Abstract
Background
Available health services data for individuals with peripheral artery disease (PAD) are often from studies of those eligible for or undergoing intervention. Knowledge of the frequency of care and mortality following an initial PAD diagnosis by setting (outpatient versus inpatient) is limited and represents an opportunity to provide new benchmark information.
Methods and Results
The purpose of this study was to characterize the frequency of care and mortality following an incident PAD diagnosis in the outpatient or inpatient setting using data from the ARIC (Atherosclerosis Risk in Communities) study cohort linked with Centers for Medicare and Medicaid Services fee‐for‐service claims data (2002–2012). Direct standardization was used to estimate age‐standardized rates of encounters and mortality. PAD was defined by billing code in any claim position. We observed 1086 incident PAD cases (873 outpatient, 213 inpatient). At 1 year after diagnosis, participants diagnosed in the outpatient setting had 2.15 (95% confidence interval [CI], 2.10–2.21) PAD‐related outpatient encounters per person‐year, and 6.4% (95% CI, 4.8–8.1) had a PAD‐related hospitalization. Conversely, participants diagnosed in the inpatient setting had 1.02 (95% CI, 0.94–1.10) PAD‐related outpatient encounters per person‐year, and 14.2% (95% CI, 9.3–18.7) had a PAD‐related rehospitalization. One‐year mortality was 7.1% (95% CI, 5.4–8.7) and 16.0% (95% CI, 11.0–21.1) among those diagnosed in outpatient and inpatient settings, respectively.
Conclusions
This study provides important data estimating frequency of care and mortality by the setting of initial PAD diagnosis. Individuals with PAD are frequent users of health care, and those diagnosed in the inpatient setting have high rates of rehospitalization and mortality.
Keywords: Medicare, mortality, peripheral artery disease, population science, utilization
Subject Categories: Peripheral Vascular Disease, Aging, Mortality/Survival, Epidemiology, Health Services
Clinical Perspective
What Is New?
Through a large population‐based cohort study linked with Medicare fee‐for‐services claims data, we provide accurate estimates of the frequency of care following a peripheral artery disease diagnosis by the location of the incident diagnosis in the outpatient or inpatient setting.
Individuals diagnosed in the inpatient setting have high rates of rehospitalization and mortality.
What Are the Clinical Implications?
Our findings provide a basis for the assessment of healthcare resource spending among peripheral artery disease patients and a comparison, by demographics and comorbid conditions, of disease trajectories associated with clinically manifest peripheral artery disease diagnosed in the inpatient or outpatient setting.
Peripheral artery disease (PAD) is a prevalent and disabling atherosclerotic disorder that disproportionately affects older adults.1, 2, 3, 4 Up to 25% of patients with symptomatic PAD may progress to limb‐threatening clinical manifestations that are associated with high healthcare costs and frequent PAD‐related procedures.5, 6, 7 In particular, hospitalization costs associated with PAD‐related revascularization and limb amputation procedures account for more than $11 billion annually in the United States and are similarly high in several European countries.8 Long‐term health outcomes, including cardiac events and mortality, following these procedures are poor.6, 9
Although the prognosis for patients following a PAD‐related procedure is well described,10, 11 little is known about the outpatient and inpatient clinical care and outcomes following an initial PAD diagnosis. In particular, postdiagnosis care for individuals with PAD—including initial diagnosis in the outpatient setting through clinic visits, admissions, and use of procedures—has not been described. Administrative claims data, which contain diagnostic and procedure codes from inpatient and outpatient encounters, provide an opportunity to estimate the frequency of PAD‐related care from the healthcare setting of first diagnosis through follow‐up care in both the outpatient and inpatient settings.
The primary objective of this study was to characterize the frequency of care for study participants following an incident PAD diagnosis in the outpatient or inpatient setting. Our secondary objective was to estimate mortality associated with an initial PAD diagnosis by setting of diagnosis (ie, outpatient or inpatient). To accomplish these aims, we used data from the biracial ARIC (Atherosclerosis Risk in Communities) study cohort12 linked with Centers for Medicare and Medicaid Services (CMS) claims data for the years 2000–2012.
Methods
Availability of data and material, as well as detailed policies for accessing ARIC data, can be found online ( https://www2.cscc.unc.edu/aric/).13
Study Population
The ARIC cohort study
The ARIC cohort, established to examine the etiology of atherosclerosis and its clinical manifestations, includes 15 792 participants (aged 45–64 years at baseline) enrolled between 1987 and 1989. The ARIC cohort was selected by probability sampling from 4 US communities: Washington County, Maryland; Forsyth County, North Carolina; the city of Jackson, Mississippi; and the suburb cities of Minneapolis, Minnesota.12 Other details about the study population have been published previously.12
Those eligible for inclusion in this study were ARIC participants enrolled continuously for at least 2 years in Medicare Parts A and B through a fee‐for‐service (FFS) plan from 2000 to 2012. Data were collected on cohort participants at 5 clinic examinations and through annual follow‐up telephone interviews. Information from ARIC visits 1 to 4, which occurred at 3‐year intervals and concluded in 1999, is included in the present study. Information collected through the final telephone interview before diagnosis was used to define comorbid conditions.
Linkage of cohort data with administrative claims
Data for ARIC cohort participants were linked with CMS claims for the years 1991–2012 using a finder file that included each participant's social security number, sex, and date of birth. A total of 14 899 Medicare‐eligible ARIC study participants were identified, of which 14 702 ARIC cohort identifiers (98.7% match) were matched successfully.
Information concerning ARIC study participant enrollment in FFS Medicare was obtained from monthly indicators of enrollment in Part A, Part B, and Medicaid buy‐in available from annual CMS Medicare Beneficiary Summary files. Continuous enrollment periods were created to indicate uninterrupted CMS Medicare FFS coverage, defined as enrollment in CMS Medicare Part A and Part B and lack of enrollment in a Medicare Advantage (health maintenance organization) plan. Enrollment status before 2000 was not considered for this analysis. Study participants with missing enrollment information and those with continuous and exclusive Medicare Advantage enrollment were excluded from the study. Given limited representation, participants aged <65 years and those of race other than black or white were excluded. For study participants with >1 continuous FFS enrollment period (n=349), the longest FFS period was selected to give the best opportunity to capture relevant claims.
Demographics and comorbidities
Participant race, sex, education level, and family income were self‐reported at the ARIC baseline visit (1987–1989). We used age at time of entry into the ARIC cohort and date of PAD healthcare event ascertained from the claims to calculate age at the time of the incident PAD event. Participant information regarding comorbid conditions was available from 4 ARIC clinic visits and annual telephone follow‐up surveys. Comorbidity status was determined at the time of diagnosis. Diabetes mellitus was defined as self‐reported history of physician‐diagnosed diabetes mellitus at any of the 4 clinic visits or annual telephone survey, usage of diabetes mellitus medication during the 2 weeks before a visit, fasting blood glucose level ≥126 mg/dL, or nonfasting blood glucose ≥200 mg/dL. Kidney failure was defined as an estimated glomerular filtration rate <15.0 mL/min per 1.73 m2 using the creatinine‐based CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) equation and data from the 4 clinic visits.14 Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg at any of the 4 clinic visits, use of antihypertensive medication during the 2 weeks before any of the clinic visits, or self‐report in the annual follow‐up interview. Hyperlipidemia was defined as total cholesterol ≥240 mg/dL at any of the 4 clinic visits or self‐report in the annual follow‐up interview. Smoking status was self‐reported at each clinic visit and is defined as any history (current and former smokers) or no history (never smokers) for the purposes of this study. Body mass index was calculated as weight in kilograms divided by height squared (in meters) derived at ARIC visit 4 (1996–1998). Obesity was defined as body mass index ≥30.0. History of coronary heart disease, stroke, and heart failure before incident PAD diagnosis was based on self‐report at baseline and adjudication of hospitalized events. Self‐rated health via self‐report questionnaire was defined as poor, fair, good, or excellent, and the lowest rating from any assessment until the time of PAD diagnosis was used. Adequate access to care was defined as any outpatient claim identified within 1 year of the incident PAD diagnosis date.
Ascertainment of PAD encounters in the claims
PAD‐related outpatient office visits were identified (1) from the physician claims (carrier) files as claims with PAD‐related diagnostic and billing codes for new and established office visits and preventive medicine visits and (2) from facility claims (outpatient) files as claims with PAD‐related codes for visits to Federally Qualified Healthcare centers. Hospitalizations (eg, inpatient visits and procedures) were identified from the Medicare Provider Analysis and Review records. PAD occurrence was defined using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) and Healthcare Common Procedure Coding System (HCPCS) adapted from previous PAD‐related administrative studies (Table S1).15, 16, 17 Relevant PAD codes in any listed position were counted for inpatient and outpatient visits. Provider specialty codes were used to identify outpatient visits to primary care providers and cardiology visits. All discharge ICD‐9‐CM codes associated with the incident PAD hospitalizations were grouped into categories of comorbid conditions using definitions provided by the CMS Chronic Conditions Data Warehouse. Claim position was examined because of concerns about potential upcoding, in which a PAD‐related code might be added to the end of a claim to increase billing.18
Cohort construction
An incident inpatient PAD diagnosis was defined as a hospitalization with a PAD code in any of the 25 diagnosis or procedure positions at any time during the study period. An outpatient PAD diagnosis was defined as ≥2 claims within 12 consecutive months with a PAD‐related ICD‐9‐CM; Current Procedural Terminology, Fourth Edition; HCPCS; or Federally Qualified Healthcare Revenue Center code in any of the 12 diagnosis positions or 6 procedure positions; the claims had to occur ≥1 day apart, and the incident date was defined as the date of the second claim.17 If a singular outpatient event preceded an inpatient event within 365 days, the event was classified as an inpatient PAD diagnosis with the incident event date as the inpatient date of discharge. Single PAD‐related outpatient events occurring with no PAD‐related hospitalizations or PAD‐related outpatient events within 365 days were not considered incident PAD events.
A cohort was constructed of ARIC participants enrolled in Medicare as FFS beneficiaries who had an incident inpatient or outpatient PAD diagnosis. PAD cases detected in the first 2 years of enrollment (ie, a 2‐year look‐back period) were excluded as prevalent cases to minimize misclassification of incident events.19 This 2‐year look‐back method has been shown to reduce misclassification of incidence of chronic diseases to <10%.19 In addition, FFS eligibility for at least 2 years after the incident diagnosis was required to allow for adequate follow‐up time. Consequently, the analytical study population included ARIC study participants with a PAD diagnosis from 2002 to 2010, with follow‐up extending through an administrative censoring date of December 31, 2012.
Statistical Analyses
Direct standardization was used to estimate age‐standardized rates of inpatient and outpatient encounters with 95% confidence intervals (CIs) following an initial PAD diagnosis. The denominator for rate estimates included cohort participants’ time in continuous FFS enrollment following an initial PAD diagnosis. Estimates were age‐standardized to reflect the age, race, and sex distribution of the 2005 Medicare population aged ≥67 years. Age categories for standardization included 67 to 69, 70 to 74, 75 to 79, and ≥80 years at the time of PAD diagnosis. Estimates were calculated by initial‐diagnosis setting (inpatient, outpatient) and within‐diagnosis setting in strata of race and sex. CIs that did not overlap were determined to represent different estimates, although no formal statistical tests were performed.
Analyses for time to initial hospitalization (for outpatient PAD diagnosis) or rehospitalization (for inpatient PAD diagnosis) accounted for death as a competing risk using the cumulative incidence function.20, 21 Estimates were calculated by initial‐ and within‐diagnosis settings stratified by race and sex. The end of follow‐up was determined by death, enrollment in Medicare Advantage, or the date of December 31, 2012 (end of the study observation period). Beneficiaries’ death dates were obtained from the Master Beneficiary Summary File. Propensity score models were used to adjust for confounding in mortality estimates using standardized mortality ratio weighting22; estimates for individuals diagnosed with PAD in the inpatient setting were weighted to reflect the distribution of covariates among individuals diagnosed with PAD in the outpatient setting. Covariates included age, sex, race, income, education, diabetes mellitus, smoking history, hyperlipidemia, hypertension, obesity, coronary heart disease, stroke, heart failure, kidney failure, self‐rated health, and adequate access to care in the year before diagnosis. The distribution of propensity scores was examined, and nonoverlapping propensity scores were trimmed from mortality analyses (n=21). All analyses were completed using SAS version 9.4 (SAS Institute). Written informed consent was obtained from all participants, and all ARIC field center institutional review boards approved the ARIC study.
Results
The final analytic sample included 11 652 ARIC participants with 86 228 person‐years of FFS enrollment time. The median length of the FFS enrollment period was 6.3 years. We observed 1086 incident diagnoses of PAD during the eligibility period (2002–2010), of which 873 (80.4%) were in the outpatient setting and 213 (19.6%) were in the inpatient setting. Table 1 describes participants’ demographics and comorbid conditions stratified by initial PAD diagnosis setting. Also included in Table 1 are similar data for ARIC participants who did not have a PAD diagnosis during the study observation period (n=10 566).
Table 1.
Characteristics of FFS Participants Without an Incident PAD Diagnosis (n=10 566) and Those With an Incident PAD Diagnosis in the Outpatient Setting (n=873) or the Inpatient Setting (n=213): ARIC, 2002–2010
| PAD Status | |||
|---|---|---|---|
| No PAD (n=10 566) | Outpatient Incident PAD (n=873) | Inpatient Incident PAD (n=213) | |
| Age at diagnosis, y, mean, SD | NA | 74.9 (4.9) | 74.4 (4.6) |
| Sex, female | 57.4 (56.4–58.3) | 57.6 (54.3–60.9) | 47.0 (40.1–53.9) |
| Race, black | 27.9 (27.1–28.8) | 27.3 (24.3–30.4) | 26.4 (20.6–32.9) |
| Median household income | |||
| Low (<$35 000) | 34.5 (33.6–35.4) | 53.8 (50.4–57.2) | 55.2 (48.2–62.0) |
| Mid ($35 000–$49 999) | 19.4 (18.7–20.2) | 19.2 (16.6–22.0) | 17.5 (12.6–23.2) |
| High (≥$50 000) | 46.1 (45.2–47.1) | 27.0 (24.1–30.1) | 27.3 (21.5–33.9) |
| Education, less than high school | 22.2 (21.4–23.0) | 28.2 (25.2–31.3) | 28.3 (22.3–34.9) |
| Diabetes mellitusa | 20.0 (19.2–20.7) | 47.1 (43.7–50.5) | 26.3 (20.5–32.7) |
| Smoking historyb | 66.4 (65.5–67.3) | 67.1 (63.9–70.2) | 74.7 (68.3–80.3) |
| Hyperlipidemiac | 70.9 (70.1–71.8) | 75.8 (72.9–78.6) | 73.2 (66.8–79.1) |
| Hypertensiond | 52.4 (51.4–53.4) | 87.5 (85.1–89.6) | 70.0 (63.3–76.0) |
| Obesitye | 39.8 (38.8–40.7) | 50.2 (46.8–53.5) | 50.0 (42.9–56.7) |
| History of CHDf | 11.6 (11.0–12.2) | 15.4 (13.0–17.9) | 24.9 (19.2–31.3) |
| History of strokeg | 8.3 (7.8–8.9) | 9.4 (7.5–11.5) | 17.4 (12.5–23.1) |
| History of heart failureh | 16.2 (15.5–16.9) | 17.4 (15.0–20.0) | 35.7 (29.3–42.5) |
| End‐stage renal diseasei | 0.7 (0.5–0.9) | 0.2 (0.0–0.8) | 0.9 (0.1–3.4) |
| Self‐rated health, poorj | 27.0 (26.1–27.8) | 27.6 (24.7–30.7) | 35.2 (28.8–42.0) |
Data are shown as percentage (95% confidence interval) except as noted. ARIC indicates Atherosclerosis Risk in Communities; CHD, coronary heart disease; FFS, fee‐for‐service; NA, not assessed; PAD, peripheral artery disease.
Diabetes mellitus is defined as self‐reported history of physician‐diagnosed diabetes mellitus at any of the 4 clinic visits, use of diabetes mellitus medication within 2 weeks before a visit, fasting glucose ≥126 mg/dL, or nonfasting blood glucose ≥200 mg/dL.
Smoking history is defined as any history or no history.
Hyperlipidemia is defined as total cholesterol ≥240 mg/dL.
Hypertension is defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or antihypertensive medication usage within 2 weeks before any of the 4 clinic visits.
Obesity is defined as body mass index ≥30.
History of CHD is defined as history of myocardial infarction, coronary revascularization during any time during follow‐up but before the incident PAD diagnosis date.
History of stroke is defined as prevalent or incident stroke before the incident PAD diagnosis date,
History of heart failure is defined as prevalent or incident heart failure before the incident PAD diagnosis date.
End‐stage renal disease is defined as an estimated glomerular filtration rate <15.0 mL/min/1.73 m2 using the CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) equation.
Self‐rated health is defined as poor, fair, good, or excellent.
Participants with a PAD diagnosis were more likely than those without a PAD diagnosis to have hypertension (84% versus 52%), diabetes mellitus (43% versus 20%), obesity (50% versus 40%), and an income of <$35 000 per year (54% versus 35%; Table S2). Characteristics by race and sex are presented for those diagnosed in the outpatient setting (Table S3) and the inpatient setting (Table S4).
Compared with those with incident PAD diagnosed in the outpatient setting, a larger proportion of study participants with incident PAD diagnosed in the inpatient setting reported a history of major circulatory system disorders, including coronary heart disease (25% versus 15%), stroke (17% versus 9%), and heart failure (36% versus 17%). Those with incident PAD diagnosed in the outpatient setting were more likely to be female (58% versus 47%) and have a history of diabetes mellitus (47% versus 26%) and hypertension (88% versus 70%) compared with those with incident PAD diagnosed in the inpatient setting. There was no difference in participant age at time of diagnosis between diagnosis settings. Although representing a small proportion of all study participants, those with an incident inpatient diagnosis were more likely to undergo an endovascular or surgical procedure (19.8% versus 6.0%). Of the 213 participants with an inpatient incident PAD diagnosis, 34 had an endovascular or surgical procedure at the time of diagnosis. Eight participants presented for limb amputation. Of the 873 diagnosed in the outpatient setting, 52 had an endovascular or surgical procedure at the time of diagnosis. No study participants presented for limb amputation in the outpatient setting.
Among the 213 participants diagnosed with PAD in the inpatient setting, 37 (17%) were hospitalized with a primary diagnosis of PAD (Table 2). A PAD‐related code was present in the first 3 or 5 positions, respectively, in 71 (33%) and 110 (52%) of the 213 incident hospitalizations. Participants diagnosed with PAD in the inpatient setting frequently had a concomitant code (Table S5) for ischemic heart disease (39%), diabetes mellitus (24%), chronic kidney disease (17%), heart failure (17%), and atrial fibrillation (12%). For 38% (81/213) of the incident PAD hospitalizations, the primary discharge diagnosis was a circulatory system–related condition (including PAD). Respiratory conditions (10%), digestive system diseases (9%), musculoskeletal diseases (7%), and neoplasms (7%) were the most common noncardiovascular disease primary discharge diagnoses.
Table 2.
Primary Diagnoses and Comorbid Conditions for Incident PAD Hospitalizations: ARIC Study, 2002–2010 (n=213)
| Primary Discharge Diagnosis Grouped by ICD‐9‐CM Chapter | Inpatient Incident PAD | |
|---|---|---|
| n | % | |
| Diseases of the circulatory system (390–459) | 81 | 38.0% |
| PAD‐related code in primary position | 37 | 17.4% |
| Non–circulatory system disorders (001–389, 580–999, V01–V89, E800–E999; procedures 00–99) | 132 | 62.0% |
| Comorbid conditions and proceduresa (ICD‐9‐CM code[s]) | ||
| Ischemic heart disease | 83 | 39.0% |
| Myocardial infarction | 7 | 3.3% |
| Atrial fibrillation | 26 | 12.2% |
| Heart failure | 36 | 16.9% |
| Stroke | 13 | 6.1% |
| Chronic kidney disease | 37 | 17.4% |
| Diabetes mellitus | 51 | 23.9% |
ARIC indicates Atherosclerosis Risk in Communities; ICD‐9‐CM, International Classification of Diseases, Ninth Revision, Clinical Modification; PAD, peripheral artery disease.
ICD‐9‐CM codes for each condition and procedure are listed in Table S3.
Encounters Following Outpatient Incident PAD Diagnosis
Among individuals diagnosed with PAD in the outpatient setting, median time to first subsequent PAD‐related outpatient encounter was 346 days. We observed an age‐standardized rate of 2.15 (95% CI, 2.10–2.21) PAD‐related outpatient encounters per person‐year over the course of study follow‐up (2002–2012). There were no differences in rates of PAD‐related outpatient encounters per person‐year by race or sex (Figure 1). The highest rate of all‐cause outpatient encounters was for primary care providers (age‐standardized rate: 6.36 visits per person‐year; 95% CI, 6.26–6.45; Figure 2). By comparison, the age‐standardized rate of outpatient cardiology encounters was 1.94 (95% CI, 1.89–2.0) visits per person‐year. Cardiology encounters per person‐year were lower among black patients (1.20; 95% CI, 1.12–1.28) compared with white patients (2.18; 95% CI, 2.12–2.25) and lower among female patients (1.48; 95% CI, 1.43–1.55) compared with male patients (2.27; 95% CI, 2.21–2.35; Figure 2).
Figure 1.
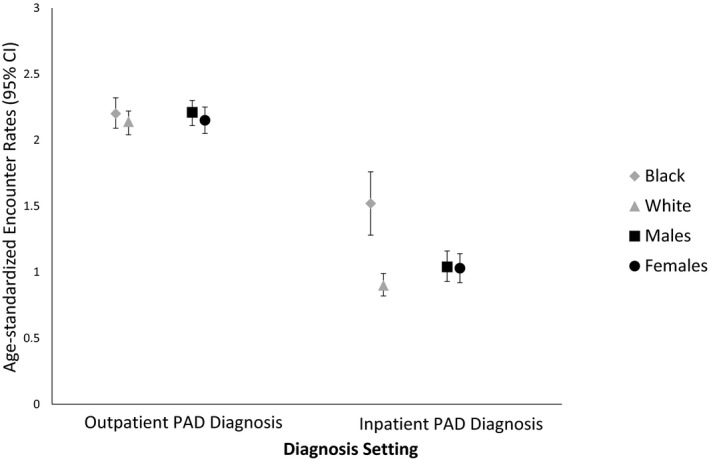
Age‐standardized rates of race‐ and sex‐specific peripheral artery disease (PAD)–related outpatient encounters (per person‐year) following a PAD diagnosis by diagnosis setting: ARIC (Atherosclerosis Risk in Communities) study, 2002–2012. Estimates are standardized to the 2005 Medicare population. CI indicates confidence interval.
Figure 2.
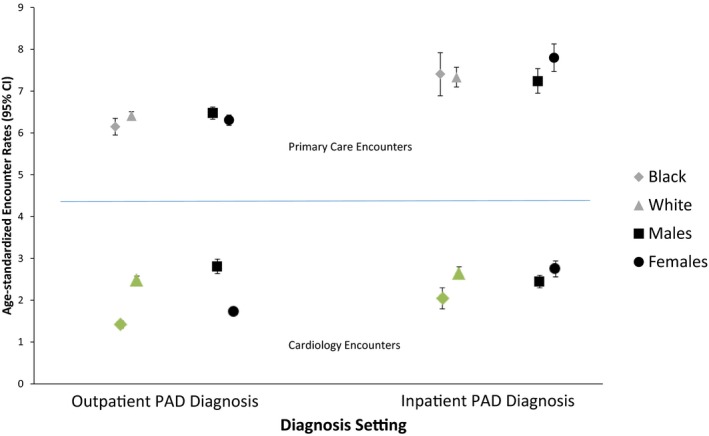
Age‐standardized rates of race‐ and sex‐specific outpatient primary care encounters (per person‐year) and cardiology encounters following a peripheral artery disease diagnosis by diagnosis setting: ARIC (Atherosclerosis Risk in Communities) study, 2002–2012. Estimates are standardized to the 2005 Medicare population. CI indicates confidence interval.
Cumulative 1‐year incidence of first PAD‐related and first all‐cause hospitalization among those with PAD diagnosed in the outpatient setting was 6.4% (95% CI, 4.8–8.1) and 32.2% (95% CI, 29.0–35.2), respectively (Table 3). Incidence of first PAD‐related hospitalization and all‐cause hospitalization did not differ by sex or race.
Table 3.
Age‐Standardizeda Cumulative Incidence of First PAD and All‐Cause Hospitalizations (95% CI) at 1 Year and 2 Years Following Incident PAD Diagnosis Among Participants Diagnosed in the Outpatient Setting: ARIC Study (2002–2012)
| Outpatient Incident PAD (n=873) | ||||
|---|---|---|---|---|
| First PAD Hospitalization At 1 y | First PAD Hospitalization At 2 y | First All‐Cause Hospitalization At 1 y | First All‐Cause Hospitalization At 2 y | |
| Overall | 6.4 (4.8–8.1) | 9.5 (7.6–11.5) | 32.2 (29.0–35.2) | 48.4 (44.9–51.6) |
| Black | 7.6 (4.2–10.9) | 10.7 (6.7–14.6) | 38.3 (31.8–44.2) | 52.6 (45.7–58.6) |
| White | 6.0 (4.1–7.8) | 9.1 (6.8–11.3) | 29.8 (26.2–33.3) | 46.8 (42.7–50.5) |
| Male | 9.0 (6.0–11.8) | 11.8 (8.4–15.0) | 35.3 (30.2–40.0) | 49.4 (44.0–54.3) |
| Female | 4.6 (2.7–6.4) | 7.9 (5.5–10.2) | 29.9 (25.8–33.8) | 47.6 (43.0–51.8) |
ARIC indicates Atherosclerosis Risk in Communities; CI, confidence interval; PAD, peripheral artery disease.
Standardized to reflect age, race, and sex distribution of the 2005 Medicare population; age strata included 67–69, 70–74, 75–79, and ≥80 y.
Encounters Following Inpatient Incident PAD Diagnosis
Individuals with PAD diagnosed in the inpatient setting had a median time to first PAD‐related outpatient visit of 849 days with an age‐standardized rate of 1.02 (95% CI, 0.94–1.10) PAD‐related outpatient encounters per person‐year (Figure 1). Median time to first PAD‐related visit was significantly lower (465 days) among those with a PAD diagnosis in the first 3 positions on the incident inpatient record. Age‐standardized rates of PAD‐related outpatient encounters per person‐year among black and white patients were 1.52 (95% CI, 1.28–1.74) and 0.90 (95% CI, 0.82–0.99), respectively. The highest rates of non–PAD‐related outpatient encounters were for primary care providers (7.43 visits per person‐year; 95% CI, 7.21–7.64; Figure 2). The age‐standardized rate of non–PAD‐related cardiology care was 2.29 (95% CI, 2.17–2.40) encounters per person‐year (Figure 2). Outpatient cardiology encounters per person‐year were 1.76 (95% CI, 1.53–2.0) among black patients and 2.32 (95% CI, 2.20–2.45) among white patients.
Cumulative incidence of first PAD‐related and first all‐cause rehospitalization at 1 year among those with PAD diagnosed in the inpatient setting was 14.2% (95% CI, 9.3–18.7) and 43.4% (95% CI, 36.3–49.7), respectively (Table 4). We did not observe significant differences in the rates of the cumulative incidence of all‐cause re‐hospitalizations by sex or race.
Table 4.
Age‐Standardizeda Cumulative Incidence of First PAD and All‐Cause Hospitalizations (95% CI) at 1 Year and 2 Years After Incident PAD Diagnosis Among Participants Diagnosed in the Inpatient Setting: ARIC Study (2002–2012)
| Inpatient Incident PAD (n=213) | ||||
|---|---|---|---|---|
| First PAD Rehospitalization at 1 y | First PAD Rehospitalization at 2 y | First All‐Cause Rehospitalization at 1 y | First All‐Cause Rehospitalization at 2 y | |
| Overall | 14.2 (9.3–18.7) | 20.0 (14.4–25.2) | 43.4 (36.3–49.7) | 61.3 (54.1–67.4) |
| Black | 21.4 (9.9–31.5) | 30.7 (17.4–41.9) | 55.1 (40.0–66.4) | 75.4 (60.9–84.5) |
| White | 11.6 (6.4–16.5) | 16.2 (10.2–21.9) | 38.7 (30.5–45.9) | 55.8 (47.2–63.0) |
| Male | 14.3 (7.6–20.6) | 21.7 (13.6–29.0) | 44.6 (34.6–53.1) | 60.5 (50.2–68.7) |
| Female | 13.9 (6.9–20.4) | 18.1 (10.1–25.3) | 41.8 (31.3–50.6) | 61.8 (51.0–70.2) |
ARIC indicates Atherosclerosis Risk in Communities; CI, confidence interval; PAD, peripheral artery disease.
Standardized to reflect age, race, and sex distribution of the 2005 Medicare population; Age strata included 67–69, 70–74, 75–79, and ≥80 y.
Mortality Following Incident PAD Diagnosis
Overall age‐standardized mortality following diagnosis of PAD in any setting was 8.9% (95% CI, 7.2–10.5) at 1 year after diagnosis (Table 5). Propensity score–adjusted mortality at 1 year (Figure 3) was 6.3% (95% CI, 4.8–7.7) and 14.7% (95% CI, 9.9–19.3) among those with incident outpatient and inpatient PAD diagnoses, respectively. Propensity score–adjusted mortality estimates were similar to age‐standardized estimates.
Table 5.
Mortality at 1 Year and 2 Years After Incident PAD Diagnosis in the Outpatient (n=873) or Inpatient (n=213) Setting: ARIC Study, 2002–2010
| 1‐y Mortality (95% CI) | 2‐y Mortality (95% CI) | |||
|---|---|---|---|---|
| Age‐Standardized Modela | Full Modelb | Age‐Standardized Modela | Full Modelb | |
| Overall (n=1086) | 8.9 (7.2–10.5) | 7.8 (6.1–9.2) | 16.6 (14.4–18.7) | 15.1 (13.0–17.2) |
| Incident outpatient (n=873) | 7.1 (5.4–8.7) | 6.3 (4.8–7.7) | 15.3 (12.9–17.6) | 13.7 (11.4–15.9) |
| Incident inpatient (n=213) | 16.0 (11.0–21.1) | 14.7 (9.9–19.3) | 21.5 (15.8–27.2) | 19.9 (14.3–25.5) |
ARIC indicates Atherosclerosis Risk in Communities; CI, confidence interval; PAD, peripheral artery disease.
Standardized to reflect age, race, and sex distribution of the 2005 Medicare population; age strata included 67–69, 70–74, 75–79, and ≥80 y.
Full adjusted model includes age, race, sex, income, education, diabetes mellitus, smoking, hyperlipidemia, hypertension, obesity, coronary heart disease, stroke, heart failure, end‐stage renal disease, disease severity, self‐rated health, and any‐cause office visit in 1 year before diagnosis. Propensity score models use 202 inpatient and 862 outpatient incident PAD events because of nonoverlap of 22 observations.
Figure 3.

Propensity score‐adjusted cumulative mortality by setting of peripheral artery disease (PAD) diagnosis. Red line indicates inpatient PAD diagnosis. Blue line indicates outpatient PAD diagnosis. The propensity score model includes age, race, sex, income, education, diabetes mellitus, smoking, hyperlipidemia, hypertension, obesity, coronary heart disease, stroke, heart failure, end‐stage renal disease, disease severity, any‐cause office visit within 1 year before diagnosis, and self‐rated health. The modeling strategy is described by Brookhart et al16.
Discussion
This study is among the first to examine the frequency of healthcare encounters following a PAD diagnosis and to present findings by setting of the initial PAD diagnosis. We found that in a population of Medicare beneficiaries, most incident PAD was initially diagnosed in the outpatient setting. PAD‐related hospitalizations at 1 year were rare among those with an incident outpatient PAD diagnosis. Outpatient encounters with primary care providers, cardiologists, and PAD‐related visits were relatively frequent compared with national rates published by the National Ambulatory Medical Care Survey, the only study identified that presented encounter rates in the Medicare population.23 Few differences for follow‐up encounters were observed in stratified analyses, although we did observe that black patients experienced lower rates of follow‐up cardiology encounters compared with white patients. Participants with an inpatient incident PAD diagnosis had a poorer short‐term prognosis and a higher rate of all‐cause rehospitalization than those diagnosed in the outpatient setting.
Characteristics and existing comorbid conditions of ARIC study participants with PAD identify risk factors similar to those noted in other studies of PAD24, 25; however, the rich covariate detail available in the ARIC study, from clinic visits, interviews, and self‐report questionnaires, provided supplemental data that are often unavailable to health services researchers. In comparison to a recent claims‐based study, we identified a higher prevalence of risk factors, such as diabetes mellitus (43% versus 16%) and hypertension (84% versus 54%),15 among those with PAD. We further confirmed the findings of a German study that identified an inverse association between income and PAD incidence26 and extended this association to our US‐based study. Socioeconomic disparities in the development of PAD are similar to disparities observed in the development of other cardiovascular diseases and suggest a need for more interventions targeted toward those with low incomes.
Although guidelines concerning appropriate timing of healthcare encounters following an incident PAD diagnosis have not been established, most guideline recommendations for care following a hospitalized cardiovascular event suggest contact with a provider within 6 weeks.27, 28 The observed long median time to first PAD‐related outpatient visit following an incident diagnosis in the inpatient setting (465 days among those with a PAD code in the first 3 positions) suggests that those with an inpatient PAD diagnosis may constitute a population of individuals not receiving appropriate postacute care.29
Although we observed that study participants with inpatient PAD diagnoses had few postdiagnosis PAD‐related outpatient encounters, our findings suggest that those with an incident PAD diagnosis in either setting are high users of outpatient healthcare services overall. In comparison to a census‐based Medicare‐aged general population (including all diagnoses) derived from a National Ambulatory Health Care Survey (NAMCS), participants with a PAD diagnosis identified in the present study had, on average, 2 times the number of postdiagnosis outpatient encounters per person‐year (31.6 versus 14.4).23 The ARIC study participants with a PAD diagnosis also experienced more than twice the rate of encounters per person‐year with primary care providers than the general population of the NAMCS, possibly because of the coexisting comorbidity burden.
Previous studies have suggested that diagnosis of CVD in the inpatient setting, compared with the outpatient setting, portends worse outcomes.30, 31 Results of the current study extend this observation to PAD. We found that mortality, as well as the proportion of patients with hospitalizations, was higher among participants with a PAD diagnosis in the inpatient setting compared with the outpatient setting. Although participants from these 2 settings of diagnosis differ significantly by a variety of comorbid conditions, and their respective risk profiles are likely drive much of the observed mortality gap, findings from our study suggest that identification of PAD in the outpatient setting is favorable with respect to existing comorbidity burden and survival. The mortality gap might be due to the early detection of the disease in the outpatient setting; however, claims data do not include information about disease severity—an important area of potential future research.
Of note, participants diagnosed with PAD in the inpatient setting in our study had mortality comparable to an analysis that examined death among ARIC participants after incident heart failure hospitalization.32 PAD, however, remains understudied, and awareness of it remains poor in comparison to other cardiovascular diseases.33 Although PAD can be a later stage disease that presents after other cardiac events such as myocardial infarction and stroke,34 only 25% of all participants diagnosed with PAD in our study had existing atherosclerotic cardiovascular disease. Consequently, many patients present with PAD as their first atherosclerotic cardiovascular disease event, and identifying these patients earlier and better managing progression of their disease could be beneficial in delaying myocardial infarction, stroke, and other major circulatory system disorders.35
Strengths and Limitations
While our observations regarding differences in postdiagnosis PAD‐related follow‐up care by diagnosis setting are meaningful, several factors could influence these findings. First, nearly half of the inpatient incident PAD diagnoses included a PAD code in the 11th position or beyond, implying that PAD was only a distal cause of the hospitalization or that the PAD code represented prevalent disease. Second, the greater prevalence of diabetes mellitus and hypertension—chronic comorbid conditions that may necessitate frequent outpatient follow‐up—identified among participants diagnosed with PAD in the outpatient versus the inpatient setting suggests that the 2 groups of study participants with a PAD diagnosis may utilize health care differently.36 Finally, our assessment of postdiagnosis follow‐up care was constrained for the inpatient setting by a relatively high proportion of participants discharged to a nursing home (13%), where follow‐up specialty care is unlikely.
Because this analysis was based on inpatient and outpatient provider visits among CMS Medicare enrollees in FFS programs, our estimates are not generalizable to Medicare beneficiaries enrolled in managed care programs, who have been reported to be healthier than those in FFS.37 Generalizability of this study is further limited because our estimates reflect cohort survivors in a closed cohort. Administrative claims data reflect billing practices, and diagnostic coding found in claims data is not always accurate in relation to documented diagnoses or procedures. Last, our comparison of mortality by setting is limited by challenges in our inability to control for residual confounding because of our use of administrative claims. Although supplementing the administrative data with cohort information should help mitigate confounding bias, we have no information for important clinical factors, such as anatomical disease or symptomatic severity.
An important strength of this study is the inclusion of outpatient as well as inpatient clinical encounters in the assessment of the frequency of encounters following an incident PAD diagnosis. Furthermore, prior studies have provided limited information about the healthcare encounters for individuals with PAD, stratified by the setting of healthcare delivery (inpatient versus outpatient). These estimates are age‐standardized, and look‐back periods for incidence are in accordance with recent recommendations,19 providing further strength for this study.
Conclusions
This study addresses an important gap in existing literature by providing accurate estimates of the frequency of care following a PAD diagnosis by the location of the incident diagnosis in the outpatient or inpatient setting. Individuals with PAD are high users of healthcare services and experience high postdiagnosis mortality. Our findings provide a basis for the assessment of healthcare resource spending among PAD patients and a comparison, by demographics and comorbid conditions, of disease trajectories associated with clinically manifest PAD diagnosed in the inpatient or outpatient setting.
Sources of Funding
CK was funded by the National Research Service Award predoctoral traineeship from the National Heart, Lung, and Blood Institute, sponsored by the Cardiovascular Epidemiology Program, University of North Carolina at Chapel Hill (5T32HL007055); a National Research Service Award predoctoral traineeship from the Agency for Healthcare Research and Quality, sponsored by the Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill (T32‐HS000032); and a grant from the Agency for Healthcare Research and Quality Research Dissertation Program (1R36HS023728‐01). The ARIC (Atherosclerosis Risk in Communities) study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Disclosures
None.
Supporting information
Table S1. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM); Current Procedural Terminology, Fourth Edition (CPT‐4); Healthcare Common Procedure Coding System (HCPCS); and Federally Qualified Healthcare Revenue Center (FQHC) Codes Used to Identify Peripheral Artery Disease and Provider Specialty Visits in Claims
Table S2. Characteristics of Fee‐For‐Service Participants Without an Incident Peripheral Artery Disease (PAD) Diagnosis (n=10 566) and Those With an Incident PAD Diagnosis in the Outpatient Setting (n=873) or Inpatient Setting (n=213): ARIC (Atherosclerosis Risk in Communities) Study, 2002–2010
Table S3. Characteristics of Fee‐For‐Service Participants With an Incident Peripheral Artery Disease Diagnosis in the Outpatient Setting (n=873) by Sex and Race: ARIC (Atherosclerosis Risk in Communities) Study, 2002–2010
Table S4. Characteristics of Fee‐For‐Service Participants With an Incident Peripheral Artery Disease Diagnosis in the Inpatient Setting (n=213) by Sex and Race: ARIC (Atherosclerosis Risk in Communities) Study, 2002–2010
Table S5. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) Codes Used to Identify Concomitant Diseases
Acknowledgments
The authors thank the staff and participants of the ARIC (Atherosclerosis Risk in Communities) study for their important contributions.
(J Am Heart Assoc. 2018;7:e007332 DOI: 10.1161/JAHA.117.007332.)29654201
References
- 1. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the national health and nutrition examination survey, 1999–2000. Circulation. 2004;110:738–743. [DOI] [PubMed] [Google Scholar]
- 2. Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S, Kori S. The effect of novel cardiovascular risk factors on the ethnic‐specific odds for peripheral arterial disease in the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2006;48:1190–1197. [DOI] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, deFerranti S , Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire VDK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 4. Sigvant B, Wiberg‐Hedman K, Bergqvist D, Rolandsson O, Andersson B, Persson E, Wahlberg E. A population‐based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg. 2007;45:1185–1191. [DOI] [PubMed] [Google Scholar]
- 5. Dormandy JA, Mahlr M, Ascady G, Balsano F, De Leeuw P, Blombery P, Bousser MG, Clement D, Coffman J, Deutshinoff A, Bletry O, Hampton J, Mahler F, Ohlin P, Rieger H, Stranden E, Turple AGG, Urai L, Verstraete M. Fate of the patient with chronic limb ischaemia. J Cardiovasc Surg. 1989;30:50–57. [PubMed] [Google Scholar]
- 6. Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, Steg G, Bhatt DL, Hirsch AT. Vascular hospitalization rates and costs in patients with peripheral artery disease in the United States. Circ Cardiovasc Qual Outcomes. 2010;3:642–651. [DOI] [PubMed] [Google Scholar]
- 7. Sigvant B, Lundin F, Wahlberg E. The risk of disease progression in peripheral arterial disease is higher than expected: a meta‐analysis of mortality and disease progression in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2016;51:395–403. [DOI] [PubMed] [Google Scholar]
- 8. Smolderen KG, Wang K, de Pouvourville G, Bruggenjurgen B, Rother J, Zeymer U, Parhofer KG, Steg PG, Bhatt DL, Magnuson EA; REACH Registry Investigators . Two‐year vascular hospitalisation rates and associated costs in patients at risk of atherothrombosis in France and Germany: highest burden for peripheral arterial disease. Eur J Vasc Endovasc Surg. 2012;43:198–207. [DOI] [PubMed] [Google Scholar]
- 9. Sigvant B, Hasvold P, Kragsterman B, Falkenberg M, Johansson S, Thuresson M, Nordanstig J. Cardiovascular outcomes in patients with peripheral arterial disease as an initial or subsequent manifestation of atherosclerotic disease: results from a Swedish nationwide study. J Vasc Surg. 2017;66:507–514.e1 [DOI] [PubMed] [Google Scholar]
- 10. Taylor SM, Kalbaugh CA, Blackhurst DW, Cass AL, Trent EA, Langan EM III, Youkey JR. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44:747–755; discussion 755–746. [DOI] [PubMed] [Google Scholar]
- 11. Taylor SM, Kalbaugh CA, Healy MG, Cass AL, Gray BH, Langan EM III, Cull DL, Carsten CG III, York JW, Snyder BA, Youkey JR. Do current outcomes justify more liberal use of revascularization for vasculogenic claudication? A single center experience of 1,000 consecutively treated limbs. J Am Coll Surg. 2008;206:1053–1062; discussion 1062–1054. [DOI] [PubMed] [Google Scholar]
- 12. Investigators TA . The atherosclerosis risk in communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13. ARIC study data. Available at: https://www2.cscc.unc.edu/aric/. Accessed March 3, 2015.
- 14. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF III, Feldman HI, Kusek JW, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nehler MR, Duval S, Diao L, Annex BH, Hiatt WR, Rogers K, Zakharyan A, Hirsch AT. Epidemiology of peripheral arterial disease and critical limb ischemia in an insured population. J Vasc Surg. 2014;60:686–695. [DOI] [PubMed] [Google Scholar]
- 16. Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalbaugh C, Kucharska‐Newton A, Wruck L, Lund JL, Selvin E, Matsushita K, Bengtson LGS, Heiss G, Loehr L. Peripheral artery disease prevalence and incidence estimated from both outpatient and inpatient settings among medicare fee‐for‐service beneficiaries in the atherosclerosis risk in communities (ARIC) study. J Am Heart Assoc. 2017;6: e003796 DOI: 10.1161/JAHA.116.003796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brunt CS. CPT fee differentials and visit upcoding under Medicare Part B. Health Econ. 2011;20:831–841. [DOI] [PubMed] [Google Scholar]
- 19. Griffiths RI, O'Malley CD, Herbert RJ, Danese MD. Misclassification of incident conditions using claims data: impact of varying the period used to exclude pre‐existing disease. BMC Med Res Methodol. 2013;13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley; 1980. [Google Scholar]
- 21. Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 22. Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes. 2013;6:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National ambulatory medical care survey summary tables, 2012. 2015.
- 24. Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC working group. Transatlantic inter‐society consensus. J Vasc Surg. 2000;31:S1–S296. [PubMed] [Google Scholar]
- 25. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter‐society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. 2007;45:S5–S67. [DOI] [PubMed] [Google Scholar]
- 26. Kroger K, Dragano N, Stang A, Moebus S, Mohlenkamp S, Mann K, Siegrist J, Jockel KH, Erbel R; Heinz Nixdorf Recall Study Investigator Group . An unequal social distribution of peripheral arterial disease and the possible explanations: results from a population‐based study. Vasc Med. 2009;14:289–296. [DOI] [PubMed] [Google Scholar]
- 27. Grady KL, Dracup K, Kennedy G, Moser DK, Piano M, Stevenson LW, Young JB. Team management of patients with heart failure: a statement for healthcare professionals from the cardiovascular nursing council of the American Heart Association. Circulation. 2000;102:2443–2456. [DOI] [PubMed] [Google Scholar]
- 28. Lloyd‐Jones D, Adams R, Brown T, Carnethon M, Dai S, De Simone G, Ferguson T, Ford E, Furie K, Gillespie C, Go AS, Greenlund K, Haase N, Halpern S, Ho P, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie PD, Stafford R, Thom T, Wasserthiel‐Smoller S, Wong N, Wylie‐Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Executive summary: heart disease and stroke statistics—2010 update. Circulation. 2010;121:948–954. [DOI] [PubMed] [Google Scholar]
- 29. Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. [DOI] [PubMed] [Google Scholar]
- 30. Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries: 1993–2007. Circ Cardiovasc Qual Outcomes. 2012;5:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ezekowitz JA, Kaul P, Bakal JA, Quan H, McAlister FA. Trends in heart failure care: has the incidence diagnosis of heart failure shifted from the hospital to the emergency department and outpatient clinics? Eur J Heart Fail. 2010;13:142–147. [DOI] [PubMed] [Google Scholar]
- 32. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the atherosclerosis risk in communities study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 33. Hirsch AT, Criqui MH, Treat‐Jacobson D, Regensteiner JC, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. J Am Med Assoc. 2001;286:1317–1324. [DOI] [PubMed] [Google Scholar]
- 34. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, Goto S, Liau CS, Richard AJ, Rother J, Wilson PWF. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. J Am Med Assoc. 2006;295:180–189. [DOI] [PubMed] [Google Scholar]
- 35. Criqui MH, Ninomiya JK, Wingard DL, Ji M, Fronek A. Progression of peripheral arterial disease predicts cardiovascular disease morbidity and mortality. J Am Coll Cardiol. 2008;52:1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sheehan P, Edmonds M, Januzzi JLJ, Regensteiner JC, Sanders L, Sykes M. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341. [DOI] [PubMed] [Google Scholar]
- 37. Landon BE, Zaslavsky AM, Bernard SL, Cioffi MJ, Cleary PD. Comparison of performance of traditional medicare vs managed care. J Am Med Assoc. 2004;291:1744–1752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM); Current Procedural Terminology, Fourth Edition (CPT‐4); Healthcare Common Procedure Coding System (HCPCS); and Federally Qualified Healthcare Revenue Center (FQHC) Codes Used to Identify Peripheral Artery Disease and Provider Specialty Visits in Claims
Table S2. Characteristics of Fee‐For‐Service Participants Without an Incident Peripheral Artery Disease (PAD) Diagnosis (n=10 566) and Those With an Incident PAD Diagnosis in the Outpatient Setting (n=873) or Inpatient Setting (n=213): ARIC (Atherosclerosis Risk in Communities) Study, 2002–2010
Table S3. Characteristics of Fee‐For‐Service Participants With an Incident Peripheral Artery Disease Diagnosis in the Outpatient Setting (n=873) by Sex and Race: ARIC (Atherosclerosis Risk in Communities) Study, 2002–2010
Table S4. Characteristics of Fee‐For‐Service Participants With an Incident Peripheral Artery Disease Diagnosis in the Inpatient Setting (n=213) by Sex and Race: ARIC (Atherosclerosis Risk in Communities) Study, 2002–2010
Table S5. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) Codes Used to Identify Concomitant Diseases


