Abstract
Background
Subclinical atherosclerosis identification remains challenging; abdominal visceral adiposity may improve risk stratification beyond traditional cardiovascular risk factors. Hypertriglyceridemic waist, a visceral adiposity marker combining elevated triglycerides (≥2 mmol/L) and waist circumference (≥90 cm), has been related to carotid atherosclerosis, although associations with high‐risk features, including lipid‐rich necrotic core (LRNC), remain unknown. We tested the hypothesis that hypertriglyceridemic waist is an independent marker of high‐risk atherosclerosis features.
Methods and Results
In this cross‐sectional study including 467 white men (mean age, 45.9±14.8 years; range 19.4–77.6 years), carotid atherosclerosis characteristics were examined by magnetic resonance imaging and associations with hypertriglyceridemic waist and benefits beyond Framingham Risk Score (FRS) and Pathobiological Determinants of Atherosclerosis in Youth (PDAY) were determined. Subclinical carotid atherosclerosis was present in 61.9% of participants, whereas 50.1% had LRNC. Hypertriglyceridemic waist was associated with carotid maximum wall thickness (P=0.014), wall volume (P=0.025), normalized wall index (P=0.004), and Carotid Atherosclerosis Score (derived from wall thickness and LRNC; P=0.049). Hypertriglyceridemic waist was associated with carotid LRNC volume beyond FRS (P=0.037) or PDAY (P=0.015), contrary to waist circumference alone (both P>0.05). Although 69.7% and 62.0% of participants with carotid atherosclerosis and/or LRNC were not high‐risk by FRS or PDAY, respectively, hypertriglyceridemic waist correctly reclassified 9.7% and 4.5% of them, respectively. Combining hypertriglyceridemic waist with FRS (net reclassification improvement=0.17; P<0.001) or PDAY (net reclassification improvement=0.05; P=0.003) was superior to each score alone in identifying individuals with carotid atherosclerosis and/or LRNC.
Conclusions
Hypertriglyceridemic waist is an independent marker of carotid high‐risk atherosclerosis features in men, improving on FRS and PDAY risk score.
Keywords: atherosclerosis, cardiovascular disease, cardiovascular disease risk factors, carotid artery, magnetic resonance imaging, visceral fat
Subject Categories: Magnetic Resonance Imaging (MRI), Atherosclerosis, Risk Factors
Clinical Perspective
What Is New?
This study documents, for the first time, the independent association between hypertriglyceridemic waist and lipid‐rich necrotic core volume of the carotid artery, a high‐risk atherosclerosis feature.
What Are the Clinical Implications?
Hypertriglyceridemic waist, an office‐based clinical tool easily accessible at low cost, may improve the identification of individuals with carotid artery atherosclerosis and/or evidence of lipid‐rich necrotic core in the carotid artery, beyond traditional risk factors.
Adding the evaluation of hypertriglyceridemic waist to traditional risk screening provided superior classification of carotid atherosclerosis risk of white men.
Cardiovascular disease at large, and more specifically atherosclerosis, remains a critical cause of morbidity and mortality worldwide.1 More than half of cardiovascular patients have no more than one traditional risk factor,2 making it difficult to establish optimal screening strategies. The direct appraisal of atherosclerosis requires costly imaging modalities and analytical skills, and thus remains beyond the scope of screening the asymptomatic population that may benefit most. Poor lifestyle habits, including overconsumption of processed food and sedentary lifestyles, have contributed to the emergence of key nontraditional mediators of cardiovascular risk.3, 4 Visceral adipose tissue (VAT) is one such important key mediator, influencing glucose metabolism, blood pressure, lipid profile, and inflammatory profile.5 Even in apparently healthy young adults devoid of traditional risk factors, whether of normal weight or overweight, VAT has been related to a worse cardiometabolic profile, including a deteriorated lipid profile and elevated fasting glucose.6 More recently, excess abdominal adiposity and its concomitant poor metabolic profile have been linked to the presence of subclinical carotid atherosclerosis in middle‐aged men.7, 8, 9
An important drawback related to VAT quantification is the necessity to use costly imaging modalities, such as magnetic resonance imaging (MRI) or methods involving radiation, like computed tomography. Clinical measurements of abdominal adiposity, including waist circumference, show significant correlations with VAT,10 in addition to being important risk markers of cardiovascular events.11 However, although the waist circumference predicts increased morbidity/mortality within every body mass index (BMI) category, it does not distinguish subcutaneous from visceral abdominal adiposity. We previously demonstrated that the hypertriglyceridemic waist phenotype, defined as an elevated fasting triglyceride level (≥2.0 mmol/L in men) combined with an increased waist circumference (≥90 cm in men), could represent a simple office‐based clinical tool informing on excess VAT, superior to the waist girth used alone.12, 13 This emerging marker has been shown in several studies to be a useful screening tool for various populations, identifying those with a deteriorated metabolic profile and higher risk for cardiovascular disease.13, 14, 15 In multiethnic populations, the hypertriglyceridemic waist was also related to subclinical carotid atherosclerosis, although not significantly after adjusting for traditional risk factors.16
Beyond global burden and simple artery morphological characteristics, high‐risk atherosclerosis is generally characterized by lipid‐rich necrotic core (LRNC), intraplaque hemorrhage, and calcification of the artery wall17; such tissue characteristics are good predictors of cardiovascular events.18 Although hypertriglyceridemic waist has been related to subclinical carotid atherosclerosis, its association with high‐risk atherosclerosis features, including LRNC, remains unknown. We aimed to test the hypothesis that the hypertriglyceridemic waist is associated with high‐risk atherosclerosis features, as determined by comprehensive volumetric coverage of the carotid arteries. The objective of the present study was, therefore, to investigate whether hypertriglyceridemic waist is associated with high‐risk carotid atherosclerosis features, as determined by MRI in a sample of men exhibiting a broad spectrum of cardiovascular risk.
Methods
Study Population
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. White men, free of symptomatic or known carotid artery disease, were recruited between February 1, 2008 and September 30, 2016 at the Institut Universitaire de Cardiologie et de Pneumologie de Québec—Université Laval (Québec City, Québec, Canada). Men without clinical coronary artery disease (CAD) were recruited by solicitations through the media and word of mouth. Those experiencing CAD were recruited at the time of their outpatient visit or during hospitalization for CAD. Participants were considered CAD free when exempt from a history of cardiovascular disease, exempt from typical CAD symptoms and signs, and free of relevant medication. In addition, the absence of CAD was confirmed by a normal resting ECG and a negative maximum exercise treadmill stress test result when >34 years (modified Bruce protocol; TMX 425; Trackmaster, Newton, KS).19 Conversely, CAD was considered to be present when patients had a typical clinical presentation, including typical angina (chest pain) or equivalent in diabetic patients (shortness of breath) leading to/and further confirmed by coronary angiography in a clinical setting, as per standard American College of Cardiology/American Heart Association recommendations.20 Therefore, clinical CAD was confirmed by invasive coronary angiography in all. Subjects with contraindications to MRI were excluded (1.8% of recruited participants), as were those with a history of cerebrovascular disease. The Institutional Research Ethics Board approved the study, and all participants provided signed informed consent.
Risk Factor Evaluation
For all participants, measurements were performed and data were collected at the time of study entry. Data on age, cardiovascular medication, and risk factors (obesity, diabetes mellitus, hypertension, dyslipidemia, smoking, and family history of cardiovascular disease in a first‐degree relative) were collected. Resting blood pressure measurements, fasting blood draw, and anthropometric measurements (weight, height, and waist and hip circumferences) were performed according to standardized procedures.6, 13 The waist circumference was measured at the end of normal expiration, at the middistance between the last rib and the top of the iliac crest, ensuring that the participant did not contract abdominal muscles. A positive hypertriglyceridemic waist was determined by the simultaneous presence of fasting triglyceride concentration ≥2.0 mmol/L and waist circumference ≥90 cm.13 The identification of the metabolic syndrome was made according to the presence of at least 3 of the following criteria: resting blood pressure ≥130/85 mm Hg, waist circumference ≥102 cm, high‐density lipoprotein cholesterol (HDL‐C) <1.0 mmol/L, triglycerides ≥1.7 mmol/L, and fasting glucose ≥5.55 mmol/L.21 The presence of traditional cardiovascular risk factors was defined as follows: (1) obesity was defined by BMI ≥30 kg/m2; (2) hypertension was defined by a resting systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg or the use of antihypertensive therapies; (3) dyslipidemia was defined by a low‐density lipoprotein cholesterol concentration ≥5 mmol/L or the use of lipid‐lowering drugs; (4) smoking included both current and former smokers; (5) diabetes mellitus was defined as current use of oral hypoglycemic agents or insulin, by the patient chart, or as fasting plasma glucose ≥7.0 mmol/L; and (6) family history of cardiovascular disease in a first‐degree relative included the mother, father, brother, and sister, as reported by the participant. The integrated 10‐year Framingham Risk Score (FRS), informing on the estimated risk of cardiovascular events, has been calculated for all participants.22 Furthermore, we computed the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) risk score; given the wide range of age of our population, the PDAY risk score was computed from the modifiable risk factors, as previously reported.23, 24
MRI Protocol and Image Analysis
Adipose tissue evaluation
At the time of study entry, ECG‐gated 1.5‐T MRI (Philips Achieva 1.5 Tesla; Philips Healthcare, Best, the Netherlands) was performed with a dedicated cardiac coil for volumetric quantification of cardiac adipose tissue, including both epicardial and pericardial fat. The body coil was used for volumetric quantification of abdominal subcutaneous adipose tissue (SAT) and VAT as well as to estimate hepatic fat fraction. Axial T1‐weighted (T1W) spin echo sequence (slice thickness=5 mm; repetition time [TR]=750 ms; echo time [TE]=6–8 ms; resolution=0.67×0.67 mm) was performed at the level of the mitral valve to quantify cardiac fat during diastole. Axial T1W slice was also acquired at the level of L4‐L5 intervertebral space for VAT and SAT quantification (slice thickness=5 mm; TR=750 ms; TE=6–8 ms; resolution=0.78×0.78 mm).6 For hepatic fat measurement, axial T1W slice was acquired at the level of L1‐L2 lumbar intervertebral space (slice thickness=5 mm; TR=750 ms; TE=6–8 ms; resolution=0.78×0.78 mm). All acquisitions were performed with and then without fat saturation to confirm that the disappearing signal is in fact fat.
Image analysis was performed off‐line in a standardized core laboratory (Laboratoire d'Imagerie Cardiovasculaire Avancée) using dedicated software (QMass MR; Medis, Leiden, the Netherlands) by trained technicians blinded to the study hypothesis and patient data. On fat‐enhanced images, the pericardial space was identified as the dark stripe separating the bright epicardial fat within from the pericardial fat without, and thus cardiac fat was readily traced manually. On fat‐enhanced images, the SAT contour was well delineated and manually traced. To minimize observer bias and maximize reproducibility of VAT quantification, a standardized region of interest was positioned in the homogeneous adipose tissue, and all pixels with similar signal intensities were automatically classified as VAT by the software. Cardiac and abdominal fat volumes were reported for a standardized length of 5 mm (mm3/5 mm). Hepatic fat fraction was calculated as the difference between the signal from the non–fat‐saturated image and the signal from the fat‐saturated image/the signal from the non–fat‐saturated image, and reported as a percentage. Detailed method is available in a prior publication.6
Carotid artery evaluation
At the time of study entry, vector cardiography‐gated 1.5‐T MRI, using a system coupled to a dedicated bilateral phased‐array carotid coil, was performed (Machnet B.V., Roden, the Netherlands). Centered on the carotid bulb, high‐resolution MRI was performed at 12 contiguous parallel short‐axis slice locations, simultaneously on the right and left carotid arteries (slice thickness=2 mm; slice gap=0 mm; resolution=0.63×0.63 mm), providing full contiguous coverage of 24 mm per side (total carotid coverage of 48 mm). Coregistered sequences were obtained for each slice location in black blood T1W turbo spin echo (TE=8 ms; TR=800 ms), proton density–weighted (PDW) turbo spin echo (TE=12 ms; TR=2400 ms) and T2‐weighted (T2W) turbo spin echo (TE=50 ms; TR=2400 ms), and time‐of‐flight (TOF) spoiled gradient echo (TE=3 ms; TR=20 ms).
Integrated analysis was performed simultaneously on these 4 sequences (QPlaque; Medis, Leiden, the Netherlands). The inner intima and outer adventitia borders were delineated on each carotid slice, and volumetric measurements were determined by modified Simpson's rule (Figure 1).25 According to a validated gray intensity scale, each component area was delineated on all slices.25, 26 LRNC is isointense/hyperintense on T1W, hypointense on T2W, and isointense/hypointense on PDW and TOF.26, 27, 28 Loose fibrous matrix is isointense/hypointense on T1W, hyperintense on T2W and PDW, and isointense on TOF.26 The calcification is hypointense on the 4 sequences.26 Intraplaque hemorrhage is hyperintense on T1W and TOF and hyperintense/isointense/hypointense on T2W and PDW.26 Fibrous tissue is isointense on T1W, T2W, and PDW; and hypointense on TOF.26 Volumetric measurement of each component was determined by the modified Simpson's rule.25 To compare volumetric carotid measurements between participants in the event in which occasional slices could not be analyzed because of artifacts (<2% of analyzed slices), volumetric data were normalized for the number of analyzed slices and reported for a standardized length of 4 mm (mm3/4 mm). The maximum and minimum carotid wall thicknesses were automatically determined, and atherosclerosis was considered to be present when the maximum/minimum wall thickness ratio was ≥2 on at least 3 contiguous slices.29 The normalized wall index (wall volume/vessel volume) was calculated as a measure of percentage atheroma that allows standardization despite interindividual variation in vessel size.30 Carotid Atherosclerosis Score (CAS) has been previously reported as a marker of high‐risk atherosclerotic plaque.31 For both left and right carotid arteries, CAS was computed from the maximum wall thickness and the maximum percentage of LRNC as follows: CAS=1 if maximum wall thickness ≤2 mm; otherwise, if maximum wall thickness >2 mm, CAS was set according to the maximum percentage of LRNC; CAS=2 if maximum percentage of LRNC <20%; CAS=3 if maximum percentage of LRNC was ≥20% and ≤40%; and CAS=4 if maximum percentage of LRNC >40%.31 We determined the maximum CAS per individual as the highest value between the right and left carotid arteries, whereas CAS was the average of both sides. The evaluation was performed on the common and internal segments, including the proximal and distal bulbs, as previously described.30
Figure 1.
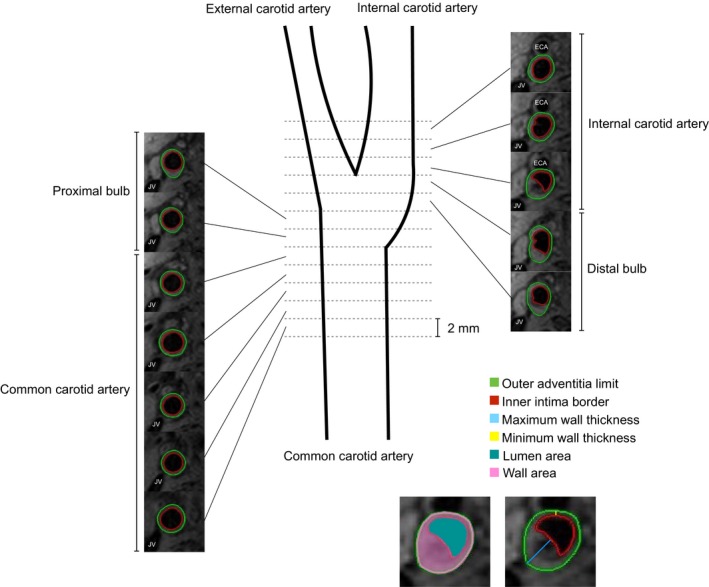
Schematic diagram of carotid coverage by magnetic resonance imaging. A single carotid artery with T1‐weighted sequence is presented for clarity, whereas bilateral imaging was performed through T1‐, T2‐, and proton density–weighted and time‐of‐flight analyses. Centered on the bifurcation, 12 contiguous slices (2 mm) were acquired on the right and left carotid arteries, covering 24 mm per side. Acquisition covered the common (including the proximal bulb) and internal (including the distal bulb) carotid segments. For each slice, the inner intima (red) and outer adventitia (green) borders were outlined, determining the wall (pink) and lumen (teal) areas. The maximum (blue) and minimum (yellow) wall thicknesses were determined. ECA indicates external carotid artery; JV, jugular vein. Definitions derived from Underhill et al.30
Statistical Analyses
Three groups were generated: (1) Hypertriglyceridemic Waist (HTGW)−/− : waist circumference <90 cm and triglycerides <2.0 mmol/L; (2) HTGW+/−: waist circumference ≥90 cm and triglycerides <2.0 mmol/L; and (3) HTGW+/+: waist circumference ≥90 cm and triglycerides ≥2.0 mmol/L. Participants in the HTGW−/− and HTGW+/− groups were devoid of hypertriglyceridemic waist, whereas only those in the HTGW+/+ group had hypertriglyceridemic waist. Participants with waist circumference <90 cm and triglyceride level ≥2.0 mmol/L (n=7; 1% of those screened) were excluded, considering that this combination is rare and represents unique metabolic features that would bias analysis in this study; analyses on the overall population were repeated, including these participants, yielding similar results. Continuous variables were expressed as mean±SD, whereas categorical variables were expressed as a percentage. Kruskal‐Wallis test was performed to compare clinical and carotid markers between the 3 HTGW groups. Student t test was performed to compare clinical markers between those with versus without the hypertriglyceridemic waist, whereas the χ2 test was performed to compare the prevalence of risk factors between them. Univariable and multivariable logistic regressions were performed to determine associations between hypertriglyceridemic waist and cardiovascular markers, while adjusting for FRS or traditional risk factors (smoking, hypertension, dyslipidemia, diabetes mellitus, obesity, and family history of cardiovascular disease). Univariable and multivariable linear regressions were performed to determine the associations between carotid atherosclerosis characteristics and hypertriglyceridemic waist, while adjusting for FRS or traditional risk factors, as mentioned previously. φ and Pearson correlations were used to determine the strength of the associations when applicable. The net reclassification improvement (NRI) was computed to verify how well the addition of hypertriglyceridemic waist to the FRS or PDAY reclassifies participants in identifying the presence of carotid atherosclerosis (maximum/minimum wall thickness ratio ≥2 on at least 3 contiguous slices) and/or evidence of carotid LRNC (as a combined outcome or each considered apart), compared with traditional risk score alone. The performance of the combination of hypertriglyceridemic waist with each risk score was compared with each risk score alone by the McNemar test. Finally, the reproducibility of carotid measurements has been verified in a random sample of 10% of the population. The reproducibility was excellent including Lin's concordance correlation coefficient of 0.98 (Bland‐Altman 95% confidence interval [CI], −68.41 to 151.71) for wall volume and 0.91 (Bland‐Altman 95% CI, −24.42 to 25.61) for LRNC volume. All statistical analyses were performed with Stata 15.1 (StataCorp LP, College Station, TX), and 2‐tailed P<0.05 was considered statistically significant for all analyses.
Results
Population Characteristics
This cross‐sectional study included 467 white men free of symptomatic or known carotid artery disease, with a mean age of 45.9±14.8 years (range, 19.4–77.6 years). The population presented a broad range of cardiovascular risk, as indicated in Table S1. Although the mean FRS was 14.5±15.8%, our cohort included 53.7% of participants at low risk (FRS, <10%), 19.5% at moderate risk (FRS, 10%–19%), and 26.8% at high risk (FRS, ≥20%). Furthermore, 6.4% were diabetic, 12.7% were obese, 20.1% had dyslipidemia, 22.9% had hypertension, 33.8% were smokers, and 57.6% presented a family history of cardiovascular disease. All in all, 24.6% of the population presented none of the described traditional risk factors, whereas 21.1% had at least 3 cardiovascular risk factors. The prevalence of clinical CAD was 29.3%, including 11.8% with an acute myocardial infarction and 17.6% with chronic stable angina. A hypertriglyceridemic waist was observed in 10.5% of the population and was more prevalent among those with clinical CAD in comparison to those without (19.0% in patients with CAD versus 7.0% in CAD‐free subjects; P<0.001). In addition, men with the hypertriglyceridemic waist phenotype presented greater FRS (P=0.005) and greater PDAY risk score (P<0.001) compared with those without.
We compared features of the 3 cardiovascular risk profiles: HTGW−/−, HTGW+/−, and HTGW+/+ (Table 1). First, the mean BMI was in the overweight category (≥25 kg/m2) for both HTGW+/− and HTGW+/+ groups, whereas it was in the normal weight category for the HTGW−/− group. However, although SAT was similar in both the HTGW+/− and the HTGW+/+ groups, VAT was significantly greater in the HTGW+/+ group compared with the HTGW+/− group, in addition to being greater in the HTGW+/− group in comparison to the HTGW−/− group. Likewise, fasting glucose, triglycerides, and total cholesterol/HDL‐C ratio progressed significantly from HTGW−/− to HTGW+/− to HTGW+/+, whereas HDL‐C incrementally decreased significantly across the groups.
Table 1.
Characteristics of Hypertriglyceridemic Waist Phenotype Groups
| Characteristics | HTGW−/− (n=196) | HTGW+/− (n=222) | HTGW+/+ (n=49) | P Value |
|---|---|---|---|---|
| Age, y | 38.2±13.9 | 51.9±13.1 | 49.8±11.0 | <0.001* |
| Anthropometry and adiposity distribution | ||||
| Body mass index, kg/m2 | 23.2±2.3 | 28.0±2.9 | 28.9±3.3 | <0.001* , † |
| Waist circumference, cm | 81.6±5.5 | 99.7±7.5 | 102.3±8.4 | <0.001* , † |
| Hip circumference, cm | 95.8±5.3 | 102.5±6.0 | 102.4±6.6 | <0.001* |
| SAT, mm3/5 mm | 124.4±56.2 | 235.8±75.8 | 233.7±74.8 | <0.001* |
| VAT, mm3/5 mm | 54.7±31.5 | 152.4±73.6 | 185.9±87.6 | <0.001* , † |
| Cardiac adipose tissue, mm3/5 mm | 12.2±4.6 | 26.6±13.5 | 26.7±10.1 | <0.001* |
| Epicardial adipose tissue, mm3/5 mm | 9.2 ±2.5 | 13.4±4.2 | 13.4 ±3.3 | <0.001* |
| Pericardial adipose tissue, mm3/5 mm | 2.9±3.5 | 13.2±10.9 | 13.3±8.5 | <0.001* |
| Hepatic fat fraction, % | 5.0±4.0 | 9.1±9.0 | 13.6±10.3 | <0.001* , † |
| Cardiometabolic profile | ||||
| Systolic blood pressure, mm Hg | 118±10 | 124±17 | 129±20 | <0.001* |
| Diastolic blood pressure, mm Hg | 72±8 | 77±11 | 80±10 | <0.001* |
| Fasting glucose, mmol/L | 5.02±0.60 | 5.49±0.89 | 5.98±1.58 | <0.001* , † |
| Total cholesterol, mmol/L | 4.40±0.82 | 4.46±1.13 | 4.97±0.93 | 0.002† |
| Triglycerides, mmol/L | 0.89±0.37 | 1.19±0.40 | 2.94±1.02 | <0.001* , † |
| HDL cholesterol, mmol/L | 1.47±0.32 | 1.26±0.31 | 1.00±0.23 | <0.001* , † |
| LDL cholesterol, mmol/L | 2.60±0.80 | 2.75±1.06 | 2.72±0.86 | 0.39 |
| Total cholesterol/HDL cholesterol ratio | 3.12±0.83 | 3.64±0.98 | 5.16±1.38 | <0.001* , † |
| Metabolic syndrome, % | 0.5 | 14.8 | 64.6 | <0.001* , † |
| Prior coronary artery disease, % | 11.2 | 40.1 | 53.1 | <0.001* |
| 10‐y Framingham Risk Score, % | 6.7±9.9 | 20.1±16.9 | 20.6±16.2 | <0.001* |
| Low risk (<10%), % | 80.0 | 36.1 | 27.1 | <0.001* |
| Moderate risk (10%–19%), % | 11.8 | 24.2 | 29.2 | |
| High risk (≥20%), % | 8.2 | 39.7 | 43.8 | |
| PDAY risk score, % | ||||
| Low risk (<1) | 45.9 | 11.1 | 2.1 | <0.001* , † |
| Moderate risk (1–4) | 41.3 | 36.6 | 18.8 | |
| High risk (≥5) | 12.8 | 52.3 | 79.2 | |
| Cardiovascular risk factors, % | ||||
| Obesity | 0.0 | 19.5 | 32.7 | <0.001* , † |
| Hypertension | 8.7 | 31.5 | 40.8 | <0.001* |
| Diabetes mellitus | 2.6 | 8.6 | 12.2 | 0.009* |
| Dyslipidemia | 8.2 | 28.4 | 30.6 | <0.001* |
| Family history of cardiovascular disease | 42.4 | 70.6 | 60.4 | <0.001* |
| Current or former smoker | 17.9 | 43.2 | 55.1 | <0.001* |
| Risk factor count, % | ||||
| 0 | 48.0 | 8.5 | 2.1 | <0.001* |
| 1 | 35.7 | 32.4 | 31.3 | |
| 2 | 9.7 | 30.1 | 22.9 | |
| ≥3 | 6.6 | 29.2 | 43.8 | |
| Cardiovascular therapy at study entry,% | ||||
| Antiplatelet agent | 6.6 | 25.2 | 18.4 | <0.001* |
| Oral anticoagulant | 0.0 | 1.4 | 2.0 | 0.21 |
| Lipid‐lowering agent | 6.1 | 26.1 | 26.5 | <0.001* |
| β Blockers | 2.6 | 16.2 | 10.2 | <0.001* |
| Calcium channel blockers | 0.5 | 5.9 | 4.1 | 0.011* |
| Angiotensin‐converting enzyme inhibitors | 1.0 | 7.7 | 6.1 | 0.005* |
| Angiotensin II receptor blocker | 1.5 | 5.0 | 4.1 | 0.15 |
Values are expressed as mean±SD for continuous variables or percentage for categorical variables (P value for Kruskal‐Wallis test). HTGW−/−: waist circumference <90 cm and triglycerides <2.0 mmol/L; HTGW+/−: waist circumference ≥90 cm and triglycerides <2.0 mmol/L; and HTGW+/+: waist circumference ≥90 cm and triglycerides ≥2.0 mmol/L. HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein; PDAY, Pathobiological Determinants of Atherosclerosis in Youth; SAT, subcutaneous abdominal adipose tissue; VAT, visceral abdominal adipose tissue.
*P<0.05 between HTGW−/− and HTGW+/−; † P<0.05 between HTGW+/− and HTGW+/+.
Carotid Atherosclerosis Features
In the present study, 61.9% of men had subclinical carotid atherosclerosis, according to the previously proposed definition of a maximum‐to‐minimum wall thickness ≥2 on at least 3 consecutive slices,29 whereas 50.1% had presence of LRNC in the carotid artery wall. On the other hand, the prevalence of intraplaque hemorrhage (0.7%) and of calcification (13.3%) in the carotid artery wall was relatively low, whereas 19.6% of participants had loose matrix. Complete carotid artery characteristics of the study sample are presented in Table S2. Hypertriglyceridemic waist phenotype was significantly more prevalent among those with carotid atherosclerosis in comparison to those without (12.8% versus 6.7%; P=0.038). Furthermore, those with hypertriglyceridemic waist exhibited significantly greater LRNC volume compared with those without (4.87±6.58 versus 2.93±4.81 mm3/4 mm; P=0.012). CAS was calculated from the maximum wall thickness and percentage of LRNC.31 Only 7.4% of participants had a CAS of 1, whereas 66.5% had a CAS of 2 and 26.1% had a CAS of 3; none of our participants had a CAS of 4. We observed that participants with a hypertriglyceridemic waist presented a greater CAS compared with those without (P=0.049).
Carotid characteristics of the HTGW−/−, HTGW+/−, and HTGW+/+ groups are presented in Table 2. In comparison to the HTGW−/− group, the HTGW+/− group presented a significantly greater carotid maximum wall thickness (P<0.001), wall volume (P<0.001), normalized wall index (P<0.001), and CAS (P=0.002). Furthermore, the HTGW+/− group exhibited a significantly greater carotid calcification volume compared with the HTGW−/− group (P=0.002). The prevalence of carotid calcification (P<0.001) and loose matrix (P=0.031) was also significantly greater in the HTGW+/− versus the HTGW−/− groups. We observed that the HTGW+/+ group presented a greater volume of LRNC compared with the HTGW+/− group, but the difference did not reach significance (4.87±6.58 versus 3.30±5.21 mm3/4 mm; P=0.08).
Table 2.
Carotid Atherosclerosis Features of Hypertriglyceridemic Waist Phenotype Groups
| Variable | HTGW−/− (n=196) | HTGW+/− (n=222) | HTGW+/+ (n=49) | P Value |
|---|---|---|---|---|
| Presence of carotid atherosclerosis, % | 57.1 | 63.1 | 75.5 | 0.05 |
| Wall volume, mm3/4 mm | 127.75±23.14 | 151.35±36.18 | 151.51±29.10 | <0.001* |
| Maximum wall thickness, mm | 1.81±0.43 | 2.11±0.55 | 2.16±0.54 | <0.001* |
| Normalized wall index‡ | 0.54±0.07 | 0.60±0.09 | 0.61±0.09 | <0.001* |
| LRNC volume, mm3/4 mm | 2.51±4.28 | 3.30±5.21 | 4.87±6.58 | 0.024 |
| Calcification volume, mm3/4 mm | 0.06±0.36 | 0.29±0.99 | 0.26±0.65 | <0.001* |
| Intraplaque hemorrhage volume, mm3/4 mm | 0.00±0.00 | 0.12±1.24 | 0.06±0.42 | 0.22 |
| Loose matrix volume, mm3/4 mm | 0.45±1.54 | 0.85±2.34 | 0.57±1.35 | 0.08* |
| Maximum % of LRNC | 6.16±8.37 | 7.30±8.09 | 9.09±9.50 | 0.046 |
| CAS, % | 1.9±0.5 | 2.0±0.4 | 2.1±0.4 | <0.001* |
| 1 | 11.9 | 4.6 | 2.1 | 0.06* |
| 2 | 65.0 | 67.9 | 66.7 | |
| 3 | 23.2 | 27.5 | 31.3 | |
| 4 | 0.0 | 0.0 | 0.0 | |
| Presence of LRNC, % | 44.9 | 52.3 | 61.7 | 0.08 |
| Presence of calcification, % | 6.2 | 18.3 | 19.2 | <0.001* |
| Presence of intraplaque hemorrhage, % | 0.0 | 0.9 | 2.1 | 0.21 |
| Presence of loose matrix, % | 15.0 | 23.4 | 21.3 | 0.09* |
Values are expressed as mean±SD for continuous variables or percentage for categorical variables (P value for Kruskal‐Wallis test). HTGW−/−: waist circumference <90 cm and triglycerides <2.0 mmol/L; HTGW+/−: waist circumference ≥90 cm and triglycerides <2.0 mmol/L; and HTGW+/+: waist circumference ≥90 cm and triglycerides ≥2.0 mmol/L. CAS indicates Carotid Atherosclerosis Score; LRNC, lipid‐rich necrotic core.
*P<0.05 between HTGW−/− and HTGW+/−; † P<0.05 between HTGW+/− and HTGW+/+. ‡Normalized for the vessel volume.
Hypertriglyceridemic Waist and Clinical Markers
Detailed associations between hypertriglyceridemic waist and cardiovascular risk markers are presented in Table 3. Hypertriglyceridemic waist was associated positively and significantly with several key markers of cardiovascular risk (Figure 2), including BMI (odds ratio [OR], 1.26; P<0.001), systolic (OR, 1.02; P=0.002) and diastolic (OR, 1.04; P=0.004) blood pressures, fasting glucose (OR, 1.74; P<0.001), total cholesterol (OR, 1.68; P<0.001), and total cholesterol/HDL‐C ratio (OR, 3.59; P<0.001). Hypertriglyceridemic waist phenotype was negatively and significantly associated with HDL‐C (OR, 0.01; P<0.001). Hypertriglyceridemic waist was also associated with FRS (OR, 1.02; P=0.006), PDAY risk score (OR, 1.27; P<0.001), the number of traditional risk factors (OR, 1.54; P<0.001), and the presence of metabolic syndrome (OR, 20.94; P<0.001; φ=0.50). This office‐based clinical tool was associated positively and significantly with adiposity distribution measured by MRI: SAT (OR, 1.01; P<0.001), VAT (OR, 1.01; P<0.001), hepatic fat fraction (OR, 1.08; P<0.001), and cardiac adipose tissue (OR, 1.04; P=0.001), including both epicardial fat (OR, 1.11; P=0.002) and pericardial fat (OR, 1.04; P=0.002). Hypertriglyceridemic waist informs about excessive ectopic abdominal fat, remaining associated with VAT even after adjusting for FRS (OR, 1.01; P<0.001; 95% CI, 1.01–1.02), PDAY risk score (OR, 1.00; P=0.050; 95% CI, 1.00–1.01), or traditional risk factors (smoking, hypertension, dyslipidemia, diabetes mellitus, obesity, and family history of cardiovascular disease) (OR, 1.01; P<0.001; 95% CI, 1.00–1.01). Hypertriglyceridemic waist was also associated with hepatic fat fraction after adjusting for FRS (OR, 1.06; P=0.001; 95% CI, 1.02–1.11), PDAY risk score (OR, 1.04; P=0.046; 95% CI, 1.00–1.09), or traditional risk factors (OR, 1.06; P=0.006; 95% CI, 1.02–1.11). Although hypertriglyceridemic waist demonstrated a strong and positive association with almost all markers of the cardiovascular risk profile, the presence of clinical CAD was also associated with hypertriglyceridemic waist (OR, 3.13; P<0.001; φ=0.18), even after adjusting for FRS (OR, 2.73; P=0.014; 95% CI, 1.23–6.08). However, the presence of CAD was no longer associated with hypertriglyceridemic waist after adjusting for PDAY risk score (OR, 1.77; P=0.10; 95% CI, 0.91–3.44).
Table 3.
Associations Between the Hypertriglyceridemic Waist and Clinical Risk Markers
| Variable | Univariable | Adjusted for 10‐y Framingham Risk Score | ||||
|---|---|---|---|---|---|---|
| OR | P Value | 95% CI | OR | P Value | 95% CI | |
| Age, y | 1.02 | 0.06 | 1.00–1.04 | 1.00 | 0.83 | 0.96–1.03 |
| Body mass index, kg/m2 | 1.26 | <0.001 | 1.16–1.36 | 1.24 | <0.001 | 1.14–1.35 |
| SAT, mm3/5 mm | 1.01 | <0.001 | 1.00–1.01 | 1.01 | 0.001 | 1.00–1.01 |
| VAT, mm3/5 mm | 1.01 | <0.001 | 1.01–1.01 | 1.01 | <0.001 | 1.01–1.02 |
| Cardiac adipose tissue, mm3/5 mm | 1.04 | 0.001 | 1.02–1.06 | 1.03 | 0.017 | 1.01–1.06 |
| Epicardial adipose tissue, mm3/5 mm | 1.11 | 0.002 | 1.04–1.19 | 1.08 | 0.06 | 1.00–1.17 |
| Pericardial adipose tissue, mm3/5 mm | 1.04 | 0.002 | 1.02–1.07 | 1.04 | 0.028 | 1.00–1.07 |
| Hepatic fat fraction, % | 1.08 | <0.001 | 1.03–1.12 | 1.06 | 0.001 | 1.02–1.11 |
| Systolic blood pressure, mm Hg | 1.02 | 0.002 | 1.01–1.04 | 1.02 | 0.015 | 1.00–1.04 |
| Diastolic blood pressure, mm Hg | 1.04 | 0.004 | 1.01–1.07 | 1.04 | 0.010 | 1.01–1.06 |
| Fasting glucose, mmol/L | 1.74 | <0.001 | 1.34–2.26 | 1.62 | 0.001 | 1.21–2.15 |
| Total cholesterol, mmol/L | 1.68 | <0.001 | 1.26–2.24 | 1.95 | <0.001 | 1.44–2.63 |
| HDL cholesterol, mmol/L | 0.01 | <0.001 | 0.00–0.04 | 0.01 | <0.001 | 0.00–0.04 |
| LDL cholesterol, mmol/L | 1.05 | 0.76 | 0.77–1.43 | 1.22 | 0.22 | 0.89–1.69 |
| Total cholesterol/HDL cholesterol ratio | 3.59 | <0.001 | 2.58–4.97 | 3.95 | <0.001 | 2.77–5.63 |
| 10‐y Framingham risk score, % | 1.02 | 0.006 | 1.01–1.04 | ··· | ··· | ··· |
| PDAY risk score | 1.28 | <0.001 | 1.19–1.37 | 1.28 | <0.001 | 1.19–1.37 |
| Metabolic syndrome | 20.94 | <0.001 | 10.50–41.76 | 21.01 | <0.001 | 10.07–43.84 |
| Prior coronary artery disease | 3.13 | <0.001 | 1.71–5.71 | 2.73 | 0.014 | 1.23–6.08 |
| Obesity | 4.22 | <0.001 | 2.15–8.29 | 3.80 | <0.001 | 1.90–7.62 |
| Hypertension | 2.62 | 0.002 | 1.42–4.86 | 1.96 | 0.08 | 0.93–4.14 |
| Diabetes mellitus | 2.28 | 0.09 | 0.89–5.90 | 1.22 | 0.73 | 0.39–3.74 |
| Dyslipidemia | 1.89 | 0.06 | 0.98–3.64 | 1.23 | 0.62 | 0.54–2.82 |
| Family history of cardiovascular disease | 1.14 | 0.67 | 0.62–2.10 | 0.93 | 0.83 | 0.49–1.79 |
| Current or former smoker | 2.69 | 0.001 | 1.48–4.90 | 2.10 | 0.06 | 0.98–4.50 |
| Risk factor count | 1.54 | <0.001 | 1.26–1.89 | 1.65 | 0.002 | 1.20–2.26 |
Data are expressed as OR, P value, and 95% CI using logistic regressions. CI indicates confidence interval; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; OR, odds ratio; PDAY: Pathobiological Determinants of Atherosclerosis in Youth; SAT, subcutaneous abdominal adipose tissue; VAT, visceral abdominal adipose tissue.
Figure 2.
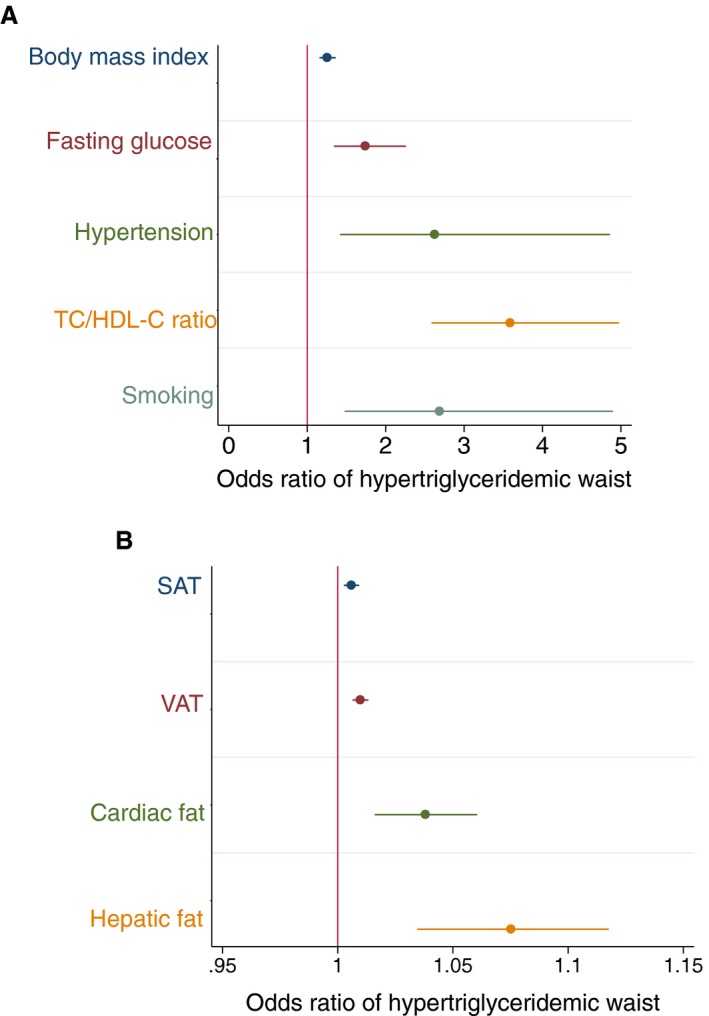
Associations between the hypertriglyceridemic waist and cardiovascular risk factors (A) or adipose tissues (abdominal subcutaneous adipose tissue [SAT], abdominal visceral adipose tissue [VAT], and cardiac fat volumes have been reported as mm3/5 mm, whereas hepatic fat has been reported as a percentage; B). Body mass index has been computed from the participant's weight in kilograms and participant's height in meters (kg/m2). Blood glucose level has been measured in mmol/L after 12 hours of fasting. Graphics are expressed in odds ratio with 95% confidence interval. HDL‐C indicates high‐density lipoprotein cholesterol (mmol/L); TC, total cholesterol (mmol/L).
Hypertriglyceridemic Waist and Carotid Atherosclerosis Features
Detailed associations between hypertriglyceridemic waist and carotid parameters are presented in Table 4. Hypertriglyceridemic waist was positively and significantly associated with the presence of subclinical carotid atherosclerosis (OR, 2.03; P=0.041; φ=0.10; Figure 3). Hypertriglyceridemic waist was associated significantly with most markers of carotid atherosclerosis burden and risk, including maximum wall thickness (R 2=0.01, P=0.014, r=0.11), artery wall volume (R 2=0.01, P=0.025, r=0.10), normalized wall index (R 2=0.02, P=0.004, r=0.13), and CAS (R 2=0.01, P=0.049, r=0.09). Hypertriglyceridemic waist was positively and significantly associated with the volume of carotid LRNC (R 2=0.01, P=0.012, r=0.12; 95% CI, 0.43–3.46), and this association remained significant after adjusting for FRS (P=0.037; 95% CI, 0.10–3.19) or traditional risk factors (P=0.019; 95% CI, 0.31–3.48). Conversely, although waist circumference alone was also associated with the volume of carotid LRNC (R 2=0.01, P=0.036, r=0.10), significance was lost when adjusted for FRS (P=0.38). We then examined if these relationships remained associated beyond the risk of coronary artery atherosclerosis by the integrated PDAY risk score. The association between waist circumference and the volume of carotid artery LRNC was no longer significant after adjusting for the risk of subclinical coronary artery atherosclerosis (P=0.12). However, we observed that the hypertriglyceridemic waist phenotype was associated with the volume of carotid artery LRNC beyond the risk of subclinical coronary artery atherosclerosis, as estimated by the PDAY risk score (P=0.015; 95% CI, 0.40–3.65).
Table 4.
Associations Between Atherosclerosis Features and the Hypertriglyceridemic Waist
| Variable | Univariable | Adjusted for 10‐y Framingham Risk Score | ||||
|---|---|---|---|---|---|---|
| R 2 | P Value | 95% CI | R 2 | P Value | 95% CI | |
| Wall volume, mm3/4 mm | 0.01 | 0.025 | 1.43 to 21.11 | 0.20 | 0.29 | −4.18 to 14.00 |
| Maximum wall thickness, mm | 0.01 | 0.014 | 0.04 to 0.35 | 0.18 | 0.18 | −0.05 to 0.25 |
| Normalized wall index | 0.02 | 0.004 | 0.01 to 0.06 | 0.26 | 0.11 | −0.00 to 0.04 |
| LRNC volume, mm3/4 mm | 0.01 | 0.012 | 0.43 to 3.46 | 0.03 | 0.037 | 0.10 to 3.19 |
| Calcification volume, mm3/4 mm | 0.001 | 0.50 | −0.15 to 0.31 | 0.11 | 0.82 | −0.25 to 0.20 |
| Intraplaque hemorrhage volume, mm3/4 mm | 0.00 | 0.99 | −0.26 to 0.26 | 0.02 | 0.72 | −0.32 to 0.22 |
| Loose matrix volume, mm3/4 mm | 0.00 | 0.77 | −0.68 to 0.50 | 0.04 | 0.42 | −0.84 to 0.35 |
| Maximum % of LRNC, % | 0.01 | 0.07 | −0.16 to 4.81 | 0.02 | 0.16 | −0.73 to 4.32 |
| Carotid Atherosclerosis Score | 0.01 | 0.049 | 0.00 to 0.28 | 0.05 | 0.20 | −0.05 to 0.23 |
Data are expressed as R 2, P value, and 95% CI using linear regressions. CI indicates confidence interval; LRNC, lipid‐rich necrotic core.
Figure 3.
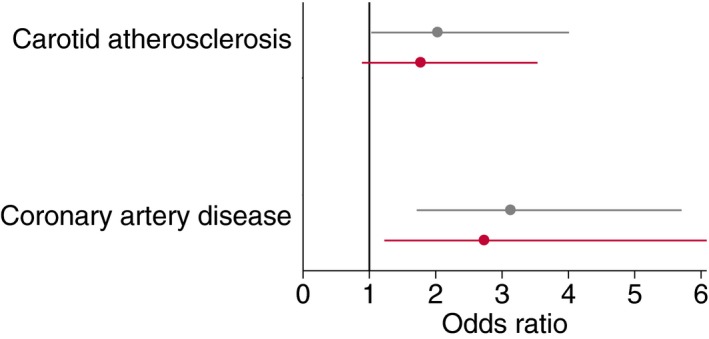
Association between the hypertriglyceridemic waist and carotid atherosclerosis or coronary artery disease (odds ratio with a 95% confidence interval). Black, univariable analysis; red, adjusted for the 10‐year Framingham Risk Score.
Net Reclassification Improvement
In the present study, 69.7% of participants having subclinical carotid atherosclerosis and/or evidence of carotid LRNC were classified at low/moderate risk, according to FRS (ie, calculated risk, <20%), and 62.0% were classified according to PDAY (ie, calculated risk, <5%). Among them, 9.7% and 4.5%, respectively, presented a hypertriglyceridemic waist phenotype that would have correctly reclassified them as higher risk. Ultimately, combining the hypertriglyceridemic waist with FRS was superior to FRS alone in identifying individuals with subclinical carotid atherosclerosis and/or evidence of LRNC (NRI=0.17, P<0.001; NRIevent=[22–0]/119=0.18; NRInonevent=[0–5]/335=−0.01). The combination of hypertriglyceridemic waist with FRS remained superior to FRS alone in identifying the presence of subclinical carotid atherosclerosis and/or LRNC in both CAD‐free (NRI=0.32, P<0.001; NRIevent=[11–0]/32=0.34; NRInonevent=[0–5]/291=−0.02) or CAD (NRI=0.13, P<0.001; NRIevent=[11–0]/87=0.13; NRInonevent=[0–0]/44=0) groups taken separately, highlighting potential benefit specifically in those without known CAD. The added value of hypertriglyceridemic waist over waist circumference alone when combined to the FRS is provided by improved classification of those without carotid LRNC or carotid atherosclerosis (Table 5). Similarly to FRS, the combination of hypertriglyceridemic waist with the PDAY risk score presented a significant NRI to identify individuals with carotid atherosclerosis and/or evidence of LRNC in the carotid artery in comparison to PDAY score alone (NRI=0.05, P=0.003; NRIevent=[9–0]/171=0.05; NRInonevent=[0–1]/281=−0.004). Contrary to FRS, with PDAY, no added value of hypertriglyceridemic waist over waist circumference alone is observed for the mere presence of atherosclerosis, but added value is observed for the prediction of LRNC. Therefore, hypertriglyceridemic waist is of particular interest for the prediction of higher‐risk atherosclerosis characterized by LRNC.
Table 5.
NRI of Hypertriglyceridemic Waist versus Waist Circumference Combined to FRS and PDAY Risk Score for Carotid Artery Atherosclerosis and LRNC
| NRI (NRIevent/NRInon‐event) | |
|---|---|
| Presence of carotid atherosclerosis | |
| FRS+hypertriglyceridemic waist | 0.04 (−0.27/0.31)* |
| PDAY risk score+hypertriglyceridemic waist | −0.02 (−0.23/0.21)* |
| Evidence of carotid LRNC | |
| FRS+hypertriglyceridemic waist | 0.18 (−0.22/0.40)* |
| PDAY risk score+hypertriglyceridemic waist | 0.11 (−0.18/0.29)* |
FRS indicates Framingham risk score; LRNC, lipid‐rich necrotic core; NRI, net reclassification improvement; PDAY, Pathobiological Determinants of Atherosclerosis in Youth.
*P<0.05.
Discussion
We investigated, for the first time, the associations between the hypertriglyceridemic waist phenotype, a simple office‐based measurement, and atherosclerosis presence, burden, and tissue characteristics by comprehensive volumetric carotid artery imaging with magnetic resonance. Our sample was representative of a wide spectrum of cardiovascular risk, ranging from young adults without cardiovascular disease and at low risk through patients with CAD confirmed by invasive coronary angiography. Hypertriglyceridemic waist was positively associated with the presence of carotid atherosclerosis and also with markers of carotid atherosclerosis burden. Moreover, hypertriglyceridemic waist was independently associated with the volume of carotid artery LRNC, a marker of high‐risk atherosclerosis. Ultimately, the addition of hypertriglyceridemic waist to the FRS or PDAY risk score correctly reclassified individuals with carotid atherosclerosis and/or LRNC by MRI who were initially considered low risk by these risk scores. Therefore, the hypertriglyceridemic waist may improve on current strategies on the basis of traditional risk factors alone.
Hypertriglyceridemic Waist and Cardiovascular Risk
We have previously suggested that excess visceral/ectopic adipose tissue accumulation is the missing link to explain the association between the constellation of metabolic dysfunction of the metabolic syndrome and cardiovascular disease, beyond the contribution of traditional risk factors.3, 32, 33 However, the direct measurement of VAT requires costly imaging and analysis modalities that make it less practical for use in screening larger populations in the context of prevention. A clinical measurement of abdominal adiposity, such as the waist circumference that is easily accessible at low cost, shows significant correlations with abdominal VAT measurements.10 Furthermore, waist circumference is reported as an important risk marker of cardiovascular events.11 Although obesity (BMI, ≥30 kg/m2) is recognized as a traditional risk factor that must be considered in the primary screening for cardiovascular risk, it appears that an elevated waist girth informs on the presence of a poor cardiometabolic profile and predicts cardiovascular risk and mortality beyond the BMI alone.34 Although an elevated waist circumference indicates increased cardiovascular risk, it does not clearly discriminate SAT from VAT, although it is only the latter that portends the important umbrella of metabolic disorders and poor clinical outcomes of the ectopic fat.35 Patients with increased VAT typically present an increased concentration of small and dense low‐density lipoprotein particles,36 associated with an elevated fasting triglyceride concentration.37 This increased concentration of high‐risk particles is predictive of an augmented risk of CAD, even in apparently healthy men.38 Furthermore, small low‐density lipoprotein size is associated with the progression of coronary atherosclerosis documented by angiography.39 This observation has been validated by several studies, suggesting that triglyceride level is a simple marker of proatherogenic low‐density lipoprotein particles.13, 37 Lemieux et al were the first to suggest the combination of an elevated waist circumference and an increased triglyceride level as a warning sign of higher cardiovascular risk.13 The presence of an elevated waist circumference simultaneously with an elevated triglyceride concentration could indicate inability to store surplus energy in SAT because of dysfunctional adipose tissue.5 Thereby, the hypertriglyceridemic waist phenotype is a simple clinical marker of the presence of dysfunctional fat, such as VAT,35 and thus of metabolic disorders.40 In agreement with previous observations, the present study shows that the hypertriglyceridemic waist is positively associated with visceral adiposity and with cardiac and hepatic fat. Furthermore, the relationships between the hypertriglyceridemic waist and abdominal VAT or hepatic fat are independent from traditional risk factors, remaining significant after adjusting for FRS and PDAY. As postulated several years ago, the hypertriglyceridemic phenotype has proved itself to be an effective marker to identify men at higher coronary risk via an atherogenic metabolic profile.13, 41, 42 We observed that those with the hypertriglyceridemic waist phenotype exhibited a greater FRS compared with those without, consistent with previous studies.43 Furthermore, the hypertriglyceridemic waist exhibited a strong and positive association with almost all individual markers of cardiovascular risk, and was also associated with the presence of clinical CAD, even after adjusting for FRS.
Hypertriglyceridemic Waist and Carotid Atherosclerosis Burden
Atherosclerosis is a systemic disease, and subclinical atherosclerosis evaluated in the carotid artery is an excellent indicator of global atherosclerosis burden.29 The direct examination of artery features informs on the real burden of atherosclerotic disease, especially in the vicinity of the carotid bulb or bifurcation.44 The hypertriglyceridemic waist phenotype has been associated with various atherosclerosis burden markers, including coronary calcium score in patients with type 2 diabetes mellitus14 and with carotid intima‐media thickness in the chronic kidney disease population.15 In an apparently healthy multiethnic population, the hypertriglyceridemic waist was also associated with carotid atherosclerotic plaques identified by the intima‐media thickness, but the association was not significant after adjusting for traditional risk factors.16 A major drawback of the carotid intima‐media thickness measurement with ultrasound is poor reproducibility.45 MRI presents superior reproducibility compared with other noninvasive imaging modalities across the full spectrum of atherosclerosis severity (from early to severe disease) and is by far the most promising method to measure carotid atherosclerosis burden and features.46 To the best of our knowledge, this study is the first to investigate the association between carotid atherosclerosis burden examined by MRI and the hypertriglyceridemic waist phenotype. Our results indicate that the hypertriglyceridemic waist is significantly more prevalent among those with subclinical carotid atherosclerosis in comparison to those without. Furthermore, carotid maximum wall thickness, a marker widely used to inform about the presence of atherosclerosis,47 was positively associated with the hypertriglyceridemic waist. In comparison to the maximum wall thickness, carotid artery wall volume is more suitable to better inform about the true burden of atherosclerosis, because it measures the whole circumference of the artery over its entire length of interest. Moreover, the normalized wall index is a useful marker of atherosclerosis burden, taking into account individual morphological differences by normalizing the artery wall volume to the vessel volume. We observed that the hypertriglyceridemic waist is positively and significantly associated with all of these markers of carotid atherosclerosis burden.
Hypertriglyceridemic Waist and High‐Risk Carotid Atherosclerosis Features
LRNC is arguably the most important unique feature of high‐risk atherosclerotic plaque.17, 18 We observed that the volume of carotid LRNC was associated with the waist circumference, but the association was no longer significant when adjusted for FRS or PDAY, indicating that waist circumference alone is not an independent marker of high‐risk atherosclerosis features. However, our results have shown that those with a hypertriglyceridemic waist phenotype presented a significantly greater carotid LRNC volume compared with those without. Moreover, carotid LRNC volume was positively and significantly associated with the hypertriglyceridemic waist, and this association remained significant even after adjusting for the risk of cardiovascular events or the risk of subclinical coronary artery atherosclerosis. Therefore, although no significant difference was observed between the HTGW+/− and HTGW+/+ groups for carotid artery characteristics, the hypertriglyceridemic waist remains an independent marker of high‐risk carotid atherosclerosis features and thus a superior clinical marker compared with waist circumference alone. This was further confirmed by NRI for high‐risk atherosclerosis features beyond clinical risk scores alone. CAS, computed from the maximum wall thickness and the maximum percentage of LRNC, has been validated to stratify the severity of atherosclerosis disease.31 In our population, CAS was positively associated with the hypertriglyceridemic waist phenotype, further supporting the clinical utility of the hypertriglyceridemic waist. Although no participant exhibited a CAS of 4, such high CAS level is rare as the reported prevalence is on the order of 5% in patients with significant carotid stenosis.31 Intraplaque hemorrhage is another high‐risk atherosclerosis feature. However, although intraplaque hemorrhage has been correlated with cardiovascular disease progression, its prevalence remains low in comparison to the incidence of cardiovascular events,48 a finding corroborated by the present study. This low prevalence of intraplaque hemorrhage may likely explain the absence of association. Calcification and loose fibrous matrix are both important features of atherosclerosis, although some inconsistencies remain as to their role in risk assessment. Indeed, although the role of calcification in atherosclerosis burden is well validated,49 its role in plaque vulnerability is not clear; although it has been suggested to promote plaque rupture,50 calcification has also been demonstrated to mitigate plaque stress.51 Loose fibrous matrix, resulting from healed plaque rupture,26, 52 has been associated with symptomatic plaque,53 while also related to stable plaque characteristics.54 All participants included in the present study were devoid of symptomatic carotid artery disease, and no association was observed between the hypertriglyceridemic waist and the loose fibrous matrix or calcification volumes. However, the prevalence of calcification and loose fibrous matrix in the carotid artery was significantly greater in the HTGW+/− group compared with the HTGW−/− group. Furthermore, the HTGW+/− group exhibited a significantly greater burden of carotid calcification in comparison to the HTGW−/− group. Nevertheless, considering the inconsistencies about the increased risk associated with these atherosclerosis components, further studies are needed.
This is the first documented investigation of the relationship between hypertriglyceridemic waist and the presence and burden of high‐risk carotid atherosclerosis features. We observed that approximately two thirds of participants with carotid atherosclerosis and/or LRNC were at low/moderate risk by FRS or PDAY, and therefore, unsuspected for carotid atherosclerosis or high‐risk features. The addition of the hypertriglyceridemic waist assessment to the usual screening for traditional risk factors would correctly reclassify an additional 4.5% to 9.7% of individuals at a negligible cost, considering the standard lipid profile already measures triglycerides. According to the present study, the combination of FRS or PDAY and the hypertriglyceridemic waist phenotype significantly reclassified those with subclinical carotid atherosclerosis and/or evidence of LRNC in their carotid artery with an appreciable NRI compared with FRS or PDAY alone. Furthermore, the combination of hypertriglyceridemic waist with FRS or PDAY remained superior to FRS or PDAY alone in identifying subclinical carotid atherosclerosis and/or LRNC in the CAD‐free or CAD groups; because we observed a greater NRI in the CAD‐free group, hypertriglyceridemic waist is perhaps even more valuable in asymptomatic individuals, otherwise unsuspected for atherosclerosis. Of particular interest, hypertriglyceridemic waist combined to either FRS or PDAY improved identification of the high‐risk atherosclerosis feature LRNC beyond waist circumference alone combined to these same risk scores, highlighting its potential value in primary care. In this regard, our results suggest that this simple tool could potentially improve office‐based cardiovascular risk assessment.
Study Limitations
The design of the study is cross‐sectional and although we observed associations between carotid atherosclerosis characteristics and the hypertriglyceridemic waist, the study design does not allow the establishment of causality between these variables. Participants were enrolled over a significant period of time, which may have influenced the coronary disease management and therapies of recruited coronary patients over those years. All participants included in this study were recruited on a volunteer basis, which may have influenced the results. Nevertheless, this study has been rigorously designed to compare atherosclerosis characteristics across a large spectrum of risk and performing the recruitment in the population at large (by media and word of mouth) is expected to have attenuated the impact of a potential selection bias. Finally, the study sample included men recruited from a single center: A larger multicenter study, including women and diverse ethnic groups, will be needed to investigate whether these observations apply to the general population.
Conclusions
Hypertriglyceridemic waist is strongly associated with the presence and burden of subclinical carotid atherosclerosis in men across a wide spectrum of cardiovascular risk. Beyond waist circumference alone, hypertriglyceridemic waist is an independent marker of high‐risk carotid atherosclerosis features. Ultimately, the addition of hypertriglyceridemic waist to the FRS or the PDAY risk score improves the identification of carotid atherosclerosis and high‐risk features and allows the reclassification of individuals thought to be at low risk. This simple office‐based tool may improve cardiovascular risk stratification beyond traditional risk factors alone.
Sources of Funding
This study was supported by the Canadian Institutes of Health Research, Ottawa (Ontario, Canada); the Heart and Stroke Foundation of Canada, Ottawa (Ontario, Canada); the Fonds de Recherche du Québec—Santé (FRQS), Montréal (Québec, Canada); and the Fondation de l'Institut Universitaire de Cardiologie et de Pneumologie de Québec, Québec City (Québec, Canada). LeBlanc is supported by a PhD award from the FRQS, Montréal (Québec, Canada) and by the Faculté de Médecine, Université Laval, Québec City (Québec, Canada). Coulombe is supported by the Mach‐Gaensslen Foundation of Canada, Ottawa (Ontario, Canada) and by the Faculté de Médecine, Université Laval, Québec City (Québec, Canada). Larose is a Senior Clinical Research Scholar supported by the FRQS, Montréal (Québec, Canada) and holds the Laval University Chair of Research and Innovation in Cardiovascular Imaging (Québec, Canada).
Disclosures
Pibarot reports significant research grants from Cardiac Phoenix, Edwards Life Science, and Medtronic. The remaining authors have no disclosures to report.
Supporting information
Table S1. Characteristics of the Population
Table S2. Atherosclerosis Features of the Carotid Artery*
(J Am Heart Assoc. 2018;7:e008139 DOI: 10.1161/JAHA.117.008139.)29654193
References
- 1. GBD 2013 Mortality and Causes of Death Collaborators . Global, regional, and national age‐sex specific all‐cause and cause‐specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akosah KO, Schaper A, Cogbill C, Schoenfeld P. Preventing myocardial infarction in the young adult in the first place: how do the National Cholesterol Education Panel III guidelines perform? J Am Coll Cardiol. 2003;41:1475–1479. [DOI] [PubMed] [Google Scholar]
- 3. Despres JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126:1301–1313. [DOI] [PubMed] [Google Scholar]
- 4. Maltais ML, Leblanc S, Archambault‐Therrien C, Jean B, Bobeuf F, Dionne IJ. Various sources of animal protein intake and their association with muscle mass index and insulin resistance in overweight postmenopausal women. Int J Nutr Metab. 2013;5:17–21. [Google Scholar]
- 5. Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. [DOI] [PubMed] [Google Scholar]
- 6. De Larochellière E, Côté J, Gilbert G, Bibeau K, Ross MK, Dion‐Roy V, Pibarot P, Despres JP, Larose E. Visceral/epicardial adiposity in nonobese and apparently healthy young adults: association with the cardiometabolic profile. Atherosclerosis. 2014;234:23–29. [DOI] [PubMed] [Google Scholar]
- 7. Koskinen J, Kahonen M, Viikari JS, Taittonen L, Laitinen T, Ronnemaa T, Lehtimaki T, Hutri‐Kahonen N, Pietikainen M, Jokinen E, Helenius H, Mattsson N, Raitakari OT, Juonala M. Conventional cardiovascular risk factors and metabolic syndrome in predicting carotid intima‐media thickness progression in young adults: the cardiovascular risk in young Finns study. Circulation. 2009;120:229–236. [DOI] [PubMed] [Google Scholar]
- 8. Camhi SM, Katzmarzyk PT, Broyles ST, Srinivasan SR, Chen W, Bouchard C, Berenson GS. Subclinical atherosclerosis and metabolic risk: role of body mass index and waist circumference. Metab Syndr Relat Disord. 2011;9:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lear SA, Humphries KH, Kohli S, Frohlich JJ, Birmingham CL, Mancini GB. Visceral adipose tissue, a potential risk factor for carotid atherosclerosis: results of the Multicultural Community Health Assessment Trial (M‐CHAT). Stroke. 2007;38:2422–2429. [DOI] [PubMed] [Google Scholar]
- 10. Pouliot MC, Despres JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. [DOI] [PubMed] [Google Scholar]
- 11. Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S; INTERHEART Investigators . Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29:932–940. [DOI] [PubMed] [Google Scholar]
- 12. Despres JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes‐Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. [DOI] [PubMed] [Google Scholar]
- 13. Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Almeras N, Bergeron J, Gaudet D, Tremblay G, Prud'homme D, Nadeau A, Despres JP. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102:179–184. [DOI] [PubMed] [Google Scholar]
- 14. Sam S, Haffner S, Davidson MH, D'Agostino RB Sr, Feinstein S, Kondos G, Perez A, Mazzone T. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diabetes Care. 2009;32:1916–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhe X, Bai Y, Cheng Y, Xiao H, Wang D, Wu Y, Huang X, Tian X, Wang T. Hypertriglyceridemic waist is associated with increased carotid atherosclerosis in chronic kidney disease patients. Nephron Clin Pract. 2012;122:146–152. [DOI] [PubMed] [Google Scholar]
- 16. Gasevic D, Carlsson AC, Lesser IA, Mancini GJ, Lear SA. The association between “hypertriglyceridemic waist” and sub‐clinical atherosclerosis in a multiethnic population: a cross‐sectional study. Lipids Health Dis. 2014;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Virani SS, Ballantyne CM. From plaque burden to plaque composition: toward personalized risk assessment. JACC Cardiovasc Imaging. 2017;10:250–252. [DOI] [PubMed] [Google Scholar]
- 18. Zavodni AE, Wasserman BA, McClelland RL, Gomes AS, Folsom AR, Polak JF, Lima JA, Bluemke DA. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi‐Ethnic Study of Atherosclerosis (MESA). Radiology. 2014;271:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boyer M, Levesque V, Poirier P, Marette A, Mathieu P, Despres JP, Larose E, Arsenault BJ. Impact of a 1‐year lifestyle modification program on plasma lipoprotein and PCSK9 concentrations in patients with coronary artery disease. J Clin Lipidol. 2016;10:1353–1361. [DOI] [PubMed] [Google Scholar]
- 20. King SB III, Smith SC Jr, Hirshfeld JW Jr, Jacobs AK, Morrison DA, Williams DO, Feldman TE, Kern MJ, O'Neill WW, Schaff HV, Whitlow PL, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: 2007 Writing Group to Review New Evidence and Update the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention, Writing on Behalf of the 2005 Writing Committee. Circulation. 2008;117:261–295. [DOI] [PubMed] [Google Scholar]
- 21. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr; International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity . Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 22. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 23. Gidding SS, Rana JS, Prendergast C, McGill H, Carr JJ, Liu K, Colangelo LA, Loria CM, Lima J, Terry JG, Reis JP, McMahan CA. Pathobiological determinants of atherosclerosis in youth (PDAY) risk score in young adults predicts coronary artery and abdominal aorta calcium in middle age: the CARDIA study. Circulation. 2016;133:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McMahan CA, Gidding SS, Viikari JS, Juonala M, Kahonen M, Hutri‐Kahonen N, Jokinen E, Taittonen L, Pietikainen M, McGill HC Jr, Raitakari OT. Association of pathobiologic determinants of atherosclerosis in youth risk score and 15‐year change in risk score with carotid artery intima‐media thickness in young adults (from the Cardiovascular Risk in Young Finns Study). Am J Cardiol. 2007;100:1124–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Larose E, Yeghiazarians Y, Libby P, Yucel EK, Aikawa M, Kacher DF, Aikawa E, Kinlay S, Schoen FJ, Selwyn AP, Ganz P. Characterization of human atherosclerotic plaques by intravascular magnetic resonance imaging. Circulation. 2005;112:2324–2331. [DOI] [PubMed] [Google Scholar]
- 26. Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–239. [DOI] [PubMed] [Google Scholar]
- 27. Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, Small R, Davies JW, Kerwin WS, Hatsukami TS. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid‐rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051–2056. [DOI] [PubMed] [Google Scholar]
- 28. Dong L, Kerwin WS, Ferguson MS, Li R, Wang J, Chen H, Canton G, Hatsukami TS, Yuan C. Cardiovascular magnetic resonance in carotid atherosclerotic disease. J Cardiovasc Magn Reson. 2009;11:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LeBlanc S, Bibeau K, Bertrand OF, Levesque V, Deschenes St‐Pierre B, Pibarot P, Despres JP, Larose E. Carotid versus coronary atherosclerosis burdens in acute compared with chronic symptomatic coronary artery disease. Can J Physiol Pharmacol. 2017;95:1–10. [DOI] [PubMed] [Google Scholar]
- 30. Underhill HR, Yuan C, Terry JG, Chen H, Espeland MA, Hatsukami TS, Saam T, Chu B, Yu W, Oikawa M, Takaya N, Yarnykh VL, Kraft R, Carr JJ, Maldjian J, Tang R, Crouse JR III. Differences in carotid arterial morphology and composition between individuals with and without obstructive coronary artery disease: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2008;10:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu D, Hippe DS, Underhill HR, Oikawa‐Wakayama M, Dong L, Yamada K, Yuan C, Hatsukami TS. Prediction of high‐risk plaque development and plaque progression with the carotid atherosclerosis score. JACC Cardiovasc Imaging. 2014;7:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist‐to‐hip ratio as predictors of cardiovascular events: meta‐regression analysis of prospective studies. Eur Heart J. 2007;28:850–856. [DOI] [PubMed] [Google Scholar]
- 33. Cheng VY, Dey D, Tamarappoo B, Nakazato R, Gransar H, Miranda‐Peats R, Ramesh A, Wong ND, Shaw LJ, Slomka PJ, Berman DS. Pericardial fat burden on ECG‐gated noncontrast CT in asymptomatic patients who subsequently experience adverse cardiovascular events. JACC Cardiovasc Imaging. 2010;3:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lassale C, Tzoulaki I, Moons KGM, Sweeting M, Boer J, Johnson L, Huerta JM, Agnoli C, Freisling H, Weiderpass E, Wennberg P, van der A DL, Arriola L, Benetou V, Boeing H, Bonnet F, Colorado‐Yohar SM, Engström G, Eriksen AK, Ferrari P, Grioni S, Johansson M, Kaaks R, Katsoulis M, Katzke V, Key TJ, Matullo G, Melander O, Molina‐Portillo E, Moreno‐Iribas C, Norberg M, Overvad K, Panico S, Quiros JR, Saieva C, Skeie G, Steffen A, Stepien M, Tjønneland A, Trichopoulou A, Tumino R, van der Schouw YT, Verschuren WMM, Langenberg C, Di Angelantonio E, Riboli E, Wareham NJ, Danesh J, Butterworth AS. Separate and combined associations of obesity and metabolic health with coronary heart disease: a pan‐European case‐cohort analysis. Eur Heart J. 2017;39:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lemieux I, Poirier P, Bergeron J, Almeras N, Lamarche B, Cantin B, Dagenais GR, Despres JP. Hypertriglyceridemic waist: a useful screening phenotype in preventive cardiology? Can J Cardiol. 2007;23(suppl B):23B–31B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tchernof A, Lamarche B, Prud'Homme D, Nadeau A, Moorjani S, Labrie F, Lupien PJ, Despres JP. The dense LDL phenotype: association with plasma lipoprotein levels, visceral obesity, and hyperinsulinemia in men. Diabetes Care. 1996;19:629–637. [DOI] [PubMed] [Google Scholar]
- 37. McNamara JR, Jenner JL, Li Z, Wilson PW, Schaefer EJ. Change in LDL particle size is associated with change in plasma triglyceride concentration. Arterioscler Thromb. 1992;12:1284–1290. [DOI] [PubMed] [Google Scholar]
- 38. Arsenault BJ, Lemieux I, Despres JP, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM. Cholesterol levels in small LDL particles predict the risk of coronary heart disease in the EPIC‐Norfolk prospective population study. Eur Heart J. 2007;28:2770–2777. [DOI] [PubMed] [Google Scholar]
- 39. Vakkilainen J, Steiner G, Ansquer JC, Aubin F, Rattier S, Foucher C, Hamsten A, Taskinen MR; DAIS Group . Relationships between low‐density lipoprotein particle size, plasma lipoproteins, and progression of coronary artery disease: the Diabetes Atherosclerosis Intervention Study (DAIS). Circulation. 2003;107:1733–1737. [DOI] [PubMed] [Google Scholar]
- 40. Ren Y, Luo X, Wang C, Yin L, Pang C, Feng T, Wang B, Zhang L, Li L, Yang X, Zhang H, Zhao J, Hu D. Prevalence of hypertriglyceridemic waist and association with risk of type 2 diabetes mellitus: a meta‐analysis. Diabetes Metab Res Rev. 2016;32:405–412. [DOI] [PubMed] [Google Scholar]
- 41. Czernichow S, Bruckert E, Bertrais S, Galan P, Hercberg S, Oppert JM. Hypertriglyceridemic waist and 7.5‐year prospective risk of cardiovascular disease in asymptomatic middle‐aged men. Int J Obes (Lond). 2007;31:791–796. [DOI] [PubMed] [Google Scholar]
- 42. Tanko LB, Bagger YZ, Qin G, Alexandersen P, Larsen PJ, Christiansen C. Enlarged waist combined with elevated triglycerides is a strong predictor of accelerated atherogenesis and related cardiovascular mortality in postmenopausal women. Circulation. 2005;111:1883–1890. [DOI] [PubMed] [Google Scholar]
- 43. Blackburn P, Lemieux I, Almeras N, Bergeron J, Cote M, Tremblay A, Lamarche B, Despres JP. The hypertriglyceridemic waist phenotype versus the National Cholesterol Education Program‐Adult Treatment Panel III and International Diabetes Federation clinical criteria to identify high‐risk men with an altered cardiometabolic risk profile. Metabolism. 2009;58:1123–1130. [DOI] [PubMed] [Google Scholar]
- 44. Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Timmins LH, Binongo JN, Golub LJ, Corban MT, Finn AV, Oshinski JN, Quyyumi AA, Giddens DP, Samady H. Association of coronary wall shear stress with atherosclerotic plaque burden, composition, and distribution in patients with coronary artery disease. J Am Heart Assoc. 2012;1:e002543 DOI: 10.1161/JAHA.112.002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lorenz MW, Polak JF, Kavousi M, Mathiesen EB, Volzke H, Tuomainen TP, Sander D, Plichart M, Catapano AL, Robertson CM, Kiechl S, Rundek T, Desvarieux M, Lind L, Schmid C, DasMahapatra P, Gao L, Ziegelbauer K, Bots ML, Thompson SG; PROG‐IMT Study Group . Carotid intima‐media thickness progression to predict cardiovascular events in the general population (the PROG‐IMT collaborative project): a meta‐analysis of individual participant data. Lancet. 2012;379:2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Duivenvoorden R, de Groot E, Elsen BM, Lameris JS, van der Geest RJ, Stroes ES, Kastelein JJ, Nederveen AJ. In vivo quantification of carotid artery wall dimensions: 3.0‐Tesla MRI versus B‐mode ultrasound imaging. Circ Cardiovasc Imaging. 2009;2:235–242. [DOI] [PubMed] [Google Scholar]
- 47. Selwaness M, Bos D, van den Bouwhuijsen Q, Portegies ML, Ikram MA, Hofman A, Franco OH, van der Lugt A, Wentzel JJ, Vernooij MW. Carotid atherosclerotic plaque characteristics on magnetic resonance imaging relate with history of stroke and coronary heart disease. Stroke. 2016;47:1542–1547. [DOI] [PubMed] [Google Scholar]
- 48. Sun J, Balu N, Hippe DS, Xue Y, Dong L, Zhao X, Li F, Xu D, Hatsukami TS, Yuan C. Subclinical carotid atherosclerosis: short‐term natural history of lipid‐rich necrotic core–a multicenter study with MR imaging. Radiology. 2013;268:61–68. [DOI] [PubMed] [Google Scholar]
- 49. Wayhs R, Zelinger A, Raggi P. High coronary artery calcium scores pose an extremely elevated risk for hard events. J Am Coll Cardiol. 2002;39:225–230. [DOI] [PubMed] [Google Scholar]
- 50. Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress‐induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103:14678–14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. [DOI] [PubMed] [Google Scholar]
- 52. Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. [DOI] [PubMed] [Google Scholar]
- 53. Saam T, Cai J, Ma L, Cai YQ, Ferguson MS, Polissar NL, Hatsukami TS, Yuan C. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology. 2006;240:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Doherty JR, Dahl JJ, Kranz PG, El Husseini N, Chang HC, Chen NK, Allen JD, Ham KL, Trahey GE. Comparison of acoustic radiation force impulse imaging derived carotid plaque stiffness with spatially registered MRI determined composition. IEEE Trans Med Imaging. 2015;34:2354–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the Population
Table S2. Atherosclerosis Features of the Carotid Artery*


