Abstract
Background
We aimed to evaluate a novel method of atrial fibrillation (AF) screening using an iPhone camera to detect and analyze photoplethysmographic signals from the face without physical contact by extracting subtle beat‐to‐beat variations of skin color that reflect the cardiac pulsatile signal.
Methods and Results
Patients admitted to the cardiology ward of the hospital for clinical reasons were recruited. Simultaneous facial and fingertip photoplethysmographic measurements were obtained from 217 hospital inpatients (mean age, 70.3±13.9 years; 71.4% men) facing the front camera and with an index finger covering the back camera of 2 independent iPhones before a 12‐lead ECG was recorded. Backdrop and background light intensity was monitored during signal acquisition. Three successive 20‐second (total, 60 seconds) recordings were acquired per patient and analyzed for heart rate regularity by Cardiio Rhythm (Cardiio Inc, Cambridge, MA) smartphone application. Pulse irregularity in ≥1 photoplethysmographic readings or 3 uninterpretable photoplethysmographic readings were considered a positive AF screening result. AF was present on 12‐lead ECG in 34.6% (n=75/217) patients. The Cardiio Rhythm facial photoplethysmographic application demonstrated high sensitivity (95%; 95% confidence interval, 87%–98%) and specificity (96%; 95% confidence interval, 91%–98%) in discriminating AF from sinus rhythm compared with 12‐lead ECG. The positive and negative predictive values were 92% (95% confidence interval, 84%–96%) and 97% (95% confidence interval, 93%–99%), respectively.
Conclusions
Detection of a facial photoplethysmographic signal to determine pulse irregularity attributable to AF is feasible. The Cardiio Rhythm smartphone application showed high sensitivity and specificity, with low negative likelihood ratio for AF from facial photoplethysmographic signals. The convenience of a contact‐free approach is attractive for community screening and has the potential to be useful for distant AF screening.
Keywords: atrial fibrillation, mobile apps, photoplethysmography, screening, smartphone
Subject Categories: Atrial Fibrillation
Clinical Perspective
What Is New?
It is feasible to accurately detect atrial fibrillation without physical contact by analyzing photoplethysmographic signals from a person's face using a smartphone camera.
What Are the Clinical Implications?
The convenience of a contact‐free approach to detect atrial fibrillation is attractive for community screening and has the potential to be useful for screening over a distance using telemedicine.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting >8.8 million adults worldwide.1 Prevalence of AF sharply increases with age, and with our rapidly aging population, prevalence of AF is expected to double by 2060.2, 3 Cardioembolic stroke is one of the most common complications of AF, and at least 1 in 3 strokes is directly attributable to AF.4, 5 One of the clinical challenges is to identify AF and initiate stroke prophylaxis before the occurrence of stroke. However, detection of AF can be difficult because it is often asymptomatic and intermittent in duration. In a recent study, AF was first diagnosed at the time of stroke in nearly 1 in 5 cases.6
Screening can identify asymptomatic AF for stroke prophylaxis, but the best method of AF screening is not established. In a systematic review, single time‐point screening of a general population aged >65 years detected new AF in 1.4%.7 Current guidelines recommend opportunistic screening by pulse palpation, followed by confirmatory 12‐lead ECG or single‐lead ECG rhythm strip in patients >65 years of age.8, 9 Limitations of screening by pulse palpation include variable diagnostic accuracy10 and the time to perform a 12‐lead ECG.
In recent years, several handheld devices, including stand‐alone and smartphone‐based devices and applications, have been developed for point‐of‐care AF screening.11, 12 The AliveCor heart monitor (AliveCor, San Francisco, CA) is a Food and Drug Administration–approved handheld single‐lead ECG device attached to a smartphone with an AF detection application. Initial validation study of the AliveCor for AF detection has shown high sensitivity of 98% and specificity of 97%.13 However, the diagnostic performance of the AliveCor as a screening test in a “real‐world” primary care setting was lower, with sensitivity of 98% (95% confidence interval [CI], 92%–100%) and specificity of 91% (95% CI, 89%–93%) in the SEARCH‐AF (Screening Education And Recognition in Community pHarmacies of Atrial Fibrillation) study using the same algorithm.12
In addition to handheld ECG devices, smartphones are capable of detecting pulsatile photoplethysmographic signals related to cardiac‐induced fluctuations in tissue blood volume using the built‐in cameras and LED (light‐emitting diode) smartphone flash.14, 15 To date, the photoplethysmographic signal is typically recorded by placing a finger over the smartphone camera lens, which measures changes in reflected light intensity from the LED caused by blood volume changes in the fingertip. Recent studies have demonstrated both the Cardiio Rhythm and PulseSMART finger photoplethysmographic‐based smartphone applications have high sensitivity (92.7% and 97.0%, respectively) and specificity (99.7% and 93.5%, respectively) in discriminating an irregular pulse during AF from sinus rhythm.16, 17
Facial video recording using the smartphone camera is a novel method of detecting a pulsatile facial photoplethysmographic signal without physical contact,18, 19, 20 with the potential for distant screening. A small proof‐of‐concept study supported the feasibility of such a contact‐free method for AF detection,19 but the performance of facial photoplethysmography for the detection of AF in a prospective study has not been reported. The primary aim of this study was to evaluate the performance of a smartphone application, Cardiio Rhythm, in detecting AF from facial photoplethysmographic signals acquired without physical contact, with the patient using 12‐lead ECG as the reference standard. In addition, we compared the AF detection performance of facial photoplethysmography with fingertip photoplethysmography, the typical method for acquiring photoplethysmographic signals using a smartphone.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
Inpatients at the Prince of Wales Hospital cardiology ward were recruited between 1 April and 30 November 2016. These patients were admitted for clinical reasons. The in‐hospital environment provided a controlled setting and made it more feasible to perform reference 12‐lead ECG measurements. Informed consent was obtained from all patients, and the study was approved by the local institutional review board, Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (reference no. 2016.550).
Clinical characteristics were recorded, including age, sex, and history of heart failure, hypertension, stroke, and coronary artery disease. Stroke prevention therapy, including oral anticoagulants, antiplatelet therapy, and left atrial appendage occlusion, was recorded. CHA2DS2‐VASc score (congestive heart failure, hypertension, age, diabetes mellitus, previous stroke/transient ischemic attack, female sex, and vascular disease) was calculated on the basis of clinical data. Body height and weight were measured under standard anthropometry procedures, and body mass index was calculated as weight (in kilograms) divided by height (in square meters). Blood pressure measurements were taken using an automatic blood pressure monitor (Tango M2; SunTech Medical, Inc, NC) before AF measurements. The facial skin color of participants was evaluated using the von Luschan skin color chart (range, 1–36, with 1 being a lightest skin color and 36 being a darkest skin color; Figure S1).21
Study Setup for AF Measurement
Two iPhone 6S units (Apple Inc, Cupertino, CA) installed with the Cardiio Rhythm application (beta version; Cardiio, Inc, Cambridge, MA) were used for simultaneous facial and fingertip photoplethysmographic detection. A 12‐lead ECG (Mortara ELI 150c; Milwaukee, WI) was performed after photoplethysmographic measurements. The background light intensity was measured in unit of lux during signal acquisition.
Cardiio Rhythm Smartphone Application
Cardiio Rhythm application is a novel smartphone application that measures the rhythm of the heart through recording pulsatile photoplethysmographic signal from either the fingertip or the face without physical contact. The camera detects subtle beat‐to‐beat variations of skin color on the basis of the amount of reflected light that changes, according to the arterial blood volume pulsations. Photoplethysmographic waveforms were sampled at 30 Hz, and each measurement recorded 512 samples (≈17 seconds). Photoplethysmographic waveforms were filtered by using a bandpass filter (0.7–4.0 Hz) to remove baseline wander and high‐frequency noise. Detection of AF was based on an irregularly irregular pattern in the photoplethysmographic waveform attributable to AF. Briefly, the algorithm computed repeating patterns in the photoplethysmographic waveform on the basis of autocorrelation analysis and classified the patterns using a previously trained support vector machine.22 Each photoplethysmographic recording was encrypted and wirelessly transmitted to a secure cloud server to compute the likelihood of a “regular” or an “irregular” pulse, expressed as a percentage (100% being the highest likelihood) (Figure 1A and 1B). When the Cardiio Rhythm application was unable to interpret the photoplethysmographic signal, the reading was defined as “uninterpretable”. Three consecutive uninterpretable photoplethysmographic readings are rare with normal sinus rhythm and can be attributable to variability in photoplethysmographic wavelength and amplitude during AF, leading to uninterpretable photoplethysmographic signals. In this study, a positive test screening for AF was defined as either of the following: (1) detection of an irregular heart rhythm in ≥1 photoplethysmographic measurements or (2) 3 consecutive uninterpretable photoplethysmographic measurements. All other combinations were categorized as a negative test screening for AF.
Figure 1.
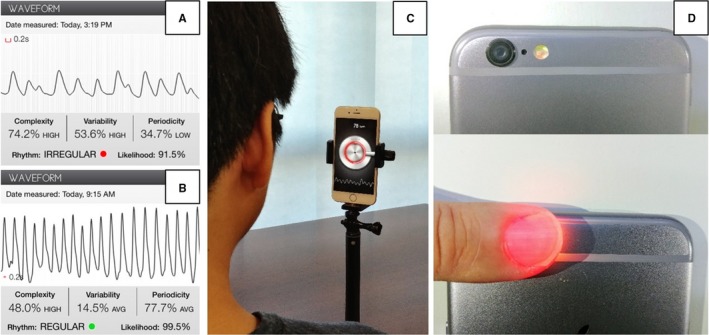
Examples of photoplethysmographic (PPG) recordings analyzed by Cardiio Rhythm application from patients in atrial fibrillation (A) and sinus rhythm (B). C, Setup to acquire PPG signals from face by using the front camera. D, Obtaining PPG signals from fingertip by using the back camera.
The Cardiio Rhythm application uses the same heart rate measurement algorithm as the Cardiio: Heart Rate Monitor application that has previously been validated for measuring heart rate at rest and after exercise against 12‐lead ECG23 and against a Food and Drug Administration–cleared pulse oximeter.24
Facial Photoplethysmographic Detection
Each participant was asked to sit in front of an iPhone placed upright on a desk ≈30 cm away. A large circle that displayed the front camera's field of view appeared on the iPhone screen once the Cardiio Rhythm application was activated and the participant was instructed to position his/her entire face within the circle during the measurement session (Figure 1C). The participant was instructed to hold the head still and not talk during each measurement. Continuous pulsatile photoplethysmographic signal from the face, detected by the camera, was displayed in real time on the bottom of the iPhone screen. A new measurement would automatically restart when poor photoplethysmographic signal quality was detected. Three consecutive 20‐second (total of 60 seconds) measurements were performed for each patient.
Fingertip Photoplethysmographic Detection
The finger photoplethysmographic signal was measured simultaneously with the facial photoplethysmographic measurement. Patients were instructed to cover the camera on the back of a second iPhone with their left index finger (Figure 1D). Continuous pulsatile photoplethysmographic signal from the fingertip detected by the camera was displayed in real time on the bottom of the iPhone screen. Again, 3 consecutive 20‐second (total of 60 seconds) measurements were performed for each patient.
ECG (12 Lead)
A 12‐lead ECG was performed immediately after facial and finger photoplethysmographic measurements to serve as the reference standard. The ECG recordings were analyzed for the presence of AF by a cardiologist (B.P.Y.) blinded to the photoplethysmographic results. Patients with an implanted pacemaker were excluded if the pacemaker was configured in active pacing mode.
Statistical Analysis
Continuous and discrete variables were presented as means±SD or median (interquartile range) and as numbers and percentages, respectively. Independent t test and Mann‐Whitney U tests were performed to test the difference between means and medians, respectively. χ2 Test and Fisher's exact test were performed to determine differences in categorical variables between groups. The diagnostic accuracy of both facial and finger photoplethysmography for AF detection was determined using 12‐lead ECG as the reference standard. The calculations of sensitivity, specificity, predictive value, likelihood ratio, diagnostic odds ratio, and prevalence for AF diagnosis were performed by using 2×2 contingency tables as simple proportions, with corresponding 95% CIs. Cohen's κ coefficients were calculated to measure the agreement between finger and facial photoplethysmographic detection for AF. A κ value of >0.8 indicated excellent agreement.25 Univariate logistic regression models were performed to explore differences between characteristics of participants and facial photoplethysmographic detection failure. Multivariate logistic regression analysis was then performed to determine predictors of facial photoplethysmographic detection failure, controlling for age, sex, facial skin color, background light intensity, stroke risk scores, antithrombotic treatment received, systolic blood pressure, and resting heart rate. Last, a backward stepwise multivariate logistic analysis was used. All analyses were 2 tailed, and P<0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS statistical software (IBM SPSS Statistics for Windows, version 22.0; IBM Corporation).
We calculated a sample size of 211 was needed to test the performance of the diagnostic test, with a false‐positive result of <5% with 95% confidence on the basis of an estimated AF prevalence of 30% in our study population and an expected test sensitivity and specificity of 95%.
Results
Study Population
Of the 233 patients enrolled, 217 were included in the final analysis. A total of 16 patients were excluded because of the presence of an active pacemaker (n=12) or because they declined to complete all measurements (n=4) (Figure 2). The mean age of subjects was 70.3±13.9 years, and 71.4% were men. The median von Luschan skin color among patients was 24 (interquartile range, 21–25); 4.1% of patients were of darker skin color (von Luschan scale score, >27); the lightest skin color was 6 and the darkest was 31 on the von Luschan scale. A 12‐lead ECG showed AF was present in 75 participants (34.6%) at the time of study. There were 4 participants (1.8%) who were newly diagnosed with AF. Table 1 showed the characteristics of the patients according to the presence of AF. The AF group had a higher CHA2DS2‐VASc score of 4.5±2.0 compared with non‐AF group (P<0.01); 66.6% were either receiving oral anticoagulation therapy or had undergone left atrial appendage occlusion for stroke prevention. The AF group was more likely to have a history of transient ischemic attack or stroke, congestive heart failure, and hypertension (all P<0.05) compared with non‐AF group.
Figure 2.
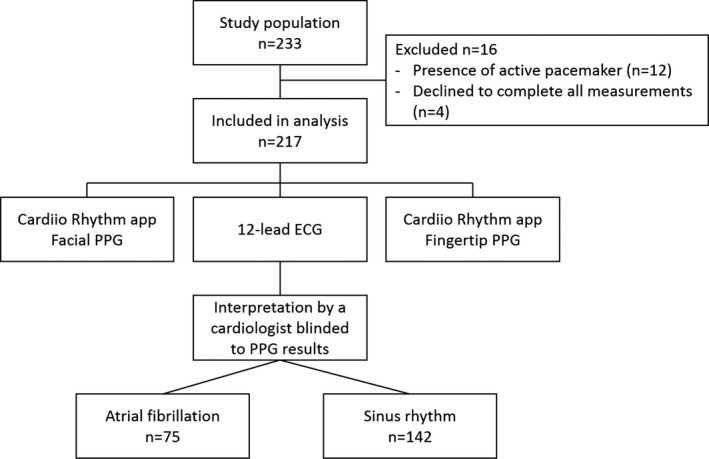
Flowchart of study participants. PPG indicates photoplethysmography.
Table 1.
Characteristics of the Study Participants According to the Presence of AF
| Characteristics | AF Absent | AF Present | All | P Value |
|---|---|---|---|---|
| (n=142) | (n=75) | (N=217) | ||
| Age, mean±SD, y | 67.8±15.0 | 75.0±10.0 | 70.3±13.9 | <0.01 |
| Male sex, n (%) | 101 (71.1) | 54 (72) | 155 (71.4) | 0.89 |
| von Luschan skin color, median (IQR) | 24 (21–25) | 24 (20–25) | 24 (21–25) | 0.89 |
| BMI, mean±SD, kg/m2 | 24.7±5.0 | 24.4±3.7 | 24.6±4.6 | 0.71 |
| SBP, mean±SD, mm Hg | 132.3±21.0 | 132.9±21.8 | 132.5±21.2 | 0.83 |
| DBP, mean±SD, mm Hg | 74.0±13.7 | 75.7±13.5 | 74.6±13.6 | 0.39 |
| Heart rate at rest, mean±SD, bpm | 67.9±11.6 | 80.9±17.2 | 72.4±15.1 | <0.001 |
| Light intensity, median (IQR), lux | 127 (99–199) | 159 (121–199) | 151 (99–199) | 0.14 |
| Stroke risk scores | ||||
| CHA2DS2‐VASc score, mean±SD | 3.1±1.9 | 4.5±2.0 | 3.6±2.1 | <0.01 |
| CHA2DS2‐VASc score ≥2, n (%) | 108 (76.1) | 70 (93.3) | 178 (82.0) | <0.01 |
| Comorbidities, n (%) | ||||
| History of AF | 46 (32.4) | 71 (94.7) | 117 (53.9) | <0.01 |
| Diabetes mellitus | 45 (31.7) | 31 (41.3) | 76 (35.0) | 0.16 |
| Vascular disease | 74 (52.1) | 36 (48.0) | 110 (50.7) | 0.56 |
| TIA or stroke | 16 (11.3) | 25 (33.3) | 41 (18.9) | <0.01 |
| Congestive heart failure | 27 (19.0) | 42 (56.0) | 69 (31.8) | <0.01 |
| Pacemaker | 4 (2.8) | 3 (4.0) | 7 (3.2) | 0.70 |
| Hypertension | 77 (54.2) | 53 (70.7) | 130 (59.9) | 0.02 |
| Antithrombotic treatment, n (%) | ||||
| None | 98 (69.0) | 13 (17.3) | 111 (51.2) | <0.01 |
| Antiplatelet therapy | 23 (16.2) | 36 (48.0) | 59 (27.2) | <0.01 |
| Oral anticoagulants | 23 (16.2) | 40 (53.3) | 63 (29.0) | <0.01 |
| Vitamin K antagonists | 12 (8.5) | 22 (29.3) | 34 (15.7) | <0.01 |
| New oral anticoagulants | 11 (7.7) | 18 (24.0) | 29 (13.4) | <0.01 |
| LAAO | 0 (0) | 10 (13.3) | 10 (4.6) | <0.01 |
AF indicates atrial fibrillation; BMI, body mass index; bpm, beats per minute; DBP, diastolic blood pressure; IQR, interquartile range; LAAO, left atrial appendage occlusion; SBP, systolic blood pressure; and TIA, transient ischemic attack.
Facial Photoplethysmography Versus 12‐Lead ECG
All 217 facial photoplethysmographic classification results produced by the Cardiio Rhythm application were analyzed. The Cardiio Rhythm application identified an irregular pulse in 77 subjects (35.5%) and a regular pulse in 140 subjects (64.5%) (Table 2). Facial photoplethysmography yielded a sensitivity of 94.7% (95% CI, 87.1%–97.9%) and specificity of 95.8% (95% CI, 91.1%–98.1%) (Table 3). The Cardiio Rhythm application produced an accuracy of 95.4% and κ of 0.90 (95% CI, 0.84–0.96). False‐positive results (n=6/217 [2.8%]) were caused by premature ventricular contraction (n=2), premature atrial complexes (n=1), bradycardia (n=2), and right bundle branch block (n=1). In this study population, the positive likelihood ratio was 22.4 (95% CI, 10.2–49.1) and the negative likelihood ratio was 0.06 (95% CI, 0.02–0.15). The positive and negative predictive values were 92.2% (95% CI, 84.4%–96.3%) and 97.1% (95% CI, 92.9%–98.9%), respectively.
Table 2.
Diagnostic Accuracy of Cardiio Rhythm Facial and Fingertip Photoplethysmography in Detecting AF
| Variable | AF Present on 12‐Lead ECG | AF Absent on 12‐Lead ECG | Total |
|---|---|---|---|
| Cardiio Rhythm facial photoplethysmography positive | 71 (32.7) | 6 (2.8) | 77 |
| Cardiio Rhythm facial photoplethysmography negative | 4 (1.8) | 136 (62.7) | 140 |
| Total | 75 | 142 | 217 |
| Cardiio Rhythm fingertip photoplethysmography positive | 71 (32.7) | 10 (4.6) | 81 |
| Cardiio Rhythm fingertip photoplethysmography negative | 4 (1.8) | 132 (60.8) | 136 |
| Total | 75 | 142 | 217 |
Data are given as number (percentage). The percentages were the number in each cell divided by the total number of tests.
AF indicates atrial fibrillation.
Table 3.
Diagnostic Test Results of Facial and Fingertip Photoplethysmography in Detecting AF
| Variable | Facial Photoplethysmography | Fingertip Photoplethysmography | ||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Sensitivity, % | 94.7 | 87.1–97.9 | 94.7 | 87.1–97.9 |
| Specificity, % | 95.8 | 91.1–98.1 | 93.0 | 87.5–96.1 |
| Positive predictive value, % | 92.2 | 84.4–96.3 | 87.7 | 79.6–92.8 |
| Negative predictive value, % | 97.1 | 92.9–98.9 | 97.1 | 92.7–98.9 |
| Positive likelihood ratio | 22.4 | 10.2–49.1 | 13.4 | 7.4–24.5 |
| Negative likelihood ratio | 0.06 | 0.02–0.15 | 0.06 | 0.02–0.15 |
| Diagnostic odds ratio | 402.3 | 109.9–1472.3 | 234.3 | 70.9–773.9 |
| Prevalence, % | 34.6 | 28.3–41.3 | 34.6 | 28.3–41.3 |
| Cohen's κ coefficient | 0.90 | 0.84–0.96 | 0.86 | 0.79–0.93 |
| Predictive accuracy, %a | 95.4 | 93.5 | ||
AF indicates atrial fibrillation; and CI, confidence interval.
The sum of true positives and true negatives divided by the total number of tests.
Fingertip Photoplethysmography Versus 12‐Lead ECG
From 217 fingertip photoplethysmographic results classified by the Cardiio Rhythm application, 81 (37.3%) were labeled as an irregular pulse and 136 (62.7%) were labeled as a regular pulse (Table 2). Fingertip photoplethysmographic results had a sensitivity of 94.7% (95% CI, 87.1%–97.9%) and specificity of 93.0% (95% CI, 87.5%–96.1%) (Table 3). An accuracy of 93.5% and κ of 0.86 (95% CI, 0.79–0.93) were obtained. In this study population, the positive likelihood ratio was 13.4 (95% CI, 7.4–24.5) and the negative likelihood ratio was 0.06 (95% CI, 0.02–0.15). A positive predictive value of 87.7% (95% CI, 79.6%–92.8%) and a negative predictive value of 97.1% (95% CI, 92.7%–98.9%) were estimated.
Facial Versus Fingertip Photoplethysmography
The agreement between facial and fingertip photoplethysmographic detection was excellent. Table 4 showed that 203 facial photoplethysmographic results matched the fingertip photoplethysmographic results (93.5%), with κ of 0.86 (95% CI, 0.79–0.93).
Table 4.
Contingency Table Comparing the Test Screening Results Between Facial and Fingertip Photoplethysmography
| Variable | Cardiio Facial Photoplethysmography Positive | Cardiio Facial Photoplethysmographic Negative | Total |
|---|---|---|---|
| Cardiio fingertip photoplethysmography positive | 131 (60.4) | 5 (2.3) | 136 |
| Cardiio fingertip photoplethysmography negative | 9 (4.1) | 72 (33.2) | 81 |
| Total | 140 | 77 | 217 |
Data are given as number (percentage). The percentages were the number in each cell divided by the total number of tests.
Consecutive Uninterpretable Photoplethysmographic Signals
Three consecutive uninterpretable photoplethysmographic readings was considered a positive test screening for AF in this study. There was 1 case of false positive with 1 patient (0.5%) who had 3 consecutive uninterpretable finger photoplethysmographic signals, but regular pulse by facial photoplethysmography and sinus rhythm on 12‐lead ECG. The likely explanation for this case was loose contact between the fingertip and camera, causing poor‐quality photoplethysmographic signals. All 9 patients (4.1%) with 3 consecutive undetectable facial photoplethysmograms were considered to have an irregular pulse by finger photoplethysmography and were confirmed to be in AF on 12‐lead ECG. In the univariate logistic regression analysis, facial skin color (range, 6–31 in our study) and background light intensity (range, 37–968 lux) were not associated with consecutive uninterpretable facial photoplethysmographic recordings (P=0.89 and P=0.79, respectively), suggesting that skin color and light intensity did not affect the success rate of facial photoplethysmographic acquisition. In the backward stepwise multivariate logistic regression analysis, subjects with systolic blood pressure <120 mm Hg (odds ratio, 6.8; 95% CI, 1.3–34.6; P=0.02) and increased resting heart rate (odds ratio, 1.05; 95% CI, 1.0–1.1; P=0.02) were associated with an increased likelihood of Cardiio Rhythm application in producing 3 consecutive uninterpretable facial photoplethysmographic readings; results were confirmed by the nonautomated model.
Discussion
This is the first study reporting the diagnostic accuracy of a contact‐free photoplethysmographic‐based method for AF screening. The Cardiio Rhythm algorithm was able to accurately diagnose AF on the basis of facial photoplethysmographic signals, with a high sensitivity and specificity in a controlled setting with respect to the reference diagnosis of a 12‐lead ECG. The diagnostic accuracy of the Cardiio Rhythm facial photoplethysmography was comparable to the fingertip photoplethysmography in this study. High sensitivity, low likelihood ratio, and convenience of a contact‐free approach are attractive qualities for potential application in large‐scale community AF screening.
The diagnostic performance of the Cardiio Rhythm facial photoplethysmography to detect AF is also comparable to other screening methods and devices. Pulse palpation has a sensitivity of 92% but only a modest specificity of 82%, which means more false‐positive results.26 A recent systematic review and meta‐analysis found that modified sphygmomanometers have a pooled sensitivity of 98% and specificity of 92%, whereas non–12‐lead ECGs have a sensitivity of 91% and a specificity of 95% in detecting an irregular pulse and suspected AF.27 Recently, several studies have reported the use of smartphone cameras to differentiate between AF and sinus rhythm using fingertip photoplethysmographic signals.16, 28, 29 In a study by McManus et al, fingertip photoplethysmographic signal recorded using an iPhone 4S in 76 patients before and after cardioversion had a sensitivity of 96% and specificity of 97% in discriminating AF from sinus rhythm.28 In another study using the same algorithm and iPhone 4S in 80 inpatients and outpatients yielded a reduced sensitivity and specificity of 90% and 85%.29 The diagnostic performance of the Cardiio Rhythm fingertip photoplethysmographic algorithm was first reported by Chan et al in 1013 outpatients compared with the AliveCor's single‐lead ECG automated algorithm.16 The diagnostic sensitivity of the Cardiio Rhythm fingertip photoplethysmography for AF detection was 92.9%, which was higher than that of the AliveCor automated algorithm of 71.4%. The algorithm used in the AliveCor application at that time had been optimized to provide high specificity (95% CI, 99.4%–99.6%) at the expense of sensitivity (95% CI, 66.7%–71.4%), because this device was marketed to individuals rather than health professionals.30, 31 The specificity of the Cardiio Rhythm application (97.7%) was comparable to that of the AliveCor automated algorithm (99.4%).
Smartphones are increasingly accessible by the elderly population. It is estimated that 19 million people worldwide use mobile health devices, and the number is increasing, which offers great opportunities for AF screening through smartphone applications.32 Both finger‐ and facial‐based methods for smartphone AF detection are attractive because both preclude the need for additional hardware, wires, or electrodes. On the other hand, drawbacks of the finger‐based method include the need to teach patients to apply just the right amount of fingertip pressure on the camera/flash to ensure a noise‐free photoplethysmographic recording, the difficulty in obtaining photoplethysmographic recordings from patients with tremors or cold extremities, and the tendency for the flash to heat up, making it uncomfortable for longer periods of monitoring. In contrast, the facial photoplethysmographic approach offers an alternative that is more comfortable, hygienic (no physical contact required), and easy to use. The Cardiio Rhythm smartphone application could be applied in pharmacies, primary care clinics, or self‐testing at home. Further studies are required to investigate the feasibility of self‐screening by unsupervised patients. Furthermore, this mobile technology could be self‐administered to facilitate follow‐up of patients after cardioversion or AF radiofrequency ablation for recurrence of AF. Because the range of background light intensities in a home setting is likely to be wider than those encountered in this study setting of hospital wards, further work is needed to establish the minimum lighting conditions necessary for successful facial photoplethysmographic acquisition.
Limitations
Limitations of all photoplethysmographic‐based devices include the need for a confirmatory ECG for suspected AF.11 Atrial or ventricular extrasystoles are well‐known causes of false‐positive results for photoplethysmographic devices that use RR‐interval variability analysis. High false‐positive rates may limit their use as a mass screening tool because of unnecessary follow‐up ECG and healthcare resources. The lower false‐positive rate associated with facial photoplethysmography (4.2%) compared with finger photoplethysmography (7.0%) suggests the former may be a better AF screening method. Second, there were several detection failures for photoplethysmographic signals using Cardiio Rhythm application because of poor signal quality. Possible causes included poor lighting, poor skin perfusion, darker skin tone, low cardiac output state, fingertip loosing contact with the camera, misalignment of face to camera, and excessive movement during signal acquisition. This limitation may be compounded if the device was used by individuals in the home setting rather than a supervised and controlled setting, such as in this study. For example, the rate of detection failure may increase if the smartphone is held by hand instead of being placed on a stand in a fixed position because of accentuated motion artifacts. The fact that all detection failures for facial photoplethysmographic signals were confirmed AF on 12‐lead ECG suggested that the software noise filter was not always able to detect poorer signal quality because of variability in pulse volume. Last, there was a short delay between photoplethysmographic measurements and the 12‐lead ECG. Although unlikely, it is possible that an AF episode captured by photoplethysmography may have spontaneously terminated before the 12‐lead ECG was recorded or that an AF episode captured by the 12‐ECG only started after the photoplethysmographic recordings were performed. Other heart rhythms, such as ectopics, which were the main reason for false‐positive results, might also have changed in that time period. Future versions of the AF detection algorithm should aim to reduce poor photoplethysmographic signal quality and differentiate between AF and extrasystoles.
Conclusion
The Cardiio Rhythm facial photoplethysmographic application is a user‐friendly stand‐alone smartphone application that may provide an inexpensive means to detect AF without physical contact. We tested a beta version of the application that demonstrated high accuracy, sensitivity, and specificity under a controlled setting. Further research is needed to determine the performance of the Cardiio Rhythm application in real‐world clinical practice, and by the general public in diverse settings.
Author Contributions
B.P. Yan, M.Z. Poh, Y.C. Poh, B. Freedman, O.T.L. To, and W.H.S. Lai designed the study. W.H.S. Lai, S. C.H. Chan, L.H. Chan, K.M. Lam, H.W. Lau, C.M. Ng, L.Y. Tai, and K.W. Yip were responsible for the recruitment of subjects and data collection. B.P. Yan, C.K.Y. Chan, W.H.S. Lai, and M.Z. Poh contributed to data analysis. B.P. Yan and C.K.Y. Chan drafted the article. All authors reviewed the article and approved the final version for publication.
Sources of Funding
This study was supported by grant to B.P. Yan and B. Freedman from the Hong Kong Research Grants Council—General Research Fund (reference no. 14118314). Cardiio Inc provided the iPhones for study purposes.
Disclosures
Y.C. Poh and M.Z. Poh are cofounders and employees of Cardiio Inc and have an ownership stake in the company. The Cardiio Rhythm AF detection technology is patent pending. There are no other potential conflicts of interest relevant to this study.
Supporting information
Figure S1. The von Luschan skin color chart.
(J Am Heart Assoc. 2018;7:e008585 DOI: 10.1161/JAHA.118.008585.)29622592
Preliminary work related to this study (Abstract 17351; Circulation. 2016;134:A17351‐A) was presented at the American Heart Association Scientific Sessions, November 12 to 16, 2016, in New Orleans, LA.
References
- 1. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014;11:639–654. [DOI] [PubMed] [Google Scholar]
- 2. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949–953. [DOI] [PubMed] [Google Scholar]
- 3. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JC, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. [DOI] [PubMed] [Google Scholar]
- 5. Moran PS, Teljeur C, Ryan M, Smith SM. Systematic screening for the detection of atrial fibrillation. Cochrane Database Syst Rev. 2016;6:CD009586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borowsky LH, Regan S, Chang Y, Ayres A, Greenberg SM, Singer DE. First diagnosis of atrial fibrillation at the time of stroke. Cerebrovasc Dis. 2017;43:192–199. [DOI] [PubMed] [Google Scholar]
- 7. Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation: a systematic review. Thromb Haemost. 2013;110:213–222. [DOI] [PubMed] [Google Scholar]
- 8. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 9. Hobbs FR, Taylor CJ, Jan Geersing G, Rutten FH, Brouwer JR. European Primary Care Cardiovascular Society Swg. European Primary Care Cardiovascular Society (EPCCS) consensus guidance on stroke prevention in atrial fibrillation (SPAF) in primary care. Eur J Prev Cardiol. 2016;23:460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sudlow M, Rodgers H, Kenny RA, Thomson R. Identification of patients with atrial fibrillation in general practice. BMJ. 1999;318:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, Albert CM, Anderson CS, Antoniou S, Benjamin EJ, Boriani G, Brachmann J, Brandes A, Chao TF, Conen D, Engdahl J, Fauchier L, Fitzmaurice DA, Friberg L, Gersh BJ, Gladstone DJ, Glotzer TV, Gwynne K, Hankey GJ, Harbison J, Hillis GS, Hills MT, Kamel H, Kirchhof P, Kowey PR, Krieger D, Lee VWY, Levin LA, Lip GYH, Lobban T, Lowres N, Mairesse GH, Martinez C, Neubeck L, Orchard J, Piccini JP, Poppe K, Potpara TS, Puererfellner H, Rienstra M, Sandhu RK, Schnabel RB, Siu CW, Steinhubl S, Svendsen JH, Svennberg E, Themistoclakis S, Tieleman RG, Turakhia MP, Tveit A, Uittenbogaart SB, Van Gelder IC, Verma A, Wachter R, Yan BP; AF‐Screen Collaborators . Screening for atrial fibrillation: a report of the AF‐SCREEN international collaboration. Circulation. 2017;135:1851–1867. [DOI] [PubMed] [Google Scholar]
- 12. Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, Bennett AA, Briffa T, Bauman A, Martinez C, Wallenhorst C, Lau JK, Brieger DB, Sy RW, Freedman SB. Feasibility and cost‐effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies: the SEARCH‐AF study. Thromb Haemost. 2014;111:1167–1176. [DOI] [PubMed] [Google Scholar]
- 13. Lau JK, Lowres N, Neubeck L, Brieger DB, Sy RW, Galloway CD, Albert DE, Freedman SB. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165:193–194. [DOI] [PubMed] [Google Scholar]
- 14. Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28:R1–R39. [DOI] [PubMed] [Google Scholar]
- 15. Jonathan E, Leahy M. Investigating a smartphone imaging unit for photoplethysmography. Physiol Meas. 2010;31:N79–N83. [DOI] [PubMed] [Google Scholar]
- 16. Chan PH, Wong CK, Poh YC, Pun L, Leung WW, Wong YF, Wong MM, Poh MZ, Chu DW, Siu CW. Diagnostic performance of a smartphone‐based photoplethysmographic application for atrial fibrillation screening in a primary care setting. J Am Heart Assoc. 2016;5:e003428 DOI: 10.1161/JAHA.116.003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McManus DD, Chong JW, Soni A, Saczynski JS, Esa N, Napolitano C, Darling CE, Boyer E, Rosen RK, Floyd KC, Chon KH. PULSE‐SMART: pulse‐based arrhythmia discrimination using a novel smartphone application. J Cardiovasc Electrophysiol. 2016;27:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poh MZ, McDuff DJ, Picard RW. Non‐contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt Express. 2010;18:10762–10774. [DOI] [PubMed] [Google Scholar]
- 19. Couderc JP, Kyal S, Mestha LK, Xu B, Peterson DR, Xia X, Hall B. Detection of atrial fibrillation using contactless facial video monitoring. Heart Rhythm. 2015;12:195–201. [DOI] [PubMed] [Google Scholar]
- 20. Poh MZ, McDuff DJ, Picard RW. Advancements in noncontact, multiparameter physiological measurements using a webcam. IEEE Trans Biomed Eng. 2011;58:7–11. [DOI] [PubMed] [Google Scholar]
- 21. Treesirichod A, Chansakulporn S, Wattanapan P. Correlation between skin color evaluation by skin color scale chart and narrowband reflectance spectrophotometer. Indian J Dermatol. 2014;59:339–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vaid J, Poh M‐Z, Saleh A, Kalantarian S, Poh YKC, Rafael A, Ruskin J. Arrhythmias and Clinical EP: diagnostic accuracy of a novel mobile application (Cardiio Rhythm) for detecting atrial fibrillation. J Am Coll Cardiol. 2015;65:A361. Presented in ACC.15 Scientific Sessions. 2015; San Diego, CA. [Google Scholar]
- 23. Yan BP, Chan CK, Li CK, To OT, Lai WH, Tse G, Poh YC, Poh MZ. Resting and postexercise heart rate detection from fingertip and facial photoplethysmography using a smartphone camera: a validation study. JMIR Mhealth Uhealth. 2017;5:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Poh MZ, Poh YC. Validation of a standalone smartphone application for measuring heart rate using imaging photoplethysmography. Telemed J E Health. 2017;23:678–683. [DOI] [PubMed] [Google Scholar]
- 25. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 26. Hobbs FD, Fitzmaurice DA, Mant J, Murray E, Jowett S, Bryan S, Raftery J, Davies M, Lip G. A randomised controlled trial and cost‐effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over: the SAFE study. Health Technol Assess. 2005;9:iii–iv, ix–x, 1–74. [DOI] [PubMed] [Google Scholar]
- 27. Taggar JS, Coleman T, Lewis S, Heneghan C, Jones M. Accuracy of methods for detecting an irregular pulse and suspected atrial fibrillation: a systematic review and meta‐analysis. Eur J Prev Cardiol. 2016;23:1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McManus DD, Lee J, Maitas O, Esa N, Pidikiti R, Carlucci A, Harrington J, Mick E, Chon KH. A novel application for the detection of an irregular pulse using an iPhone 4S in patients with atrial fibrillation. Heart Rhythm. 2013;10:315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krivoshei L, Weber S, Burkard T, Maseli A, Brasier N, Kuhne M, Conen D, Huebner T, Seeck A, Eckstein J. Smart detection of atrial fibrillation. Europace. 2017;19:753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Desteghe L, Raymaekers Z, Lutin M, Vijgen J, Dilling‐Boer D, Koopman P, Schurmans J, Vanduynhoven P, Dendale P, Heidbuchel H. Performance of handheld electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace. 2017;19:29–39. [DOI] [PubMed] [Google Scholar]
- 31. Albert DE. Performance of hand‐held electrocardiogram devices to detect atrial fibrillation in a cardiology and geriatric ward setting. Europace. 2017;19:1408. [DOI] [PubMed] [Google Scholar]
- 32. Olgun Kucuk H, Kucuk U, Yalcin M, Isilak Z. Time to use mobile health devices to diagnose paroxysmal atrial fibrillation. Int J Cardiol. 2016;222:1061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The von Luschan skin color chart.


