Abstract
Background
Low 25‐hydroxyvitamin D levels are associated with an increased risk of cardiovascular events, but the effect of vitamin D supplementation on markers of vascular function associated with major adverse cardiovascular events is unclear.
Methods and Results
We conducted a systematic review and individual participant meta‐analysis to examine the effect of vitamin D supplementation on flow‐mediated dilatation of the brachial artery, pulse wave velocity, augmentation index, central blood pressure, microvascular function, and reactive hyperemia index. MEDLINE, CINAHL, EMBASE, Cochrane Central Register of Controlled Trials, and http://www.ClinicalTrials.gov were searched until the end of 2016 without language restrictions. Placebo‐controlled randomized trials of at least 4 weeks duration were included. Individual participant data were sought from investigators on included trials. Trial‐level meta‐analysis was performed using random‐effects models; individual participant meta‐analyses used a 2‐stage analytic strategy, examining effects in prespecified subgroups. 31 trials (2751 participants) were included; 29 trials (2641 participants) contributed data to trial‐level meta‐analysis, and 24 trials (2051 participants) contributed to individual‐participant analyses. Vitamin D3 daily dose equivalents ranged from 900 to 5000 IU; duration was 4 weeks to 12 months. Trial‐level meta‐analysis showed no significant effect of supplementation on macrovascular measures (flow‐mediated dilatation, 0.37% [95% confidence interval, −0.23 to 0.97]; carotid‐femoral pulse wave velocity, 0.00 m/s [95% confidence interval, −0.36 to 0.37]); similar results were obtained from individual participant data. Microvascular function showed a modest improvement in trial‐level data only. No consistent benefit was observed in subgroup analyses or between different vitamin D analogues.
Conclusions
Vitamin D supplementation had no significant effect on most markers of vascular function in this analysis.
Keywords: endothelial function, paricalcitol, systematic review, vascular function, vitamin D
Subject Categories: Cardiovascular Disease
Clinical Perspective
What Is New?
This is the first individual participant data meta‐analysis examining the effect of vitamin D analogues on markers of vascular function that are surrogates for cardiovascular events.
No consistent effect was found at trial level or on analysis of individual participant‐level data of supplementation on measures of endothelial function, arterial stiffness, or central blood pressure.
No subgroup benefited consistently on analysis of individual participant data.
What Are the Clinical Implications?
This analysis did not find convincing evidence of benefit from Vitamin D supplementation on a range of markers of vascular function.
Introduction
Low circulating levels of 25‐hydroxyvitamin D (25(OH)D) have been associated with a wide range of illness states and physiological derangements. Within the field of cardiometabolic medicine, low 25(OH)D levels have been associated with higher levels of blood pressure (BP), with diabetes mellitus, stroke, myocardial infarction, and heart failure1, 2 in observational studies. Vitamin D affects hundreds of gene targets and has effects on a wide variety of cell types and organ systems, including the heart and vascular system.3, 4 Several pathophysiological pathways have been postulated to explain the observed associations between low 25(OH)D levels and cardiovascular disease, including effects on arterial stiffness, endothelial function, cytokine secretion, vascular endothelial growth factor, and cellular calcium influx.4
Despite a sound rationale for improved cardiovascular health with vitamin D supplementation, results from intervention trials have been less encouraging. A recent individual participant data (IPD) meta‐analysis reported that vitamin D supplementation had no significant effect on BP,5 even in those participants with low baseline 25(OH)D levels or with high baseline BP. Similarly, only marginal effects were observed on glycemic control in a meta‐analysis of vitamin D supplementation in participants with diabetes mellitus.6 Meta‐analyses of cardiovascular outcomes show no effect of vitamin D supplementation on myocardial infarction or stroke, but suggest a possible effect in reducing new diagnoses of heart failure.7 It is important to note that these meta‐analyses include mostly trials performed in participants at risk for falls or with osteoporosis and may therefore not be generalizable. Several large trials of vitamin D supplementation with adequate power to detect reductions in cardiovascular events are due to report over the next few years, but the first of these trials did not report any reduction in cardiovascular event rates in a population of older people in New Zealand.8
These results call into question the causal link between vitamin D status and vascular health. Results from trials investigating the effect of vitamin D supplementation on aspects of vascular health other than BP have shown mixed results. Arterial stiffness and endothelial function measures are validated markers of cardiovascular disease risk and major adverse cardiac events, but the beneficial impact of vitamin D supplementation on these markers is unclear. We therefore performed a systematic review with meta‐analysis of trial‐level and individual participant‐level data to ascertain whether (1) vitamin D supplementation improves measures of arterial stiffness and endothelial function and (2) certain subgroups of individuals are more likely to benefit.
Methods
Data Sharing Statement
To preserve the rights of data owners, and as agreed with those who contributed data sets for this analysis, the data, analytical methods, and study materials will not be made available to other researchers for the purposes of reproducing the results or replicating the procedure.
Review Design and Search Strategy
We conducted a systematic review according to a prespecified protocol, which was registered on the PROSPERO database of systematic reviews. The protocol is accessible at: (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42012002816). Ethics committee approval was not required because no new data were collected as part of this review. We included randomized controlled trials, which compared vitamin D or analogues with placebo, with a minimum exposure period of 4 weeks. The following databases were searched from inception to end of December 2016: Medline, EMBASE, CINAHL, ClinicalTrials.gov, and the Cochrane central register of controlled trials. Gray literature was sought using Google, and references of included studies were hand‐searched for further candidate trials. Only trials where a full published trial report was available were included; trials published in abstract form only were excluded.
Trial Selection
Trials with changes in the following vascular markers were included: brachial artery flow‐mediated dilatation; reactive hyperemia index measures using finger plethysmography; pulse wave velocity (PWV) and pulse wave analysis; central aortic BP derived from peripheral artery tonometry; microvascular function measured using acetylcholine iontophoresis; and laser Doppler perfusion imaging. Studies with any baseline 25(OH)D level were eligible for inclusion. The following interventions were eligible for inclusion: vitamin D2 (ergocalciferol), vitamin D3 (cholecalciferol), calcitriol (1,25 hydroxyvitamin D3), 1‐alpha‐vitamin D, paricalcitol, and doxerocalciferol. Control groups receiving placebo were used and those receiving placebo plus cointervention were included, provided both arms of the study received the cointervention. A minimum of 4 weeks of therapy was necessary for inclusion to ensure sufficient time for vascular markers to change. Studies from both primary and secondary care or population settings were included; no restrictions were placed on sex or ethnicity. Studies recruiting participants less than 16 years old were not included, but in contrast to our previous review,5 we did include studies of participants on renal replacement therapy (hemodialysis or peritoneal dialysis) given their very high cardiovascular risk and the current interest in using vitamin D supplementation therapy in this group.
Data Extraction
Data were extracted independently by 2 researchers (L.A.B. and M.D.W.) and differences resolved by consensus. Baseline trial population data were identified, including age, sex, ethnicity, diabetes mellitus, kidney function, history of cardiovascular events, history of hypertension, baseline BP, and baseline use of angiotensin‐converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and statins. For each measure of vascular function, we recorded change in the outcome in each group between baseline and the last follow‐up visit. Study authors were contacted if data were incomplete or ambiguous in primary reports.
IPD Collection
Lead authors for each included trial were contacted and invited to contribute individual‐level participant data. Data were anonymized and transferred using a standard template before cleaning and incorporation in the final data set. Individual‐level participant data were sought for age, sex, body mass index, baseline and follow‐up 25(OH)D level, baseline medication use including ACE inhibitors and angiotensin receptor blockers, baseline estimated glomerular filtration rate (eGFR), total cholesterol, serum calcium and parathyroid hormone (PTH), presence of diabetes mellitus and previous vascular events, baseline and follow up BP and cholesterol, and baseline and follow‐up measures of vascular function.
Risk of Bias Assessment
Risk of bias was evaluated by 2 authors independently, with discrepancies resolved by consensus. We assessed each included study for risk for bias using the following fields from a risk of bias checklist9: quality of random allocation concealment, intention‐to‐treat analysis, blinding of outcome assessors, treatment and control group comparability, clear definition of inclusion and exclusion criteria, participant blinding to allocation, and description of withdrawals and dropouts. Funnel plots were generated and inspected for evidence of publication bias, supplemented by Egger's test for funnel plot asymmetry.
Statistical Analysis
Meta‐analysis at the trial level was performed using RevMan 5.3 software (Cochrane Collaboration). For all analyses, random‐effects and fixed‐effects meta‐analyses using a weighted least‐squares approach were performed. For outcomes measured with the same technique and same units (most brachial‐artery flow‐mediated dilatation [FMD] measures, reactive hyperemia index, augmentation index [AIx], central BP, and subgroups of PWV), results were expressed as mean difference between groups. For comparisons where dissimilar units were combined, results were expressed as standardized mean difference (SMD). Heterogeneity was assessed using the I2 statistic. Trial‐level meta‐regression was undertaken for FMD, PWV, and augmentation index outcomes, regressing treatment effect on daily dose equivalent (for trials using vitamin D3) and trial duration in months. Metaregression was not used for other outcomes because there were too few to produce reliable results. Metaregression was undertaken using Comprehensive Meta Analysis tools software (version 3; Biostat, Englewood, NJ).
A 2‐stage analysis was used for IPD.10 For each trial, or subgroup within each trial, mean outcome values at follow‐up in each group were calculated and adjusted for baseline outcome values using ANCOVA (SPSS version 24; IBM, Armonk, NY). These values were then combined using RevMan software as described above. For those trials using more than 1 type or dose of vitamin D, the vitamin D arms were analyzed as a single arm. The following prespecified subgroup analyses were performed: diabetes mellitus versus no diabetes mellitus; baseline systolic BP of no greater than 140 mm Hg versus greater than 140 mm Hg; diastolic BP of no greater than 90 mm Hg versus greater than 90 mm Hg; baseline PTH level of above versus below median level for the IPD set; baseline adjusted serum calcium level and baseline total cholesterol above versus below median level for the individual participant data set; estimated glomerular filtration rate (eGFR) 60 mL/min per 1.73 m2 or above versus subgroups of eGFR below 60 mL/min per 1.73 m2; baseline 25(OH)D level of less than 25, 25 to 50, and greater than 50 nmol/L; and baseline ACE inhibitor versus no ACE inhibitor use. For analyses of ACE inhibitor use, participants taking angiotensin receptor blockers were excluded given their similar, but not identical, biological effects. Subgroups for analysis were selected on the basis of possible mechanisms by which vitamin D might act (eg, through effects on the renin‐angiotensin system or by suppressing PTH),4 to explore groups thought to be most likely to benefit (eg, high BP, low 25(OH)D levels), and to identify disease populations that might be targeted by future studies (diabetes mellitus, chronic kidney disease [CKD]). For CKD, subgroups of eGFR 45 to 59, 30 to 44, 15 to 29, and <15 mL/min per 1.73 m2 were used where sufficient numbers of participants were available for the outcome; for outcomes with small numbers of participants, these subgroups were collapsed into a category eGFR <60 mL/min per 1.73 m2. Participants on dialysis were included in the <15 mL/min per 1.73 m2 subgroup.
Results
A total of 31 trials, involving 2751 participants, were eligible for inclusion in the review.11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41 Of these, 29 trials (2641 participants) had data suitable for inclusion in the trial‐level meta‐analyses; IPD were obtained from 24 trials (2051 participants). The PRISMA flowchart is shown in Figure. One study that did not include data in the published article suitable for trial‐level meta‐analysis36 provided IPD data and was included in the IPD analyses. Study size ranged from 24 to 305 participants; vitamin D3 was the most common intervention, being used in 23 of 31 (74%) of trials. The daily dose equivalent given in trials of vitamin D3 ranged from 900 to 5000 IU, and the duration of administration ranged from 4 weeks to 12 months. Paricalcitol was the only activated vitamin D analogue used in studies included in this review. Table 1 shows which vascular outcomes were measured in each included trial, and baseline trial characteristics are shown in Table 2.
Figure 1.
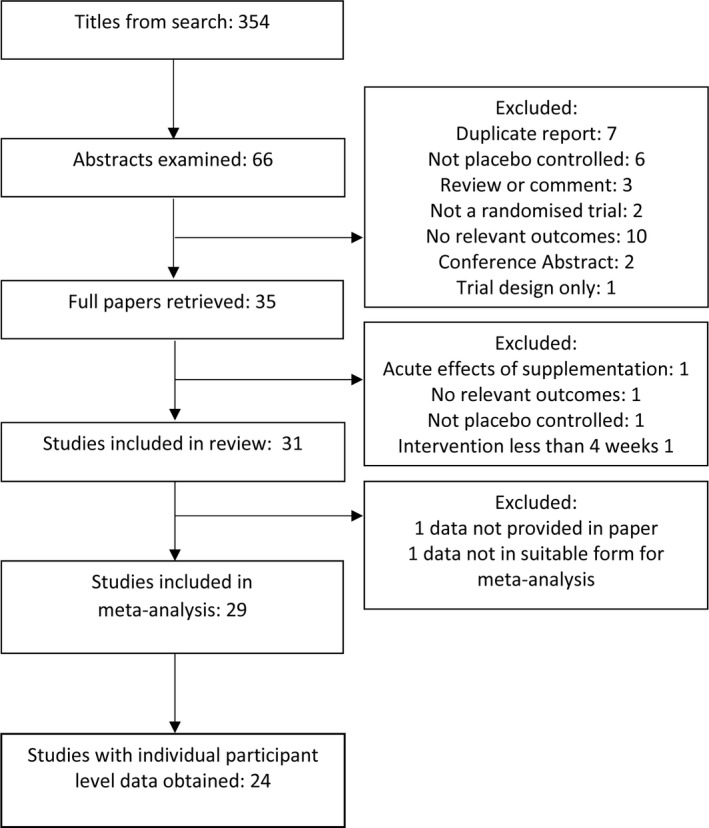
PRISMA diagram showing trial selection.
Table 1.
Measurements From Included Studies
| Title and Year | Preparation Tested | FMD | PWV | AIx | RHI | Central BP | Microvascular Function | Included in IPD Analysis? |
|---|---|---|---|---|---|---|---|---|
| Alborzi 200811 | Paricalcitol | X | No | |||||
| Sugden 200812 | Vitamin D2 | X | Yes | |||||
| Witham 201013 | Vitamin D3 | X | Yes | |||||
| Harris 201114 | Vitamin D3 | X | Yes | |||||
| Gepner 201215 | Vitamin D3 | X | X | X | X | Yes | ||
| Larsen 201216 | Vitamin D3 | X | X | X | Yes | |||
| Marckmann 201217 | Vitamin D3 | X | X | X | Yes | |||
| Sokol 201218 | Vitamin D2 | X | Yes | |||||
| Stricker 201219 | Vitamin D3 | X | X | X | Yes | |||
| Witham 201220 | Vitamin D2 | X | Yes | |||||
| Breslavsky 201321 | Vitamin D3 | X | Yes | |||||
| Hewitt 201322 | Vitamin D3 | X | No | |||||
| Witham 201323 | Vitamin D3 | X | X | Yes | ||||
| Witham 201324 | Vitamin D3 | X | Yes | |||||
| Witham 201325 | Vitamin D3 | X | X | X | X | X | Yes | |
| Yiu 201326 | Vitamin D3 | X | X | No | ||||
| Dreyer 201427 | Vitamin D2 | X | X | Yes | ||||
| Martins 201428 | Vitamin D3 | X | No | |||||
| Mose 201429 | Vitamin D3 | X | X | X | Yes | |||
| Ryu 201430 | Vitamin D3 | X | X | No | ||||
| Zoccali 201431 | Paricalcitol | X | Yes | |||||
| Garg 201532 | Vitamin D3 | X | X | Yes | ||||
| Pilz 201533 | Vitamin D3 | X | Yes | |||||
| Thethi 201534 | Paricalcitol | X | No | |||||
| Witham 201535 | Vitamin D3 | X | X | X | Yes | |||
| Barchetta 201636 | Vitamin D3 | X | Yes | |||||
| Borgi 201737 | Vitamin D2 | X | No | |||||
| Bressendorff 201638 | Vitamin D3 | X | X | X | Yes | |||
| Dalan 201639 | Vitamin D3 | X | X | Yes | ||||
| Forouhi 201640 |
Vitamin D2 Vitamin D3 |
X | Yes | |||||
| Hin 201741 | Vitamin D3 | X | X | Yes |
AIx indicates augmentation index; BP, blood pressure; FMD, flow‐mediated dilatation of the brachial artery; IPD, individual participant data; PWV, pulse wave velocity; RHI, reactive hyperemia index.
Table 2.
Details of Included Studies
| Study | N | Latitude | Study Population | Mean Age, y | % Male | 25(OH)D Range for Inclusion (nmol/L) | Mean Baseline 25(OH)D (nmol/L) | Mean Baseline SBP (mm Hg) | Control | Intervention | Duration |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alborzi,11 USA, 2008 | 24 | 40°N | CKD and on ACE‐I or ARB | 70 | 83 | No restriction | 34 | 125.4 (24 hours BP) | Placebo |
Paricalcitol 1 μg daily Paricalcitol 2 μg daily |
1 mo |
| Sugden,12 Scotland, 2008 | 34 | 56°N | Type 2 diabetes mellitus | 64 | 53 | <50 | 38 | 141 | Placebo |
Ergocalciferol 100 000 IU single dose |
8 wks |
| Witham,13 Scotland, 2010 | 61 | 56°N | Type 2 diabetes mellitus | 65 | 67 | <100 | 45 | 146 | Placebo |
100 000 IU Cholecalciferol 200 000 IU cholecalciferol single dose |
16 wks |
| Harris,14 USA, 2011 | 45 | 33°N | Black adults with no overt cardiovascular, pulmonary or metabolic disease | 30 | 47 | No restriction | 36 | 124 | Placebo | Cholecalciferol 60 000 IU/4 weekly | 16 wks |
| Gepner,15 USA, 2012 | 114 | 43°N | Healthy community dwelling postmenopausal females | 64 | 0 | >25 and <150 | 78 | 119.4 | Placebo | Cholecalciferol 2500 IU/day | 4 mo |
| Larsen,16 Denmark, 2012 | 130 | 56°N | Hypertension | 61 | 31 | No restriction | 58 | 143 | Placebo | Cholecalciferol 3000 IU/day | 20 wks |
| Marckmann,17 Denmark, 2012 | 52 | 55°N | CKD | 67 | 75 | <50 | 33 | 138 | Placebo | Cholecalciferol 40 000 IU weekly | 8 wks |
| Sokol,18 USA, 2012 | 90 | 41°N | Angiographically confirmed coronary artery disease | 56 | 73 | <50 | ? | 133 | Placebo | Ergocalciferol 50 000 IU weekly | 12 wks |
| Stricker,19 Switzerland, 2012 | 62 | 46°N | Chronic peripheral vascular disease and vitamin D deficiency | 74 | 61 | <75 | 42 | 137 | Placebo | Cholecalciferol (vitamin D3) 100 000 IU single dose | 1 mo |
| Witham,20 Scotland, 2012 | 58 | 56°N | Older adults with previous stroke | 67 | 72 | <75 | 38 | 128 | Placebo | 100 000 IU Ergocalciferol single dose | 16 wks |
| Breslavsky,21 Israel, 2013 | 47 | 32°N | Type 2 diabetes mellitus with cardiovascular risk factors | 66 | 47 | No restriction | 30 | 153 | Placebo | Cholecalciferol 1000 IU daily | 12 mo |
| Hewitt,22 Australia, 2013 | 60 | 34°S | Adults on hemodialysis | ? | 48 | <60 | 43 | 152 | Placebo | Cholecalciferol 50 000 IU weekly for 8 wks, then monthly for 4 mo | 6 mo |
| Witham,23 Scotland, 2013 | 159 | 56°N | Isolated systolic hypertension in over 70 year olds | 77 | 52 | <75 | 45 | 163 | Placebo | Cholecalciferol 100 000 IU 3 monthly | 12 mo |
| Witham,24 Scotland, 2013 | 75 | 56°N | Recent myocardial infarction | 66 | 69 | No restriction | 47 | 127.5 | Placebo | Cholecalciferol 100 000 IU/2 monthly | 6 mo |
| Witham,25 Scotland, 2013 | 50 | 56°N | South‐East Asian women living in UK for 10 y | 41 | 0 | <75 | 27 | 120 | Placebo | Cholecalciferol 100 000 IU single dose | 8 wks |
| Yiu,26 Hong Kong, 2013 | 100 | 22°N | Type 2 diabetes mellitus with suboptimal vitamin D status | 65 | 50 | <75 | 54 | 146 | Placebo | Cholecalciferol 5000 IU daily | 12 wks |
| Dreyer,27 England, 2014 | 38 | 51°N | CKD stage 3 to 4 | 47 | 74 | <40 | 25 | 116 | Placebo | Ergocalciferol 50 000 IU weekly for 1 mo, then monthly for 5 mo | 6 mo |
| Martins,28 USA, 2014 | 130 | 34°N | Overweight blacks with hypertension | 18 to 70 | 61 | 25 to 63 | 43 | 127 | Placebo | Cholecalciferol 100 000 IU monthly | 3 mo |
| Mose,29 Denmark, 2014 | 50 | 56°N | Hemodialysis or peritoneal dialysis | 68 | 64 | No restriction | 39 | 136 | Placebo | Cholecalciferol 3000 IU daily | 6 mo |
| Ryu,30 Korea, 2014 | 62 | 38°N | Type 2 diabetes mellitus aged 30 to 69 y | 56 | ? | <50 | 29 | 130 | Placebo+200 mg calcium daily | Cholecalciferol 2000 IU daily+200 mg calcium daily | 24 wks |
| Zoccali,31 Italy, 2014 | 88 | 39°N | CKD stage 3 to 4 and PTH >65 pg/mL | 63 | 65 | No restriction | 36 | 126 | Placebo | Paricalcitol 2 μg daily | 12 wks |
| Garg,32 India, 2015 | 32 | 29°N | Women aged 18 to 35 y with polycystic ovary syndrome | 22 | 0 | No restriction | 36 | Not known | Placebo+1.5 g metformin daily | 120 000 IU Cholecalciferol monthly+1.5 g metformin daily | 6 mo |
| Pilz,33 Austria, 2015 | 188 | 47°N | Hypertension | 60 | 53 | <75 | 53 | 143 | Placebo | Cholecalciferol 2800 IU daily | 8 wks |
| Thethi,34 USA, 2015 | 55 | 30°N | CKD 3 to 4 and type 2 diabetes mellitus | 63 | 67 | No restriction | NA | 136 | Placebo | Paricalcitol 1 μg daily | 12 wks |
| Witham,35 Scotland, 2015 | 50 | 56°N | Chronic fatigue syndrome | 49 | 24 | <75 | 46 | 128.5 | Placebo | Cholecalciferol 100 000 IU/2 monthly | 6 mo |
| Barchetta.36 Italy. 2016 | 55 | 42°N | Type 2 diabetes mellitus with nonalcoholic fatty liver disease | 58 | 70 | No restriction | NA | 131 | Placebo | Cholecalciferol 2000 IU daily | 24 wks |
| Borgi,37 USA, 2017 | 93 | 42°N | Overweight or obese nonhypertensives with vitamin D deficiency | 37 | ? | <50 | NA | 118 | Placebo | Ergocalciferol 50 000 IU weekly | 8 wks |
| Bressendorff,38 Denmark, 2016 | 40 | 56°N | Normotensive adults with vitamin D deficiency | 43 | 58 | <50 | 32 | 118 | Placebo | Cholecalciferol 3000 IU daily | 16 wks |
| Dalan,39 Singapore, 2016 | 64 | 1°N | Multiethnic group with type 2 diabetes mellitus | 54 | 52 | No restriction | 45 | 139 | Placebo | Cholecalciferol 2000 to 4000 IU daily depending on baseline 25(OH)D and response | 16 wks |
| Forouhi,40 England, 2016a | 160 | 52°N | Adults with elevated risk of type 2 diabetes mellitus | 53 | 57 | No restriction | 52 | 128 | Placebo |
Ergocalciferol 100 000 IU monthly Cholecalciferol 100 000 IU monthly |
4 mo |
| Hin,41 England, 2017 | 305 | 52°N | Aged ≥65 y | 72 | 51 | No restriction | 50 | 131 | Placebo |
Cholecalciferol 2000 IU daily Cholecalciferol 4000 IU daily |
12 mo |
25(OH)D indicates 25‐hydroxyvitamin D; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; CKD, chronic kidney disease; NA, not available; SBP, systolic blood pressure.
Data from 1 of 2 centers included as vascular function measured only at 1 center.
Risk of Bias Assessment
Overall risk of bias was low. Most trials (27 of 31) reported clear evidence of effective allocation concealment, and most trials reported clear evidence for masking of participants (30 of 31), healthcare professionals (30 of 31), and outcomes assessors (29 of 31). Groups were comparable at baseline in 27 of 31 trials, dropouts were clearly described in 27 of 31 trials, but analysis was clearly by intention to treat in only 16 of 31 trials. A full description of the quality assessment for each trial is shown in Table 3. Funnel plots showed no asymmetry for any of the vascular outcomes; Egger's test was calculated only for those outcomes with at least 10 trials to ensure reliability; this was nonsignificant for FMD (P=0.18), AIx (P=0.32), and PVW (P=0.70).
Table 3.
Risk of Bias Assessment of Included Studies
| Title and Year | Quality of Allocation Concealment | Analysis on Intention to Treat | No. and Description of Dropouts | Blinding—Participants | Blinding—Health Care Providers | Blinding—Outcome Assessors | Comparable Treatment and Placebo Groups |
|---|---|---|---|---|---|---|---|
| Alborzi 200811 | + | + | + | + | + | + | − |
| Sugden 200812 | + | − | + | + | + | + | + |
| Witham 201013 | + | U | + | + | + | + | + |
| Harris 201114 | + | U | + | + | + | + | + |
| Gepner 201215 | + | + | + | + | + | + | + |
| Larsen 201216 | + | − | + | + | + | + | + |
| Marckmann 201217 | + | − | + | + | + | + | + |
| Sokol 201218 | + | + | U | + | + | + | + |
| Stricker 201219 | + | + | + | + | + | + | − |
| Witham 201220 | + | U | + | + | + | + | + |
| Breslavsky 201321 | + | − | + | U | U | U | + |
| Hewitt 201322 | + | U | + | + | + | + | + |
| Witham 201323 | + | − | + | + | + | + | + |
| Witham 201324 | + | + | + | + | + | + | + |
| Witham 201325 | + | + | + | + | + | + | + |
| Yiu 201326 | + | + | + | + | + | + | + |
| Dreyer 201427 | + | + | + | + | + | + | + |
| Martins 201428 | U | + | + | + | + | + | + |
| Mose 201429 | + | U | + | + | + | + | + |
| Ryu 201430 | U | − | + | + | + | + | + |
| Zoccali 201431 | + | + | + | + | + | + | + |
| Garg 201532 | U | − | + | + | + | + | + |
| Pilz 201533 | + | + | + | + | + | + | + |
| Thethi 201534 | U | U | U | + | + | U | + |
| Witham 201535 | + | + | + | + | + | + | + |
| Barchetta 201636 | + | − | + | + | + | + | + |
| Borgi 201737 | + | + | − | + | + | + | + |
| Bressendorff 201638 | + | U | + | + | + | + | + |
| Dalan 201639 | + | + | U | + | + | + | − |
| Forouhi 201640 | + | + | + | + | + | + | + |
| Hin 201741 | + | + | + | + | + | + | + |
− indicates high risk of bias; +, low risk of bias; U, unclear risk of bias.
Trial‐Level Data
Meta‐analysis of trial‐level data showed no significant treatment effect of vitamin D analogues on FMD (mean difference, 0.5%; 95% confidence interval [CI], −0.1 to 1.1; P=0.12), PVW (SMD, 0.02; 95% CI, −0.13 to 0.17; P=0.81), AIx (mean difference, 0.0%; 95% CI, −1.3 to 1.3; P=0.98), reactive hyperemia index (mean difference, 0.02 units; 95% CI, −0.11 to 0.14; P=0.79), or central BP. Microvascular function measured by laser Doppler iontophoresis (SMD, 0.43; 95% CI, 0.09–0.76; P=0.01) showed a modest improvement with vitamin D supplementation. Results are shown in Table 4. Fixed‐effects analyses showed similar point estimates, but narrower CIs, leading to a significant treatment effect for all vitamin D analogues on FMD (mean difference, 0.5%; 95% CI, 0.1–0.9; P=0.02). On subgroup analysis by treatment type, only paricalcitol showed a significant treatment benefit on FMD (mean difference, 1.7%; 95% CI, 0.6–2.8; P=0.002); analysis for interaction showed no significant difference between the paricalcitol treatment effect and that for vitamin D3 (P=0.62) or vitamin D2 (P=0.17). No significant difference was evident in the effects of daily dosing versus intermittent dosing either for FMD (mean difference, 0.85% [95% CI, 0.01–1.69] for daily dosing versus 0.26% [95% CI, −0.58 to 1.11] for intermittent dosing; P=0.06) or for PWV (SMD, 0.10 [95% CI, −0.04 to 0.23] for daily dosing versus −0.04 [95% CI, −0.34 to 0.26] for intermittent dosing; P=0.40). Metaregression results for daily dose equivalent and for trial duration are shown in Table 5. No association between these factors and treatment effect for PWV or AIx was found, but higher dose and shorter trial length were associated with a slightly greater treatment effect for FMD.
Table 4.
Trial‐Level Analysis of Effect Size: Vitamin D Supplementation and Markers of Vascular Function
| Outcome | Intervention | No. of Studies | n | Random‐Effects | Fixed‐Effects | I2 | ||
|---|---|---|---|---|---|---|---|---|
| Treatment Effect (95% CI) | P Value | Treatment Effect (95% CI) | P Value | |||||
| FMD (%) | All | 12 | 785 | 0.49 (−0.13 to 1.11) | 0.12 | 0.48 (0.06–0.90) | 0.02 | 46% |
| D3 | 7 | 495 | 0.17 (−0.49 to 0.84) | 0.61 | 0.19 (−0.30 to 0.67) | 0.45 | 38% | |
| D2 | 3 | 163 | 0.79 (−1.04 to 2.62) | 0.40 | 0.91 (−0.39 to 2.21) | 0.17 | 45% | |
| Paricalcitol | 2 | 103 | 1.72 (0.63–2.82) | 0.002 | 1.72 (0.63–2.82) | 0.002 | 0% | |
| AIx (%) | All | 14 | 1030 | 0.0 (−1.3 to 1.3) | 0.98 | 0.0 (−1.1 to 1.1) | 0.98 | 25% |
| D3 | 14 | 1030 | 0.0 (−1.3 to 1.3) | 0.98 | 0.0 (−1.1 to 1.1) | 0.98 | 25% | |
| D2 | 0 | ··· | ··· | ··· | ··· | ··· | ··· | |
| Paricalcitol | 0 | ··· | ··· | ··· | ··· | ··· | ··· | |
| RHI, units | All | 3 | 217 | 0.02 (−0.11 to 0.14) | 0.79 | 0.02 (−0.11 to 0.14) | 0.79 | 0% |
| D3 | 2 | 130 | 0.02 (−0.18 to 0.21) | 0.86 | 0.04 (−0.10 to 0.18) | 0.61 | 37% | |
| D2 | 1 | 87 | −0.05 (−0.30 to 0.20) | 0.70 | −0.05 (−0.30 to 0.20) | 0.70 | ··· | |
| Paricalcitol | 0 | ··· | ··· | ··· | ··· | |||
| PWV (all; SMD) | All | 16 | 1333 | 0.04 (−0.11 to 0.20) | 0.60 | 0.04 (−0.07 to 0.15) | 0.50 | 44% |
| D3 | 15 | 1304 | 0.05 (−0.11 to 0.21) | 0.52 | 0.04 (−0.07 to 0.15) | 0.45 | 47% | |
| D2 | 2a | 138 | −0.24 (−0.57 to 0.10) | 0.17 | −0.24 (−0.57 to 0.10) | 0.17 | 0% | |
| Paricalcitol | 0 | ··· | ··· | ··· | ··· | ··· | ··· | |
| PWV (carotid‐femoral only; m/s)a | All | 10 | 674 | 0.04 (−0.32 to 0.41) | 0.81 | −0.01 (−0.20 to 0.21) | 0.94 | 58% |
| D3 | 10 | 674 | 0.00 (−0.32 to 0.41) | 0.81 | −0.01 (−0.20 to 0.21) | 0.94 | 58% | |
| D2 | 1 | 107 | −0.53 (−1.34 to 0.28) | 0.20 | −0.53 (−1.34 to 0.28) | 0.20 | ··· | |
| Paricalcitol | 0 | ··· | ··· | ··· | ··· | ··· | ··· | |
| PWV (others; SMD) | All | 6 | 659 | 0.11 (−0.06 to 0.28) | 0.22 | 0.11 (−0.04 to 0.27) | 0.15 | 8% |
| D3 | 5 | 630 | 0.12 (−0.06 to 0.30) | 0.19 | 0.13 (−0.03 to 0.29) | 0.11 | 14% | |
| D2 | 1 | 29 | −0.20 (−0.93 to 0.53) | 0.59 | −0.20 (−0.93 to 0.53) | 0.59 | ··· | |
| Paricalcitol | 0 | ··· | ··· | ··· | ··· | ··· | ··· | |
| Microvascular function (SMD) | All | 3 | 140 | 0.43 (0.09–0.76) | 0.01 | 0.43 (0.09–0.76) | 0.01 | 0% |
| D3 | 2 | 111 | 0.37 (−0.01 to 0.75) | 0.05 | 0.37 (−0.01 to 0.75) | 0.05 | 0% | |
| D2 | 1 | 29 | 0.65 (−0.10 to 1.41) | 0.09 | 0.65 (−0.10 to 1.41) | 0.09 | ··· | |
| Paricalcitol | 0 | ··· | ··· | ··· | ··· | ··· | ··· | |
| Central SBP, mm Hg | All | 5 | 324 | −1.5 (−5.6 to 2.6) | 0.46 | −1.2 (−3.8 to 1.4) | 0.36 | 47% |
| D3 | 5 | 324 | −1.5 (−5.6 to 2.6) | 0.46 | −1.2 (−3.8 to 1.4) | 0.36 | 47% | |
| D2 | 0 | ··· | ··· | ··· | ··· | ··· | ··· | |
| Paricalcitol | 0 | ··· | ··· | ··· | ··· | ··· | ··· | |
| Central DBP, mm Hg | All | 5 | 324 | −0.8 (−2.2 to 0.6) | 0.28 | −0.8 (−2.2 to 0.6) | 0.28 | 0% |
| D3 | 5 | 324 | −0.8 (−2.2 to 0.6) | 0.28 | −0.8 (−2.2 to 0.6) | 0.28 | 0% | |
| D2 | 0 | ··· | ··· | ··· | ··· | ··· | ··· | |
| Paricalcitol | 0 | ··· | ··· | ··· | ··· | ··· | ··· | |
AIx indicates augmentation index; CI, confidence interval; D2, vitamin D2 (ergocalciferol); D3, vitamin D3 (cholecalciferol); DBP, diastolic blood pressure; FMD, flow‐mediated dilatation of the brachial artery; PWV, pulse wave velocity; RHI, reactive hyperemia index; SBP, systolic blood pressure; SMD, standardized mean difference.
Data from Forouhi et al40 contain comparisons of D3 vs placebo and D2 vs placebo. Only D3 analysis was included in “All” category for PWV analyses.
Table 5.
Results of Trial‐Level Metaregression
| Outcome | Moderator Variable | No. of Trials | Random‐Effects | Fixed‐Effects | Regression Coefficient Units | ||
|---|---|---|---|---|---|---|---|
| Regression Coefficient (95% CI) | P Value | Regression Coefficient (95% CI) | P Value | ||||
| FMD (%) | Trial duration | 11 | −0.06 (−0.11 to −0.01) | 0.03 | −0.06 (−0.10 to −0.01) | 0.009 | % per mo |
| Daily dose equivalent | 6 | 0.14 (0.01–0.27) | 0.03 | 0.14 (0.01–0.27) | 0.03 | % per 1000 units D3 | |
| PWV (SMD) | Trial duration | 16 | 0.01 (−0.04 to 0.06) | 0.77 | 0.02 (−0.01 to 0.05) | 0.18 | SD per mo |
| Daily dose equivalent | 15 | 0.07 (−0.08 to 0.23) | 0.35 | 0.06 (−0.05 to 0.17) | 0.29 | SD per 1000 units D3 | |
| AIx (%) | Trial duration | 14 | −0.01 (−0.05 to 0.04) | 0.73 | −0.00 (−0.04 to 0.03) | 0.84 | % per mo |
| Daily dose equivalent | 14 | 0.10 (−0.06 to 0.25) | 0.22 | 0.09 (−0.04 to 0.23) | 0.18 | % per 1000 units D3 | |
AIx indicates augmentation index; CI, confidence interval; FMD, flow‐mediated dilatation of the brachial artery; PWV, pulse wave velocity; SMD, standardized mean difference.
Individual Participant Data
Similarly, meta‐analysis of IPD showed no significant treatment effect on any of the vascular outcomes studied; no effect was evident when PWV analyses were confined to studies using carotid‐femoral PWV. The main results for IPD analysis are shown in Table 6. For most analyses, heterogeneity as shown by the I2 statistic was low to moderate. Prespecified subgroup analysis of IPD data for each vascular outcome (Tables 7, 8, 9, 10, 11, 12 through 13) did not show any subgroup consistently deriving significant benefit from vitamin D or analogues; treatment/subgroup interaction analyses suggested that participants with eGFR <60 mL/min per 1.73 m2 may be less likely to show improvements in FMD or aortic diastolic BP with treatment, and those with 25(OH)D levels <25 nmol/L may be more likely to show improvements in reactive hyperemia index and AIx with treatment. Participants with diabetes mellitus appeared to have a significantly greater rise in aortic BP than those without diabetes mellitus. For outcomes in both trial‐level and IPD analysis that were performed using SMD, standardizing SDs are given in Table 14 to facilitate interpretation at the individual trial level.
Table 6.
IPD Analysis of Effect Size: Vitamin D Supplementation and Markers of Vascular Function
| Outcome | No. of Studies | n | Mean Baseline Value Across All Groups (SD) | Random Effects | Fixed Effects | I2 | ||
|---|---|---|---|---|---|---|---|---|
| Treatment Effect (95% CI) | P Value | Treatment Effect (95% CI) | P Value | |||||
| FMD (%) | 10 | 655 | 5.6 (3.6) | −0.03 (−0.78 to 0.71) | 0.93 | −0.17 (−0.58 to 0.25) | 0.44 | 63% |
| AIx (%) | 11 | 832 | 27 (16) | 0.1 (−1.4 to 1.6) | 0.91 | 0.0 (−1.0 to 1.1) | 0.96 | 39% |
| RHI, units | 3 | 220 | 1.65 (0.81) | −0.02 (−0.12 to 0.08) | 0.68 | −0.02 (−0.12 to 0.08) | 0.68 | 0% |
| PWV (all; SMD) | 13 | 1154 | ND | −0.01 (−0.16 to 0.13) | 0.85 | −0.04 (−0.15 to 0.08) | 0.56 | 25% |
| PWV (carotid‐femoral only; m/s) | 9 | 652 | 7.9 (2.8) | −0.01 (−0.31 to 0.30) | 0.96 | −0.04 (−0.25 to 0.17) | 0.70 | 44% |
| PWV (others; SMD) | 4 | 502 | ND | −0.02 (−0.20 to 0.16) | 0.83 | −0.05 (−0.36 to 0.26) | 0.75 | 0% |
| Microvascular function (SMD) | 3 | 129 | ND | 0.36 (0.01–0.71) | 0.05 | 0.36 (0.01–0.71) | 0.13 | 0% |
| Central SBP, mm Hg | 7 | 400 | 120.7 (23.9) | −0.6 (−3.2 to 1.9) | 0.63 | −0.4 (−2.4 to 1.6) | 0.67 | 31% |
| Central DBP, mm Hg | 7 | 400 | 76.5 (9.9) | −0.4 (−1.5 to 0.7) | 0.48 | −0.4 (−1.5 to 0.7) | 0.48 | 0% |
AIx indicates augmentation index; CI, confidence interval; DBP, diastolic blood pressure; FMD, flow‐mediated dilatation of the brachial artery; IPD, individual participant data; ND, not done because of heterogeneity of measurement methods; PWV, pulse wave velocity; RHI, reactive hyperemia index; SBP, systolic blood pressure; SMD, standardized mean difference.
Table 7.
IPD Subgroup Analyses for FMD
| Subgroup | No. of Studies | n | Mean Baseline Value (%) (SD) | Random Effects | Fixed Effects | I2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Effect (%) (95% CI) | P Value | P for Interaction | Treatment Effect (%) (95% CI) | P Value | P for Interaction | |||||
| SBP >140 mm Hg | 9 | 242 | 5.2 (3.2) | 0.0 (−1.1 to 1.0) | 0.94 | 0.88 | −0.1 (−0.6 to 0.5) | 0.82 | 1.0 | 56% |
| SBP ≤140 mm Hg | 9 | 376 | 5.9 (3.8) | 0.1 (−0.9 to 1.0) | 0.90 | −0.1 (−0.6 to 0.5) | 0.79 | 58% | ||
| Baseline 25(OH)D <25 nmol/L | 6 | 89 | 5.7 (4.0) | −0.3 (−1.8 to 1.2) | 0.65 | ··· | −0.1 (−1.0 to 0.9) | 0.92 | ··· | 43% |
| Baseline 25(OH)D 25 to 50 nmol/L | 9 | 292 | 5.3 (3.3) | −0.1 (−1.2 to 1.0) | 0.87 | 0.83 | −0.1 (−0.7 to 0.5) | 0.66 | 1.0 | 66% |
| Baseline 25(OH)D >50 nmol/L | 8 | 222 | 5.4 (3.4) | −0.2 (−1.1 to 0.7) | 0.66 | 0.91 | −0.2 (−0.9 to 0.4) | 0.47 | 0.87 | 41% |
| DM | 6 | 198 | 5.2 (3.5) | 0.1 (−1.1 to 1.4) | 0.82 | 0.47 | 0.4 (−0.5 to 1.2) | 0.40 | 0.27 | 37% |
| No DM | 7 | 454 | 5.7 (3.5) | −0.4 (−0.9 to 0.1) | 0.14 | −0.3 (−1.2 to 0.6) | 0.55 | 63% | ||
| No ACEi or ARB | 9 | 254 | 6.5 (3.6) | 0.1 (−2.0 to 2.2) | 0.92 | ··· | −0.6 (−1.3 to 0.1) | 0.09 | ··· | 88% |
| ACEi, no ARB | 7 | 171 | 5.4 (3.5) | −0.4 (−1.7 to 1.0) | 0.58 | 0.69 | −0.2 (−1.0 to 0.6) | 0.64 | 0.46 | 47% |
| ACEi or ARB | 7 | 297 | 5.0 (3.4) | −0.7 (−1.9 to 0.5) | 0.28 | 0.52 | −0.7 (−1.4 to −0.1) | 0.03 | 0.84 | 60% |
| PTH >5.0 pmol/L | 8 | 254 | 5.4 (3.6) | −0.3 (−1.5 to 0.9) | 0.61 | 0.39 | −0.8 (−1.5 to −0.2) | 0.02 | 0.02 | 62% |
| PTH ≤5.0 pmol/L | 7 | 230 | 5.7 (3.1) | 0.3 (−0.3 to 1.0) | 0.30 | 0.3 (−0.3 to 1.0) | 0.30 | 0% | ||
| Ca >2.30 mmol/L | 7 | 226 | 6.0 (3.4) | 0.3 (−0.3 to 1.0) | 0.30 | 0.19 | 0.3 (−0.3 to 1.0) | 0.30 | 0.08 | 0% |
| Ca ≤2.30 mmol/L | 7 | 371 | 5.2 (3.4) | −0.5 (−1.5 to 0.5) | 0.30 | −0.5 (−1.1 to 0.1) | 0.09 | 56% | ||
| eGFR ≥60 mL/min per 1.73 m2 | 6 | 382 | 5.6 (3.4) | 0.5 (−0.2 to 1.2) | 0.18 | ··· | 0.6 (0.0–1.2) | 0.04 | ··· | 27% |
| eGFR 45 to 59 mL/min per 1.73 m2 | 5 | 59 | 5.3 (3.1) | −0.1 (−2.2 to 2.1) | 0.94 | 0.60 | 0.3 (−1.0 to 1.5) | 0.66 | 0.67 | 55% |
| eGFR 30 to 44 mL/min per 1.73 m2 | 3 | 38 | 4.5 (2.9) | −1.0 (−2.7 to 0.7) | 0.27 | 0.11 | −1.0 (−2.7 to 0.7) | 0.27 | 0.08 | 0% |
| eGFR 15 to 29 mL/min per 1.73 m2 | 1 | 24 | 3.4 (3.0) | −3.1 (−5.7 to −0.5) | 0.02 | 0.009 | −3.1 (−5.7 to −0.5) | 0.02 | 0.007 | ··· |
| eGFR <15 mL/min per 1.73 m2 a | 0 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· |
| Total cholesterol ≥4.60 mmol/L | 8 | 223 | 5.4 (3.4) | −0.9 (−1.9 to 0.1) | 0.07 | 0.23 | −1.0 (−1.6 to −0.4) | 0.002 | 0.06 | 51% |
| Total cholesterol <4.60 mmol/L | 8 | 283 | 5.6 (3.7) | 0.1 (−1.1 to 1.4) | 0.83 | −0.2 (−0.8 to 0.4) | 0.60 | 69% | ||
25(OH)D indicates 25‐hydroxyvitamin D; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; Ca, calcium; CI, confidence interval; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; FMD, flow‐mediated dilatation of the brachial artery; IPD, individual participant data; PTH, parathyroid hormone; SBP, systolic blood pressure.
Or on dialysis.
Table 8.
IPD Subgroup Analyses for PWV
| Subgroup | No. of Studies | N | Mean Baseline Value (m/s) (SD)a | Random Effects | Fixed Effects | I2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Effect (95% CI) (SMD) | P Value | P for Interaction | Treatment Effect (95% CI) (SMD) | P Value | P for Interaction | |||||
| SBP >140 mm Hg | 9 | 396 | 8.9 (3.2) | −0.1 (−0.3 to 0.1) | 0.21 | 0.49 | −0.1 (−0.3 to 0.1) | 0.21 | 0.43 | 0% |
| SBP ≤140 mm Hg | 11 | 728 | 7.5 (2.6) | 0.0 (−0.2 to 0.2) | 1.00 | −0.0 (−0.2 to 0.1) | 0.62 | 40% | ||
| Baseline 25(OH)D <25 nmol/L | 11 | 157 | 7.6 (2.8) | 0.2 (−0.2 to 0.5) | 0.36 | ··· | 0.2 (−0.2 to 0.5) | 0.36 | ··· | 0% |
| Baseline 25(OH)D 25 to 50 nmol/L | 12 | 508 | 8.0 (3.2) | −0.1 (−0.3 to 0.2) | 0.67 | 0.17 | −0.1 (−0.3 to 0.1) | 0.38 | 0.15 | 39% |
| Baseline 25(OH)D >50 nmol/L | 10 | 482 | 7.8 (2.6) | 0.0 (−0.2 to 0.2) | 0.81 | 0.33 | 0.0 (−0.2 to 0.2) | 0.76 | 0.33 | 12% |
| DM | 6 | 130 | 8.5 (4.5) | 0.1 (−0.3 to 0.4) | 0.65 | 0.30 | 0.1 (−0.3 to 0.4) | 0.65 | 0.30 | 0% |
| No DM | 13 | 1021 | 7.8 (2.5) | −0.1 (−0.2 to 0.1) | 0.32 | −0.1 (−0.2 to 0.1) | 0.32 | 0% | ||
| No ACEi or ARB | 9 | 399 | 7.7 (3.1) | −0.1 (−0.3 to 0.2) | 0.70 | ··· | −0.1 (−0.3 to 0.1) | 0.44 | ··· | 21% |
| ACEi, no ARB | 7 | 203 | 8.5 (3.3) | −0.2 (−0.5 to 0.1) | 0.17 | 0.62 | −0.2 (−0.5 to 0.1) | 0.17 | 0.62 | 0% |
| ACEi or ARB | 8 | 364 | 8.1 (3.2) | 0.0 (−0.3 to 0.2) | 0.68 | 0.58 | 0.0 (−0.3 to 0.2) | 0.68 | 0.58 | 0% |
| PTH >5.0 pmol/L | 12 | 482 | 7.9 (2.9) | −0.1 (−0.3 to 0.1) | 0.16 | 0.49 | −0.1 (−0.3 to 0.1) | 0.16 | 0.49 | 0% |
| PTH ≤5.0 pmol/L | 12 | 594 | 7.9 (3.2) | 0.0 (−0.2 to 0.2) | 0.97 | 0.0 (−0.2 to 0.2) | 0.95 | 12% | ||
| Ca >2.30 mmol/L | 9 | 456 | 7.8 (3.5) | 0.0 (−0.2 to 0.2) | 0.91 | 0.12 | 0.0 (−0.2 to 0.2) | 0.91 | 0.12 | 0% |
| Ca ≤2.30 mmol/L | 10 | 496 | 8.0 (2.6) | −0.2 (−0.3 to 0.0) | 0.11 | −0.2 (−0.3 to 0.0) | 0.10 | 2% | ||
| eGFR ≥60 mL/min per 1.73 m2 | 10 | 897 | 7.8 (2.3) | 0.0 (−0.2 to 0.1) | 0.57 | ··· | 0.0 (−0.2 to 0.1) | 0.55 | ··· | 5% |
| eGFR 45 to 59 mL/min per 1.73 m2 | 6 | 76 | 7.2 (4.8) | 0.3 (−0.3 to 0.9) | 0.35 | 0.34 | 0.2 (−0.3 to 0.7) | 0.41 | 0.45 | 12% |
| eGFR 30 to 44 mL/min per 1.73 m2 | 3 | 27 | 8.8 (2.0) | −0.8 (−1.6 to 0.1) | 0.07 | 0.07 | −0.8 (−1.6 to 0.1) | 0.07 | 0.07 | 0% |
| eGFR 15 to 29 mL/min per 1.73 m2 | 2 | 8 | 13.7 (1.1) | 0.1 (−2.0 to 2.2) | 0.95 | 0.93 | 0.1 (−2.0 to 2.2) | 0.95 | 0.93 | 0% |
| eGFR <15 mL/min per 1.73 m2 b | 2 | 54 | 9.0 (4.9) | 0.4 (−0.1 to 1.0) | 0.12 | 0.17 | 0.4 (−0.1 to 1.0) | 0.12 | 0.17 | 0% |
| Total cholesterol ≥4.60 mmol/L | 11 | 651 | 7.8 (2.3) | −0.1 (−0.3 to 0.1) | 0.24 | 0.54 | −0.1 (−0.3 to 0.1) | 0.24 | 0.49 | 0% |
| Total cholesterol <4.60 mmol/L | 11 | 398 | 7.7 (3.0) | 0.0 (−0.2 to 0.3) | 0.83 | 0.0 (−0.2 to 0.2) | 0.87 | 23% | ||
25(OH)D indicates 25‐hydroxyvitamin D; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; Ca, calcium; CI, confidence interval; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IPD, individual participant data; PTH, parathyroid hormone; PWV, pulse wave velocity; SBP, systolic blood pressure; SMD, standardized mean difference.
Data only from studies measuring baseline carotid‐femoral PWV.
Or on dialysis.
Table 9.
IPD Subgroup Analyses for AIx
| Subgroup | No. of Studies | n | Mean Baseline Value (%) (SD) | Random Effects | Fixed Effects | I2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Effect (%) (95% CI) | P Value | P for Interaction | Treatment Effect (%) (95% CI) | P Value | P for Interaction | |||||
| SBP >140 mm Hg | 9 | 214 | 33.3 (16.6) | 0.8 (−1.1 to 2.7) | 0.39 | 0.31 | 0.8 (−1.1 to 2.7) | 0.39 | 0.26 | 0% |
| SBP ≤140 mm Hg | 11 | 615 | 24.9 (14.6) | −0.6 (−2.5 to 1.4) | 0.57 | −0.5 (−1.7 to 0.7) | 0.44 | 52% | ||
| Baseline 25(OH)D <25 nmol/L | 8 | 93 | 24.9 (14.9) | −4.3 (−11.0 to 2.4) | 0.20 | ··· | −4.8 (−7.8 to −1.7) | 0.002 | ··· | 76% |
| Baseline 25(OH)D 25 to 50 nmol/L | 10 | 357 | 25.8 (16.7) | 0.1 (−1.9 to 2.1) | 0.92 | 0.22 | 0.0 (−1.5 to 1.5) | 0.99 | 0.006 | 30% |
| Baseline 25(OH)D >50 nmol/L | 8 | 342 | 28.6 (15.2) | 0.5 (−1.1 to 2.0) | 0.58 | 0.17 | 0.5 (−1.1 to 2.0) | 0.58 | 0.002 | 0% |
| DM | 7 | 163 | 26.1 (15.6) | 2.0 (−2.6 to 6.6) | 0.40 | 0.37 | 0.4 (−2.2 to 3.1) | 0.75 | 0.68 | 61% |
| No DM | 9 | 666 | 27.3 (15.7) | −0.2 (−1.4 to 1.1) | 0.80 | −0.2 (−1.3 to 0.9) | 0.73 | 11% | ||
| No ACEi or ARB | 9 | 209 | 23.1 (14.3) | −0.2 (−2.0 to 1.7) | 0.86 | ··· | −0.2 (−2.0 to 1.6) | 0.85 | ··· | 4% |
| ACEi, no ARB | 7 | 137 | 25.4 (12.6) | 0.3 (−1.6 to 2.2) | 0.79 | 0.71 | 0.3 (−1.6 to 2.2) | 0.79 | 0.71 | 0% |
| ACEi or ARB | 7 | 243 | 26.4 (12.7) | −0.1 (−2.1 to 1.9) | 0.94 | 0.94 | −0.4 (−1.9 to 1.2) | 0.63 | 0.87 | 27% |
| PTH >5.0 pmol/L | 9 | 282 | 25.3 (15.9) | 0.1 (−2.4 to 2.5) | 0.96 | 0.89 | −0.3 (−2.1 to 1.5) | 0.73 | 0.62 | 43% |
| PTH ≤5.0 pmol/L | 9 | 441 | 27.9 (16.9) | 0.3 (−1.3 to 1.8) | 0.72 | 0.3 (−1.3 to 1.8) | 0.72 | 0% | ||
| Ca >2.30 mmol/L | 5 | 286 | 28.3 (18.7) | 0.0 (−3.6 to 3.6) | 1.00 | 0.45 | −0.1 (−3.4 to 3.2) | 0.96 | 0.39 | 11% |
| Ca ≤2.30 mmol/L | 6 | 265 | 26.2 (13.3) | 1.6 (−0.4 to 3.6) | 0.12 | 1.6 (−0.4 to 3.6) | 0.12 | 0% | ||
| eGFR ≥60 mL/min per 1.73 m2 | 9 | 678 | 27.3 (15.7) | −0.3 (−1.7 to 1.2) | 0.34 | ··· | −0.2 (−1.4 to 0.9) | 0.72 | ··· | 25% |
| eGFR 45 to 59 mL/min per 1.73 m2 | 6 | 64 | 28.3 (16.7) | −5.4 (−11.7 to 0.9) | 0.09 | 0.36 | −11.7 (−12.2 to −11.3) | <0.001 | <0.001 | 86% |
| eGFR 30 to 44 mL/min per 1.73 m2 | 2 | 17 | 29.7 (18.2) | −0.8 (−8.8 to 7.2) | 0.85 | 0.43 | −0.8 (−8.8 to 7.2) | 0.85 | 0.88 | 0% |
| eGFR 15 to 29 mL/min per 1.73 m2 | 0 | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· | ··· |
| eGFR <15 mL/min per 1.73 m2 a | 2 | 51 | 22.9 (12.8) | 6.6 (2.1–11.2) | 0.005 | 0.40 | 6.6 (2.1–11.2) | 0.005 | 0.005 | 0% |
| Total cholesterol ≥4.60 mmol/L | 9 | 434 | 27.8 (15.6) | −0.5 (−2.7 to 1.7) | 0.66 | 0.95 | 0.0 (−1.4 to 1.5) | 1.00 | 0.61 | 39% |
| Total cholesterol <4.60 mmol/L | 9 | 298 | 26.4 (16.2) | −0.4 (−2.5 to 1.7) | 0.73 | −0.6 (−2.4 to 1.2) | 0.52 | 20% | ||
25(OH)D indicates 25‐hydroxyvitamin D; ACEi, angiotensin‐converting enzyme inhibitor; Aix, augmentation index; ARB, angiotensin receptor blocker; Ca, calcium; CI, confidence interval; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IPD, individual participant data; PTH, parathyroid hormone; SBP, systolic blood pressure.
Or on dialysis.
Table 10.
IPD Subgroup Analyses for RHI
| Subgroup | No. of Studies | n | Mean Baseline Value (Units) (SD) | Random Effects | Fixed Effects | I2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Effect (Units) (95% CI) | P Value | P for Interaction | Treatment Effect (Units) (95% CI) | P Value | P for Interaction | |||||
| SBP >140 mm Hg | 3 | 73 | 1.71 (0.94) | −0.21 (−0.52 to 0.10) | 0.18 | 0.21 | −0.14 (−0.33 to 0.05) | 0.16 | 0.20 | 49% |
| SBP ≤140 mm Hg | 3 | 147 | 1.62 (0.74) | 0.01 (0.11–0.14) | 0.85 | 0.01 (0.11–0.14) | 0.85 | 0% | ||
| Baseline 25(OH)D <25 nmol/L | 1 | 21 | 1.82 (0.68) | 0.23 (−0.10 to 0.56) | 0.18 | ··· | 0.23 (−0.10 to 0.56) | 0.18 | ··· | ··· |
| Baseline 25(OH)D 25 to 50 nmol/L | 3 | 153 | 1.71 (0.83) | −0.02 (−0.19 to 0.16) | 0.86 | 0.19 | 0.02 (−0.09 to 0.14) | 0.67 | 0.24 | 46% |
| Baseline 25(OH)D >50 nmol/L | 2 | 40 | 1.33 (0.76) | −0.21 (−0.42 to 0.00) | 0.05 | 0.03 | −0.21 (−0.42 to 0.00) | 0.05 | 0.03 | 0% |
| DM | 3 | 110 | 1.23 (0.74) | 0.01 (−0.10 to 0.11) | 0.91 | 0.17 | 0.01 (−0.10 to 0.11) | 0.91 | 0.17 | 0% |
| No DM | 2 | 101 | 2.07 (0.63) | −0.15 (−0.35 to 0.06) | 0.16 | −0.15 (−0.35 to 0.06) | 0.16 | 0% | ||
| No ACEi or ARB | 3 | 49 | 1.57 (0.79) | 0.42 (−0.72 to 1.57) | 0.47 | ··· | 0.47 (0.29 to 0.66) | <0.001 | ··· | 97% |
| ACEi, no ARB | 3 | 128 | 1.75 (0.82) | −0.00 (−0.16 to 0.15) | 0.96 | 0.48 | −0.00 (−0.14 to 0.13) | 0.96 | <0.001 | 27% |
| ACEi or ARB | 3 | 171 | 1.67 (0.81) | −0.05 (−0.21 to 0.11) | 0.54 | 0.43 | −0.04 (−0.15 to 0.07) | 0.50 | <0.001 | 46% |
| PTH >5.0 pmol/L | 3 | 117 | 1.77 (0.77) | −0.02 (−0.17 to 0.13) | 0.80 | 0.85 | −0.02 (−0.17 to 0.13) | 0.80 | 0.85 | 0% |
| PTH ≤5.0 pmol/L | 3 | 102 | 1.52 (0.84) | 0.00 (−0.14 to 0.14) | 1.00 | 0.00 (−0.14 to 0.14) | 1.00 | 0% | ||
| Ca >2.30 mmol/L | 3 | 127 | 1.50 (0.81) | −0.08 (−0.21 to 0.05) | 0.21 | 0.22 | −0.08 (−0.21 to 0.05) | 0.21 | 0.22 | 0% |
| Ca ≤2.30 mmol/L | 3 | 93 | 1.86 (0.76) | 0.05 (−0.11 to 0.21) | 0.55 | 0.05 (−0.11 to 0.21) | 0.55 | 0% | ||
| eGFR ≥60 mL/min per 1.73 m2 | 3 | 192 | 1.69 (0.80) | −0.04 (−0.14 to 0.07) | 0.48 | 0.81 | −0.04 (−0.14 to 0.07) | 0.48 | 0.77 | 0% |
| eGFR <60 mL/min per 1.73 m2 | 2 | 27 | 1.41 (0.84) | −0.10 (−0.58 to 0.39) | 0.69 | 0.00 (−0.25 to 0.25) | 1.00 | 54% | ||
| Total cholesterol ≥4.60 mmol/L | 3 | 60 | 1.44 (0.85) | −0.04 (−0.38 to 0.30) | 0.81 | 1.00 | 0.00 (−0.19 to 0.18) | 0.98 | 0.73 | 43% |
| Total cholesterol <4.60 mmol/L | 3 | 141 | 1.72 (0.81) | −0.04 (−0.16 to 0.09) | 0.57 | −0.04 (−0.16 to 0.09) | 0.57 | 0% | ||
25(OH)D indicates 25‐hydroxyvitamin D; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; Ca, calcium; CI, confidence interval; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IPD, individual participant data; PTH, parathyroid hormone; RHI, reactive hyperemia index; SBP, systolic blood pressure.
Table 11.
IPD Subgroup Analyses for Microvascular Function
| Subgroup | No. of Studies | n | Random Effects | Fixed Effects | I2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Effect (SMD) (95% CI) | P Value | P for Interaction | Treatment Effect (SMD) (95% CI) | P Value | P for Interaction | ||||
| SBP >140 mm Hg | 1 | 21 | 0.08 (−0.80 to 0.96) | 0.86 | 0.47 | 0.08 (−0.80 to 0.96) | 0.86 | 0.47 | ··· |
| SBP ≤140 mm Hg | 3 | 106 | 0.44 (0.05–0.82) | 0.03 | 0.44 (0.05–0.82) | 0.03 | 0% | ||
| Baseline 25(OH)D <25 nmol/L | 3 | 46 | 0.05 (−0.54 to 0.64) | 0.86 | ··· | 0.05 (−0.54 to 0.64) | 0.86 | ··· | 0% |
| Baseline 25(OH)D 25 to 50 nmol/L | 3 | 56 | 0.20 (−0.39 to 0.79) | 0.51 | 0.72 | 0.20 (−0.34 to 0.74) | 0.47 | 0.71 | 11% |
| Baseline 25(OH)D >50 nmol/L | 2 | 26 | 1.04 (0.14–1.93) | 0.02 | 0.04 | 1.04 (0.14–1.93) | 0.02 | 0.04 | 0% |
| DM | 1 | 18 | 0.41 (−0.54 to 1.35) | 0.40 | 0.91 | 0.41 (−0.54 to 1.35) | 0.40 | 0.91 | ··· |
| No DM | 3 | 111 | 0.35 (−0.03 to 0.73) | 0.07 | 0.35 (−0.03 to 0.73) | 0.07 | 0% | ||
| No ACEi or ARB | 2 | 70 | 0.19 (−0.28 to 0.66) | 0.44 | ··· | 0.19 (−0.28 to 0.66) | 0.44 | ··· | 0% |
| ACEi, no ARB | 2 | 29 | 0.37 (−0.38 to 1.13) | 0.33 | 0.69 | 0.37 (−0.38 to 1.13) | 0.33 | 0.69 | 0% |
| ACEi or ARB | 2 | 57 | 0.65 (0.11–1.18) | 0.02 | 0.21 | 0.65 (0.11–1.18) | 0.02 | 0.21 | 0% |
| PTH >5.0 pmol/L | 3 | 70 | 0.24 (−0.24 to 0.72) | 0.33 | 0.54 | 0.24 (−0.24 to 0.72) | 0.33 | 0.54 | 0% |
| PTH ≤5.0 pmol/L | 2 | 54 | 0.47 (−0.08 to 1.02) | 0.09 | 0.47 (−0.08 to 1.02) | 0.09 | 0% | ||
| Ca >2.30 mmol/L | 2 | 25 | −0.45 (−1.25 to 0.35) | 0.27 | 0.10 | −0.45 (−1.25 to 0.35) | 0.27 | 0.10 | 0% |
| Ca ≤2.30 mmol/L | 2 | 37 | 0.43 (−0.23 to 1.09) | 0.20 | 0.43 (−0.23 to 1.09) | 0.20 | 0% | ||
| eGFR ≥60 mL/min per 1.73 m2 | 2 | 86 | 0.22 (−0.21 to 0.64) | 0.32 | 0.46 | 0.22 (−0.21 to 0.64) | 0.32 | 0.46 | 0% |
| eGFR <60 mL/min per 1.73 m2 | 2 | 43 | 0.50 (−0.11 to 1.11) | 0.11 | 0.50 (−0.11 to 1.11) | 0.11 | 0% | ||
| Total cholesterol ≥4.60 mmol/L | 3 | 66 | 0.05 (−0.47 to 0.57) | 0.85 | 0.32 | 0.05 (−0.45 to 0.54) | 0.85 | 0.31 | 7% |
| Total cholesterol <4.60 mmol/L | 3 | 61 | 0.42 (−0.10 to 0.94) | 0.11 | 0.42 (−0.10 to 0.94) | 0.11 | 0% | ||
25(OH)D indicates 25‐hydroxyvitamin D; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; Ca, calcium; CI, confidence interval; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IPD, individual participant data; PTH, parathyroid hormone; SBP, systolic blood pressure; SMD, standardized mean difference.
Table 12.
IPD Subgroup Analyses for Aortic SBP
| Subgroup | No. of Studies | n | Mean Baseline Value (mm Hg) (SD) | Random Effects | Fixed Effects | I2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Effect (95% CI) (mm Hg) | P Value | P for Interaction | Treatment Effect (95% CI) (mm Hg) | P Value | P for Interaction | |||||
| SBP >140 mm Hg | 5 | 79 | 141.4 (26.3) | 0.4 (−8.8 to 9.6) | 0.93 | 0.95 | 0.4 (−5.5 to 6.3) | 0.90 | 0.82 | 57% |
| SBP ≤140 mm Hg | 7 | 319 | 115.8 (19.1) | 0.1 (−3.3 to 3.5) | 0.96 | −0.3 (−2.4 to 1.9) | 0.82 | 51% | ||
| Baseline 25(OH)D <25 nmol/L | 6 | 77 | 114.2 (28.7) | −2.7 (−9.8 to 4.4) | 0.46 | ··· | −1.7 (−5.9 to 2.5) | 0.42 | ··· | 50% |
| Baseline 25(OH)D 25 to 50 nmol/L | 7 | 148 | 121.9 (24.7) | −2.9 (−6.0 to 0.3) | 0.08 | 0.96 | −2.9 (−6.0 to 0.3) | 0.08 | 0.65 | 0% |
| Baseline 25(OH)D >50 nmol/L | 6 | 171 | 123.0 (20.1) | 0.7 (−3.8 to 5.1) | 0.76 | 0.43 | 1.2 (−1.9 to 4.2) | 0.45 | 0.27 | 24% |
| DM | 4 | 45 | 119.3 (35.3) | 9.4 (−0.2 to 19.1) | 0.06 | 0.17 | 7.9 (0.3 to 15.4) | 0.04 | 0.006 | 26% |
| No DM | 7 | 355 | 120.9 (22.0) | −1.6 (−13.8 to 10.6) | 0.80 | −3.0 (−5.1 to −1.0) | 0.004 | 97% | ||
| No ACEi or ARB | 5 | 138 | 111.8 (24.1) | −6.1 (−11.8 to −0.4) | 0.04 | ··· | −5.8 (−9.1 to −2.1) | <0.001 | ··· | 51% |
| ACEi, no ARB | 3 | 78 | 130.4 (22.1) | −5.4 (−10.8 to 0.1) | 0.05 | 0.87 | −5.4 (−10.8 to 0.1) | 0.05 | 0.9 | 0% |
| ACEi or ARB | 4 | 173 | 132.6 (23.7) | −3.1 (−7.1 to 0.8) | 0.12 | 0.40 | −3.1 (−7.1 to 0.8) | 0.12 | 0.32 | 0% |
| PTH >5.0 pmol/L | 6 | 168 | 121.0 (27.5) | −1.0 (−4.3 to 2.3) | 0.55 | 0.52 | −1.0 (−4.3 to 2.3) | 0.55 | 0.67 | 0% |
| PTH ≤5.0 pmol/L | 6 | 154 | 123.1 (24.0) | 2.8 (−8.2 to 13.8) | 0.62 | −1.9 (−5.6 to 1.8) | 0.31 | 85% | ||
| Ca >2.30 mmol/L | 2 | 30 | 118.4 (20.0) | 0.3 (−5.5 to 6.0) | 0.93 | 0.61 | 0.3 (−5.5 to 6.0) | 0.93 | 0.61 | 0% |
| Ca ≤2.30 mmol/L | 3 | 120 | 121.9 (25.9) | 2.0 (−0.9 to 5.0) | 0.18 | 2.0 (−0.9 to 5.0) | 0.18 | 0% | ||
| eGFR ≥60 mL/min per 1.73 m2 | 5 | 300 | 120.0 (17.7) | −1.3 (−4.8 to 2.2) | 0.47 | 0.70 | −0.6 (−2.8 to 1.5) | 0.56 | 0.92 | 59% |
| eGFR <60 mL/min per 1.73 m2 | 4 | 44 | 130.9 (20.2) | 0.8 (−9.2 to 10.9) | 0.87 | −0.9 (−6.3 to 4.6) | 0.76 | 69% | ||
| Total cholesterol ≥4.60 mmol/L | 5 | 158 | 122.2 (21.6) | −2.3 (−5.8 to 1.2) | 0.20 | 0.68 | −2.3 (−5.8 to 1.2) | 0.20 | 0.76 | 0% |
| Total cholesterol <4.60 mmol/L | 5 | 138 | 122.5 (27.5) | −0.7 (−7.5 to 6.1) | 0.83 | −1.5 (−5.3 to 2.4) | 0.45 | 66% | ||
25(OH)D indicates 25‐hydroxyvitamin D; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; Ca, calcium; CI, confidence interval; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IPD, individual participant data; PTH, parathyroid hormone; SBP, systolic blood pressure.
Table 13.
IPD Subgroup Analyses for Aortic DBP
| Subgroup | No. of Studies | n | Mean Baseline Value (mm Hg) (SD) | Random Effects | Fixed Effects | I2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Effect (95% CI) | P Value | P for Interaction | Treatment Effect (95% CI) | P Value | P for Interaction | |||||
| SBP >140 mm Hg | 5 | 79 | 79.8 (11.1) | −0.7 (−3.3 to 2.0) | 0.62 | 0.95 | −0.7 (−3.3 to 2.0) | 0.62 | 0.95 | 0% |
| SBP ≤140 mm Hg | 7 | 320 | 75.7 (9.3) | −0.6 (−1.9 to 0.6) | 0.33 | −0.6 (−1.9 to 0.6) | 0.33 | 0% | ||
| Baseline 25(OH)D <25 nmol/L | 6 | 77 | 75.8 (9.5) | 1.0 (−2.4 to 4.5) | 0.56 | ··· | 1.0 (−2.4 to 4.5) | 0.56 | ··· | 0% |
| Baseline 25(OH)D 25 to 50 nmol/L | 7 | 151 | 77.2 (10.0) | −0.7 (−2.8 to 1.3) | 0.48 | 0.41 | −0.7 (−2.8 to 1.3) | 0.48 | 0.41 | 0% |
| Baseline 25(OH)D >50 nmol/L | 6 | 171 | 76.3 (10.0) | −0.5 (−2.0 to 1.0) | 0.52 | 0.43 | −0.5 (−2.0 to 1.0) | 0.52 | 0.43 | 0% |
| DM | 4 | 45 | 74.0 (11.5) | 3.8 (−1.6 to 9.1) | 0.17 | 0.12 | 2.8 (0.1 to 5.4) | 0.04 | 0.02 | 68% |
| No DM | 7 | 355 | 76.9 (9.6) | −0.6 (−1.9 to 0.6) | 0.29 | −0.6 (−1.9 to 0.6) | 0.29 | 0% | ||
| No ACEi or ARB | 6 | 152 | 75.4 (9.8) | −0.5 (−2.6 to 1.5) | 0.61 | ··· | −0.5 (−2.6 to 1.5) | 0.61 | ··· | 0% |
| ACEi, no ARB | 4 | 92 | 79.3 (11.4) | 0.6 (−1.9 to 3.1) | 0.62 | 0.50 | 0.6 (−1.9 to 3.1) | 0.62 | 0.50 | 0% |
| ACEi or ARB | 4 | 173 | 78.4 (10.5) | −0.4 (−2.2 to 1.4) | 0.69 | 0.94 | −0.4 (−2.2 to 1.4) | 0.69 | 0.94 | 0% |
| PTH >5.0 pmol/L | 6 | 169 | 77.3 (10.2) | 0.1 (−2.3 to 2.5) | 0.93 | 0.62 | 0.1 (−2.3 to 2.5) | 0.93 | 0.59 | 0% |
| PTH ≤5.0 pmol/L | 6 | 154 | 77.2 (10.4) | −0.7 (−2.7 to 1.3) | 0.52 | −0.7 (−2.4 to 1.0) | 0.39 | 17% | ||
| Ca >2.30 mmol/L | 2 | 31 | 79.3 (9.8) | 0.4 (−4.7 to 5.5) | 0.88 | 0.94 | 0.4 (−4.7 to 5.5) | 0.88 | 0.94 | 0% |
| Ca ≤2.30 mmol/L | 3 | 120 | 77.0 (10.2) | 0.2 (−1.6 to 1.9) | 0.86 | 0.2 (−1.6 to 1.9) | 0.86 | 0% | ||
| eGFR ≥60 mL/min per 1.73 m2 | 5 | 300 | 77.3 (9.2) | −0.6 (−1.8 to 0.7) | 0.35 | 0.07 | −0.6 (−1.8 to 0.7) | 0.35 | 0.03 | 0% |
| eGFR <60 mL/min per 1.73 m2 | 4 | 45 | 75.5 (9.2) | 4.6 (−0.9 to 10.1) | 0.10 | 2.9 (−0.1 to 5.9) | 0.06 | 46% | ||
| Total cholesterol ≥4.60 mmol/L | 5 | 158 | 77.3 (9.5) | −0.6 (−2.6 to 1.3) | 0.52 | 0.76 | −0.6 (−2.6 to 1.3) | 0.52 | 0.89 | 0% |
| Total cholesterol <4.60 mmol/L | 5 | 138 | 74.5 (9.8) | 0.0 (−3.3 to 3.4) | 0.98 | −0.8 (−3.0 to 1.3) | 0.45 | 49% | ||
25(OH)D indicates 25‐hydroxyvitamin D; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; Ca, calcium; CI, confidence interval; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; IPD, individual participant data; PTH, parathyroid hormone; SBP, systolic blood pressure.
Table 14.
Standardizing SDs Pertaining to Trials Combined Using SMD Method
| Study | Trial Level | IPD (Pooled SD) | ||
|---|---|---|---|---|
| PWV (m/s) (Vitamin D, Placebo) | Microvascular Function (Units) (Vitamin D, Placebo) | PWV (m/s) | Microvascular Function (Units) | |
| Gepner 201215 | 0.9, 1.1 | ··· | 1.0 | ··· |
| Larsen 201216 | 0.9, 1.4 | ··· | 1.2 | ··· |
| Marckmann 201217 | 2.6, 2.6 | ··· | 3.1 | ··· |
| Stricker 201219 | ··· | 16.4, 12.4 | ··· | 13.4 |
| Hewitt 201322 | 5.3, 4.8 | ··· | ··· | ··· |
| Witham 201323 | 1.4, 1.3 | ··· | 1.2 | ··· |
| Witham 201325 | 1.2, 1.6 | 1.1, 3.3 | 1.2 | 1.8 |
| Yiu 201326 | 3.4, 4.1 | ··· | ··· | ··· |
| Dreyer 201427 | 1.1, 0.8 | 727, 391 | 1.0 | 591 |
| Mose 201429 | 2.1, 2.4 | ··· | 2.3 | ··· |
| Ryu 201430 | 1.4, 1.8 | ··· | ··· | ··· |
| Garg 201532 | 1.3, 1.2 | ··· | 1.1 | ··· |
| Pilz 201533 | 1.6, 1.6 | ··· | 1.9 | ··· |
| Witham 201535 | 2.5, 1.6 | ··· | 0.9 | ··· |
| Bressendorff 201638 | 0.9, 0.4 | ··· | 1.1 | ··· |
| Forouhi 201640 | 2.1, 1.7 | ··· | 1.7 | ··· |
| Hin 201741 | 1.4, 1.4 | ··· | 3.7 | ··· |
IPD indicates individual participant data; PWV, pulse wave velocity; SMD, standardized mean difference.
Discussion
The present meta‐analysis found little evidence to support the hypothesis that supplementation of vitamin D or use of vitamin D analogues can improve markers of cardiovascular health. Our results were broadly consistent across a range of vascular markers and interventions, and subgroup analyses using IPD did not identify a subgroup that was more likely to benefit from treatment—this remained true even for those participants with the lowest 25(OH)D levels, with high baseline BP, and with higher baseline PTH levels. Random‐effects and fixed‐effects analyses gave very similar results in the majority of analyses. Our results are consistent with our previous work that failed to find a beneficial effect of vitamin D therapy on BP5 and are also in accord with 2 recent, smaller meta‐analyses examining arterial stiffness and endothelial function.42, 43 One further recent meta‐analysis, examining only FMD, showed a slightly greater benefit (treatment effect of vitamin D was 1.27% for FMD),44 perhaps attributable to differences in both study selection and the data used; our analysis had the benefit of access to IPD, which allowed us to verify the accuracy of published data and data used in previous meta‐analyses. The results are also consistent with recent data suggesting no effect of vitamin D supplementation on plasma N‐terminal pro‐B‐type natriuretic peptide levels or echocardiographic indices in older people after 12 months of therapy.45
Despite the large number of participants included in this analysis, it is not possible to completely refute the possibility that vitamin D or its analogues could still have a modest benefit on vascular health. The markers measured in studies included in this meta‐analysis are subject to changes attributed to differences in environment, diet, smoking, medications, and operator skill; such factors require careful use of protocols to standardize measurement and reduce variability.46, 47 The upper limit of the 95% CIs in our analyses encompasses a 1% improvement in FMD, a 1% improvement in AIx, a 0.3‐m/s improvement in PWV, and a 5.6 mm Hg improvement in aortic systolic BP. A 5 mm Hg reduction in aortic systolic BP would be consistent with significant clinical benefit, and a 1% improvement in FMD would be consistent with an 8% to 13% reduction in cardiovascular event rates.48, 49 A trial published too recently to be included in this systematic review suggested a large improvement in FMD in participants with nondialyzed CKD,50 and it therefore remains possible that individuals with nondialyzed CKD, particularly with baseline low 25(OH)D levels, might benefit, although results from trials enrolling nondialyzed CKD participants that we included in this review showed improvement in FMD in only 1 of 3 trials.11, 31, 34 Similarly, a recently published substudy using monthly high‐dose vitamin D3 showed an improvement in aortic BP and arterial stiffness measures in those with baseline 25(OH)D levels <50 nmol/L; effect sizes were consistent with our IPD analysis findings for this subgroup. No significant improvements were observed in the overall trial group, however.51
Pooled observational data show that a loge difference in PWV (≈2.7 m/s) corresponds to a 35% to 45% increase in the risk of a cardiovascular event. A 0.3‐m/s improvement in PWV is therefore unlikely to be associated with a clinically important reduction in cardiovascular events.52 Furthermore, it is still possible that agents such as paricalcitol might provide a greater magnitude of benefit to selected markers such as FMD. Paricalcitol is an active analogue of vitamin D (ie, it does not require further hydroxylation before binding to and activating the vitamin D receptor), and it is possible that this pharmacological difference from vitamin D2 or D3 might account for the observed result. It is, however, more likely that this result is attributed to the play of chance given the large number of comparisons contained in our analysis.
A modest improvement in microvascular function with vitamin D was noted in the trial‐level analysis, although this was of smaller magnitude in the IPD analyses and did not reach significance. The clinical significance of such an improvement in microvascular function is less clear than for changes in macrovascular markers, given that there are few long‐term prognostic studies evaluating microvascular markers. Differences in the physiological control of small and large blood vessels, particularly the role of local metabolic factors in determining microvascular tone, may underpin the difference in response to vitamin D observed here.
A number of limitations of our analysis require discussion. Despite the large number of participants, power for subgroup analyses was limited by the available data; most trials measured only 1 or 2 vascular outcomes, and some baseline variables were not collected in all trials. Caution is warranted in overinterpreting the results of positive associations in the IPD subgroup analyses; the large number of comparisons poses a risk of type I statistical error. Conversely, our decision to combine results from active treatment arms in trials with more than 1 active treatment arm risks diluting the apparent size of any treatment effect, although the impact of this is likely to be minimal given the small number of trials with more than 1 active treatment arm. For some outcomes, heterogeneity of measurement techniques required use of SMDs. Use of SMD limits the clinical utility of the results, and the heterogeneity of measurements means that translating SMD results to clinically meaningful values is challenging. However, use of SMD does at least allow some inferences about possible effect direction and magnitude to be obtained. Despite an extensive series of hypothesis‐driven subgroup analyses and metaregressions to examine potential causes for heterogeneity, we were unable to identify subgroups of patients more likely to benefit from intervention, and heterogeneity in our IPD subgroup analyses remained high. Some of this heterogeneity may be attributable to the small number of trials in each analysis, but other, unmeasured sources of real difference between trials may still exist.
Although the risk of bias in most trials was low, only half of the included trials analyzed data by intention to treat, and the inclusion of trials with non‐intention‐to‐treat analyses will tend to inflate observed effect sizes. It is also possible that not all eligible trials were found or included; for some trials, published trial reports had not been produced or could not be obtained from the authors. New trials continue to be published in this area, but most continue to use small numbers of participants and are likely to have limited impact on our conclusions. Not all authors were willing to share their IPD, and not all trial reports contained sufficient information to allow data to be extracted for meta‐analysis. A further limitation is inherent in the populations studied; most populations contained only a minority of participants with 25(OH)D levels below 25 nmol/L, a group that would be thought to be most likely to benefit. Similarly, some trials were conducted in groups without overt vascular disease, where again the possibilities for improving vascular function might have been limited. Most trials were conducted in white populations, which potentially limits the generalizability of the findings. In particular, few blacks were enrolled in the included studies; this group have particularly low 25(OH)D levels when living at high latitudes and may be more likely to show a reduction in BP with vitamin D supplementation.53
A range of vitamin D doses were used in the included trials; debate continues as to what dose of vitamin D is optimum or indeed what the target level of 25(OH)D should be. If a level of 75 nmol/L is regarded as optimum as has been suggested from observational studies,54 doses at the upper end of the range included in this analysis are required to reach this level.40, 55, 56 Metaregression of vitamin D dose versus treatment effect suggested that higher doses of vitamin D were associated with a slightly greater treatment effect for FMD, but not for PWV or AIx. We found no evidence that daily dosing was more efficacious than intermittent dosing, despite previous work that has suggested that daily dosing provides more‐consistent tissue exposure to the parent compound, facilitating uptake and autocrine activation,57 and evidence that daily dosing may be more efficacious in some conditions (eg, respiratory disease).58 Our results are consistent with our previous analysis that did not find a difference between daily and intermittent dosing on BP.5 A final explanation that requires consideration is that the duration of therapy in most trials may simply have been too short to produce biological effects—particularly those trials intervening for only a few weeks. This explanation is plausible for outcomes such as arterial stiffness if biological effects are mediated by changes in vascular calcification, but seems less so for outcomes such as FMD and reactive hyperemia index, where other interventions are known to alter these parameters within days or weeks. Further evidence against this hypothesis is provided by the metaregression results, which suggest that longer trial duration was associated with a smaller treatment effect for FMD.
The results of these analyses add to the growing body of evidence suggesting that vitamin D supplementation may not have any beneficial effects on cardiovascular health. The lack of effect on vitamin D supplementation on BP in most studies to date5, 53 and the lack of effect on vascular markers observed in the current analysis suggests that associations between 25(OH)D levels and cardiovascular events observed in observational studies may not be causal. Not all observational studies have been prospective in nature, and the degree of adjustment for confounders has been variable. There are several reasons why assumptions about causality may be incorrect, including reverse causality (where overt or preclinical illness leads to lower 25(OH)D levels through mechanisms such as immobility, obesity, or inflammation59, 60), and confounding by shared risk factors for both cardiovascular disease and low 25(OH)D levels; obesity, inactivity, smoking, and advanced age are all known to be associated with lower 25(OH)D levels, and such confounding is notoriously difficult to fully adjust for. Existing evidence from meta‐analyses of vascular events in osteoporosis trials using vitamin D does not support an effect of vitamin D in lowering cardiovascular event rates,1, 7 with the possible exception of heart failure, and the first of a new wave of large, population‐based vitamin D trials has recently reported, again showing no effect of vitamin D supplementation on cardiovascular event rates.8 Randomized trials of relatively short duration cannot exclude a benefit of vitamin D supplementation over the span of a lifetime; observational designs including Mendelian randomization studies61 may still be the only way to shed light on very long exposures to vitamin D, although even these designs are subject to bias and confounding.
A number of other large vitamin D trials are due to report over the next 3 to 4 years,62 and most of these include cardiovascular events as key outcomes—outcomes which our analysis did not focus on. Existing evidence does not support the use of vitamin D to reduce cardiovascular risk, and the results of our analysis do not suggest a specific target group that is particularly likely to benefit from vitamin D supplementation, although we found no evidence of a deleterious effect on cardiovascular function. The relative lack of representation of some groups, particularly nonwhite groups, tempers the generalizability of this conclusion. In the absence of a subgroup with clear benefit, and with large trial reports expected soon, further small‐scale trials examining surrogate vascular end points are unlikely to advance this field of research significantly.
Disclosures
None.
Acknowledgments
Professor Forouhi acknowledges support from MRC Epidemiology Unit core funding (MC_UU_12015/5).
(J Am Heart Assoc. 2018;7:e008273 DOI: 10.1161/JAHA.117.008273.)29848497
References
- 1. Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2:76–89. [DOI] [PubMed] [Google Scholar]
- 2. Theodoratou E, Tzoulaki I, Zgaga L, John PA. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta‐analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones G, Strugnell SA, DeLuca HF. Current understanding of the molecular actions of vitamin D. Physiol Rev. 1998;78:1193–1231. [DOI] [PubMed] [Google Scholar]
- 4. Beveridge LA, Witham MD. Vitamin D and the cardiovascular system. Osteoporos Int. 2013;24:2167–2180. [DOI] [PubMed] [Google Scholar]
- 5. Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HM, Alvarez JA, Boxer RS, Dalbeni A, Gepner AD, Isbel NM, Larsen T, Nagpal J, Petchey WG, Stricker H, Strobel F, Tangpricha V, Toxqui L, Vaquero MP, Wamberg L, Zittermann A, Witham MD. Effect of vitamin D supplementation on blood pressure: a systematic review and meta‐analysis incorporating individual patient data. JAMA Intern Med. 2015;175:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta‐analysis. Diabet Med. 2012;29:e142–e150. [DOI] [PubMed] [Google Scholar]
- 7. Ford JA, MacLennan GS, Avenell A, Bolland M, Grey A, Witham M; RECORD Trial Group . Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta‐analysis. Am J Clin Nutr. 2014;100:746–755. [DOI] [PubMed] [Google Scholar]
- 8. Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J, Murphy J, Khaw KT, Camargo CA Jr. Effect of monthly high‐dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol. 2017;2:608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51:1235–1241. [DOI] [PubMed] [Google Scholar]
- 10. Riley RD, Kauser I, Bland M, Thijs L, Staessen JA, Wang J, Gueyffier F, Deeks JJ. Meta‐analysis of randomised trials with a continuous outcome according to baseline imbalance and availability of individual participant data. Stat Med. 2013;32:2747–2766. [DOI] [PubMed] [Google Scholar]
- 11. Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: a randomized double‐blind pilot trial. Hypertension. 2008;52:249–255. [DOI] [PubMed] [Google Scholar]
- 12. Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. [DOI] [PubMed] [Google Scholar]
- 13. Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53:2112–2119. [DOI] [PubMed] [Google Scholar]
- 14. Harris RA, Pedersen‐White J, Guo DH, Stallmann‐Jorgensen IS, Keeton D, Huang Y, Shah Y, Zhu H, Dong Y. Vitamin D3 supplementation for 16 weeks improves flow‐mediated dilation in overweight African‐American adults. Am J Hypertens. 2011;24:557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gepner AD, Ramamurthy R, Krueger DC, Korcarz CE, Binkley N, Stein JH. A prospective randomized controlled trial of the effects of vitamin D supplementation on cardiovascular disease risk. PLoS One. 2012;7:e36617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsen T, Mose FH, Bech JN, Hansen AB, Pedersen EB. Effect of cholecalciferol supplementation during winter months in patients with hypertension: a randomized, placebo‐controlled trial. Am J Hypertens. 2012;25:1215–1222. [DOI] [PubMed] [Google Scholar]
- 17. Marckmann P, Agerskov H, Thineshkumar S, Bladbjerg EM, Sidelmann JJ, Jespersen J, Nybo M, Rasmussen LM, Hansen D, Scholze A. Randomized controlled trial of cholecalciferol supplementation in chronic kidney disease patients with hypovitaminosis D. Nephrol Dial Transplant. 2012;27:3523–3531. [DOI] [PubMed] [Google Scholar]
- 18. Sokol SI, Srinivas V, Crandall JP, Kim M, Tellides G, Lebastchi AH, Yu Y, Gupta AK, Alderman MH. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc Med. 2012;17:394–404. [DOI] [PubMed] [Google Scholar]
- 19. Stricker H, Tosi BF, Guidicelli‐Nicolosi S, Limoni C, Colucci G. Effect of a single, oral, high‐dose vitamin D supplementation on endothelial function in patients with peripheral arterial disease: a randomised controlled pilot study. Eur J Vasc Endovasc Surg. 2012;44:307–312. [DOI] [PubMed] [Google Scholar]
- 20. Witham MD, Dove FJ, Sugden JA, Doney AS, Struthers AD. The effect of vitamin D replacement on markers of vascular health in stroke patients—a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2012;22:864–870. [DOI] [PubMed] [Google Scholar]
- 21. Breslavsky A, Frand J, Matas Z, Boaz M, Barnea Z, Shargorodsky M. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin Nutr. 2013;32:970–975. [DOI] [PubMed] [Google Scholar]
- 22. Hewitt NA, O'Connor AA, O'Shaughnessy DV, Elder GJ. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Witham MD, Price RJ, Struthers AD, Donnan PT, Messow CM, Ford I, McMurdo ME. Cholecalciferol treatment to reduce blood pressure in older patients with isolated systolic hypertension: the VitDISH randomized controlled trial. JAMA Intern Med. 2013;173:1672–1679. [DOI] [PubMed] [Google Scholar]
- 24. Witham MD, Dove FJ, Khan F, Lang CC, Belch JJ, Struthers AD. Effects of vitamin D supplementation on markers of vascular function after myocardial infarction—a randomised controlled trial. Int J Cardiol. 2013;167:745–749. [DOI] [PubMed] [Google Scholar]
- 25. Witham MD, Adams F, Kabir G, Kennedy G, Belch JJ, Khan F. Effect of short‐term vitamin D supplementation on markers of vascular health in South Asian women living in the UK—a randomised controlled trial. Atherosclerosis. 2013;230:293–299. [DOI] [PubMed] [Google Scholar]
- 26. Yiu YF, Yiu KH, Siu CW, Chan YH, Li SW, Wong LY, Lee SW, Tam S, Wong EW, Lau CP, Cheung BM, Tse HF. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis. 2013;227:140–146. [DOI] [PubMed] [Google Scholar]
- 27. Dreyer G, Tucker AT, Harwood SM, Pearse RM, Raftery MJ, Yaqoob MM. Ergocalciferol and microcirculatory function in chronic kidney disease and concomitant vitamin d deficiency: an exploratory, double blind, randomised controlled trial. PLoS One. 2014;9:e99461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martins D, Meng YX, Tareen N, Artaza J, Lee JE, Farodolu C, Gibbons G, Norris K. The effect of short term vitamin D supplementation on the inflammatory and oxidative mediators of arterial stiffness. Health (Irvine Calif). 2014;6:1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mose FH, Vase H, Larsen T, Kancir AS, Kosierkiewic R, Jonczy B, Hansen AB, Oczachowska‐Kulik AE, Thomsen IM, Bech JN, Pedersen EB. Cardiovascular effects of cholecalciferol treatment in dialysis patients—a randomized controlled trial. BMC Nephrol. 2014;15:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ryu OH, Chung W, Lee S, Hong KS, Choi MG, Yoo HJ. The effect of high‐dose vitamin D supplementation on insulin resistance and arterial stiffness in patients with type 2 diabetes. Korean J Intern Med. 2014;29:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zoccali C, Curatola G, Panuccio V, Tripepi R, Pizzini P, Versace M, Bolignano D, Cutrupi S, Politi R, Tripepi G, Ghiadoni L, Thadhani R, Mallamaci F. Paricalcitol and endothelial function in chronic kidney disease trial. Hypertension. 2014;64:1005–1011. [DOI] [PubMed] [Google Scholar]
- 32. Garg G, Kachhawa G, Ramot R, Khadgawat R, Tandon N, Sreenivas V, Kriplani A, Gupta N. Effect of vitamin D supplementation on insulin kinetics and cardiovascular risk factors in polycystic ovarian syndrome: a pilot study. Endocr Connect. 2015;4:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pilz S, Gaksch M, Kienreich K, Grübler M, Verheyen N, Fahrleitner‐Pammer A, Treiber G, Drechsler C, Ó Hartaigh B, Obermayer‐Pietsch B, Schwetz V, Aberer F, Mader J, Scharnagl H, Meinitzer A, Lerchbaum E, Dekker JM, Zittermann A, März W, Tomaschitz A. Effects of vitamin D on blood pressure and cardiovascular risk factors: a randomized controlled trial. Hypertension. 2015;65:1195–1201. [DOI] [PubMed] [Google Scholar]
- 34. Thethi TK, Bajwa MA, Ghanim H, Jo C, Weir M, Goldfine AB, Umpierrez G, Desouza C, Dandona P, Fang‐Hollingsworth Y, Raghavan V, Fonseca VA. Effect of paricalcitol on endothelial function and inflammation in type 2 diabetes and chronic kidney disease. J Diabetes Complications. 2015;29:433–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Witham MD, Adams F, McSwiggan S, Kennedy G, Kabir G, Belch JJ, Khan F. Effect of intermittent vitamin D3 on vascular function and symptoms in chronic fatigue syndrome—a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2015;25:287–294. [DOI] [PubMed] [Google Scholar]
- 36. Barchetta I, Del Ben M, Angelico F, Di Martino M, Fraioli A, La Torre G, Saulle R, Perri L, Morini S, Tiberti C, Bertoccini L, Cimini FA, Panimolle F, Catalano C, Baroni MG, Cavallo MG. No effects of oral vitamin D supplementation on non‐alcoholic fatty liver disease in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled trial. BMC Med. 2016;14:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Borgi L, McMullan C, Wohlhueter A, Curhan GC, Fisher ND, Forman JP. Effect of vitamin D on endothelial function: a randomized, double‐blind, placebo‐controlled trial. Am J Hypertens. 2017;30:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bressendorff I, Brandi L, Schou M, Nygaard B, Frandsen NE, Rasmussen K, Ødum L, Østergaard OV, Hansen D. The effect of high dose cholecalciferol on arterial stiffness and peripheral and central blood pressure in healthy humans: a randomized controlled trial. PLoS One. 2016;11:e0160905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dalan R, Liew H, Assam PN, Chan ES, Siddiqui FJ, Tan AW, Chew DE, Boehm BO, Leow MK. A randomised controlled trial evaluating the impact of targeted vitamin D supplementation on endothelial function in type 2 diabetes mellitus: the DIMENSION trial. Diab Vasc Dis Res. 2016;13:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Forouhi NG, Menon RK, Sharp SJ, Mannan N, Timms PM, Martineau AR, Rickard AP, Boucher BJ, Chowdhury TA, Griffiths CJ, Greenwald SE, Griffin SJ, Hitman GA. Effects of vitamin D2 or D3 supplementation on glycaemic control and cardiometabolic risk among people at risk of type 2 diabetes: results of a randomized double‐blind placebo‐controlled trial. Diabetes Obes Metab. 2016;18:392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hin H, Tomson J, Newman C, Kurien R, Lay M, Cox J, Sayer J, Hill M, Emberson J, Armitage J, Clarke R. Optimum dose of vitamin D for disease prevention in older people: BEST‐D trial of vitamin D in primary care. Osteoporos Int. 2017;28:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hussin AM, Ashor AW, Schoenmakers I, Hill T, Mathers JC, Siervo M. Effects of vitamin D supplementation on endothelial function: a systematic review and meta‐analysis of randomised clinical trials. Eur J Nutr. 2017;56:1095–1104. [DOI] [PubMed] [Google Scholar]
- 43. Upala S, Sanguankeo A, Congrete S, Jaruvongvanich V. Effect of cholecalciferol supplementation on arterial stiffness: a systematic review and meta‐analysis. Scand Cardiovasc J. 2016;50:230–235. [DOI] [PubMed] [Google Scholar]
- 44. Mazidi M, Karimi E, Rezaie P, Vatanparast H. The impact of vitamin D supplement intake on vascular endothelial function; a systematic review and meta‐analysis of randomized controlled trials. Food Nutr Res. 2017;61:1273574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tomson J, Hin H, Emberson J, Kurien R, Lay M, Cox J, Hill M, Arnold L, Leeson P, Armitage J, Clarke R. Effects of vitamin D on blood pressure, arterial stiffness and cardiac function in older people after 1 year: BEST‐D trial. J Am Heart Assoc. 2017;6:e005707 DOI: 10.1161/JAHA.117.005707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Onkelinx S, Cornelissen V, Goetschalckx K, Thomaes T, Verhamme P, Vanhees L. Reproducibility of different methods to measure the endothelial function. Vasc Med. 2012;17:79–84. [DOI] [PubMed] [Google Scholar]
- 47. Ghiadoni L, Faita F, Salvetti M, Cordiano C, Biggi A, Puato M, Di Monaco A, De Siati L, Volpe M, Ambrosio G, Gemignani V, Muiesan ML, Taddei S, Lanza GA, Cosentino F. Assessment of flow‐mediated dilation reproducibility: a nationwide multicenter study. J Hypertens. 2012;30:1399–1405. [DOI] [PubMed] [Google Scholar]
- 48. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: a meta‐analysis. Int J Cardiovasc Imaging. 2010;26:631–640. [DOI] [PubMed] [Google Scholar]
- 49. Ras RT, Streppel MT, Draijer R, Zock PL. Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol. 2013;168:344–351. [DOI] [PubMed] [Google Scholar]
- 50. Kumar V, Yadav AK, Lal A, Kumar V, Singhal M, Billot L, Gupta KL, Banerjee D, Jha V. A randomized trial of vitamin D supplementation on vascular function in CKD. J Am Soc Nephrol. 2017;28:3100–3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sluyter JD, Camargo CA Jr, Stewart AW, Waayer D, Lawes CMM, Toop L, Khaw KT, Thom SAM, Hametner B, Wassertheurer S, Parker KH, Hughes AD, Scragg R. Effect of monthly, high‐dose, long‐term vitamin D supplementation on central blood pressure parameters: a randomized controlled trial substudy. J Am Heart Assoc. 2017;6:e006802 DOI: 10.1161/JAHA.117.006802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton‐Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Forman JP, Scott JB, Ng K, Drake BF, Suarez EG, Hayden DL, Bennett GG, Chandler PD, Hollis BW, Emmons KM, Giovannucci EL, Fuchs CS, Chan AT. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61:779–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bischoff‐Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson‐Hughes B. Estimation of optimal serum concentrations of 25‐hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. [DOI] [PubMed] [Google Scholar]
- 55. Gallagher JC, Sai A, Templin T, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med. 2012;156:425–437. [DOI] [PubMed] [Google Scholar]
- 56. Aloia JF, Patel M, Dimaano R, Li‐Ng M, Talwarr SA, Mikhail M, Pollack S, Yeh JK. Vitamin D intake to attain a desired serum 25‐hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87:1952–1958. [DOI] [PubMed] [Google Scholar]
- 57. Hollis BW, Wagner CL. The role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98:4619–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov‐Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki‐Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S Jr, Stelmach I, Kumar GT, Urashima M, Camargo CA Jr. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta‐analysis of individual participant data. BMJ. 2017;356:i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reid D, Toole BJ, Knox S, Talwar D, Harten J, O'Reilly DS, Blackwell S, Kinsella J, McMillan DC, Wallace AM. The relation between acute changes in the systemic inflammatory response and plasma 25‐hydroxyvitamin D concentrations after elective knee arthroplasty. Am J Clin Nutr. 2011;93:1006–1011. [DOI] [PubMed] [Google Scholar]
- 60. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. [DOI] [PubMed] [Google Scholar]
- 61. Afzal S, Nordestgaard BG. Vitamin D, hypertension, and ischemic stroke in 116 655 individuals from the general population: a genetic study. Hypertension. 2017. Jul 31. pii: HYPERTENSIONAHA.117.09411. DOI: 10.1161/hypertensionaha.117.09411. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 62. Kupferschmidt K. Uncertain verdict as vitamin D goes on trial. Science. 2012;337:1476–1478. [DOI] [PubMed] [Google Scholar]


