Abstract
Background
Contemporary data on patients presenting with acute limb ischemia (ALI), who are selected for treatment with endovascular peripheral vascular interventions (PVI), are limited. Our study examined outcomes following endovascular PVI in patients with ALI by comparing with patients treated for chronic critical limb ischemia using a regional quality improvement registry.
Methods and Results
Of the 11 035 patients in the Vascular Study Group of New England PVI database (2010–2014), we identified 365 patients treated for lower extremity ALI who were 5:1 frequency matched (by procedure year and arterial segments treated) to 1808 patients treated for critical limb ischemia. ALI patients treated with PVI had high burden of atherosclerotic risk factors and were more likely to have had prior ipsilateral revascularizations. ALI patients were less likely to be treated with self‐expanding stents and more likely to undergo thrombolysis than patients with critical limb ischemia. In multivariable analysis, ALI was associated with higher technical failure (odds ratio 1.7, 95% confidence interval, 1.1%–2.5%), increased rate of distal embolization (odds ratio 2.7, 95% confidence interval, 1.5%–4.9%), longer length of stay (means ratio 1.6, 95% confidence interval, 1.4%–1.8%), and higher in‐hospital mortality (odds ratio 2.8, 95% confidence interval, 1.3%–5.9%). ALI was not associated with risk of major amputation or mortality at 1 year.
Conclusions
In a multicenter cohort of patients treated with PVI, we found that ALI patients selected for treatment with endovascular techniques experienced greater short‐term adverse events but similar long‐term outcomes as their critical limb ischemia counterparts. Further studies are needed to refine the selection of ALI patients who are best served by PVI.
Keywords: intervention, peripheral artery disease, registry
Subject Categories: Peripheral Vascular Disease, Quality and Outcomes
Clinical Perspective
What Is New?
Using a prospective, multicenter regional registry, we compared the treatment and outcomes of patients with acute limb ischemia (ALI) versus chronic limb ischemia who were treated with endovascular peripheral vascular interventions (PVI).
Patients with ALI treated with PVI had a high prevalence of atherosclerotic risk factors and prior limb revascularization.
Patients with ALI treated with PVI had higher in‐hospital event rates including technical failure, distal embolization and mortality, but similar major amputation and mortality at 1 year compared with their chronic limb ischemia counterparts.
Female sex, smoking, prior cardiovascular disease, emergent case, and thrombolysis were associated with higher in‐hospital adverse outcomes among ALI patients.
What Are the Clinical Implications?
In a large observational cohort of patients treated with endovascular therapy, contemporary endovascular techniques result in greater short‐term adverse events but equivalent long‐term outcomes in ALI patients compared with chronic limb ischemia.
Further studies are needed to improve the risk stratification and selection of ALI patients who will benefit from endovascular therapy.
Introduction
Patients with peripheral arterial disease are at risk of acute limb ischemia (ALI), a challenging vascular emergency caused by an abrupt occlusion of the main arterial supply to the involved extremity.1 This condition is associated with high morbidity and mortality as prolonged ischemia can threaten tissue viability and potentially result in a loss of limb or life.2, 3 Thus, prompt diagnosis followed by rapid restoration of blood flow to the ischemic extremity are paramount in the management of ALI.4
Traditionally, ALI has been treated with immediate systemic anticoagulation and subsequent urgent open vascular revascularization using thromboembolectomy or bypass procedure. Yet, the risks of perioperative adverse events and postoperative death associated with operative revascularization performed in the setting of ALI remain high.5 With the advent of endovascular techniques and following the publication of randomized controlled trials in the 1990s suggesting that percutaneous thrombolysis is a less‐invasive yet comparable alternative to operative revascularization,6, 7, 8, 9, 10, 11, 12 ALI has been increasingly managed with endovascular peripheral vascular interventions (PVI) rather than open surgical approaches.13, 14
Despite this recent shift toward PVI over the past few decades, contemporary data on patients presenting with lower extremity ALI, who are selected for treatment with PVI, from large studies are sparse. Therefore, the objectives of this study were to characterize this specific subset of patients presenting with lower extremity ALI and elucidate not only the treatment modalities but also clinical outcomes following PVI by using a regional quality improvement registry. We specifically sought to use the Vascular Study Group of New England PVI database to provide “real world” contemporary data in order to further evaluate the outcomes following PVI in patients with lower extremity ALI by comparing with patients treated for chronic critical limb ischemia (CLI).
Methods
Study Design
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure; however, the data may be available to investigators through the Vascular Study Group of New England (VSGNE). The VSGNE is a regional cooperative quality improvement initiative developed in 2002 to prospectively collect and study outcomes in patients undergoing vascular and endovascular surgery in participating academic and community hospitals across all 6 New England states. The VSGNE registry collects data by procedure type, and we specifically used the VSGNE PVI database as the outcomes of ALI from its infra‐inguinal bypass database have been previously reported.5 Details regarding this registry have been previously described15 and are available at http://www.vsgne.org. The Institutional Review Board at Boston University School of Medicine waived the need for patient informed consent and approved the use of de‐identified data for this study.
Patient Population
We identified all consecutive patients who underwent PVI by surgeons practicing at more than 30 academic and community hospitals in the New England area between 2010 and 2014. Indications for PVI are classified as asymptomatic, claudication, rest pain, tissue loss, and acute ischemia. Figure 1 shows the flowchart for the patient selection process for our study. Patients who underwent PVI procedures for indication of ALI (indication for index procedure listed as acute ischemia; n=367) were included in this study and compared with patients treated for CLI (indication for index procedure listed as rest pain or tissue loss; n=3649), as these patients with advanced peripheral arterial disease were more likely to be managed with similar endovascular techniques as patients presenting with ALI. Patients with aneurysmal disease, asymptomatic disease, or claudication were excluded. The study also excluded patients with ALI who were listed to have undergone “elective” PVI and those with insufficient outcome data. Finally, patients who underwent concomitant PVI and leg bypass are not included in this study as these patients are included in the VSGNE infrainguinal bypass database and not included in the PVI database.
Figure 1.
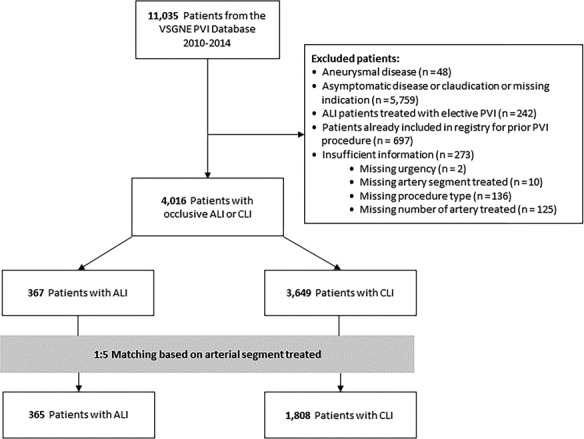
Flowchart of the patient selection process for the study. Each patient who underwent PVI for ALI was matched with 5 patients who underwent PVI for CLI for arterial segment treated: aortoiliac (aorta/iliac/common femoral arteries), femoropopliteal (superficial femoral/profunda/popliteal arteries), and infrapopliteal (anterior tibial/tibioperoneal trunk/posterior tibial/peroneal arteries) segments. ALI indicates acute limb ischemia; CLI, chronic critical limb ischemia; PVI, peripheral vascular interventions; VSGNE, Vascular Study Group of New England.
Outcome and Variable Definitions
In our analysis, we reviewed patient demographics, pre‐existing medical comorbidities, periprocedural details, and postprocedural outcomes. More than 100 demographic and clinical variables were collected prospectively by trained abstractors for each procedure and entered in the VSGNE registry. Definitions of medical comorbidities and index procedural details within the VSGNE have been previously published.15 Our primary outcome measures included major amputation and mortality up to 1 year postoperatively. In‐person follow‐up was required for the assessment of re‐interventions, such as major amputation. Follow‐up for mortality was based on data abstracted from Social Security records.15, 16 We also evaluated in‐hospital outcomes, such as technical failure, postprocedural complications (specifically arterial dissection, arterial perforation, distal embolization, puncture site hematoma, and access site occlusion), postprocedural hospital length of stay (LOS), and in‐hospital mortality as secondary end points.
Statistical Analysis
The aim of the study was to compare outcomes based on the indication (ALI versus CLI) of index procedure among patients who underwent PVI. Cohort frequency group matching was performed to select patients for comparison between the 2 groups. Each patient who underwent PVI for ALI was matched with 5 patients who underwent PVI for CLI. The matching factors were procedure year and arterial segments treated: aortoiliac (aorta/iliac/common femoral arteries), femoropopliteal (superficial femoral/profunda/popliteal arteries), and infrapopliteal (anterior tibial/tibioperoneal trunk/posterior tibial/peroneal arteries) segments. These factors uniquely defined patient strata for matching procedure. These matches were selected randomly from each stratum of CLI patients with the same values of matching variables. The ratio of 5 (CLI) to 1 (ALI) in the cohort match was based on the available set of patients and chosen to reduce potential bias caused by covariates when estimating effects from observational data.17 As shown in Figure 1, the final cohort after frequency group matching included a total of 2173 patients who underwent PVI, of which 365 patients had an indication for ALI and 1808 patients had an indication for CLI. All analyses were performed in the matched cohorts.
We compared the baseline patient demographics, clinical parameters, and procedural variables of the 2 groups by using the χ2 test for categorical variables and the Student t test for continuous variables. Those variables with P<0.05 and those considered to be clinically significant were entered into the multivariable regression models. The following variables were considered as possible confounders: age, sex, current smoking status, diabetes mellitus, chronic obstructive pulmonary disease, dialysis, use of aspirin and statin, and previous ipsilateral PVI and/or leg bypass. Logistic regression was used for technical failure, distal embolization, and in‐hospital mortality. Cox proportional hazard model was used to compare 1‐year major amputation and 1‐year mortality. Gamma regression was used to analyze postprocedural hospital LOS once early hospital deaths were excluded.
We then explored factors independently predictive of technical failure, distal embolization, hospital LOS, and in‐hospital mortality among ALI patients treated with PVI (excluding the CLI patients from the model). The following factors were considered: age per 5 years, sex, current smoking status, diabetes mellitus, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, use of aspirin and statin, previous ipsilateral PVI, and procedural characteristics (including arterial segment treated, emergency status, and procedure type). Logistic regression was used to predict technical failure, distal embolization, and in‐hospital mortality. Gamma regression was used to predict postprocedural hospital LOS. Analysis was performed with SAS 9.2 Software (SAS Institute Inc, Cary, NC). P<0.05 was defined as statistically significant.
Results
Patient Characteristics
After matching, a total of 2173 patients who underwent PVI procedures were included in the overall study cohort. Baseline patient demographics, medical comorbidities, and procedural details are demonstrated in Table 1. The mean age of the patients was 69±12 years, and 57% were male. Ninety percent of patients were white, and 35% were current smokers. Thirty‐three percent and 16% of patients had a history of previous ipsilateral PVI and leg bypass, respectively. Treatment types included percutaneous transluminal angioplasty (79%), self‐expanding stents (41%), balloon‐expanding stents (16%), stent grafts (11%), and thrombolysis (8%).
Table 1.
Demographics, Clinical, and Procedural Variables of ALI and CLI Patients
| Variables | Overall (n=2173) | ALI (n=365) | CLI (n=1808) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y | 69±12 | 67±12 | 70±12 | <0.001 |
| Male sex, n (%) | 1240 (57) | 195 (53) | 1045 (58) | 0.124 |
| White race, n (%) | 1948 (90) | 334 (91) | 1614 (89) | 0.201 |
| Current smoking, n (%) | 784 (35) | 173 (47) | 575 (32) | <0.001 |
| Clinical parameters | ||||
| Hypertension, n (%) | 1945 (89) | 317 (87) | 1628 (90) | 0.069 |
| Diabetes mellitus, n (%) | 1238 (57) | 164 (45) | 1074 (59) | <0.001 |
| CAD, n (%) | 777 (36) | 129 (35) | 648 (36) | 0.851 |
| CHF, n (%) | 515 (24) | 79 (22) | 436 (24) | 0.311 |
| COPD, n (%) | 579 (27) | 122 (33) | 457 (25) | 0.001 |
| Dialysis, n (%) | 243 (11) | 25 (7) | 218 (12) | 0.004 |
| Renal insufficiency, n (%) | 168 (9) | 29 (9) | 139 (9) | 0.944 |
| Aspirin use, n (%) | 1642 (76) | 248 (68) | 1394 (77) | <0.001 |
| Statin use, n (%) | 1481 (68) | 224 (61) | 1257 (70) | 0.002 |
| β‐Blocker use, n (%) | 1420 (65) | 227 (62) | 1193 (66) | 0.161 |
| Chronic anticoagulant use, n (%) | 341 (16) | 66 (18) | 275 (15) | 0.167 |
| Previous ipsilateral PVI, n (%) | 724 (33) | 141 (39) | 583 (32) | 0.019 |
| Previous ipsilateral bypass, n (%) | 340 (16) | 75 (20) | 265 (15) | 0.005 |
| Urgency | ||||
| Elective, n (%) | 1269 (58) | 0 (0) | 1269 (70) | <0.001 |
| Urgent, n (%) | 781 (36) | 259 (71) | 522 (29) | <0.001 |
| Emergent, n (%) | 123 (6) | 106 (29) | 17 (1) | <0.001 |
| Procedural details | ||||
| Arterial segment treateda | ||||
| Aortoiliac, n (%) | 983 (45) | 166 (46) | 817 (45) | 0.919 |
| Femoropopliteal, n (%) | 1088 (50) | 183 (50) | 905 (50) | 0.977 |
| Infrapopliteal, n (%) | 727 (34) | 123 (34) | 604 (33) | 0.914 |
| Number of arteries treated | ||||
| 1, n (%) | 1002 (46) | 175 (48) | 827 (46) | 0.454 |
| >2, n (%) | 1171 (54) | 190 (52) | 981 (54) | 0.454 |
| Procedure type | ||||
| PTA, n (%) | 1710 (79) | 286 (78) | 1424 (79) | 0.863 |
| Self‐expand stent, n (%) | 885 (41) | 120 (33) | 765 (42) | 0.001 |
| Balloon‐expand stent, n (%) | 339 (16) | 59 (16) | 280 (16) | 0.745 |
| Stent graft, n (%) | 240 (11) | 48 (13) | 192 (11) | 0.159 |
| Thrombolysis, n (%) | 175 (8) | 90 (25) | 85 (5) | <0.001 |
| Cryoplasty, n (%) | 11 (1) | 2 (1) | 9 (1) | 0.902 |
| Cutting balloon, n (%) | 95 (4) | 24 (7) | 71 (4) | 0.024 |
| Laser atherectomy, n (%) | 34 (2) | 8 (2) | 26 (1) | 0.290 |
| Mechanical atherectomy | ||||
| Orbital, n (%) | 169 (8) | 28 (8) | 141 (8) | 0.934 |
| Excisional, n (%) | 37 (2) | 4 (1) | 33 (2) | 0.326 |
ALI indicates acute limb ischemia; CAD, coronary artery disease; CHF, congestive heart failure; CLI, chronic critical limb ischemia; COPD, chronic obstructive pulmonary disease; PTA, percutaneous transluminal angioplasty; PVI, peripheral vascular interventions.
Aortoiliac, aorta/iliac/common femoral arteries; femoropopliteal, superficial femoral/profunda/popliteal arteries; infrapopliteal, anterior tibial/tibial‐peroneal trunk/posterior tibial/peroneal artery.
Of the 2173 patients, 365 patients underwent PVI for indication of ALI, whereas 1808 patients underwent PVI for indication of CLI in the matched set. ALI patients who were treated with PVI were still an older group of patients with the mean age of 67±12 years and had a high burden of atherosclerotic risk factors, such as current tobacco use, hypertension, and diabetes mellitus. Several patients have had previous ipsilateral revascularizations, including PVI or bypass. In terms of endovascular procedure types, a large majority (78%) underwent percutaneous transluminal angioplasty, and about 25% were treated with thrombolysis (Table 1).
Compared with CLI patients, ALI patients treated with PVI were more likely to be current smokers and less likely to be treated with aspirin and statin. They were also more likely to have had prior ipsilateral revascularizations, including PVI (39% versus 32%, P=0.019) and leg bypass (20% versus 15%, P=0.005). Furthermore, ALI patients were less likely to be treated with self‐expanding stents (33% versus 42%, P=0.001) and more likely to undergo thrombolysis (25% versus 5%, P<0.001) than CLI patients (Table 1).
In‐Hospital and 1‐Year Outcomes
Comparison of in‐hospital outcomes between the ALI and CLI groups are illustrated in Figure 2. ALI patients treated with PVI experienced worse in‐hospital outcomes as compared with CLI patients. Specifically, these patients had higher technical failure (11% versus 8%, P=0.029) with increased rate of distal embolization (5% versus 2%, P=0.001). They also had longer postprocedural hospital LOS (mean 6±8 days versus 4±6 days, P<0.001) and higher in‐hospital mortality (4% versus 1%, P=0.001). No significant differences in the rates of arterial dissection, arterial perforation, puncture site hematoma, and access site occlusion were identified between the 2 groups.
Figure 2.
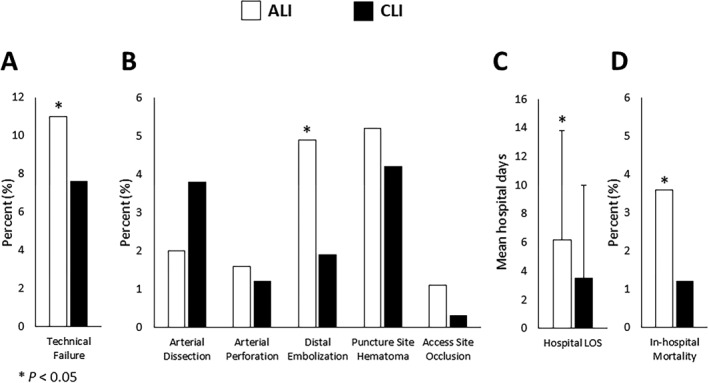
Comparison of in‐hospital outcomes, including (A) technical failure, (B) postprocedural complications, (C) postprocedural hospital length of stay, and (D) in‐hospital mortality between ALI and CLI groups. ALI indicates acute limb ischemia, white bar; CLI, chronic critical limb ischemia, black bar; LOS, length of stay.
Kaplan–Meier survival curves for amputation‐free survival and overall survival at 1‐year follow‐up are presented in Figure 3. No differences in 1‐year outcomes were found between the ALI and CLI groups with respect to rates of major amputation (6% versus 6%, P=0.7) and mortality (15% versus 15%, P=0.9).
Figure 3.
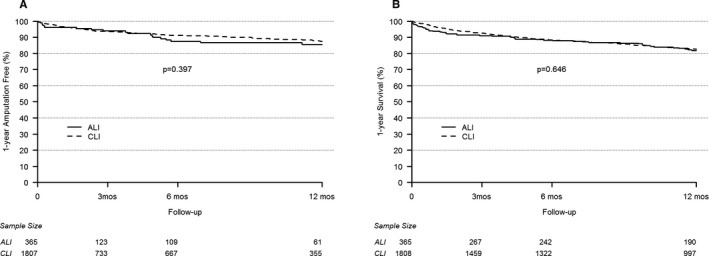
Kaplan–Meier survival curves for (A) amputation‐free survival and (B) overall survival at 1 year. ALI indicates acute limb ischemia, solid line; CLI, chronic critical limb ischemia, dotted line.
On multivariable analysis as shown in Figure 4, ALI continued to be associated with worse in‐hospital outcomes, including higher technical failure (odds ratio [OR] 1.7, 95% confidence interval [CI], 1.1%–2.5%, P=0.008), increased rate of distal embolization (OR 2.7, 95% CI, 1.5%–4.9%, P=0.001), longer postprocedural hospital LOS (mean ratio 1.6, 95% CI, 1.4%–1.8%, P<0.001), and higher in‐hospital mortality (OR 2.8, 95% CI, 1.3%–5.9%, P=0.007). However, there was still no association between ALI and 1‐year major amputation (hazard ratio 1.2, 95% CI, 0.8%–2.0%, P=0.353) or 1‐year mortality (hazard ratio 1.2, 95% CI, 0.9%–1.6%, P=0.2).
Figure 4.
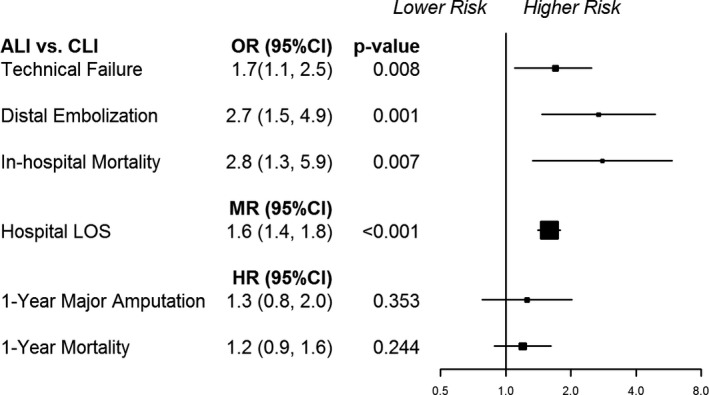
Multivariable analysis of in‐hospital and 1‐year outcomes. ALI indicates acute limb ischemia; CI, confidence interval; CLI, chronic critical limb ischemia; HR, hazard ratio; LOS, length of stay; MR, mean ratio; OR, odds ratio.
Independent Predictors of In‐Hospital Adverse Events Among ALI Patients
Table 2 demonstrates variables that were independently associated with increased odds of in‐hospital adverse events following PVI among ALI patients. The female sex (OR 2.4, 95% CI, 1.1%–5.0%, P=0.026), history of coronary artery disease (OR 2.4, 95% CI, 1.0–5.7, P=0.044), emergent case (OR 2.4, 95% CI, 1.0–5.6, P=0.038), and thrombolysis use (OR 2.7, 95% CI, 1.3–6.0, P=0.011) were predictive of higher technical failure. Use of self‐expanding stent was protective against technical failure (OR 0.2, 95% CI, 0.1–0.5, P=0.001). Use of thrombolysis (OR 10.3, 95% CI, 3.0%–35.1%, P<0.001), stent graft (OR 5.6, 95% CI, 1.1%–28.7%, P=0.038), and balloon‐expanding stent (OR 5.6, 95% CI, 1.1%–29.5%, P=0.043) were associated with increased rate of distal embolization. History of congestive heart failure (mean ratio 1.4, 95% CI, 1.1%–1.7%, P=0.008), emergent case (mean ratio 1.4, 95% CI, 1.1%–1.7%, P=0.004), and aortoiliac disease (mean ratio 1.7, 95% CI, 1.3%–2.1%, P<0.001) were associated with longer postprocedural hospital LOS. Finally, emergent case (OR 5.5, 95% CI, 1.4%–22.2%, P=0.017) was an independent predictor of increased in‐hospital mortality following PVI among ALI patients.
Table 2.
Predictors of In‐Hospital Adverse Events in ALI Patients Treated With PVI
| Variables | Technical Failure | Distal Embolization | Hospital LOS | In‐Hospital Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | MR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age per 5 y | 0.9 | 0.7 to 1.0 | 0.084 | 1.0 | 0.8 to 1.4 | 0.716 | 1.0 | 0.9 to 1.0 | 0.189 | 1.4 | 1.0 to 2.1 | 0.086 |
| Female sex | 2.4 | 1.1 to 5.0 | 0.026 | 2.1 | 0.7 to 6.8 | 0.200 | 1.0 | 0.8 to 1.2 | 0.860 | 1.9 | 0.5 to 8.0 | 0.370 |
| Current smoker | 0.5 | 0.2 to 1.2 | 0.135 | 1.5 | 0.4 to 6.1 | 0.548 | 0.9 | 0.7 to 1.1 | 0.325 | 4.8 | 0.9 to 26.8 | 0.070 |
| Diabetes mellitus | 0.8 | 0.4 to 2.0 | 0.697 | 2.1 | 0.6 to 7.0 | 0.242 | 1.1 | 0.9 to 1.4 | 0.336 | 1.6 | 0.4 to 6.8 | 0.556 |
| CAD | 2.4 | 1.0 to 5.7 | 0.044 | 0.4 | 0.1 to 1.8 | 0.245 | 1.0 | 0.8 to 1.2 | 0.950 | 1.6 | 0.3 to 7.3 | 0.571 |
| CHF | 1.2 | 0.4 to 3.0 | 0.739 | 0.8 | 0.2 to 3.9 | 0.800 | 1.4 | 1.1 to 1.7 | 0.008 | 4.8 | 1.0 to 23.7 | 0.054 |
| COPD | 0.4 | 0.2 to 1.1 | 0.075 | 0.6 | 0.2 to 2.5 | 0.495 | 1.0 | 0.8 to 1.2 | 0.630 | 0.5 | 0.1 to 2.3 | 0.398 |
| Aspirin use | 0.8 | 0.4 to 2.1 | 0.728 | 1.3 | 0.4 to 4.9 | 0.657 | 1.1 | 0.9 to 1.3 | 0.526 | 0.5 | 0.1 to 2.1 | 0.327 |
| Statin use | 0.8 | 0.3 to 1.8 | 0.594 | 1.5 | 0.4 to 5.4 | 0.538 | 0.8 | 0.7 to 1.0 | 0.058 | 0.8 | 0.2 to 4.0 | 0.822 |
| Previous PVI | 0.8 | 0.4 to 1.9 | 0.706 | 0.4 | 0.1 to 1.6 | 0.208 | 0.8 | 0.6 to 1.0 | 0.032 | 0.1 | 0.1 to 0.8 | 0.033 |
| Emergent case | 2.4 | 1.0 to 5.6 | 0.038 | 1.8 | 0.5 to 6.1 | 0.342 | 1.4 | 1.1 to 1.7 | 0.004 | 5.5 | 1.4 to 22.2 | 0.017 |
| PTA | 0.9 | 0.3 to 2.9 | 0.830 | 1.2 | 0.3 to 4.5 | 0.808 | 1.2 | 0.9 to 1.6 | 0.138 | 0.9 | 0.2 to 4.6 | 0.876 |
| Self‐expand stent | 0.2 | 0.1 to 0.5 | 0.001 | 1.8 | 0.5 to 6.1 | 0.327 | 1.0 | 0.8 to 1.2 | 0.914 | 0.3 | 0.1 to 1.7 | 0.171 |
| Balloon‐expand stent | 0.4 | 0.1 to 1.6 | 0.175 | 5.6 | 1.1 to 29.5 | 0.043 | 0.8 | 0.6 to 1.2 | 0.316 | 0.6 | 0.1 to 5.1 | 0.660 |
| Stent graft | 0.3 | 0.1 to 1.1 | 0.078 | 5.6 | 1.1 to 28.7 | 0.038 | 0.9 | 0.7 to 1.2 | 0.542 | 1.0 | 0.1 to 8.1 | 0.971 |
| Cutting balloon | 1.3 | 0.3 to 5.7 | 0.709 | 1.0 | 0.1 to 7.0 | 0.986 | 1.0 | 0.6 to 1.6 | 0.901 | 2.8 | 0.2 to 38.4 | 0.447 |
| Mechanical atherectomy | 0.6 | 0.1 to 2.1 | 0.382 | 1.8 | 0.3 to 10.8 | 0.532 | 1.0 | 0.7 to 1.4 | 0.879 | 1.3 | 0.2 to 11.2 | 0.812 |
| Thrombolysis | 2.7 | 1.3 to 6.0 | 0.011 | 10.3 | 3.0 to 35.1 | <0.001 | 0.9 | 0.8 to 1.2 | 0.601 | 1.1 | 0.2 to 5.7 | 0.866 |
| Aortoiliac | 0.8 | 0.3 to 2.0 | 0.649 | 0.3 | 0.1 to 1.2 | 0.085 | 1.7 | 1.3 to 2.1 | <0.001 | 2.4 | 0.4 to 13.6 | 0.331 |
ALI indicates acute limb ischemia; CAD, coronary artery disease; CHF, congestive heart failure; CI, confidence interval; COPD, chronic obstructive pulmonary disease; LOS, length of stay; MR, mean ratio; OR, odds ratio; PTA, percutaneous transluminal angioplasty; PVI, peripheral vascular interventions.
Discussion
In the present study, we examined the clinical characteristics, treatment modalities, and outcomes of patients treated with endovascular PVI for the indication of lower extremity ALI in a contemporary regional registry. We found that patients presenting with ALI who are selected for treatment with PVI were individuals with high burden of atherosclerotic risk factors. ALI patients were likely to have had prior ipsilateral revascularizations, including PVI and leg bypass, as compared with CLI patients. Procedurally, both ALI and CLI patients were treated frequently with percutaneous transluminal angioplasty, and ALI patients were more likely to undergo thrombolysis than CLI patients. Following PVI, ALI patients experienced worse in‐hospital outcomes—specifically technical failure, distal embolization, postprocedural hospital LOS, and in‐hospital mortality—but equivalent rates of major amputation and mortality at 1 year as compared with their CLI counterparts. Finally, we found that female sex, current smoking status, history of coronary artery disease or congestive heart failure, emergent case, and thrombolysis use were independent predictors of worse in‐hospital outcomes among ALI patients.
Clinical characteristics of ALI patients selected for treatment with PVI described in our study parallel those presented in prior literature emerging in the past few years. Baril et al reviewed data from the 1998–2009 Medicare Provider Analysis & Review (MedPAR) files and demonstrated that patients hospitalized for management of ALI had high burden of known atherosclerotic risk factors and noted a significant rise in the prevalence of these comorbidities, including diabetes mellitus, hypertension, hyperlipidemia, and atrial fibrillation over the past decade.14 Additionally, past studies showed that patients presenting with ALI often have prior history of previous ipsilateral revascularizations, consistent with underlying advanced peripheral arterial disease in these patients.1, 18, 19, 20, 21
Recent single‐center studies suggest favorable outcomes of ALI patients treated with PVI. In this comprehensive multicenter registry, we found a technical failure rate of 11% among ALI patients treated with PVI, which is comparable to those previously reported.18, 20, 21 One study found a technical failure rate of 1.6%,19 but this lower rate may reflect the difference in endovascular treatment techniques used. Some single‐center studies18, 20, 21 utilized catheter‐direct thrombolysis with recombinant tissue plasminogen activator±percutaneous mechanical thrombectomy as the initial intervention in all of their ALI patients, while Linnemann et al19 included ALI patients treated by a multitude of techniques, such as mechanical thrombectomy, catheter thrombus aspiration, local thrombolysis, angioplasty, stent placement, and atherectomy. Our in‐hospital and 1‐year mortality of 4% and 15% were comparable to 3% to 4% and 14.3% to 18% presented from these single‐center studies.18, 19, 20, 21, 22 Interestingly, our 6% major amputation rate at 1 year after PVI was significantly lower than the rates of 13% to 32% as previously reported.18, 20, 21, 22 This may be because of the heterogeneity in patient selection and Rutherford class severity among the studies. Overall, our findings suggest that, in a real‐world setting with a diverse patient population, PVI for selected ALI patients result in favorable outcomes.
In the present study, predictors of worse in‐hospital outcomes following PVI among ALI patients were evaluated. Like past studies, we found that female sex,18 history of coronary artery disease,19 and thrombolysis use18, 21 were factors independently associated with worse in‐hospital outcomes. Sex‐related differences in outcomes of peripheral arterial disease have been previously described, and it demonstrated that female patients were less likely to be hospitalized for management of peripheral arterial disease, were more likely to be admitted emergently, and experienced higher postprocedural mortality rates as compared with male patients.23 These findings suggest that female patients present for medical attention with a more advanced disease at presentation and require emergent interventions that lead to a more complicated hospital course. Since patients with coronary artery disease have high burden of atherosclerotic risk factors, it should come as no surprise that patients with established coronary artery disease experience inferior in‐hospital outcomes. Finally, thrombolysis use in ALI patients was also associated with worse in‐hospital outcomes. We speculate that this finding is because of the increased thrombus burden or greater thrombus resistance to thrombolysis, indicating underlying advanced disease in ALI patients.
Our study extends the prior work by comparing a specific subset of ALI patients who were selected for treatment with PVI in a comprehensive multicenter registry. We compared outcomes of ALI patients with CLI patients given overall similar patterns of endovascular treatments. ALI patients experienced worse in‐hospital outcomes following PVI, analogous to the published outcomes for infra‐inguinal bypass previously reported from the VSGNE.5 Our findings may be attributed to the increased thrombus burden as seen in patients with ALI as compared with those with CLI, resulting in higher technical failure and rate of distal embolization. In addition, ALI is often rapidly progressive, requiring urgent interventions that may underlie the longer postprocedural hospital LOS and increased hospital mortality. Interestingly, we found that patients presenting with ALI experienced comparable long‐term outcomes at 1 year, specifically rates of major amputation and mortality, as their CLI counterparts. However, the rates of major amputation (6%) and mortality (15%) at 1 year in ALI patients following PVI from the present study were lower than those previously reported following infra‐inguinal bypass for ALI (22.4% major amputation and 20.9% mortality at 1 year).5 These apparent variances may reflect the differences in ALI patients selected for PVI compared with those selected for bypass. Overall, our findings indicate favorable 1‐year outcomes in ALI patients selected for treatment with PVI.
This study has several limitations. First, this is an observational analysis of the PVI database in the VSGNE registry; therefore, the findings presented in this study do not reflect all ALI patients treated in the region as the registry is organized by procedure type rather than the presenting diagnosis. However, this is one of the largest reported series in the past decade describing ALI patients who were selected for treatment by contemporary endovascular techniques. In addition, selected variables are not captured in the registry, including the cause of the occlusive disease (an embolus versus an in‐situ thrombosis), degree of underlying vascular disease burden, and severity of clinical presentation (ie, Fontaine Class). Additionally, incidence of postprocedural bleeding and stroke, which are common complications reported in association with thrombolysis in prior literature, were not collected, making direct comparison of the present data with other studies difficult. Given the fact that a majority of the patients were white, the generalizability of the results to patients of other racial groups should be performed with caution. As shown, patients with CLI differ in several clinical characteristics from patients with ALI that may account for differences in short‐term outcomes. However, many patients with ALI who are treated with PVI have underlying advanced PAD and have similar treatment approaches, making a comparison of clinical value. It would also be of interest to compare outcomes of patients with ALI treated with PVI compared with open surgery. However, the VSGNE registry collects data separately based on procedure type, limiting its ability to make comparisons between open and endovascular treatments. Finally, long‐term follow‐up variables, such as major amputation at 1 year, were self‐reported in the VSGNE registry, with 1‐year follow‐up available in ≈20% of patients in our study. Therefore, the low rate of major amputation at 1 year presented in this study may perhaps in part be attributed to underreporting. However, even with the limitations listed above, the utility of the VSGNE database has been validated through regularly scheduled audits,15 and its strength lies in its comprehensive repository of specific variables collected and its regular adjudication process.
Conclusion
In a large observational cohort of patients who underwent PVI, we found that contemporary endovascular techniques result in greater short‐term adverse events but equivalent long‐term outcomes in ALI patients as compared with CLI patients. Further studies are needed to improve the risk stratification and selection of ALI patients who are appropriate and best served by treatment with PVI.
Sources of Funding
Dr Hamburg is supported by National Institutes of Health (NIH) grants HL102299, HL109790, HL81587, and HL11539. Dr Inagaki received support from National Institutes of Health (NIH) training grant in Immunobiology of Trauma T32 GM86308.
Disclosures
None.
Supporting information
Appendix S1. Vascular Study Group of New England Investigators.
(J Am Heart Assoc. 2018;7:e004782 . DOI: 10.1161/JAHA.116.004782.)29650705
References
- 1. Bonaca MP, Gutierrez JA, Creager MA, Scirica BM, Olin J, Murphy SA, Braunwald E, Morrow DA. Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: results from the trial to assess the effects of vorapaxar in preventing heart attack and stroke in patients with atherosclerosis‐thrombolysis in myocardial infarction 50 (TRA2 degrees P‐TIMI 50). Circulation. 2016;133:997–1005. [DOI] [PubMed] [Google Scholar]
- 2. Eliason JL, Wainess RM, Proctor MC, Dimick JB, Cowan JA Jr, Upchurch GR Jr, Stanley JC, Henke PK. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg. 2003;238:382–389; discussion 389–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kempe K, Starr B, Stafford JM, Islam A, Mooney A, Lagergren E, Corriere MA, Edwards MS. Results of surgical management of acute thromboembolic lower extremity ischemia. J Vasc Surg. 2014;60:702–707. [DOI] [PubMed] [Google Scholar]
- 4. Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, Hiratzka LF, Murphy WR, Olin JW, Puschett JB, Rosenfield KA, Sacks D, Stanley JC, Taylor LM Jr, White CJ, White J, White RA, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Hunt SA, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B; American Association for Vascular Surgery; Society for Vascular Surgery; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society of Interventional Radiology; ACC/AHA Task Force on Practice Guidelines Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease; American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; Vascular Disease Foundation . ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (writing committee to develop guidelines for the management of patients with peripheral arterial disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; Transatlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. [DOI] [PubMed] [Google Scholar]
- 5. Baril DT, Patel VI, Judelson DR, Goodney PP, McPhee JT, Hevelone ND, Cronenwett JL, Schanzer A; Vascular Study Group of New E . Outcomes of lower extremity bypass performed for acute limb ischemia. J Vasc Surg. 2013;58:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nilsson L, Albrechtsson U, Jonung T, Ribbe E, Thorvinger B, Thorne J, Astedt B, Norgren L. Surgical treatment versus thrombolysis in acute arterial occlusion: a randomised controlled study. Eur J Vasc Surg. 1992;6:189–193. [DOI] [PubMed] [Google Scholar]
- 7. Ouriel K, Shortell CK, DeWeese JA, Green RM, Francis CW, Azodo MV, Gutierrez OH, Manzione JV, Cox C, Marder VJ. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021–1030. [DOI] [PubMed] [Google Scholar]
- 8. Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220:251–266; discussion 266‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ouriel K, Veith FJ, Sasahara AA. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23:64–73; discussion 74‐75. [DOI] [PubMed] [Google Scholar]
- 10. Weaver FA, Comerota AJ, Youngblood M, Froehlich J, Hosking JD, Papanicolaou G. Surgical revascularization versus thrombolysis for nonembolic lower extremity native artery occlusions: results of a prospective randomized trial. The STILE Investigators. Surgery versus thrombolysis for ischemia of the lower extremity. J Vasc Surg. 1996;24:513–521; discussion 521‐523. [DOI] [PubMed] [Google Scholar]
- 11. Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105–1111. [DOI] [PubMed] [Google Scholar]
- 12. Wang JC, Kim AH, Kashyap VS. Open surgical or endovascular revascularization for acute limb ischemia. J Vasc Surg. 2016;63:270–278. [DOI] [PubMed] [Google Scholar]
- 13. Korabathina R, Weintraub AR, Price LL, Kapur NK, Kimmelstiel CD, Iafrati MD, Ali Tahir SM. Twenty‐year analysis of trends in the incidence and in‐hospital mortality for lower‐extremity arterial thromboembolism. Circulation. 2013;128:115–121. [DOI] [PubMed] [Google Scholar]
- 14. Baril DT, Ghosh K, Rosen AB. Trends in the incidence, treatment, and outcomes of acute lower extremity ischemia in the United States Medicare population. J Vasc Surg. 2014;60:669–677.e662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cronenwett JL, Likosky DS, Russell MT, Eldrup‐Jorgensen J, Stanley AC, Nolan BW; Vsgnne . A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE). J Vasc Surg. 2007;46:1093–1101; discussion 1101‐1012. [DOI] [PubMed] [Google Scholar]
- 16. Hill ME, Rosenwaike I. The Social Security Administration's Death Master File: the completeness of death reporting at older ages. Soc Secur Bull. 2001;64:45–51. [PubMed] [Google Scholar]
- 17. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kashyap VS, Gilani R, Bena JF, Bannazadeh M, Sarac TP. Endovascular therapy for acute limb ischemia. J Vasc Surg. 2011;53:340–346. [DOI] [PubMed] [Google Scholar]
- 19. Linnemann B, Sutter T, Sixt S, Rastan A, Schwarzwaelder U, Noory E, Buergelin K, Beschorner U, Zeller T. Elevated cardiac troponin T contributes to prediction of worse in‐hospital outcomes after endovascular therapy for acute limb ischemia. J Vasc Surg. 2012;55:721–729. [DOI] [PubMed] [Google Scholar]
- 20. Byrne RM, Taha AG, Avgerinos E, Marone LK, Makaroun MS, Chaer RA. Contemporary outcomes of endovascular interventions for acute limb ischemia. J Vasc Surg. 2014;59:988–995. [DOI] [PubMed] [Google Scholar]
- 21. Taha AG, Byrne RM, Avgerinos ED, Marone LK, Makaroun MS, Chaer RA. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg. 2015;61:147–154. [DOI] [PubMed] [Google Scholar]
- 22. Genovese EA, Chaer RA, Taha AG, Marone LK, Avgerinos E, Makaroun MS, Baril DT. Risk factors for long‐term mortality and amputation after open and endovascular treatment of acute limb ischemia. Ann Vasc Surg. 2016;30:82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egorova N, Vouyouka AG, Quin J, Guillerme S, Moskowitz A, Marin M, Faries PL. Analysis of gender‐related differences in lower extremity peripheral arterial disease. J Vasc Surg. 2010;51:372–378.e371; discussion 378–379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Vascular Study Group of New England Investigators.


