Abstract
Background
Mineralocorticoid receptor antagonist (MRA) therapy may be beneficial to patients with atrial fibrillation (AF), but little is known about their use in patients with AF and subsequent outcomes.
Methods and Results
In order to better understand MRA use and subsequent outcomes, we performed a retrospective cohort study of the contemporary ORBIT‐AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) registry. AF progression and cardiovascular outcomes were compared using propensity‐matched Cox proportional hazards modeling according to MRA use at baseline and new MRA use at follow‐up versus patients with no MRA use. Among 7012 patients with nonpermanent AF, 320 patients were taking MRA at enrollment, and 416 patients initiated MRA use during follow‐up. The mean patient age was 72.5 years, 56.3% were men, and 70.4% had paroxysmal AF. Among all patients taking MRAs, 434 (59.0%) had heart failure, 655 (89.0%) had hypertension, and 380 (51.6%) had both. After adjustment, new MRA use was not associated with reduced AF progression (hazard ratio, 1.18; 95% confidence interval, 0.88–1.58; P=0.27) but showed a trend towards lower risk of stroke, transient ischemic attack, or systemic embolism (hazard ratio, 0.17; 95% confidence interval, 0.02–1.23; P=0.08). Results were similar for a comparison of new MRA users and baseline MRA users compared with nonusers.
Conclusions
In community‐based outpatients with AF, the majority of MRA use was for heart failure and hypertension. MRA use also trended towards lower adjusted stroke risk. Future studies should test the hypothesis that MRA use may decrease the risk of stroke in patients with AF.
Keywords: atrial fibrillation, mineralocorticoid antagonist, stroke
Subject Categories: Atrial Fibrillation, Ischemic Stroke
Clinical Perspective
What Is New?
In patients with nonpermanent atrial fibrillation, mineralocorticoid receptor antagonist use in patients with atrial fibrillation is common and is driven by heart failure and hypertension.
Neither new nor baseline use of mineralocorticoid receptor antagonists was associated with reduction in atrial fibrillation progression.
Despite a higher rate of comorbid diseases in patients with MRA use, we observed a trend towards lower risk of stroke, transient ischemic attack, and systemic embolism.
What Are the Clinical Implications?
This raises the hypothesis that mineralocorticoid receptor antagonist use may reduce thromboembolic events in patients with atrial fibrillation, as has been observed with renin‐angiotensin‐aldosterone system antagonism in other disease states.
The renin‐angiotensin‐aldosterone system has long been considered a potential upstream target for the treatment and prevention of atrial fibrillation (AF). Renin‐angiotensin‐aldosterone system activation leads to myocardial oxidative stress, fibrosis, and electrical remodeling,1, 2 and promotes other profibrillatory conditions such as heart failure (HF) and hypertension. Several meta‐analyses of angiotensin‐converting enzyme inhibitor (ACEI) and angiotensin receptor blocker (ARB) therapy have suggested that these agents can improve freedom from AF, both in the primary and secondary prevention settings.3, 4, 5 However, results have been mixed and seem to favor these agents mostly in the primary prevention of AF. Consequently, current guidelines only recommend ACEI or ARB therapy for primary prevention of AF in systolic HF (class IIa) and hypertension (class IIb).6 In other clinical settings, ACEI and ARB therapy has also been shown to reduce the risk of stroke, such as in the LIFE (Losartan Intervention for End Point Reduction in Hypertension),7 HOPE (Heart Outcomes Prevention Evaluation),8 and PROGRESS (Perindopril Protection Against Recurrent Stroke Study)9 trials. In the recently presented RACE 3 (Routine versus Aggressive Upstream Rhythm Control for Prevention of Early Persistent Atrial Fibrillation in Heart Failure Study),10 upstream therapy with ACEIs/ARBs, statins, mineralocorticoid receptor antagonists (MRAs), and cardiac rehabilitation were more effective in maintaining sinus rhythm at 1 year than standard therapy in patients with HF. This study underlined the potential utility of upstream therapy on the reduction of risk factors in patients with early persistent AF and HF.
MRAs, which directly target the action of aldosterone, may be a more effective target for renin‐angiotensin‐aldosterone system inhibition than ACEIs and ARBs for the prevention and treatment of AF for multiple reasons. First, AF is associated with increased aldosterone levels,11, 12 overexpression of mineralocorticoid receptors,12 and aldosterone‐mediated electrical remodeling.13 Also, in the setting of HF, mechanisms of aldosterone escape where aldosterone activity is increased in the absence of detectable angiotensin irregularities have also been described.14 In the SPIR‐AF (Spironolactone–β‐Blocker±Enalapril Treatment on Occurrence of Symptomatic Atrial Fibrillation Episodes in Patients With a History of Paroxysmal Atrial Fibrillation) study,15 randomization to spironolactone resulted in less frequent episodes of AF in patients with paroxysmal AF. MRA therapy has also been associated with decreased AF recurrence after catheter ablation of persistent AF,16 improved success in cardioversion of persistent AF,17 and lower rates of AF in patients with HF.18, 19 Yet, little is known about current MRA use in patients with AF. In this analysis, we utilized the ORBIT‐AF registry to describe MRA use in a contemporary AF population and subsequent outcomes. We hypothesized that MRA use would be associated with slower AF progression, as well as decreased mortality, stroke, new‐onset HF, and cardiovascular hospitalization.
Methods
Study Population
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The ORBIT‐AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation)20 is a contemporary registry of patients with AF from 176 heterogeneous outpatient practices in the United States. The rationale and design of the ORBIT‐AF registry have been previously published.20 Briefly, patients 18 years and older with ECG‐documented AF were eligible, while patients with a life expectancy <6 months or AF secondary to reversible conditions (eg, acute pulmonary embolism or thyroid storm) were excluded. Baseline data including demographic information, medical history, type of AF, HF history, and pharmacotherapy and treatment history were captured at enrollment. Additionally, follow‐up data including change in AF type, stroke or other thromboembolism events, cardiovascular and all‐cause death, new‐onset HF, and cardiovascular hospitalization were also obtained every 6 months. Patients were enrolled from June 2010 through August 2011. For the purpose of this analysis, patients with information regarding MRA use at baseline and with at least one 6‐month follow‐up were included in the study. Because of concerns over the inability to modify disease substrate, patients with permanent AF were excluded. MRA use included spironolactone or eplerenone. Eligible patients were further classified as baseline users of MRA therapy (baseline use) or new initiators of MRA therapy (new use) during follow‐up. The ORBIT‐AF registry was approved by Duke's institutional review board, and participating sites obtained approval from local institutional review boards as needed before entering patient data. Patients provided informed consent as part of the ORBIT‐AF registry.
Outcome Measures
The primary outcome was AF progression. Secondary outcomes included all‐cause death, cardiovascular death, new‐onset HF, first cardiovascular hospitalization and composite stroke, transient ischemic attack (TIA), and systemic embolism. All outcomes were ascertained at every 6‐month follow‐up.
Consistent with consensus nomenclature, paroxysmal AF was defined as recurrent AF episodes that terminated spontaneously within 7 days, persistent AF as recurrent AF that was sustained for more than 7 days, and permanent AF as continuous AF in which the presence of the AF was accepted by patient and physician. Assessment of AF type was made by the site investigator according to consensus definitions and updated with each follow‐up.21 As previously mentioned, because the primary end point was AF progression, patients with either permanent AF or new‐onset AF at baseline were excluded. As previously described,21 AF progression was defined as a binary outcome (“same or better” and “worsening”), where any change in baseline AF status from paroxysmal or persistent AF to a more advanced status (persistent or permanent) was defined as “worsening.”
Statistical Analysis
Baseline characteristics of patients on and off MRA therapy are presented as frequencies and percentages for categorical variables, and median (25th percentile–75th percentile) for continuous variables. Characteristics were compared using chi‐square tests for categorical variables and the Wilcoxon rank‐sum test for continuous variables. These comparisons were made twice, once in patients with HF at baseline, and once in those without HF at baseline (Table 1). The event rates of each outcome were recorded. For AF progression, the proportion of patients with worsening AF was compared using pooled logistic regression, and an odds ratio was computed. For all other outcomes, event rates were described per 100 patient‐years and compared with Cox proportional hazards modeling. In order to address site variance, in all models, we included a robust covariance estimate to account for correlation within each site. When specifically considering the new‐onset HF outcomes, only patients without HF at baseline were compared.
Table 1.
Baseline Characteristics According to MRA Use, Stratified by HF Status in Patients With AF
| Characteristic | No HF (n=4952) | HF (n=2060) | ||||
|---|---|---|---|---|---|---|
| No MRA (n=4850) | MRA (n=102) | P Value | No MRA (n=1842) | MRA (n=218) | P Value | |
| Age, y | 73 (65–80) | 74 (64–82) | 0.5563 | 76 (67–82) | 73 (64–82) | 0.0242 |
| Male | 2686 (55.38) | 47 (46.08) | 0.0615 | 1083 (58.79) | 130 (59.63) | 0.8120 |
| White | 4399 (90.70) | 95 (93.14) | 0.8955 | 1629 (88.44) | 191 (87.61) | 0.4888 |
| SBP, mm Hg | 127 (118–138) | 122 (110–139) | 0.0721 | 123 (112–136) | 120 (104–130) | <0.0001 |
| DBP, mm Hg | 74 (68–80) | 72 (66–80) | 0.2211 | 70 (62–80) | 70 (60–78) | 0.0065 |
| HR | 69 (61–78) | 72 (64–80) | 0.0097 | 70 (63–80) | 72 (64–80) | 0.0758 |
| BMI, kg/m2 | 29.0 (25.5–33.6) | 30.1 (24.5–36.4) | 0.2917 | 29.3 (25.1–34.9) | 31.99 (9.40) | 0.1261 |
| CAD history | 1340 (27.63) | 31 (30.39) | 0.5371 | 980 (53.20) | 117 (53.67) | 0.8961 |
| Hypertension | 3854 (79.46) | 96 (94.12) | 0.0003 | 1592 (86.43) | 190 (87.16) | 0.7660 |
| Diabetes mellitus | 1164 (24.00) | 27 (26.47) | 0.5634 | 699 (37.95) | 95 (43.58) | 0.1063 |
| PVD | 478 (9.86) | 7 (6.86) | 0.3142 | 343 (18.62) | 38 (17.43) | 0.6687 |
| Hyperlipidemia | 3355 (69.18) | 70 (68.63) | 0.9056 | 1401 (76.06) | 170 (77.98) | 0.5280 |
| CKD | 1364 (30.82) | 42 (45.65) | 0.0024 | 807 (46.11) | 112 (52.83) | 0.0642 |
| NYHA functional status | N/A | <0.0001 | ||||
| Class I | ··· | ··· | 643 (35.10) | 49 (22.58) | ||
| Class II | ··· | ··· | 817 (44.60) | 95 (43.78) | ||
| Class III | ··· | ··· | 342 (18.67) | 67 (30.88) | ||
| Class IV | ··· | ··· | 30 (1.64) | 6 (2.76) | ||
| eGFR (MDRD), mg/dL | 70.3 (56.7–85.1) | 62.0 (50.2–72.7) | 0.0003 | 62.4 (47.8–78.5) | 58.7 (45.4–73.9) | 0.0137 |
| Hemoglobin, g/dL | 13.7 (12.5–14.8) | 13.8 (12.5–14.9) | 0.7385 | 13.0 (11.7–14.2) | 13.0 (11.5–14.2) | 0.9382 |
| LVEF | 60 (55–65) | 56 (50–62) | 0.0479 | 50 (40–60) | 40 (30–55) | <0.0001 |
| AAD use | 1850 (38.14) | 39 (38.24) | 0.9851 | 634 (34.42) | 64 (29.36) | 0.1354 |
| OAC (warfarin or dabigatran) use | 3387 (69.84) | 78 (76.47) | 0.1479 | 1415 (76.82) | 178 (81.65) | 0.1071 |
| Cardiologist as provider | 3811 (78.58) | 84 (82.35) | 0.3571 | 1498 (81.32) | 192 (88.07) | 0.0141 |
Values are expressed as number (percentage) or median (25th percentile–75th percentile). AAD indicates antiarrhythmic drug; AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, heart rate; LVEF, left ventricular ejection fraction; MDRD, Modification of Diet in Renal Disease; MRA, mineralocorticoid antagonist; N/A, not available; NYHA, New York Heart Association; OAC, oral anticoagulation; PVD, peripheral artery disease; SBP, systolic blood pressure.
Two models were run for each outcome. First, the outcomes of new MRA users (no MRA use at baseline who initiated MRA during follow‐up) were compared against outcomes of patients who did not receive MRA therapy at baseline or through the duration of the study. A second comparison was made between outcomes of a composite group consisting of new and baseline use of MRA users against outcomes of patients who did not receive MRA therapy at baseline or through the duration of the study.
For each comparison, 2 analyses were constructed to analyze the association between MRA use and each outcome. First, an unadjusted model was constructed. Second, a propensity‐matched analysis was performed. Pooled logistic regression was performed in order to determine the propensity for MRA use at each 6‐month visit. The list of covariates used to model MRA use propensity included age, HF status and New York Heart Association functional class, systemic blood pressure, left atrial diameter, and AF type. We also included interaction terms for HF and intraventricular conduction pattern, HF and left atrial diameter, and HF and AF type. These models were also used to describe factors associated with MRA use at baseline and during follow‐up. A full list of covariates is shown in Table S1. For new MRA users, covariates were updated as of the visit before the initiation of MRA use. For baseline MRA users, only baseline data were used. Continuous covariates were tested for linearity, and any nonlinear associations were accounted for using linear splines. Missing data for baseline covariates were handled using single imputation, with imputed values obtained by the Markov chain Monte Carlo method. If a follow‐up visit was missed but a subsequent visit was not, data for the missing visit were imputed by carrying forward the measurements from the previous visit. Propensity matching was performed at each visit, aiming for a 5‐to‐1 match of nonusers to MRB users. The difference in propensities was no larger than a caliper of 20% of a standard deviation. If 5 matches were not found, a minimum of 3 were required to remain in the propensity‐matched analysis. A table comparing the standardized differences of covariates after matching is shown in Tables S2 through S5.
All analyses were performed using SAS software (version 9.3, SAS Institute Inc).
Results
Cohort Formation
A total of 10 137 patients were enrolled from 176 sites in ORBIT‐AF. In total, 3125 patients were excluded from this analysis: 2 were missing MRA use information at baseline, 2830 had permanent AF at baseline, and 293 patients did not have follow‐up. Thus, 7012 patients were included in the final cohort, of which 320 patients were taking MRAs at baseline and 416 patients had newly initiated MRA use during follow‐up. In outcomes analyses, 14 patients had missing information on MRA use during follow‐up and were excluded from further analyses. For comparisons of AF progression, 438 patients with new‐onset AF were excluded. For comparisons of new‐onset HF, 2060 patients with baseline HF were excluded. Overall, the mean follow‐up was 2.3±0.8 years for all patients and 1.5±0.7 years for new MRA users after initiation of therapy.
Baseline Characteristics
The baseline characteristics of patients according to MRA use are presented in Table 1, stratified by HF status. The mean age was 73 years and 56% were men. Among all patients taking MRAs, 434 (59.0%) had HF, 655 (89.0%) had hypertension, and 380 (51.6%) had both. Patients on MRA therapy at baseline were more likely to have HF (68% versus 28%, P<0.001). Predictors of MRA use are shown in Table S1. Among those without HF, 94% of patients taking MRAs had hypertension, compared with 79% of patients not on MRA therapy (P<0.001). In patients without HF, MRA users were observed to have higher heart rates (independent of rhythm), more often had hypertension and chronic kidney disease, and had lower left ventricular ejection fractions. In patients with HF, MRA users at baseline were younger and had lower systolic and diastolic blood pressure, better New York Heart Association functional class, lower left ventricular ejection fraction, and lower estimated glomerular filtration rate.
Propensity Matching and Predictors of MRA Use
For patients with baseline MRA use and patients who newly initiated MRA, the propensity for MRA use was modeled separately. Propensity‐matched cohorts were generated for new MRA users alone, and for new MRA users and baseline MRA users combined. In the group of new MRA users, 258 of 416 patients were matched with 1287 patients not on MRA at baseline or during follow‐up. In these groups, roughly 52% of patients had HF, 75% had paroxysmal AF, 56% were rate controlled, and 74% were on oral anticoagulation. In the combined group of baseline and new MRA users, 534 of 736 MRA users were matched with 2658 nonusers. In these groups, roughly 58% had HF, 71% had paroxysmal AF, 59% were rate controlled, and 76% were on oral anticoagulation. Propensity‐matched patients were well matched on numerous covariates, including HF status, AF type, AF management, and oral anticoagulation. A list of all matched covariates and standardized differences are reported in Tables S2 through S5. Predictors of baseline and new MRA use are shown in Tables S6 and S7.
Outcomes
In an unadjusted comparison of new MRA users and nonusers (Table 2), MRA users had a higher incidence of all‐cause death, were more likely to develop new‐onset HF during follow‐up, and were more likely to experience a cardiovascular hospitalization. After an adjusted comparison using propensity matching of new MRA users and nonusers (Table 3), all of the observed associations weakened with the exception of the outcome of stroke, TIA, or other embolic events, which persisted in a trend favoring MRA use (hazard ratio, 0.17; 95% confidence interval, 0.02–1.23 [P=0.079]) (Figure 1).
Table 2.
Incidence Rate of Outcomes in New MRA Users Compared With Nonusers
| Outcome | Overall (N=5650) | MRA Use | |
|---|---|---|---|
| Never Used (n=5390) | New Use (n=260) | ||
| AF progression, No. (%) (N=5620)a | 1241 (22.08) | 1186 (22.13) | 55 (21.15) |
| All‐cause death | 372 (3.39) | 351 (3.32) | 21 (5.39) |
| Cardiovascular death | 134 (1.22) | 126 (1.19) | 8 (2.06) |
| First stroke, non‐CNS embolism, or TIA | 142 (1.31) | 141 (1.35) | 1 (0.26) |
| New‐onset HF (N=4174)b | 79 (0.98) | 75 (0.95) | 4 (2.34) |
| First cardiovascular hospitalization | 1376 (14.54) | 1306 (14.28) | 70 (22.08) |
Event rates per 100 patient‐years of follow‐up. CNS indicates central nervous system; TIA, transient ischemic attack.
Among the 5620 patients in the atrial fibrillation (AF) progression analysis, 260 were new mineralocorticoid antagonist (MRA) users and 5360 were not.
Among the 4174 patients in the new‐onset heart failure (HF) analysis, 121 were new MRA users and 4053 were not.
Table 3.
Unadjusted and Propensity‐Matched Association Between New MRA Use and Outcomes
| Outcome | Unadjusted | Propensity‐Matched | ||
|---|---|---|---|---|
| HR or OR (95% CI) | P Value | HR or OR (95% CI) | P Value | |
| AF progressiona | 1.36 (0.99–1.88) | 0.0598 | 1.18 (0.88–1.58) | 0.2731 |
| All‐cause death | 1.82 (1.21–2.72) | 0.0038 | 1.09 (0.67–1.79) | 0.7303 |
| Cardiovascular death | 1.87 (0.90–3.87) | 0.0941 | 0.97 (0.43–2.15) | 0.9319 |
| First stroke, non‐CNS embolism, or TIA | 0.19 (0.03–1.41) | 0.1055 | 0.17 (0.02–1.23) | 0.0792 |
| New‐onset HF | 2.61 (1.02–6.66) | 0.0443 | 1.73 (0.54–5.57) | 0.3587 |
| First cardiovascular hospitalization | 1.49 (1.16–1.92) | 0.0016 | 1.09 (0.85–1.39) | 0.5020 |
AF indicates atrial fibrillation; CI, confidence interval; CNS, central nervous system; HF, heart failure; HR, hazard ratio; MRA, mineralocorticoid antagonist; TIA, transient ischemic attack.
Odds ratio (OR) reported.
Figure 1.
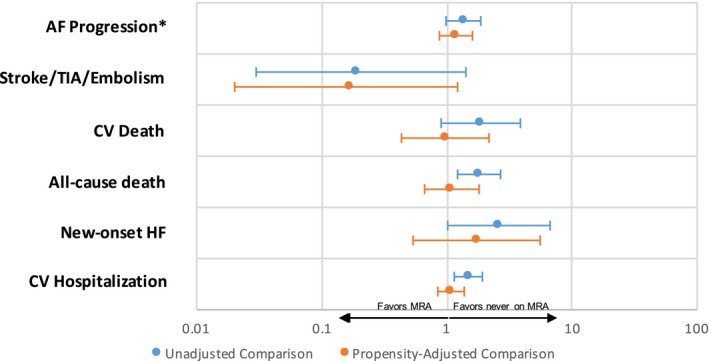
Associations between new mineralocorticoid antagonist (MRA) use and outcomes in unadjusted (blue, top line) and propensity‐matched (orange, bottom line) patients. Ratios <1 (to the left) favor patients on MRA therapy. Ratios >1 (to the right) favor patients never on MRA therapy. *Odds ratios are reported, with others as hazard ratios. AF indicates atrial fibrillation; CV, cardiovascular; HF, heart failure; TIA, transient ischemic attack.
Similar results were seen in the comparison between combined new and baseline MRA users versus nonusers (Tables 4 and 5). In the unadjusted comparison of new and baseline users combined, MRA users were more likely to have progression of AF, more likely to experience death from any cause as well as cardiovascular death, more likely to be diagnosed with HF during follow‐up, and more likely to experience cardiovascular hospitalization (Figure 2). In the propensity‐matched comparison, all of these associations weakened except for stroke, TIA, or other embolism, which numerically continued to favor MRA use (hazard ratio, 0.60; 95% confidence interval, 0.31–1.16 [P=0.13]).
Table 4.
Incidents Rates of Outcomes by MRA Use (New and Baseline Use Combined)
| Outcome | Overall (N=7012) | MRA Use | |
|---|---|---|---|
| Never Used (n=6432) | Baseline+New Use (n=580) | ||
| AF progression, No. (%) (N=6442)a | 1795 (27.86) | 1635 (27.81) | 160 (28.47) |
| All‐cause death | 785 (4.99) | 701 (4.79) | 84 (7.62) |
| Cardiovascular death | 301 (1.92) | 257 (1.76) | 44 (4.02) |
| Frist stroke, non‐CNS embolism, or TIA | 209 (1.35) | 198 (1.37) | 11 (1.01) |
| New‐onset HF (N=4947)b | 135 (1.21) | 123 (1.14) | 12 (3.00) |
| First cardiovascular hospitalization | 2146 (16.75) | 1946 (16.30) | 200 (22.94) |
Event rates per 100 patient‐years of follow‐up. CNS indicates central nervous system; TIA, transient ischemic attack.
Among the 6442 patients in the atrial fibrillation (AF) progression analysis, 562 were mineralocorticoid antagonist (MRA) users and 5880 were not.
Among the 4947 patients in the new‐onset heart failure (HF) analysis, 223 were MRA users and 4724 were not.
Table 5.
Unadjusted and Propensity‐Matched Association Between All MRA Use and Outcomes
| Outcome | Unadjusted | Propensity‐Matched | ||
|---|---|---|---|---|
| HR or OR (95% CI) | P Value | HR or OR (95% CI) | P Value | |
| AF progressiona | 1.36 (1.10–1.67) | 0.0047 | 1.11 (0.92–1.34) | 0.2685 |
| All‐cause death | 1.86 (1.45–2.39) | <0.0001 | 1.07 (0.84–1.37) | 0.5791 |
| Cardiovascular death | 2.64 (1.81–3.84) | <0.0001 | 1.29 (0.90–1.84) | 0.1614 |
| First stroke, non‐CNS embolism, or TIA | 0.75 (0.44–1.31) | 0.3146 | 0.60 (0.31–1.16) | 0.1300 |
| New‐onset HF | 2.82 (1.46–5.46) | 0.0020 | 2.40 (1.19–4.88) | 0.0150 |
| First cardiovascular hospitalization | 1.42 (1.18–1.71) | 0.0002 | 1.00 (0.86–1.18) | 0.9551 |
AF indicates atrial fibrillation; CI, confidence interval; CNS, central nervous system; HF, heart failure; HR, hazard ratio; MRA, mineralocorticoid antagonist; TIA, transient ischemic attack.
Odds ratio (OR) reported.
Figure 2.
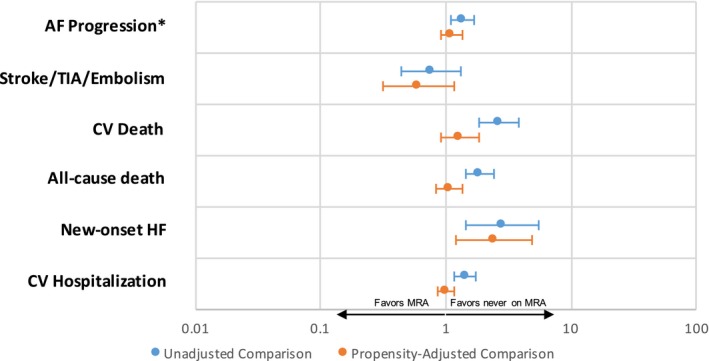
Associations between combined (new and baseline) mineralocorticoid antagonist (MRA) use and outcomes in unadjusted (blue, top line) and propensity‐matched (orange, bottom line) patients. Ratios <1 (to the left) favor patients on MRA therapy. Ratios >1 (to the right) favor patients never on MRA therapy. *Odds ratios are reported, with others as hazard ratios. AF indicates atrial fibrillation; CV, cardiovascular; TIA, transient ischemic attack.
Discussion
In this contemporary analysis of MRA use in 7012 patients with AF, there are 3 major findings. First, MRA use in patients with AF was driven by HF and hypertension. Second, neither new nor baseline MRA use was associated with reduction in AF progression. Finally, despite a higher rate of comorbid diseases in patients with MRA, we observed a trend towards lower risk of stroke, TIA, or systemic embolism. This raises the hypothesis that MRA may reduce thromboembolic events in patients with AF, as has been observed with renin‐angiotensin‐aldosterone system antagonism in other disease states.
In this nationwide cohort, among patients taking MRA therapy at baseline, 68% had HF. Of those on MRA therapy without HF, almost all of the patients (94%) had hypertension. These results suggest that these 2 conditions are the primary drivers for MRA use in our population, consistent with current guidelines. Additionally, patients taking MRAs were more likely to have chronic kidney disease and higher heart rates. All of these factors have been linked to worse outcomes in AF, and likely account for the increased incidence of death, AF progression, and cardiovascular hospitalization seen in our unadjusted comparisons of MRA users and nonusers.
In the context of these results, our observed trend toward lower incidence of stroke, TIA, and other embolic events with MRA use would not be expected to be as a result of confounding. Despite the many characteristics of MRA users that portend poorer prognosis, a trend toward lower stroke risk was seen even in unadjusted comparisons of MRA users versus nonusers. In comparisons adjusted for MRA‐use propensity, while other associations were weakened, the trend towards decreased stroke risk with MRA use was strengthened and persisted across analyses of new MRA and baseline MRA users. It is important to emphasize that the trend towards decreased stroke risk with MRA use observed in this study is hypothesis generating and should be tested in future investigations. To our knowledge, there are few data on the association between MRA use and stroke risk in patients with AF, with only 2 prior studies on ACEI and ARB therapy in AF. In the LIFE study, subgroup analysis of patients with hypertension who developed new‐onset AF were less likely to have a stroke in the ARB group compared with the atenolol group (hazard ratio, 0.49; 95% confidence interval, 0.29–0.86 [P=0.01]).22 However, in the SPORTIF (Stroke Prevention Using an Oral Direct Thrombin Inhibitor in Atrial Fibrillation) trial, ACEI and ARB therapy were not associated with a reduction in stroke risk (hazard ratio, 0.95; 95% confidence interval, 0.68–1.32).23 There were several differences between these 2 trials. The LIFE study analyzed patients with hypertension without AF who developed new‐onset AF during the study, while the SPORTIF analysis was performed in patients with AF at high risk for stroke, who were all on oral anticoagulation. In the current analysis, roughly three quarters of our patients had paroxysmal AF, and roughly the same proportion were on oral anticoagulation. This hypothesis should be further explored as additional therapies are needed to target residual stroke risk in patients who are already receiving oral anticoagulation. For example, patients with a CHA2DS2VASc score of ≥5 who were being actively treated with an oral anticoagulant continued to have a high risk of stroke at >5 events per 100 patient‐years.24
Finally, the current study showed no association between MRA use and slowing of AF progression, despite other clinical evidence suggesting improved rhythm control with MRA.10, 15, 16, 17 There are several differences between this and other studies. First, the current study is the only one performed in an outpatient setting with inclusion of patients with HF. Other observational studies have focused on patients after acute interventions for AF, such as in patients with long‐standing persistent AF undergoing catheter ablation16 or in patients with persistent AF undergoing cardioversion.17 In the SPIR‐AF randomized controlled trial, both inpatients and outpatients were included, and patients with HF were excluded. Another difference between this and other studies was the end point. Whereas other studies tracked freedom from AF15, 16, 17, 19 or hospitalizations for AF,25 we measured AF progression from changes in physician‐reported AF type over time. End points from other studies may occur earlier in the natural progression of AF, whereas the end point of AF progression may tend to be later in the disease process. Consequently, the absence of any apparent association with reduced AF progression may be explained by the fact that disease substrate may already be too advanced to harness any potential benefit of MRA therapy. Despite this concern, some studies have shown reduction in recurrent AF with MRA therapy in patients with persistent AF.10, 16, 17
Limitations
The current observational study was performed in a voluntary registry and may be subject to selection and reporting bias. Also, study data were comprised primarily of chart‐abstracted, physician‐documented study forms, and were dependent on the accuracy and quality of this documentation. Additionally, only a limited number of patients in the registry used MRA therapy, limiting the power of our results. In the analysis of stroke outcomes in particular, low event rates further limited power. Finally, MRA use was not randomized in this study, and associations may be subject to unmeasured confounding. However, in the adjusted comparisons, adjustments for all known confounders were made (Table S1). Moreover, the association between MRA use and higher patient comorbidity would be expected to confound towards increased stroke rates rather than the trend in reduction that was observed.
Conclusions
MRA therapy in patients with AF is driven by HF and hypertension. While there is no evidence that MRA use slows AF progression in the current study, our results suggest that MRA use may be associated with decreased risk of stroke. Future clinical studies should explore the hypothesis that MRA therapy may reduce residual stroke risk in patients with AF.
Sources of Funding
This project was supported (in part) by funding from the Agency of Healthcare Research and Quality through cooperative agreement number 1U19 HS021092. The ORBIT‐AF registry is sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ.
Disclosures
Fudim is supported by an American Heart Association grant (17MCPRP33460225) and National Institutes of Health T32 grant (5T32HL007101) and serves as a consultant for Coridea, Cibiem, Galvani, and GE Healthcare. Piccini receives funding for clinical research from ARCA biopharma, Boston Scientific, Gilead, Janssen Pharmaceuticals, ResMed, Spectranetics, and St. Jude Medical and serves as a consultant for Allergan, Amgen, GlaxoSmithKline, Johnson & Johnson, Medtronic, and Spectranetics. Allen received research grants from the National Institutes of Health, the American Heart Association, and the Patient‐Centered Outcomes Research Institute, and serves as a consultant for Janssen, Novartis, and Boston Scientific. Fonarow serves as a consultant for Amgen, Boston Scientific, Janssen, Johnson & Johnson, Medtronic, Novartis, St. Jude Medical, Takeda, The Medicines Company, and ZS Pharma. The remaining authors have no conflicts of interest to report.
Supporting information
Table S1. Propensity Score Covariate List
Table S2. Standardized Differences of Propensity‐Matched Pairs: New MRA Use
Table S3. Standardized Differences of Propensity‐Matched Pairs: New MRA Use (New‐Onset HF‐Matched Pairs)
Table S4. Standardized Differences of Propensity‐Matched Pairs: New and Baseline MRA Use Combined
Table S5. Standardized Differences of Propensity‐Matched Pairs: New and Baseline MRA Use Combined (New‐Onset HF‐Matched Pairs)
Table S6. Predictors of New MRA Use
Table S7. Predictors of New and Baseline MRA Use
(J Am Heart Assoc. 2018;7:e007987 DOI: 10.1161/JAHA.117.007987.)29654203
References
- 1. Mayyas F, Alzoubi KH, Van Wagoner DR. Impact of aldosterone antagonists on the substrate for atrial fibrillation: aldosterone promotes oxidative stress and atrial structural/electrical remodeling. Int J Cardiol. 2013;168:5135–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL; American College of Cardiology/American Heart Association Task Force on Practice Guidelines, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association and Heart Rhythm Society . ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 guidelines for the management of patients with atrial fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. [DOI] [PubMed] [Google Scholar]
- 3. Chaugai S, Meng WY, Sepehry AA. Effects of RAAS blockers on atrial fibrillation prophylaxis: an updated systematic review and meta‐analysis of randomized controlled trials. J Cardiovasc Pharmacol Ther. 2016;21:388–404. [DOI] [PubMed] [Google Scholar]
- 4. Han M, Zhang Y, Sun S, Wang Z, Wang J, Xie X, Gao M, Yin X, Hou Y. Renin‐angiotensin system inhibitors prevent the recurrence of atrial fibrillation: a meta‐analysis of randomized controlled trials. J Cardiovasc Pharmacol. 2013;62:405–415. [DOI] [PubMed] [Google Scholar]
- 5. Khatib R, Joseph P, Briel M, Yusuf S, Healey J. Blockade of the renin‐angiotensin‐aldosterone system (RAAS) for primary prevention of non‐valvular atrial fibrillation: a systematic review and meta analysis of randomized controlled trials. Int J Cardiol. 2013;165:17–24. [DOI] [PubMed] [Google Scholar]
- 6. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JJ, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:2246–2280. [DOI] [PubMed] [Google Scholar]
- 7. Lindholm LH, Ibsen H, Dahlöf B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe‐Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004. [DOI] [PubMed] [Google Scholar]
- 8. Bosch J, Yusuf S, Pogue J, Sleight P, Lonn E, Rangoonwala B, Davies R, Ostergren J, Probstfield J. Use of ramipril in preventing stroke: double blind randomised trial. BMJ. 2002;324:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Group PC . Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. [DOI] [PubMed] [Google Scholar]
- 10. Rienstra M, Hobbelt AH, Alings M, Tijssen JGP, Smit MD, Brügemann J, Geelhoed B, Tieleman RG, Hillege HL, Tukkie R, Van Veldhuisen DJ, Crijns HJGM, Van Gelder IG; RACE 3 Investigators . Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial Eur Heart J. 2018. Available at: https://academic.oup.com/eurheartj/advance-article-abstract/doi/10.1093/eurheartj/ehx739/4786620?redirectedFrom=fulltext. Accessed March 21, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Amir O, Amir RE, Paz H, Mor R, Sagiv M, Lewis BS. Aldosterone synthase gene polymorphism as a determinant of atrial fibrillation in patients with heart failure. Am J Cardiol. 2008;102:326–329. [DOI] [PubMed] [Google Scholar]
- 12. Berglund H, Boukter S, Theodorsson E, Vallin H, Edhag O. Raised plasma concentrations of atrial natriuretic peptide are independent of left atrial dimensions in patients with chronic atrial fibrillation. Br Heart J. 1990;64:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tsai CT, Chiang FT, Tseng CD, Hwang JJ, Kuo KT, Wu CK, Yu CC, Wang YC, Lai LP, Lin JL. Increased expression of mineralocorticoid receptor in human atrial fibrillation and a cellular model of atrial fibrillation. J Am Coll Cardiol. 2010;55:758–770. [DOI] [PubMed] [Google Scholar]
- 14. Struthers AD. The clinical implications of aldosterone escape in congestive heart failure. Eur J Heart Fail. 2004;6:539–545. [DOI] [PubMed] [Google Scholar]
- 15. Dabrowski R, Borowiec A, Smolis‐Bak E, Kowalik I, Sosnowski C, Kraska A, Kazimierska B, Wozniak J, Zareba W, Szwed H. Effect of combined spironolactone‐β‐blocker ± enalapril treatment on occurrence of symptomatic atrial fibrillation episodes in patients with a history of paroxysmal atrial fibrillation (SPIR‐AF study). Am J Cardiol. 2010;106:1609–1614. [DOI] [PubMed] [Google Scholar]
- 16. Ito Y, Yamasaki H, Naruse Y, Yoshida K, Kaneshiro T, Murakoshi N, Igarashi M, Kuroki K, Machino T, Xu D, Kunugita F, Sekiguchi Y, Sato A, Tada H, Aonuma K. Effect of eplerenone on maintenance of sinus rhythm after catheter ablation in patients with long‐standing persistent atrial fibrillation. Am J Cardiol. 2013;111:1012. [DOI] [PubMed] [Google Scholar]
- 17. Kim SK, Pak HN, Park JH, Ko KJ, Lee JS, Choi JI, Choi DH, Kim YH. Clinical and serological predictors for the recurrence of atrial fibrillation after electrical cardioversion. Europace. 2009;11:1632–1638. [DOI] [PubMed] [Google Scholar]
- 18. Swedberg K, Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Shi H, Vincent J, Pitt B. Eplerenone and atrial fibrillation in mild systolic heart failure. J Am Coll Cardiol. 2012;59:1598–1603. [DOI] [PubMed] [Google Scholar]
- 19. Gao X, Peng L, Adhikari CM, Lin J, Zuo Z. Spironolactone reduced arrhythmia and maintained magnesium homeostasis in patients with congestive heart failure. J Card Fail. 2007;13:170–177. [DOI] [PubMed] [Google Scholar]
- 20. Piccini JP, Fraulo ES, Ansell JE, Fonarow GC, Gersh BJ, Go AS, Hylek EM, Kowey PR, Mahaffey KW, Thomas LE, Kong MH, Lopes RD, Mills RM, Peterson ED. Outcomes registry for better informed treatment of atrial fibrillation: rationale and design of ORBIT‐AF. Am Heart J. 2011;162:606–612.e1. [DOI] [PubMed] [Google Scholar]
- 21. Holmqvist F, Kim S, Steinberg BA, Reiffel JA, Mahaffey KW, Gersh BJ, Fonarow GC, Naccarelli GV, Chang P, Freeman JV, Kowey PR, Thomas L, Peterson ED, Piccini JP. Heart rate is associated with progression of atrial fibrillation, independent of rhythm. Heart. 2015;101:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlöf B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Devereux RB. Angiotensin II receptor blockade reduces new‐onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End point reduction in hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–719. [DOI] [PubMed] [Google Scholar]
- 23. Lip GYH, Frison L, Grind M; on Behalf of the SI and the SESC . Angiotensin converting enzyme inhibitor and angiotensin receptor blockade use in relation to outcomes in anticoagulated patients with atrial fibrillation. J Intern Med. 2007;261:577–586. [DOI] [PubMed] [Google Scholar]
- 24. Piccini JP, Stevens SR, Chang Y, Singer DE, Lokhnygina Y, Go AS, Patel MR, Mahaffey KW, Halperin JL, Breithardt G, Hankey GJ, Hacke W, Becker RC, Nessel CC, Fox KA, Califf RM; Committee RAS and Investigators . Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once‐daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127:224–232. [DOI] [PubMed] [Google Scholar]
- 25. Williams RS, deLemos JA, Dimas V, Reisch J, Hill JA, Naseem RH. Effect of spironolactone on patients with atrial fibrillation and structural heart disease. Clin Cardiol. 2011;34:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Propensity Score Covariate List
Table S2. Standardized Differences of Propensity‐Matched Pairs: New MRA Use
Table S3. Standardized Differences of Propensity‐Matched Pairs: New MRA Use (New‐Onset HF‐Matched Pairs)
Table S4. Standardized Differences of Propensity‐Matched Pairs: New and Baseline MRA Use Combined
Table S5. Standardized Differences of Propensity‐Matched Pairs: New and Baseline MRA Use Combined (New‐Onset HF‐Matched Pairs)
Table S6. Predictors of New MRA Use
Table S7. Predictors of New and Baseline MRA Use


