Abstract
Background
Studies have shown that chronic total occlusion (CTO) in a noninfarct‐related artery in patients with ST‐segment–elevation myocardial infarction is linked to increased mortality. It remains unclear whether staged revascularization of a noninfarct‐related artery CTO in patients with ST‐segment–elevation myocardial infarction translates to improved outcomes. We performed a meta‐analysis to compare outcomes between patients presenting with ST‐segment–elevation myocardial infarction with concurrent CTO who underwent percutaneous coronary intervention of noninfarct‐related artery CTO versus those who did not.
Method and Results
We conducted an electronic database search of all published data. The primary end point was major adverse cardiovascular events. Secondary end points were all‐cause mortality, cardiovascular mortality, myocardial infarction, repeat revascularization with either percutaneous coronary intervention or coronary artery bypass grafting, stroke, and heart failure readmission. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed. Random effects model was used and heterogeneity was considered if I 2 >25. Six studies (n=1253 patients) were included in the analysis. There was a significant difference in major adverse cardiovascular events (OR, 0.54; 95% CI, 0.32–0.91), cardiovascular mortality (OR, 0.43; 95% CI, 0.20–0.95), and heart failure readmissions (OR, 0.57; 95% CI, 0.36–0.89), favoring the patients in the CTO percutaneous coronary intervention group. No significant differences were observed between the 2 groups for all‐cause mortality (OR, 0.47; 95% CI, 0.22–1.00), myocardial infarction (OR, 0.78; 95% CI, 0.41–1.46), repeat revascularization (OR, 1.13; 95% CI, 0.56–2.27), and stroke (OR, 0.51; 95% CI, 0.20–1.33).
Conclusions
In this meta‐analysis, CTO percutaneous coronary intervention of the noninfarct‐related artery in patients presenting with ST‐segment–elevation myocardial infarction was associated with a significant reduction in major adverse cardiovascular events, cardiovascular mortality, and heart failure readmissions.
Keywords: chronic total occlusion, meta‐analysis, percutaneous coronary intervention, ST‐segment–elevation myocardial infarction
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Percutaneous Coronary Intervention, Revascularization
Clinical Perspective
What Is New?
Our findings suggest that routine staged percutaneous coronary intervention (PCI) of a concurrent chronic total occlusion in a noninfarct‐related artery after successful primary PCI in patients with ST‐segment–elevation myocardial infarction is feasible and safe.
Staged PCI of a concurrent chronic total occlusion is associated with reduction in mortality, major adverse cardiovascular events, and heart failure readmissions without increased risk of stroke and myocardial infarction.
What Are the Clinical Implications?
When clinicians decide the treatment strategy after primary PCI of the culprit artery in patients with ST‐segment–elevation myocardial infarction with concurrent chronic total occlusion in a noninfarct‐related artery, our results suggest that staged PCI of the chronic occlusion is a more appropriate treatment strategy.
Further larger randomized controlled trials are needed to fully understand the role of chronic total occlusion revascularization in patients with ST‐segment–elevation myocardial infarction.
Acute ST‐segment–elevation myocardial infarction (STEMI) is typically caused by thrombotic occlusion of a coronary artery. The treatment of choice for STEMI is percutaneous coronary intervention (PCI) to restore blood flow to the occluded infarct‐related artery.1, 2 Approximately 50% of patients presenting with STEMI are found to have multivessel coronary artery disease (CAD) with concomitant stenotic lesions in noninfarct‐related arteries (nIRAs) and ≈12% to 13% are found to have a chronic total occlusion (CTO) in an nIRA.3, 4 Prior studies have shown that the presence of a CTO in an nIRA in patients with STEMI is linked to an increase in short‐ and long‐term mortality.4, 5 Moreover, it has been suggested that the presence of a CTO is associated with worse outcomes compared with the presence of multivessel CAD without a CTO.3, 5, 6 Although there is evidence that multivessel revascularization in patients with STEMI might be beneficial,7 it remains unclear whether revascularization via PCI of an nIRA CTO, and in particular in patients with STEMI, is translated to improved outcomes. The current literature includes several small studies which suggest that successful revascularization of CTO lesions is associated with a lower risk of death, stroke, and coronary artery bypass grafting; less recurrent angina; and improvement of left ventricular ejection fraction (LVEF).8, 9 However, a recent randomized prospective trial did not find benefit for patients with STEMI who had PCI of an nIRA CTO.10 We therefore performed a meta‐analysis to compare outcomes between patients presenting with STEMI who underwent PCI revascularization of nIRA CTO versus those who did not.
Methods
The authors declare that all supporting data are available within the article and its online supplementary material.
A protocol for this systematic review was created, which we posted online and registered in PROSPERO (CRD42017065380). We followed the guidelines outlined by PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses).11
Search Strategy
We conducted a literature search of PubMed Central, Embase, the Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, Google Scholar databases and the scientific session abstracts in Circulation, the Journal of the American College of Cardiology, and the European Heart Journal. Oral presentations at scientific sessions were included using the respective websites: Transcatheter Cardiovascular Therapeutics (http://www.tctmd.com), EuroPCR (http://www.europcr.com), American College of Cardiology (http://www.acc.org), American Heart Association (http://www.heart.org), and European Society of Cardiology (http://www.escardio.org). Moreover, we performed manual searches of reference lists that included studies, reviews, editorials, and letters, as well as related conference proceedings. Last accessed as up to date: December 30, 2017.
Search term keywords were: “randomized controlled trial,” “nonrandomized studies,” “myocardial infarction,” “ST‐segment–elevation myocardial infarction,” “acute coronary syndromes,” “multivessel,” “chronic total occlusion,” “nonculprit,” “staged,” “percutaneous coronary intervention,” and “revascularization,” as well as various combinations of these terms. No language restriction was implemented. Only adult human studies were included.
Inclusion Criteria
Studies that fulfilled the following specifications were included: (1) randomized controlled trials (RCTs) or nonrandomized studies of patients who presented with acute STEMI and were found to have concurrent CTO in an nIRA during the primary PCI for STEMI; and (2) direct comparison provided between patients with successful revascularization of CTO lesions in the nIRA and patients with failed or nonattempted revascularization (non‐PCI group) of CTO lesions in the nIRA. If data were not provided as treated, the outcomes were analyzed as intention to treat.
Exclusion criteria were pregnancy; age younger than 18 years; diagnoses of non‐STEMI, unstable angina, or chronic ischemic heart disease; and/or not meeting the above‐mentioned inclusion criteria.
Two reviewers (W.O. and P.V.) independently searched the studies and collected data. Data were extracted using standardized protocol and reporting forms. Disagreements were resolved by consensus or, if necessary, by a third party (M.W. and D.M.). Two reviewers (P.V. and D.B.) independently assessed the risk of bias of RCTs using standard criteria defined in the Cochrane Handbook for Systematic Reviews of Interventions 12 and the Newcastle‐Ottawa Scale for nonrandom controlled studies.13
Study End Points
The primary end point was the incidence of major adverse cardiovascular events (MACE). Secondary end points were all‐cause mortality, cardiovascular mortality, new myocardial infarction (MI), repeat revascularization (RRV) either with PCI or coronary artery bypass grafting, stroke, and heart failure (HF) readmission. Trial‐specific definitions were also used for individual end points.
Statistical Analysis
The collected data were summarized across treatment arms using the odds ratio (OR) random effect models along with a stratified Fisher exact test. We evaluated heterogeneity of effects using the I 2 statistic (defined as I 2 >25%). To address publication bias, we used 4 methods: funnel plots, Begg‐Mazumdar test, Egger test, and the Duval and Tweedie test. Meta‐regression analyses were performed to determine whether the effects of mortality were modulated by prespecified study‐level factors including age, male sex, diabetes mellitus (DM), hypertension, hyperlipidemia, smoking, LVEF, 3‐vessel CAD, and CTO of the left anterior descending artery (LAD). All variables except for age were represented as proportions in the studies. Meta‐regression was performed with unrestricted maximum‐likelihood method (inverse variance‐weighted regression) on the OR log‐transformed before being used as independent variables in linear meta‐regression analyses. Sensitivity analyses were performed using the leave‐one‐study‐out method in order to address the influence of each study by testing whether deleting each individually would significantly change the pooled results of the meta‐analysis. Additionally, chronological cumulative analyses were used to test whether the effect size and precision would shift based on technical advancement of stents, CTO equipment, antithrombotic therapy, and CTO strategies. Finally, we performed Mantel–Haenszel fixed effect model analysis to test whether the overall effects change with this statistical analysis. The statistical analysis was performed using Comprehensive Meta‐Analysis Software version 2.0 (Biostat, Inc).
Study Selection and Characteristics
The search strategy identified a total of 527 potential articles (Figure 1). After removing duplicates and articles that did not meet inclusion criteria, we screened 94 titles and abstracts. Of these, 16 were selected for further review. Ultimately, 5 observational studies and 1 RCT satisfied all inclusion criteria.10, 14, 15, 16, 17, 18 All selected studies were published in journals as full English articles. Overall, the studies enrolled a total of 1253 patients. Among this population, 692 patients underwent successful revascularization of CTO lesions in the nIRA (PCI group) and 561 patients failed revascularization of CTO lesions in the nIRA or revascularization was not attempted. The follow‐up ranged from in‐hospital discharge to up to 5 years. Patients in the PCI group were slightly younger (mean age 64 years) compared with patients in the non‐PCI group (mean age 66 years). At the time of STEMI presentation, patients in the PCI group were more likely to have the culprit lesion in the LAD as compared with patients in the non‐PCI group (38.8% versus 33.6%), and were more likely to have multivessel disease (51.3% versus 48%, respectively). The CTO lesions were staged up to 30 days after primary PCI. The average ejection fraction between the 2 groups differed by only 1.2% in favor of the PCI group. Study characteristics are shown in Tables 1 and 2, and inclusion and exclusion criteria of the selected studies are shown in Table S1.
Figure 1.
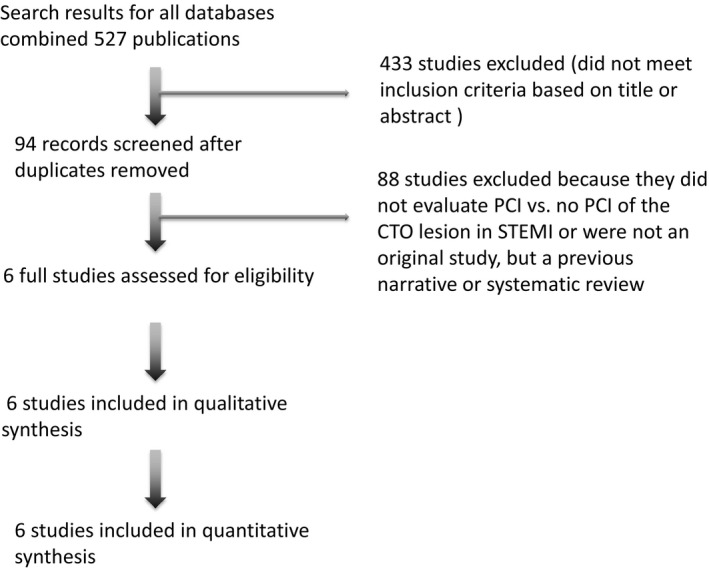
Flow chart of the literature review. From 527 studies identified from the initial search, a total of 6 studies were included after screening titles and reviewing the full articles of potentially relevant studies. CTO indicates chronic total occlusion; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction.
Table 1.
Design and Outcomes of the Studies Included in the Meta‐Analysis
| Author/Year | Design | Total Patients, No. | Follow‐Up | Primary Outcomes | MACE Definition |
|---|---|---|---|---|---|
| Yang 201114 | Single center, retrospective | 136 | 2 y | Cardiac mortality and occurrence of MACE | Cardiac death, recurrent myocardial infarction, repeat revascularization (PCI and/or CABG), and heart failure rehospitalization |
| Shi 201415 | Single center, retrospective | 148 | 3 y | Survival and occurrence of MACE | Cardiac death, recurrent myocardial infarction, repeat revascularization (PCI and/or CABG), and rehospitalization because of heart failure |
| Valenti 201416 | Multicenter registry, retrospective | 169 | 1 y | 1‐ and 3‐y cardiac survival | Not reported |
| Watanabe 201617 | Multicenter registry, retrospective | 121 | 4 y | All‐cause death | Not reported |
| Deng 201718 | Single center, retrospective | 377 | 1 y | Composite of all‐cause death, nonfatal myocardial infarction, ischemia‐driven coronary revascularization, and hospitalization for heart failure at 1 y | All‐cause death, nonfatal myocardial infarction, ischemia‐driven coronary revascularization, and hospitalization for heart failure |
| Henriques 201610 | Multicenter RCT | 302 | 4 mo | LVEF and LVEDV, assessed by cardiac MRI at 4 mo | Cardiac death, myocardial infarction, and CABG |
CABG indicates coronary artery bypass grafting; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; MRI, magnetic resonance imaging; PCI, percutaneous coronary intervention; RCT, randomized controlled trials.
Table 2.
Baseline Clinical and Demographic Characteristics of the Studies Included in the Meta‐Analysis
| Author/Year | Age, y | Male, % | Hypertension, % | DM, % | Hyperlipidemia, % | Smoking, % | Prior MI, % | 3 Vessel, % | EF, % | CTO LAD% | CTO LCx | CTO RCA | Staged CTO After Primary PCI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yang 201114 | PCI | 66 | 82 | 70 | 36 | 20 | 39 | 26 | 68 | 46 | 38 | 33 | 36 | 7 to 10 d |
| Non PCI | 69 | 82 | 76 | 37 | 22 | 37 | 33 | 65 | 47 | 37 | 39 | 29 | ||
| Shi 201415 | PCI | N/A | 78 | 65 | 23 | 55 | 45 | 28 | 51 | N/A | 36 | 30 | 34 | 7 to 10 d |
| Non PCI | N/A | 83 | 69 | 23 | 58 | 40 | 33 | 48 | N/A | 42 | 29 | 29 | ||
| Valenti 201416 | PCI | 64 | 85 | 55 | 17 | 36 | 50 | 19 | 59 | 36 | 33 | 33 | 34 | Up to 30 d |
| Non PCI | 69 | 73 | 67 | 15 | 41 | 30 | 29 | 48 | 38 | 17 | 29 | 55 | ||
| Watanabe 201617 | PCI | 66 | 84 | 73 | 37 | N/A | 42 | 10 | N/A | 48 | 31 | 36 | 33 | 4 to 17 d |
| Non PCI | 67 | 79 | 84 | 23 | N/A | 47 | 19 | N/A | 49 | 27 | 44 | 29 | ||
| Deng 201718 | PCI | 65 | 79 | 78 | 33 | 80 | 58 | 32 | 33 | 49 | 31 | 33 | 36 | 7 to 28 d |
| Non PCI | 68 | 79 | 74 | 28 | 73 | 52 | 35 | 33 | 50 | 39 | 29 | 32 | ||
| Henriques 201610 | PCI | 60 | 89 | 40 | 15 | 35 | 52 | 13 | 42 | 41 | 24 | 32 | 43 | Within 7 d |
| Non PCI | 60 | 82 | 45 | 16 | 34 | 49 | 16 | 44 | 42 | 35 | 24 | 51 |
CTO indicates chronic total occlusion; DM, diabetes mellitus; EF, ejection fraction; LAD, left anterior descending artery; LCx, left circumflex artery; MI, myocardial infarction; N/A, not available; PCI, percutaneous coronary interventions; RCA, right coronary artery.
Quantitative Data Synthesis
Efficacy Outcomes
Major adverse cardiovascular events
A total of 199 MACE were reported: 17.6% (98/556) in the PCI group and 24.8% (101/407) in the non‐PCI group. Overall, there was a significant difference in MACE favoring patients in the PCI group over patients in the non‐PCI group (OR, 0.54; 95% confidence interval [CI], 0.32–0.91 [P=0.02]) (Figure 2). Stratified Fisher exact test analysis was also significant, favoring the PCI group (OR, 0.64; 95% CI, 0.47–0.88 [P=0.008]).
Figure 2.
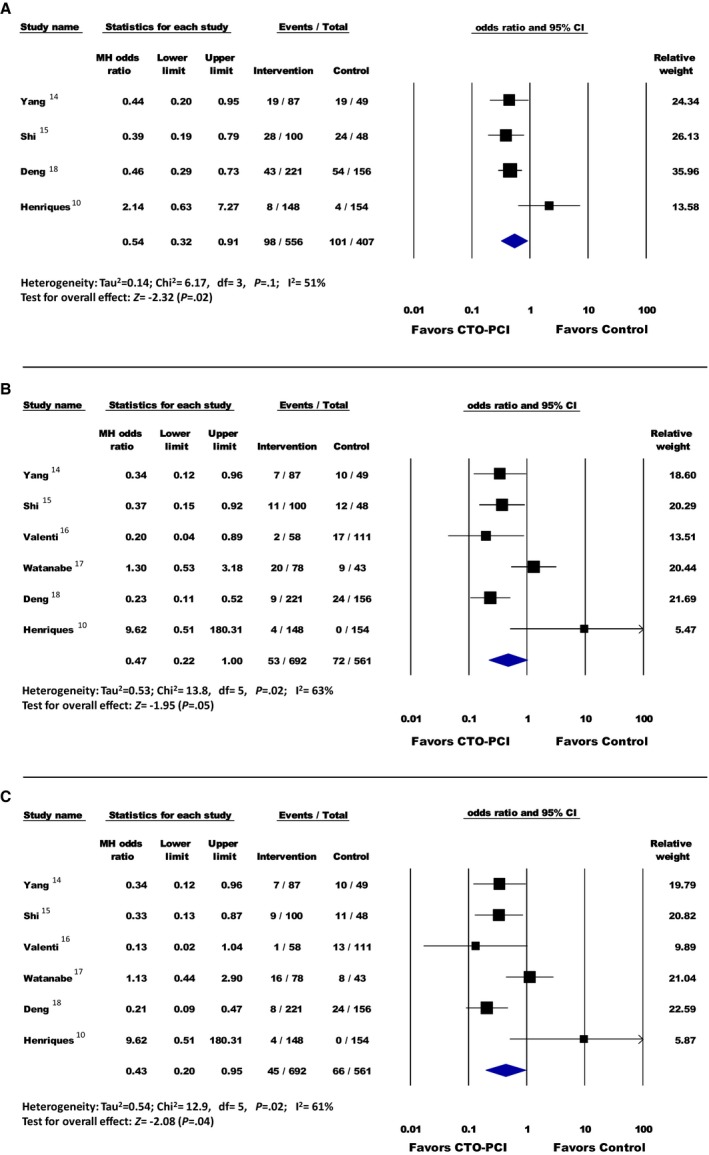
A, Major adverse cardiovascular events; (B) all‐cause mortality; (C) cardiovascular mortality. Forest plot reporting the odds ratios in patients with ST‐segment–elevation myocardial infarction (STEMI) with percutaneous coronary intervention (PCI) of the chronic total occlusion (CTO) lesion vs no PCI of CTO lesion. Diamond indicates overall summary estimate for the analysis (width of the diamond represents the 95% confidence interval [CI]); width of the shaded square represents the size of the population).
All‐cause mortality
A total of 125 all‐cause mortality events were reported: 7.6% (53/692) in the PCI group and 12.8% (72/561) in the non‐PCI group. Overall, there was no significant difference in all‐cause mortality between the groups (OR, 0.47; 95% CI, 0.22–1.00 [P=0.05]) (Figure 2). Stratified Fisher exact test analysis was significant, favoring the PCI group (OR, 0.56; 95% CI, 0.38–0.81 [P=0.003]).
Cardiovascular mortality
A total of 79 cardiovascular mortality events were reported: 6.5% (45/692) in the PCI group and 11.7% (66/561) in the non‐PCI group. Overall, there was a significant difference favoring patients in the PCI group over patients in the non‐PCI group (OR, 0.43; 95% CI, 0.20–0.95 [P=0.04]) (Figure 2). Stratified Fisher exact test analysis was also significant, favoring the PCI group (OR, 0.52; 95% CI, 0.35–0.77 [P=0.001]).
New Myocardial Infarction
A total of 34 new MI cases were reported: 3.3% (23/692) in the PCI group and 3.3% (19/561) in the non‐PCI group. Overall, there was no significant difference between the groups (OR, 0.78; 95% CI, 0.41–1.46 [P=0.43]) (Figure 3). Stratified Fisher exact test analysis was also not significant between groups (OR, 0.98; 95% CI, 0.52–1.82 [P=0.98]).
Figure 3.
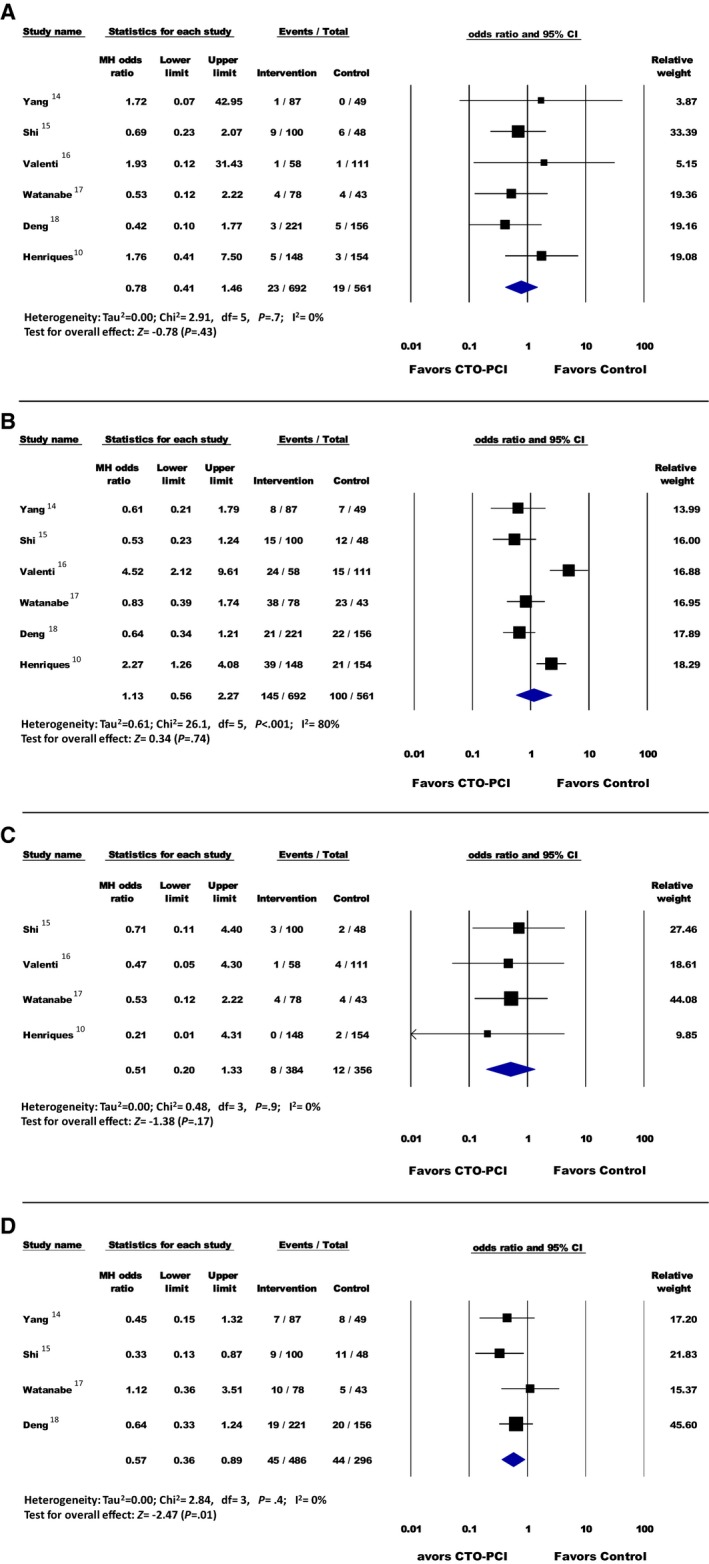
A, Myocardial infarction; (B) repeat revascularization; (C) stroke; (D) heart failure readmission. Forest plot reporting the odds ratios in patients with ST‐segment–elevation myocardial infarction (STEMI) with percutaneous coronary intervention (PCI) of the chronic total occlusion (CTO) lesion vs no PCI of CTO lesion. Diamond indicates overall summary estimate for the analysis (width of the diamond represents the 95% confidence interval [CI]); width of the shaded square, size of the population).
Repeat revascularization
A total of 202 RRVs were reported: 20.9% (145/692) in the PCI group and 17.8% (100/561) in the non‐PCI group. Overall, there was no significant difference between the groups (OR, 1.13; 95% CI, 0.56–2.27 [P=0.74]) (Figure 3). Stratified Fisher exact test analysis was also not significant between groups (OR, 1.22; 95% CI, 0.92–1.62 [P=0.18]).
Stroke
A total of 20 strokes were reported: 2.1% (8/384) in the PCI group and 3.4% (12/356) in the non‐PCI group. Overall, there was no significant difference between the groups (OR, 0.51; 95% CI, 0.20–1.33 [P=0.17]) (Figure 3). Stratified Fisher exact test analysis was also not significant between groups (OR, 0.60; 95% CI, 0.24–1.51 [P=0.39]).
Heart Failure Readmission
A total of 50 hospitalizations from HF were reported: 9.8% (45/486) in the PCI group and 17.1% (44/296) in the non‐PCI group. Overall, there was a significant difference in HF readmissions favoring patients in the PCI group over patients in the non‐PCI group (OR, 0.57; 95% CI, 0.36–0.89 [P=0.01]) (Figure 3). Stratified Fisher exact test analysis was also significant, favoring the PCI group (OR, 0.58; 95% CI, 0.37–0.91 [P=0.02]).
Sensitivity Analysis
Sensitivity analysis involving the removal of each of the studies sequentially demonstrated that if some of the studies were removed from the analysis, they influenced the summary risk estimates for cardiovascular MACE, cardiovascular mortality, and HF, making the overall results nonsignificant (Figure S1). MACE, cardiovascular, and HF readmission changed significantly (P<0.05) in the overall final effect in the chronologic cumulative analysis for each outcome before inclusion of all studies in the final effect summary (Figure S2). Sensitivity analysis with a fixed model did not change the significance of the final effect estimates for the analyzed outcomes except for all‐cause mortality, which also became significant, favoring PCI of CTO vessels (OR, 0.43; 95% CI, 0.28–0.66) (Figure S3).
Meta‐regression
Meta‐regression coefficients effects on cardiovascular mortality were not statistically significant for mean age, male sex, DM, hypertension, hyperlipidemia, smoking, LVEF, 3‐vessel CAD, or CTO of the LAD (Figure S4).
Bias
Funnel plot did not show asymmetry suggesting bias for all outcomes except for MI (Figure S5). However, after quantifying the observed bias with other methods, there was no evidence of publication bias (Begg‐Mazumdar test and Egger test P>0.05 for all outcomes explored [Figure S6]). The individual study quality appraisals of the included studies are summarized in Tables S2 and S3.
Discussion
To our knowledge, this is the first meta‐analysis assessing the efficacy of successful PCI of an nIRA CTO in patients presenting with STEMI. This meta‐analysis has 3 main findings. First, there is a reduction in the composite end point of MACE with CTO PCI of the nIRA in patients with STEMI after primary PCI. Second, we demonstrated a reduction in cardiovascular mortality and HF readmission when PCI is implemented successfully in CTO nIRA as compared with the failed/nonattempted approach. Last, the risk for stroke, MI, and RRV was not remarkably different between the successful CTO‐PCI group and the non‐PCI group. Although all‐cause mortality did not significantly differ between groups, this discrepancy could be explained by the noncardiovascular mortality observed in the non‐PCI group, while cardiovascular mortality favored the CTO PCI group. As a result, all‐cause mortality was unchanged in the 2 study groups. Unfortunately, noncardiovascular death can be influenced by cardiovascular disease as well as many other comorbidities, and determination of causes of death can be difficult, particularly in patients with multiple organ dysfunction. Therefore, interpretation of all‐cause mortality in such patients is controversial.
In their meta‐analysis, O'Connor et al19 demonstrated that the presence of coronary CTO in the nIRA in patients presenting with STEMI is associated with increased short‐ and long‐term all‐cause mortality. The 2011 American College of Cardiology Foundation/American Heart Association/Society for Cardiovascular Angiography and Intervention guidelines for PCI suggest that CTO PCI is reasonable when performed by operators with appropriate expertise (class IIa, level B).20 Although the association between CTO and worse outcomes has been well‐established, it is unknown whether revascularization of the nIRA CTO in patients with STEMI is actually translated into improved outcomes. Our study further extends these conclusions to patients with STEMI who underwent PCI in nIRA CTO as shown in another meta‐analysis of contemporary RCTs in patients with STEMI with multivessel CAD.7 The idea that patients with acute STEMI and concurrent nIRA CTO would demonstrate clinical benefit from CTO PCI was generated from the apparent 2‐fold increased mortality and morbidity rates among patients with STEMI and multivessel CAD and CTO.4, 5, 21 Even among patients without STEMI, increased mortality has been attributed to the presence of CTOs in nIRAs.3, 6 One of the possible mechanisms of increased mortality in patients with CTO could be a larger infarct size caused by the acute occlusion of a donor artery to CTO. The decreased flow of the donor artery might result in myocardial injury and necrosis in the myocardial area in which the myocardial perfusion was dependent on the collateral flow from the infarct‐related artery. It has also been reported that the presence of multivessel CAD is associated with adverse outcomes compared with single‐vessel CAD in patients with STEMI, mainly attributed to the increased mortality caused by HF.22 The presence of a CTO was associated with reduced residual LVEF and with further deterioration of LVEF during follow‐up.3 It is challenging to discern whether CTO in an nIRA is only a result of multiple cardiovascular comorbidities or whether it exacerbates mortality in patients with STEMI, but it might potentially be a modifiable factor in the improvement of mortality. The meta‐regression performed in our analysis of successful CTO PCI did not show an association between cardiovascular mortality and known cardiovascular risk factors for adverse outcomes in patients with STEMI.
Contemporary research from George et al23 also demonstrated a mortality benefit for CTO revascularization in a registry investigation of >13 000 patients in the United Kingdom. Possible explanations for the underlying mechanism of the clinical benefit of opening CTO lesions include the improvement in blood flow in the peri‐infarct area and recovery of contractile function of the hibernated areas perfused by in the CTO territory. Other possible mechanisms include an increase in electrical stability with the associated reduction of fatal arrhythmia, and an increased tolerance to further coronary ischemic events. The overall benefit of a successful PCI could be translated to improvement of LVEF, avoiding left ventricular remodeling, and subsequent worsening LVEF and development of HF.
The results of our analysis do not mirror those of the only RCT to evaluate this topic. The EXPLORE (Evaluating Xience and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST‐Segment–Elevation Myocardial Infarction) trial randomized patients presenting with acute STEMI and concurrent CTO in an nIRA to receive either early revascularization (within 1 week) or conservative (non‐PCI) therapy.10 They reported a relatively high level of successful CTO PCI (77%). However, there was no significant improvement in cardiac deaths, recurrent MI, MACE, or LVEF. The recently reported findings of the DECISION‐CTO (Optimal Medical Therapy With or Without Stenting For Coronary Chronic Total Occlusion) trial by Park et al24 call into question the value of CTO PCI in general. The DECISION‐CTO study evaluated outcomes in patients without ACS who underwent revascularization of CTO. They demonstrated that optimal medical therapy was noninferior to CTO PCI in 834 patients randomized to each arm. However, they excluded patients with LVEF <30%—a group of patients who may derive the most benefit from CTO PCI. Of note, this trial was stopped early secondary to slow enrollment. EuroCTO (Randomized Multicentre Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Coronary Occlusions) showed a high procedural success rate of CTO PCI with an overall low procedural risk. Also, there was an improvement in clinical symptoms in patients treated with PCI compared with optimal medical therapy based on the Seattle Angina Questionnaire, subscales of physical limitation, and angina frequency. The PCI group also showed a trend towards improved quality of life and significantly greater absolute freedom from angina.25
Our meta‐analysis did not demonstrate significant benefit with respect to stroke, new MI, and RRV among patients who underwent successful CTO PCI. Possible hypotheses to explain the absence of clinical benefit in revascularization of these lesions could be that myocardial injury resulting from the presence of CTOs is long‐standing, reducing the chance for viability, and that remodeling has likely already occurred, thus, minimizing the benefit from reperfusion. A number of factors should be considered when selecting patients for CTO‐PCI, including not only the presence of symptoms and extent of ischemia but also the degree of myocardial viability. Using cardiovascular magnetic resonance imaging (MRI), a subgroup analysis of the EXPLORE trial showed that benefit of CTO PCI in dysfunctional but viable segments of myocardium, as compared with nonviable myocardium where no improvement was observed after CTO PCI compared with no CTO PCI.26 Further research is needed to evaluate the use of viability in patients with STEMI with nonculprit CTO lesions and the effect of PCI on clinical outcome.
The way of dealing with nIRA lesions in patients with STEMI remains a target of controversy because of a paucity of randomized data and conflicting results in several observational studies. CTO is the most complex and challenging coronary lesion for PCI. There is a need for accurate risk stratification in patients who potentially might benefit from PCI of nIRA CTOs lesions. Despite the progress in CTO interventions, certain complications still persist. Procedure‐related mortality and MI have been reported as 1% and 5%, respectively, despite the evolution of PCI techniques and equipment, and adjunctive pharmacological therapy.27 The preparation and experience of the operators having a thorough understanding of potential complications and the availability of dedicated equipment to treat these complications will improve the rate of successful revascularization and minimize the risks.
Limitations
Our meta‐analysis has several limitations. First, this is a meta‐analysis of RCTs and observational study data. Potential biases are likely to be greater for observational studies compared with RCTs; therefore, results should always be interpreted with caution when they are included in reviews and meta‐analyses. The presence of treatment selection bias is a major criticism against most observational studies, and may threaten the validity of study results when the sickest patients are more likely to receive one treatment strategy over another. In the absence of prospective allocation of patients to treatment strategies, there is an inherent bias that favors survival in those who live beyond the initial treatment to undergo staged treatment. The inherent bias and unmeasured confounding elements of observational studies may influence the study results despite multiple sensitivity analyses.28 Second, this is a meta‐analysis performed on study‐level data. Third, the definitions, design, treatment exposure, protocols, reporting of adverse outcomes, and risk of enrolled patients differed across studies. These limitations might explain some of the observed heterogeneity for the different outcomes. Fourth, the selection criteria for PCI were diverse between studies. Fifth, crossover treatment was not reported consistently; it might have a significant unrecognized impact on the overall outcomes in nonattempted versus failed PCI. Sixth, in some studies, there was a considerable loss to follow‐up, with only a few studies providing detailed outcomes. Therefore, the long‐term risks and benefits of CTO PCI are not well‐established by these studies. Last, the available PCI equipment and stents used in some of the included studies are not the contemporary technologies available for CTO lesions; therefore, results using the newer‐generation technologies might be different from our results. Despite these limitations, the consistency of the magnitude and direction of the overall effect, and the stability of the results after the sensitivity analyses support the robustness of the conclusions and make the overall estimates justified.
Conclusions
In this meta‐analysis, CTO PCI of the nIRA in patients presenting with STEMI was associated with a significant reduction of MACE, cardiovascular mortality, and HF readmissions. However, CTO PCI was not associated with a significant improvement in stroke, new MI, and RRV among patients who underwent successful PCI of the CTO lesion. Further larger RCTs are needed to fully understand the role of CTO revascularization in patients with STEMI.
Disclosures
None.
Supporting information
Table S1. Inclusion and Exclusion Criteria of Studies
Table S2. Risk of Bias Across Individual Observational Studies
Table S3. Risk of Bias Across Individual Randomized Controlled Trials
Figure S1. Sensitivity analysis with removal of each study 1 at a time.
Figure S2. Cumulative analysis for each outcome.
Figure S3. Sensitivity analysis with fixed effect model.
Figure S4. Meta‐regression analysis by representative plots.
Figure S5. Funnel plots for each outcome.
Figure S6. Quantification of bias for each outcome using Begg and Mazumdar rank correlation, Egger regression intercept, and Duval and Tweedie trim and fill test.
(J Am Heart Assoc. 2018;7:e008415 DOI: 10.1161/JAHA.117.008415.)29654206
References
- 1. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 2. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group . 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting Without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 3. Claessen BE, van der Schaaf RJ, Verouden NJ, Stegenga NK, Engstrom AE, Sjauw KD, Kikkert WJ, Vis MM, Baan J Jr, Koch KT, de Winter RJ, Tijssen JG, Piek JJ, Henriques JP. Evaluation of the effect of a concurrent chronic total occlusion on long‐term mortality and left ventricular function in patients after primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2009;2:1128–1134. [DOI] [PubMed] [Google Scholar]
- 4. Park DW, Clare RM, Schulte PJ, Pieper KS, Shaw LK, Califf RM, Ohman EM, Van de Werf F, Hirji S, Harrington RA, Armstrong PW, Granger CB, Jeong MH, Patel MR. Extent, location, and clinical significance of non‐infarct‐related coronary artery disease among patients with ST‐elevation myocardial infarction. JAMA. 2014;312:2019–2027. [DOI] [PubMed] [Google Scholar]
- 5. van der Schaaf RJ, Vis MM, Sjauw KD, Koch KT, Baan J Jr, Tijssen JG, de Winter RJ, Piek JJ, Henriques JP. Impact of multivessel coronary disease on long‐term mortality in patients with ST‐elevation myocardial infarction is due to the presence of a chronic total occlusion. Am J Cardiol. 2006;98:1165–1169. [DOI] [PubMed] [Google Scholar]
- 6. Claessen BE, Dangas GD, Weisz G, Witzenbichler B, Guagliumi G, Mockel M, Brener SJ, Xu K, Henriques JP, Mehran R, Stone GW. Prognostic impact of a chronic total occlusion in a non‐infarct‐related artery in patients with ST‐segment elevation myocardial infarction: 3‐year results from the HORIZONS‐AMI trial. Eur Heart J. 2012;33:768–775. [DOI] [PubMed] [Google Scholar]
- 7. Villablanca PA, Briceno DF, Massera D, Hlinomaz O, Lombardo M, Bortnick AE, Menegus MA, Pyo RT, Garcia MJ, Mookadam F, Ramakrishna H, Wiley J, Faggioni M, Dangas GD. Culprit‐lesion only versus complete multivessel percutaneous intervention in ST‐elevation myocardial infarction: a systematic review and meta‐analysis of randomized trials. Int J Cardiol. 2016;220:251–259. [DOI] [PubMed] [Google Scholar]
- 8. Christakopoulos GE, Christopoulos G, Carlino M, Jeroudi OM, Roesle M, Rangan BV, Abdullah S, Grodin J, Kumbhani DJ, Vo M, Luna M, Alaswad K, Karmpaliotis D, Rinfret S, Garcia S, Banerjee S, Brilakis ES. Meta‐analysis of clinical outcomes of patients who underwent percutaneous coronary interventions for chronic total occlusions. Am J Cardiol. 2015;115:1367–1375. [DOI] [PubMed] [Google Scholar]
- 9. Hoebers LP, Claessen BE, Elias J, Dangas GD, Mehran R, Henriques JP. Meta‐analysis on the impact of percutaneous coronary intervention of chronic total occlusions on left ventricular function and clinical outcome. Int J Cardiol. 2015;187:90–96. [DOI] [PubMed] [Google Scholar]
- 10. Henriques JP, Hoebers LP, Ramunddal T, Laanmets P, Eriksen E, Bax M, Ioanes D, Suttorp MJ, Strauss BH, Barbato E, Nijveldt R, van Rossum AC, Marques KM, Elias J, van Dongen IM, Claessen BE, Tijssen JG, van der Schaaf RJ; EXPLORE Trial Investigators . Percutaneous intervention for concurrent chronic total occlusions in patients with STEMI: the EXPLORE trial. J Am Coll Cardiol. 2016;68:1622–1632. [DOI] [PubMed] [Google Scholar]
- 11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. [DOI] [PubMed] [Google Scholar]
- 12. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (Updated March 2011). Cochrane Handbook. John Wiley & Sons: Chichester; 2011. [Google Scholar]
- 13. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Available at: http://www.Ohri.Ca/programs/clinical_epidemiology/oxford.Asp. Accessed January 10, 2018.
- 14. Yang ZK, Zhang RY, Hu J, Zhang Q, Ding FH, Shen WF. Impact of successful staged revascularization of a chronic total occlusion in the non‐infarct‐related artery on long‐term outcome in patients with acute ST‐segment elevation myocardial infarction. Int J Cardiol. 2013;165:76–79. [DOI] [PubMed] [Google Scholar]
- 15. Shi G, He P, Liu Y, Lin Y, Yang X, Chen J, Zhou Y, Tan N. Evaluation of the effect of concurrent chronic total occlusion and successful staged revascularization on long‐term mortality in patients with ST‐elevation myocardial infarction. ScientificWorldJournal. 2014;2014:756080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Valenti R, Marrani M, Cantini G, Migliorini A, Carrabba N, Vergara R, Cerisano G, Parodi G, Antoniucci D. Impact of chronic total occlusion revascularization in patients with acute myocardial infarction treated by primary percutaneous coronary intervention. Am J Cardiol. 2014;114:1794–1800. [DOI] [PubMed] [Google Scholar]
- 17. Watanabe H, Morimoto T, Shiomi H, Furukawa Y, Nakagawa Y, Ando K, Kadota K, Kimura T. Chronic total occlusion in a non‐infarct‐related artery is closely associated with increased five‐year mortality in patients with ST‐segment elevation acute myocardial infarction undergoing primary percutaneous coronary intervention (from the CREDO‐Kyoto AMI registry). EuroIntervention. 2017;12:e1874–e1882. [DOI] [PubMed] [Google Scholar]
- 18. Deng J, Wang X, Shi Y, Zhao X, Han Y. Prognostic value of the age, creatinine, and ejection fraction score for non‐infarct‐related chronic total occlusion revascularization after primary percutaneous intervention in acute ST‐elevation myocardial infarction patients: a retrospective study. J Interv Cardiol. 2018;31:33–40. [DOI] [PubMed] [Google Scholar]
- 19. O'Connor SA, Garot P, Sanguineti F, Hoebers LP, Unterseeh T, Benamer H, Chevalier B, Hovasse T, Morice MC, Lefevre T, Louvard Y. Meta‐analysis of the impact on mortality of noninfarct‐related artery coronary chronic total occlusion in patients presenting with ST‐segment elevation myocardial infarction. Am J Cardiol. 2015;116:8–14. [DOI] [PubMed] [Google Scholar]
- 20. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. [DOI] [PubMed] [Google Scholar]
- 21. Zhang HP, Zhao Y, Li H, Tang GD, Ai H, Zheng NX, Liu JH, Sun FC. Impact of chronic total occlusion in a noninfarct‐related artery on clinical outcomes in patients with acute ST‐elevation myocardial infarction undergoing primary percutaneous coronary intervention. Medicine (Baltimore). 2016;95:e2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Schaaf RJ, Timmer JR, Ottervanger JP, Hoorntje JC, de Boer MJ, Suryapranata H, Zijlstra F, Dambrink JH. Long‐term impact of multivessel disease on cause‐specific mortality after ST elevation myocardial infarction treated with reperfusion therapy. Heart. 2006;92:1760–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. George S, Cockburn J, Clayton TC, Ludman P, Cotton J, Spratt J, Redwood S, de Belder M, de Belder A, Hill J, Hoye A, Palmer N, Rathore S, Gershlick A, Di Mario C, Hildick‐Smith D; British Cardiovascular Intervention Society; National Institute for Cardiovascular Outcomes Research . Long‐term follow‐up of elective chronic total coronary occlusion angioplasty: analysis from the U.K. Central Cardiac Audit Database. J Am Coll Cardiol. 2014;64:235–243. [DOI] [PubMed] [Google Scholar]
- 24. Park S. Drug‐eluting stent versus optimal medical therapy in patients with coronary chronic total occlusion: DECISION CTO randomized trial. Presented at: ACC 2017. Washington, DC; March 18, 2017. [Google Scholar]
- 25. Werner G. A randomized multicentre trial to evaluate the utilization of revascularization or optimal medical therapy for the treatment of chronic total coronary occlusions. Presented at: EuroPCR 2017. Paris, France; May 18, 2017. [Google Scholar]
- 26. Elias J, van Dongen IM, Hoebers LP, Ouweneel DM, Claessen B, Ramunddal T, Laanmets P, Eriksen E, van der Schaaf RJ, Ioanes D, Nijveldt R, Tijssen JG, Hirsch A, Henriques JPS; EXPLORE Investigators . Improved recovery of regional left ventricular function after PCI of chronic total occlusion in STEMI patients: a cardiovascular magnetic resonance study of the randomized controlled EXPLORE trial. J Cardiovasc Magn Reson. 2017;19:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stone GW, Reifart NJ, Moussa I, Hoye A, Cox DA, Colombo A, Baim DS, Teirstein PS, Strauss BH, Selmon M, Mintz GS, Katoh O, Mitsudo K, Suzuki T, Tamai H, Grube E, Cannon LA, Kandzari DE, Reisman M, Schwartz RS, Bailey S, Dangas G, Mehran R, Abizaid A, Moses JW, Leon MB, Serruys PW. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part II. Circulation. 2005;112:2530–2537. [DOI] [PubMed] [Google Scholar]
- 28. Yeh RW, Drachman DE. Culprit only, multivessel, or staged multivessel intervention in STEMI: new insights or insurmountable methodologic obstacles? Catheter Cardiovasc Interv. 2014;84:923–924. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion and Exclusion Criteria of Studies
Table S2. Risk of Bias Across Individual Observational Studies
Table S3. Risk of Bias Across Individual Randomized Controlled Trials
Figure S1. Sensitivity analysis with removal of each study 1 at a time.
Figure S2. Cumulative analysis for each outcome.
Figure S3. Sensitivity analysis with fixed effect model.
Figure S4. Meta‐regression analysis by representative plots.
Figure S5. Funnel plots for each outcome.
Figure S6. Quantification of bias for each outcome using Begg and Mazumdar rank correlation, Egger regression intercept, and Duval and Tweedie trim and fill test.


