Abstract
Background
Cardiac rehabilitation (CR) referral is recommended for eligible patients, regardless of sex or race. It is unclear whether inequality in CR referral practices was associated with patients’ long‐term survival.
Methods and Results
We linked the American Heart Association Get With The Guidelines Coronary Artery Disease registry with Medicare claims data for 48 993 coronary artery disease patients from 365 hospitals across the United States between 2003 and 2009. We used generalized estimation equations to estimate the association between CR referral and mortality accounting for clustering within hospitals. Between 2003 and 2009, only 40% of eligible patients received CR referrals. Females were 12% less likely to receive CR referral compared with males. Black, Hispanic, and Asian patients were 20%, 36%, and 50% less likely, respectively, to receive CR referral than white patients. CR referral was associated with 40% lower 3‐year all‐cause mortality. Women and minorities who received CR referral at hospital discharge had significantly lower mortality compared with those who did not (odds ratios=0.61 [95% confidence interval, 0.56–0.66] for women, 0.75 [95% confidence interval, 0.63–0.88] for black, 0.62 [95% confidence interval, 0.50–0.79] for Hispanic, and 0.63 [95% confidence interval, 0.46–0.85] for Asian patients). Seven percent of the black versus white mortality gap could potentially be reduced by equitable CR referral.
Conclusions
CR referral rates at hospital discharge remained low. Gaps in receiving CR referral at hospital discharge were large for women and minorities, and the mortality gap could potentially be reduced through elimination of inequality in CR referral.
Keywords: cardiac rehabilitation, cardiovascular disease, epidemiology, mortality
Subject Categories: Epidemiology, Cardiovascular Disease, Race and Ethnicity, Secondary Prevention, Mortality/Survival
Clinical Perspective
What Is New?
Disparities in receiving cardiac rehabilitation referral at hospital discharge were associated with lower long‐term survival.
What Are the Clinical Implications?
Sex and race disparities in cardiovascular health could potentially be reduced through higher rates of cardiac rehabilitation referral.
Introduction
Cardiovascular disease accounts for one third of all deaths in the United States,1 and 31% of all deaths globally.2, 3 Cardiac rehabilitation (CR), which emphasizes smoking cessation, physical activity, and weight management, can improve physical function, psychosocial well‐being, and quality of life for myocardial infarction (MI) survivors.4, 5, 6 Despite the many benefits of CR, referral and participation rates are surprisingly low.7, 8, 9 The Million Hearts Initiatives from the Centers for Disease Control and Prevention set a goal to achieve >70% participation in CR/secondary prevention programs by the year 2022.10 The American Heart Association and the American College of Cardiology recommend CR referral before hospital discharge as a Class I recommendation and listed assessing the impact of better CR referral, initiation, and participation on population outcomes as priority areas.11 In order to participate in a CR program, patients must first receive a referral from their physician. CR referral is recommended for eligible patients regardless of their sex, race, or geographic location. Prior studies suggest that the most easily overcome barrier to CR participation is the lack of CR referral at hospital discharge.9, 12, 13, 14, 15, 16 It is unclear whether inequality in CR referral practices by physicians was associated with patients’ long‐term survival.
Prior research on CR mainly focused on 2 areas: (1) identifying predictors for CR referral and initiation8, 9, 17, 18, 19, 20, 21 and (2) estimating the effects of CR participation with subsequent outcomes, such as mortality.22, 23, 24 For (1), the majority of evidence was collected from white males.25 Evidence from females and minorities was limited. For (2), studies were focused on actual CR participation and mainly based on trials26 or administrative databases.27 However, the administrative databases did not include information regarding CR referral. Furthermore, CR referral and participation could be influenced by patients’ underlying health status. Information on detailed clinical conditions as well as quality of care received is limited in claims databases. Can inequality in CR referral at hospital discharge lead to subsequent differences in long‐term mortality? The answer to this question is currently unknown and could provide insights into reducing cardiovascular health disparities. The magnitude of potential improvement in mortality that may be achieved by reducing the sex and racial gaps in referral rates remains to be determined.
The Get With The Guidelines–Coronary Artery Disease (GWTG‐CAD) program is a national program organized by the American Heart Association.28, 29 It is a large, multicenter, observational registry started in 2000 to support and facilitate improvement of the quality of care for patients with cardiovascular disease. CR referral, physical activity, weight management, and smoking cessation counseling recommendations were part of the quality of care performance measures. By linking this large registry in the United States with Medicare claims data containing diverse patient and hospital characteristics, we have a unique opportunity to address the abovementioned knowledge gaps. We hypothesized that (1) receipt of CR referral at hospital discharge would be associated with lower 1‐ and 3‐year mortality; (2) there should be no differences in CR referral rates by sex, race, and regional differences; and (3) elimination of gaps in CR referral could potentially be associated with reduced sex and racial disparities in cardiovascular health.
Methods
In accordance with existing policies regarding data availability in the GWTG database, the data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
We linked 125 135 patients in the GWTG‐CAD registry with Medicare inpatient data from 551 hospitals across the United States. The GWTG Program uses a web‐based Patient Management Tool (Outcome Sciences Inc, Cambridge, MA) to collect clinical data and provide decision support with real‐time online reporting features. The GWTG‐CAD program enrolls patients hospitalized with a confirmed diagnosis of CAD (International Classification of Diseases Ninth Revision [ICD‐9] codes 410–414 included). Trained data abstractors at participating hospitals in GWTG‐CAD collected detailed information on baseline demographic and clinical characteristics, in‐hospital care processes and outcomes, and discharge treatment using a standardized set of data elements and definitions. Using an internet‐based system, data quality was monitored to assure the completeness and accuracy of the submitted data. Outcome Sciences, Inc. serves as the data collection and coordination center for GWTG. Hospital participation in the GWTG‐CAD registry was voluntary and nationwide, and information included met ICD‐9, Diagnosis code 410 to 414 (diagnosis of acute MI, unstable angina, chronic stable angina, and ischemic heart disease) inclusion criteria or criteria for symptomatic peripheral vascular disease. Case findings were based on clinical identification of patients with qualifying clinical diagnoses or ICD‐9, identification with clinical verification from data abstraction. Participating hospitals submit clinical data regarding patients’ in‐hospital care and outcomes.
The Medicare linkage includes Part A (inpatient) claims and the associated denominator file from 2003 to 2009, which linked with data from the GWTG‐CAD registry.30, 31 The study population for this analysis was from hospitals in the GWTG‐CAD registry consisting of 125 134 patients in the database linked to Centers for Medicare and Medicaid Services from 551 hospitals in the United States from January 2003 to December 2009 (Figure 1). We included those that had at least 75% complete data on medical history, had a principal cardiac or principal CAD diagnosis (as recorded in the GWTG‐CAD registry), were 65 years older with a GWTG‐CAD registry hospitalization linked to Medicare, were discharged between January 1, 2003 and December 31, 2009, were discharged alive, but did not leave against medical advice and were not discharged or transferred to either another short‐term hospital or hospice or discharge destination missing (as recorded in the GWTG‐CAD registry), were enrolled in fee‐for‐service Medicare at discharge, and had available information on sex, race, and region. If multiple hospitalizations existed for a patient, the first hospitalization was selected as the index hospitalization for this analysis (Figure 1). Our final analytic sample consisted of 48 993 patients, aged 65 years or older, from 365 hospitals.
Figure 1.
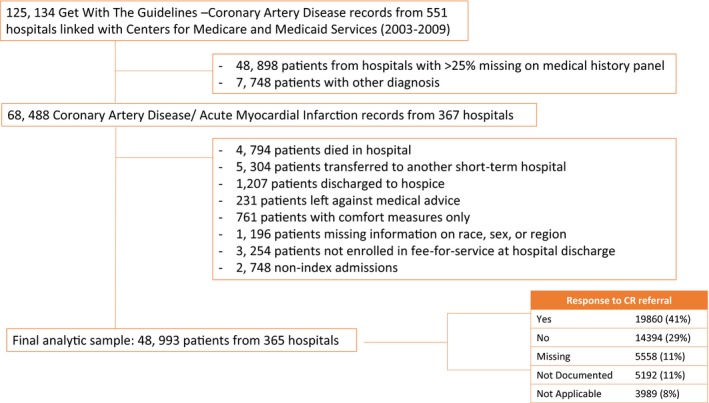
Inclusion and exclusion criteria. CR indicates cardiac rehabilitation.
Measures and Methods of Ascertainment
CR referral and other risk interventions
Outpatient CR program information was collected through the standard performance management form, and extracted from medical records by professionally trained personnel. It is part of the information captured by the GWTG registry data collection form. It was coded as “yes, no, not documented, or not applicable.” For all of our analyses, we included eligible patients as our denominator. There were 3989 (8%) patients with not applicable recorded for the outpatient CR program variable. For each eligible patient (n=48 993), information was extracted regarding whether the patients received outpatient CR referral, and whether patients received physical activity recommendation. For patients who were overweight or obese (n=28 063, body mass index ≥25 kg/m2), receipt of weight management was recorded as part of the CAD quality of care measure. For patients who were current smokers (n=7007), information on whether smoking cessation counseling was given was also recorded as part of the GWTG performance measure.
Sex, race/ethnicity, and geographic region
Our primary exposures of interest include sex (male or female), race/ethnicity (white, black, Asian, Hispanic, other), and geographic region (Northeast, Midwest, South, West). Information was extracted from medical records that were collected during patient hospitalization through patient self‐reporting and recorded separately by trained hospital personnel.
Mortality
Our outcomes were 1‐ and 3‐year all‐cause mortality. Death events were ascertained on the basis of the death date recorded in the denominator/vital status file.
Covariates
In our multivariable analysis, we considered the following variables to be potential confounders for our main analysis and mediation analysis, including patients’ sociodemographic and lifestyle information, insurance status, medical history, cardiac diagnosis, hospital characteristics, medications before hospital admission, medications prescribed at hospital discharge, as well as quality of care received during hospitalization. Quality of care was measured using the GWTG‐CAD quality of care composite performance score, which is a composite performance measure documenting 100% compliance (ie, defect‐free care). It includes adherence to 6 measures, including angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker treatment for patients with left ventricular systolic dysfunction, β‐blockers at discharge, lipid‐lowering medications, aspirin within 24 hours of admission and at discharge, and smoking cessation. All covariate information was extracted from medical records as well the GWTG‐CAD performance measures form.
Statistical Analyses
We first examined outpatient CR referral rates for the overall sample, and by sex, race, geographic region, and calendar year. For outpatient CR referral and physical activity recommendations, we performed analyses among all CAD patients. For weight management and smoking cessation recommendations, we restricted our analyses to eligible patients only (patients with body mass index ≥25 kg/m2 for weight management, and smokers for smoking cessation). We also calculated the proportion of CR‐referred patients who were white men for each follow‐up year.
To examine the generalizability of our findings, we compared patient and hospital characteristics among those included and excluded from our analytic sample. The Pearson χ2 test was used to compare binary or nominal categorical variables, and the Kruskal–Wallis test was used to compare continuous variables or ordinal categorical variables. Percent standardized differences between groups are also provided (standardized difference×100).32
We first estimated the associations between CR referral with 1‐ and 3‐year mortality using a generalized estimation equation model accounting for clustering within hospital. We tested interaction by sex, race, and region using likelihood ratio tests. To estimate the effect of CR on mortality independent of insurance status and quality of care received, we further adjusted for patients’ insurance status, medication use before and after hospital discharge, as well as quality of care received during hospitalization. We examined whether there were sex, racial, and regional differences in CR and other risk intervention recommendation rates using a generalized estimation equation model accounting for clustering within hospitals.
To examine the proportion of sex, racial, and regional gap in mortality that could potentially be reduced through optimal CR referral, we performed a causal mediation analysis.33, 34 We estimated the association between sex, race, and region with 1‐ and 3‐year mortality using the generalized estimation equation model. The proportion of the disparity in sex/race‐mortality relation that could potentially be reduced through CR referral was computed, on the risk difference scale, as [odds ratio (OR)NDE×(ORNIE−1)]/[ORNDE×ORNIE−1]×100 (NDE, Natural Direct Effect; NIE, Natural Indirect Effect).33
As a sensitivity analysis, we modified logistic regressions in the original MEDIATION SAS macro to include the generalized estimation equation statement accounting for within‐hospital correlations. We also performed sensitivity analysis to estimate the influence of unmeasured confounding factors on our effect estimates.35
This study was approved by an institutional review board at the American Heart Association–GWTG committee. All participating institutions were required to comply with local regulatory and privacy guidelines and, if required, to secure institutional review board approval. Because data were used primarily at the local site for quality improvement, sites were granted a waiver of informed consent under the common rule. Quintiles (Cambridge, MA) served as the registry coordinating center. The Duke Clinical Research Institute (Durham, NC) served as the data analysis center, and institutional review board approval was granted to analyze aggregate de‐identified data for research purposes.
Results
Among 48 993 patients from 365 fully participating GWTG‐CAD sites, 40.5% patients were referred by their physician to an outpatient rehabilitation program at discharge, and 77.1% received a physical activity recommendation (Table 1). Among 28 063 patients who were overweight, 82.7% received weight management recommendation. Among 7007 smokers, 87.6% received smoking cessation recommendations. As part of the quality of care performance measures of the AHA‐GWTG program, the CR referral rate remained low from 2003 to 2009. We observed little improvement in CR referral from 34% in 2003 to 43% in 2009 (Figure 2). Greater improvements were observed for smoking cessation, from 70% in 2003 to 98% in 2009. Similarly, improvements were seen for physical activity and weight management recommendations. Among 19 860 CR referred patients, 50% were white males, with level referral rates of 48% in 2003 to 52% in 2009.
Table 1.
CR Referral and Other Risk Intervention Rates in Patients With CAD at Hospital Discharge
| N Total (%) | Outpatient CR Referral | Physical Activity Recommendations | Weight Managementa | Smoking Cessationb | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | ||
| N eligible/overall | 48 993 (100.00) | 48 993 (100.00) | 48 993 (100.00) | 28 063 (57.28) | 7007 (14.30) |
| N yes/N eligible | 48 993 (100.00) | 19 860 (40.54) | 37 764 (77.08) | 23 199 (82.67) | 6140 (87.63) |
| Sex | |||||
| Male | 25 830 (52.72) | 11 267 (43.62) | 20 360 (78.82) | 13 232 (83.63) | 3508 (87.31) |
| Female | 23 163 (47.28) | 8593 (37.10) | 17 404 (75.14) | 9967 (81.42) | 2632 (88.06) |
| Race | |||||
| White | 40 573 (82.81) | 17 169 (42.32) | 31 319 (77.19) | 19 364 (82.68) | 5052 (87.94) |
| Black | 2775 (5.66) | 1012 (36.47) | 2042 (73.59) | 1296 (79.95) | 437 (91.81) |
| Hispanic | 2476 (5.05) | 752 (30.37) | 2023 (81.70) | 1291 (86.59) | 314 (85.56) |
| Asian | 1191 (2.43) | 265 (22.25) | 861 (72.29) | 335 (78.64) | 90 (73.17) |
| Other | 1978 (4.04) | 662 (33.47) | 1519 (76.79) | 913 (82.55) | 247 (83.45) |
| Region | |||||
| Northeast | 9232 (18.84) | 2739 (29.67) | 6794 (73.59) | 4316 (83.66) | 1156 (88.72) |
| Midwest | 13 781 (28.13) | 7370 (53.48) | 10 905 (79.13) | 7053 (82.95) | 1628 (87.15) |
| South | 17 433 (35.58) | 6505 (37.31) | 13 556 (77.76) | 8254 (81.93) | 2450 (89.06) |
| West | 8547 (17.45) | 3246 (37.98) | 6509 (76.16) | 3576 (82.66) | 906 (83.50) |
BMI indicates body mass index; CAD, coronary artery disease; CR, cardiac rehabilitation.
Among patients with BMI >25 kg/m2 only.
Among smokers only.
Figure 2.
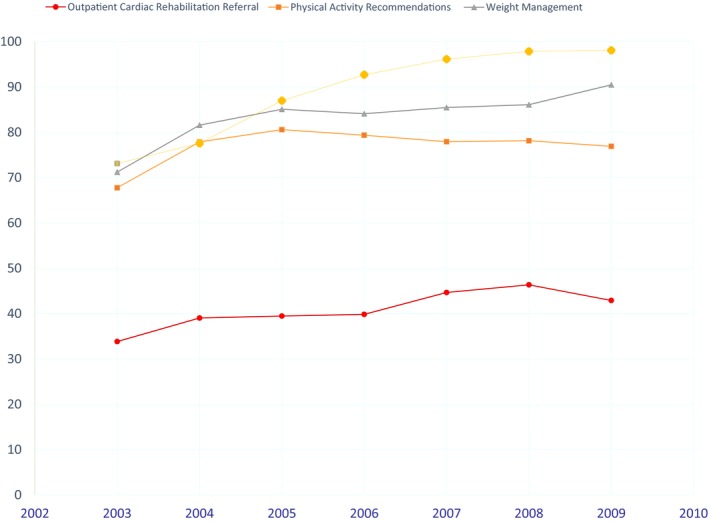
Time trends of cardiac rehabilitation referral rate and other risk intervention rates at hospital discharge from 2003 to 2009.
Patient and hospital characteristics were overall similar comparing those included with those excluded from our analytic sample (Table S1). Patients who received CR referral tended to be younger, had higher body mass index, and were more likely to receive coronary angiography or percutaneous coronary intervention during hospitalization, less likely to have in‐hospital procedures panel missing, and were more likely to be located in the Midwest region (Table 2).
Table 2.
Patients’ Characteristics by CR Referral Status at Hospital Discharge
| Variable | Overall | Referred | Not Referred | P Value | Standardized Difference, % |
|---|---|---|---|---|---|
| N=48 993 | N=19 860 | N=29 133 | |||
| Demographics | |||||
| Age, ya | 77 (71–83) | 75 (70–80) | 78 (71–84) | <0.0001 | 35.3 |
| Sex | <0.0001 | 13.5 | |||
| Female | 23 163 (47.3) | 8593 (43.3) | 14 570 (50.0) | ||
| Male | 25 830 (52.7) | 11 267 (56.7) | 14 563 (50.0) | ||
| Race | <0.0001 | ||||
| White | 40 573 (82.8) | 17 169 (86.5) | 23 404 (80.3) | 16.5 | |
| Black | 2775 (5.7) | 1012 (5.1) | 1763 (6.1) | 4.2 | |
| Hispanic | 2476 (5.1) | 752 (3.8) | 1724 (5.9) | 9.9 | |
| Asian | 1191 (2.4) | 265 (1.3) | 926 (3.2) | 12.4 | |
| Other | 1978 (4.0) | 662 (3.3) | 1316 (4.5) | 6.1 | |
| Insurance | <0.0001 | ||||
| No insurance/ND/UTD | 914 (2.0) | 341 (1.8) | 573 (2.2) | 2.6 | |
| Medicare | 27 996 (62.0) | 11 250 (59.8) | 16 746 (63.6) | 7.9 | |
| Medicaid | 2662 (5.9) | 816 (4.3) | 1846 (7.0) | 11.6 | |
| Other | 13 572 (30.1) | 6409 (34.1) | 7163 (27.2) | 14.9 | |
| Cardiac diagnosis | <0.0001 | ||||
| Heart failure with CAD | 13 919 (28.4) | 5454 (27.5) | 8465 (29.1) | 3.5 | |
| Confirmed AMI—non‐STEMI | 4590 (9.4) | 2492 (12.5) | 2098 (7.2) | 18.0 | |
| Confirmed AMI—STEMI | 22 524 (46.0) | 8141 (41.0) | 14 383 (49.4) | 16.9 | |
| Unstable angina | 2756 (5.6) | 1245 (6.3) | 1511 (5.2) | 4.7 | |
| Coronary artery disease | 5204 (10.6) | 2528 (12.7) | 2676 (9.2) | 11.4 | |
| Year | <0.0001 | 14.5 | |||
| 2009 | 3084 (6.3) | 1323 (6.7) | 1761 (6.0) | ||
| 2008 | 6680 (13.6) | 3096 (15.6) | 3584 (12.3) | ||
| 2007 | 7270 (14.8) | 3247 (16.3) | 4023 (13.8) | ||
| 2006 | 7105 (14.5) | 2830 (14.2) | 4275 (14.7) | ||
| 2005 | 9500 (19.4) | 3749 (18.9) | 5751 (19.7) | ||
| 2004 | 8076 (16.5) | 3152 (15.9) | 4924 (16.9) | ||
| 2003 | 7278 (14.9) | 2463 (12.4) | 4815 (16.5) | ||
| Medical history | |||||
| Atrial fib/atrial flutter | 5482 (11.7) | 1810 (9.4) | 3672 (13.3) | <0.0001 | 12.3 |
| COPD or asthma | 7967 (17.0) | 3025 (15.7) | 4942 (17.9) | <0.0001 | 5.9 |
| Diabetes mellitus | 15 743 (33.6) | 6181 (32.1) | 9562 (34.6) | <0.0001 | 5.4 |
| Hyperlipidemia | 23 065 (49.2) | 10 747 (55.8) | 12 318 (44.6) | <0.0001 | 22.5 |
| Hypertension | 34 850 (74.4) | 14 404 (74.8) | 20 446 (74.1) | 0.08 | 1.6 |
| Peripheral vascular disease (PVD) | 5460 (11.6) | 2116 (11.0) | 3344 (12.1) | 0.0002 | 3.5 |
| CAD | 8459 (18.0) | 3592 (18.6) | 4867 (17.6) | 0.004 | 2.6 |
| Prior MI | 9891 (21.1) | 3940 (20.5) | 5951 (21.6) | 0.003 | 2.7 |
| Cerebrovascular accident/transient ischemic attack | 5255 (11.2) | 1738 (9.0) | 3517 (12.7) | <0.0001 | 12.0 |
| Implantable cardioverter defibrillator | 261 (0.6) | 105 (0.5) | 156 (0.6) | 0.77 | 0.3 |
| Heart failure | 8443 (18.0) | 2633 (13.7) | 5810 (21.0) | <0.0001 | 19.6 |
| Anemia | 1394 (3.0) | 405 (2.1) | 989 (3.6) | <0.0001 | 8.9 |
| Pacemaker—biventricular/resync/cardiac resynchronization | 739 (1.6) | 260 (1.3) | 479 (1.7) | 0.001 | 3.1 |
| Dialysis (chronic) | 911 (1.9) | 251 (1.3) | 660 (2.4) | <0.0001 | 8.1 |
| Renal insufficiency | 5411 (11.5) | 1811 (9.4) | 3600 (13.0) | <0.0001 | 11.6 |
| Depression | 1241 (2.6) | 464 (2.4) | 777 (2.8) | 0.007 | 2.5 |
| Prior PCI or CABG | 2186 (4.7) | 947 (4.9) | 1239 (4.5) | 0.03 | 2.0 |
| Valvular heart disease | 277 (0.6) | 118 (0.6) | 159 (0.6) | 0.61 | 0.5 |
| Medical history panel missing | 2121 (4.3) | 595 (3.0) | 1526 (5.2) | <0.0001 | 11.3 |
| Smoking | 7007 (14.6) | 3193 (16.4) | 3814 (13.4) | <0.0001 | 8.6 |
| Measures | |||||
| Systolic blood pressure, mm Hga | 123 (110–138) | 122 (110–136) | 124 (110–140) | <0.0001 | 9.5 |
| BMIa | 26.63 (23.49–30.41) | 27.2 (24.2–30.86) | 26.3 (23.03–30.07) | <0.0001 | 15.2 |
| Ejection fractiona | 50 (38–60) | 50 (40–60) | 50 (35–60) | <0.0001 | 6.5 |
| Ejection fraction <40% or moderate or severe dysfunction | 10 973 (25.9) | 4132 (23.0) | 6841 (28.1) | <0.0001 | 11.5 |
| Medications before admission | |||||
| ACE‐inhibitors | 4936 (32.0) | 2180 (31.7) | 2756 (32.2) | 0.56 | 0.9 |
| Aspirin | 7706 (49.9) | 3592 (52.3) | 4114 (48.0) | <0.0001 | 8.5 |
| β‐Blocker | 3202 (20.7) | 1397 (20.3) | 1805 (21.1) | 0.26 | 1.8 |
| Lipid‐lowering agents | 7663 (49.6) | 3547 (51.6) | 4116 (48.1) | <0.0001 | 7.2 |
| Prior medication missing | 33 558 (68.5) | 12 991 (65.4) | 20 567 (70.6) | <0.0001 | 11.1 |
| In‐hospital procedures | |||||
| No procedure | 6402 (15.1) | 1036 (5.5) | 5366 (22.7) | <0.0001 | 51.1 |
| Cardiac cath/coronary angiography | 28 025 (66.0) | 13 917 (73.8) | 14 108 (59.8) | <0.0001 | 30.1 |
| Cardioversion | 156 (0.4) | 89 (0.5) | 67 (0.3) | 0.0015 | 3.1 |
| CABG or cardiac value surgery | 5467 (12.9) | 3550 (18.8) | 1917 (8.1) | <0.0001 | 31.7 |
| PCI or PCI with stent | 22 056 (52.0) | 11 602 (61.5) | 10 454 (44.3) | <0.0001 | 35.0 |
| CRT‐P or CRT‐D | 88 (0.2) | 36 (0.2) | 52 (0.2) | 0.50 | 0.7 |
| Implantable cardioverter defibrillator only | 162 (0.4) | 70 (0.4) | 92 (0.4) | 0.75 | 0.3 |
| Dialysis or ultrafiltration, or unspecified | 135 (0.3) | 40 (0.2) | 95 (0.4) | 0.0005 | 3.4 |
| Mechanical ventilation | 1093 (2.6) | 673 (3.6) | 420 (1.8) | <0.0001 | 11.1 |
| Right cardiac catheterization | 671 (1.6) | 441 (2.3) | 230 (1.0) | <0.0001 | 10.7 |
| In‐hospital procedures panel missing | 6545 (13.4) | 1006 (5.1) | 5539 (19.0) | <0.0001 | 43.9 |
| Medications at discharge | |||||
| ACE inhibitors | 27 505 (56.9) | 11 949 (60.7) | 15 556 (54.3) | <0.0001 | 13.0 |
| Aspirin | 43 971 (90.0) | 18 743 (94.6) | 25 228 (86.8) | <0.0001 | 26.9 |
| β‐Blockers | 41 997 (86.4) | 17 564 (88.9) | 24 433 (84.7) | <0.0001 | 12.6 |
| Lipid‐lowering agents | 38 484 (80.6) | 17 303 (88.3) | 21 181 (75.2) | <0.0001 | 34.2 |
| Hospital characteristics | |||||
| Number of bedsa | 342 (226–527) | 373 (265–536) | 323 (208–507) | <0.0001 | 7.6 |
| Region | <0.0001 | ||||
| West | 8547 (17.4) | 3246 (16.3) | 5301 (18.2) | 4.9 | |
| South | 17 433 (35.6) | 6505 (32.8) | 10 928 (37.5) | 10.0 | |
| Midwest | 13 781 (28.1) | 7370 (37.1) | 6411 (22.0) | 33.6 | |
| Northeast | 9232 (18.8) | 2739 (13.8) | 6493 (22.3) | 22.2 | |
| Academic hospital | 28 873 (59.0) | 12 567 (63.4) | 16 306 (56.0) | <0.0001 | 15.2 |
| Rural location | 6020 (12.5) | 1704 (8.6) | 4316 (15.2) | <0.0001 | 20.4 |
| Primary PTCA performed for AMI | 44 180 (91.3) | 18 737 (95.3) | 25 443 (88.6) | <0.0001 | 24.8 |
| Cardiac surgery performed on‐site | 39 751 (82.7) | 17 240 (88.1) | 22 511 (79.0) | <0.0001 | 24.6 |
| Heart transplants performed at site | 5541 (11.6) | 1739 (8.9) | 3802 (13.4) | <0.0001 | 14.5 |
ACE indicates angiotensin‐converting enzyme; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CR, cardiac rehabilitation; CRT, cardiac resynchronization therapy; IQR, interquartile range; MI, myocardial infarction; ND, not documented; non‐STEMI, non‐ST‐elevation myocardial infarction; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; PVD, peripheral vascular disease; STEMI, ST‐elevation myocardial infarction; UD, undetermined.
Continuous variables presented as median (IQR).
CR referral and other risk interventions were all significantly inversely associated with 1‐ and 3‐year all‐cause mortality (Table 3). The significant inverse associations remained after we further adjusted for patients’ insurance status, medications, as well as quality of care received. We found significant effect modification by sex for physical activity recommendation (P for interaction=0.0009, Table 4), and for smoking cessation counseling (P for interaction=0.04, Table 4) for 1‐year mortality, but no significant interactions for 3‐year mortality. Overall, the significant inverse association with mortality by CR referral was consistently seen for both male and female patients, and for all racial groups (Table 4).
Table 3.
Associations Between CR Referral and Other Risk Interventions at Hospital Discharge With 1‐ and 3‐Y All‐Cause Mortality
| Deaths | Unadjusted | Adjusteda | Adjustedb | ||||
|---|---|---|---|---|---|---|---|
| N (%) | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| At 1 y | |||||||
| Cardiac rehabilitation referral | |||||||
| Yes | 1958 (9.86) | 0.38 (0.34, 0.42) | <0.0001 | 0.52 (0.48, 0.56) | <0.0001 | 0.56 (0.52, 0.60) | <0.0001 |
| No | 6474 (22.22) | Reference | Reference | Reference | |||
| Activity recommendations | |||||||
| Yes | 5682 (15.05) | 0.60 (0.55, 0.64) | <0.0001 | 0.67 (0.62, 0.72) | <0.0001 | 0.71 (0.66, 0.77) | <0.0001 |
| No | 2750 (24.49) | Reference | Reference | Reference | |||
| Weight managementc | |||||||
| Yes | 2627 (11.32) | 0.55 (0.50, 0.61) | <0.0001 | 0.65 (0.58, 0.73) | <0.0001 | 0.69 (0.62, 0.78) | <0.0001 |
| No | 945 (19.43) | Reference | Reference | Reference | |||
| Smoking cessationd | |||||||
| Yes | 859 (13.99) | 0.58 (0.49, 0.70) | <0.0001 | 0.65 (0.53, 0.81) | <0.0001 | 0.66 (0.50, 0.88) | 0.004 |
| No | 192 (22.15) | Reference | Reference | Reference | |||
| At 3 y | |||||||
| CR referral | |||||||
| Yes | 4330 (21.80) | 0.42 (0.39, 0.46) | <0.0001 | 0.56 (0.53, 0.60) | <0.0001 | 0.60 (0.57, 0.64) | <0.0001 |
| No | 11 527 (39.57) | Reference | Reference | Reference | |||
| Activity recommendations | |||||||
| Yes | 11 095 (29.38) | 0.62 (0.57, 0.67) | <0.0001 | 0.68 (0.63, 0.73) | <0.0001 | 0.72 (0.67, 0.77) | <0.0001 |
| No | 4762 (42.41) | Reference | Reference | Reference | |||
| Weight managementc | |||||||
| Yes | 5524 (23.81) | 0.60 (0.54, 0.65) | <0.0001 | 0.69 (0.63, 0.76) | <0.0001 | 0.73 (0.67, 0.80) | <0.0001 |
| No | 1709 (35.14) | Reference | Reference | Reference | |||
| Smoking cessationd | |||||||
| Yes | 1787 (29.10) | 0.68 (0.58, 0.79) | <0.0001 | 0.79 (0.66, 0.95) | 0.01 | 0.69 (0.54, 0.88) | 0.003 |
| No | 327 (37.72) | Reference | Reference | Reference | |||
ACE indicates angiotensin‐converting enzyme; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CR, cardiac rehabilitation; GWTG‐CAD, Get With The Guidelines–Coronary Artery Disease; MI, myocardial infarction; NSTEMI, non‐ST‐elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; PVD, peripheral vascular disease; STEMI, ST‐elevation myocardial infarction.
Adjusted for patients sociodemographic and lifestyles information (age, calendar year, BMI, smoking status), patients’ medical history (atrial fibrillation/atrial flutter, COPD or asthma, diabetes mellitus, hyperlipidemia, hypertension, PVD, CAD, prior MI, cerebrovascular accident/transient ischemic attack, implantable cardioverter defibrillator, heart failure, anemia, pacemaker—biventricular/resync/cardiac resynchronization therapy, dialysis [chronic], renal insufficiency, depression, prior PCI or CABG, valvular heart disease, systolic blood pressure at discharge, ejection fraction <40% or moderate or severe dysfunction), cardiac diagnosis (STEMI, NSTEMI, AMI unspecified, unstable angina, CAD), and hospital characteristics (geographic region, rural location, teaching status, number of beds, primary PTCA, cardiac surgery, heart transplants site).
Further adjusted for patients’ insurance status (Medicaid, Medicare, other, No insurance/Undetermined/Missing), medication use before hospital admission (ACE inhibitors, β‐blocker, aspirin, lipid‐lowering agents), medication use at hospital discharge (ACE inhibitors, β‐blocker, aspirin, lipid‐lowering agents), and the GWTG‐CAD quality of care composite performance score.
Among patients with BMI >25 kg/m2 only.
Among smokers only.
Table 4.
Associations of 1‐ or 3‐Y Mortality and Risk Interventions by Sex and Race
| Adjusted OR (95% CI) | P Value | P for Interaction | |
|---|---|---|---|
| 1‐y mortality | |||
| CR referral | |||
| Sex | |||
| Female | 0.58 (0.53, 0.63) | <0.0001 | 0.17 |
| Male | 0.54 (0.49, 0.59) | <0.0001 | |
| Race | |||
| White | 0.55 (0.51, 0.59) | <0.0001 | 0.06 |
| Black | 0.73 (0.60, 0.89) | 0.001 | |
| Hispanic | 0.49 (0.38, 0.63) | <0.0001 | |
| Asian | 0.47 (0.27, 0.80) | 0.005 | |
| Other | 0.66 (0.46, 0.95) | 0.02 | |
| Activity recommendations | |||
| Sex | |||
| Female | 0.78 (0.72, 0.86) | <0.0001 | 0.0009 |
| Male | 0.65 (0.59, 0.71) | <0.0001 | |
| Race | |||
| White | 0.70 (0.65, 0.76) | <0.0001 | 0.52 |
| Black | 0.78 (0.60, 1.00) | 0.05 | |
| Hispanic | 0.80 (0.62, 1.03) | 0.08 | |
| Asian | 0.67 (0.43, 1.04) | 0.07 | |
| Other | 0.85 (0.64, 1.12) | 0.24 | |
| Weight managementa | |||
| Sex | |||
| Female | 0.74 (0.65, 0.85) | <0.0001 | 0.10 |
| Male | 0.65 (0.57, 0.75) | <0.0001 | |
| Race | |||
| White | 0.68 (0.61, 0.77) | <0.0001 | 0.78 |
| Black | 0.65 (0.46, 0.91) | 0.01 | |
| Hispanic | 0.73 (0.49, 1.09) | 0.11 | |
| Asian | 0.84 (0.39, 1.83) | 0.66 | |
| Other | 0.93 (0.58, 1.48) | 0.74 | |
| Smoking cessationb | |||
| Sex | |||
| Female | 0.87 (0.60, 1.26) | 0.46 | 0.04 |
| Male | 0.57 (0.42, 0.78) | 0.0004 | |
| Race | |||
| White | 0.67 (0.50, 0.89) | 0.006 | 0.77 |
| Black | 0.54 (0.24, 1.22) | 0.13 | |
| Hispanic | 0.88 (0.38, 2.04) | 0.76 | |
| Asian | 1.27 (0.46, 3.55) | 0.64 | |
| Other | 0.62 (0.25, 1.51) | 0.29 | |
| 3‐y mortality | |||
| CR referral | |||
| Sex | |||
| Female | 0.61 (0.56, 0.66) | <0.0001 | 0.67 |
| Male | 0.60 (0.55, 0.64) | <0.0001 | |
| Race | |||
| White | 0.60 (0.56, 0.64) | <0.0001 | 0.06 |
| Black | 0.75 (0.63, 0.88) | 0.0005 | |
| Hispanic | 0.62 (0.50, 0.79) | <0.0001 | |
| Asian | 0.63 (0.46, 0.85) | 0.002 | |
| Other | 0.53 (0.42, 0.67) | <0.0001 | |
| Activity recommendations | |||
| Sex | |||
| Female | 0.74 (0.68, 0.81) | <0.0001 | 0.29 |
| Male | 0.70 (0.65, 0.77) | <0.0001 | |
| Race | |||
| White | 0.71 (0.66, 0.77) | <0.0001 | 0.47 |
| Black | 0.80 (0.66, 0.98) | 0.03 | |
| Hispanic | 0.69 (0.54, 0.88) | 0.002 | |
| Asian | 0.73 (0.53, 1.00) | 0.05 | |
| Other | 0.87 (0.67, 1.13) | 0.29 | |
| Weight managementa | |||
| Sex | |||
| Female | 0.73 (0.65, 0.82) | <0.0001 | 0.95 |
| Male | 0.74 (0.66, 0.82) | <0.0001 | |
| Race | |||
| White | 0.72 (0.65, 0.79) | <0.0001 | 0.61 |
| Black | 0.73 (0.56, 0.95) | 0.02 | |
| Hispanic | 0.86 (0.59, 1.24) | 0.41 | |
| Asian | 0.73 (0.39, 1.37) | 0.32 | |
| Other | 1.01 (0.62, 1.63) | 0.98 | |
| Smoking cessationb | |||
| Sex | |||
| Female | 0.72 (0.52, 0.99) | 0.04 | 0.87 |
| Male | 0.70 (0.52, 0.93) | 0.01 | |
| Race | |||
| White | 0.70 (0.54, 0.90) | 0.005 | 0.88 |
| Black | 0.81 (0.39, 1.70) | 0.58 | |
| Hispanic | 0.80 (0.36, 1.78) | 0.58 | |
| Asian | 0.90 (0.53, 1.54) | 0.71 | |
| Other | 0.55 (0.22, 1.33) | 0.18 | |
Adjusted for patients’ sociodemographic and lifestyles information (age, calendar year, BMI, smoking status), patients’ medical history (atrial fibrillation/atrial flutter, COPD or asthma, diabetes mellitus, hyperlipidemia, hypertension, peripheral vascular disease [PVD], CAD, prior MI, cerebrovascular accident/transient ischemic attack, implantable cardioverter defibrillator, heart failure, anemia, pacemaker—biventricular/resync/cardiac resynchronization therapy, dialysis [chronic], renal insufficiency, depression, prior PCI or CABG, valvular heart disease, systolic blood pressure at discharge, ejection fraction <40% or moderate or severe dysfunction), cardiac diagnosis (STEMI, NSTEMI, AMI unspecified, unstable angina, CAD), hospital characteristics (geographic region, rural location, teaching status, number of beds, primary PTCA, cardiac surgery, heart transplants site), patients’ insurance status (Medicaid, Medicare, other, No insurance/Undetermined/Missing), medication use before hospital admission (ACE inhibitors, β‐blocker, aspirin, lipid‐lowering agents), medication use at hospital discharge (ACE inhibitors, β‐blocker, aspirin, lipid‐lowering agents), and the GWTG‐CAD quality of care composite performance score. ACE indicates angiotensin‐converting enzyme; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CR, cardiac rehabilitation; MI, myocardial infarction; NSTEMI, non‐ST‐elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; PTCA; percutaneous transluminal coronary angioplasty; PVD, peripheral vascular disease; STEMI, ST‐elevation myocardial infarction.
Among patients with BMI >25 kg/m2 only.
Among smokers only.
We observed significant sex, racial, and regional differences in CR referral at hospital discharge (Table 5). Females were 12% less likely to receive CR referral compared with males (OR=0.88, 95% confidence interval [CI], 0.85–0.92, Table 5). Compared with white patients, black, Hispanic, and Asian patients were 20%, 36%, and 50% less likely to receive CR referral (P for interaction=0.008). Patients in the Midwest region were 2.5 times more likely to receive CR referral compared with those in the Northeast region. Compared with males, females were less likely to receive physical activity recommendations (OR=0.91, 95% CI, 0.86–0.96). No difference was observed for weight management and smoking cessation.
Table 5.
Sex, Racial, and Regional Differences in CR Referral and Other Risk Interventions
| N Events (%) | Unadjusted OR (95% CI) | P Value | Adjusted OR (95% CI)a | P Value | |
|---|---|---|---|---|---|
| CR referral | |||||
| Sex | |||||
| Female | 8593 (37.10) | 0.79 (0.76, 0.83) | <0.0001 | 0.88 (0.85, 0.92) | <0.0001 |
| Male | 11 267 (43.62) | Reference | Reference | ||
| Race | 0.04 | 0.008 | |||
| Black | 1012 (36.47) | 0.87 (0.78, 0.97) | 0.01 | 0.80 (0.66, 0.98) | 0.02 |
| Hispanic | 752 (30.37) | 1.00 (0.90, 1.10) | 0.94 | 0.64 (0.47, 0.88) | 0.005 |
| Asian | 265 (22.25) | 1.06 (0.90, 1.24) | 0.49 | 0.50 (0.28, 0.90) | 0.02 |
| Other | 662 (33.47) | 1.06 (0.87, 1.28) | 0.56 | 0.88 (0.67, 1.14) | 0.32 |
| White | 17 169 (42.32) | Reference | Reference | ||
| Region | 0.002 | 0.01 | |||
| Midwest | 7370 (53.48) | 2.34 (1.45, 3.78) | 0.0005 | 2.51 (1.38, 4.58) | 0.002 |
| South | 6505 (37.31) | 1.26 (0.79, 1.99) | 0.33 | 1.29 (0.73, 2.30) | 0.38 |
| West | 3246 (37.98) | 1.29 (0.77, 2.14) | 0.33 | 1.58 (0.80, 3.14) | 0.19 |
| Northeast | 2739 (29.67) | Reference | Reference | ||
| Activity recommendations | |||||
| Sex | |||||
| Female | 17 404 (75.14) | 0.91 (0.88, 0.95) | <0.0001 | 0.91 (0.86, 0.96) | 0.001 |
| Male | 20 360 (78.82) | Reference | Reference | ||
| Race | 0.17 | 0.25 | |||
| Black | 2042 (73.59) | 0.95 (0.88, 1.02) | 0.17 | 0.77 (0.59, 1.02) | 0.06 |
| Hispanic | 2023 (81.70) | 1.04 (0.97, 1.12) | 0.30 | 1.28 (0.92, 1.77) | 0.14 |
| Asian | 861 (72.29) | 1.13 (1.00, 1.27) | 0.05 | 1.16 (0.61, 2.19) | 0.64 |
| Other | 1519 (76.79) | 1.07 (0.84, 1.37) | 0.56 | 1.21 (0.87, 1.67) | 0.25 |
| White | 31 319 (77.19) | Reference | Reference | ||
| Region | 0.12 | 0.96 | |||
| Midwest | 10 905 (79.13) | 1.27 (0.82, 1.97) | 0.28 | 1.02 (0.60, 1.73) | 0.95 |
| South | 13 556 (77.76) | 1.55 (1.04, 2.31) | 0.03 | 0.97 (0.53, 1.78) | 0.92 |
| West | 6509 (76.16) | 1.10 (0.71, 1.70) | 0.67 | 1.11 (0.62, 1.99) | 0.72 |
| Northeast | 6794 (73.59) | Reference | Reference | ||
| Weight managementb | |||||
| Sex | |||||
| Female | 9967 (81.42) | 0.93 (0.89, 0.97) | 0.0008 | 0.96 (0.91, 1.01) | 0.11 |
| Male | 13 232 (83.63) | Reference | Reference | ||
| Race | 0.23 | 0.15 | |||
| Black | 1296 (79.95) | 1.00 (0.93, 1.08) | 0.97 | 1.03 (0.93, 1.14) | 0.61 |
| Hispanic | 1291 (86.59) | 1.11 (1.01, 1.21) | 0.02 | 1.16 (1.02, 1.31) | 0.02 |
| Asian | 335 (78.64) | 1.08 (0.92, 1.26) | 0.35 | 1.14 (0.94, 1.39) | 0.17 |
| Other | 913 (82.55) | 0.95 (0.85, 1.07) | 0.42 | 0.99 (0.87, 1.13) | 0.92 |
| White | 19 364 (82.68) | Reference | Reference | ||
| Region | 0.24 | 0.30 | |||
| Midwest | 7053 (82.95) | 1.10 (0.68, 1.79) | 0.68 | 1.50 (0.74, 3.04) | 0.25 |
| South | 8254 (81.93) | 1.52 (0.98, 2.37) | 0.06 | 1.70 (0.86, 3.39) | 0.12 |
| West | 3576 (82.66) | 1.28 (0.78, 2.09) | 0.32 | 2.11 (0.99, 4.49) | 0.05 |
| Northeast | 4316 (83.66) | Reference | Reference | ||
| Smoking cessationa | |||||
| Sex | |||||
| Female | 2632 (88.06) | 1.03 (0.94, 1.14) | 0.51 | 1.07 (0.93, 1.24) | 0.33 |
| Male | 3508 (87.31) | Reference | Reference | ||
| Race | 0.17 | 0.65 | |||
| Black | 437 (91.81) | 1.12 (0.89, 1.41) | 0.34 | 1.09 (0.74, 1.61) | 0.67 |
| Hispanic | 314 (85.56) | 0.96 (0.71, 1.29) | 0.76 | 0.96 (0.61, 1.49) | 0.84 |
| Asian | 90 (73.17) | 0.66 (0.45, 0.99) | 0.04 | 0.73 (0.47, 1.12) | 0.15 |
| Other | 247 (83.45) | 0.81 (0.62, 1.05) | 0.10 | 0.89 (0.62, 1.27) | 0.51 |
| White | 5052 (87.94) | Reference | Reference | ||
| Region | 0.08 | 0.03 | |||
| Midwest | 1628 (87.15) | 0.73 (0.44, 1.21) | 0.21 | 0.56 (0.29, 1.08) | 0.08 |
| South | 2450 (89.06) | 1.08 (0.69, 1.70) | 0.73 | 1.04 (0.55, 1.95) | 0.90 |
| West | 906 (83.50) | 0.68 (0.41, 1.11) | 0.11 | 0.72 (0.38, 1.37) | 0.32 |
| Northeast | 1156 (88.72) | Reference | Reference | ||
ACE indicates angiotensin‐converting enzyme; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CR, cardiac rehabilitation; GWTG‐CAD, Get With The Guidelines–Coronary Artery Disease; MI, myocardial infarction; NSTEMI, non‐ST‐elevation myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; PTCA, percutaneous transluminal coronary angioplasty; PVD, peripheral vascular disease; STEMI, ST‐elevation myocardial infarction.
Among smokers only.
Among patients with BMI >25 kg/m2 only.
Adjusted for patients sociodemographic and lifestyles information (age, calendar year, BMI, smoking status), patients’ medical history (atrial fibrillation/atrial flutter, COPD or asthma, diabetes mellitus, hyperlipidemia, hypertension, peripheral vascular disease [PVD], CAD, prior MI, cerebrovascular accident/transient ischemic attack, implantable cardioverter defibrillator, heart failure, anemia, pacemaker—biventricular/resync/cardiac resynchronization therapy, dialysis [chronic], renal insufficiency, depression, prior PCI or CABG, valvular heart disease, systolic blood pressure at discharge, ejection fraction <40% or moderate or severe dysfunction), cardiac diagnosis (STEMI, NSTEMI, AMI unspecified, unstable angina, CAD), hospital characteristics (geographic region, rural location, teaching status, number of beds, primary PTCA, cardiac surgery, heart transplants site), patients’ insurance status (Medicaid, Medicare, other, No insurance/Undetermined/Missing), medication use before hospital admission (ACE inhibitors, β‐blocker, aspirin, lipid‐lowering agents), medication use at hospital discharge (ACE inhibitors, β‐blocker, aspirin, lipid‐lowering agents), and the GWTG‐CAD quality of care composite performance score.
To examine the proportion of sex, racial, and regional gap in mortality that could be reduced through equitable CR referral, we first examined the association between sex, race, and region with 1‐ and 3‐year mortality (Table S2). We found a racial difference in mortality (P for interaction=0.004 for 1‐year mortality and 0.001 for 3‐year mortality). Compared with white patients, black patients were 1.3 times more likely to die at year 1 and year 3 post hospital discharge (OR=1.31, 95% CI, 1.16–1.47 for 1‐year mortality, OR=1.36, 95% CI: 1.23–1.50 for 3‐year mortality). This elevated mortality risk among black patients persisted regardless of referral to outpatient CR, having received activity recommendations, or weight management at discharge. Mediation analysis results indicated that CR referral was a significant mediator for the association between sex and race with mortality (P for natural indirect effect <0.0001 for women, 0.003 for black, 0.0002 for Hispanic, and 0.002 for Asians patients, Table S3). Results were similar after we accounted for within‐hospital correlations. Seven percent (95% CI, 6–12%) of the black–white mortality gap could potentially be reduced through equitable CR referral.
For the observed inverse associations of CR referral with 3‐year mortality (OR=0.60) to be completely because of unmeasured confounders, the unmeasured confounders would need to be associated with both the CR referral and the mortality by a risk ratio of 3.0‐fold each, above and beyond the measured confounders. The unmeasured confounders needed to be associated with both the risk interventions and the mortality by a risk ratio of 2.3‐fold each for physical activity and weight management and 1.8‐fold each for smoking cessation, to completely explain away the observed lower mortality.
Discussion
In this large cardiovascular disease registry of patients in the United States linked with a database that included long‐term mortality, receipt of CR referral at hospital discharge was significantly inversely associated with all‐cause mortality. Despite improvements in other risk interventions, CR referral rates remained low, particularly for females, black, Hispanics, and Asian patients, who were 12%, 20%, 36%, and 50% less likely to receive CR referral, respectively. Eliminating inequality in CR referral at hospital discharge could potentially reduce long‐term mortality in these patient populations. Specifically, the odds of mortality could be reduced by 40% for women, 25% for black, 38% for Hispanic, and 37% for Asian patients. Seven percent of the black versus white mortality gap could potentially be reduced by equitable CR referral.
The largest randomized trial of CR among patients with MI demonstrated significant benefits in the subsequent risk of nonfatal MI and other cardiovascular outcomes.4, 36 Meta‐analyses suggested that CR participation can reduce all‐cause mortality by 15% to 28% for patients with MI4, 37, 38, 39 and 45% to 47% for patients with percutaneous coronary intervention.40 The effect was estimated to be comparable to improvements associated with aspirin, β‐blocker, angiotensin‐converting enzyme inhibitor, statin, and anticoagulant therapy.5, 41, 42, 43 However, the generalizability of these results was limited because the majority of trial participants were highly selected low‐risk white males, with few females, minorities, or elderly patients, and with short duration of follow‐up.4 Observational studies with long‐term mortality were sparse and mainly focused on CR participation. Prior studies lack detailed clinical and treatment information, data on severity of medical conditions, and information about other quality of care/risk interventions that patients received, which serve as important factors for CR referral and participation.4, 22, 41, 44 Most prior studies were based on claims databases that do not contain information on CR referral. It is unknown whether patients who did not participate in CR therapy did not receive a physician referral, or chose not to participate after referral receipt. Suaya et al used a landmark propensity score analysis as well as 2 instrumental variables derived from the Medicare beneficiary database to estimate the association between CR attendance and mortality. They provided key evidence for a strong inverse association between CR attendance and mortality.22 However, it was unclear how many non‐CR attendance patients did not actually receive CR referral. Hammill et al restricted their analyses in Medicare beneficiaries by examining among patients with at least 1 claim for early outpatient CR services. They reported a significant dose–response relationship between numbers of CR sessions attended and long‐term mortality at 4 years.27
Our study builds upon previous studies by looking at an upstream factor for CR participation: receipt of CR referral at hospital discharge. We assessed the association between inequality in CR referral on patients’ long‐term mortality, a question that has not been previously addressed and could potentially provide beneficial information to policy makers, health professionals, researchers, and patients. The GWTG‐CAD database has detailed and rich information on CR referral, as well as other lifestyle intervention recommendations. By linking the GWTG‐CAD with an outpatient database, we were afforded a unique opportunity to examine the association between CR referral with long‐term cardiovascular mortality above and beyond quality of care received.41, 45 The American College of Cardiology and the American Heart Association have issued a Class I recommendation that certain cardiovascular patients (acute MI, percutaneous coronary intervention, coronary artery bypass grafting, heart valve repair/replacement, heart transplant, coronary artery disease, and angina) should be referred to a CR program before hospital discharge. CR participation rates are alarmingly low, particularly with respect to women and minorities.20 Aragam et al reported a minimal influence of patient demographic factors on rates of CR referral among patients with percutaneous coronary intervention.8 In our study, among eligible cardiovascular patients, we found that females were 15% less likely to receive CR referral than males. Compared with white patients, minorities, particularly Asian patients, were 54% less likely to receive CR referral. Despite great improvements in the institution of other risk interventions as part of quality measures, CR referral remained alarmingly low from 2003 to 2009. In our study, we showed that CR referral at hospital discharge was significantly associated with reduced mortality for women and minorities, a group of patients that have traditionally being understudied in the literature. Prior studies suggested that the most significant predictor and easily overcome barrier is the lack of CR referral at hospital discharge.8 We expanded upon prior study by assessing the degree to which the mortality disparity could be reduced by reducing inequality in CR referral at hospital discharge, using a causal mediation analysis approach. In our study, we found that reducing inequality in CR referral between black and white patients could narrow the mortality gap by 7% in the long term. To the best of our knowledge, no prior study has examined whether and to what extent CR could help reduce cardiovascular health disparities. We also provided evidence that CR referral benefits were independent of other guideline‐based therapies, treatments, and quality of care measures.
The major limitation of our study is that we did not have linkage with Medicare Part B files and do not have information about actual CR enrollment and adherence. Future studies with both CR referral and participation data are needed. Our results were limited because the data were available only up to the year 2009. Beatty et al reported an ≈7% improvement in CR referral from 2007 to 2012 based on the ACTION registry‐GWTG data.46 Future studies using more recent data on CR referral and any association with mortality are needed. Although our sample consisted of a large number of medical centers located all over the United States, participation in GWTG was purely voluntary, and thus these findings may not be reflective of centers that are not included in this quality initiative. Data were collected by medical chart review and depend upon the accuracy and completeness of documentation. Counseling regarding lifestyle interventions might have been provided but not recorded in the medical record. The improvements in performance and quality measures over time might have been influenced by factors other than GWTG‐CAD participation such as secular trends. Because CR rehabilitation referral, physical activity recommendation, weight management, and smoking cessation counseling are part of the GWTG‐quality performance and quality of care measures, CR referral and physical activity recommendations may have some overlap. In practice, physical therapy is also used for CR‐eligible patients who have difficulty accessing CR programs, are disabled, with multiple comorbidities, frail, or have limited cognitive function. Since we do not have data on actual levels of activity, this is not possible to explore further. However, our study indicated that despite the improvements in physical activity recommendations at GWTG database, the CR referral rate remained low from 2003 to 2009. Lastly, there are many reasons a clinician takes into consideration when giving a CR referral. A patient could be too old/sick to benefit from CR (eg, frailty, multiple comorbidities, impaired cognition), or too healthy and may not need CR, or may receive CR referral from an outpatient setting, or may have no access to CR programs.21, 47, 48 Our findings may be impacted by residual measured and unmeasured confounding, particularly by these important patient‐centered factors related to CR referral. Future research incorporating these patient factors in the GWTG and other related registry databases is needed. From our sensitivity analyses, we believe any residual confounding from these factors was unlikely to be strong. Information on race was collected through patients’ self‐report and thus subject to misclassification. However, the misclassification was highly likely to be nondifferential and attenuate the effect estimates toward the null. We believe our study represents a best scenario because participating hospitals are part of the GWTG quality of care improvement program, which aims to improve quality of care.
Our study has several strengths. To the best of our knowledge, there are no other data sources at the national level that follow the continuum of CR from referral at hospital discharge to long‐term outcomes, so linking GWTG data to Medicare data provides a unique opportunity. Second, the analysis of other lifestyle interventions such as physical activity, weight, and smoking cessation within the same study provides an important context for understanding the magnitude of effect of CR referral. Third, the quantification of the inequality in CR referral on subsequent disparities in mortality reveals an important opportunity to save lives by providing equitable care. As far as we are aware, our study provided the first evidence that inequality in receiving CR referral at hospital discharge was associated with patients’ long‐term survival. This information could provide insights on ways to reduce sex and race disparities in cardiovascular health. Our study included patients who were underrepresented in previous studies, including females, black, Hispanic, and Asian patients, and those with more comorbidities. We provided novel insights into the proportion of health disparity that could potentially be reduced through equitable CR referral. Our mediation analysis approach enabled us to address these critical knowledge gaps. Detailed clinical information on ejection fraction, body mass index, smoking, medical treatment, insurance status, and quality of care measures in this unique database also enabled us to control potential confounding more adequately. Additional strengths of our study are the availability of detailed demographic and clinical information before CR referral, and complete ascertainment of long‐term mortality. We used rigorous statistical analytical methods to account for clustering within hospitals and also examined the robustness of our results to unmeasured and residual confounding. With additional adjustment for quality of care received during hospitalization, our results remained the same and showed that the association between CR referral and lower mortality was independent of quality of care received. Our sensitivity analysis showed that this observed association is highly unlikely to be completely caused by other unmeasured factors. Other strengths of our study include long‐term follow‐up and a large sample size with diverse patients and hospital characteristics.
In conclusion, receipt of CR referral at hospital discharge was associated with lower 1‐ and 3‐year mortality. Substantial sex and racial gaps existed for CR referral, especially for females, black, Hispanic, and Asian patients. Eliminating the disparity in CR referral by adopting a policy of providing referrals to all cardiovascular patients at hospital discharge could potentially reduce the racial disparity in long‐term mortality by 7%. CR referral rates urgently need improvement, and national targeted CR quality improvement interventions should be supported.
Sources of Funding
The Get With The Guidelines–Coronary Artery Disease (GWTG–CAD) program was provided by the American Heart Association. The GWTG‐CAD program was supported in part through the American Heart Association Pharmaceutical Roundtable and an unrestricted educational grant from Merck.
Disclosures
Dr Bhatt discloses the following relationships—Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Cleveland Clinic, Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Ironwood, Ischemix, Lilly, Medtronic, Pfizer, Regeneron, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott); Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, Takeda. Dr Fonarow reports being a GWTG Steering Committee member. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Characteristics of Patients and Hospitals Included and Excluded From the Analytic Sample
Table S2. Associations Between Sex, Race, and Geographic Region With 1‐ and 3‐Y All‐Cause Mortality
Table S3. Mediation Analysis by Cardiac Rehabilitation Referral
Acknowledgments
Quintiles, the data collection coordination center for the American Heart Association/American Stroke Association Get With The Guidelines® programs, serves as the data collection (through their Patient Management Tool) and coordination center for GWTG. The Duke Clinical Research Institute (DCRI) serves as the data analysis center and has an agreement to analyze the aggregate de‐identified data for research purposes.
(J Am Heart Assoc. 2018;7:e008088 DOI: 10.1161/JAHA.117.008088.)29626153
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, AlMazroa MA, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker‐Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Abdulhak AB, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FGR, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez‐Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo J‐P, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Memish ZA, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KMV, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez‐Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui‐Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh P‐H, Yip P, Zabetian A, Zheng Z‐J, Lopez AD, Murray CJL. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim GB. Public health: global burden of cardiovascular disease. Nat Rev Cardiol. 2014;11:248. [DOI] [PubMed] [Google Scholar]
- 4. Martin B‐J, Hauer T, Arena R, Austford LD, Galbraith PD, Lewin AM, Knudtson ML, Ghali WA, Stone JA, Aggarwal SG. Cardiac rehabilitation attendance and outcomes in coronary artery disease patients. Circulation. 2012;126:677–687. [DOI] [PubMed] [Google Scholar]
- 5. Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CW. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124:2951–2960. [DOI] [PubMed] [Google Scholar]
- 6. Poffley A, Thomas E, Grace SL, Neubeck L, Gallagher R, Niebauer J, O'Neil A. A systematic review of cardiac rehabilitation registries. Eur J Prev Cardiol. 2017;24:1596–1609. DOI: 10.1177/2047487317724576. [DOI] [PubMed] [Google Scholar]
- 7. Doll JA, Hellkamp A, Ho PM, Kontos MC, Whooley MA, Peterson ED, Wang TY. Participation in cardiac rehabilitation programs among older patients after acute myocardial infarction. JAMA Intern Med. 2015;175:1700–1702. [DOI] [PubMed] [Google Scholar]
- 8. Aragam KG, Dai D, Neely ML, Bhatt DL, Roe MT, Rumsfeld JS, Gurm HS. Gaps in referral to cardiac rehabilitation of patients undergoing percutaneous coronary intervention in the United States. J Am Coll Cardiol. 2015;65:2079–2088. [DOI] [PubMed] [Google Scholar]
- 9. Brown TM, Hernandez AF, Bittner V, Cannon CP, Ellrodt G, Liang L, Peterson ED, Pina IL, Safford MM, Fonarow GC. Predictors of cardiac rehabilitation referral in coronary artery disease patients: findings from the American Heart Association's Get With The Guidelines Program. J Am Coll Cardiol. 2009;54:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, Shepard DS, Thomas RJ. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the Million Hearts Cardiac Rehabilitation Collaborative. Mayo Clin Proc. 2017;92:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Labarthe DR, Goldstein LB, Antman EM, Arnett DK, Fonarow GC, Alberts MJ, Hayman LL, Khera A, Sallis JF, Daniels SR, Sacco RL, Li S, Ku L, Lantz PM, Robinson JG, Creager MA, Van Horn L, Kris‐Etherton P, Bhatnagar A, Whitsel LP. Evidence‐based policy making: assessment of the American Heart Association's strategic policy portfolio: a policy statement from the American Heart Association. Circulation. 2016;133:e615–e653. [DOI] [PubMed] [Google Scholar]
- 12. Barber K, Stommel M, Kroll J, Holmes‐Rovner M, McIntosh B. Cardiac rehabilitation for community‐based patients with myocardial infarction: factors predicting discharge recommendation and participation. J Clin Epidemiol. 2001;54:1025–1030. [DOI] [PubMed] [Google Scholar]
- 13. Gravely‐Witte S, Leung YW, Nariani R, Tamim H, Oh P, Chan VM, Grace SL. Effects of cardiac rehabilitation referral strategies on referral and enrollment rates. Nat Rev Cardiol. 2010;7:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scott LB. Referral to outpatient cardiac rehabilitation: intervention research at the patient, provider, and health system levels. Nat Clin Pract Cardiovasc Med. 2008;5:671–672. [DOI] [PubMed] [Google Scholar]
- 15. Pasquali SK, Alexander KP, Lytle BL, Coombs LP, Peterson ED. Testing an intervention to increase cardiac rehabilitation enrollment after coronary artery bypass grafting. Am J Cardiol. 2001;88:1415–1416, a6. [DOI] [PubMed] [Google Scholar]
- 16. Grace SL, Abbey SE, Shnek ZM, Irvine J, Franche RL, Stewart DE. Cardiac rehabilitation II: referral and participation. Gen Hosp Psychiatry. 2002;24:127–134. [DOI] [PubMed] [Google Scholar]
- 17. Aragam KG, Moscucci M, Smith DE, Riba AL, Zainea M, Chambers JL, Share D, Gurm HS. Trends and disparities in referral to cardiac rehabilitation after percutaneous coronary intervention. Am Heart J. 2011;161:544–551.e2. [DOI] [PubMed] [Google Scholar]
- 18. Suaya JA, Shepard DS, Normand S‐LT, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. [DOI] [PubMed] [Google Scholar]
- 19. Dunlay SM, Witt BJ, Allison TG, Hayes SN, Weston SA, Koepsell E, Roger VL. Barriers to participation in cardiac rehabilitation. Am Heart J. 2009;158:852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peters AE, Keeley EC. Trends and predictors of participation in cardiac rehabilitation following acute myocardial infarction: data from the Behavioral Risk Factor Surveillance System. J Am Heart Assoc. 2018;7:e007664 DOI: 10.1161/JAHA.117.007664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roblin D, Diseker RA III, Orenstein D, Wilder M, Eley M. Delivery of outpatient cardiac rehabilitation in a managed care organization. J Cardiopulm Rehabil. 2004;24:157–164. [DOI] [PubMed] [Google Scholar]
- 22. Suaya JA, Stason WB, Ades PA, Normand SL, Shepard DS. Cardiac rehabilitation and survival in older coronary patients. J Am Coll Cardiol. 2009;54:25–33. [DOI] [PubMed] [Google Scholar]
- 23. Rauch B, Davos CH, Doherty P, Saure D, Metzendorf MI, Salzwedel A, Voller H, Jensen K, Schmid JP. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: a systematic review and meta‐analysis of randomized and non‐randomized studies—the Cardiac Rehabilitation Outcome Study (CROS). Eur J Prev Cardiol. 2016;23:1914–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pack QR, Goel K, Lahr BD, Greason KL, Squires RW, Lopez‐Jimenez F, Zhang Z, Thomas RJ. Participation in cardiac rehabilitation and survival after coronary artery bypass graft surgery: a community‐based study. Circulation. 2013;128:590–597. [DOI] [PubMed] [Google Scholar]
- 25. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS. Exercise‐based cardiac rehabilitation for coronary heart disease: Cochrane systematic review and meta‐analysis. J Am Coll Cardiol. 2016;67:1–12. [DOI] [PubMed] [Google Scholar]
- 26. Santiago de Araujo Pio C, Marzolini S, Pakosh M, Grace SL. Effect of cardiac rehabilitation dose on mortality and morbidity: a systematic review and meta‐regression analysis. Mayo Clin Proc. 2017;92:1644–1659. [DOI] [PubMed] [Google Scholar]
- 27. Hammill BG, Curtis LH, Schulman KA, Whellan DJ. Relationship between cardiac rehabilitation and long‐term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation. 2010;121:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewis WR, Peterson ED, Cannon CP, Super DM, LaBresh KA, Quealy K, Liang L, Fonarow GC. An organized approach to improvement in guideline adherence for acute myocardial infarction: results with the Get With The Guidelines Quality Improvement Program. Arch Intern Med. 2008;168:1813–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boyer NM, Laskey WK, Cox M, Hernandez AF, Peterson ED, Bhatt DL, Cannon CP, Fonarow GC. Trends in clinical, demographic, and biochemical characteristics of patients with acute myocardial infarction from 2003 to 2008: a report from the American Heart Association Get With The Guidelines Coronary Artery Disease Program. J Am Heart Assoc. 2012;1:e001206 DOI: 10.1161/JAHA.112.001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brennan JM, Peterson ED, Messenger JC, Rumsfeld JS, Weintraub WS, Anstrom KJ, Eisenstein EL, Milford‐Beland S, Grau‐Sepulveda MV, Booth ME, Dokholyan RS, Douglas PS. Linking the National Cardiovascular Data Registry CathPCI Registry with Medicare claims data: validation of a longitudinal cohort of elderly patients undergoing cardiac catheterization. Circ Cardiovasc Qual Outcomes. 2012;5:134–140. [DOI] [PubMed] [Google Scholar]
- 32. Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. SAS Paper 335‐2012. 2012; Cleveland, OH. [Google Scholar]
- 33. Valeri L, VanderWeele TJ. SAS macro for causal mediation analysis with survival data. Epidemiology. 2015;26:e23–e24. [DOI] [PubMed] [Google Scholar]
- 34. VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology. 2014;25:473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268–274. [DOI] [PubMed] [Google Scholar]
- 36. Giannuzzi P, Temporelli PL, Marchioli R, Maggioni AP, Balestroni G, Ceci V, Chieffo C, Gattone M, Griffo R, Schweiger C, Tavazzi L, Urbinati S, Valagussa F, Vanuzzo D. Global secondary prevention strategies to limit event recurrence after myocardial infarction: results of the GOSPEL study, a multicenter, randomized controlled trial from the Italian Cardiac Rehabilitation Network. Arch Intern Med. 2008;168:2194–2204. [DOI] [PubMed] [Google Scholar]
- 37. Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise‐based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2001:Cd001800. [DOI] [PubMed] [Google Scholar]
- 38. Clark AM, Hartling L, Vandermeer B, McAlister FA. Meta‐analysis: secondary prevention programs for patients with coronary artery disease. Ann Intern Med. 2005;143:659–672. [DOI] [PubMed] [Google Scholar]
- 39. Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:945–950. [PubMed] [Google Scholar]
- 40. Goel K, Lennon RJ, Tilbury RT, Squires RW, Thomas RJ. Impact of cardiac rehabilitation on mortality and cardiovascular events after percutaneous coronary intervention in the community. Circulation. 2011;123:2344–2352. [DOI] [PubMed] [Google Scholar]
- 41. Bittner V. Cardiac rehabilitation: call to action for healthcare providers. Circulation. 2012;126:671–673. [DOI] [PubMed] [Google Scholar]
- 42. Thomas RJ, King M, Lui K, Oldridge N, Pina IL, Spertus J, Bonow RO, Estes NA III, Goff DC, Grady KL, Hiniker AR, Masoudi FA, Radford MJ, Rumsfeld JS, Whitman GR. AACVPR/ACC/AHA 2007 performance measures on cardiac rehabilitation for referral to and delivery of cardiac rehabilitation/secondary prevention services endorsed by the American College of Chest Physicians, American College of Sports Medicine, American Physical Therapy Association, Canadian Association of Cardiac Rehabilitation, European Association for Cardiovascular Prevention and Rehabilitation, Inter‐American Heart Foundation, National Association of Clinical Nurse Specialists, Preventive Cardiovascular Nurses Association, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2007;50:1400–1433. [DOI] [PubMed] [Google Scholar]
- 43. Ford ES, Ajani UA, Croft JB, Critchley JA, Labarthe DR, Kottke TE, Giles WH, Capewell S. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. [DOI] [PubMed] [Google Scholar]
- 44. Witt BJ, Jacobsen SJ, Weston SA, Killian JM, Meverden RA, Allison TG, Reeder GS, Roger VL. Cardiac rehabilitation after myocardial infarction in the community. J Am Coll Cardiol. 2004;44:988–996. [DOI] [PubMed] [Google Scholar]
- 45. Polk DM, O'Gara PT. Closing the treatment gap for cardiac rehabilitation. JAMA Intern Med. 2015;175:1702–1703. [DOI] [PubMed] [Google Scholar]
- 46. Beatty AL, Li S, Thomas L, Amsterdam EA, Alexander KP, Whooley MA. Trends in referral to cardiac rehabilitation after myocardial infarction: data from the National Cardiovascular Data Registry 2007 to 2012. J Am Coll Cardiol. 2014;63:2582–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gurewich D, Prottas J, Bhalotra S, Suaya JA, Shepard DS. System‐level factors and use of cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2008;28:380–385. [DOI] [PubMed] [Google Scholar]
- 48. Gaalema DE, Higgins ST, Shepard DS, Suaya JA, Savage PD, Ades PA. State‐by‐state variations in cardiac rehabilitation participation are associated with educational attainment, income, and program availability. J Cardiopulm Rehabil Prev. 2014;34:248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of Patients and Hospitals Included and Excluded From the Analytic Sample
Table S2. Associations Between Sex, Race, and Geographic Region With 1‐ and 3‐Y All‐Cause Mortality
Table S3. Mediation Analysis by Cardiac Rehabilitation Referral


