Abstract
Background
The American Heart Association has defined metrics of ideal cardiovascular health known as Life's Simple 7 (LS7) to prevent cardiovascular disease. We examined the association between LS7 and incident atrial fibrillation (AF) in a biracial cohort of middle‐ and older‐aged adults without known cardiovascular disease.
Methods and Results
This analysis included 13 182 ARIC (Atherosclerosis Risk in Communities) study participants (mean baseline age=54±5.7 years; 56% women; 25% black) free of AF and cardiovascular disease. An overall LS7 score was calculated as the sum of the LS7 component scores and classified as inadequate (0‐4), average (5‐9), or optimal (10‐14) cardiovascular health. The primary outcome was incident AF, identified primarily by ECG and hospital discharge coding of AF through December 31, 2014. A total of 2266 (17%) incident AF cases were detected over a median follow‐up of 25.1 years. Compared with the inadequate category (n=1057), participants in the average (n=8629) and optimal (n=3496) categories each had a lower risk of developing AF in a multivariable Cox proportional hazards model (hazard ratio 0.59, 95% confidence interval 0.51, 0.67 for average; and hazard ratio 0.38, 95% confidence interval 0.32, 0.44 for optimal). In a similar model, a 1‐point‐higher LS7 score was associated with a 12% lower risk of incident AF (hazard ratio 0.88, 95% confidence interval 0.86, 0.89).
Conclusions
A higher LS7 score is strongly associated with a lower risk of AF in individuals without baseline cardiovascular disease. Determining whether interventions that improve the population's cardiovascular health also reduce AF incidence is needed.
Keywords: atrial fibrillation, prevention, risk factors/global assessment, risk prediction
Subject Categories: Atrial Fibrillation, Primary Prevention, Epidemiology, Statements and Guidelines
Clinical Perspective
What Is New?
In a population without baseline cardiovascular disease, better cardiovascular health, as measured by the American Heart Association's Life's Simple 7 metric, was associated with reduced risk of incident atrial fibrillation (AF).
Compared with individuals with inadequate cardiovascular health, individuals with average and optimal cardiovascular health had a 37% and 57% lower risk of developing AF, respectively.
A 1‐point‐higher Life's Simple 7 score was associated with an 11% lower risk of incident AF.
What Are the Clinical Implications?
These findings suggest that the American Heart Association's Life's Simple 7 metric may also be an effective tool to help promote AF prevention in addition to cardiovascular disease prevention.
Formal evaluation of whether population‐based efforts to improve Life's Simple 7 health behaviors and risk factors decrease AF incidence is needed.
The American Heart Association Strategic Planning Task Force and Statistics Committee developed the Life's Simple 7 (LS7), which consists of 7 modifiable health behaviors and biological factors (smoking, body mass index [BMI], physical activity, diet, total cholesterol, blood pressure, and fasting blood glucose), as part of its 2020 impact goal to improve the cardiovascular health of all Americans by 20% while reducing deaths from cardiovascular disease (CVD) and stroke by 20%.1 Attainment of ideal cardiovascular health has been associated with a reduced incidence of coronary heart disease, stroke, and heart failure (HF).2, 3, 4, 5, 6
Atrial fibrillation (AF) is the most commonly presenting cardiac arrhythmia in clinical practice, affecting over 2 million people in the United States.7 In a study of more than 5000 middle‐ and older‐aged individuals without baseline CVD, nearly 10% developed AF over a median follow‐up of only 8.6 years.8 AF is a major source of cardiovascular morbidity and mortality and incurs a high healthcare burden.9, 10, 11, 12 AF and other CVDs share many common risk factors, and poor control of some individual LS7 components has been associated with an increased AF risk.9, 13, 14, 15, 16
There are limited studies, however, on whether a better LS7 score is also associated with a reduced risk of AF and no studies in populations free of baseline CVD.17 Demonstrating such a relationship between LS7 and AF in this population can further heighten interest toward achievement of the American Heart Association's strategic 2020 goals and refine estimates of the impact of achieving such goals. Therefore, the purpose of this study was to examine the association between LS7 and incident AF in a subgroup of ARIC (Atherosclerosis Risk in Communities) participants without baseline CVD.
Methods
Study Population and Design
The ARIC study included 15 792 men and women aged 45 to 64 years sampled from 4 US communities in 1987‐1989.18 Trained interviewers administered a questionnaire to collect information on demographic characteristics, health behaviors, medical history, and medication use. Fasting blood samples were also collected. Participants were reexamined in 1990‐1992 (93% response), 1993‐1995 (86%), 1996‐1998 (80%), and 2011‐2013 (65%) and followed for cardiovascular events. Institutional review boards at each participating institution (University of Minnesota, Johns Hopkins University, University of North Carolina, and University of Mississippi Medical Center) approved the study, and all participants gave written informed consent at each study visit. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
After excluding participants with CVD or AF (defined below) at baseline (n=1887), with missing data for any of the LS7 components (n=635), and, due to small numbers, race other than black or white and the few black participants from Minneapolis and Washington County (n=87), the remaining sample size was 13 182. The current study is a prospective analysis of these participants to determine the relationship of the LS7, assessed at baseline (1987‐1989), and incident AF with follow‐up through 2014.
Life's Simple 7
LS7 components were assessed at each study visit except for diet, which was only assessed at baseline and visit 3. Cigarette smoking status was categorized as current, former, or never. Former smokers were further classified according to quit date (>12 months versus ≤12 months). Field center staff directly measured weight and standing height, and BMI was calculated as measured weight in kilograms divided by standing height in meters squared. Systolic blood pressure and diastolic blood pressure were measured 3 times, and the average of the last 2 measurements was used. Total blood cholesterol concentration was assessed by enzymatic procedures.19 Blood glucose levels were measured by the modified hexokinase/glucose‐6‐phosphate dehydrogenase method.
Physical activity was measured using the modified Baecke questionnaire, which defines 3 semicontinuous indices ranging from 1 (low) to 5 (high) for physical activity in sports, during leisure time, and at work.20, 21 Responses were subsequently converted to minutes per week of moderate or vigorous exercise based on metabolic equivalent values and the number of months annually a participant partook in the activity.22
Usual dietary intake was determined at baseline using a semiquantitative 66‐item food‐frequency questionnaire administered by trained interviewers to improve accuracy and completeness.23, 24 Frequency and portion size of each food item were multiplied by nutritional content from US Department of Agriculture sources to estimate intake of micro‐ and macronutrients. The healthy diet score was calculated as the sum of the scores for each of 5 individual components: fruits and vegetables (≥4.5 cups per day), fish (≥2 3.5‐oz servings per week), fiber‐rich whole grains (≥3 1‐oz‐equivalent serving per day), sodium (<1500 mg/day), sugar‐sweetened beverages (≤450 kcal/week). The range is from 0 to 5, with a lower score being unhealthy.
Each individual component was categorized as poor, intermediate, or ideal according to the American Heart Association's LS7 criteria.1 Ideal levels of health factors were: nonsmoker or quit >1 year ago; body mass index <25 kg/m2; blood pressure <120/80 mm Hg; total cholesterol <200 mg/dL; fasting blood glucose <100 mg/dL; ≥150 min/week of physical activity; and a healthy diet score (≥4 components). Study participants who were treated to target levels for hypercholesterolemia, hypertension, or diabetes mellitus were classified as intermediate for the respective health factor. An overall LS7 score ranging from 0 to 14 was calculated as the sum of the LS7 component scores (2 points for ideal, 1 point for intermediate, and 0 for poor). This score was classified as inadequate (0‐4), average (5‐9), or optimum (10‐14) cardiovascular health.
Atrial Fibrillation
Incident AF was defined as in previous ARIC analyses.25 Study participants underwent electrocardiographic recordings at baseline and at each follow‐up exam. ECGs were read at the Epidemiology Coordinating and Research Centre at the University of Alberta (Edmonton, Alberta, Canada) during the initial phases of the study and at the Epidemiological Cardiology Research Center at the Wake Forest School of Medicine (Winston‐Salem, NC) during later phases. All ECG recordings automatically coded as AF were visually rechecked by a trained cardiologist to confirm the diagnosis. Individuals with ECG‐diagnosed AF at the initial examination were considered to have baseline AF and excluded from the analysis. A trained abstractor obtained and recorded all International Classification of Diseases, Ninth Revision, Clinical Modification (ICD?9?CM) hospital discharge diagnoses from each participant's hospitalizations reported in the annual follow‐up interview. AF was defined as the presence of ICD‐9‐CM code 427.31 or 427.32 in the discharge codes. AF hospitalization diagnoses occurring simultaneously with heart revascularization surgery or other cardiac surgery involving heart valves or septa, without evidence of AF in subsequent hospitalizations or study exams, were excluded. ARIC participants were also labeled as AF cases if the underlying cause of death was AF. The incidence date of AF was defined as the date for the first ECG showing AF or the first hospital discharge with an AF diagnosis. Follow‐up for incident AF was complete through December 31, 2014.
Covariates
All covariates were assessed at visit 1 (1987‐1989) and included age (years), sex, race, ARIC field center, education level (<high school degree, ≥high school degree), alcohol consumption (never/current/former and drinks/week), medication use (antidiabetic, antihypertensive, and lipid‐lowering therapy), ECG‐defined left ventricular hypertrophy,26 and CVD. Education level was determined from interviews. Medication use (antidiabetic, antihypertensive, aspirin, and lipid‐lowering therapy) was ascertained by asking participants to bring containers of current medications, and then all medication names were documented. CVD at baseline included the presence of coronary heart disease, HF, or stroke. Coronary heart disease was defined as a self‐reported history of physician‐diagnosed myocardial infarction, prior coronary revascularization, or a previous myocardial infarction by ECG. HF was defined as the reported use of HF medication or the presence of HF according to the Gothenburg criteria.27 Stroke was defined as a self‐reported history of physician‐diagnosed stroke.
Statistical Analysis
Baseline characteristics were compared across LS7 health categories (inadequate, average, or optimal). Categorical variables are reported as frequency and percentage, and continuous variables are reported as mean±standard deviation. Statistical significance for categorical variables was tested using the chi‐squared method and either the Student t test or analysis of variance procedure for continuous variables.
The cumulative incidence function was used to plot the cumulative incidence of AF by LS7 categories (ie, poor, intermediate, optimal), accounting for the competing risk of death. Age‐standardized cumulative incidence estimates were computed for AF by LS7 categories using the 2010 United States population as the standard. Follow‐up time was defined as the time from the baseline visit to AF development, death, loss to follow‐up, or end of follow‐up (December 31, 2014). Cox proportional hazards models were used to compute the hazard ratios (HR) and 95% confidence intervals (CI) for incident AF for each individual LS7 component (referent category=poor). HRs for incident AF were also calculated according to number of ideal components (0‐1 [ref.], 2‐3, or 4+ ideal components), per 1 ideal higher component score, across overall LS7 score categories (optimal [9‐14] or average [5‐8] versus inadequate [0‐4]), and per 1‐unit higher overall LS7 score. Multivariable models were constructed as follows: model 1 adjusted for age, sex, race, education, and ARIC study site; model 2 adjusted for model 1 covariates with the addition of alcohol consumption and left ventricular hypertrophy. A competing risk model was not used in our Cox regression analysis, as the main purpose of this analysis was to determine the etiologic association between LS7 categories and incident AF. We evaluated for effect modification by sex and race using a stratification technique and comparing models with and without interaction terms. Because data for all LS7 components were available at baseline and visit 3, we determined how changes in LS7 score between these 2 visits affected AF risk. Change in LS7 score was defined as a change in overall LS7 score categories (referent category=no change). Multivariable models were constructed as above with additional adjustment for baseline LS7 score. Participants with complete data for both study visits and free of AF at visit 3 were eligible for this analysis. The test statistic developed by Grambsch and Therneau was used to test the proportional hazards assumption, and this was not violated in our analyses.28 Statistical significance for all comparisons including interactions was defined as P<0.05. SAS Version 9.4 (SAS Institute, Cary, NC) was used for all analyses.
Results
A total of 13 182 participants free of baseline CVD with data on all LS7 measures and no previous AF were included in the final analysis. Mean age was 54±5.7 years, 25% were black, and 44% male. The mean LS7 score at baseline was 7.9±2.4 (black 6.7±2.3; white 8.3±2.2; P<0.001), and 2266 (17%) participants developed AF over a median follow‐up of 25.1 years. Sociodemographic and clinical characteristics across baseline LS7 categories are compared in Table 1. Older age, male sex, black race, less education, higher BMI, higher systolic blood pressure, higher diastolic blood pressure, higher total cholesterol, higher fasting glucose, smoking, less physical activity, an unhealthy diet, and left ventricular hypertrophy were all associated with poorer cardiovascular health, whereas current alcohol use was associated with better cardiovascular health.
Table 1.
Baseline Characteristics of ARIC Participants Without Known Cardiovascular Disease According to Life's Simple 7 Health Categorya
| Characteristic | Inadequate, LS7=0 to 4 (n=1057) | Average, LS7=5 to 9 (n=8629) | Optimal, LS7=10 to 14 (n=3496) | P Valueb |
|---|---|---|---|---|
| Age, y | 55 (5.6) | 54 (5.7) | 53 (5.7) | <0.001 |
| Male, n (%) | 437 (41) | 4131 (48) | 1258 (36) | <0.001 |
| Black, n (%) | 567 (5.4) | 2320 (27) | 390 (11) | <0.001 |
| Education <12 y, n (%) | 775 (73) | 4990 (58) | 1428 (41) | <0.001 |
| Body mass index, kg/m2 | 32 (5.4) | 28 (5.2) | 25 (3.4) | <0.001 |
| Systolic blood pressure, mm Hg | 137 (21) | 123 (18) | 111 (13) | <0.001 |
| Diastolic blood pressure, mm Hg | 81 (13) | 75 (11) | 69 (8.7) | <0.001 |
| Total cholesterol, mg/dL | 245 (43) | 218 (41) | 196 (34) | <0.001 |
| Glucose, mg/dL | 141 (70) | 108 (37) | 95 (13) | <0.001 |
| Smoking status, n (%) | <0.001 | |||
| Never | 527 (50) | 2514 (29) | 333 (9) | |
| Former | 314 (30) | 2884 (33) | 972 (28) | |
| Current | 216 (20) | 3231 (38) | 2191 (63) | |
| Physical activity, n (%) | <0.001 | |||
| None | 938 (89) | 3658 (43) | 221 (6) | |
| Intermediate | 31 (3) | 378 (4) | 125 (4) | |
| Ideal | 88 (8) | 4593 (53) | 3150 (90) | |
| Healthy diet score, n (%) | <0.001 | |||
| Poor | 800 (76) | 4687 (54) | 1086 (31) | |
| Intermediate | 247 (23) | 3601 (42) | 1934 (55) | |
| Ideal | 10 (1) | 341 (4) | 476 (14) | |
| Alcohol consumption, % | <0.001 | |||
| Never | 328 (31) | 2134 (25) | 823 (23) | |
| Former | 255 (24) | 1583 (18) | 474 (14) | |
| Current | 474 (45) | 4912 (57) | 2199 (63) | |
| Left ventricular hypertrophy, % | 86 (8) | 253 (3) | 32 (1) | <0.001 |
ARIC indicates Atherosclerosis Risk in Communities; LS7, Life's Simple 7.
Continuous variables are expressed as mean (SD). Categorical variables are n (%).
Statistical significance for categorical variables tested using the chi‐squared method, and for continuous variables the analysis of variance procedure was used.
Blood glucose and physical activity were the most common ideal health components in the study population, and an ideal healthy dietary pattern was the least common (Figure 1). The risk of incident AF according to the individual LS7 components, expressed as poor, intermediate, or ideal, evaluated in separate models is shown in Figure 2. Compared with participants in the poor category of their respective components, those with ideal levels of physical activity and those with either intermediate or ideal levels of body mass index, blood pressure, fasting glucose, and smoking were at a significantly lower risk of incident AF after adjustment for age, sex, race, education, study site, alcohol use, and left ventricular hypertrophy (Figure 2). No associations with incident AF were observed for healthy diet score or total cholesterol (Figure 2).
Figure 1.
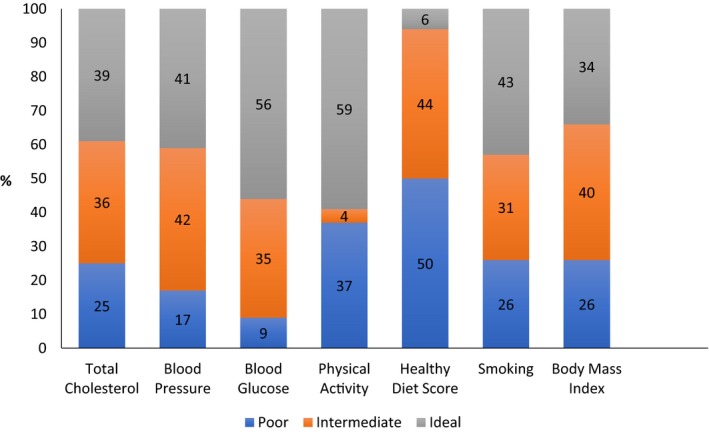
Distribution of Life's Simple 7 components among ARIC (Atherosclerosis Risk in Communities) participants at baseline, 1987‐1989 (n=13 182).
Figure 2.
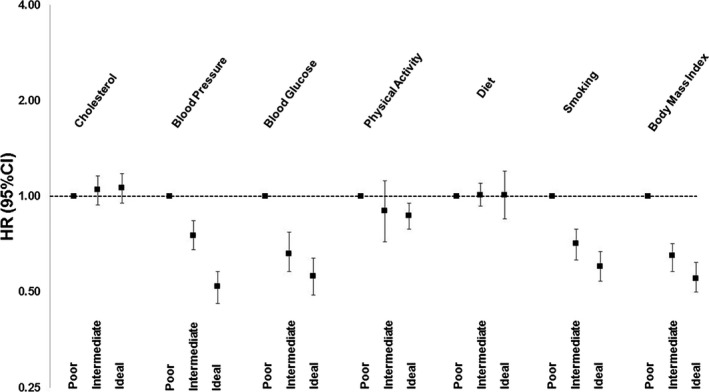
Hazard ratios (HR) and 95% confidence intervals (CI) of incident atrial fibrillation according to individual Life's Simple 7 (LS7) components. All hazard ratios are adjusted for age, sex, race, education, ARIC (Atherosclerosis Risk in Communities) study site, alcohol consumption, and left ventricular hypertrophy. The referent category (farthest left) for the individual LS7 components represents poor cardiovascular health. Error bars seen in this figure represent 95% confidence intervals.
The majority of participants had 2 or 3 ideal health components (Table 2). A much smaller proportion of the population was at the extremes of this spectrum, with 357 (2.7%) achieving 6 or 7 ideal health components and 478 (3.6%) lacking any ideal components. Twelve percent (n=42) of those with at least 6 ideal health components at baseline developed AF during follow‐up compared with 23% (n=109) of study participants with 0 ideal health components. Compared with participants with 0 or 1 ideal health components, those with either 2 to 3 ideal health components or at least 4 ideal health components were at a significantly lower risk of incident AF in adjusted analysis (Table 2). A 1‐component higher number of ideal health metrics was associated with a 16% lower risk of incident AF in adjusted analysis (HR=0.83; 95% CI 0.81, 0.86).
Table 2.
Associations Between Number of Ideal Life's Simple 7 Components and Incident Atrial Fibrillationa
| n AF/N at Risk (%) | Incidence Rate Per 1000 Person‐Years | Model 1b HR (95% CI) | Model 2c HR (95% CI) | |
|---|---|---|---|---|
| Number of ideal LS7 components | ||||
| 0 to 1 | 554/2482 (22.3%) | 11 | Ref | Ref |
| 2 to 3 | 1220/6637 (18.4%) | 8.7 | 0.73 (0.66, 0.81) | 0.75 (0.68, 0.83) |
| 4+ | 492/4063 (12.1%) | 5.3 | 0.47 (0.41, 0.53) | 0.50 (0.44, 0.56) |
| Per ideal component increase | 0.82 (0.79, 0.85) | 0.83 (0.81, 0.86) | ||
AF indicates atrial fibrillation; CI, confidence interval; HR, hazard ratio; LS7, Life's Simple 7.
Results of Cox proportional hazards models.
Model 1 adjusted for age, sex, race, education, and ARIC (Atherosclerosis Risk in Communities) study site.
Model 2 adjusted for model 1+alcohol consumption and left ventricular hypertrophy.
Figure 3 shows the cumulative incidence of AF according to LS7 category. In multivariable adjusted analyses, participants in the average and optimal categories had a 37% and 57% respective lower risk of developing AF compared with the inadequate category (HR=0.59; 95% CI 0.51, 0.67 for intermediate and HR=0.38; 95% CI 0.32, 0.44 for ideal) (Table 3). A 1‐point‐higher LS7 score was associated with a 12% lower risk of incident AF (HR=0.88; 95% CI 0.86, 0.89). Associations did not significantly differ in sex‐ or race‐stratified analyses (Table 4).
Figure 3.
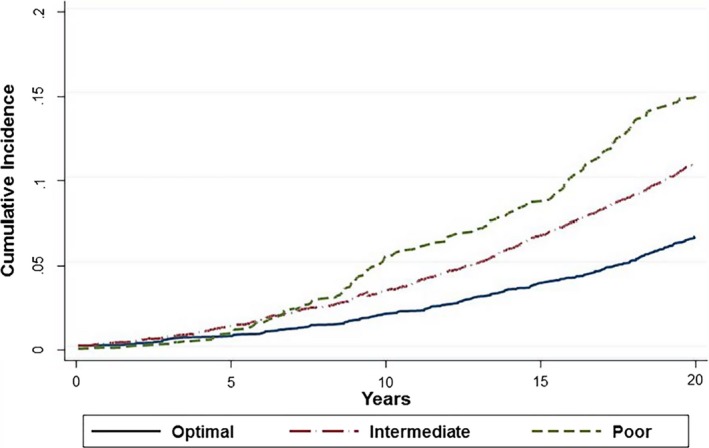
Cumulative incidence of atrial fibrillation by Life's Simple 7 Score adjusted for competing risk of death.
Table 3.
Associations Between Overall Life's Simple 7 Score and Incidence of Atrial Fibrillationa
| n AF/N at Risk (%) | Incidence Rate per 1000 Person Yearsb | Model 1c HR (95% CI) | Model 2d HR (95% CI) | |
|---|---|---|---|---|
| LS7 health categories | ||||
| Inadequate (0‐4 points) | 238/1057 (22.5%) | 12 | Ref | Ref |
| Average (5‐9 points) | 1591/8629 (18.4%) | 8.8 | 0.56 (0.49, 0.65) | 0.59 (0.51, 0.67) |
| Optimal (10‐14 points) | 437/3496 (13.5%) | 5.9 | 0.35 (0.30, 0.41) | 0.38 (0.32, 0.44) |
| LS7 score per unit increase | 0.87 (0.85, 0.89) | 0.88 (0.86, 0.89) | ||
AF indicates atrial fibrillation; CI, confidence interval; HR, hazard ratio; LS7, Life's Simple 7.
Results of multivariable Cox proportional hazards models.
Standardized using the 2010 United States population.
Model 1 adjusted for age, sex, race, education, and ARIC (Atherosclerosis Risk in Communities) study site.
Model 2 adjusted for model 1+alcohol consumption and left ventricular hypertrophy.
Table 4.
Associations Between Overall Life's Simple 7 Score and Incident Atrial Fibrillation Stratified by Race and Sexa
| n AF/N at Risk (%) | Model 1b HR (95% CI) | Model 2c HR (95% CI) | n AF/N at Risk (%) | Model 1b HR (95% CI) | Model 2c HR (95% CI) | Interaction P Valued | |
|---|---|---|---|---|---|---|---|
| Black Participants (n=3277) | White Participants (n=9905) | ||||||
| LS7 health categories | |||||||
| Inadequate (0‐4 points) | 100/567 (17.6%) | Ref | Ref | 138/490 (28.1%) | Ref | Ref | |
| Average (5‐9 points) | 276/2320 (11.9%) | 0.53 (0.42, 0.67) | 0.56 (0.45, 0.71) | 1315/6309 (20.8%) | 0.59 (0.49, 0.70) | 0.60 (0.51, 0.72) | |
| Optimal (10‐14 points) | 30/390 (7.7%) | 0.34 (0.22, 0.51) | 0.40 (0.26, 0.61) | 407/3106 (13.1%) | 0.36 (0.30, 0.44) | 0.38 (0.31, 0.46) | 0.75 |
| LS7 score per unit increase | 0.85 (0.81, 0.89) | 0.87 (0.83, 0.91) | 0.87 (0.85, 0.89) | 0.88 (0.86, 0.90) | 0.53 | ||
| Male participants (n=5826) | Female participants (n=7356) | ||||||
| LS7 health categories | |||||||
| Inadequate (0‐4 points) | 114/437 (26.0%) | Ref | Ref | 124/620 (20.0%) | Ref | Ref | |
| Average (5‐9 points) | 853/4131 (20.6%) | 0.54 (0.45, 0.66) | 0.56 (0.46, 0.68) | 738/4498 (16.4%) | 0.59 (0.48, 0.72) | 0.62 (0.51, 0.76) | |
| Optimal (10‐14 points) | 207/1258 (16.4%) | 0.36 (0.29, 0.46) | 0.38 (0.30, 0.49) | 230/2238 (10.3%) | 0.34 (0.27, 0.43) | 0.38 (0.30, 0.48) | 0.46 |
| LS7 score per unit increase | 0.89 (0.86, 0.91) | 0.89 (0.87, 0.92) | 0.85 (0.83, 0.88) | 0.86 (0.84, 0.89) | 0.11 | ||
AF indicates atrial fibrillation; CI, confidence interval; HR, hazard ratio; LS7, Life's Simple 7.
Results of multivariable Cox regression models.
Model 1 adjusted for age, race, education, and study site.
Model 2 adjusted for model 1+alcohol consumption and left ventricular hypertrophy.
Interactions tested using model 2.
Table 5 shows that an improvement in overall LS7 score category over time was also associated with a reduced risk of incident AF (HR=0.84; 95% CI 0.72, 0.97). The risk reduction was greatest for individuals with an inadequate score at baseline (Table 5). Of the 10 482 participants included in this analysis, 7336 (70%) had no change in LS7 category, 1944 (19%) observed a decrease in category, and 1202 (11%) improved their score.
Table 5.
Change in Life's Simple 7 Category Between Visit 1 and Visit 3 and Risk of Incident Atrial Fibrillation (N=10 482)a
| All (N=10 482) | Baseline LS7 Category | |||
|---|---|---|---|---|
| Poor (N=678) | Intermediate (N=6737) | Ideal (N=3067) | ||
| Change in LS7 category | ||||
| Decrease | 1.03 (0.91, 1.18) | ··· | 1.13 (0.93, 1.37) | 1.09 (0.88, 1.35) |
| No change | Ref | Ref | Ref | Ref |
| Increase | 0.84 (0.72, 0.97) | 0.56 (0.41, 0.77) | 0.93 (0.77, 1.12) | ··· |
Data presented as HR (95% CI). CI indicates confidence interval; HR, hazard ratio; LS7, Life's Simple 7.
Results of multivariable Cox proportional hazards model adjusted for baseline LS7 score, age, sex, race, education, ARIC (Atherosclerosis Risk in Communities) study site, alcohol consumption, and left ventricular hypertrophy. Covariates included were updated using data from visit 3.
Discussion
In this cohort of individuals without baseline CVD, a higher LS7 score was associated with a reduced risk of developing AF. Individuals with average and optimal cardiovascular health had a 41% and 62% respective lower risk of developing AF compared with those with inadequate health. Associations did not significantly differ by race or sex. Although achievement of ideal cardiovascular health was rare in this population, an increase of 1 ideal health component was still associated with a 17% lower risk of AF. More importantly, an improvement of only 1 component of the LS7 by 1 level (ie, poor to intermediate or intermediate to ideal) resulted in a 12% lower risk of AF. Therefore, any improvement in the population distribution of LS7 components may considerably reduce overall AF incidence.
Better health status for blood pressure, fasting glucose, tobacco use, and BMI were all associated with a reduced risk of AF in individual LS7 component analysis. This is consistent with prior literature documenting hypertension, diabetes mellitus, smoking, and obesity as established risk factors for the development of AF.29, 30, 31, 32, 33 Ideal physical activity also protected against AF. Although the majority of evidence suggests physical activity reduces AF risk as well, that relationship is more complicated than these for other established risk factors and depends on many factors including individual susceptibility, the environment, and degree of activity.30, 34 In a meta‐analysis of more than 20 studies and 600 000 participants, moderate‐intensity exercise was associated with a lower risk of developing AF, whereas high‐intensity exercise reduced AF risk in women but increased AF risk in men.35 We did not separately assess risk of AF according to physical activity intensity, and these differences can not be excluded in this cohort as well. Similar to our study, prior literature has not demonstrated a reliable association between either total cholesterol or dietary components and AF.36, 37, 38, 39, 40, 41, 42 No previous studies have explored whether specific dietary patterns might be associated with the risk of AF.
Although a higher LS7 score was also associated with a reduced risk of AF in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study, this was demonstrated only in individuals with optimal cardiovascular health, and the association was not as robust as that seen in the present study.17 The relationships of individual LS7 components with AF were not consistent with what has been previously established. Only blood pressure and BMI were associated with incident AF. The significant limitations in study design may explain differences in results. AF was ascertained predominantly by self‐report at a single follow‐up visit almost 10 years after the baseline exam. As a result, sensitivity for detecting AF was diminished because participant recall may not have been accurate and better methods such as hospitalization records were not available. Over two‐thirds of study participants were excluded, and longitudinal time‐to‐event analyses could not be performed. Additionally, 15% of study individuals had baseline CVD, and results may not be directly applicable to asymptomatic populations. Our results build on the established literature by addressing these important limitations and improving generalizability of results.
Nearly 16 million US individuals are projected to have AF by 2050.43 AF accounts for up to 15% of all strokes and carries an in‐hospital mortality of 1%.44, 45 Annual costs for AF treatment are estimated at over 6.5 billion dollars and are driven primarily by hospitalizations, which have increased by 23% from 2000 to 2010.12, 45 Despite the growing public health challenge that AF poses, effective prevention strategies are lacking, and this has been highlighted by reports from the National Heart, Lung, and Blood Institute and European Heart Rhythm Association.29, 30 It is still unknown whether observational data on the associations between lifestyle habits and AF can be translated into effective preventive methods, and existing data have not been fully utilized to discern the potential of a variety of therapeutic maneuvers on reducing incident AF.29 Although the purpose of the LS7 was to reduce incident CVD by improving overall cardiovascular health in this process, our findings indicate it may also represent a valuable prevention strategy for AF. The LS7 is an easily interpretable and accessible tool that both patients and practitioners can focus on for attainment of cardiovascular health goals. More importantly, it can be readily measured with existing and future data and may make tracking improvements in AF prevention efforts easier.
Our study has limitations. The method of AF detection requiring either hospitalization or being present on routine ECG is not sensitive for cases of paroxysmal AF that were either asymptomatic or did not require hospitalization, and these cases were missed. The LS7 was measured at baseline only for a follow‐up period of more than 2 decades. The food frequency questionnaire is not an ideal instrument for capturing dietary intake, especially sodium, and this may have affected assessment of dietary factors and risk of AF. Finally, this is an observational study, and causality cannot be inferred on the basis of these findings.
In conclusion, in this large cohort of middle‐aged US adults without baseline CVD, better cardiovascular health, as reflected by the LS7 score, was associated with a substantially reduced risk of AF. Although the American Heart Association's LS7 metric was designed for the purposes of improving primary CVD prevention, our findings suggest that it may also be an effective tool to help promote AF prevention as well in this population. Formal evaluation of whether population‐based efforts to improve LS7 health behaviors and risk factors decrease AF incidence is needed.
Sources of Funding
The Atherosclerosis Risk in Communities study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). W.T.O. is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number F32HL134290. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health. Additional support was provided by American Heart Association grant 16EIA26410001 (A.A.). L.Y.C. is supported by grant R01 HL126637 from the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
(J Am Heart Assoc. 2018;7:e008424 DOI: 10.1161/JAHA.117.008424.)29650711
References
- 1. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 2. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kulshreshtha A, Vaccarino V, Judd S, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life's simple 7 and risk of incident stroke: REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Stroke. 2013;44:1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2012;125:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nayor M, Enserro DM, Vasan RS, Xanthakis V. Cardiovascular health status and incidence of heart failure in the Framingham Offspring Study. Circ Heart Fail. 2016;9:e002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, Wang TJ, Tofler G, Vasan RS. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–1683. [DOI] [PubMed] [Google Scholar]
- 7. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selvy JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. [DOI] [PubMed] [Google Scholar]
- 8. O'Neal WT, Efird JT, Nazarian S, Alonso A, Michos ED, Szklo M, Heckbert SR, Soliman EZ. Mitral annular calcification progression and the risk of atrial fibrillation: results from MESA. Eur Heart J Cardiovasc Imaging. 2018;19:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort: the Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 10. Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham Study. N Engl J Med. 1982;306:1018–1022. [DOI] [PubMed] [Google Scholar]
- 11. Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008; 92:17–40, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. [DOI] [PubMed] [Google Scholar]
- 13. Huxley RR, Filion KB, Konety S, Alonso A. Meta‐analysis of cohort and case–control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. 2011;108:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heeringa J, Kors JA, Hofman A, van Rooij FJ, Witteman JC. Cigarette smoking and risk of atrial fibrillation: the Rotterdam Study. Am Heart J. 2008;156:1163–1169. [DOI] [PubMed] [Google Scholar]
- 15. Chamberlain AM, Agarwal SK, Folsom AR, Duval S, Soliman EZ, Ambrose M, Eberly LE, Alonso A. Smoking and incidence of atrial fibrillation: results from the Atherosclerosis Risk in Communities (ARIC) Study. Heart Rhythm. 2011;8:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huxley RR, Misialek JR, Agarwal SK, Loehr LR, Soliman EZ, Chen LY, Alonso A. Physical activity, obesity, weight change, and risk of atrial fibrillation: the Atherosclerosis Risk in Communities Study. Circ Arrhythm Electrophysiol. 2014;7:620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garg PK, O'Neal WT, Ogunsua A, Thacker EL, Howard G, Soliman EZ, Cushman M. The American Heart Association's Life Simple 7 and risk of atrial fibrillation: the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. Am J Cardiol. 2018;121:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The ARIC Investigators . The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19. Siedel J, Hagele EO, Ziegenhorn J, Wahlefeld AW. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29:1075–1080. [PubMed] [Google Scholar]
- 20. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. [DOI] [PubMed] [Google Scholar]
- 21. Folsom AR, Arnett DK, Hutchinson RG, Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease in middle‐aged women and men. Med Sci Sports Exerc. 1997;29:901–909. [DOI] [PubMed] [Google Scholar]
- 22. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR Jr, Schmitz KH, Emplaincourt PO, Jacobs DR Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. [DOI] [PubMed] [Google Scholar]
- 23. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. [DOI] [PubMed] [Google Scholar]
- 24. Stevens J, Metcalf PA, Dennis BH, Tell GS, Shimakawa T, Folsom AR. Reliability of a food frequency questionnaire by ethnicity, gender, age and education. Nutr Res. 1996;16:735–745. [Google Scholar]
- 25. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas EJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okwuosa TM, Soliman EZ, Lopez F, Williams KA, Alonso A, Ferdinand KC. Left ventricular hypertrophy and cardiovascular disease risk prediction and reclassification in blacks and whites: the Atherosclerosis Risk in Communities Study. Am Heart J. 2015;169:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eriksson H, Caidahl K, Larsson B, Ohlson LO, Welin L, Wilhelmsen L, Svardsudd K. Cardiac and pulmonary causes of dyspnoea: validation of a scoring test for clinical‐epidemiological use: the Study of Men Born in 1913. Eur Heart J. 1987;8:1007–1014. [DOI] [PubMed] [Google Scholar]
- 28. Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 29. Benjamin EJ, Chen P, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd‐Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a National Heart, Lung, and Blood Institute workshop. Circulation. 2009;116:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gorenek B, Pelliccia A, Benjamin EJ, Boriani G, Crijns HJ, Fogel RI, Van Gelder IC, Halle M, Kudaiberieva G, Lane DA, Larsen TB, Lip GY, Lochen M, Marin F, Niebauer J, Sanders P, Tokgozoglu L, Vos MA, Van Wagoner DR. European Heart Rhythm Association (EHRA)/European Association of Cardiovascular Prevention and Rehabilitation (EACPR) position paper on how to prevent atrial fibrillation endorsed by the Heart Rhythm Society (HRS) and Asia Pacific Heart Rhythm Society (APHRS). Eur J Prev Cardiol. 2017;24:4–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens CJ, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Agarwal SK, McManus DD, Ellinor PT, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kaab S, Couper D, Harris TB, Soliman EZ, Stricker BH, Gudnason V, Heckbert SR, Benjamin EJ. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE‐AF consortium. J Am Heart Assoc. 2013;2:e000102 DOI: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Huxley RR, Lopez FL, Folsom AR, Agarwal SK, Loehr LR, Soliman EZ, Maclehose R, Konety S, Alonso A. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2011;123:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128:2470–2477. [DOI] [PubMed] [Google Scholar]
- 34. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 35. Mohanty S, Mohanty P, Tamaki M, Natale V, Gianni C, Trivedi C, Gokoglan Y, Di Biase L, Natale A. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta‐analysis. J Cardiovasc Electrophysiol. 2016;27:1021–1029. [DOI] [PubMed] [Google Scholar]
- 36. Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, Ambrose M, Alonso A. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol. 2011;107:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB, Newton‐Cheh C, Yamamoto JF, Magnani JW, Tadros TM, Kannel WB, Wang TJ, Ellinor PT, Wolf PA, Vasan RS, Benjamin EJ. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community‐based cohort study. Lancet. 2009;373:739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shen J, Johnson VM, Sullivan LM, Jacques PF, Magnani JW, Lubitz SA, Pandey S, Levy D, Vasan RS, Quatromoni PA, Junyent M, Ordovas JM, Benjamin EJ. Dietary factors and incident atrial fibrillation: the Framingham Heart Study. Am J Clin Nutr. 2011;93:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gronroos NN, Alonso A. Diet and risk of atrial fibrillation. Circ J. 2010;74:2029–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Menezes AR, Lavie CJ, DiNicolantonio JJ, O'Keefe J, Morin DP, Khatib S, Milani RV. Atrial fibrillation in the 21st century: a current understanding of risk factors and primary prevention strategies. Mayo Clin Proc. 2013;88:394–409. [DOI] [PubMed] [Google Scholar]
- 41. Alonso A, Yin X, Roetker NS, Magnani JW, Kronmal RA, Ellinor PT, Chen LY, Lubitz SA, McClelland RL, McManus DD, Soliman EZ, Huxley RR, Nazarian S, Szklo M, Heckbert SR, Benjamin EJ. Blood lipids and the incidence of atrial fibrillation: the Multi‐Ethnic Study of Atherosclerosis and the Framingham Heart Study. J Am Heart Assoc. 2014;3:e001211 DOI: 10.1161/JAHA.114.001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lopez FL, Agarwal SK, Maclehose RF, Soliman EZ, Sharrett AR, Huxley RR, Konety S, Ballantyne CM, Alonso A. Blood lipid levels, lipid‐lowering medications, and the incidence of atrial fibrillation: the Atherosclerosis Risk in Communities Study. Circ Arrhythm Electrophysiol. 2012;5:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. [DOI] [PubMed] [Google Scholar]
- 44. Wolf PA, Mitchell JB, Baker CS, Kannel WB, D'Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med. 1998;158:229–234. [DOI] [PubMed] [Google Scholar]
- 45. Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles‐Gonzalez JF, Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371–2379. [DOI] [PubMed] [Google Scholar]


