Abstract
Background
Adults with repaired coarctation of the aorta (CoA) have reduced long‐term survival compared with the general population. This study aimed to determine whether CoA is independently associated with premature ischemic and hemorrhagic stroke in the contemporary era.
Methods and Results
This was a cross‐sectional study utilizing the National Inpatient Sample database from 2005 to 2014. We hypothesized that patients with CoA are hospitalized with ischemic and hemorrhagic stroke at a younger age compared with the general population. To test this hypothesis, we compared the age at stroke in patients with and without a diagnosis of CoA using simple and multivariable weighted linear regression. Among 4 894 582 stroke discharges, 207 had a diagnosis of CoA. Patients with CoA had strokes at significantly younger age compared with patients without CoA: 18.9 years younger for all‐cause stroke (P<0.001), 15.9 years younger for ischemic stroke (P<0.001), and 28.5 years younger for hemorrhagic stroke (P<0.001), after adjusting for potential confounders. There was no significant difference in the proportion of ischemic strokes between those with and without CoA (79.2% versus 83.0%, P=0.50). However, CoA patients had a higher proportion of subarachnoid hemorrhage (11.8% versus 4.8%, P=0.039) than those without CoA. Among patients who had a hemorrhagic stroke, the prevalence of unruptured intracranial aneurysms was higher in patients with CoA compared with those without CoA (23.3% versus 2.5%, P=0.002).
Conclusions
Patients with CoA have both ischemic and hemorrhagic strokes at significantly younger ages compared with the general population.
Keywords: adult congenital heart disease, coarctation of the aorta, intracranial aneurysm, stroke
Subject Categories: Congenital Heart Disease, Cerebrovascular Disease/Stroke, Cerebral Aneurysm, Intracranial Hemorrhage, Ischemic Stroke
Clinical Perspective
What Is New?
Patients with coarctation of the aorta (CoA) have ischemic stroke 15.9 years and hemorrhagic stroke 28.5 years younger than the general population, after adjusting for other risk factors.
As in the general population, ischemic stroke is the most common cause of stroke in patients with CoA.
Among all patients with stroke, those with CoA were more likely to have a subarachnoid hemorrhage (11.8% versus 4.8%) and to have an unruptured intracranial aneurysm (9.7% versus 1.1%) than those without CoA.
What Are the Clinical Implications?
Traditional risk factor reduction alone may not be sufficient to reduce the premature burden of stroke in patients with CoA.
Our findings support the American College of Cardiology/American Heart Association 2008 Guidelines for the Management of Adult Congenital Heart Disease recommendations for lifelong surveillance and treatment of hypertension and to screen all patients with CoA for intracranial aneurysms.
Further research is needed to investigate appropriate surveillance and treatment strategies to reduce the premature morbidity of stroke.
Introduction
Despite adequate relief of aortic arch obstruction, adults with coarctation of the aorta (CoA) have reduced long‐term survival compared with the general population.1, 2, 3 Case reports and single‐center series suggest that stroke may be an important contributor to premature morbidity and mortality in CoA.3, 4, 5, 6, 7, 8, 9, 10 Proposed mechanisms include long‐term vascular dysfunction and a high prevalence of systemic hypertension and intracranial aneurysms.11, 12, 13 However, there are limited data on age at, risk factors for, or type of stroke (ischemic versus hemorrhagic) in adults with CoA. The Quebec Congenital Heart Disease Registry reported an increased hazard of stroke in patients with left‐sided lesions, but they did not further specify CoA.14 A study from the Swedish Patient Register found an increased risk of ischemic stroke in CoA. However, there were few events and this study did not include analysis of hemorrhagic stroke.15 Studies limited to tertiary medical centers are limited by the relatively low incidence of stroke and variable follow‐up duration of CoA patients.16 Therefore, evaluating the burden of stroke in adults with CoA necessitates the use of population‐based data. There are no existing US population‐based studies of stroke in CoA. Prospective registries or longitudinal databases of sufficient size or with adequate follow‐up duration are also not available. Therefore, we utilized the National Inpatient Sample (NIS) database, the largest all‐payer inpatient database representing >95% of the US population.17 Our primary aim was to determine whether CoA is independently associated with premature stroke in the contemporary era. Our secondary aims were to delineate the proportions of hemorrhagic and ischemic stroke and the prevalence of unruptured intracranial aneurysms.
Methods
Because of limitations of the NIS data use agreement and availability of the data directly from the Agency for Healthcare Research and Quality, the data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
A cross‐sectional study utilizing discharge data from the NIS database, provided by the Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality, was performed for the period 2005 to 2014. Each year of the NIS includes data from an estimated 35 million annual discharges.17 Patients ≥18 years of age were included in our study. Our primary hypothesis was that patients with CoA are hospitalized with all‐cause, ischemic, and hemorrhagic stroke at a younger age compared with the general population. Secondary outcomes included proportion of stroke types, prevalence of unruptured intracranial aneurysm, mortality, and discharge disposition in those with and without CoA. Because of the use of publicly available anonymized data, the Boston Children's Hospital Institutional Review Board exempted the study and waived the requirement for informed consent.
Definitions
CoA was defined as International Classification of Diseases, Ninth Revision (ICD‐9) code 747.1.18 Observations were excluded if there was a concomitant ICD‐9 code indicating infective endocarditis (421.x), complex congenital heart disease (Table 1), or pregnancy as designated by Healthcare Cost and Utilization Project. Ischemic stroke was defined by ICD‐9 codes of 433.x1, 434.x (excluding 434.x0, “without mention of infarction”), or 436.x.19 Hemorrhagic stroke was defined by ICD‐9 code 430.x (subarachnoid hemorrhage) or 431.x (intracerebral hemorrhage).20 Previously validated covariate ICD‐9 definitions are shown in Table 2.21, 22, 23, 24, 25, 26, 27, 28 Unruptured intracranial aneurysm was defined by ICD‐9 code 437.3. Median income quartiles based on ZIP code are provided by Healthcare Cost and Utilization Project; the upper limit of quartile 1 is 150% of the federal poverty level. Per Healthcare Cost and Utilization Project, race includes both race and ethnicity; in the case that the source data supplied both, ethnicity takes precedence over race.17 For example, if a patient is coded as being both black and Hispanic, then race is defined as Hispanic.
Table 1.
International Classification of Diseases, Ninth Revision (ICD‐9) Codes for Complex Congenital Heart Disease
| ICD‐9 | Diagnosis |
|---|---|
| 745.0 | Common truncus |
| 745.1 | Transposition of great vessels |
| 745.2 | Tetralogy of Fallot |
| 745.3 | Common ventricle |
| 745.4 | Ventricular septal defect |
| 745.6 | Endocardial cushion defects |
| 745.7 | Cor biloculare |
| 746.0 | Anomalies of pulmonary valve |
| 746.1 | Tricuspid atresia and stenosis |
| 746.2 | Ebstein's anomaly |
| 746.5 | Congenital mitral stenosis |
| 746.6 | Congenital mitral insufficiency |
| 746.7 | Hypoplastic left heart syndrome |
| 746.8 | Other congenital anomalies of heart (includes Shone's) |
| 747.3 | Anomalies of pulmonary artery |
| 747.4 | Anomalies of great veins |
Table 2.
International Classification of Diseases, Ninth Revision (ICD‐9) Covariate Definitions
| Covariate | ICD‐9 Codes |
|---|---|
| Anticoagulant or antiplatelet agent (long‐term use) | V586.1, V586.3 |
| Atrial fibrillation or flutter | 427.3 |
| Chronic kidney disease | 585, 585.1, 585.2, 585.3, 585.4, 585.5, 585.6, 585.9, 792.5, V420, V451, V451.1, V451.2, V560, V561, V562, V563.1, V563.2, V568 |
| Coronary artery disease | 414.0, 414.2, 414.3, 414.8, 414.9 |
| Diabetes mellitus | 249.00, 250.00, 250.01, 790.2, 790.21, 790.22, 790.29, 791.5, 791.6, V458.5, V539.1, V654.6, 249.01, 249.10, 249.11, 249.20, 249.21, 249.30, 249.31, 249.40, 249.41, 249.50, 249.51, 249.60, 249.61, 249.70, 249.71, 249.80, 249.81, 249.90, 249.91, 250.02, 250.03, 250.10, 250.11, 250.12, 250.13, 250.20, 250.21, 250.22, 250.23, 250.30, 250.31, 250.32, 250.33, 250.40, 250.41, 250.42, 250.43, 250.50, 250.51, 250.52, 250.53, 250.60, 250.61, 250.62, 250.63, 250.70, 250.71, 250.72, 250.73, 250.80, 250.81, 250.82, 250.83, 250.90, 250.91, 250.92, 250.93 |
| Ever‐smoker | 305.1 or V15.82 |
| Heart failure | 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, or 428 |
| Hyperlipidemia | 272.0, 272.1, 272.2, 272.3, 272.4 |
| Hypertension | 401.0, 401.1, 401.9, 402.00, 402.01, 402.10, 402.11, 402.90, 402.91, 403.0, 403.00, 403.01, 403.1, 403.10, 403.11, 403.9, 403.90, 403.91, 404.0, 404.00, 404.01, 404.02. 404.03, 404.1, 404.10, 404.11, 404.12, 404.13, 404.9, 404.90, 404.91, 404.92, 404.93, 405.01, 405.09, 405.11, 405.19, 405.91, 405.99, 437.2 |
Statistical Analysis
To test our primary hypothesis, we compared the age at the time of stroke hospitalization in patients with and without a diagnosis of CoA using simple and multivariable weighted linear regression. Covariates included sex, race, long‐term anticoagulation or antiplatelet use, atrial fibrillation or flutter, chronic kidney disease, coronary artery disease, diabetes mellitus, heart failure, hyperlipidemia, hypertension, tobacco use, and median income by ZIP code. Sample weights were applied to patient‐level discharge observations to generate a nationally representative estimate of US hospitalizations per recommendations from the Healthcare Cost and Utilization Project Methods Series.29 To account for a change in sampling frame in 2012, revised trend weights supplied by the Agency for Healthcare Research and Quality were used. Stata statistical software (version 14.2) svy commands were used to account for sample weights and clustering in the NIS survey design.
In both primary and secondary analyses, 2 models were evaluated, 1 excluding and 1 including potential intermediaries in the causal pathway (hypertension, heart failure, anticoagulation, atrial fibrillation or flutter, and chronic kidney disease). Interaction between hypertension and CoA was also assessed using an interaction term for hypertension. Since income was missing in 2.2% of observations and race in 14.7%, multiple imputation was used to impute the incomplete observations based on the values of all other covariates (Stata mi commands). Multinomial regression was used for both variables, and 10 imputed data sets were created. Weighted linear regression models were run for each of the imputed data sets and combined to give the final results. Relationships between secondary outcomes and patient characteristics were assessed using logistic regression. For all analyses, the statistical significance level was set at 0.05 and hypothesis tests were 2‐sided.
Sensitivity Analyses
In 2009, the number of secondary diagnosis codes used in the NIS database increased from 15 to 25. To evaluate the potential effect of this change, we explored inclusion of only the first 15 diagnostic codes for all years, as compared with 15 diagnostic codes from 2005 to 2008 and 25 diagnostic codes from 2009 to 2014. Codes for bicuspid aortic valve (BAV), which is present in >50% of patients with CoA, has limited accuracy in administrative databases.30, 31 It may be coded as either bicuspid aortic valve/congenital aortic insufficiency (746.4) or congenital aortic stenosis (746.3). To determine the impact of inclusion of observations with BAV on the independent association of CoA with age at stroke, we repeated the analyses adjusting for the presence of these codes.
Results
Among 4 894 582 discharges with a primary diagnosis of stroke, 207 had a concomitant, secondary diagnosis of CoA. Characteristics of these patients are summarized in Table 3. Patients with and without a CoA diagnosis were similar with respect to sociodemographic characteristics and most clinical comorbidities. However, CoA patients were less likely to have diabetes mellitus (16.9% versus 34.3%, P=0.023) and more likely to be Hispanic (23.2% versus 7.9%, P<0.001). Hypertension was highly prevalent in patients with and without CoA (86.4% versus 78.7%, P=0.22).
Table 3.
Characteristics of Patients With Stroke
| Variable | CoA (%) (N=207) | Non‐CoA (%) (N=4 894 582) | P Value |
|---|---|---|---|
| Female | 88 (42.5) | 2 582 648 (52.8) | 0.18 |
| Anticoagulation/antiplatelet use | 17 (8.3) | 393 080 (8.0) | 0.95 |
| Atrial fibrillation or flutter | 27 (13.2) | 1 073 518 (21.9) | 0.17 |
| Chronic kidney disease | 15 (7.1) | 553 109 (11.3) | 0.40 |
| Coronary artery disease | 51 (24.5) | 1 117 803 (22.8) | 0.80 |
| Diabetes mellitus | 35 (16.9) | 1 679 633 (34.3) | 0.023 |
| Heart failure | 39 (18.7) | 639 178 (13.1) | 0.28 |
| Hyperlipidemia | 91 (43.8) | 2 205 330 (45.1) | 0.87 |
| Hypertension | 179 (86.4) | 3 853 648 (78.7) | 0.22 |
| Tobacco use | 59 (28.5) | 1 194 034 (24.4) | 0.54 |
| Race | 0.007a | ||
| White | 122 (61.6) | 2 611 344 (69.6) | |
| Black | 20 (10.2) | 623 224 (16.3) | |
| Hispanic | 46 (23.2) | 296 274 (7.9) | |
| Asian or Pacific Islander | – | 113 386 (3.0) | |
| Native American | – | 18 164 (0.5) | |
| Other | b | 102 909 (2.7) | |
| Median income by ZIP code | 0.34 | ||
| Quartile 1 | 81 (41.3) | 1 111 341 (29.2) | |
| Quartile 2 | 45 (21.5) | 965 552 (26.3) | |
| Quartile 3 | 29 (16.4) | 880 522 (23.7) | |
| Quartile 4 | 43 (20.9) | 807 885 (20.8) |
CoA indicates coarctation of the aorta.
Significance because of higher percent Hispanic in CoA group. Total numbers (N) are weighted estimates rounded to the nearest whole number. There were no CoA patients of Asian, Pacific Islander, or Native American race, denoted by “–.”
Healthcare Cost and Utilization Project data use agreement prohibits reporting of fewer than 11 observations.
CoA and Age at Stroke
A comparison of age at stroke in patients with and without a diagnosis of CoA is shown in Figure 1. The median age at stroke was significantly lower in CoA patients (52 years; interquartile range 31–64) compared with those without CoA (72 years; interquartile range 60–82; P<0.001). The association between CoA and age at stroke remained significant in multivariable models adjusting for sociodemographic and clinical comorbidities (Table 4). In addition, there was no change in the association after inclusion of potential intermediaries in the model (Table 4). The interaction term for hypertension and CoA was not significant (P=0.41), and therefore was excluded from the final models.
Figure 1.
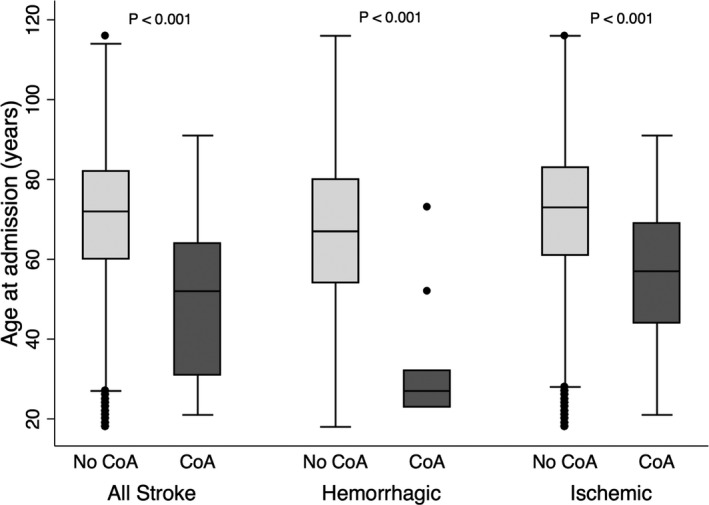
Age at stroke in patients with and without CoA. Box‐plot diagram showing that patients with CoA have stroke at a significantly younger age compared with those without CoA. The central line is the median and the box ranges from the 25th to 75th percentile with bars encompassing 95% confidence interval. P values are derived from univariate weighted linear regression. CoA indicates coarctation of the aorta.
Table 4.
Multivariable Linear Models of Relationship Between Coarctation of the Aorta and Age at Stroke Without and With Potential Intermediaries
| Primary Diagnosis | β for CoA (y) | 95% CI | P Value |
|---|---|---|---|
| All stroke | |||
| Model 1 | −19.0 | −23.1, −14.9 | <0.001 |
| Model 2 | −18.9 | −22.8, −15.0 | <0.001 |
| Ischemic stroke | |||
| Model 1 | −15.7 | −21.1, −11.2 | <0.001 |
| Model 2 | −15.9 | −20.1, −11.6 | |
| Hemorrhagic stroke | |||
| Model 1 | −29.8 | −39.5, −20.0 | <0.001 |
| Model 2 | −28.5 | −37.8, −19.2 | <0.001 |
Model 1: adjusted for sex, coronary artery disease, diabetes mellitus, hyperlipidemia, tobacco use, race, and income. Model 2: adjusted for all Model 1 variables plus: atrial fibrillation or flutter, long‐term anticoagulation or antiplatelet use, chronic kidney disease, heart failure, and hypertension. CoA indicates coarctation of the aorta; CI, confidence interval.
Types of Stroke
The relative proportions of stroke types are depicted in Figure 2. There was no significant difference in the proportion of ischemic strokes between patients with and without CoA (79.2% versus 83.0%, P=0.50). However, CoA patients had a higher proportion of subarachnoid hemorrhage (11.8% versus 4.8%, P=0.039) than those without CoA (Figure 2). Patients with CoA had both ischemic and hemorrhagic stroke at a younger age than those without CoA (57 versus 73 years and 27 versus 67 years, respectively, P<0.001) (Figure 1). The median age of subarachnoid hemorrhage in patients with CoA was 23 years, 35 years younger than in those without CoA (P<0.001). The association between CoA and age at ischemic and hemorrhagic stroke remained significant in multivariable models adjusting for sociodemographic and clinical comorbidities (Table 4). Inclusion of potential intermediaries in the model (atrial fibrillation or flutter, long‐term anticoagulation or antiplatelet use, chronic kidney disease, heart failure, and hypertension) did not significantly attenuate the association of CoA with age at ischemic or hemorrhagic stroke (Table 4).
Figure 2.
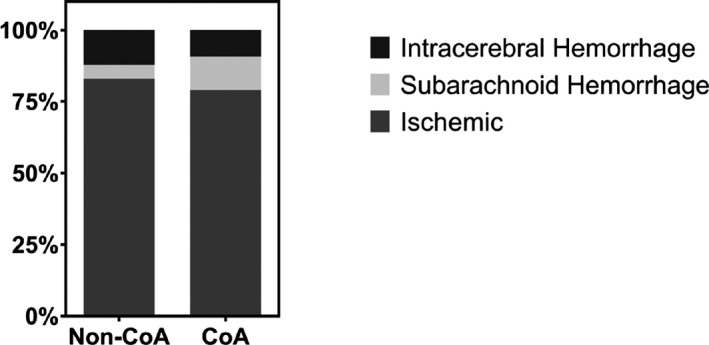
Proportion of stroke type in patients with and without CoA. Stacked bar graphs show the relative proportions of stroke types (intracerebral hemorrhage, subarachnoid hemorrhage, and ischemic) between those with and without CoA. CoA indicates coarctation of the aorta.
Intracranial Aneurysm
As summarized in Figure 3, unruptured intracranial aneurysms were more prevalent in stroke patients with CoA than in those without CoA (9.7% versus 1.1%, P<0.001). Among patients who had a hemorrhagic stroke, the prevalence of unruptured intracranial aneurysms was higher in patients with CoA compared with those without CoA (23.3% versus 2.5%, P=0.002). On further subanalysis, 40% of CoA patients who had a subarachnoid hemorrhage were found to have an unruptured intracranial aneurysm. None of the CoA patients with intracerebral hemorrhage had an unruptured aneurysm.
Figure 3.
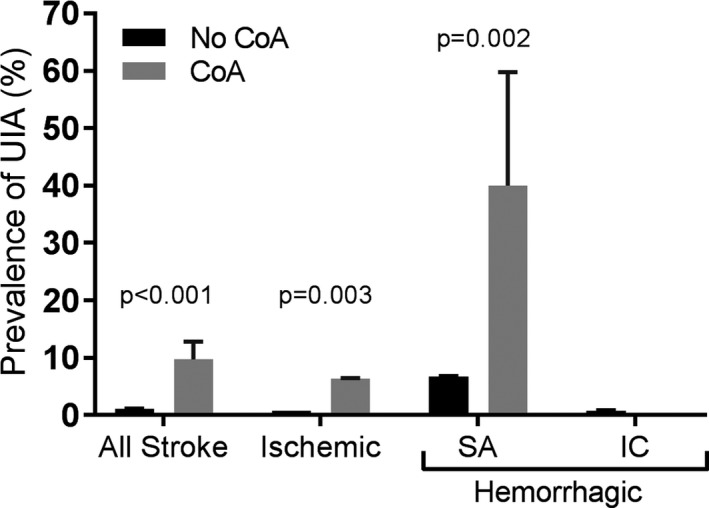
Prevalence of unruptured intracranial aneurysm. Prevalence of unruptured intracranial aneurysms in patients with and without coarctation of the aorta who have had stroke. Hemorrhagic stroke is subdivided into subarachnoid and intracerebral hemorrhage. P values are derived from univariate weighted logistic regression. Bars represent standard errors. CoA indicates coarctation of the aorta; IC, intracranial hemorrhage; SA, subarachnoid hemorrhage.
In‐Hospital Mortality and Disposition
The overall in‐hospital mortality was 4.9% for ischemic stroke and 24.6% for hemorrhagic stroke. There were no in‐hospital deaths in the CoA group. There were no statistically significant differences in hospital discharge disposition between those with and without CoA. Following ischemic stroke, 41.6% of patients with CoA and 43.1% of patients without CoA were discharged to a skilled nursing or intermediate care facility (P=0.86). Following hemorrhagic stroke, 31.8% of patients with CoA and 38.9% of patients without CoA were discharged to a skilled nursing or intermediate care facility (P=0.66).
Sensitivity Analyses
To explore whether the increase in the number of diagnostic codes from 15 to 25 in 2009 may have confounded our results, we explored the inclusion of only the first 15 codes for all years. The association between CoA and age at admission for stroke remained unchanged (β −18.7, 95% confidence interval, −24.2, −13.3, P<0.001). Therefore, all reported analyses included 15 diagnostic codes from 2005 to 2008 and 25 diagnostic codes from 2009 to 2014.
Only 6.7% of patients with CoA had a code for BAV or congenital aortic stenosis, which is significantly less than the established prevalence of BAV of at least 50% in patients with CoA.30 To determine the impact of inclusion of observations with codes for BAV or congenital aortic stenosis on the independent association of CoA with age at stroke, we repeated the analyses adjusting for the presence of these codes. There was no significant change in the association of CoA with age at stroke; therefore, reported analyses do not adjust for BAV.
Discussion
Our results indicate that patients with CoA in the United States have ischemic and hemorrhagic stroke at a substantially younger age than the general population, even after accounting for traditional cerebrovascular risk factors. To the best of our knowledge, this is the largest study to date examining ischemic and hemorrhagic stroke in adults with CoA.
CoA and Age At Stroke
Our finding that patients with CoA have ischemic strokes at a younger age than the general population is consistent with prior studies.14, 15 Lanz et al reported a median age of 49.9 years for ischemic stroke for patients with all types of congenital heart disease and an increased risk among patients with left‐sided lesions, though they did not further specify CoA.14 Mandalenakis et al found that a diagnosis of CoA conferred a significantly increased risk of ischemic stroke compared with the general population.15 However, presumably because of limited sample size with only 9 events, they did not account for potential confounders in the CoA subgroup. Inclusion of potential confounders and intermediaries in our models did not significantly attenuate the ß estimate for CoA, supporting the independent association of CoA with age at stroke.
While there are multiple single‐center reports of individual patients with CoA who sustained a hemorrhagic stroke during the second and third decades of life, to the best of our knowledge, this is the first population‐based study of hemorrhagic stroke in CoA patients in the contemporary era.3, 9 We found that CoA patients were 28 years younger at the time of admission for hemorrhagic stroke compared with those without CoA, independent of other risk factors. These results are consistent with those of Lanz et al, who reported that the age‐ and sex‐standardized incidence rate of hemorrhagic stroke in all types of congenital heart disease was 5 to 6 times higher compared with the general population. The high in‐hospital mortality rate in patients with hemorrhagic stroke seen in our data set is consistent with prior studies.32 Our finding that none of the patients with CoA died during hospitalization may be related to the significantly younger age of patients with CoA.
In our analyses, we considered only isolated CoA, excluding those with complex associated lesions, such as congenital mitral valve disease. We were unable to completely account for the effect of BAV in this population, which is present in >50% of CoA patients, because of the known limitations of accurately identifying patients with BAV in administrative databases.31 However, when we adjusted for BAV in our sensitivity analysis, there was no change in our estimate of the association of CoA with age at ischemic or hemorrhagic stroke.
Notably, the association of CoA with age at stroke was not significantly attenuated by inclusion of hypertension in the model, nor with the inclusion of an interaction term. These results suggest that blood pressure management alone may not be sufficient to mitigate the risk of stroke in this population. However, we cannot exclude the role that hypertension may play in the pathogenesis of stroke in this patient population because it is a well‐established risk factor for intracranial aneurysm formation and rupture in the general population and is present in 86% of CoA patients with stroke.12, 33 Although CoA is a known risk factor for hypertension, the prevalence of hypertension in our data set was similar in patients with or without CoA. This is not unexpected in a population of stroke patients, because hypertension is a known risk factor for stroke, independent of CoA.
There is the potential for selection bias, because only those patients who survive long enough to have a stroke are included in the analyses. Therefore, younger age of stroke in those with CoA may in small part be because of early mortality of patients with CoA compared with the general population. However, prior analyses have found only a 5‐ to 10‐year reduction in life expectancy for patients with CoA, in large part because of cerebrovascular disease.4 Therefore, it is unlikely that underlying differences in population age distributions explain our findings of CoA patients having all‐cause stroke at ages 20 years younger and hemorrhagic stroke at ages 28 years younger than the general population.
There are multiple proposed contributors to the early age of stroke in patients with CoA. Long‐term vascular dysfunction and increased carotid intima‐medial thickness may increase the risk of ischemic stroke.13, 34, 35, 36, 37 In addition, CoA patients are more likely to be male and have hyperlipidemia.38, 39 There is a high prevalence (10%–12%) of intracranial aneurysms, which may predispose to hemorrhagic stroke.11, 12 The high prevalence of systemic hypertension (>50%) increases the risk of both hemorrhagic and ischemic stroke.40, 41
Intracranial Aneurysm
Our findings that patients with CoA have hemorrhagic stroke 28 years younger than the general population, independent of risk factors such as hypertension or anticoagulation use, and have a high prevalence of unruptured intracranial aneurysms (40% in those with subarachnoid hemorrhage) suggest that the cause of hemorrhagic stroke in patients with CoA may be a result of early development and subsequent rupture of intracranial aneurysms. Risk factor reduction alone may not sufficiently reduce the risk of early hemorrhagic stroke. Independent of the cause of the initial stroke, the presence of an unruptured aneurysm may pose increased risk for a subsequent event if untreated. Notably, the prevalence of unruptured intracranial aneurysm in CoA patients in the entire stroke cohort (9.7%) was similar to the reported prevalence of 10% to 12% in cross‐sectional surveillance studies.11, 12
Limitations
Many of the limitations of this study are intrinsic to the use of an administrative database. First, we were unable to determine type or timing of CoA repair or the presence of residual arch obstruction, which may contribute to the age at stroke. Second, information regarding medication use for comorbidities such as hypertension was not available. Third, body mass index could not be ascertained given the poor predictive value of ICD‐9 codes for obesity.42 Fourth, it is likely that CoA is not routinely coded in all admitted patients with CoA, resulting in an underestimation of its prevalence, though this would not affect the estimates of age at stroke. Finally, it should be noted that we are reporting the difference in age at stroke between those with and without CoA, adjusting for other comorbidities, but not the absolute or relative risk of stroke among those with CoA.
Conclusions
Questions remain regarding the optimal strategy to reduce the premature morbidity and mortality related to stroke in patients with CoA. Our findings suggest that traditional risk factor reduction alone may not be sufficient and other approaches should be investigated. Nevertheless, our results support the recommendation of the 2008 guidelines for the management of adult congenital heart disease to regularly monitor patients with repaired CoA for resting and exercise‐induced systemic hypertension, to treat aggressively when hypertension and other established risk factors are identified, and to screen for intracranial aneurysms.1
In conclusion, patients with CoA in the United States have ischemic and hemorrhagic stroke at substantially younger ages compared with the general population. Further research is needed to investigate appropriate surveillance and treatment strategies to reduce the premature morbidity of stroke in patients with CoA.
Sources of Funding
Funding for the project was provided by NIH/NHLBI T32 HL007572‐32 (Pickard) and the Matthew's Hearts of Hope research grant (Pickard).
Disclosures
JJG has received salary support from grants from Novartis Pharmaceutical Corporation and Eli Lilly and Company to the Brigham and Women's Hospital and is a consultant to Aetion, Inc. and to Optum, Inc., all for unrelated work. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2018;7:e009072 DOI: 10.1161/JAHA.118.009072.)29858370
References
- 1. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, Del Nido P, Fasules JW, Graham TP, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation. 2008;118:2395–2451. [DOI] [PubMed] [Google Scholar]
- 2. Brown ML, Burkhart HM, Connolly HM, Dearani JA, Cetta F, Li Z, Oliver WC, Warnes CA, Schaff HV. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol. 2013;62:1020–1025. [DOI] [PubMed] [Google Scholar]
- 3. Choudhary P, Canniffe C, Jackson DJ, Tanous D, Walsh K, Celermajer DS. Late outcomes in adults with coarctation of the aorta. Heart. 2015;101:1190–1195. [DOI] [PubMed] [Google Scholar]
- 4. Diller G‐P, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Li W, Babu‐Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow‐up at a large tertiary centre. Circulation. 2015;132:2118–2125. [DOI] [PubMed] [Google Scholar]
- 5. Presbitero P, Demarie D, Villani M, Perinetto EA, Riva G, Orzan F, Bobbio M, Morea M, Brusca A. Long term results (15–30 years) of surgical repair of aortic coarctation. Br Heart J. 1987;57:462–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toro‐Salazar OH, Steinberger J, Thomas W, Rocchini AP, Carpenter B, Moller JH. Long‐term follow‐up of patients after coarctation of the aorta repair. Am J Cardiol. 2002;89:541–547. [DOI] [PubMed] [Google Scholar]
- 7. Hoimyr H, Christensen TD, Emmertsen K, Johnsen SP, Riis A, Hansen OK, Hjortdal VE. Surgical repair of coarctation of the aorta: up to 40 years of follow‐up. Eur J Cardiothorac Surg. 2006;30:910–916. [DOI] [PubMed] [Google Scholar]
- 8. Maron BJ, Humphries JO, Rowe RD, Mellits ED. Prognosis of surgically corrected coarctation of the aorta. A 20‐year postoperative appraisal. Circulation. 1973;47:119–126. [DOI] [PubMed] [Google Scholar]
- 9. Cohen M, Fuster V, Steele PM, Driscoll D, McGoon DC. Coarctation of the aorta. Long‐term follow‐up and prediction of outcome after surgical correction. Circulation. 1989;80:840–845. [DOI] [PubMed] [Google Scholar]
- 10. Celermajer DS, Greaves K. Survivors of coarctation repair: fixed but not cured. Heart. 2002;88:113–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Curtis SL, Bradley M, Wilde P, Aw J, Chakrabarti S, Hamilton M, Martin R, Turner M, Stuart AG. Results of screening for intracranial aneurysms in patients with coarctation of the aorta. AJNR Am J Neuroradiol. 2012;33:1182–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Egbe AC, Padang R, Brown RD, Khan AR, Luis SA, Huston J, Akintoye E, Connolly HM. Prevalence and predictors of intracranial aneurysms in patients with bicuspid aortic valve. Heart. 2017;103:1508–1514. [DOI] [PubMed] [Google Scholar]
- 13. Gardiner HM, Celermajer DS, Sorensen KE, Georgakopoulos D, Robinson J, Thomas O, Deanfield JE. Arterial reactivity is significantly impaired in normotensive young adults after successful repair of aortic coarctation in childhood. Circulation. 1994;89:1745–1750. [DOI] [PubMed] [Google Scholar]
- 14. Lanz J, Brophy JM, Therrien J, Kaouache M, Guo L, Marelli AJ. Stroke in adults with congenital heart disease: incidence, cumulative risk, and predictors. Circulation. 2015;132:2385–2394. [DOI] [PubMed] [Google Scholar]
- 15. Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Hansson P, Dellborg M. Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc. 2016;5:e003071 DOI: 10.1161/JAHA.115.003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gurvitz M, Valente AM, Broberg C, Cook S, Stout K, Kay J, Ting J, Kuehl K, Earing M, Webb G, Houser L, Opotowsky A, Harmon A, Graham D, Khairy P, Gianola A, Verstappen A, Landzberg M. Prevalence and predictors of gaps in care among adult congenital heart disease patients: HEART‐ACHD (The Health, Education, and Access Research Trial). J Am Coll Cardiol. 2013;61:2180–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anon . NIS Database Documentation. Healthc Cost Util Proj (HCUP) 2017. Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp. Accessed April 10, 2018.
- 18. Broberg C, McLarry J, Mitchell J, Winter C, Doberne J, Woods P, Burchill L, Weiss J. Accuracy of administrative data for detection and categorization of adult congenital heart disease patients from an electronic medical record. Pediatr Cardiol. 2015;36:719–725. [DOI] [PubMed] [Google Scholar]
- 19. Andrade SE, Harrold LR, Tjia J, Cutrona SL, Saczynski JS, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):100–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. [DOI] [PubMed] [Google Scholar]
- 21. Quan H, Khan N, Hemmelgarn BR, Tu K, Chen G, Campbell N, Hill MD, Ghali WA, McAlister FA. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54:1423–1428. [DOI] [PubMed] [Google Scholar]
- 22. Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf. 2012;21:154–162. [DOI] [PubMed] [Google Scholar]
- 23. Quan H, Li B, Saunders LD, Parsons GA, Nilsson CI, Alibhai A, Ghali WA; IMECCHI Investigators . Assessing validity of ICD‐9‐CM and ICD‐10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43:1424–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 25. Khokhar B, Jette N, Metcalfe A, Cunningham CT, Quan H, Kaplan GG, Butalia S, Rabi D. Systematic review of validated case definitions for diabetes in ICD‐9‐coded and ICD‐10‐coded data in adult populations. BMJ Open. 2016;6:e009952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiley LK, Shah A, Xu H, Bush WS. ICD‐9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc. 2013;20:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen J, Dharmarajan K, Wang Y, Krumholz HM. National trends in heart failure hospital stay rates, 2001 to 2009. J Am Coll Cardiol. 2013;61:1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure—associated hospitalizations in the United States. J Am Coll Cardiol. 2013;61:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Houchens R, Ross D, Elixhauser A, Jiang J. Nationwide Inpatient Sample (NIS) Redesign Final Report. HCUP Methods Ser Rep # 2014‐04 ONLINE April 4, 2014 US Agency Healthc Res Qual 2014. Available at: http://www.hcup-us.ahrq.gov/reports/methods/methods.jsp. Accessed April 10, 2018.
- 30. Roos‐Hesselink JW, Schölzel BE, Heijdra RJ, Spitaels SEC, Meijboom FJ, Boersma E, Bogers AJJC, Simoons ML. Aortic valve and aortic arch pathology after coarctation repair. Heart. 2003;89:1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khan A, Ramsey K, Ballard C, Armstrong E, Burchill LJ, Menashe V, Pantely G, Broberg CS. Limited accuracy of administrative data for the identification and classification of adult congenital heart disease. J Am Heart Assoc. 2018;7:e007378 DOI: 10.1161/JAHA.117.007378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith EE, Shobha N, Dai D, Olson DM, Reeves MJ, Saver JL, Hernandez AF, Peterson ED, Fonarow GC, Schwamm LH. A risk score for in‐hospital death in patients admitted with ischemic or hemorrhagic stroke. J Am Heart Assoc. 2013;2:e005207 DOI: 10.1161/JAHA.112.005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greving JP, Wermer MJH, Brown RD, Morita A, Juvela S, Yonekura M, Ishibashi T, Torner JC, Nakayama T, Rinkel GJE, Algra A. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. 2014;13:59–66. [DOI] [PubMed] [Google Scholar]
- 34. Luijendijk P, Lu H, Heynneman FB, Huijgen R, De Groot EE, Vriend JWJ, Vliegen HW, Groenink M, Bouma BJ, Mulder BJM. Increased carotid intima‐media thickness predicts cardiovascular events in aortic coarctation. Int J Cardiol. 2014;176:776–781. [DOI] [PubMed] [Google Scholar]
- 35. Xu J, Shiota T, Omoto R, Zhou X, Kyo S, Ishii M, Rice MJ, Sahn DJ. Intravascular ultrasound assessment of regional aortic wall stiffness, distensibility, and compliance in patients with coarctation of the aorta. Am Heart J. 1997;134:93–98. [DOI] [PubMed] [Google Scholar]
- 36. Mivelaz Y, Leung MT, Zadorsky MT, De Souza AM, Potts JE, Sandor GGS. Noninvasive assessment of vascular function in postoperative cardiovascular disease (coarctation of the aorta, tetralogy of Fallot, and transposition of the great arteries). Am J Cardiol. 2016;118:597–602. [DOI] [PubMed] [Google Scholar]
- 37. Voges I, Kees J, Jerosch‐Herold M, Gottschalk H, Trentmann J, Hart C, Gabbert DD, Pardun E, Pham M, Andrade AC, Wegner P, Kristo I, Jansen O, Kramer H‐H, Rickers C. Aortic stiffening and its impact on left atrial volumes and function in patients after successful coarctation repair: a multiparametric cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2016;18:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michalski AM, Richardson SD, Browne ML, Carmichael SL, Canfield MA, Vanzutphen AR, Anderka MT, Marshall EG, Druschel CM. Sex ratios among infants with birth defects, National Birth Defects Prevention Study, 1997–2009. Am J Med Genet Part A. 2015;167:1071–1081. [DOI] [PubMed] [Google Scholar]
- 39. Roifman I, Therrien J, Ionescu‐Ittu R, Pilote L, Guo L, Kotowycz MA, Martucci G, Marelli AJ. Coarctation of the aorta and coronary artery disease: fact or fiction? Circulation. 2012;126:16–21. [DOI] [PubMed] [Google Scholar]
- 40. Hager A, Kanz S, Kaemmerer H, Schreiber C, Hess J. Coarctation Long‐term Assessment (COALA): significance of arterial hypertension in a cohort of 404 patients up to 27 years after surgical repair of isolated coarctation of the aorta, even in the absence of restenosis and prosthetic material. J Thorac Cardiovasc Surg. 2007;134:738–745. [DOI] [PubMed] [Google Scholar]
- 41. Ou P, Bonnet D, Auriacombe L, Pedroni E, Balleux F, Sidi D, Mousseaux E. Late systemic hypertension and aortic arch geometry after successful repair of coarctation of the aorta. Eur Heart J. 2004;25:1853–1859. [DOI] [PubMed] [Google Scholar]
- 42. Kuhle S, Kirk SFL, Ohinmaa A, Veugelers PJ. Comparison of ICD code‐based diagnosis of obesity with measured obesity in children and the implications for health care cost estimates. BMC Med Res Methodol. 2011;11:173. [DOI] [PMC free article] [PubMed] [Google Scholar]


