina Questionnaire angina frequency, Seattle Angina Questionnaire angina‐related physical limitation, Seattle Angina Questionnaire angina‐related quality of life, SF‐12 physical component summary, SF‐12 mental component summary, EQ‐5D utility index, and EQ‐5D visual analog score) as the dependent variables in these models. Explanatory variables in the adjusted model included patient sex, 1‐month omega‐3/‐6 FA biomarkers, age, race, education, current smoking, hypertension, diabetes mellitus, body mass index, and the corresponding 1‐month health status score. For each dependent variable and each omega‐3/‐6 FA biomarker, 2 models were developed: model 1 included only the omega‐3/‐6 FA biomarker, and model 2 included the omega‐3/‐6 FA biomarker and all other covariates. We also tested interactions between sex and omega‐3/‐6 biomarkers to assess whether the relationship of these biomarkers with health outcomes differed by sex. To account for missing data because of loss of follow‐up at 12 months, we conducted an inverse propensity weighting analysis. Propensity scores were generated using logistic regression to estimate the probability of being observed at 12 months, incorporating baseline demographic and clinical characteristics as predictors.24 We then used the inverse of the propensity scores to weight the observed responses when estimating 12‐month health status.25 We used the Bonferroni correction to account for multiple comparisons. As we considered 7 health outcomes and four exposures in the regression analysis, we used a P value of 0.002 as the Bonferroni corrected threshold for statistical significance. In a sensitivity analysis, we estimated the models in men and women separately.
Since 14.7% of the sample had missing data on covariates, we used a multiple imputation approach in R 3.10 (the R Foundation for Statistical Computing) to estimate missing data so as to retain all patients in the final models. In all the above analyses, we considered a 2‐sided P<0.05 as statistically significant. SAS 9.3 (SAS Institute Inc., Cary, NC) and R 3.10 were used to analyze the most recent version of the VIRGO database.
Results
Sample Characteristics
A total of 2219 adults with AMI in VIRGO were included in the current analysis (725 men and 1494 women) (Table 1). Male and female participants had similar median age (48 years for men and 49 years for women, interquartile range of 44 to 52 years for both sexes). The majority of participants were white (78%), married (52%), employed (64%), had education beyond high school (59%), and health insurance (79%). Half of the participants presented with STEMI and >31% had prior heart disease. The most common cardiovascular risk factors among all participants were high waist circumference (71% of participants), hypertension (64%), smoking (55%), and dyslipidemia (51%).
Table 1.
Baseline Characteristics of Study Participants, Stratified By Sex
| Characteristics | Overall (N=2219) | Men (n=725) | Women (n=1494) | P Value |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age, median (IQR), y | 49 (44–52) | 48 (44–52) | 49 (44–52) | 0.05 |
| Race, n (%) | ||||
| White | 1720 (77.7%) | 610 (84.3%) | 1110 (74.4%) | <0.01 |
| Black | 358 (16.2%) | 60 (8.3%) | 298 (20.0%) | |
| Other | 137 (6.2%) | 54 (7.5%) | 83 (5.6%) | |
| Married/living with a partner as if married, n (%) | 1161 (52.4%) | 430 (59.3%) | 731 (49.0%) | <0.01 |
| Education, n (%) | ||||
| <High school | 42 (1.9%) | 11 (1.5%) | 31 (2.1%) | 0.64 |
| High school | 858 (38.9%) | 279 (38.7%) | 579 (39.0%) | |
| >High school | 1304 (59.2%) | 431 (59.8%) | 873 (58.9%) | |
| Ability to pay for medication, n (%) | ||||
| Health insurance | 1738 (78.6%) | 560 (77.7%) | 1178 (79.0%) | 0.47 |
| Employed (work full or part time) | 1419 (63.9%) | 533 (73.5%) | 886 (59.3%) | <0.01 |
| Finances at end of month | <0.01 | |||
| Some money left over | 704 (31.8%) | 291 (40.2%) | 413 (27.7%) | |
| Just enough to make ends meet | 798 (36%) | 251 (34.7%) | 547 (36.6%) | |
| Not enough to make ends meet | 715 (32.3%) | 182 (25.1%) | 533 (35.7%) | |
| AMI severity, n (%) | ||||
| AMI type | ||||
| NSTEMI | 1109 (50.0%) | 312 (43.0%) | 797 (53.3%) | <0.01 |
| STEMI | 1110 (50.0%) | 413 (57.0%) | 697 (46.7%) | |
| GRACE risk score >99 | 194 (8.9%) | 61 (8.5%) | 133 (9.1%) | 0.67 |
| Left ventricular ejection fraction <40% | 214 (10.1%) | 70 (10.0%) | 144 (10.1%) | 0.97 |
| Hemodynamic instability | 166 (7.5%) | 57 (7.9%) | 109 (7.3%) | 0.70 |
| Present >6 h after symptom onset | 959 (43.4%) | 272 (37.6%) | 687 (46.2%) | <0.01 |
| Comorbidities and CVD risk factors, n (%) | ||||
| Prior heart disease (CAD, angina, heart failure) | 690 (31.1%) | 214 (29.5%) | 476 (31.9%) | 0.26 |
| Hypertension | 1441 (64.9%) | 456 (62.9%) | 985 (65.9%) | 0.16 |
| Diabetes mellitus | 664 (29.9%) | 153 (21.1%) | 511 (34.2%) | <0.01 |
| Dyslipidemia | 1128 (50.9%) | 397 (54.9%) | 731 (49.0%) | 0.01 |
| Obesity (BMI ≥30 kg/m2) | 1162 (52.4%) | 346 (47.7%) | 816 (54.6%) | <0.01 |
| High waist circumference (women >88 cm, men >102 cm) | 1270 (71.1%) | 321 (53.3%) | 949 (80.1%) | <0.01 |
| Current smoking | 1211 (54.6%) | 391 (54.0%) | 820 (54.9%) | 0.73 |
| Physical activity | ||||
| Recommended | 849 (38.5%) | 313 (43.4%) | 536 (36.1%) | <0.01 |
| Insufficient | 654 (29.6%) | 192 (26.6%) | 462 (31.1%) | |
| Inactive | 703 (31.9%) | 216 (30%) | 487 (32.8%) | |
| Depression, median (IQR) | 6 (2–12) | 5 (2–9) | 7 (3–13) | <0.01 |
| Reperfusion in‐hospital, n (%) | ||||
| None | 859 (42.1%) | 227 (34.1%) | 632 (45.9%) | <0.01 |
| Fibrinolytic therapy | 98 (4.8%) | 47 (7.1%) | 51 (3.7%) | |
| PCI | 1085 (53.1%) | 391 (57.8%) | 694 (50.4%) | |
| Discharge medication, n (%) | ||||
| Statin use | ||||
| High‐intensity statin | 868 (39.1%) | 310 (42.8%) | 558 (37.3%) | 0.15 |
| Low‐ and moderate‐intensity statin | 1167 (52.6%) | 381 (52.5%) | 786 (52.6%) | |
| None | 184 (8.3%) | 34 (4.7%) | 150 (10.1%) | |
| Aspirin | 2142 (98.2%) | 712 (98.9%) | 1430 (97.9%) | 0.13 |
| Beta‐blockers | 2031 (97.1%) | 638 (98.5%) | 1348 (96.4%) | 0.01 |
| ACE inhibitors or ARBs | 1416 (70.8%) | 512 (77.3%) | 904 (67.5%) | <0.01 |
All percentages are calculated by excluding missing, don't know and patient refused. P‐value was testing for differences in patient baseline characteristics between men and women. ACE indicates angiotensin‐converting enzyme; AMI, acute myocardial infarction; ARBs, Angiotensin II Receptor Blockers; BMI, body mass index; CAD, coronary artery disease; CVD, cardiovascular disease; GRACE, Global Registry of Acute Coronary Events; IQR, interquartile range; NSTEMI, non–ST‐elevation AMI; STEMI, ST‐elevation AMI.
Compared with men, women in the study were more likely to be black, single, and unemployed (P<0.01 for all, Table 1). Women were also more likely than men to have diabetes mellitus, obesity, a high waist circumference, and longer wait times from AMI symptom onset to hospital arrival. However, women were less likely to have dyslipidemia or present with STEMI. In addition, women were less likely to receive in‐hospital reperfusion treatment than men, and were less likely to receive statin, beta‐blockers, and angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers at discharge. Smoking status and other AMI clinical severity indicators (such as GRACE risk score >99, hemodynamic instability, and left ventricular ejection fraction<40%) were similar between men and women in the study.
Omega‐3 and ‐6 Fatty Acids in Young AMI Patients at 1 Month
Overall, the distributions of omega‐3 FA and AA biomarkers were skewed to the right (Figure 2). The median EPA in young AMI patients was 0.30% (interquartile range: 0.20–0.50), DHA was 2.80% (2.00–4.00), and AA was 0.40% (0.20–0.50) at 1 month (Table 2). The median omega‐3 index (the sum of EPA and DHA divided by the total phospholipid fatty acids), omega‐3/omega‐6 ratio, and EPA/AA ratio among all participants were 3.20% (2.20–4.50), 0.24 (0.19–0.30), and 1.00 (0.50–1.33), respectively. Compared with a healthy population, AMI patients had much lower omega‐3 FA and AA levels (Table S2). Specifically, the mean (SD) for EPA, omega‐3 index, and EPA/AA ratio in the VIRGO study was 0.42% (0.44), 3.56% (1.86), and 1.15 (1.19) respectively, compared with 1.03% (1.11), 4.35% (2.71), and 1.91 (2.51) in the healthy population.
Figure 2.
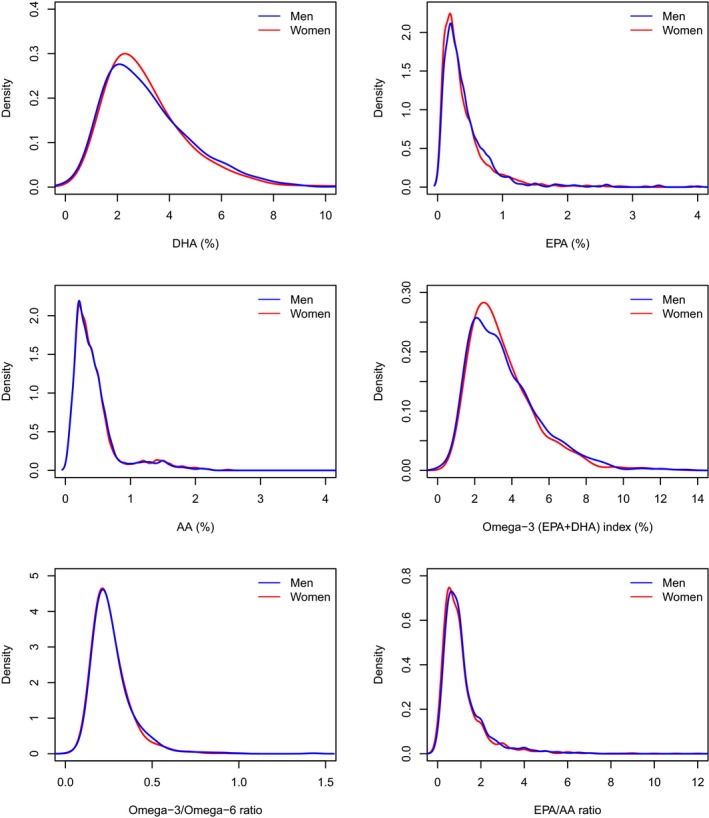
Distributions of omega‐3 and ‐6 fatty acids at 1 month after discharge for AMI, by sex (men=blue, women=red). AA indicates arachidonic acid; AMI, acute myocardial infarction; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Table 2.
Sex Differences in Omega‐3 and ‐6 Fatty Acids at 1 Month After Discharge
| Overall (N=2183) | Men (n=693) | Women (n=1490) | P Value | |
|---|---|---|---|---|
| DHA, median (IQR), % | 2.80 (2.00–4.00) | 2.90 (1.90–4.10) | 2.80 (2.00–3.90) | 0.68 |
| EPA, median (IQR), % | 0.30 (0.20–0.50) | 0.30 (0.20–0.50) | 0.30 (0.20–0.50) | 0.02 |
| AA, median (IQR), % | 0.40 (0.20–0.50) | 0.40 (0.20–0.50) | 0.30 (0.20–0.50) | 0.98 |
| Omega‐3 index, median (IQR), % | 3.20 (2.20–4.50) | 3.20 (2.10–4.60) | 3.10 (2.20–4.40) | 0.54 |
| Omega‐3/omega‐6 ratio, median (IQR) | 0.24 (0.19–0.30) | 0.24 (0.19–0.31) | 0.23 (0.19–0.30) | 0.14 |
| EPA/AA ratio, median (IQR) | 1.00 (0.50–1.33) | 1.00 (0.50–1.50) | 0.83 (0.50–1.33) | 0.02 |
All percentages are calculated by excluding missing, don't know, and patient refused. P‐value was testing for the differences in omega‐3 and ‐6 FA profiles between men and women at 1 month after discharge. AA indicates arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IQR, interquartile range.
Sex Differences in Omega‐3 and ‐6 Fatty Acids in Young AMI Patients At 1 Month
Overall, there were no significant sex differences in 1‐month omega‐3 FAs and AA among young AMI patients (Figure 2, Table 2, and Table 3). At 1 month after AMI discharge, the majority of the omega‐3 FA and AA measures, including DHA, omega‐3 index, omega‐3/omega‐6 ratio, AA, were similar for men and women with the exception of EPA levels. Women had significantly lower EPA and EPA/AA ratio compared with men, but the absolute difference was small (≈0.01 percentage points [Table 2]). The median EPA/AA ratio was 0.83 (0.50–1.33) for women and 1.00 (0.50–1.50) for men (P=0.02). Subgroup analysis among patients with STEMI and NSTEMI, respectively, showed results consistent with the main analysis: omega‐3 and‐6 FA levels were largely similar between men and women.
Table 3.
Sex Differences in Omega‐3 and ‐6 Fatty Acids at 1 Month After Discharge, Stratified By STEMI vs NSTEMI
| Patients With STEMI | Overall (N=1110) | Men (n=413) | Women (=697) | P Value |
|---|---|---|---|---|
| DHA, median (IQR), % | 2.80 (2.00–4.10) | 2.90 (2.00–4.30) | 2.80 (2.00–4.00) | 0.13 |
| EPA, median (IQR), % | 0.30 (0.20–0.50) | 0.30 (0.20–0.50) | 0.30 (0.20–0.50) | 0.01 |
| AA, median (IQR), % | 0.40 (0.20–0.50) | 0.40 (0.20–0.50) | 0.40 (0.20–0.50) | 0.23 |
| Omega‐3 index, median (IQR), % | 3.20 (2.20–4.50) | 3.30 (2.20–4.80) | 3.10 (2.20–4.40) | 0.08 |
| Omega‐3/omega‐6 ratio, median (IQR) | 0.24 (0.19–0.30) | 0.24 (0.20–0.31) | 0.24 (0.19–0.30) | 0.10 |
| EPA/AA ratio, median (IQR) | 1.00 (0.50–1.33) | 1.00 (0.50–1.33) | 1.00 (0.50–1.25) | 0.16 |
| Patients With NSTEMI | Overall (N=1109) | Men (N=312) | Women (N=797) | P Value |
|---|---|---|---|---|
| DHA, median (IQR), % | 2.70 (1.90–3.90) | 2.60 (1.80–3.83) | 2.80 (2.00–3.90) | 0.21 |
| EPA, median (IQR), % | 0.30 (0.20–0.50) | 0.30 (0.20–0.50) | 0.30 (0.20–0.50) | 0.44 |
| AA, median (IQR), % | 0.30 (0.20–0.50) | 0.30 (0.20–0.50) | 0.30 (0.20–0.50) | 0.15 |
| Omega‐3 index, median (IQR), % | 3.10 (2.20–4.40) | 3.00 (2.00–4.50) | 3.10 (2.20–4.30) | 0.25 |
| Omega‐3/omega‐6 ratio, median (IQR) | 0.23 (0.19–0.30) | 0.23 (0.19–0.31) | 0.23 (0.19–0.30) | 0.79 |
| EPA/AA ratio, median (IQR) | 0.88 (0.50–1.50) | 1.00 (0.50–1.50) | 0.83 (0.50–1.40) | 0.04 |
All percentages are calculated by excluding missing, don't know, and patient refused. P‐value was for testing differences in omega‐3/omega‐6 FA profiles between men and women at 1 month after discharge. AA indicates arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IQR, interquartile range; NSTEMI, non–ST‐elevation AMI; STEMI, ST‐elevation AMI.
Association Between 1‐Month Omega‐3/‐6 Fatty Acids and 12‐Month Health Status
Associations between 1‐month omega‐3/‐6 FA levels and 12‐month health status were shown in Table 4. In unadjusted models, only EPA was positively associated with 12‐month SF‐12 Physical Component Summary score using the Bonferroni corrected statistical threshold. Following adjustment for potential confounders, including age, sex, race, smoking, hypertension, diabetes mellitus, body mass index, and the corresponding health status score at 1 month, the coefficients of omega‐3 and ‐6 FAs attenuated and none was significant using the Bonferroni corrected statistical threshold. Interaction terms between sex and omega‐3 FAs and AA were not statistically significant in any of the fully adjusted models for 12‐month health status, indicating that the association of 1‐month omega‐3 FAs and AA with 12‐month health outcomes did not vary between men and women (Table S3).
Table 4.
Mean Difference in 12‐Month Health Status Outcomes Associated 1‐Month Omega‐3/‐6 Fatty Acids (Per 1 Percentage Point Increase)
| Health Status Outcome | Omega‐3 Index, % | DHA, % | EPA, % | AA, % | ||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted Model, Point Estimate (P Value) | Fully Adjusted Model, Point Estimate (P Value) | Unadjusted Model, Point Estimate (P Value) | Fully Adjusted Model, Point Estimate (P Value) | Unadjusted Model, Point Estimate (P Value) | Fully Adjusted Model, Point Estimate (P Value) | Unadjusted Model, Point Estimate (P Value) | Fully Adjusted Model, Point Estimate (P Value) | |
| SAQ angina frequency | 0.36 (0.07) | 0.20 (0.28) | 0.33 (0.15) | 0.19 (0.38) | 2.06 (0.02) | 1.11 (0.16) | 1.07 (0.29) | 1.25 (0.18) |
| SAQ physical limitation | 0.07 (0.74) | 0.11 (0.56) | 0.06 (0.82) | 0.05 (0.82) | 1.87 (0.03) | 1.25 (0.12) | 0.63 (0.53) | 1.27 (0.18) |
| SAQ quality of life | 0.47 (0.09) | 0.34 (0.17) | 0.46 (0.15) | 0.36 (0.21) | 2.16 (0.06) | 1.38 (0.19) | 1.84 (0.18) | 2.20 (0.07) |
| SF‐12 PCS score | 0.29 (0.05) | 0.02 (0.87) | 0.21 (0.22) | 0.005 (0.97) | 2.25 (0.003) | 0.20 (0.71) | −0.73 (0.32) | −0.55 (0.39) |
| SF‐12 MCS score | 0.18 (0.16) | 0.02 (0.86) | 0.18 (0.22) | 0.02 (0.91) | 0.83 (0.12) | 0.62 (0.22) | −0.49 (0.43) | −0.17 (0.77) |
| EQ‐5D utility index score | 0.005 (0.04) | 0.002 (0.26) | 0.005 (0.07) | 0.002 (0.32) | 0.02 (0.03) | 0.009 (0.30) | −0.007 (0.55) | 0.003 (0.78) |
| EQ‐5D visual analog scale | 0.53 (0.03) | 0.30 (0.18) | 0.57 (0.04) | 0.39 (0.12) | 1.79 (0.08) | 0.03 (0.97) | 0.52 (0.67) | 1.05 (0.33) |
Data were shown as mean difference in the health status measures per 1 percentage point increase in omega‐3/‐6 fatty acids and the corresponding P value for statistical significance. Full models were adjusted for patient age, sex, race, current smoking, hypertension, diabetes mellitus, body mass index, and corresponding health status score at 1 month. P values were testing for mean differences in 12‐month health status outcomes associated with each 1 percentage point increase in 1‐month omega‐3/‐6 fatty acids. We used the Bonferroni correction to account for multiple comparisons. As we considered 7 health outcomes and four exposures in the regression analysis, we used a P value of 0.002 as the Bonferroni corrected threshold for statistical significance. AA indicates arachidonic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; EQ‐5D, EuroQol; MCS, Mental Component Summary; PCS, Physical Component Summary; SF‐12, Short Form‐12.
Discussion
To our knowledge, this is the first study investigating potential sex differences in omega‐3 FAs and AA and their relationships to patient‐reported outcomes among young patients with AMI. Our data showed that, in contrast to reports from the general population, omega‐3 FAs and AA at 1‐month post‐AMI were largely similar between young men and women with recent AMIs. Moreover, omega‐3 FAs and AA at 1‐month after AMI were generally not associated with 12‐month patient‐reported health status after adjusting for patient demographic, clinical characteristics, and the corresponding 1‐month health status score. Additionally, there was no evidence of sex difference in this association.
This study extends the existing literature in several important ways. First, our finding of no evidence of sex differences in omega‐3 FAs and AA does not support the hypothesis that low omega‐3 FAs and AA explain why young women with AMI have poorer health status outcomes than men. Previous studies that examined omega‐3 FA profiles in patients with AMI have not examined differences between men and women.26 In this prespecified study of VIRGO, we assessed a wide range of omega‐3 FA and AA biomarkers and all of them were not associated with any significant or clinically meaningful differences by sex. Notably, these results differ from those of similarly aged general populations. Women in the general population have higher circulating DHA concentrations, but lower circulating EPA and DPA concentrations compared with men and this difference is independent of dietary intake.6, 7, 8, 9 This disparity does not exist in young women with AMI, perhaps because of the selection of patients who have had an AMI.
Second, our study is among the first to quantify the associations between omega‐3 FAs and a wide range of patient‐reported health outcomes at 12 months after AMI. While positive associations were observed in the unadjusted models, the results attenuated and became statistically non‐significant after adjusting for demographic, clinical characteristics, and 1‐month health status. In recent years, there has been a growing interest in the potential health benefits of omega‐3 FA supplementation for primary and secondary prevention of cardiovascular disease. By far, the majority of research that showed benefits of omega‐3 FA supplementations has focused on clinical outcomes as opposed to patient‐reported health outcomes.10, 11, 12 In younger patients with AMI, the event rates of clinical outcomes are much lower (eg, mortality rate is <2% in the VIRGO study), and improving patients’ recovery in symptoms, functioning status, and quality of life is a major goal of post‐AMI care. Therefore, it is particularly important to assess the role of omega‐3 FAs on patient‐reported health outcomes in this population. Our findings of no independent association between omega‐3/‐6 FAs and patient‐reported health outcomes call into question treatment with omega‐3/‐6 FA supplementation in young patients with AMI, which is recommended by the current guidelines.27
The current guidelines recommend omega‐3 FA supplementation for secondary prevention of cardiovascular diseases largely based on evidence from 5 clinical randomized trials.27 However, 3 out of these 5 trials found little evidence of an effect of omega‐3 FA supplementation on its primary clinical cardiovascular disease end point.28, 29, 30 The 2 trials that suggested benefits of omega‐3 FA supplementation enrolled older patients (average age of 60) and focused only on clinical outcomes as opposed on patient‐reported outcomes.10, 31 Another 3 clinical trials of omega‐3 FA supplementations are currently ongoing, which are also focusing on older patients.32, 33, 34 A recent American Heart Association Advisory report recommends replacement of saturated fat with polyunsaturated vegetable oils that consist of high omega‐6 linoleic acid to lower cardiovascular disease risk, largely based on evidence from middle‐aged and elderly population.35 More studies are needed to assess omega‐3 and omega‐6 FA supplementations specifically in younger patients with AMI.
Finally, consistent with previous studies,26, 36, 37 we confirmed that AMI patients had lower levels of omega‐3 FAs and AA compared with the healthy population. Whether improvement in omega‐3 FAs and AA can translate into improvement in health outcomes in AMI patients is not clear. In older AMI patients, such as those in the TRIUMPH study26 where secondary prevention is more about recurrent events, improving omega‐3 and ‐6 FAs through dietary supplement may help to prevent the recurrent myocardial infarction and mortality. In younger AMI patients where the recurrent event rate is low, our study suggests that improving omega‐3 and ‐6 FA levels may have little gain in improving patient's recovery.
Our study has several limitations. First, VIRGO was an observational study that required patients to be healthy enough at baseline to participate; thus, we were unable to enroll those who were too ill to be enrolled. Second, there were some patients lost to follow‐up at 1 and 12 months after AMI in our study. If these patients were less healthy than the patients included in the analysis and were disproportionately distributed between men and women, our estimated sex difference in omega 3 FAs and AA may be over or underestimated. However, our data showed that rates of loss to follow‐up were comparable between men and women, and baseline characteristics were similar between those who were lost to follow‐up and those who were included in the analysis. We also conducted an inverse propensity weighting analysis to account for missing data because of loss to follow‐up and found similar results as the complete case analysis. Third, we only reported arachidonic acid as one of the omega‐6 FAs in the study but were not able to isolate the potential association of other individual omega‐6 FAs with patient‐reported outcomes. For instance, linoleic acid is an omega‐6 FAs of particular interest, which has been previously shown to be prognostically important.38, 39 However, linoleic acid is an important component of the omega 3 index, and we did not find a significant association between the omega 3 index and patient‐reported outcomes. Future studies are needed to define the independent association of linoleic acid alone with patient‐reported outcomes in young patients with AMI. Fourth, we could not measure omega‐3 and ‐6 FA levels before AMI. It is possible that some patients might have initiated omega‐3 FA supplement after AMI and altered their level of omega‐3 FA subsequently. However, our data showed that <2% of patients received omega‐3 FA supplementation during hospitalization and at discharge for AMI, suggesting the impact of omega‐3 FA supplementation on our results is small. Moreover, we measured omega‐3 FAs from plasma phospholipids, which reflect short‐term or recent fatty acid intake. It has limited ability to capture long‐term fatty acid intake compared with measuring fatty acid compositions from whole blood, red blood cell, and adipose tissue.40, 41, 42 Measurement methods of plasma levels of omega‐3 and ‐6 FAs are not standardized in the literature,43 which makes it difficult to identify a comparable reference population when comparing with omega‐3 FAs and AA levels in our sample. Finally, this study is deigned to assess whether sex differences in omega‐3 FAs exist in AMI patients and whether the differences in omega‐3 account for the differences in outcome after AMI. It is important to note that although we compared AMI patients with general population to show the profiles of omega‐3 FAs are different in the 2 populations, we are not making any inference on whether omega‐3 FA profiles may be associated with the risk for initial AMI.
Conclusion
The currently available data in VIRGO showed no evidence of sex differences in omega‐3 FAs and arachidonic acid in young men and women 1 month after AMI. In addition, omega‐3 FAs and arachidonic acid were not associated with 12‐month health status outcomes after adjusting for sociodemographics, comorbidities, and cardiovascular disease risk factors. Future studies evaluating the role of other acids individually such as linoleic acid in influencing health status outcomes in young patients with AMI will provide additional insights.
Sources of Funding
The VIRGO study (NCT00597922) was supported by grant R01 HL081153 from the National Heart, Lung, and Blood Institute.
Disclosures
Dr Krumholz is a recipient of research agreements from Medtronic and from Johnson & Johnson (Janssen), through Yale University, to develop methods of clinical trial data sharing; is the recipient of a grant from the Food and Drug Administration and Medtronic to develop methods for post‐market surveillance of medical devices; works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures; chairs a cardiac scientific advisory board for UnitedHealth; is a participant/participant representative of the IBM Watson Health Life Sciences Board; is on the Advisory Board of Element Science and Aetna; and is the founder of Hugo, a personal health information platform. Dr Xu works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures. Dr Spertus is supported by grants from Gilead, Genentech, Lilly, Amorcyte, and EvaHeart, and has a patent for the Seattle Angina Questionnaire with royalties paid. The remaining authors have no disclosures to report.
Supporting information
Table S1. Fatty Acids Measured in LC‐MS Assay in the VIRGO Study
Table S2. Comparison of Sex Differences in Post‐AMI Omega‐3 and ‐6 Fatty Acids in VIRGO With Those in a Healthy Population
Table S3. Mean Difference in 12‐Month Health Status Outcomes Associated 1‐Month Omega‐3/‐6 Fatty Acids (Per 1 Percentage Point Increase) in Men and Women Separately
Acknowledgments
We thank Quest Diagnostics for providing financial support for inflammatory marker measurements of study participants.
(J Am Heart Assoc. 2018;7:e008189 DOI: 10.1161/JAHA.117.008189.)29848494
References
- 1. Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex‐based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999;341:217–225. [DOI] [PubMed] [Google Scholar]
- 2. Dreyer RP, Wang Y, Strait KM, Lorenze NP, D'Onofrio G, Bueno H, Lichtman JH, Spertus JA, Krumholz HM. Gender differences in the trajectory of recovery in health status among young patients with acute myocardial infarction: results from the VIRGO study. Circulation. 2015;131:1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris WS, Von Schacky C. The Omega‐3 Index: a new risk factor for death from coronary heart disease? Prev Med. 2004;39:212–220. [DOI] [PubMed] [Google Scholar]
- 4. Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood eicosapentaenoic and docosahexaenoic acids predict all‐cause mortality in patients with stable coronary heart disease: the Heart and Soul study. Circ Cardiovasc Qual Outcomes. 2010;3:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega‐3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. [DOI] [PubMed] [Google Scholar]
- 6. Crowe FL, Skeaff CM, Green TJ, Gray AR. Serum n‐3 long‐chain PUFA differ by sex and age in a population‐based survey of New Zealand adolescents and adults. Br J Nutr. 2008;99:168–174. [DOI] [PubMed] [Google Scholar]
- 7. Bakewell L, Burdge GC, Calder PC. Polyunsaturated fatty acid concentrations in young men and women consuming their habitual diets. Br J Nutr. 2006;96:93–99. [DOI] [PubMed] [Google Scholar]
- 8. Giltay EJ, Gooren LJ, Toorians AW, Katan MB, Zock PL. Docosahexaenoic acid concentrations are higher in women than in men because of estrogenic effects. Am J Clin Nutr. 2004;80:1167–1174. [DOI] [PubMed] [Google Scholar]
- 9. Nikkari T, Luukkainen P, Pietinen P, Puska P. Fatty acid composition of serum lipid fractions in relation to gender and quality of dietary fat. Ann Med. 1995;27:491–498. [DOI] [PubMed] [Google Scholar]
- 10. GISSI‐Prevenzione Investigators . Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI‐Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 11. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega‐3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta‐analysis. JAMA. 2012;308:1024–1033. [DOI] [PubMed] [Google Scholar]
- 12. Alexander DD, Miller PE, Van Elswyk ME, Kuratko CN, Bylsma LC. A meta‐analysis of randomized controlled trials and prospective cohort studies of eicosapentaenoic and docosahexaenoic long‐chain omega‐3 fatty acids and coronary heart disease risk. Mayo Clin Proc. 2017;92:15–29. [DOI] [PubMed] [Google Scholar]
- 13. Lichtman JH, Lorenze NP, D'Onofrio G, Spertus JA, Lindau ST, Morgan TM, Herrin J, Bueno H, Mattera JA, Ridker PM, Krumholz HM. Variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: evidence for a sex‐age interaction. Arch Intern Med. 1998;158:2054–2062. [DOI] [PubMed] [Google Scholar]
- 15. Rahimi AR, Spertus JA, Reid KJ, Bernheim SM, Krumholz HM. Financial barriers to health care and outcomes after acute myocardial infarction. JAMA. 2007;297:1063–1072. [DOI] [PubMed] [Google Scholar]
- 16. Yore MM, Ham SA, Ainsworth BE, Kruger J, Reis JP, Kohl HW III, Macera CA. Reliability and validity of the instrument used in BRFSS to assess physical activity. Med Sci Sports Exerc. 2007;39:1267–1274. [DOI] [PubMed] [Google Scholar]
- 17. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 18. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. [DOI] [PubMed] [Google Scholar]
- 19. Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long‐term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. [DOI] [PubMed] [Google Scholar]
- 20. Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 21. Rabin R, de Charro F. EQ‐5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33:337–343. [DOI] [PubMed] [Google Scholar]
- 22. Superko HR, Superko AR, Lundberg GP, Margolis B, Garrett BC, Nasir K, Agatston AS. Omega‐3 fatty acid blood levels clinical significance update. Curr Cardiovasc Risk Rep. 2014;8:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Superko HR, Clarke N, Caulfield M, Redor‐Goldman M, Goldman S, Sninsky J. Plasma omega‐3 fatty acid distribution in a US population. J Clin Lipidol. 2013;7:268. [Google Scholar]
- 24. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Langkamp DL, Lehman A, Lemeshow S. Techniques for handling missing data in secondary analyses of large surveys. Acad Pediatr. 2010;10:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salisbury AC, Amin AP, Harris WS, Chan PS, Gosch KL, Rich MW, O'Keefe JH Jr, Spertus JA. Predictors of omega‐3 index in patients with acute myocardial infarction. Mayo Clin Proc. 2011;86:626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris‐Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology . Omega‐3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135:e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J; Group OS . OMEGA, a randomized, placebo‐controlled trial to test the effect of highly purified omega‐3 fatty acids on top of modern guideline‐adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. [DOI] [PubMed] [Google Scholar]
- 29. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial G . n‐3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. [DOI] [PubMed] [Google Scholar]
- 30. Galan P, Kesse‐Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S; Group SFOC . Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989;2:757–761. [DOI] [PubMed] [Google Scholar]
- 32. Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif JC, Ketchum SB, Doyle RT Jr, Murphy SA, Soni PN, Braeckman RA, Juliano RA, Ballantyne CM; Investigators R‐I . Rationale and design of REDUCE‐IT: reduction of cardiovascular events with icosapent ethyl‐intervention trial. Clin Cardiol. 2017;40:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pradhan AD, Manson JE. Update on the vitamin D and omega‐3 trial (VITAL). J Steroid Biochem Mol Biol. 2016;155:252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Institutes of Health . Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy (EVAPORATE). 2017. Available at: https://clinicaltrials.gov/ct2/show/NCT02926027. Accessed September 1, 2017.
- 35. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris‐Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG, Stone NJ, Van Horn LV, American Heart Association . Dietary fats and cardiovascular disease: a presidential advisory from the American Heart Association. Circulation. 2017;136:e1–e23. [DOI] [PubMed] [Google Scholar]
- 36. Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long‐chain n‐3 fatty acids and the risk of sudden death. N Engl J Med. 2002;346:1113–1118. [DOI] [PubMed] [Google Scholar]
- 37. Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197:821–828. [DOI] [PubMed] [Google Scholar]
- 38. Harris WS. The omega‐3 index as a risk factor for coronary heart disease. Am J Clin Nutr. 2008;87:1997S–2002S. [DOI] [PubMed] [Google Scholar]
- 39. Harris WS. The omega‐3 index: clinical utility for therapeutic intervention. Curr Cardiol Rep. 2010;12:503–508. [DOI] [PubMed] [Google Scholar]
- 40. Rise P, Eligini S, Ghezzi S, Colli S, Galli C. Fatty acid composition of plasma, blood cells and whole blood: relevance for the assessment of the fatty acid status in humans. Prostaglandins Leukot Essent Fatty Acids. 2007;76:363–369. [DOI] [PubMed] [Google Scholar]
- 41. Baylin A, Kim MK, Donovan‐Palmer A, Siles X, Dougherty L, Tocco P, Campos H. Fasting whole blood as a biomarker of essential fatty acid intake in epidemiologic studies: comparison with adipose tissue and plasma. Am J Epidemiol. 2005;162:373–381. [DOI] [PubMed] [Google Scholar]
- 42. Sun Q, Ma J, Campos H, Hankinson SE, Hu FB. Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. Am J Clin Nutr. 2007;86:74–81. [DOI] [PubMed] [Google Scholar]
- 43. Smith CE, Follis JL, Nettleton JA, Foy M, Wu JH, Ma Y, Tanaka T, Manichakul AW, Wu H, Chu AY, Steffen LM, Fornage M, Mozaffarian D, Kabagambe EK, Ferruci L, Chen YD, Rich SS, Djousse L, Ridker PM, Tang W, McKnight B, Tsai MY, Bandinelli S, Rotter JI, Hu FB, Chasman DI, Psaty BM, Arnett DK, King IB, Sun Q, Wang L, Lumley T, Chiuve SE, Siscovick DS, Ordovas JM, Lemaitre RN. Dietary fatty acids modulate associations between genetic variants and circulating fatty acids in plasma and erythrocyte membranes: meta‐analysis of nine studies in the CHARGE consortium. Mol Nutr Food Res. 2015;59:1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Fatty Acids Measured in LC‐MS Assay in the VIRGO Study
Table S2. Comparison of Sex Differences in Post‐AMI Omega‐3 and ‐6 Fatty Acids in VIRGO With Those in a Healthy Population
Table S3. Mean Difference in 12‐Month Health Status Outcomes Associated 1‐Month Omega‐3/‐6 Fatty Acids (Per 1 Percentage Point Increase) in Men and Women Separately


