Abstract
Background
We examined a large community‐based sample of patients with atrial fibrillation (AF) and valvular heart disease (VHD) (excluding prosthetic valves) with a goal to compare outcomes among patients with AF, with and without VHD, taking warfarin, dabigatran, and rivaroxaban.
Methods and Results
We identified Medicare beneficiaries enrolled in Medicare Part D benefit plan from 2011 to 2013 with newly diagnosed AF (18 137 patients with VHD [dabigatran, 1979; rivaroxaban, 2027; warfarin, 14 131] and 85 596 patients without VHD [dabigatran, 13 522; rivaroxaban, 14 257; warfarin, 57 817]). Primary outcomes of all‐cause mortality, ischemic strokes, major bleeding, and myocardial infarction were compared across the 3 anticoagulants using 3‐way propensity‐matched samples. After propensity matching, a total of 5871 patients with VHD and 40 221 patients without VHD and AF were studied. Both dabigatran and rivaroxaban were associated with significantly lower risk of death in patients with VHD with AF (dabigatran versus warfarin: hazard ratio, 0.71; 95% confidence interval, 0.52–0.98; P=0.038; rivaroxaban versus warfarin: hazard ratio, 0.68; 95% confidence interval, 0.49–0.95; P=0.022). Nongastrointestinal bleeding was significantly reduced with dabigatran and rivaroxaban versus warfarin in those with VHD (dabigatran versus warfarin: hazard ratio, 0.17; 95% confidence interval, 0.06–0.49; P=0.001; rivaroxaban versus warfarin: hazard ratio, 0.37; 95% confidence interval, 0.17–0.84; P=0.017). Ischemic stroke and gastrointestinal bleeding rates did not differ between rivaroxaban, dabigatran, and warfarin in patients with VHD. The effects of the 3 anticoagulants on outcomes were comparable in patients with and without VHD and with AF.
Conclusions
In this cohort of Medicare beneficiaries with VHD (excluding patients with prosthetic valves) and new‐onset AF between 2011 and 2013, novel oral non–vitamin K anticoagulants were safe and effective options for prevention of systemic thromboembolism.
Keywords: anticoagulation, atrial fibrillation arrhythmia, valvular disease
Subject Categories: Atrial Fibrillation, Arrhythmias
Clinical Perspective
What Is New?
After propensity‐matching analysis, non–vitamin K antagonist oral anticoagulants were associated with reduced all‐cause mortality risk compared with warfarin in patients with and without valvular heart disease and with atrial fibrillation.
Ischemic stroke rates were similar between anticoagulants in patients with valvular heart disease, whereas rivaroxaban was associated with lower stroke rates than warfarin in the patients without valvular heart disease.
Nongastrointestinal bleeding risk was lower with non–vitamin K antagonist oral anticoagulants than warfarin in patients with and without valvular heart disease.
What Are the Clinical Implications?
Therefore, clinicians have >1 anticoagulation option available for patients without hemodynamically significant valvular disease requiring surgery.
Introduction
Increased thromboembolic risk attributable to development of atrial thrombi occurs with any form of atrial fibrillation (AF); hence, long‐term oral anticoagulation is recommended for most patients with AF. Anticoagulation reduces the thromboembolic risk by approximately two thirds, irrespective of baseline risk.1 However, the use of all antithrombotic agents increased the risk of bleeding, with intracranial hemorrhage being the most serious bleeding complication. The therapeutic armamentarium for primary and secondary prevention of thromboembolic events among patients with AF has expanded to include non–vitamin K antagonist oral anticoagulants (NOACs). In patients with AF, anticoagulation with any of the approved NOACs (dabigatran, rivaroxaban, apixaban, and edoxaban) is associated with similar or lower rates of both ischemic stroke and major bleeding and less than half risk of intracranial hemorrhage compared with adjusted dose warfarin in randomized controlled trials.2, 3, 4, 5
Most of the clinical trials of antithrombotic therapy in patients with AF have excluded patients with mechanical valves, mitral stenosis, and rheumatic heart disease. Valvular heart disease (VHD) coexists in >50% of patients with AF and is associated with a higher risk of thromboembolic events, independent of the underlying cardiac rhythm.6 Among patients with mitral valve stenosis or prosthetics valves, AF portends high thromboembolic risk and vitamin K antagonists are indicated for stroke and systemic embolism prevention.7 Some patients with VHD have been included in the NOAC trials. In the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial, 26% of patients had a history of moderate or severe VHD (most of them with mitral regurgitation) or previous valve surgery.8 Although these patients had higher rates of stroke and systemic embolism than those without, there was no evidence of differential effects of apixaban compared with warfarin on stroke, major bleeding, and all‐cause mortality between patients with and without VHD. In a post hoc analysis of the ROCKET‐AF (Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation),9 14.1% had significant VHD and 5.3% had prior valvular procedures. Among patients with VHD, the rates of systemic thromboembolism and all‐cause mortality were similar, but major bleeding risk was significantly higher with rivaroxaban versus warfarin. Furthermore, a recent analysis of the RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy) trial demonstrated that 21.8% of patients with AF had VHD (excluding prosthetic valves and significant mitral stenosis) and that the presence of VHD did not affect the comparison between dabigatran and warfarin.10
We hypothesized that NOACs are prescribed to patients with VHD, despite lack of robust data. Therefore, we examined a large community‐based sample of patients with AF and VHD (excluding prosthetic valves) with a goal to do the following: (1) compare all‐cause mortality, stroke, and major bleeding risk among patients taking warfarin, dabigatran, and rivaroxaban; and (2) evaluate differences in outcomes between patients with AF with and without VHD.
Methods
Data Source
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The study was conducted using the Centers for Medicare and Medicaid Services patient records and linking data sources, including Beneficiary Base and Chronic Conditions segments, Part A (Inpatient), Part B (Carrier) Standard Analytic Files, and Part D Pharmacy Drug Event files for 2010 through 2013; and Part D files from January 1, 2010, through December 31, 2013. The study was granted a waiver of consent by the University of Iowa (Iowa City, IA) institutional review board because it involves analysis of existing data and the involved individuals did not receive a test material (ie, drug or device) as participants in the study.
Patient Population
We performed a retrospective cohort analysis of claims data for adult Medicare beneficiaries (aged >65 years) who were newly diagnosed with AF between November 1, 2011, and October 31, 2013, and initiated dabigatran 150 mg BID, rivaroxaban 20 mg QD, or warfarin within 90 days after AF diagnosis. New AF was defined on the basis of previously established algorithms (ie, 1 inpatient claim or 2 outpatient claims within 90 days with International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code 427.31 as primary or first secondary diagnosis, with no AF diagnoses during the prior 12 months).11, 12 We excluded patients if they were <66 years at the time of diagnosis (to ensure at least 12 months of Medicare eligibility before diagnosis), were enrolled in a Medicare managed care program during the observation period, or were not enrolled in a Part D drug prescription plan at the time of AF diagnosis. Furthermore, we identified patients with VHD on the basis of ICD‐9‐CM codes 395.x, 396.x, 398.9, 424.1, 7463, and 7464 (aortic valve disease); 394.x, 396.x, 398.9, 424.0, 7465, and 7466 (mitral valve disease); 397.0, 398.9, 424.2, and 746.1 (tricuspid valve disease); and 397.1, 424.3, 746.00, 746.02, and 746.09 (pulmonary valve disease). We required 1 primary or secondary inpatient diagnosis or 2 primary outpatient diagnoses to define valve disease. Patients with no inpatient or outpatient valve diagnoses were nonvalvular patients. Patients with ambiguous criteria (eg, 1 outpatient diagnosis and no inpatient diagnoses) were excluded (n=37 022). We excluded patients with bioprosthetic or mechanical valves on the basis of ICD‐9 codes V422 and V433.
Covariates
Data on patient‐level characteristics, such as patient demographics, comorbid conditions, concurrent medication use, and prior health services use, were extracted from Medicare enrollment data and inpatient and carrier claims. Comorbid diseases were identified by ICD‐9‐CM diagnoses in inpatient and outpatient claims during the 12 months preceding AF diagnosis using algorithms defined by Elixhauser et al.13 We identified additional comorbidities of importance to AF outcomes, including the following: other dysrhythmias (ICD‐9‐CM codes 427.X, excluding 427.3), cardiomyopathy (ICD‐9 codes 425.X), cardiac conduction disorder (eg, bundle branch block; ICD‐9 codes 426.X), and previous implantable cardiac device (eg, pacemaker; ICD‐9 codes V45.0 and V53.3). The CHA2DS2‐VASc stroke risk score (1 point each for congestive heart failure diagnosis, female sex, hypertension diagnosis, diabetes mellitus diagnosis, aged 65–75 years, and vascular disease diagnosis; 2 points each for aged >75 years and prior stroke or transient ischemic attack) was calculated.14 A modified hypertension, abnormal renal and liver functions, stroke, bleeding, labile international normalized ratio, elderly, drugs, or alcohol score was used to represent bleeding risk15 (international normalized ratio history was not reflected in our score because laboratory values were not available). We applied a previously validated comorbidity score, as defined by Gagne and colleagues, to quantify comorbidity burden.16 Furthermore, we extracted data pertaining to health care use (number of inpatient hospital days, skilled nursing facility stay, and extended care stay) and medication use (insulin, statins, β blockers, calcium channel blockers, aspirin, clopidogrel, proton pump inhibitors, and nonsteroidal anti‐inflammatory drug use). We also calculated medication adherence as proportion of days covered over the initial 180 days of anticoagulant use.17
End Points
The main end points in this study are the following: (1) all‐cause mortality; (2) stroke, including ischemic stroke or transient ischemic attack; (3) gastrointestinal bleeding; (4) any bleeding; (5) nongastrointestinal bleeding; and (6) acute myocardial infarction on the basis of the primary ICD‐9‐CM diagnosis on inpatient standard analytical files claims for short‐term care stays.13
Statistical Analysis
We created separate cohorts for patients with and without VHD. Within each group, we compared 3 treatment groups: patients treated with dabigatran 150 mg BID (dabigatran group), patients treated with rivaroxaban 20 mg QD (rivaroxaban group), and patients treated with warfarin (warfarin group). We compared demographic characteristics, comorbid diseases, and medication use among patients taking different anticoagulants, by the use of χ2 test for categorical variables and ANOVA or Kruskal‐Wallis test (as appropriate) for continuous variables. We performed 3‐way propensity‐matching method, as described by Rassen and colleagues,18 to create groups of patients receiving dabigatran, rivaroxaban, or warfarin who were balanced with respect to patient covariates and also had clinical equipoise (patients included in the matched samples were plausible candidates for all 3 anticoagulants). Propensity matching was conducted separately for patients with and without VHD. Success of the matching algorithm was evaluated by comparing standardized differences in demographic variables, comorbid diseases, and medication use. The propensity‐matched samples were used to calculate event rates/patient year of follow‐up for the studied outcomes for the 3 anticoagulant groups in VHD and non‐VHD separately. Moreover, we performed inverse probability of treatment weighting (IPTW) for the treatment groups among patients with and without VHD. Consistent with Crump et al,19 we restricted the analytic sample to those subjects whose propensity for each drug lay in the interval from 0.05 to 0.95 to avoid the influence of extreme values. Results may be slightly different with IPTW compared with propensity matching, as found in other studies.20, 21 IPTW methods can be sensitive to the influence of patients who receive unexpected treatments, particularly if treatment effects differ in these patients.22 Moreover, IPTW estimates average treatment effect among all patients, whereas our matching algorithm estimates the average treatment effect among patients who are equal candidates for all 3 drugs.23
We used multivariable Cox proportional hazards regression models with dependent variables being time from medication initiation to specific event to evaluate the relative hazard of each event, while further controlling for patient characteristics. The results of regression analyses were reported as hazard ratios (HRs) with 95% confidence intervals (CIs) for dabigatran versus warfarin, rivaroxaban versus warfarin, and rivaroxaban versus dabigatran. Analysis of patient outcomes censored patients for medication cessation. All analyses were conducted with the use of SAS, with 2‐tailed level of significance set at 0.05.
Results
Our analysis included 20 525 patients with VHD (dabigatran, 2132; rivaroxaban, 2170; warfarin, 16 223) and 85 596 patients without VHD (dabigatran, 13 522; rivaroxaban, 14 257; warfarin, 57 817). We identified significant differences in baseline characteristics across the 3 anticoagulant groups in patients with and without VHD before propensity matching (Table 1). After propensity‐match analysis, we identified 5871 patients with VHD (1957 in each anticoagulant group) and 40 221 patients without VHD (13 407 in each group). Among patients with valvular disease, mean follow‐up periods to death or medication cessation were 233, 196, and 233 days for dabigatran, rivaroxaban, and warfarin, respectively. Follow‐up periods were slightly longer for patients without valvular disease (248, 211, and 247 days for dabigatran, rivaroxaban, and warfarin). All standardized differences in demographic characteristics, comorbid conditions, medications, and healthcare use between anticoagulant groups were <10%, which are the recommended criteria (Table 2).24
Table 1.
Characteristics of Study Patients Taking Dabigatran, Rivaroxaban, or Warfarin Before Propensity Matching
| Characteristics | Nonvalvular AF | Valvular AF | ||||||
|---|---|---|---|---|---|---|---|---|
| Dabigatran | Rivaroxaban | Warfarin | P Value | Dabigatran | Rivaroxaban | Warfarin | P Value | |
| No. of patients | 13 522 | 14 257 | 57 817 | … | 1979 | 2027 | 14 131 | … |
| Age, mean (SD), y | 75.5 (6) | 75.4 (6) | 77.8 (7) | 0.07 | 77 (7) | 77 (7) | 80 (7) | 0.1 |
| Female sex, % | 47 | 50 | 55 | 0.02 | 62 | 60 | 67 | <0.01 |
| Race, % | ||||||||
| White | 90 | 90 | 87 | <0.001 | 90 | 91 | 88 | 0.03 |
| Black | 3.5 | 3 | 5.5 | 4 | 3 | 5.5 | ||
| Hispanic | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Other | 6.6 | 6.3 | 7.6 | 6 | 5 | 6.7 | ||
| Comorbid conditions, % | ||||||||
| Hypertension | 84 | 84 | 86 | <0.001 | 90 | 89 | 91 | <0.01 |
| Diabetes mellitus | 33 | 34 | 38 | <0.001 | 33 | 33 | 35 | <0.01 |
| Heart failure | 19 | 19 | 29 | <0.001 | 47 | 44 | 60 | <0.001 |
| Previous MI | 7 | 7 | 11 | <0.001 | 15 | 16 | 21 | <0.01 |
| Depression | 11 | 10 | 14 | <0.001 | 15 | 17 | 17 | <0.01 |
| COPD | 26 | 26 | 32 | <0.001 | 46 | 44 | 52 | 0.04 |
| PVD | 16 | 16 | 22 | <0.001 | 25 | 25 | 32 | 0.02 |
| Neurological disorder | 6 | 6 | 10 | <0.001 | 9 | 12 | 13 | 0.01 |
| Renal disease | 8 | 7 | 19 | <0.001 | 12 | 12 | 30 | <0.001 |
| Liver disease | 4 | 4 | 4 | <0.001 | 4 | 6 | 6 | <0.01 |
| Dementia | 2 | 2 | 4 | <0.001 | 3 | 3 | 5 | <0.01 |
| Electrolyte imbalance | 16 | 16 | 26 | <0.001 | 3 | 3 | 4 | 0.04 |
| Weight loss | 3 | 4 | 7 | <0.001 | 5 | 7 | 10 | 0.02 |
| ICD | 4 | 5 | 6 | <0.01 | 5 | 6 | 7 | <0.01 |
| Hypothyroidism | 22 | 21 | 23 | <0.01 | 26 | 27 | 28 | <0.01 |
| Dysrhythmias | 29 | 30 | 32 | 0.01 | 41 | 42 | 45 | <0.01 |
| Cardiomyopathy | 5 | 5 | 7 | <0.01 | 14 | 15 | 17 | <0.01 |
| Prior cerebral infarction | 5 | 6 | 9 | <0.01 | 10 | 9 | 13 | 0.02 |
| Previous bleeding, % | ||||||||
| Prior gastrointestinal hemorrhage | 24 | 24 | 26 | <0.01 | 32 | 33 | 35 | 0.02 |
| Prior intracranial hemorrhage | 0.4 | 0.4 | 0.7 | <0.001 | 0.6 | 0.4 | 1 | <0.001 |
| Previous major bleeding | 29 | 30 | 32 | <0.01 | 38 | 39 | 42 | 0.02 |
| Comorbidity scores, mean (SD) | ||||||||
| GAGNE comorbidity score | 2.7 (2) | 2.7 (2) | 3.7 (3) | 0.03 | 4.2 (2.5) | 4.2 (2.6) | 5.5 (3) | 0.07 |
| CHA2DS2‐VASc score | 4.1 (1.6) | 4.1 (1.6) | 4.6 (1.7) | <0.01 | 5 (1.6) | 5 (1.7) | 5.6 (1.6) | 0.2 |
| HAS‐BLED score | 1.6 (0.8) | 1.6 (0.7) | 1.8 (0.9) | 0.04 | 1.8 (0.8) | 1.8 (0.8) | 2 (1) | 0.1 |
| Medications in prior 90 d, % | ||||||||
| Statins | 44 | 45 | 42 | <0.01 | 43 | 43 | 43 | <0.001 |
| Clopidogrel | 4.4 | 4.5 | 5.7 | <0.001 | 7 | 6 | 7 | <0.001 |
| Proton pump inhibitors | 20 | 20 | 21 | <0.001 | 20 | 21 | 23 | <0.01 |
| NSAIDs | 13 | 13 | 12 | <0.001 | 12 | 12 | 11 | <0.01 |
| Prior health services use | ||||||||
| Prior acute inpatient hospital stay, mean (SD), d | 2 (4) | 1.9 (5) | 4 (8) | <0.001 | 4.8 (6) | 4.9 (7) | 7.9 (10) | 0.3 |
| No. of prescriptions, mean (SD) | 8.8 (6) | 8.8 (6) | 9.4 (6) | <0.001 | 9.5 (6) | 9.6 (6) | 10.1 (6) | 0.2 |
| Prior stay in skilled nursing facility, % | 2.1 | 1.9 | 6 | <0.001 | 4 | 5 | 8 | <0.01 |
| AF diagnosed as inpatient, % | 43 | 41 | 51 | <0.001 | 88 | 86 | 87 | <0.01 |
CHA2DS2‐VASc, 1 point each for congestive heart failure diagnosis, female sex, hypertension diagnosis, diabetes mellitus diagnosis, aged 65 to 75 years, and vascular disease diagnosis; 2 points each for aged >75 years and prior stroke or transient ischemic attack. HAS‐BLED, 1 point each for hypertension diagnosis, renal disease, liver disease, stroke history, prior major bleeding, labile international normalized ratio, aged >65 years, medication use predisposing to bleeding, and alcohol or drug use history. AF indicates atrial fibrillation; COPD, chronic obstructive pulmonary disease; HAS‐BLED, hypertension, abnormal renal and liver functions, stroke, bleeding, labile international normalized ratio, elderly, drugs, or alcohol; ICD, internal cardioverter defibrillator; MI, myocardial infarction; and PVD, peripheral vascular disease.
Table 2.
Standardized Differences After Propensity Matching
| Variable | Nonvalvular AF | Valvular AF | ||||
|---|---|---|---|---|---|---|
| Dabigatran vs Rivaroxaban | Dabigatran vs Warfarin | Rivaroxaban vs Warfarin | Dabigatran vs Rivaroxaban | Dabigatran vs Warfarin | Rivaroxaban vs Warfarin | |
| Age | 1.37 | −2.32 | −1.03 | −0.26 | 0.41 | 0.16 |
| Female sex | −1.21 | −0.78 | −1.99 | 0.1 | 6.47 | 6.56 |
| Race | ||||||
| White | 1.54 | −3.19 | −1.79 | 1.58 | −3.08 | −1.68 |
| Black | −1.82 | 1.87 | 0.3 | −2.18 | 0.48 | −1.49 |
| Other | −0.55 | 2.46 | 1.96 | −0.22 | 3.53 | 3.45 |
| Comorbid conditions | ||||||
| Hypertension | 0.65 | 0.12 | 0.79 | −2.48 | 3.29 | 0.68 |
| Diabetes mellitus | −0.6 | 2.63 | 2.04 | 0.76 | −1.18 | −0.43 |
| Heart failure | 0.51 | 2.69 | 3.16 | −3.28 | 0.93 | −2.38 |
| Previous MI | 1.52 | 0.08 | 1.43 | 3.41 | −3.21 | 0 |
| Depression | −0.9 | 1.07 | 0.23 | 3.46 | 0.28 | 3.66 |
| COPD | 0.89 | 1.62 | 2.48 | −5.65 | 2.56 | −3.08 |
| PVD | 0.61 | 1.45 | 2.02 | 2.47 | 3.87 | 6.23 |
| Neurological disorder | 0.53 | −0.03 | 0.44 | 5.87 | 1.47 | 6.88 |
| Renal disease | −1.84 | 1.86 | 0.42 | 1.24 | −3.85 | −2.82 |
| Liver disease | 0.95 | 1.38 | 2.28 | 3.32 | −0.95 | 2.23 |
| Dementia | −1 | 0.25 | −0.56 | 0.3 | −0.76 | −0.51 |
| Electrolyte imbalance | 0.91 | 1.43 | 2.26 | 2.2 | 2.03 | 4.15 |
| Weight loss | 0.64 | −0.1 | 0.44 | 6.27 | 0.39 | 5.87 |
| ICD | 2.5 | 0.89 | 3.17 | 1.97 | −2.52 | −0.61 |
| Hypothyroidism | −0.45 | 2.3 | 1.86 | 0.46 | 2.87 | 3.31 |
| Dysrhythmias | 1.39 | 2.17 | 3.53 | 1.14 | 1.14 | 2.27 |
| Cardiomyopathy | 1.48 | 1.46 | 2.8 | 1.73 | 1.27 | 2.94 |
| Prior cerebral infarction | −1.36 | 2.06 | 0.88 | −2.26 | 5 | 2.98 |
| Previous bleeding | ||||||
| Prior gastrointestinal hemorrhage | 0.58 | 2.15 | 2.71 | 0.87 | −0.54 | 0.32 |
| Prior intracranial hemorrhage | −0.58 | 0.69 | 0.2 | −1.47 | −1.16 | −2.44 |
| Previous major bleeding | 0.07 | 2.78 | 2.85 | 1.89 | −0.94 | 0.94 |
| Comorbidity scores | ||||||
| GAGNE comorbidity score | 0.79 | 2.96 | 3.65 | 1.2 | −0.21 | 0.89 |
| CHA2DS2‐VASc score | 0.5 | 2.64 | 3.15 | 1.76 | 2.76 | 4.5 |
| HAS‐BLED score | −1.34 | 3.33 | 2.1 | 3.8 | −2.78 | 0.84 |
| Medications in prior 90 d | ||||||
| Statins | 0.27 | 0.72 | 0.99 | 0.41 | −0.31 | 0.1 |
| Clopidogrel | 0.11 | 1.3 | 1.39 | −0.41 | −4.6 | −5.05 |
| Proton pump inhibitors | 0.23 | 0.48 | 0.7 | 1.65 | −5.76 | −4.08 |
| NSAIDs | −0.88 | 1.58 | 0.68 | 0.47 | −1.76 | −1.28 |
| Prior health services use | ||||||
| Prior acute inpatient hospital stay | 1 | −0.4 | 0.33 | 2.56 | −1.1 | 0.97 |
| No. of prescriptions | −0.29 | 0.93 | 0.65 | 1.58 | −0.11 | 1.45 |
| Prior stay in skilled nursing facility | −0.64 | −0.72 | −1.2 | 1.48 | 2.31 | 3.56 |
| AF diagnosed as inpatient | −2.66 | 4.6 | 1.97 | −4.26 | −0.31 | −4.47 |
Data are given as percentage standardized difference. CHA2DS2‐VASc, 1 point each for congestive heart failure diagnosis, female sex, hypertension diagnosis, diabetes mellitus diagnosis, aged 65 to 75 years, and vascular disease diagnosis; 2 points each for aged >75 years and prior stroke or transient ischemic attack. HAS‐BLED, 1 point each for hypertension diagnosis, renal disease, liver disease, stroke history, prior major bleeding, labile international normalized ratio, aged >65 years, medication use predisposing to bleeding, and alcohol or drug use history. AF indicates atrial fibrillation; COPD, chronic obstructive pulmonary disease; HAS‐BLED, hypertension, abnormal renal and liver functions, stroke, bleeding, labile international normalized ratio, elderly, drugs, or alcohol; ICD, internal cardioverter defibrillator; MI, myocardial infarction; and PVD, peripheral vascular disease.
Outcomes
The rates of each outcome are expressed as events per patient‐day of follow‐up and presented in Table 3. Before propensity‐match analysis, the stroke rates were higher in the VHD group versus the non‐VHD group (2.4 versus 1.7 events/100 patient‐years). Moreover, the all‐cause mortality (9.5 versus 4.6 events/100 patient‐years) and gastrointestinal bleeding (6 versus 3 events/100 patient‐years) were approximately twice as frequent in patients with VHD as in patients without VHD. The rates of intracranial bleeding were similar in patients with and without VHD (0.56 versus 0.42 events/100 patient‐years) (Tables 3 and 4).
Table 3.
Event Rates/100 Patient‐Years of Follow‐Up (Number of Events) Before Propensity Matching
| Variable | Nonvalvular Atrial Fibrillation | Valvular Atrial Fibrillation | ||||
|---|---|---|---|---|---|---|
| Dabigatran (n=13 522) | Rivaroxaban (n=14 257) | Warfarin (n=57 817) | Dabigatran (n=2132) | Rivaroxaban (n=2170) | Warfarin (n=16 223) | |
| All‐cause mortality | 2.3 (209) | 2.8 (227) | 5.6 (2080) | 4.9 (66) | 5.6 (64) | 10.7 (1023) |
| Stroke | 1.4 (129) | 1.2 (99) | 1.9 (696) | 1.6 (22) | 2.1 (24) | 2.6 (246) |
| Any bleeding | 3.0 (270) | 4.2 (339) | 4.5 (1657) | 5.7 (76) | 8.0 (90) | 7.3 (688) |
| Gastrointestinal bleeding | 2.6 (238) | 3.7 (300) | 3.4 (1250) | 5.5 (73) | 7.1 (81) | 6.0 (561) |
| Nongastrointestinal bleeding | 0.4 (35) | 0.5 (40) | 1.1 (425) | 0.3 (4) | 0.8 (9) | 1.4 (137) |
| Myocardial infarction | 0.7 (68) | 0.8 (68) | 1.1 (398) | 1.5 (20) | 1.00 (11) | 2.0 (187) |
Table 4.
Hazard of Outcomes in Matched Cohorts of Valvular and Nonvalvular AF
| Valvular AF | Nonvalvular AF |
|---|---|
| All‐cause mortality | |
| Dabigatran vs warfarin: 0.71 (0.52–0.98; P=0.038) | Dabigatran vs warfarin: 0.68 (0.57–0.81; P<0.0001) |
| Rivaroxaban vs warfarin: 0.68 (0.49–0.95; P=0.022) | Rivaroxaban vs warfarin: 0.74 (0.62–0.88; P=0.0006) |
| Rivaroxaban vs dabigatran: 0.96 (0.67–1.37; P=0.82) | Rivaroxaban vs dabigatran: 1.1 (0.9–1.3; P=0.4) |
| Stroke | |
| Dabigatran vs warfarin: 1.12 (0.59–1.1; P=0.7) | Dabigatran vs warfarin: 0.86 (0.68–1.1; P=0.2) |
| Rivaroxaban vs warfarin: 1.3 (0.7–2.4; P=0.4) | Rivaroxaban vs warfarin: 0.7 (0.5–0.9; P=0.005) |
| Rivaroxaban vs dabigatran: 1.1 (0.64–2.1; P=0.62) | Rivaroxaban vs dabigatran: 0.8 (0.61–1.04; P=0.1) |
| Any bleeding | |
| Dabigatran vs warfarin: 0.93 (0.68–1.3; P=0.67) | Dabigatran vs warfarin: 0.84 (0.71–0.99; P=0.04) |
| Rivaroxaban vs warfarin: 1.1 (0.8–1.5; P=0.5) | Rivaroxaban vs warfarin: 1.08 (0.9–1.3; P=0.3) |
| Rivaroxaban vs dabigatran: 1.2 (0.86–1.6; P=0.3) | Rivaroxaban vs dabigatran: 1.28 (1.09–1.5; P=0.003) |
| Gastrointestinal bleeding | |
| Dabigatran vs warfarin: 1.27 (0.9–1.8; P=0.17) | Dabigatran vs warfarin: 1.08 (0.9–1.3; P=0.4) |
| Rivaroxaban vs warfarin: 1.4 (0.99–1.99; P=0.05) | Rivaroxaban vs warfarin: 1.37 (1.15–1.64; P=0.0005) |
| Rivaroxaban vs dabigatran: 1.1 (0.8–1.5; P=0.5) | Rivaroxaban vs dabigatran: 1.28 (1.07–1.5; P=0.005) |
| Nongastrointestinal bleeding | |
| Dabigatran vs warfarin: 0.17 (0.06–0.49; P=0.001) | Dabigatran vs warfarin: 0.34 (0.23–0.5; P<0.001) |
| Rivaroxaban vs warfarin: 0.37 (0.17–0.84; P=0.017) | Rivaroxaban vs warfarin: 0.42 (0.28–0.6; P<0.017) |
| Rivaroxaban vs dabigatran: 2.2 (0.66–7.3; P=0.2) | Rivaroxaban vs dabigatran: 1.2 (0.76–1.9; P=0.4) |
| Myocardial infarction | |
| Dabigatran vs warfarin: 1.4 (0.67–2.96; P=0.36) | Dabigatran vs warfarin: 1.04 (0.73–1.47; P=0.84) |
| Rivaroxaban vs warfarin: 1.02 (0.45–2.32; P=0.96) | Rivaroxaban vs warfarin: 1.1 (0.78–1.5; P=0.56) |
| Rivaroxaban vs dabigatran: 0.7 (0.34–1.6; P=0.4) | Rivaroxaban vs dabigatran: 1.07 (0.75–1.5; P=0.7) |
Data are given as hazard ratio (95% confidence interval). AF indicates atrial fibrillation.
In Table 4, we present the hazard of each outcome in patients taking dabigatran versus warfarin, rivaroxaban versus warfarin, and dabigatran versus rivaroxaban separately for patients with and without VHD, on the basis of multivariable Cox regression on propensity‐matched samples.
All‐cause mortality
Both dabigatran and rivaroxaban were associated with significantly lower risk of death in patients with VHD and AF (dabigatran versus warfarin: HR, 0.71; 95% CI, 0.52–0.98; P=0.038; rivaroxaban versus warfarin: HR, 0.68; 95% CI, 0.49–0.95; P=0.022) (Figure 1). Among patients without VHD, all‐cause mortality was also significantly reduced with NOACs versus warfarin (dabigatran versus warfarin: HR, 0.68; 95% CI, 0.57–0.81; P<0.0001; rivaroxaban versus warfarin: HR, 0.74; 95% CI, 0.62–0.88; P=0.0006). No significant differences in all‐cause mortality between the 2 NOACs studied were observed in patients with and without VHD and with AF (Table 5) (Figure 2).
Figure 1.
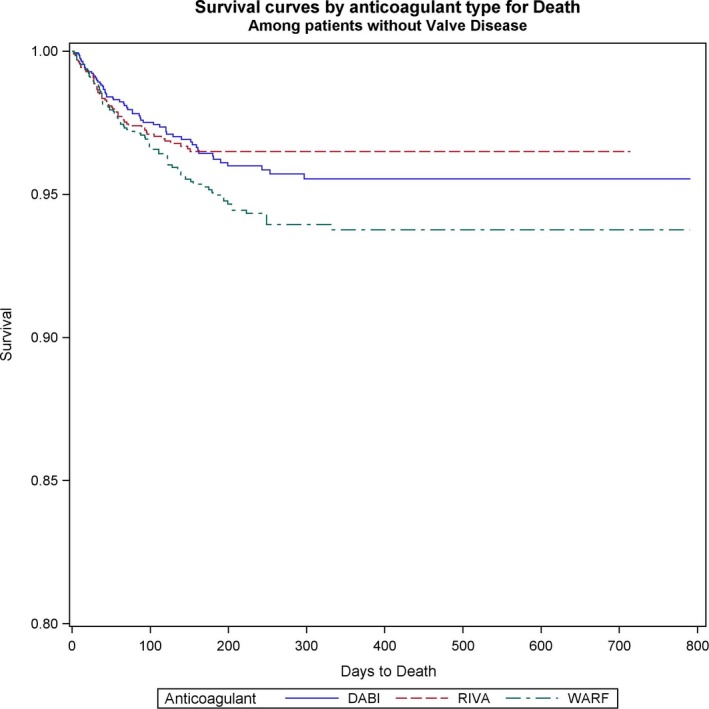
Survival curves for all‐cause mortality comparing the 3 anticoagulants (warfarin [WARF], dabigatran [DABI], and rivaroxaban [RIVA]) in patients with valvular heart disease with newly diagnosed atrial fibrillation.
Table 5.
Event Rates/100 Patient‐Years of Follow‐Up (Number of Events) in Propensity‐Matched Samples
| Variable | Nonvalvular Atrial Fibrillation | Valvular Atrial Fibrillation | ||||
|---|---|---|---|---|---|---|
| Dabigatran | Rivaroxaban | Warfarin | Dabigatran | Rivaroxaban | Warfarin | |
| Total patients | 13 407 | 13 407 | 13 407 | 1957 | 1957 | 1957 |
| All‐cause mortality | 2.2 (204) | 2.8 (220) | 3.4 (304) | 5.0 (63) | 5.7 (60) | 7.2 (90) |
| Stroke | 1.4 (127) | 1.2 (95) | 1.6 (148) | 1.6 (20) | 2.1 (22) | 1.5 (18) |
| Any bleeding | 3.0 (267) | 4.1 (314) | 3.5 (316) | 6.0 (74) | 7.9 (81) | 6.4 (79) |
| Gastrointestinal bleeding | 2.6 (235) | 3.6 (277) | 2.4 (219) | 5.8 (71) | 7.1 (73) | 4.5 (56) |
| Nongastrointestinal bleeding | 0.4 (35) | 0.5 (38) | 1.1 (101) | 0.3 (4) | 0.8 (8) | 1.9 (23) |
| Myocardial infarction | 0.7 (65) | 0.8 (64) | 0.7 (63) | 1.4 (17) | 1.1 (11) | 1.0 (12) |
Figure 2.
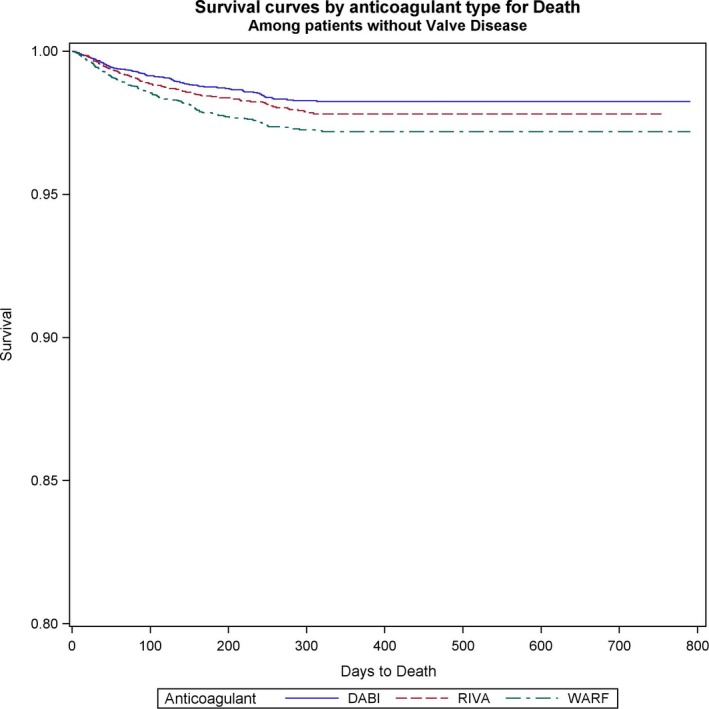
Survival curves for all‐cause mortality comparing the 3 anticoagulants (warfarin [WARF], dabigatran [DABI], and rivaroxaban [RIVA]) in patients without valvular heart disease with newly diagnosed atrial fibrillation.
Stroke
Ischemic stroke rates did not differ between rivaroxaban, dabigatran, and warfarin in patients with VHD and AF (dabigatran versus warfarin: HR, 1.12; 95% CI, 0.59–1.1; P=0.7; rivaroxaban versus warfarin: HR, 1.3; 95% CI, 0.7–2.4; P=0.4; rivaroxaban versus dabigatran: HR, 1.1; 95% CI, 0.64–2.1; P=0.62) (Figure 3). Among patients without VHD, rivaroxaban was associated with lower stroke risk than warfarin (rivaroxaban versus warfarin: HR, 0.7; 95% CI, 0.5–0.9; P=0.005). In these patients, no differences between NOACs and dabigatran versus warfarin were found (dabigatran versus warfarin: HR, 0.86; 95% CI, 0.68–1.1; P=0.2; rivaroxaban versus dabigatran: HR, 0.8; 95% CI, 0.61–1.04; P=0.1) (Figure 4).
Figure 3.
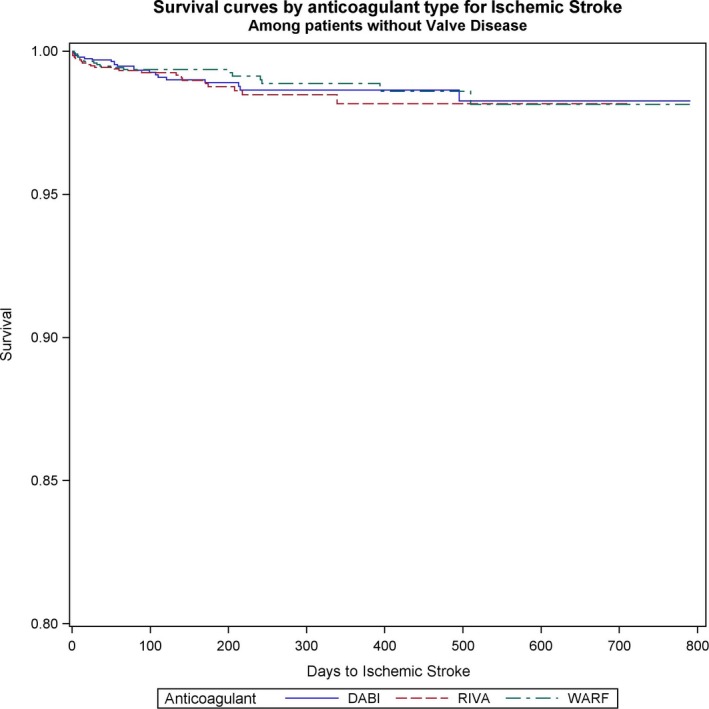
Survival curves for stroke comparing the 3 anticoagulants (warfarin [WARF], dabigatran [DABI], and rivaroxaban [RIVA]) in patients with valvular heart disease with newly diagnosed atrial fibrillation.
Figure 4.
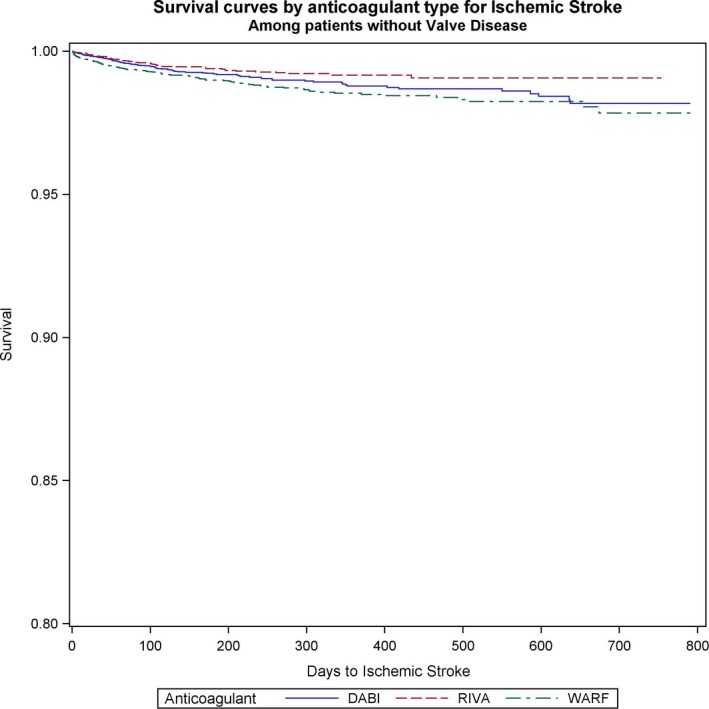
Survival curves for stroke comparing the 3 anticoagulants (warfarin [WARF], dabigatran [DABI], and rivaroxaban [RIVA]) in patients without valvular heart disease with newly diagnosed atrial fibrillation.
Bleeding
In the VHD group of patients, total bleeding events were not significantly different among the 3 anticoagulation groups (Figure S1). In the non‐VHD group, dabigatran was associated with lower risk of total bleeding events compared with warfarin (dabigatran versus warfarin: HR, 0.84; 95% CI, 0.71–0.99; P=0.04) and rivaroxaban (rivaroxaban versus dabigatran: HR, 1.28; 95% CI, 1.09–1.5; P=0.003). Rivaroxaban and warfarin had similar total bleeding risk in this group (Figure S2).
Gastrointestinal bleeding risk did not differ among the anticoagulation groups in patients with VHD and AF (Tables 3 and 4) (Figure S3). However, in patients without VHD, rivaroxaban was associated with significantly increased risk of gastrointestinal bleeding compared with warfarin (rivaroxaban versus warfarin: HR, 1.37; 95% CI, 1.15–1.64; P=0.0005) and dabigatran (rivaroxaban versus dabigatran: HR, 1.28; 95% CI, 1.07–1.5; P=0.005). Dabigatran and warfarin exhibited similar gastrointestinal bleeding risk in the non‐VHD group (Figure S4).
Nongastrointestinal bleeding was significantly reduced with NOACs versus warfarin in the VHD (dabigatran versus warfarin: HR, 0.17; 95% CI, 0.06–0.49; P=0.001; rivaroxaban versus warfarin: HR, 0.37; 95% CI, 0.17–0.84; P=0.017) (Figure 5), and non‐VHD (dabigatran versus warfarin: HR, 0.34; 95% CI, 0.23–0.5; P<0.001; rivaroxaban versus warfarin: HR, 0.42; 95% CI, 0.28–0.6; P<0.001) groups (Figure 6). We did not identify differences in nongastrointestinal bleeding between NOACs (Table 5).
Figure 5.
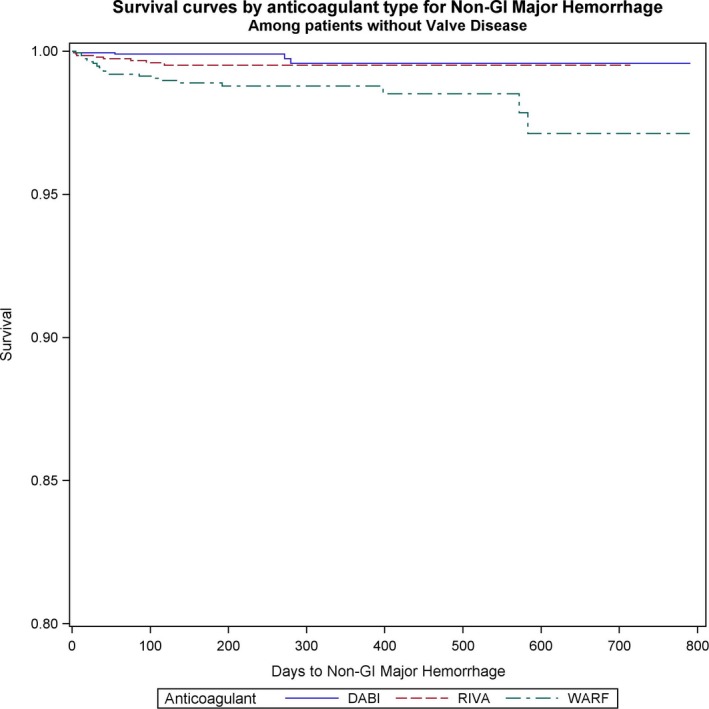
Survival curves for nongastrointestinal (non‐GI) bleeding comparing the 3 anticoagulants (warfarin [WARF], dabigatran [DABI], and rivaroxaban [RIVA]) in patients with valvular heart disease with newly diagnosed atrial fibrillation.
Figure 6.
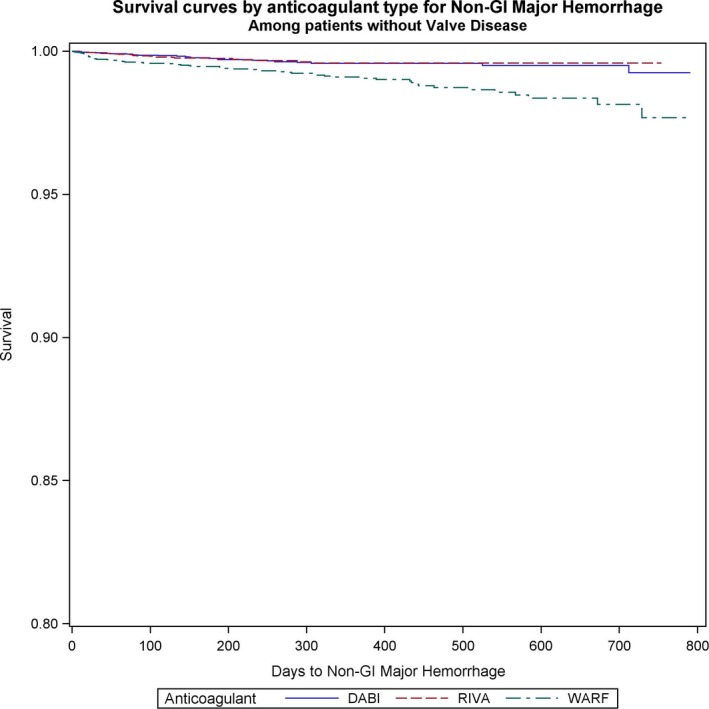
Survival curves for nongastrointestinal (non‐GI) bleeding comparing the 3 anticoagulants (warfarin [WARF], dabigatran [DABI], and rivaroxaban [RIVA]) in patients without valvular heart disease with newly diagnosed atrial fibrillation.
Acute myocardial infarction
Acute myocardial infarction did not differ significantly among dabigatran, rivaroxaban, and warfarin in any of the studied groups of patients (Tables 3 and 4) (Figures S5 and S6).
Subgroup analysis of patients with mitral valve disease
On the basis of the ICD‐9 codes 4240, 7465, 7466, 3940, and 394.X, we identified 9960 patients with mitral valve disease (55% of all valve patients). Only 323 patients had mitral stenosis on the basis of the ICD‐9 code 394.0; hence, we did not analyze these patients separately. The results for mitral valve disease were similar to these for the entire VHD population. For example, the relative hazard of death was 0.70 (P=0.049), 0.51 (P<0.01), and 0.73 (P=0.14) for dabigatran versus warfarin, rivaroxaban versus warfarin, and dabigatran versus rivaroxaban, respectively, in patients with mitral valve disease. As with the matched samples of all patients with valvular disease, there were no significant differences by anticoagulant type in the hazard of stroke, any major hemorrhage, gastrointestinal hemorrhage, or acute myocardial infarction, but the relative hazards of nongastrointestinal major hemorrhage were significantly lower for dabigatran relative to rivaroxaban and warfarin.
IPTW analysis
Results of the IPTW are shown in Table S1. For patients without VHD, conclusions with respect to the presence of significant differences and direction of difference were identical in the propensity‐matched analysis and IPTW analysis, although the magnitude of differences differs slightly. For example, relative hazards of any major hemorrhage for dabigatran versus warfarin, rivaroxaban versus warfarin, and rivaroxaban versus dabigatran were 0.84 (P=0.04), 1.08 (P=0.3), and 1.28 (P=0.003), respectively, in the propensity‐matched sample, compared with 0.88 (P=0.045), 1.06 (P=0.33), and 1.20 (P=0.018), respectively, in the IPTW analysis, with dabigatran having significantly lower bleeding rates than warfarin and rivaroxaban in both analyses.
For patients with valve disease, dabigatran and rivaroxaban were associated with significantly lower mortality compared with warfarin, which is consistent with the findings in the propensity‐matched analysis, although the reduction in mortality was larger in the IPTW analysis. We also found a significantly lower hazard of stroke with dabigatran versus warfarin (HR, 0.60; 95% CI, 0.38–0.97; P=0.04) and a significantly higher hazard of any major hemorrhage with rivaroxaban versus warfarin (HR, 1.26; 95% CI, 1.01–1.56; P=0.04) in the IPTW analyses, whereas these differences were not statistically significant in propensity‐matched analyses.
Adherence to anticoagulants
Adherence over the initial 180 days of medication use was calculated as the proportion of days covered17 and was 0.68, 0.75, and 0.84 for dabigatran, rivaroxaban, and warfarin, respectively, among patients who survived to 180 days. The proportion of days covered did not differ significantly for patients with and without valvular disease. This is consistent with previous reports of NOAC adherence on the basis of administrative data.25 Overall, 25%, 20%, and 17% of dabigatran, rivaroxaban, and warfarin users ceased the initial anticoagulant within 90 days.
Discussion
The salient findings of this analysis of a nationally representative sample of Medicare claims in patients with AF with and without VHD can be summarized as follows: (1) patients with VHD have higher comorbidity burden and higher rates of all‐cause mortality, stroke, and gastrointestinal bleeding than those without VHD; (2) after propensity‐matching analysis, NOACs were associated with reduced all‐cause mortality risk compared with warfarin in both patients with and without VHD and with AF; (3) ischemic stroke rates were similar between anticoagulants in patients with VHD, whereas rivaroxaban was associated with lower stroke rates than warfarin in the patients without VHD; (4) nongastrointestinal bleeding risk was lower with NOACs than warfarin in both patients with and without VHD. Rivaroxaban was associated with higher gastrointestinal bleeding rates than both dabigatran and warfarin in patients without VHD.
A significant percentage of patients with AF have VHD, and on the basis of the current definition of valvular AF, they are classified as patients with “nonvalvular AF.” For these patients, 4 NOACs can be used for primary and secondary prevention of thromboembolic events. The definition of valvular AF has evolved to include rheumatic mitral stenosis and mechanical valve, bioprosthetic heart valve, and mitral valve repair, according to the American College of Cardiology/American Heart Association/Heart Rhythm Society 2014 guidelines26; or mechanical heart valves and hemodynamically significant valve disease, severe enough to warrant surgical or percutaneous intervention, according to a recent consensus from the European Heart Rhythm Association.27 The randomized controlled trials of NOACs2, 3, 4, 5 excluded patients with severe mitral stenosis (ARISTOTLE, ENGAGE‐AF [Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation], RE‐LY, ROCKET‐AF), prosthetic or mechanical valves (excluded in all 4 pivotal trials), and hemodynamically significant valve disease (ROCKET‐AF and RE‐LY trial). However, 26.4% of patients in the ARISTOTLE trial, 21.8% of patients in the RE‐LY trial, and 14.1% of patients in ROCKET‐AF included those with at least moderate VHD, which, in the vast majority of cases, was mitral regurgitation. The rationale behind exclusion of severe mitral stenosis or hemodynamically significant valve disease was the increased thromboembolic risk and the need for surgical or percutaneous intervention. Furthermore, on the basis of the results of the RE‐ALIGN (Dabigatran Etexilate in Patients With Mechanical Heart Valves) trial,28 the use of dabigatran in patients with mechanical heart valves was associated with increased rates of thromboembolic and bleeding complications. In our study, we excluded patients who had valve replacement (likely attributable to hemodynamically significant valve disease) during the study period.
Previous post hoc analyses of patients with VHD included in the pivotal NOAC trials showed increased stroke and major bleeding risk among patients with VHD, similar to our study findings.8, 9, 10 The relative benefits of NOACs were comparable in patients with and without VHD, despite higher thromboembolic and bleeding risk in the VHD cohorts. We demonstrated that NOACs and warfarin had similar stroke and gastrointestinal bleeding rates, a pattern similar to the findings of the RE‐LY trial and ROCKET‐AF. In addition, we found a consistent reduction in all‐cause mortality risk with NOACs in both patients with and without VHD, which was possibly because of, at least in part, lower nongastrointestinal and intracranial bleeding with NOACs compared with warfarin. Notably, the risk of hemorrhagic stroke was significantly lower with apixaban, rivaroxaban, and dabigatran compared with warfarin, in the pivotal trials. Other potential reasons for the differences in mortality between anticoagulants are the administration of NOACs in generally healthier and more stable patients as well as lower time in therapeutic range among patients taking warfarin in the general population. We attempted to eliminate the differences in comorbidity burden between NOACs and warfarin by propensity‐score analysis, including all the available patient‐related characteristics. Furthermore, Medicare Part D (prescription benefit plan) beneficiaries may have greater ability to adhere to prescription medications and achieve acceptable times in therapeutic range compared with patients without prescription coverage.
The mechanisms leading to systemic thromboembolism vary between patients with and without valvular AF. In patients with nonvalvular AF in whom NOACs have shown reliable results, thrombi develop predominantly in the left atrial appendage because of low flow, low shear stress, and stasis.29 Patients with bioprosthetic and mechanical valves develop valve thrombosis, and in these cases, coagulation cascade is triggered by contact of the serum with artificial surfaces. For these patients, vitamin K antagonism results in inhibition not only of thrombin and factor X but also factors VII and IX, which may participate in the activation of coagulation cascade in patients with artificial valves. Despite the theoretical differences in the pathophysiological characteristics of thrombosis between patients with and without valvular AF, cases of left atrial thrombosis in severe rheumatic mitral stenosis, despite treatment with dabigatran, have been reported.30 In our study, we did not exclude patients with rheumatic valvular disease, and we believe it may be unsafe to extrapolate our findings to the group of patients with hemodynamically significant rheumatic valvular disease, who represent a minority of our patient population.
Limitations
Potential limitations of this study should also be considered. First, because of the observational nature of the study, it is impossible that unmeasured confounders could have affected our results, despite propensity‐match analysis. Second, AF and VHD were identified via ICD‐9 codes, and the reliability of ICD‐9 codes for VHD is unclear. Third, our analysis included patients aged >65 years, and the results may not be generalizable to younger patients. In addition, we did not include patients taking lower doses or dabigatran or rivaroxaban adjusted for their renal function. On the basis of the analysis by Graham and colleagues on Medicare beneficiaries with nonvalvular AF,31 19.6% of patients taking dabigatran and 26.8% of patients taking rivaroxaban received an adjusted dose. The increased risk of major gastrointestinal bleeding with rivaroxaban compared with dabigatran was evident in patients taking a lower dose, similarly to patients taking regular doses. Although we excluded these patients from our analysis, we used the 3‐way propensity matching method to create groups of patients receiving anticoagulants that were balanced with respect to patient covariates and had clinical equipoise. Therefore, patients included in the matched samples were plausible candidates for all 3 anticoagulants under study. Finally, we are lacking detailed evidence on time in therapeutic range, AF burden, and severity of VHD. The main strengths of our study are the large sample size, the application of propensity‐matched analysis, and the inclusion of patients with newly diagnosed AF who initiated anticoagulation during the study period.
Conclusion
The purpose of our study was to improve understanding of safety and efficacy of NOACs in patients with AF and VHD. In patients without prosthetic valves, dabigatran and rivaroxaban were associated with lower risk of death and nongastrointestinal bleeding and similar rates of stroke with warfarin. Therefore, clinicians have >1 anticoagulation option available for patients without hemodynamically significant valvular disease requiring surgery. Further validation of our results is warranted, especially in high thromboembolic risk patients with VHD, such as mitral stenosis and rheumatic valvular disease.
Disclosures
None.
Supporting information
Table S1. Hazard of Outcomes in Using Inverse Probability of Treatment Weights (IPTW)
Figure S1. Survival curves for any bleeding comparing the 3 anticoagulants (WARF, Warfarin; DABI, Dabigatran; RIVA, Rivaroxaban) in patients with VHD with newly diagnosed AF.
Figure S2. Survival curves for any bleeding comparing the 3 anticoagulants in patients without VHD with newly diagnosed AF.
Figure S3. Survival curves for Gastrointestinal bleeding comparing the 3 anticoagulants in patients with VHD with newly diagnosed AF.
Figure S4. Survival curves for Gastrointestinal bleeding comparing the 3 anticoagulants in patients without VHD with newly diagnosed AF.
Figure S5. Survival curves for Acute Myocardial Infarction comparing the 3 anticoagulants in patients with VHD with newly diagnosed AF.
Figure S6. Survival curves for Acute Myocardial Infarction comparing the 3 anticoagulants in patients without VHD with newly diagnosed AF.
(J Am Heart Assoc. 2018;7:e008773 DOI: 10.1161/JAHA.118.008773.)29622591
This article was handled independently by N. A. Mark Estes III, MD, as a guest editor. The editors had no role in the evaluation of the article or in the decision about its acceptance.
References
- 1. Friberg L, Rosenqvist M, Lip GY. Net clinical benefit of warfarin in patients with atrial fibrillation: a report from the Swedish atrial fibrillation cohort study. Circulation. 2012;125:2298–2307. [DOI] [PubMed] [Google Scholar]
- 2. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE‐LY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 3. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al‐Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez‐Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 4. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 5. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. [DOI] [PubMed] [Google Scholar]
- 6. Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Oliveira MM, Mairesse G, Crijns HJ, Simantirakis E, Atar D, Kirchhof P, Vardas P, Tavazzi L, Maggioni AP. A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: baseline results of EurObservational Research Programme Atrial Fibrillation (EORP‐AF) Pilot General Registry. Europace. 2014;16:308–319. [DOI] [PubMed] [Google Scholar]
- 7. Petty GW, Khandheria BK, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Predictors of cerebrovascular events and death among patients with valvular heart disease: a population‐based study. Stroke. 2000;31:2628–2635. [DOI] [PubMed] [Google Scholar]
- 8. Avezum A, Lopes RD, Schulte PJ, Lanas F, Gersh BJ, Hanna M, Pais P, Erol C, Diaz R, Bahit MC, Bartunek J, De Caterina R, Goto S, Ruzyllo W, Zhu J, Granger CB, Alexander JH. Apixaban in comparison with warfarin in patients with atrial fibrillation and valvular heart disease: findings from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. Circulation. 2015;132:624–632. [DOI] [PubMed] [Google Scholar]
- 9. Breithardt G, Baumgartner H, Berkowitz SD, Hellkamp AS, Piccini JP, Stevens SR, Lokhnygina Y, Patel MR, Halperin JL, Singer DE, Hankey GJ, Hacke W, Becker RC, Nessel CC, Mahaffey KW, Fox KA, Califf RM; for the ROCKET AF Steering Committee and Investigators . Clinical characteristics and outcomes with rivaroxaban vs. warfarin in patients with non‐valvular atrial fibrillation but underlying native mitral and aortic valve disease participating in the ROCKET AF trial. Eur Heart J. 2014;35:3377–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ezekowitz MD, Nagarakanti R, Noack H, Brueckmann M, Litherland C, Jacobs M, Clemens A, Reilly PA, Connolly SJ, Yusuf S, Wallentin L. Comparison of dabigatran and warfarin in patients with atrial fibrillation and valvular heart disease: the RE‐LY Trial (Randomized Evaluation of Long‐Term Anticoagulant Therapy). Circulation. 2016;134:589–598. [DOI] [PubMed] [Google Scholar]
- 11. Ellis ER, Culler SD, Simon AW, Reynolds MR. Trends in utilization and complications of catheter ablation for atrial fibrillation in Medicare beneficiaries. Heart Rhythm. 2009;6:1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gage BF, Boechler M, Doggette AL, Fortune G, Flaker GC, Rich MW, Radford MJ. Adverse outcomes and predictors of underuse of antithrombotic therapy in Medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–827. [DOI] [PubMed] [Google Scholar]
- 13. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 14. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 15. Pisters R, Lane DA, Niewlaat R, deVos CB, Crijns HJ, Lip GY. A novel user friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 16. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15:565–574. [DOI] [PubMed] [Google Scholar]
- 18. Rassen JA, Shelat AA, Franklin JM, Glynn RJ, Solomon DH, Schneeweiss S. Matching by propensity score in cohort studies with three treatment groups. Epidemiology. 2013;24:401–409. [DOI] [PubMed] [Google Scholar]
- 19. Crump RK, Hotz VJ, Imbens GW. Dealing with limited overlap in estimation of average treatment effects. Biometrika. 2009;96:187–199. [Google Scholar]
- 20. Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, Robins JM. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity‐based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163:262–270. [DOI] [PubMed] [Google Scholar]
- 21. Lunt M, Solomon D, Rothman K, Glynn R, Hyrich K, Symmons DP, Stürmer T; British Society for Rheumatology Biologics Register; British Society for Rheumatology Biologics Register Control Centre Consortium . Different methods of balancing covariates leading to different effect estimates in the presence of effect modification. Am J Epidemiol. 2009;169:909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brookhart MA, Wyss R, Layton JB, Stürmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown JD, Shewale AR, Talbert JC. Adherence to rivaroxaban, dabigatran, and apixaban for stroke prevention in incident treatment‐naïve nonvalvular atrial fibrillation. J Manag Care Spec Pharm. 2016;22:1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. [DOI] [PubMed] [Google Scholar]
- 27. Lip GY, Windecker S, Huber K, Kirchhof P, Marin F, Ten Berg JM, Haeusler KG, Boriani G, Capodanno D, Gilard M, Zeymer U, Lane D; Document Reviewers , Storey RF, Bueno H, Collet JP, Fauchier L, Halvorsen S, Lettino M, Morais J, Mueller C, Potpara TS, Rasmussen LH, Rubboli A, Tamargo J, Valgimigli M, Zamorano JL. Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary or valve interventions: a joint consensus document of the European Society of Cardiology Working Group on Thrombosis, European Heart Rhythm Association (EHRA), European Association of Percutaneous Cardiovascular Interventions (EAPCI) and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS) and Asia‐Pacific Heart Rhythm Society (APHRS). Eur Heart J. 2014;35:3155–3179. [DOI] [PubMed] [Google Scholar]
- 28. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt JU, Simoons ML, van de Werf F; RE‐ALIGN Investigators . Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. [DOI] [PubMed] [Google Scholar]
- 29. Leung DY, Black IW, Cranney GB, Hopkins AP, Walsh WF. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1994;24:755–762. [DOI] [PubMed] [Google Scholar]
- 30. Luis SA, Poon K, Luis C, Shukla A, Bett N, Hamilton‐Craig C. Massive left atrial thrombus in a patient with rheumatic mitral stenosis and atrial fibrillation while anticoagulated with dabigatran. Circ Cardiovasc Imaging. 2013;6:491–492. [DOI] [PubMed] [Google Scholar]
- 31. Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR, Wei Y, Liao J, Goulding MR, Mott K, Chillarige Y, MaCurdy TE, Worrall C, Kelman JA. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. 2016;176:1662–1671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Hazard of Outcomes in Using Inverse Probability of Treatment Weights (IPTW)
Figure S1. Survival curves for any bleeding comparing the 3 anticoagulants (WARF, Warfarin; DABI, Dabigatran; RIVA, Rivaroxaban) in patients with VHD with newly diagnosed AF.
Figure S2. Survival curves for any bleeding comparing the 3 anticoagulants in patients without VHD with newly diagnosed AF.
Figure S3. Survival curves for Gastrointestinal bleeding comparing the 3 anticoagulants in patients with VHD with newly diagnosed AF.
Figure S4. Survival curves for Gastrointestinal bleeding comparing the 3 anticoagulants in patients without VHD with newly diagnosed AF.
Figure S5. Survival curves for Acute Myocardial Infarction comparing the 3 anticoagulants in patients with VHD with newly diagnosed AF.
Figure S6. Survival curves for Acute Myocardial Infarction comparing the 3 anticoagulants in patients without VHD with newly diagnosed AF.


