Abstract
Background
Although choline metabolism has been associated with atherosclerotic heart disease, less research attention has been paid to the associations of choline and its oxidative metabolite betaine with cardiac arrhythmias.
Methods and Results
We evaluated associations of plasma concentrations and dietary intakes of choline and betaine with long‐term atrial fibrillation (AF) risk in a community‐based cohort, HUSK ([the Hordaland Health Study] n=6949), and validated the findings in 2 patient cohorts: the Western Norway Coronary Angiography Cohort (n=4164) and the NORVIT (Norwegian B‐Vitamin) Trial (n=3733). Information on AF was obtained from the CVDNOR (Cardiovascular Disease in Norway) project. In HUSK, WECAC (Western Norway Coronary Angiography Cohort), and NORVIT, 552, 411, and 663 AF cases were identified during a median follow‐up time of 10.9, 7.3, and, 8.7 years, respectively. Plasma concentrations of choline and betaine were significantly positively associated with later AF risk after multivariable adjustments in HUSK. Such associations were independently replicated in the 2 external prospective patient cohorts. The pooled hazard ratio was 1.13 (95% confidence interval 1.08‐1.19, P<0.001) and 1.16 (95% confidence interval 1.10‐1.22, P<0.001) per SD increment for log‐transformed choline and betaine, respectively. Moreover, dietary intake of choline was marginally associated with AF risk (pooled hazard ratio 1.29, 95% confidence interval 1.01‐1.66, fifth versus first quintile), whereas no significant association was observed between dietary betaine and AF risk.
Conclusions
Our findings indicate that plasma concentrations as well as dietary intake of choline, but not betaine, are associated with subsequent risk of AF, suggesting a potential role of choline metabolism in the pathogenesis of AF.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov.Unique identifier: NCT00671346.
Keywords: atrial fibrillation, betaine, choline, cohort studies, the CVDNOR project
Subject Categories: Atrial Fibrillation, Metabolism, Epidemiology
Clinical Perspective
What Is New?
Higher plasma concentrations of choline and betaine were associated with an increased risk of atrial fibrillation.
Dietary intake of total choline was marginally associated with atrial fibrillation risk and betaine intake not associated.
What Are the Clinical Implications?
Our findings suggest a potential role of choline metabolism in atrial fibrillation pathogenesis and may help to target individuals predisposed to develop atrial fibrillation for potential interventions.
Atrial fibrillation (AF) is the most common clinically relevant cardiac arrhythmia linked to excess morbidity and mortality.1 It has emerged as a global epidemic due to progressively increasing incidence and prevalence worldwide, with developed countries having a greater burden.2 This underscores the importance of disease prevention by identifying novel risk markers because traditional AF risk factors such as higher age, male sex, obesity, diabetes mellitus, and hypertension do not fully explain the variance in AF risk.3
Choline is an essential nutrient but can also be derived from endogenous biosynthesis.4 The total choline in foods includes free choline in addition to choline esters.5 In the mitochondria, choline is oxidized to betaine, which serves as an intracellular osmolyte and methyl donor in the folate‐independent remethylation of homocysteine to methionine, catalyzed by betaine‐homocysteine methyltransferase, a reaction producing dimethylglycine.4, 6 In addition to de novo synthesis primarily in liver and kidney, betaine is also obtained from dietary sources.4
No associations of dietary choline and betaine with cardiovascular disease (CVD) risk have been reported,7, 8, 9 whereas circulating choline and its metabolites have been associated with lifestyle diseases including atherosclerotic cardiovascular disease.4, 6, 10, 11 However, less attention has been paid to the possible associations between choline metabolites and cardiac arrhythmias, although a recent metabolomics study suggested no significant association between plasma betaine and incident AF in the population‐based Framingham Study.12
The current study therefore investigated whether plasma choline and betaine, as well as their dietary intakes, were associated with subsequent AF over long‐term follow‐up. The primary cohort consisted of participants from the general population, and the findings were sought replicated in 2 external cohorts: patients with suspected stable angina pectoris and patients hospitalized with acute myocardial infarction.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
The community‐based HUSK (Hordaland Health Study) has been described in detail elsewhere13, 14 (http://husk.b.uib.no). The present study cohort was confined to 7050 men and women who were born during 1925‐1927 or 1950‐1951 and participated in the baseline examinations in the period of April 1998 to June 1999. Of the 7050 participants, we excluded 65 who were diagnosed with AF before enrollment as well as participants with missing data on plasma biomarkers (n=36). A total of 6949 participants (3072 men and 3877 women) were included in the final analyses.
The 2 patient cohorts, WECAC (the Western Norway Coronary Angiography Cohort) and NORVIT (the Norwegian Vitamin Trial), have been described in more detail previously.11 Briefly, WECAC consisted of 5209 patients undergoing coronary angiography during 2000‐2004. Of these, 4164 patients with suspected stable angina pectoris were selected for the current study. About two‐thirds of these patients were included in WENBIT (the Western Norway B‐vitamin Intervention Trial), a randomized clinical trial investigating the effect of B‐vitamin treatment on CVD and mortality.15 NORVIT was a randomized clinical trial including 3749 patients hospitalized with acute myocardial infarction in the period 1998‐2002 and randomized to identical treatment protocols as in WENBIT.16 In WECAC, we excluded patients with a history of previous AF from the analyses, whereas we did not have information on previous AF among patients in NORVIT.
The study was performed according to the Declaration of Helsinki, and all participants provided written informed consent. The study protocol was approved by the Regional Committee for Medical and Health Research Ethics (REK 2009/825 and 2010/1880).
Biosampling and Biochemical Analyses
In HUSK, nonfasting blood samples were collected at baseline. Time since last meal was recorded. In WECAC, blood samples were drawn 1 to 3 days before or immediately after the coronary angiography procedure, and 28.3% of patients were fasting. In NORVIT, blood samples were drawn within 1 week after the index acute myocardial infarction, with no information on fasting status.
In HUSK, aliquots of serum and plasma were frozen at −80°C until later analyses. In WECAC and NORVIT, routine laboratory measurements were carried out on fresh blood samples at each recruiting hospital, whereas blood samples for specific analyses were shipped to the core biobank at Haukeland University Hospital for long‐term storage at −80°C. Study‐specific analyses for all the 3 cohorts were carried out at the Bevital A/S Laboratory, Bergen, Norway (http://www.bevital.no) on thawed samples by personnel blinded to the study outcomes.
Plasma choline and betaine were measured by liquid chromatography‐tandem mass spectrometry.17, 18 Serum folate was measured by a microbiological assay.19 Serum creatinine was measured colorimetrically using the alkaline picrate method with reagents from Roche (Basle, Switzerland).13 Plasma high‐sensitivity C‐reactive protein (CRP) was determined by an immuno‐MALDI‐MS method.20 Within‐day coefficients of variation for choline and betaine were 5.4% to 7.6%, and between‐day coefficients of variation were 3.8% to 9.5%.17 The limit of detection was 0.50 μmol/L for both choline and betaine and 0.10 mg/L for CRP. The limit of quantitation was 1.0 μmol/L for both choline and betaine and 0.16 mg/L for CRP.
Follow‐Up and Outcome Ascertainment
Cohort participants were followed from baseline to the date of AF diagnosis, death, emigration, or through December 31, 2009 (the end of follow‐up), whichever came first. Information on hospitalization with an AF diagnosis was obtained via linkage to hospital discharge diagnosis data obtained through the CVDNOR (Cardiovascular Disease in Norway) project (https://cvdnor.b.uib.no).21, 22, 23 The primary outcome was hospitalization or death attributed to AF (Code 427.3 from International Classification of Diseases, Ninth Revision [ICD‐9], I48 from ICD‐10). Information on death was collected from the Cause of Death Registry at Statistics Norway. A unique 11‐digit personal identifier was used to link baseline variables with study end points.
Dietary Intake Assessment
Dietary information in HUSK and WECAC was collected with the use of a validated self‐administered food frequency questionnaire.24, 25 The food frequency questionnaire included 169 food items and offered alternatives for frequency, number of units consumed, and portion sizes to capture the habitual dietary information during the past year. Information on the use of supplements also was obtained from the food frequency questionnaire and included in the calculations. A software system (KBS software, version 3.2; University of Oslo, Norway) was developed to calculate energy and nutrient intakes. Total choline and betaine intakes were estimated according to the US Department of Agriculture's choline database.5
Other Baseline Factors
Smoking status was based on self‐reported smoking status and corrected by plasma cotinine (ie, self‐reported nonsmokers with plasma cotinine concentrations ≥85 nmol/L were reclassified as current smokers).26 Blood pressure was measured and body mass index (BMI) calculated based on measured height and weight (kg/m2). Calculation of estimated glomerular filtration rate was based on serum creatinine levels using the Chronic Kidney Disease Epidemiology Collaboration equation.27
Statistical Analyses
Continuous and categorical variables are reported as medians (interquartile ranges) and counts (%), respectively. Differences between AF cases and noncases were compared using chi‐squared tests for categorical and Wilcoxon‐Mann‐Whitney tests for continuous variables. Log‐transformation was applied to all plasma biomarkers to normalize their distributions. Spearman partial correlation coefficients were calculated to examine relations between dietary and plasma biomarkers after control for energy intake.
Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) per 1‐SD increment of log‐transformed plasma concentrations of choline and betaine. In the minimally adjusted models we adjusted for sex and age (continuous). In the multivariable adjusted models we additionally adjusted for a priori selected AF risk factors including BMI (continuous), diabetes mellitus (yes/no), hypertension (yes/no), and smoking (yes/no). An inverse variance‐weighted, fixed‐effect meta‐analysis was used to combine the results across the 3 cohorts because no significant between‐study heterogeneity was found. In HUSK, we also calculated the associations after additional adjustment for estimated glomerular filtration rate (continuous), fasting status (yes/no), education level (≤10, 11‐13, ≥14 years), serum folate (continuous), and plasma CRP (continuous) in the models. In order to address the potential effect of atherosclerotic heart disease on the association between choline/betaine and AF risk, we performed analyses by additional adjustment for CVD/coronary heart disease at baseline in HUSK and WECAC. Potential nonlinear associations between choline/betaine and risk of AF were visualized by smoothed splines from generalized additive models.
To assess potential improvement in model discrimination and reclassification, we calculated the integrated discrimination index, and the continuous net reclassification index (>0) in logistic regression models containing the same covariates as the multivariable Cox model, with and without plasma choline/betaine.28, 29
Regarding the estimation of associations between dietary intakes of choline and betaine with AF risk, subjects with extreme values of total energy intake (ie, <1st percentile or >99th percentile) for each sex were excluded. Choline and betaine intakes were adjusted for total energy intake by using the residual method.30 The consumptions of choline and betaine were categorized into quintiles in each cohort. Multivariable models were adjusted for sex, age (continuous), BMI (continuous), diabetes mellitus (yes/no), hypertension (yes/no), smoking (yes/no), and energy intake (continuous). The results were also pooled by an inverse variance‐weighted, fixed‐effect meta‐analysis.
Statistical analyses were conducted with the SAS statistical package (Version 9.4; SAS Institute, Inc, Cary, NC), SPSS for Windows (Version 23.0, IBM, Armonk, NY), and R (R Core Team 2016, Vienna, Austria, version 3.3.1, http://www.r-project.org). All tests were 2‐sided, and P<0.05 was considered statistically significant.
Results
Population Characteristics at Baseline
In the 3 cohorts, those who developed AF during follow‐up were in general older at baseline (P<0.001), had lower estimated glomerular filtration rate, more frequently had hypertension and diabetes mellitus, and were less likely to be current smokers. Moreover, participants who subsequently developed AF had consistently higher baseline concentrations of plasma choline and betaine (Table 1). In HUSK, those who developed AF had slightly higher energy‐adjusted intake of choline than those who did not. No differences were seen for betaine intake in HUSK or for choline or betaine intake in the WECAC (Table 1).
Table 1.
Baseline Characteristics of the Study Participants in HUSK, WECAC, and NORVIT
| Characteristic | All | AF | No AF | P Valuea |
|---|---|---|---|---|
| HUSK | ||||
| Participants, n | 6949 | 552 | 6397 | |
| Age, y | 48 (47‐72) | 72 (71‐73) | 48 (47‐72) | <0.001 |
| Men, % | 44.2 | 58.0 | 42.0 | <0.001 |
| BMI, kg/m2 | 25.4 (23.1‐27.9) | 25.9 (23.5‐28.8) | 25.3 (23.1‐27.8) | <0.001 |
| Current smoking, % | 28.6 | 25.0 | 28.9 | 0.05 |
| eGFR, mL/min per 1.73 m2 | 80 (69‐90) | 72 (64‐81) | 81 (70‐91) | <0.001 |
| Hypertension, % | 42.4 | 63.2 | 40.6 | <0.001 |
| Diabetes mellitus, % | 3.8 | 7.8 | 3.4 | <0.001 |
| CVD, % | 9.9 | 23.4 | 8.8 | <0.001 |
| Plasma/serum biomarkers | ||||
| Choline, μmol/L | 9.61 (8.28‐11.1) | 10.2 (8.82‐12.1) | 9.55 (8.23‐11.0) | <0.001 |
| Betaine, μmol/L | 38.1 (31.0‐45.9) | 41.4 (34.1‐49.2) | 37.9 (30.7‐45.6) | <0.001 |
| Folate, nmol/L | 6.68 (5.08‐9.04) | 6.48 (4.92‐8.91) | 6.70 (5.11‐9.05) | 0.20 |
| CRP, mg/L | 1.56 (0.68‐3.59) | 2.29 (1.10‐5.12) | 1.50 (0.66‐3.48) | <0.001 |
| Dietary factors | ||||
| Energy, kJ/day | 7898 (6328‐9780) | 7517 (6002‐9184) | 7829 (6347‐9842) | <0.001 |
| Total choline intake, mg/dayb | 259.5 (228.0‐294.4) | 265.9 (232.1‐301.3) | 258.9 (227.8‐293.6) | 0.011 |
| Betaine intake, mg/dayb | 130.7 (112.6‐151.3) | 131.3 (113.0‐151.8) | 130.6 (112.5‐151.3) | 0.88 |
| WECAC | ||||
| Participants, n | 3809 | 411 | 3398 | |
| Age, y | 61 (54‐69) | 68 (61‐74) | 60 (54‐68) | <0.001 |
| Men, % | 71.7 | 75.9 | 71.1 | 0.043 |
| BMI, kg/m2 | 26.3 (24.2‐28.9) | 26.2 (24.2‐29.2) | 26.3 (24.2‐28.9) | 0.95 |
| Current smoking, % | 32.0 | 26.3 | 32.7 | 0.009 |
| eGFR, mL/min per 1.73 m2 | 91 (79‐100) | 85 (70‐94) | 92 (80‐100) | <0.001 |
| Hypertension, % | 46.0 | 56.9 | 44.7 | <0.001 |
| Diabetes mellitus, % | 11.5 | 15.8 | 11.0 | 0.004 |
| CHD, % | 45.3 | 48.9 | 44.9 | 0.12 |
| Plasma biomarkers | ||||
| Choline, μmol/L | 9.62 (8.21‐11.4) | 10.30 (8.68‐12.4) | 9.55 (8.15‐11.3) | <0.001 |
| Betaine, μmol/L | 38.9 (31.8‐47.3) | 39.9 (32.9‐51.2) | 38.7 (31.7‐46.9) | 0.002 |
| Dietary factors | ||||
| Energy, kJ/day | 8465 (6860‐10 387) | 8121 (6557‐9830) | 8520 (6907‐10 470) | 0.032 |
| Total choline intake, mg/dayb | 247.0 (218.0‐280.9) | 248.3 (222.2‐284.0) | 246.9 (217.6‐280.5) | 0.30 |
| Betaine intake, mg/dayb | 137.8 (118.6‐159.3) | 139.8 (123.4‐159.1) | 137.6 (117.4‐159.3) | 0.24 |
| NORVIT | ||||
| Participants, n | 3733 | 663 | 3070 | |
| Age, y | 63 (54‐73) | 70 (63‐76) | 61 (53‐71) | <0.001 |
| Men, % | 74.0 | 70.8 | 74.7 | 0.037 |
| BMI, kg/m2 | 25 (23‐28) | 26 (24‐28) | 25 (23‐28) | 0.06 |
| Current smoking, % | 67.7 | 58.6 | 69.7 | <0.001 |
| eGFR, mL/min per 1.73 m2 | 76 (64‐89) | 71 (59‐85) | 77 (65‐90) | <0.001 |
| Hypertension, % | 29.0 | 39.2 | 26.7 | <0.001 |
| Diabetes mellitus, % | 9.7 | 12.5 | 9.1 | 0.008 |
| Plasma biomarkers | ||||
| Choline, μmol/L | 10.3 (8.68‐12.2) | 11.1 (9.10‐12.9) | 10.2 (8.61‐12.0) | <0.001 |
| Betaine, μmol/L | 33.1 (27.3‐39.9) | 34.0 (28.4‐41.2) | 32.9 (27.1‐39.7) | 0.002 |
Values are given as medians (interquartile ranges) or percentages. AF indicates atrial fibrillation; BMI, body mass index; CHD, coronary heart disease; CRP, C‐reactive protein; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HUSK, Hordaland Health Study; NORVIT, Norwegian B‐Vitamin Trial; WECAC, Western Norway Coronary Angiography Cohort.
Difference between the 2 groups.
Adjusted for total energy intake. Data analyses were confined to those participants with dietary data (n=5950 in HUSK and 1899 in WECAC).
Prospective Associations of Plasma Choline and Betaine With Subsequent AF Risk
In HUSK, WECAC, and NORVIT, 552, 411, and 663 AF cases were identified during a median follow‐up time of 10.9, 7.3, and 8.7 years, respectively. Higher plasma concentrations of choline and betaine were associated with an increased risk of developing AF in unadjusted models and in models adjusted for sex, age, BMI, diabetes mellitus, hypertension, and smoking (Table 2). In HUSK, the HR was 1.45 (95% CI 1.33‐1.57, P<0.001, log‐transformed, per SD increment) for choline and 1.37 (95% CI 1.26‐1.49, P<0.001) for betaine in unadjusted models. These associations remained significant in sex‐ and age‐adjusted models and multivariable models adjusted for sex, age, BMI, diabetes mellitus, hypertension, and smoking (Table 2). The unadjusted associations of plasma concentrations of choline and betaine with AF risk were almost log‐linear. These associations were, however, attenuated after multivariable adjustment (Figure). Additional adjustment for estimated glomerular filtration rate, fasting status, education level, serum folate, and plasma CRP did not appreciably alter the results (HR 1.10, 95% CI 1.00‐1.21, P=0.048 for choline; HR 1.16, 95% CI 1.04‐1.29, P=0.006 for betaine). Analyses on WECAC and NORVIT participants showed consistent results with HUSK, although in WECAC, plasma betaine was only marginally associated with AF risk after adjustments (Table 2). In WECAC, additional adjustment for fasting status made the associations slightly stronger (HR 1.17, 95% CI 1.05‐1.30, P=0.004 for choline; HR 1.12, 95% CI 1.01‐1.25, P=0.036 for betaine).
Table 2.
Associations of Plasma Choline and Betaine With Incident AF Risk in HUSK, WECAC, and NORVITa
| Plasma Metabolite | Unadjusted | Adjusted for Sex and Age | Multivariable Modelb | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| HUSK (n=6949) | ||||||
| Choline | 1.45 (1.33‐1.57) | <0.001 | 1.12 (1.02‐1.22) | 0.013 | 1.11 (1.02‐1.22) | 0.015 |
| Betaine | 1.37 (1.26‐1.49) | <0.001 | 1.13 (1.02‐1.24) | 0.014 | 1.18 (1.07‐1.29) | 0.001 |
| WECAC (n=3809) | ||||||
| Choline | 1.37 (1.24‐1.51) | <0.001 | 1.17 (1.05‐1.29) | 0.004 | 1.14 (1.03‐1.27) | 0.013 |
| Betaine | 1.16 (1.05‐1.28) | 0.002 | 1.07 (0.96‐1.18) | 0.22 | 1.10 (0.99‐1.22) | 0.08 |
| NORVIT (n=3733) | ||||||
| Choline | 1.30 (1.22‐1.39) | <0.001 | 1.17 (1.08‐1.26) | <0.001 | 1.15 (1.06‐1.24) | <0.001 |
| Betaine | 1.14 (1.06‐1.24) | 0.001 | 1.14 (1.06‐1.24) | 0.001 | 1.18 (1.09‐1.29) | <0.001 |
| Pooled resultsc | ||||||
| Choline | 1.36 (1.30‐1.42) | <0.001 | 1.15 (1.09‐1.21) | <0.001 | 1.13 (1.08‐1.19) | <0.001 |
| Betaine | 1.22 (1.16‐1.29) | <0.001 | 1.12 (1.06‐1.18) | <0.001 | 1.16 (1.10‐1.22) | <0.001 |
AF indicates atrial fibrillation; BMI, body mass index; CI, confidence interval; HR, hazard ratio; HUSK, Hordaland Health Study; NORVIT, Norwegian B‐Vitamin Trial; WECAC, Western Norway Coronary Angiography Cohort.
HRs (95% CIs) are reported per 1‐SD increment of natural log‐transformed plasma levels.
Models were adjusted for sex, age (continuous), BMI (continuous), diabetes mellitus (yes/no), hypertension (yes/no), and smoking (yes/no).
The results across the 3 cohorts were pooled using an inverse variance–weighted, fixed‐effect meta‐analysis.
Figure 1.
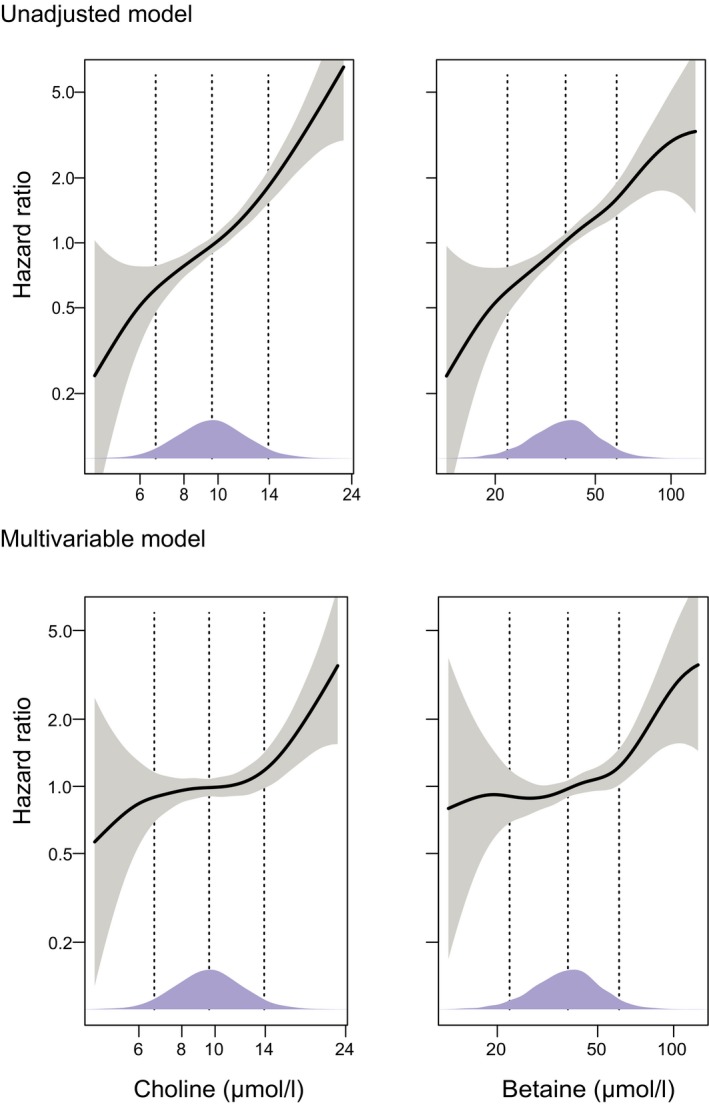
Associations of plasma choline and betaine with atrial fibrillation risk using generalized additive models in the Hordaland Health Study. Multivariable models were constructed with adjustment for sex, age, BMI (continuous), diabetes mellitus (yes/no), hypertension (yes/no), and smoking (yes/no). Both exposures were log‐transformed before entering the models. The solid lines show HRs, and the shaded areas 95% CIs. Density plots indicate the distribution of the biomarkers, and dotted lines denote the 5th, 50th, and 95th percentiles. BMI indicates body mass index; CI, confidence interval; HR, hazard ratio.
Moreover, additional adjustment for CVD at baseline gave similar associations in HUSK (HR 1.11, 95% CI 1.01‐1.21, P=0.025 for choline; HR 1.16, 95% CI 1.06‐1.28, P=0.002 for betaine). It resembled the results in WECAC after additional adjustment for coronary heart disease at baseline (HR 1.14, 95% CI 1.03‐1.27, P=0.014 for choline; HR 1.10, 95% CI 0.99‐1.22, P=0.09 for betaine).
In HUSK, the addition of plasma choline or betaine to the basic logistic regression model including sex, age, BMI, diabetes mellitus, hypertension, and smoking resulted in minor improvements in reclassification of participants at risk. The category‐free net reclassification index for the addition of choline and betaine in predicting AF was 0.02 (95% CI −0.07 to 0.11, P=0.646) and 0.14 (95% CI 0.06‐0.23, P=0.001), respectively. Similarly, the corresponding integrated discrimination index was 0.0014 (95% CI 0.000‐0.003, P=0.023) for choline and 0.003 (95% CI 0.001‐0.005, P=0.004) for betaine. In WECAC, the category‐free net reclassification index was 0.06 (95% CI −0.04 to 0.17, P=0.228) for choline and 0.06 (95% CI −0.05 to 0.16, P=0.286) for betaine, and the integrated discrimination index was 0.0019 (95% CI 0.000‐0.004, P=0.041) for choline and 0.001 (95% CI −0.001 to 0.003, P=0.219) for betaine. In NORVIT, the category‐free net reclassification index was 0.02 (95% CI −0.06 to 0.11, P=0.583) for choline and 0.14 (95% CI 0.06‐0.22, P=0.001) for betaine, and the integrated discrimination index was 0.0043 (95% CI 0.002‐0.007, P<0.001) for choline and 0.006 (95% CI 0.003‐0.009, P<0.001) for betaine.
Associations Between Dietary Intakes of Choline and Betaine and AF Risk
Only weak correlations between dietary intakes and plasma concentrations were found for choline (Spearman ρ=0.06 in HUSK and −0.05 in WECAC) and betaine (Spearman ρ=0.12 in both HUSK and WECAC) (Table 3). Dietary intake of choline was marginally associated with AF risk (pooled HR 1.29, 95% CI 1.01‐1.66, fifth versus first quintile) after adjustment for sex, age, BMI, diabetes mellitus, hypertension, and smoking, whereas no significant association was observed between dietary betaine and AF risk (Table 4).
Table 3.
Energy‐Adjusted Partial Correlation Coefficientsa Between Dietary Intakes and Plasma Concentrations of Choline and Betaine at Baseline in HUSK and WECAC
| Plasma Choline | Plasma Betaine | |
|---|---|---|
| HUSK (n=5916) | ||
| Total choline intake, mg/day | 0.06b | −0.01 |
| Betaine intake, mg/day | 0.04b | 0.12b |
| WECAC (n=1899) | ||
| Total choline intake, mg/day | −0.05b | −0.02b |
| Betaine intake, mg/day | −0.05b | 0.12b |
HUSK, Hordaland Health Study; WECAC, Western Norway Coronary Angiography Cohort.
Adjusted for sex and age (continuous).
P<0.05.
Table 4.
HRs (95% CIs) for AF Risk By Quintiles of Energy‐Adjusted Dietary Intakes of Choline and Betaine
| Quintiles of Dietary Intake | P Trend | |||||
|---|---|---|---|---|---|---|
| 1 (Low) | 2 | 3 | 4 | 5 (High) | ||
| HUSK (n=5950) | ||||||
| Total choline | ||||||
| Unadjusted | 1 | 1.15 (0.85‐1.56) | 1.28 (0.95‐1.73) | 1.29 (0.96‐1.74) | 1.48 (1.11‐1.98) | 0.006 |
| Adjusted for sex and age | 1 | 1.11 (0.82‐1.50) | 1.17 (0.87‐1.57) | 1.20 (0.89‐1.62) | 1.43 (1.07‐1.92) | 0.013 |
| Multivariable modela | 1 | 1.08 (0.80‐1.46) | 1.13 (0.84‐1.52) | 1.14 (0.85‐1.54) | 1.32 (0.99‐1.78) | 0.06 |
| Betaine | ||||||
| Unadjusted | 1 | 0.95 (0.71‐1.27) | 1.03 (0.78‐1.36) | 1.03 (0.78‐1.36) | 0.97 (0.73‐1.29) | 0.99 |
| Adjusted for sex and age | 1 | 0.90 (0.68‐1.20) | 1.02 (0.77‐1.35) | 0.97 (0.74‐1.29) | 0.95 (0.72‐1.27) | 0.95 |
| Multivariable modela | 1 | 0.94 (0.71‐1.25) | 1.06 (0.80‐1.40) | 1.00 (0.75‐1.33) | 0.99 (0.74‐1.32) | 0.90 |
| WECAC (n=1899) | ||||||
| Total choline | ||||||
| Unadjusted | 1 | 1.18 (0.76‐1.85) | 0.93 (0.58‐1.50) | 1.45 (0.94‐2.24) | 1.20 (0.76‐1.88) | 0.26 |
| Adjusted for sex and age | 1 | 1.13 (0.72‐1.77) | 0.94 (0.58‐1.51) | 1.38 (0.89‐2.12) | 1.31 (0.84‐2.07) | 0.13 |
| Multivariable modela | 1 | 1.12 (0.72‐1.77) | 0.92 (0.57‐1.48) | 1.34 (0.87‐2.07) | 1.23 (0.78‐1.93) | 0.24 |
| Betaine | ||||||
| Unadjusted | 1 | 1.76 (1.10‐2.82) | 1.55 (0.96‐2.52) | 1.57 (0.97‐2.54) | 1.47 (0.91‐2.40) | 0.32 |
| Adjusted for sex and age | 1 | 1.65 (1.03‐2.65) | 1.60 (0.98‐2.59) | 1.49 (0.92‐2.42) | 1.39 (0.85‐2.25) | 0.43 |
| Multivariable modela | 1 | 1.73 (1.08‐2.77) | 1.67 (1.03‐2.71) | 1.54 (0.95‐2.51) | 1.47 (0.90‐2.41) | 0.32 |
| Pooled resultsb | ||||||
| Total choline | ||||||
| Unadjusted | 1 | 1.16 (0.90‐1.49) | 1.17 (0.91‐1.51) | 1.34 (1.05‐1.71) | 1.39 (1.09‐1.78) | 0.004 |
| Adjusted for sex and age | 1 | 1.11 (0.86‐1.43) | 1.10 (0.85‐1.41) | 1.26 (0.98‐1.61) | 1.40 (1.10‐1.78) | 0.004 |
| Multivariable modela | 1 | 1.09 (0.85‐1.41) | 1.06 (0.82‐1.37) | 1.20 (0.94‐1.54) | 1.29 (1.01‐1.66) | 0.027 |
| Betaine | ||||||
| Unadjusted | 1 | 1.12 (0.88‐1.44) | 1.14 (0.90‐1.46) | 1.14 (0.90‐1.46) | 1.08 (0.84‐1.38) | 0.58 |
| Adjusted for sex and age | 1 | 1.06 (0.83‐1.36) | 1.14 (0.89‐1.45) | 1.08 (0.85‐1.38) | 1.05 (0.82‐1.34) | 0.71 |
| Multivariable modela | 1 | 1.11 (0.87‐1.42) | 1.18 (0.93‐1.51) | 1.12 (0.87‐1.42) | 1.10 (0.85‐1.41) | 0.51 |
AF indicates atrial fibrillation; BMI, body mass index; CI, confidence interval; HR, hazard ratio; HUSK, Hordaland Health Study; WECAC, Western Norway Coronary Angiography Cohort.
Models were adjusted for sex, age (continuous), BMI (continuous), diabetes mellitus (yes/no), hypertension (yes/no), and smoking (yes/no).
The results across the 2 cohorts were pooled using an inverse variance–weighted, fixed‐effect meta‐analysis.
Discussion
Principal Findings
In our study involving 3 prospective cohorts, higher plasma concentrations of choline and betaine were associated with an increased AF risk. The risk associations were independent of traditional AF risk factors including older age, male sex, higher BMI, hypertension, diabetes mellitus, and smoking. In contrast, dietary intake of total choline was marginally associated with AF risk, and betaine intake was not associated.
Previous Studies on Choline Metabolites and CVD Risk
Several studies have previously reported on the associations between systemic choline metabolites and CVD risk. A cross‐sectional analysis in HUSK suggested that high choline and low betaine plasma concentrations were associated with an unfavorable cardiovascular risk profile.31 A study among patients with established coronary heart disease reported that both high and low plasma betaine concentrations were associated with an increased risk of later coronary events and hospitalization for heart failure.32 In WENBIT patients with stable angina pectoris, elevated plasma choline levels were associated with an increased risk of acute myocardial infarction in nonsmoking patients.6
Regarding AF specifically, elevated plasma total homocysteine, a metabolite closely related to the choline oxidation pathway, has been associated with increased risk of AF recurrence following electrical cardioversion.33 In the Framingham Heart Study,12 no significant association between plasma betaine and risk of incident AF was reported, with age‐ and sex‐adjusted HR (95% CI) 1.03 (0.87‐1.22). In contrast, the corresponding pooled HR (95% CI) was 1.12 (1.06‐1.18) in our study. Several reasons may explain the discrepancy between the 2 findings. First, as the authors in the Framingham Heart Study stated, use of a strict threshold for corrected P values and limited power due to relatively small numbers of incident AF cases and participants may have masked moderate associations, probably including the betaine‐AF association. Second, the ascertainment of AF cases was heterogeneous. AF cases were ascertained by participant records in the Framingham Heart Study but by hospital discharge codes in our study, which possibly leads to different degrees of underestimation of new‐onset AF in the 2 studies. Third, the Framingham Heart Study used fasting plasma samples, whereas we used mostly nonfasting samples, which may somewhat contribute to the inconsistency.
Possible Mechanisms
The mechanisms behind the relationship of dietary choline and betaine with CVD risk remain elusive. One study from the United States suggested an inverse association between choline intake and risk of stroke, a major adverse event related to AF.34 However, a recent large meta‐analysis of observational data concluded that there was no relationship between dietary choline and betaine and CVD risk.35 Accordingly, in our study, the correlations between dietary and systemic choline and betaine were weak or absent, in line with previous studies.36 Moreover, we found a weak association of total choline intake, but no association of betaine intake, with incident AF. The positive relationships between systemic choline and betaine and AF risk are therefore more likely related to metabolic rather than to dietary factors.
First, the choline metabolic pathway is partially taking place inside the mitochondrion, and it is therefore interesting that experimental data in rodents suggest a causal link between increased mitochondrial oxidative stress and AF.37 Oxidative stress may impair betaine‐homocysteine methyltransferase, resulting in accumulation of circulating choline and betaine.38 Second, high plasma concentrations of choline and betaine have been associated with elevated concentrations of trimethylamine N‐oxide.39 This metabolite is related to microbiomic processing of dietary choline and to a lesser extent betaine, and systemic trimethylamine N‐oxide has been positively associated with adverse cardiovascular events.40 Third, systemic choline and betaine were shown to be related to lipid metabolism.41 Higher plasma choline may downregulate apolipoprotein A1, the major protein of high‐density lipoprotein cholesterol, which may consequently facilitate AF.42 However, associations between lipid‐related genetic variants and AF risk have recently been challenged.43 Moreover, the association between betaine and AF risk was less strong in patients with suspected stable angina pectoris than in the general population. This may due to complex interactions of choline oxidation with extensive use of medications among the WECAC patients.44 Fourth, intravascular volume status may alter the susceptibility to AF.45 Notably, betaine is an important osmolyte, ie, a regulator of cellular volume,4 suggesting that increased plasma levels could be associated with AF due to altered cellular osmotic stress. Fifth, the choline metabolism is an important determinant of methylation status in the liver, especially the flux over the betaine‐homocysteine methyltransferase enzyme.46 Several studies have suggested that AF is associated with methylation of genetic loci related to AF susceptibility.47, 48 Although any relationship between choline metabolism and methylation specifically in the myocardium is elusive, it is possible that the present findings reflect methylation status and epigenetic regulation relevant to AF development and persistence.
Strengths and Limitations
To the best of our knowledge, this is the first study examining associations of plasma and dietary choline and betaine with subsequent AF risk. The major strengths of this study include the large number of participants recruited from 3 independent cohorts with different health profiles, measurements of exposures both as plasma concentrations and dietary intakes, along with long‐term prospective follow‐up. However, the study has several limitations that merit attention. The ascertainment of AF was based on hospitalization data only, which implies that cases with asymptomatic AF without hospitalization might not have been included. In NORVIT, we had no baseline information on any previous AF, making it impossible to state whether a later diagnosis of AF was truly an incident event. However, the risk estimates in NORVIT were in general similar to those observed in HUSK and WECAC, making it less likely that the choline and betaine AF risk associations in NORVIT merely reflected persistent or recurrent AF present at baseline. Moreover, the within‐person reproducibility for neither plasma betaine nor choline is excellent.44, 49 Therefore, the risk associations explored based on baseline samples may be underestimated due to regression dilution bias.50
Conclusions
The current study indicates that higher plasma concentrations of choline and betaine are associated with an increased risk of subsequent AF, independent of traditional AF risk factors. Moreover, dietary intake of choline, but not betaine, is associated with the risk of AF. This suggests alterations in choline metabolism being implicated in AF pathogenesis.
Disclosures
None.
Acknowledgments
The authors thank Tomislav Dimoski at the Norwegian Institute of Public Health, Oslo, Norway for his contribution by developing the software necessary for obtaining data from Norwegian hospitals, conducting the data collection, and quality assurance of data in this project.
(J Am Heart Assoc. 2018;7:e008190 DOI: 10.1161/JAHA.117.008190.)29650710
References
- 1. Benjamin EJ, Chen PS, Bild DE, Mascette AM, Albert CM, Alonso A, Calkins H, Connolly SJ, Curtis AB, Darbar D, Ellinor PT, Go AS, Goldschlager NF, Heckbert SR, Jalife J, Kerr CR, Levy D, Lloyd‐Jones DM, Massie BM, Nattel S, Olgin JE, Packer DL, Po SS, Tsang TS, Van Wagoner DR, Waldo AL, Wyse DG. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129:837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rienstra M, McManus DD, Benjamin EJ. Novel risk factors for atrial fibrillation: useful for risk prediction and clinical decision making? Circulation. 2012;125:e941–e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ueland PM. Choline and betaine in health and disease. J Inherit Metab Dis. 2011;34:3–15. [DOI] [PubMed] [Google Scholar]
- 5. Patterson KY, Bhagwat SA, Williams JR, Howe JC, Holden JM, Zeisel SH, Dacosta KA, Mar M‐H. USDA Database for the Choline Content of Common Foods, Release 2 (2008). Beltsville, MD: USDA Nutrient Data Laboratory; 2015. [Google Scholar]
- 6. Schartum‐Hansen H, Pedersen ER, Svingen GF, Ueland PM, Seifert R, Ebbing M, Strand E, Bleie O, Nygard O. Plasma choline, smoking, and long‐term prognosis in patients with stable angina pectoris. Eur J Prev Cardiol. 2015;22:606–614. [DOI] [PubMed] [Google Scholar]
- 7. Dalmeijer GW, Olthof MR, Verhoef P, Bots ML, van der Schouw YT. Prospective study on dietary intakes of folate, betaine, and choline and cardiovascular disease risk in women. Eur J Clin Nutr. 2008;62:386–394. [DOI] [PubMed] [Google Scholar]
- 8. Bidulescu A, Chambless LE, Siega‐Riz AM, Zeisel SH, Heiss G. Usual choline and betaine dietary intake and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2007;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagata C, Wada K, Tamura T, Konishi K, Kawachi T, Tsuji M, Nakamura K. Choline and betaine intakes are not associated with cardiovascular disease mortality risk in Japanese men and women. J Nutr. 2015;145:1787–1792. [DOI] [PubMed] [Google Scholar]
- 10. Svingen GF, Ueland PM, Pedersen EK, Schartum‐Hansen H, Seifert R, Ebbing M, Loland KH, Tell GS, Nygard O. Plasma dimethylglycine and risk of incident acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2013;33:2041–2048. [DOI] [PubMed] [Google Scholar]
- 11. Svingen GF, Schartum‐Hansen H, Ueland PM, Pedersen ER, Seifert R, Ebbing M, Bonaa KH, Mellgren G, Nilsen DW, Nordrehaug JE, Oyen J, Nygard O. Elevated plasma dimethylglycine is a risk marker of mortality in patients with coronary heart disease. Eur J Prev Cardiol. 2015;22:743–752. [DOI] [PubMed] [Google Scholar]
- 12. Ko D, Riles EM, Marcos EG, Magnani JW, Lubitz SA, Lin H, Long MT, Schnabel RB, McManus DD, Ellinor PT, Ramachandran VS, Wang TJ, Gerszten RE, Benjamin EJ, Yin X, Rienstra M. Metabolomic profiling in relation to new‐onset atrial fibrillation (from the Framingham Heart Study). Am J Cardiol. 2016;118:1493–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vikse BE, Vollset SE, Tell GS, Refsum H, Iversen BM. Distribution and determinants of serum creatinine in the general population: the Hordaland Health Study. Scand J Clin Lab Invest. 2004;64:709–722. [DOI] [PubMed] [Google Scholar]
- 14. Refsum H, Nurk E, Smith AD, Ueland PM, Gjesdal CG, Bjelland I, Tverdal A, Tell GS, Nygard O, Vollset SE. The hordaland homocysteine study: a community‐based study of homocysteine, its determinants, and associations with disease. J Nutr. 2006;136:1731S–1740S. [DOI] [PubMed] [Google Scholar]
- 15. Ebbing M, Bleie O, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, Refsum H, Pedersen EK, Nygard O. Mortality and cardiovascular events in patients treated with homocysteine‐lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300:795–804. [DOI] [PubMed] [Google Scholar]
- 16. Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, Wang H, Nordrehaug JE, Arnesen E, Rasmussen K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–1588. [DOI] [PubMed] [Google Scholar]
- 17. Midttun O, Kvalheim G, Ueland PM. High‐throughput, low‐volume, multianalyte quantification of plasma metabolites related to one‐carbon metabolism using HPLC‐MS/MS. Anal Bioanal Chem. 2013;405:2009–2017. [DOI] [PubMed] [Google Scholar]
- 18. Holm PI, Ueland PM, Kvalheim G, Lien EA. Determination of choline, betaine, and dimethylglycine in plasma by a high‐throughput method based on normal‐phase chromatography‐tandem mass spectrometry. Clin Chem. 2003;49:286–294. [DOI] [PubMed] [Google Scholar]
- 19. Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53. [DOI] [PubMed] [Google Scholar]
- 20. Meyer K, Ueland PM. Targeted quantification of C‐reactive protein and cystatin c and its variants by immuno‐MALDI‐MS. Anal Chem. 2014;86:5807–5814. [DOI] [PubMed] [Google Scholar]
- 21. Igland J, Tell GS, Ebbing M, Nygård O, Vollset SE, Dimoski T. Cardiovascular disease in Norway 1994–2009: description of data and data quality. 2013.
- 22. Sulo G, Igland J, Vollset SE, Nygård O, Øyen N, Tell GS. Cardiovascular disease and diabetes mellitus in Norway during 1994–2009: CVDNOR—a nationwide research project. Nor Epidemiol. 2013;23:101–107. [Google Scholar]
- 23. Sulo G, Igland J, Nygard O, Vollset SE, Ebbing M, Tell GS. Favourable trends in incidence of AMI in Norway during 2001–2009 do not include younger adults: a CVDNOR project. Eur J Prev Cardiol. 2014;21:1358–1364. [DOI] [PubMed] [Google Scholar]
- 24. Andersen LF, Solvoll K, Johansson LR, Salminen I, Aro A, Drevon CA. Evaluation of a food frequency questionnaire with weighed records, fatty acids, and alpha‐tocopherol in adipose tissue and serum. Am J Epidemiol. 1999;150:75–87. [DOI] [PubMed] [Google Scholar]
- 25. Andersen LF, Veierod MB, Johansson L, Sakhi A, Solvoll K, Drevon CA. Evaluation of three dietary assessment methods and serum biomarkers as measures of fruit and vegetable intake, using the method of triads. Br J Nutr. 2005;93:519–527. [DOI] [PubMed] [Google Scholar]
- 26. Jarvis MJ, Tunstall‐Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health. 1987;77:1435–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207–112 [DOI] [PubMed] [Google Scholar]
- 29. Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S; discussion 1229S–1231S. [DOI] [PubMed] [Google Scholar]
- 31. Konstantinova SV, Tell GS, Vollset SE, Nygard O, Bleie O, Ueland PM. Divergent associations of plasma choline and betaine with components of metabolic syndrome in middle age and elderly men and women. J Nutr. 2008;138:914–920. [DOI] [PubMed] [Google Scholar]
- 32. Lever M, George PM, Elmslie JL, Atkinson W, Slow S, Molyneux SL, Troughton RW, Richards AM, Frampton CM, Chambers ST. Betaine and secondary events in an acute coronary syndrome cohort. PLoS One. 2012;7:e37883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Naji F, Suran D, Kanic V, Vokac D, Sabovic M. High homocysteine levels predict the recurrence of atrial fibrillation after successful electrical cardioversion. Int Heart J. 2010;51:30–33. [DOI] [PubMed] [Google Scholar]
- 34. Millard HR, Musani SK, Dibaba DT, Talegawkar SA, Taylor HA, Tucker KL, Bidulescu A. Dietary choline and betaine; associations with subclinical markers of cardiovascular disease risk and incidence of CVD, coronary heart disease and stroke: the Jackson Heart Study. Eur J Nutr. 2018;57:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meyer KA, Shea JW. Dietary choline and betaine and risk of CVD: a systematic review and meta‐analysis of prospective studies. Nutrients. 2017;9:711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abratte CM, Wang W, Li R, Axume J, Moriarty DJ, Caudill MA. Choline status is not a reliable indicator of moderate changes in dietary choline consumption in premenopausal women. J Nutr Biochem. 2009;20:62–69. [DOI] [PubMed] [Google Scholar]
- 37. Xie W, Santulli G, Reiken SR, Yuan Q, Osborne BW, Chen BX, Marks AR. Mitochondrial oxidative stress promotes atrial fibrillation. Sci Rep. 2015;5:11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martinez‐Vega R, Garrido F, Partearroyo T, Cediel R, Zeisel SH, Martinez‐Alvarez C, Varela‐Moreiras G, Varela‐Nieto I, Pajares MA. Folic acid deficiency induces premature hearing loss through mechanisms involving cochlear oxidative stress and impairment of homocysteine metabolism. FASEB J. 2015;29:418–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Obeid R, Awwad HM, Rabagny Y, Graeber S, Herrmann W, Geisel J. Plasma trimethylamine N‐oxide concentration is associated with choline, phospholipids, and methyl metabolism. Am J Clin Nutr. 2016;103:703–711. [DOI] [PubMed] [Google Scholar]
- 40. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients. 2013;5:3481–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alonso A, Yin X, Roetker NS, Magnani JW, Kronmal RA, Ellinor PT, Chen LY, Lubitz SA, McClelland RL, McManus DD, Soliman EZ, Huxley RR, Nazarian S, Szklo M, Heckbert SR, Benjamin EJ. Blood lipids and the incidence of atrial fibrillation: the multi‐ethnic study of atherosclerosis and the Framingham Heart Study. J Am Heart Assoc. 2014;3:e001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Norby FL, Eryd SA, Niemeijer MN, Rose LM, Smith AV, Yin X, Agarwal SK, Arking DE, Chasman DL, Chen LY, Eijgelsheim M, Engstrom G, Franco OH, Heeringa J, Hindy G, Hofman A, Lutsey PL, Magnani JW, McManus DD, Orho‐Melander M, Pankow JS, Rukh G, Schulz CA, Uitterlinden AG, Albert CM, Benjamin EJ, Gudnason V, Smith JG, Stricker BH, Alonso A. Association of lipid‐related genetic variants with the incidence of atrial fibrillation: the AFGen consortium. PLoS One. 2016;11:e0151932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Svingen GF, Schartum‐Hansen H, Pedersen ER, Ueland PM, Tell GS, Mellgren G, Njolstad PR, Seifert R, Strand E, Karlsson T, Nygard O. Prospective associations of systemic and urinary choline metabolites with incident type 2 diabetes. Clin Chem. 2016;62:755–765. [DOI] [PubMed] [Google Scholar]
- 45. Danelich IM, Lose JM, Wright SS, Asirvatham SJ, Ballinger BA, Larson DW, Lovely JK. Practical management of postoperative atrial fibrillation after noncardiac surgery. J Am Coll Surg. 2014;219:831–841. [DOI] [PubMed] [Google Scholar]
- 46. Strakova J, Gupta S, Kruger WD, Dilger RN, Tryon K, Li L, Garrow TA. Inhibition of betaine‐homocysteine S‐methyltransferase in rats causes hyperhomocysteinemia and reduces liver cystathionine β‐synthase activity and methylation capacity. Nutr Res. 2011;31:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhao G, Zhou J, Gao J, Liu Y, Gu S, Zhang X, Su P. Genome‐wide DNA methylation analysis in permanent atrial fibrillation. Mol Med Rep. 2017;16:5505–5514. [DOI] [PubMed] [Google Scholar]
- 48. Tao H, Shi KH, Yang JJ, Li J. Epigenetic mechanisms in atrial fibrillation: new insights and future directions. Trends Cardiovasc Med. 2016;26:306–318. [DOI] [PubMed] [Google Scholar]
- 49. Midttun O, Townsend MK, Nygard O, Tworoger SS, Brennan P, Johansson M, Ueland PM. Most blood biomarkers related to vitamin status, one‐carbon metabolism, and the kynurenine pathway show adequate preanalytical stability and within‐person reproducibility to allow assessment of exposure or nutritional status in healthy women and cardiovascular patients. J Nutr. 2014;144:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hutcheon JA, Chiolero A, Hanley JA. Random measurement error and regression dilution bias. BMJ. 2010;340:c2289. [DOI] [PubMed] [Google Scholar]


