Abstract
Background
Left ventricular diastolic dysfunction (DD) is common, particularly in women and older individuals, and it is associated with adverse cardiovascular outcomes. We evaluated the impact of age‐ and sex‐specific diagnostic criteria on the assessment of DD in the community‐based Framingham Heart Study.
Methods and Results
We estimated age‐ and sex‐specific reference limits for echocardiographic measures of DD in a healthy reference subsample (N=2355, mean age 44 years, 66% women). The prevalence, correlates, and association with future cardiovascular disease were compared for DD using age‐ and sex‐specific versus single cut point reference limits in a broad sample (N=6102, mean age 50 years, 56% women). Using age‐ and sex‐specific criteria, DD was present in ≈25% to 30% of individuals across age groups, and it was directly associated with a number of modifiable risk factors. In contrast, with single cut point criteria, age was the primary determinant of DD. During follow‐up (mean 7.9±2.2 years), incident cardiovascular disease occurred in 213 of 5770 individuals. Using age‐ and sex‐specific criteria, mild and moderate‐severe DD were associated with 50% (95% confidence interval, 1.09–2.05) and 65% (95% confidence interval, 1.14–2.38) higher incidences of cardiovascular disease, respectively, in age‐ and sex‐adjusted analyses. With single cut point criteria, moderate‐severe DD (hazard ratio, 1.66; 95% confidence interval, 1.05–2.61), but not mild DD (hazard ratio, 0.94; 95% confidence interval, 0.63–1.40), was associated with incident cardiovascular disease.
Conclusions
Age‐ and sex‐specific reference limits may result in DD assessments that are less dependent on age, more robustly related to modifiable risk factors, and are more closely associated with incident cardiovascular disease.
Keywords: diastolic dysfunction, echocardiography, epidemiology, heart failure, prevention
Subject Categories: Epidemiology, Cardiovascular Disease, Heart Failure
Clinical Perspective
What Is New?
Diastolic dysfunction is common in the community but its relations with modifiable cardiovascular risk factors and with overt cardiovascular disease are incompletely understood.
The prevalence of diastolic dysfunction is directly related to increasing age and varies by sex.
We developed age‐ and sex‐specific reference limits for key measures of diastolic function in a large community‐based cohort.
We then evaluated the cross‐sectional relations and prognostic significance of diastolic dysfunction using these reference limits.
What Are the Clinical Implications?
In a healthy reference sample of community‐dwelling individuals, we observed direct associations of age with E/Eʹ ratio and inverse relations with E/A ratio and Eʹ velocity.
Using age‐ and sex‐specific criteria, diastolic dysfunction is related to several modifiable risk factors regardless of age.
Our findings demonstrate different cross‐sectional relations and prospective associations for diastolic dysfunction as assessed by single cut point versus age‐ and sex‐specific criteria.
These results highlight an important limitation in the current assessment of diastolic function and support further investigations into the use of age‐ and sex‐specific reference limits to evaluate diastolic function in clinical practice.
Introduction
Left ventricular (LV) diastolic dysfunction (DD) is associated with cardiovascular disease (CVD) including heart failure (HF),1, 2 atrial fibrillation,3, 4, 5 and cardiovascular death.6 Using standard criteria,7 age is the dominant predictor of DD; indeed the prevalence of DD rises with advancing age, ranging from 27% to 43% in middle‐aged adults to becoming almost ubiquitous (88%) in those older than 85 years.1, 8, 9, 10 Whether DD represents a largely unavoidable consequence of cardiac senescence versus a result of accumulating cardiovascular risk factors remains unresolved. Determining the relative contributions of both nonmodifiable (age and sex) and modifiable risk factors to the development of DD is integral to disease prevention efforts.
Echocardiographic assessment of diastolic function has several limitations. Reduced diastolic function is considered to be an intermediate phenotype in the pathophysiology of heart failure (HF), particularly HF with preserved ejection fraction.11, 12 However, HF with preserved ejection fraction trials have repeatedly demonstrated that traditional echocardiographic features of DD are not required to develop the condition, and were absent in approximately one third of patients.13, 14, 15 Furthermore, the evidence supporting DD as an independent predictor of future HF is restricted to a small number of studies.1, 2, 16 These observations contrast with data suggesting that DD is common in asymptomatic individuals in the community, with the majority having some degree of DD by advanced age.9
Motivated by reports of strong age‐ and sex‐related differences in diastolic function, and observations of limitations in the current assessment of DD, we sought to evaluate the usefulness of age‐ and sex‐specific reference limits for DD assessment in the community. Accordingly, we hypothesized that DD characterized by age‐ and sex‐specific reference limits is less dependent on age and is more closely related to the burden of traditional risk factors and to the risk of future CVD. We tested our hypotheses in the large community‐based Framingham Heart Study by comparing the prevalence, clinical correlates, and predictive significance of DD using age‐ and sex‐specific and single cut point reference limits.
Methods
The data, analytic methods, and study materials are not currently available to other researchers for purposes of reproducing the results or replicating the procedure. The procedure for requesting data from the Framingham Heart Study can be found at https://www.framinghamheartstudy.org/.
Study Sample
The design of the Framingham Offspring and Third Generation cohorts were detailed previously.17, 18 For the present investigation, the 3021 attendees of the eighth Offspring examination (2005–2008) and 4095 attendees of the first examination of the Third Generation (2002–2005) were eligible for inclusion. Anthropometry, a cardiovascular‐focused clinical examination, comprehensive medical history, and phlebotomy were performed at each Heart Study examination. Participants were excluded for the following reasons: missing or inadequate diastolic function measures (N=355), wall motion abnormalities (N=199), LV systolic dysfunction (N=62; defined as fractional shortening ≤0.29 or 2‐dimensional evidence of mild or greater LV systolic dysfunction), moderate or greater valvular heart disease (N=148), paced rhythm (N=13), missing covariates (N=232), and atrial fibrillation at the time of examination (N=5) yielding a final broad sample of 6102 individuals eligible for the present investigation (Figure S1). To create a healthy reference sample for deriving reference limits, we excluded participants from the broad sample with prevalent CVD (N=286), hypertension (N=1727), atrial fibrillation (N=15), diabetes mellitus (N=70), dyslipidemia (N=743; defined as low‐density lipoprotein cholesterol ≥160 mg/dL, high‐density lipoprotein cholesterol <40 mg/dL, or triglycerides ≥250 mg/dL), obesity (N=503; body mass index [BMI] ≥30 kg/m2), or current smoking (N=403), resulting in a reference sample of 2355 individuals. For prospective analyses, individuals from the broad sample with prevalent CVD (N=286) or missing follow‐up time (N=46) were excluded. The Boston University Medical Center Institutional Review Board approved all study protocols, and all participants provided informed written consent.
Echocardiography
Echocardiography including M‐mode, 2‐dimensional, pulsed‐wave Doppler and tissue Doppler imaging were performed on all participants as part of a standardized protocol at the index examinations using an HP Sonos Ultrasound Machine (Philips Healthcare, Andover, MA). With the participant in the left lateral decubitus position, pulsed‐wave Doppler examination of mitral inflow was performed in the apical 4‐chamber position, and the sample volume was placed at the level of the mitral leaflet tips. Tissue Doppler assessment of peak early diastolic tissue velocity of the lateral mitral annulus (Eʹ) and transmitral Doppler flow velocities (E and A wave peak velocities) were performed. Repeated measurements of the Eʹ, E, and A waves resulted in interobserver correlation coefficients of >0.97.19
Definition of DD
We characterized individuals as having normal diastolic function, mild DD, moderate DD, or severe DD based on the three available measures of diastolic function (lateral Eʹ velocity, E/Eʹ ratio, and E/A ratio). For the single cut point analyses, we used the modified Olmstead criteria (excluding assessments of mitral inflow during the Valsalva maneuver and pulmonary venous flow patterns, which were not available for the present analyses)20, 21: normal diastolic function, E/A >0.75 and E/Eʹ <10; mild DD, E/A ≤0.75 and E/Eʹ <10; moderate DD, E/A ≤1.5 and E/Eʹ ≥10; severe DD, E/A >1.5 and E/Eʹ ≥10. For the age‐ and sex‐specific criteria, we categorized individuals as having: normal LV diastolic function, no abnormal measures; mild DD, 1 abnormal measure; moderate DD, 2 abnormal measures; and severe DD, 3 abnormal measures. We performed 2 additional sensitivity analyses using different classification schemes (Table S1).
Covariates
Blood pressure was measured on seated participants using a manual mercury column sphygmomanometer; the average of 2 readings was recorded for each participant. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or use of medications for diabetes mellitus. Current smoking (yes/no within the year prior the index examination) was assessed by self‐report. Weight in kilograms was divided by height in meters squared to calculate BMI.
Outcome Ascertainment
Framingham Heart Study participants are under longitudinal surveillance for the development of CVD outcomes, which are adjudicated by an end points review committee of 3 physicians during a consensus review of pertinent medical records, examination visits, and health history updates. For the present investigation, the outcome was a first CVD event, which was defined as a composite of fatal and nonfatal myocardial infarction, coronary insufficiency, angina, stroke or transient ischemic attack, intermittent claudication, or heart failure using traditional Framingham Heart Study criteria.22
Statistical Analysis
Baseline characteristics were presented for the broad and reference samples. Sex‐stratified quantile regression was used to estimate age‐ and sex‐specific reference limits for each diastolic function measure. Values at the 90th percentile or greater were considered abnormal for E/Eʹ ratio and values at the 10th percentile or less were considered abnormal for Eʹ and for E/A ratio.
To test for differences in categorizing the severity of DD using the 2 different criteria, we calculated the paired difference between DD prevalence (treating DD as an ordinal variable) with single cut point versus age‐ and sex‐specific criteria. A Spearman correlation coefficient was then calculated for the association of this difference and 10‐year age group.
We used stepwise regression models to evaluate the clinical correlates of DD using both the age‐ and sex‐specific and single cut point criteria. Age and sex were forced into the model, and systolic blood pressure, diastolic blood pressure, hypertension treatment status, diabetes mellitus, BMI, total/high‐density lipoprotein cholesterol, smoking, and CVD were eligible for inclusion. A P<0.10 in a univariate screen was required to enter the multivariable model, and a Bonferroni‐adjusted P<0.005 was used to determine which variables remained in the final model. Analyses were performed treating DD categories as ordinal variables (using ordinal logistic regression models) and then repeated, treating DD as the binary variable of mild or greater or moderate or greater DD (using logistic regression models).
We used Cox proportional hazards regression models to evaluate the association of each DD category with incident CVD, treating normal LV diastolic function as the referent. Two multivariable‐models were estimated for each classification scheme. The first was adjusted for age and sex. The second model was adjusted additionally for systolic blood pressure, hypertension treatment status, diabetes mellitus, BMI, total/high‐density lipoprotein cholesterol, and smoking. We tested the proportionality of hazards assumption by including an interaction of the natural log of time to CVD * DD category (P>0.05). A 2‐sided P<0.05 was used to determine statistical significance for the association of DD with incident CVD. All analyses were performed with SAS version 9.3 (Cary, NC).
Results
Clinical characteristics of the broad and reference samples are shown in Table 1 and Table S2. The mean age in the broad sample was 50±15 years, over half were women, and 32% of individuals had hypertension. The reference sample was younger (mean age 44±14 years), had a higher proportion of women (66%), and was, by definition, free of CVD or major clinical risk factors.
Table 1.
Baseline Characteristics of the Study Samples
| Characteristic | Broad Sample (N=6102) | Reference Sample (N=2355) |
|---|---|---|
| Age, y | 50±15 | 44±14 |
| Women, N (%) | 3394 (56) | 1560 (66) |
| Systolic blood pressure, mm Hg | 121±16 | 113±11 |
| Diastolic blood pressure, mm Hg | 75±10 | 72±8 |
| Heart rate, bpm | 62±10 | 61±10 |
| Body mass index, kg/m2 | 27.2±5.4 | 24.1±3.0 |
| Total cholesterol, mg/dL | 189±35 | 183±29 |
| HDL cholesterol, mg/dL | 56±17 | 62±15 |
| Triglycerides, mg/dL | 110±60 | 85±38 |
| Hypertension, N (%) | 1963 (32) | 0 |
| Hypertension treatment, N (%) | 1457 (24) | 0 |
| Current smoking, N (%) | 847 (14) | 0 |
| Prior atrial fibrillation, N (%) | 81 (1) | 0 |
| Cardiovascular disease, N (%) | 286 (5) | 0 |
| Diabetes mellitus, N (%) | 352 (6) | 0 |
| Chronic kidney disease, N (%) | 284 (4.7) | 28 (1.2) |
| Left ventricular mass, g | 159±43 | 143±37 |
| Medication use | ||
| ACE inhibitor/ARB, N (%) | 870 (14.3) | 0 (0) |
| Beta‐blocker, N (%) | 663 (10.9) | 15 (0.6) |
| Diuretic, N (%) | 636 (10.4) | 13 (0.5) |
| Left atrial dimension, cm | 3.8±0.5 | 3.6±0.4 |
| Fractional shortening, % | 37±4 | 36±4 |
| Lateral Eʹ, cm/s | 11.4±2.9 | 12.6±2.8 |
| E velocity, cm/s | 67.6±12.8 | 69.2±12.5 |
| A velocity, cm/s | 58.6±15.9 | 51.8±12.8 |
| E/Eʹ ratio | 6.3±1.9 | 5.7±1.5 |
Values represent mean ± standard deviation for continuous variables and number (%) for categorical variables. Chronic kidney disease was defined as an estimated glomerular filtration rate of <60 mL/min/1.73 m2. ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; HDL, high‐density lipoprotein.
Derivation of Age‐ and Sex‐Specific Reference Limits
Sex‐stratified quantile regression was used to estimate age‐ and sex‐specific reference limits for Eʹ velocity, E/Eʹ and E/A in the reference sample. Age was directly related to the A velocity and E/Eʹ ratio and was inversely related to the E velocity, Eʹ velocity, and E/A ratio (Figures S2 through S6). The mean value for Eʹ velocity fell below the guideline‐supported threshold7 of 10 cm/s by 70 years of age in both men and women (Figure S2A). The abnormal Eʹ threshold was reached at a younger age in the broad sample (Figure S2B). Mean E/Eʹ ratio was higher in women versus men but did not rise above the guideline‐supported threshold value7 of 8 in any age group (Figure S3). Mean E/A ratio fell to <0.75 by 80 years of age in both sexes in both the reference and the broad group (Figure S4). The cutoff values for age‐ and sex‐specific reference limits are shown by decade of age in Table 2. Age‐ and sex‐specific cutoff values differed substantially from the commonly used single cut point reference limits established by echocardiographic guidelines.7
Table 2.
10th Percentile Reference Limits According to Age‐ and Sex‐Specific Criteria
| Age | Lateral Eʹ (cm/s) | E/A Ratio | E/Eʹ Ratio |
|---|---|---|---|
| Men | |||
| 30 y | 11.00 | 1.21 | 6.33 |
| 40 y | 10.11 | 1.06 | 6.64 |
| 50 y | 9.21 | 0.91 | 6.94 |
| 60 y | 8.32 | 0.76 | 7.25 |
| 70 y | 7.42 | 0.61 | 7.55 |
| 80 y | 6.53 | 0.46 | 7.85 |
| Women | |||
| 30 y | 11.39 | 1.24 | 6.62 |
| 40 y | 10.31 | 1.10 | 7.33 |
| 50 y | 9.22 | 0.95 | 8.03 |
| 60 y | 8.14 | 0.81 | 8.73 |
| 70 y | 7.05 | 0.66 | 9.44 |
| 80 y | 5.97 | 0.51 | 10.14 |
Single cut point (guideline‐based) reference limits: Lateral Eʹ=10 cm/s, E/A ratio ≥0.8, E/Eʹ ratio ≤8. Reference limit values represent the lower reference limit for lateral Eʹ and E/A ratio and the upper reference limit for E/Eʹ ratio.
Prevalence of DD in the Broad Sample
By classifying DD using age‐ and sex‐specific criteria, 15% to 22% of the study sample had mild DD, and 10% of the sample had moderate‐severe DD throughout the age range (Figure[A]). By contrast, using single cut point criteria, DD was rare until 50 years of age, after which the prevalence and severity of DD rose steeply (Figure[B]). By 70 to <80 years of age, over half of the participants had DD, and after 80 years of age, over two thirds did. Compared with age‐ and sex‐specific criteria, the single cut point criteria categorizes more people with higher DD at older ages (r=0.24, P<0.0001).
Figure 1.
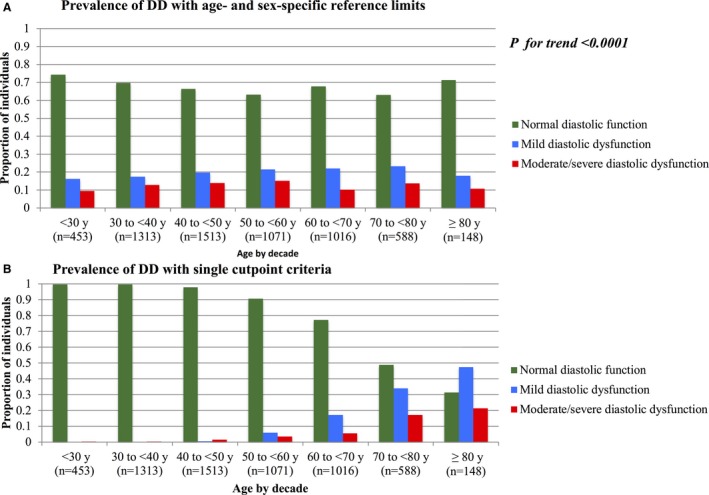
Proportion of individuals with diastolic dysfunction. A, By age‐ and sex‐specific criteria. B, By single cutpoint criteria. P for trend test represents the P‐value for the correlation of the difference in diastolic dysfunction severity between the 2 criteria and age group.
Clinical Correlates of DD
With age‐ and sex‐specific criteria, DD was associated with age, female sex, systolic and diastolic blood pressure, hypertension treatment status, BMI, total/high‐density lipoprotein cholesterol and diabetes mellitus (Table 3). Age was inversely related to DD in the multivariable‐adjusted model, but it was directly related to DD in models adjusted only for age and sex (odds ratio, 1.09 per 15.3 years; 95% confidence interval, 1.03–1.15; P=0.0017).
Table 3.
Clinical Correlates of LV Diastolic Dysfunction in the Broad Sample: Age‐ and Sex‐Specific vs Single Cut Point Criteria
| Variable | Age‐ and Sex‐Specific Criteria | Single Cut Point Criteria | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Age | 0.91 (0.84, 0.97) | 0.007 | 7.40 (6.46, 8.47) | <0.0001 |
| Female sex | 1.42 (1.26, 1.60) | <0.0001 | 1.72 (1.43, 2.07) | <0.0001 |
| Systolic blood pressure | 1.20 (1.12, 1.30) | <0.0001 | ··· | ··· |
| Diastolic blood pressure | 1.29 (1.21, 1.39) | <0.0001 | 1.37 (1.25, 1.50) | <0.0001 |
| Hypertension treatment status | 1.24 (1.07, 1.44) | 0.004 | ··· | ··· |
| Body mass index | 1.38 (1.30, 1.47) | <0.0001 | 1.36 (1.25, 1.48) | <0.0001 |
| Total/HDL cholesterol | 1.15 (1.08, 1.22) | <0.0001 | ··· | ··· |
| Diabetes mellitus | 1.68 (1.34, 2.10) | <0.0001 | ··· | ··· |
Values represent the odds of having diastolic dysfunction (treated as an ordinal variable: normal diastolic function, mild DD, moderate‐severe DD) for each 1 SD higher value (for continuous variables) or the presence of a categorical variable vs its absence. 1 SD is equal to: 15.3 years for age, 16.1 mm Hg for SBP, 9.6 mm Hg for DBP, 5.4 kg/m2 for BMI, and 1.2 for total/HDL cholesterol. Smoking and previous cardiovascular disease were included as candidate variables in the selection models but were not statistically significant. CI indicates confidence interval; HDL, high‐density lipoprotein; LV, left ventricular.
Using single cut point criteria, DD was associated with age, female sex, diastolic blood pressure, and BMI (Table 3); age had the largest effect estimate with an over 7‐fold higher odds of having DD for each 15.3 years of age. Notably, this dependence on age was most pronounced in those with mild DD (Table S3). When the odds of having moderate or greater DD were evaluated, the impact of age was reduced and the additional risk factors of systolic and diastolic blood pressure and hypertension treatment status were observed to correlate with DD (Table S4). In exploratory analyses, a higher heart rate was observed to correlate with LV DD (Table S5).
Prospective Associations of DD With Incident CVD
CVD events occurred in 213 of 5770 individuals during a mean follow‐up of 7.9±2.2 years (range, 0.1–11.7 years). Using age‐ and sex‐specific criteria, mild, and moderate‐severe DD were associated with 50% and 65% higher hazards of CVD, respectively, in age‐ and sex‐adjusted analyses (Table 4). Upon adjustment for clinical risk factors, these associations were no longer statistically significant. With single cut point criteria, mild DD was not associated with incident CVD in age‐ and sex‐adjusted or in multivariable‐adjusted analyses. Moderate‐severe DD was associated with a 66% higher hazard of CVD in age‐ and sex‐adjusted models, which was no longer statistically significant upon adjustment for clinical risk factors. In sensitivity analyses, we used additional DD classification schemes based on both single cut point and age‐ and sex‐specific reference limits with similar results (Table S6).
Table 4.
Associations of LV Diastolic Dysfunction Categories With Incident CVD
| No. Events/No. At Risk | Age‐ and Sex‐Adjusted, HR (95% CI) | P Value | Multivariable‐Adjusted, HR (95% CI) | P Value | |
|---|---|---|---|---|---|
| Age‐ and sex‐specific criteria | |||||
| 1. Normal diastolic function | 119/3906 | Referent | ··· | Referent | ··· |
| 2. Mild DD | 57/1134 | 1.50 (1.09–2.05) | 0.01 | 1.34 (0.98–1.85) | 0.07 |
| 3. Moderate‐severe DD | 37/730 | 1.65 (1.14–2.38) | 0.008 | 1.26 (0.86–1.85) | 0.24 |
| Test for trend | 1.32 (1.11–1.56) | 0.002 | 1.16 (0.97–1.38) | 0.11 | |
| 1. Normal diastolic function | 119/3906 | Referent | ··· | Referent | ··· |
| 2. Any DD | 94/1864 | 1.55 (1.19–2.04) | 0.0014 | 1.31 (0.99–1.73) | 0.06 |
| Single cut point criteria | |||||
| 1. Normal diastolic function | 150/5103 | Referent | ··· | Referent | ··· |
| 2. Mild DD | 37/451 | 0.94 (0.63–1.40) | 0.76 | 0.90 (0.60–1.33) | 0.59 |
| 3. Moderate‐severe DD | 26/216 | 1.66 (1.05–2.61) | 0.03 | 1.36 (0.86–2.15) | 0.18 |
| Test for trend | 1.22 (0.97–1.53) | 0.09 | 1.11 (0.89–1.39) | 0.37 | |
| 1. Normal diastolic function | 150/5103 | Referent | ··· | Referent | ··· |
| 2. Any DD | 63/667 | 1.14 (0.81–1.61) | 0.44 | 1.04 (0.74–1.46) | 0.82 |
Hazard ratios (HRs) represent the relative hazard for each category compared with the referent group. The multivariable model is adjusted for age, sex, systolic blood pressure, hypertension treatment status, diabetes mellitus, body mass index, total/high‐density lipoprotein cholesterol, and smoking. CI indicates confidence interval; CVD, cardiovascular disease; DD, diastolic dysfunction; LV, left ventricular.
Discussion
We investigated the prevalence, clinical correlates, and predictive significance of DD, defined with and without age‐ and sex‐specific thresholds, in a large community‐based sample. We observed several key findings. First, in prospective analyses adjusted for age and sex, the age‐ and sex‐specific criteria were associated with incident CVD regardless of DD severity. By single cut point criteria, mild DD was not associated with an increased risk of CVD. Second, using both sets of criteria, the association of DD with future CVD was no longer statistically significant upon adjustment for common CVD risk factors, suggesting that the increased risk of CVD in those with DD is at least partly attributable to shared burden of standard risk factors. Furthermore, using single cut point (age‐ and sex‐independent) criteria, age was the primary determinant of DD, and the proportion of individuals with normal LV diastolic function decreased to a minority of the sample by 70 years of age. By contrast, with age‐ and sex‐specific criteria, statistically significant relations were observed between DD and a number of clinical risk factors that were not as clearly discernible if single cut point criteria were used.
DD as a Predictor of Future CVD
We observed modest associations between DD and incident CVD in age‐ and sex‐adjusted analyses that were no longer statistically significant upon adjustment for clinical risk factors. A small number of previous studies have reported associations of DD with clinical outcomes in the community. From the Rochester Epidemiology Project, Redfield et al21 demonstrated associations of DD with overall mortality in unadjusted analyses and after adjusting for age, sex, and ejection fraction. In a follow‐up study, Kane et al1 demonstrated that DD was associated with future HF, and this association persisted upon adjustment for age, hypertension, diabetes mellitus, and baseline CAD. The most notable difference between their study and ours is that we excluded individuals with LV systolic dysfunction. Kane et al did not report whether DD was predictive of future HF after accounting for reduced systolic function. Vogel et al23 examined the natural history of preclinical DD and reported 3‐year cumulative probability of HF of 11.6% in those with DD, but their analyses were not adjusted for clinical risk factors. Therefore, although DD appears to be associated with adverse cardiovascular outcomes, its utility as an independent predictor of HF, CVD, or mortality appears modest.
A number of possible explanations exist for the limited independent associations of DD with future CVD. Traditional risk factors were strongly related with DD in our investigation (especially using age‐ and sex‐specific reference limits) and also attenuated the association with incident CVD. If DD simply represents end‐organ dysfunction resulting from the effects of CVD risk factor exposure, its independent power to predict future CVD may be limited. Furthermore, although it is often thought of as preclinical HF with preserved ejection fraction, DD is neither necessary nor sufficient to develop HF. This notion is supported by the observation that while DD is highly prevalent in asymptomatic individuals, up to one third of individuals enrolled in HF with preserved ejection fraction clinical trials do not have echocardiographic evidence of DD.13, 14, 15 Indeed, other conditions such as pulmonary, cardiometabolic, or kidney dysfunction appear to greatly modify the risk of progression to HF in those with DD.2, 23, 24 Finally, although echocardiographic measures used to assess LV diastolic function were derived largely based on hemodynamic correlations,25, 26, 27 their accuracy in diagnosing preclinical DD at the individual level is less clear.28, 29 Advancements in imaging techniques and integration of other features such as myocardial strain, ventricular‐arterial coupling, or functional capacity may prove useful in refining the noninvasive assessment of DD in the future.
Diastolic Function Declines With Age
The extent to which age‐related changes represent unavoidable sequelae of cardiac senescence versus maladaptive responses to repeated pathologic stressors (such as increased afterload and metabolic stress) remains uncertain. In the present investigation, age‐related decrements in LV diastolic function were observed in the healthy reference sample, which consisted of individuals free of CVD and overt clinical risk factors. These findings are consistent with the work of Dalen et al,30 who reported lower mean Eʹ velocity and E/A ratio and higher mean E/Eʹ in older individuals in a less strictly defined and smaller healthy reference sample. The finding of age‐related changes in diastolic function in the absence of overt CVD risk factors supports the hypothesis that normal aging itself leads to reductions in the measures commonly used to assess LV diastolic function.
In particular, the Eʹ velocity falls steadily even with healthy aging. Relying on a single cut point value of Eʹ to diagnose LV DD would be expected to result in a large proportion of older individuals being diagnosed with LV DD, which may not identify individuals that are necessarily at higher risk for CVD.
Whereas age was the primary risk factor for DD using single cut point criteria, with age‐ and sex‐specific criteria, DD was associated with a number of modifiable clinical risk factors. The inverse association of age with DD in these models likely reflects the rising burden of CVD risk factors with age, which is not fully accounted for in our analyses. One potential advantage of age‐ and sex‐specific reference limits, therefore, is that DD based on these criteria is more closely related to modifiable risk factors.
DD Differs Among Men and Women
We observed different distributions for LV diastolic function measures in men and women (this was particularly pronounced for E/Eʹ ratio) and we therefore stratified our reference limits by sex. Similar sex‐based differences in LV function and geometry have been reported previously.31, 32, 33, 34, 35 The biological basis for sex‐based variations, including potential neurohormonal contributions36 and sex‐related differences in ventricular‐arterial coupling,31, 37 warrant further study, especially in light of the higher risk of HF with preserved ejection fraction in women.
Limitations and Strengths
Several limitations of the present investigation merit consideration. Echocardiograms performed at the Framingham Heart Study were one component of a comprehensive examination day, and the time commitment for each test was thus minimized in order to facilitate participation by the largest possible number of study participants. As a result, we did not obtain several measurements previously shown to assist in the assessment of DD, including the septal Eʹ velocity, pulmonary vein inflow, longitudinal strain, mitral inflow during the Valsalva maneuver, mitral annular size, or regurgitant velocity across the tricuspid valve. Left atrial volumes were also not used for this investigation. Additional investigations incorporating these measures and adhering more closely with the 2016 guidelines for assessment of diastolic function38 would refine the criteria proposed herein. Moreover, LV diastolic function was assessed a single time. As these measures can vary widely with loading conditions and other hemodynamic factors, the single assessment might have introduced additional variability into our baseline assessments. Such a regression dilution bias would be expected to bias toward the null hypothesis of no association between DD and the outcomes evaluated and may have affected our ability to detect meaningful associations. Although the majority of the echocardiograms were performed in the morning, we did not fully account for circadian variation, which may affect diastolic function.39 The number of observed events was relatively small for each component of our composite outcome, and we were therefore unable to definitively evaluate the associations of DD with separate cardiovascular outcomes. Despite the total sample size of more than 6000 participants, relatively few individuals >70 years of age were available for the healthy reference sample as a result of the significant burden of overt CVD and CVD risk factors in this age group. Therefore, the reference limit estimates for this age category may be less precise when applied to the general population. Furthermore, the age‐ and sex‐specific criteria that we used treated E/A as a monotonic variable, which does not account for the complexities of changes in diastolic filling that occur with rising LV filling pressures. To explore whether this assumption might compromise the association of our criteria with incident CVD, we constructed 2 additional age‐ and sex‐specific criteria that were analyzed in sensitivity analyses. Both of these criteria demonstrated reduced prediction compared with the criteria used in primary analysis. Thus, future studies with higher numbers of individuals with severe diastolic dysfunction are needed to discover relations of age and sex with restrictive filling patterns. Whereas we did evaluate interobserver variability in measurements, variation within individuals in the short term also may influence the predictive utility of our proposed criteria. Given the constraints of participant burden in an epidemiological setting, we were unable to assess intraindividual variability in LV diastolic function measures. Finally, our study sample consisted of mostly white individuals of European descent. Future studies are needed to evaluate the effect of race on diastolic function.
Notwithstanding these limitations, our study has a number of important strengths. Foremost, it was conducted in a large, well‐characterized, community‐based cohort with comprehensive ascertainment of outcomes in ≈6100 individuals. We were able to determine age‐ and sex‐specific reference limits from a strictly defined healthy sample of 2355 individuals with representation from all age groups, which allowed us to calculate reference limits for abnormal diastolic function treating age as a continuous variable.
Conclusions
Our findings show that age‐ and sex‐specific reference limits demonstrate different clinical correlates and association with overt CVD compared with single cut point criteria. Additional studies are warranted to determine whether age‐ and sex‐specific reference limits should be used to assess DD in clinical practice.
Sources of Funding
This work was partially supported by the National Heart, Lung and Blood Institute's Framingham Heart Study (Contracts N01‐HC‐25195 and HHSN268201500001I) and by grants HL076784, G028321, HL070100, HL060040, HL080124, HL071039, HL077447, HL107385, HL126136, 2R01HL092577, and 2‐K24‐HL04334. Dr Nayor received support from K23‐HL138260 and training grant U10HL110337 both from the National Heart, Lung, and Blood Institute. Dr Cooper is supported by the UNCF/Merck Science Initiative. Dr Vasan is supported by an Evans Scholar award from the Department of Medicine, Boston University School of Medicine.
Disclosures
Dr Mitchell is the owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, serves as a consultant to and receives grants and honoraria from Novartis, Merck, Servier, and Philips Healthcare. The remaining authors have no disclosures to report.
Supporting information
Table S1. Additional Diastolic Dysfunction Classification Schemes Used for Sensitivity Analyses
Table S2. Composition of Study Samples by Age Decade and Sex
Table S3. Clinical Correlates of Mild or Greater Diastolic Dysfunction
Table S4. Clinical Correlates of Moderate or Greater Diastolic Dysfunction
Table S5. Clinical Correlates of LV Diastolic Dysfunction Including Heart Rate as a Predictor
Table S6. Sensitivity Analysis: Associations of Additional Age‐ and Sex‐Specific and Age‐ and Sex‐Independent Criteria With Incident CVD.
Figure S1. Flow diagram of study design.
Figure S2. Quantile regression plots for lateral E′. A, Reference sample. B, Broad sample. The horizontal red line represents the guideline recommended single cut point reference limit.
Figure S3. Quantile regression plots for E/E′ ratio. A, Reference sample. B. Broad sample. The horizontal red line represents the guideline recommended single cut point reference limit.
Figure S4. Quantile regression plots for E/A ratio. A, Reference sample. B, Broad sample. The horizontal red line represents the guideline recommended single cut point reference limit.
Figure S5. Quantile regression plots for E velocity. A, Reference sample. B, Broad sample.
Figure S6. Quantile regression plots for A velocity. A, Reference sample. B, Broad sample.
(J Am Heart Assoc. 2018;7:e008291 DOI: 10.1161/JAHA.117.008291.)29858363
References
- 1. Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lam CSP, Lyass A, Kraigher‐Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation. 2011;124:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenberg MA, Gottdiener JS, Heckbert SR, Mukamal KJ. Echocardiographic diastolic parameters and risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012;33:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, Oh JK, Leibson C, Montgomery SC, Seward JB. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol. 2002;40:1636–1644. [DOI] [PubMed] [Google Scholar]
- 5. Vasan RS, Larson MG, Levy D, Galderisi M, Wolf PA, Benjamin EJ. Doppler transmitral flow indexes and risk of atrial fibrillation (the Framingham Heart Study). Am J Cardiol. 2003;91:1079–1083. [DOI] [PubMed] [Google Scholar]
- 6. Bella JN, Palmieri V, Roman MJ, Liu JE, Welty TK, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Mitral ratio of peak early to late diastolic filling velocity as a predictor of mortality in middle‐aged and elderly adults: the Strong Heart Study. Circulation. 2002;105:1928–1933. [DOI] [PubMed] [Google Scholar]
- 7. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 8. Kuznetsova T, Herbots L, Lopez B, Jin Y, Richart T, Thijs L, Gonzalez A, Herregods MC, Fagard RH, Diez J, Staessen JA. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. [DOI] [PubMed] [Google Scholar]
- 9. Yousaf F, Collerton J, Kingston A, Kenny A, Davies K, Jagger C, Robinson L, Kirkwood TBL, Keavney B. Prevalence of left ventricular dysfunction in a UK community sample of very old people: the Newcastle 85+ study. Heart. 2012;98:1418–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mureddu GF, Agabiti N, Rizzello V, Forastiere F, Latini R, Cesaroni G, Masson S, Cacciatore G, Colivicchi F, Uguccioni M, Perucci CA, Boccanelli A. Prevalence of preclinical and clinical heart failure in the elderly: a population‐based study in Central Italy. Eur J Heart Fail. 2012;14:718–729. [DOI] [PubMed] [Google Scholar]
- 11. Ammar KA, Jacobsen SJ, Mahoney DW, Kors JA, Redfield MM, Burnett JC, Rodeheffer RJ. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115:1563–1570. [DOI] [PubMed] [Google Scholar]
- 12. Wan S‐H, Vogel MW, Chen HH. Pre‐clinical diastolic dysfunction. J Am Coll Cardiol. 2014;63:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE; I‐PRESERVE Investigators . Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. [DOI] [PubMed] [Google Scholar]
- 14. Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, Ostergren J, McKelvie RS. Diastolic dysfunction in heart failure with preserved systolic function: need for objective evidence: results from the CHARM Echocardiographic Substudy–CHARMES. J Am Coll Cardiol. 2007;49:687–694. [DOI] [PubMed] [Google Scholar]
- 15. Shah AM, Shah SJ, Anand IS, Sweitzer NK. Cardiac structure and function in heart failure with preserved ejection fraction: baseline findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist trial. Circ Heart Fail. 2014;7:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–1048. [DOI] [PubMed] [Google Scholar]
- 17. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families: the Framingham offspring study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 18. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The third generation cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 19. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, Aragam J, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Relations of central hemodynamics and aortic stiffness with left ventricular structure and function: the Framingham heart study. J Am Heart Assoc. 2016;5:e002693 DOI: 10.1161/JAHA.115.002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O'Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 22. Kannel WB, Wolf PA, Garrison RJ. The Framingham Study: An Epidemiological Investigation of Cardiovascular Disease. Section 34. Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death Using Pooled Repeated Biennial Measurements: Framingham Heart Study, 30‐Year Follow‐Up. 1987 ed. Bethesda, MD: National Heart, Lung, and Blood Institute; 1987. (NIH publication no. 87–2703). [Google Scholar]
- 23. Vogel MW, Slusser JP, Hodge DO, Chen HH. The natural history of preclinical diastolic dysfunction: a population‐based study. Circ Heart Fail. 2012;5:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre‐clinical diastolic dysfunction: a population‐based study. J Am Coll Cardiol. 2010;55:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler‐catheterization study. Circulation. 2000;102:1788–1794. [DOI] [PubMed] [Google Scholar]
- 26. Kim YJ, Sohn DW. Mitral annulus velocity in the estimation of left ventricular filling pressure: prospective study in 200 patients. J Am Soc Echocardiogr. 2000;13:980–985. [DOI] [PubMed] [Google Scholar]
- 27. Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician's Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. [DOI] [PubMed] [Google Scholar]
- 28. Santos M, Rivero J, McCullough SD, West E. E/e′ ratio in patients with unexplained dyspnea lack of accuracy in estimating left ventricular filling pressure. Circ Heart Fail. 2015;8:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss H‐P, Pauschinger M, Tschöpe C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler‐conductance catheterization study. Circulation. 2007;116:637–647. [DOI] [PubMed] [Google Scholar]
- 30. Dalen H, Thorstensen A, Vatten LJ, Aase SA, Stoylen A. Reference values and distribution of conventional echocardiographic Doppler measures and longitudinal tissue Doppler velocities in a population free from cardiovascular disease. Circ Cardiovasc Imaging. 2010;3:614–622. [DOI] [PubMed] [Google Scholar]
- 31. Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, Rodeheffer RJ. Longitudinal changes in left ventricular stiffness: a community‐based study. Circ Heart Fail. 2013;6:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tighe DA, Vinch CS, Hill JC, Meyer TE, Goldberg RJ, Aurigemma GP. Influence of age on assessment of diastolic function by Doppler tissue imaging. Am J Cardiol. 2003;91:254–257. [DOI] [PubMed] [Google Scholar]
- 33. Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, Aragam J, Benjamin EJ, Solomon SD, Vasan RS. Age‐ and sex‐based reference limits and clinical correlates of myocardial strain and synchrony: the Framingham Heart Study. Circ Cardiovasc Imaging. 2013;6:692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng S, Fernandes VRS, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age‐related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi‐Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lam CSP, Cheng S, Choong K, Larson MG, Murabito JM, Newton‐Cheh C, Bhasin S, McCabe EL, Miller KK, Redfield MM, Vasan RS, Coviello AD, Wang TJ. Influence of sex and hormone status on circulating natriuretic peptides. J Am Coll Cardiol. 2011;58:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Amer Soc of Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 39. Voutilainen S, Kupari M, Hippelainen M, Karppinen K, Ventila M. Circadian variation of left ventricular diastolic function in healthy people. Heart. 1996;75:35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Additional Diastolic Dysfunction Classification Schemes Used for Sensitivity Analyses
Table S2. Composition of Study Samples by Age Decade and Sex
Table S3. Clinical Correlates of Mild or Greater Diastolic Dysfunction
Table S4. Clinical Correlates of Moderate or Greater Diastolic Dysfunction
Table S5. Clinical Correlates of LV Diastolic Dysfunction Including Heart Rate as a Predictor
Table S6. Sensitivity Analysis: Associations of Additional Age‐ and Sex‐Specific and Age‐ and Sex‐Independent Criteria With Incident CVD.
Figure S1. Flow diagram of study design.
Figure S2. Quantile regression plots for lateral E′. A, Reference sample. B, Broad sample. The horizontal red line represents the guideline recommended single cut point reference limit.
Figure S3. Quantile regression plots for E/E′ ratio. A, Reference sample. B. Broad sample. The horizontal red line represents the guideline recommended single cut point reference limit.
Figure S4. Quantile regression plots for E/A ratio. A, Reference sample. B, Broad sample. The horizontal red line represents the guideline recommended single cut point reference limit.
Figure S5. Quantile regression plots for E velocity. A, Reference sample. B, Broad sample.
Figure S6. Quantile regression plots for A velocity. A, Reference sample. B, Broad sample.


