Abstract
Background
We sought to assess the risk of acute kidney injury (AKI) and mortality associated with intensive systolic blood pressure reduction in acute intracerebral hemorrhage.
Methods and Results
Patients with acute intracerebral hemorrhage had spontaneous cause and symptom onset within 24 hours. We excluded patients with structural causes, coagulopathy, thrombocytopenia, and preexisting end‐stage renal disease. We defined AKI using the Acute Kidney Injury Network criteria. Chronic kidney disease status was included in risk stratification and was defined by Kidney Disease Outcomes Quality Initiative staging. Maximum systolic blood pressure reduction was defined over a 12‐hour period and dichotomized using receiver operating characteristic curve analysis. Descriptive statistics were done using independent sample t tests, χ2 tests, and Mann‐Whitney U tests, whereas multivariable logistic regression analysis was used to evaluate for predictors for AKI and mortality. A total of 448 patients with intracerebral hemorrhage met inclusion criteria. Maximum systolic blood pressure reduction was dichotomized to 90 mm Hg and found to increase the risk of AKI in patients with normal renal function (odds ratio, 2.1; 95% confidence interval, 1.19–3.62; P=0.010) and chronic kidney disease (odds ratio, 3.91; 95% confidence interval, 1.26–12.15; P=0.019). The risk of AKI was not significantly different in normal renal function versus chronic kidney disease groups when adjusted for demographics, presentation characteristics, and medications associated with AKI. AKI positively predicted mortality for patients with normal renal function (odds ratio, 2.41; 95% confidence interval, 1.11–5.22; P=0.026) but not for patients with chronic kidney disease (odds ratio, 3.13; 95% confidence interval, 0.65–15.01; P=0.154).
Conclusions
These results indicate that intensive systolic blood pressure reduction with a threshold >90 mm Hg in patients with acute intracerebral hemorrhage may be an independent predictor for AKI.
Keywords: acute kidney injury, autoregulation, blood pressure, chronic kidney disease, hypertension, intracerebral hemorrhage, kidney, renal disease
Subject Categories: Intracranial Hemorrhage, Hypertension, Mortality/Survival
Clinical Perspective
What Is New?
In a large cohort of patients with acute spontaneous intracerebral hemorrhage who received intensive blood pressure reduction, we studied the relationship between maximum blood pressure reduction in the first 12 hours and clinical outcomes during hospital stay, focusing on acute kidney injury and in‐hospital death.
What Are the Clinical Implications?
In the first 12 hours, systolic blood pressure reduction of >90 mm Hg may increase risk of acute kidney injury and in‐hospital mortality, even in patients without previous kidney dysfunction.
Treatment goals that avoid acutely exceeding this amount of blood pressure reduction may protect against iatrogenically inducing acute kidney injury.
Development of acute kidney injury was strongly predictive of in‐hospital mortality.
Elevated acute blood pressure has been associated with hematoma expansion in patients with intracerebral hemorrhage (ICH).1, 2, 3 However, the ideal target for systolic blood pressure (SBP) reduction, particularly for patients presenting with extremely high SBP (≥220 mm Hg), remains unclear. The multicenter randomized INTERACT 2 (Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage Trial) showed a trend suggesting intensive SBP reduction targeted to <140 mm Hg leads to improved clinical outcome.4 As a result, this goal was adopted by the American Heart Association/American Stroke Association recommendations for acute ICH management.5 However, this blood pressure goal has been disputed by the more recent ATACH 2 (Antihypertensive Treatment of Acute Cerebral Hemorrhage 2) trial that compared an intensive SBP goal (<140 mm Hg) to a standard SBP goal (140–179 mm Hg) and found no improvement in primary outcome.6 Moreover, the ATACH 2 trial reinforced a growing body of evidence7 that suggested that intensive SBP reduction might lead to higher rates of adverse renal events.
Intensive blood pressure reduction can induce multiorgan ischemia, particularly in the heart and kidneys.8, 9, 10, 11 The identification and determination of risk factors for acute kidney injury (AKI) is particularly important because of its association with death or major disability after ICH.12 This identification is particularly important in patients with chronic kidney disease (CKD) who may be even more vulnerable to AKI.13, 14
In this study, we sought to evaluate the association between intensive SBP reduction and AKI in patients with acute ICH, the role of CKD in predicting AKI, and the role of decreased renal function in clinical outcome after ICH.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Data Collection
Institutional review board approval was obtained for the conduct of a prospective cohort study evaluating functional outcomes in adult spontaneous nontraumatic patients with ICH at a tertiary‐level university hospital. Informed consent was waived with institutional review board approval. All data were prospectively collected as per hospital registry protocol for acute ICH and retrospectively reviewed for accuracy by blinded neurologists (N.G., Y.K., and A.K.). Consecutive patients with ICH were initially identified by International Classification of Diseases (ICD−9) code, which spanned a 4‐year period, from January 2, 2011 to December 26, 2015. Inclusion criteria were as follows: spontaneous cause for ICH, adult age (≥18 years old), and symptom onset within 24 hours. Exclusion criteria were as follows: nonspontaneous causes of ICH (including traumatic ICH, metastatic hemorrhagic lesion, ICH resulting from venous sinus thrombosis, and ICH resulting from underlying vascular lesion), supratherapeutic international normalized ratio in the setting of prehospital anticoagulation or coagulopathy (threshold international normalized ratio ≥1.7), thrombocytopenia (platelets <50 000/mm3), prehospital end‐stage renal disease, and inadequate serum creatinine (SCr) recordings.
All patients with ICH were initially admitted to the intensive care unit. As per hospital protocol, patients were treated with continuous nicardipine infusion or intravenous pushes of enalapril, hydralazine, labetalol, or oral clonidine to reach a goal SBP <140 mm Hg during the first 24 hours after admission.
Demographic characteristics, medical history, premorbid Modified Rankin Scale scores, admission laboratory values, and baseline radiological and clinical parameters were prospectively collected. Baseline clinical severity was documented with National Institutes of Health Stroke Scale (NIHSS) scores by certified physicians. Clinical outcome end points included Modified Rankin Scale score at discharge, hospital length of stay, in‐hospital mortality, presence of AKI, and progression of AKI to end‐stage renal disease.
Blood Pressure Evaluation
SBP and diastolic blood pressure (DBP) were recorded hourly during intensive care unit admission. SBP and DBP were retrospectively collected for the first 12 hours after admission. Maximum SBP reduction (the difference between maximum and minimum SBP) and maximum DBP reduction (the difference between maximum and minimum DBP) were calculated.
AKI Evaluation and Resolution
To assess renal function, daily SCr was tracked over the first 3 days after admission and at discharge. AKI was defined and staged according to Acute Kidney Injury Network guidelines: either a minimum increase in SCr of ≥0.3 mg/dL or >150% of baseline SCr.15 Subjects without ≥2 SCr readings within the first 48 hours of admission were excluded. Urine output was not included in defining or staging AKI in this study because of limited data. Resolution of AKI was determined using discharge SCr and defined as discharge SCr being less than or equal to the lowest SCr recorded in the first 3 days of admission and an SCr improvement of ≥0.3 mg/dL.
Iatrogenic Nephrotoxin Evaluation
Potential iatrogenic nephrotoxic sources administered within the first 3 days of hospitalization were also evaluated and included the following: loop diuretics, thiazides, NSAIDs, antibiotics (including piperacillin/tazobactam, vancomycin, sulfamethoxazole/trimethoprim, penicillins, ampicillin, acyclovir, fluoroquinolones, and aminoglycosides), angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptor blockers, hyperosmolar agents (mannitol and hypertonic saline), and contrast from computed tomographic angiogram.
Elevated Admission SCr Evaluation: CKD Versus Prerenal AKI
Because of inaccuracy of self‐reported renal history, CKD and staging were calculated using admission SCr values and estimated glomerular filtration rate (eGFR). CKD stage was defined according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative staging guidelines.16 The algorithm in Figure 1 was used to differentiate between prerenal AKI and CKD in patients who presented with elevated admission SCr.
Figure 1.
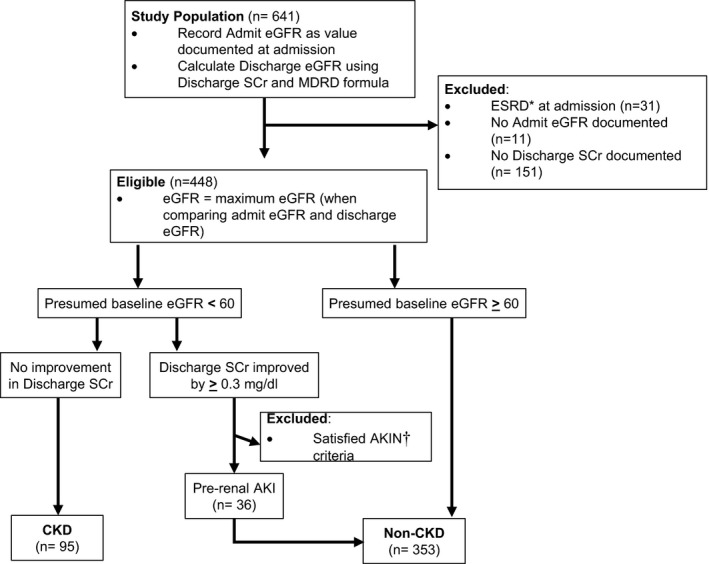
Determination of chronic kidney disease (CKD) vs prerenal acute kidney injury (AKI). The compositions of the CKD group vs the normal prehospital renal function group were determined by using estimated glomerular filtration rate (eGFR) values recorded at the time of admission and eGFR values calculated from the discharge serum creatinine (SCr) by means of the Modification of Diet in Renal Disease (MDRD) formula. The baseline eGFRs in the CKD range were monitored for improvement at discharge to screen for prerenal AKI. Those determined to have prerenal AKI were defined as having normal prehospital renal function. Subjects without discharge SCr values could not be evaluated for prerenal AKI, and admit eGFR was presumed baseline. AKIN indicates Acute Kidney Injury Network; ESRD, end‐stage renal disease.
Values for eGFR and SCr were examined at both admission and discharge. Both admission and discharge eGFR values were calculated using the Modification of Diet in Renal Disease formula from admission and discharge SCr, respectively. Patients were classified as having normal prehospital renal function if they had normal SCr limits, as delineated by the National Kidney Foundation (upper limits, 1.2 mg/dL for women and 1.4 mg/dL for men), and had baseline eGFR ≥60 mL/min per 1.73 m2 at admission.
We considered patients with prerenal AKI on admission to have normal renal function. However, we made several assumptions to differentiate between CKD and prerenal AKI in patients with elevated admission SCr. First, we assumed that “baseline eGFR” corresponded to the lowest SCr values, whether this was admission or discharge SCr. Second, prerenal AKI was assumed if SCr exceeded the normal range at admission but then improved by ≥0.3 mg/dL by discharge or if a patient's Kidney Disease Outcomes Quality Initiative CKD stage improved from admission to the time of discharge. To be considered prerenal injury, subjects’ SCr values could not meet the AKI criteria previously delineated. Third, if a patient did not have a discharge SCr value, then prerenal AKI could not be assessed and the eGFR recorded at admission was assumed to represent baseline.
Subjects with a baseline eGFR <60 mL/min per 1.73 m2 and who did not meet the inclusion criteria for prerenal AKI were classified as having CKD. CKD was classified into stages 1 to 5, as per Kidney Disease Outcomes Quality Initiative classifications. This classification was incomplete, however, because we were unable to assess persistence of renal function at ≥3 months. Patients undergoing hemodialysis or with eGFR values ≤15 mL/min per 1.73 m2 at admission were excluded from this study.
Statistical Analysis
We described our cohort using descriptive statistics and then compared the AKI versus non‐AKI cohorts using independent sample t tests, χ2 tests, and Mann‐Whitney U tests, as appropriate. Odds ratios (ORs) with 95% confidence intervals (CIs) are presented as measures of effect size. The relationship between intensive SBP reduction and AKI risk was determined first by using a receiver operating characteristic curve analysis to obtain an SBP cutoff value that maximized sensitivity and specificity (Figure 2). The obtained criterion was then used as the threshold to create a dichotomous variable for maximum SBP reduction. AKI risk assessment was also evaluated on the basis of renal function status on admission (normal prehospital renal function versus CKD) and followed logistic regression analyses; CIs were used to compare the difference of the log odds between the 2 groups. Predicted probabilities from the regression models were also used to obtain the area under the receiver operating characteristic curve for each model. To assess for the impact of AKI during hospitalization on mortality, multivariable logistic regression analyses adjusting for patient age, ICH volume, location, intraventricular hemorrhage, and admission NIHSS were used. P<0.05 was considered statistically significant. One‐sample Kolmogorov‐Smirnov test was used to evaluate for normal distribution of predictor variables. SPSS, version 22, was used for all statistical analyses.
Figure 2.
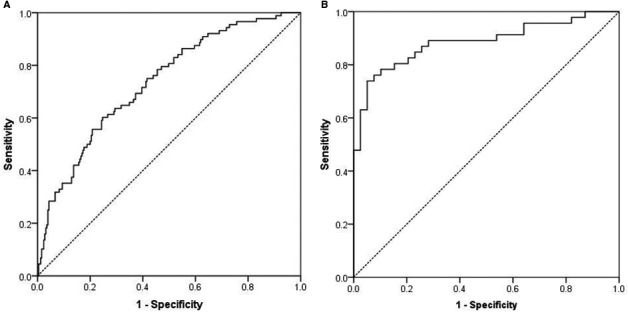
Receiver operating characteristic (ROC) curve analyses of the predictive power of systolic blood pressure (SBP) range on acute kidney injury (AKI) occurrence. A, The ROC curve for subjects with normal prehospital renal function demonstrated an SBP range ≥90 mm Hg and was associated with increased risk of AKI. B, The ROC curve for the chronic kidney disease group also showed an increased risk of AKI at the same SBP range threshold of 90 mm Hg.
Results
Study Population
A total of 641 patients with ICH were evaluated. Of these patients, 448 met inclusion criteria and could be evaluated for renal variables. Of the patients, 139 (31%) developed in‐hospital AKI. The AKI and non‐AKI groups did not significantly differ in terms of sex, body mass index, or medical history, but they did exhibit significant differences in mean age, race, ICH admission volume, history of hyperlipidemia, CKD, admission glucose levels, clinical severity at admission, presence of subcortical locations for ICH, and admission SBP and DBP (Table 1). Of those who developed AKI, 85.1% met the Acute Kidney Injury Network criteria for stage 1 AKI, whereas criteria for stages 2 and 3 were met by 9.7% and 5.2%, respectively (Table 2). AKI resolution could not be assessed for all subjects who developed AKI, but of the 106 with the appropriate laboratory data, 50.9% did meet our criteria for AKI resolution.
Table 1.
Baseline Characteristics of the Study Population Stratified by Presence of AKI During Hospitalization
| Variable | Total Sample (N=448) | AKI Group (n=139) | Non‐AKI Group (n=309) | P Value | Effect Size, Cohen's d or OR (95% CI) |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 62.0±14.0 | 58.5±13.0 | 61.8±13.5 | 0.015 | d=0.3, small |
| BMI, kg/m2 | 29.0±7.4 | 29.6±6.8 | 28.8±7.6 | 0.274 | |
| Male sex | 359 (56.0) | 83 (59.7) | 168 (54.4) | 0.292 | |
| Race | 0.002 | ||||
| White | 88 (19.2) | 13 (9.4) | 73 (23.6) | ||
| Black | 274 (61.2) | 101 (72.7) | 173 (56.0) | ||
| Hispanic | 81 (18.1) | 24 (17.3) | 7 (18.4) | ||
| Asian | 5 (1.1) | 0 | 5 (1.6) | ||
| Other | 2 (0.4) | 1 (0.7) | 1 (0.3) | ||
| Medical history | |||||
| Alcohol use | 136/425 (32.0) | 36 (27.7) | 100 (33.9) | 0.206 | |
| Smoking | 149/421 (35.4) | 39 (30.2) | 110 (37.7) | 0.141 | |
| Hypertension | 387/443 (87.4) | 124 (90.5) | 263 (85.9) | 0.182 | |
| Coronary artery disease | 53/443 (12.0) | 16 (11.8) | 37 (12.1) | 0.931 | |
| Congestive heart failure | 29/443 (6.5) | 12 (8.8) | 17 (5.5) | 0.197 | |
| Hyperlipidemia | 136/436 (31.2) | 31 (23.1) | 105 (34.8) | 0.016 | OR, 0.6 (95% CI, 0.4–0.9) |
| Statin therapy preinjury | 116/425 (27.3) | 37 (28.0) | 79 (27.0) | 0.819 | |
| History of stroke | 99/443 (22.3) | 27 (19.9) | 72 (23.5) | 0.402 | |
| Chronic kidney diseasea | 95/448 (21.2) | 50 (36.0) | 45 (14.6) | <0.001 | OR, 3.3 (95% CI, 2.1–5.3) |
| Diabetes mellitus | 163/443 (36.8) | 50 (36.8) | 113 (36.8) | 0.993 | |
| Antiplatelet therapy preinjury | 144/429 (33.6) | 43 (32.3) | 101 (34.1) | 0.716 | |
| Anticoagulant therapy preinjury | 13/431 (3.0) | 3 (2.3) | 10 (3.4) | 0.537 | |
| Admission variables | |||||
| Glucose, mg/dL | 152.6±75.7 | 168.1±75.9 | 147.6±81.1 | 0.012 | d=0.3 |
| INR | 1.1±0.4 | 1.1±0.2 | 1.1±0.5 | 0.785 | |
| Platelet count | 227.3±75.6 | 231.9±74.9 | 226.4±68.3 | 0.445 | |
| mRS score at admission, median (quartile 1–3) | 0 (0) | 0 (0) | 0 (0) | 0.140 | |
| NIHSS score at admission, median (quartile 1–3), points | 7.5 (15) | 14 (21) | 7 (13) | <0.001 | |
| ICH score | 1.5±1.4 | 1.8±1.4 | 1.3±1.1 | <0.001 | d=0.4 |
| Admission hematoma volume, cm3 | 14.0±17.9 | 15.5±17.8 | 12.0±14.0 | 0.045 | d=0.2 |
| Intracerebral hemorrhage location | 0.039 | ||||
| Basal ganglia | 168/447 (37.6) | 58 (41.7) | 110 (35.7) | ||
| Thalamus | 84/447 (18.8) | 30 (21.6) | 54 (17.5) | ||
| Pons | 24/447 (5.4) | 11 (7.9) | 13 (4.2) | ||
| Cerebellar | 37/447 (8.3) | 11 (7.9) | 26 (8.4) | ||
| Lobar/cortical | 123/447 (27.5) | 23 (17.3) | 99 (32.1) | ||
| Centrum semiovale | 3/447 (0.7) | 1 (0.7) | 2 (0.6) | ||
| Other | 8/447 (1.8) | 4 (2.9) | 4 (1.3) | ||
| Subcortical | 324/445 (72.8) | 112 (81.2) | 212 (69.1) | 0.008 | OR, 1.9 (95% CI, 1.2–3.2) |
| Admission heart rate, beats/min | 89.6±22.1 | 90.8±24.0 | 88.0±20.4 | 0.197 | |
| Admission systolic blood pressure, mm Hg | 182.6±39.9 | 201.1±42.6 | 180.7±37.5 | <0.001 | d=0.5 |
| Admission diastolic blood pressure, mm Hg | 103.4±27.6 | 116.4±31.0 | 101.7±26.2 | <0.001 | d=0.5 |
Data are given as mean±SD or number/total (percentage) unless otherwise indicated. AKI indicates acute kidney injury; BMI, body mass index; CI, confidence interval; ICH, intracerebral hemorrhage; INR, international normalized ratio; mRS, Modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; OR, odds ratio.
Extrapolated by Modification of Diet in Renal Disease equation using admission serum creatinine and glomerular filtration rate and defined by Kidney Disease Outcomes Quality Initiative staging guidelines.
Table 2.
Outcomes and Complications of the Study Population Stratified by Presence of AKI During Hospitalization
| Variable | Total Sample (N=448) | AKI Group (n=139) | Non‐AKI Group (n=309) | P Value | Effect Size, Cohen's d or OR (95% CI) |
|---|---|---|---|---|---|
| Renal injury characteristics | |||||
| AKIN stage 1 | 114/448 (25.4) | 114/134 (85.1) | NA | ||
| AKIN stage 2 | 13/448 (2.9) | 13/134 (9.7) | NA | ||
| AKIN stage 3 | 7/448 (1.6) | 7/134 (5.2) | NA | ||
| AKI resolved | NA | 54/106 (50.9) | NA | ||
| Renal failure during hospitalization | 17/447 (3.8) | 15 (10.8) | 2 (0.6) | <0.001 | OR, 18.5 (95% CI, 4.2–82.1) |
| External ventricular drain | 90/447 (20.1) | 34 (24.6) | 56 (18.1) | 0.113 | |
| Nicardipine | 295/440 (67.0) | 103 (75.7) | 192 (63.2) | 0.009 | OR, 1.8 (95% CI, 1.2–2.9) |
| Vasopressor use | 15/447 (3.4) | 12 (8.7) | 3 (1.0) | <0.001 | OR, 9.7 (95% CI, 2.7–35.0) |
| Hemicraniectomy | 39/446 (8.7) | 11 (8.0) | 28 (9.1) | 0.699 | |
| Respiratory failure | 170/448 (37.9) | 78 (56.1) | 92 (29.8) | <0.001 | OR, 3.0 (95% CI, 2.0–4.6) |
| Hematoma expansion | 50/299 (16.7) | 13 (16.5) | 37 (16.8) | 0.941 | |
| Intraventricular hemorrhage | 208/445 (46.7) | 80 (58.4) | 128 (41.6) | 0.001 | OR, 2.0 (95% CI, 1.3–3.0) |
| Disposition | <0.001 | ||||
| Home | 121/447 (27.1) | 33 (23.7) | 88 (28.6) | ||
| Rehabilitation | 137/447 (30.6) | 29 (20.9) | 108 (35.1) | ||
| Skilled nursing facility | 87/447 (19.5) | 19 (13.7) | 68 (22.1) | ||
| Hospice | 10/447 (2.2) | 3 (2.2) | 7 (2.3) | ||
| Death | 92/447 (20.6) | 55 (39.6) | 37 (12) | ||
| Poor dispositiona | 191/447 (42.7) | 77 (55.4) | 114 (37.0) | <0.001 | OR, 2.1 (95% CI, 1.4–3.2) |
| Length of stay, d | 10.2±11.3 | 11.6±12.9 | 11.8±11.5 | 0.878 | |
| NIHSS score at hospital discharge, median (quartile 1–3), points | 5 (2–20.3) | 13 (3–42) | 5 (1–13) | <0.001 | |
| mRS score at hospital discharge, median (quartile1–3) | 4 (3) | 4 (3) | 3 (2) | <0.001 | |
| Death | 92/448 (20.5) | 55 (39.6) | 37 (12.0) | <0.001 | OR, 4.8 (95% CI, 3.0–7.8) |
Data are given as number/total (percentage) or mean±SD unless otherwise indicated. AKI indicates acute kidney injury; AKIN, Acute Kidney Injury Network; CI, confidence interval; mRS, Modified Rankin Scale; NA, not applicable; NIHSS, National Institute of Health Stroke Scale; OR, odds ratio.
Poor disposition: disposition to skilled nursing facility, hospice, or death.
A comparison of nephrotoxic agents received during the first 4 days after admission showed significant differences between the AKI and non‐AKI groups for administration of vancomycin (P=0.003), piperacillin/tazobactam (P=0.012), ampicillin (P=0.018), fluoroquinolones (P=0.004), and ACEIs (P=0.001) and contrast from computed tomographic angiogram testing within the first 24 hours of hospitalization (P=0.005) (Table S1). Almost all immediate blood pressure parameters were higher in AKI subgroup, with the exception of minimum SBP and mean DBP (Table S2).
Baseline CKD was significantly different in patients who developed in‐hospital AKI. Of the subjects who developed AKI, 36.0% had preexisting CKD, whereas only 14.6% of the patients in the non‐AKI group had preexisting CKD (P<0.001) (Table 1). Because this divergence represented an association of interest, further risk analyses included stratification of baseline renal function.
AKI Risk on the Basis of Blood Pressure
Logistic regression models using in‐hospital AKI as the clinical outcome were adjusted for age, race, ICH volume, intraventricular hemorrhage, admission NIHSS score, and use of piperacillin/tazobactam, vancomycin, fluoroquinolones, and ACEIs (Table 3). In the normal prehospital renal function group, a maximum SBP reduction >90 mm Hg was associated with increased odds of AKI (OR, 2.08; 95% CI, 1.19–3.62; P=0.010). Model accuracy for the normal prehospital renal function group was 78.4%, with an area under the receiver operating characteristic curve of 0.73 (95% CI, 0.67–0.79). This SBP range was also associated with increased odds of AKI in the CKD group (OR, 3.91; 95% CI, 1.26–12.15; P=0.019). Model accuracy for the CKD model was 76.7%, with an area under the receiver operating characteristic curve of 0.86 (95% CI, 0.78–0.93). The impact of maximum SBP reduction >90 mm Hg was not significantly different between groups on the basis of the overlapping 95% CIs of the OR.
Table 3.
Multivariable Logistic Regression Analysis Predicting Development of AKI in Patients With and Without Preexisting CKD
| Variable | Normal Prehospital Renal Function Group (n=353) | CKD Group (n=95) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age | 0.98 | 0.96–1.00 | 0.140 | 1.01 | 0.97–1.06 | 0.628 |
| White race | 0.68 | 0.34–1.33 | 0.259 | 0.22 | 0.05–1.01 | 0.051 |
| Hematoma volume at admission | 1.00 | 0.98–1.02 | 0.764 | 1.02 | 0.97–1.07 | 0.417 |
| Intraventricular hemorrhage | 1.37 | 0.76–2.47 | 0.292 | 0.94 | 0.25–3.51 | 0.923 |
| NIHSS score at admission | 1.03 | 1.00–1.06 | 0.065 | 1.07 | 1.01–1.14 | 0.024 |
| CTA at admission | 0.69 | 0.37–1.27 | 0.229 | 0.39 | 0.07–2.18 | 0.280 |
| Piperacillin/tazobactam | 1.52 | 0.56–4.14 | 0.412 | 1.01 | 0.16–6.19 | 0.995 |
| Vancomycin | 0.75 | 0.31–1.82 | 0.527 | 3.30 | 0.64–17.05 | 0.154 |
| Fluoroquinolones | 2.23 | 1.08–4.59 | 0.030 | 1.54 | 0.29–8.11 | 0.610 |
| ACEI | 0.45 | 0.25–0.80 | 0.007 | 1.52 | 0.33–6.95 | 0.586 |
| Maximum systolic blood pressure reduction >90 mm Hga | 2.08 | 1.19–3.62 | 0.010 | 3.91 | 1.26–12.15 | 0.019 |
| Accuracy, % | 78.4 | 76.7 | ||||
| AUC | 0.73 | 0.67–0.79 | 0.86 | 0.78–0.93 | ||
ACEI indicates angiotensin‐converting enzyme inhibitor; AKI, acute kidney injury; AUC, area under the receiver operating characteristic curve; CI, confidence interval; CKD, chronic kidney disease; CTA, computed tomographic angiogram with contrast; NIHSS, National Institute of Health Stroke Scale; OR, odds ratio.
Maximum systolic blood pressure reduction, defined as the difference between the maximum systolic value and the minimum systolic value over the initial 12 hours of hospital admission.
Two of the parameters adjusted for in the logistic regression models also emerged as predictive of in‐hospital AKI. ACEI use emerged as a protectant against AKI but only in subjects with normal prehospital renal function. Admission NIHSS was a predictor of AKI for only those with CKD at admission. Maximum SBP reduction dichotomized at >90 mm Hg emerged as a consistent predictor of AKI for patients with both normal renal function (OR, 2.08; 95% CI, 1.19–3.62; P=0.010) and CKD (OR, 3.91; 95% CI, 1.26–12.15; P=0.019).
Mortality Risk
Adjusted logistic regression models, stratified by CKD status, were performed to assess mortality risk (Table 4). CKD status did play a role for AKI association with in‐hospital mortality. For patients with normal prehospital renal function, AKI was positively associated with higher odds of in‐hospital mortality (OR, 2.41; 95% CI, 1.11–5.22; P=0.026). Model accuracy for the normal prehospital renal function group was 90.2% with an area under the receiver operating characteristic curve of 0.89 (95% CI, 0.84 – 0.94). However, the association between AKI and in‐hospital mortality was not statistically significant in patients with CKD (P=0.154). Model accuracy for the CKD model was 89.3% with an area under the receiver operating characteristic curve of 0.96 (95%CI, 0.92–1.00). For patients with normal prehospital renal function, other predictors of mortality included age, admission hematoma volume, and admission NIHSS. For patients with CKD, predictors of mortality included admission hematoma volume and NIHSS (Table 4). A strong interaction (Wald, 45.544; df=1; P<0.001) of renal function on the association of admission NIHSS with mortality was identified.
Table 4.
Multivariable Logistic Regression Analysis Predicting In‐Hospital Mortality in Patients With and Without Preexisting CKD
| Variable | Normal Prehospital Renal Function Group (n=353) | CKD Group (n=95) | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age | 1.04 | 1.01–1.07 | 0.010 | 0.99 | 0.93–1.05 | 0.682 |
| Hematoma volume at admission | 1.02 | 1.00–1.05 | 0.012 | 1.11 | 1.02–1.21 | 0.022 |
| Subcortical location | 1.58 | 0.59–4.32 | 0.373 | 2.72 | 0.30–24.73 | 0.375 |
| Intraventricular hemorrhage | 1.49 | 0.66–3.36 | 0.334 | 1.69 | 0.34–8.34 | 0.521 |
| NIHSS score at admission | 1.16 | 1.11–1.21 | <0.001 | 1.14 | 1.06–1.24 | 0.001 |
| Acute kidney injury during hospitalization | 2.41 | 1.11–5.22 | 0.026 | 3.13 | 0.65–15.01 | 0.154 |
| Accuracy, % | 90.2 | 89.3 | ||||
| AUC | 0.89 | 0.84–0.94 | 0.96 | 0.92–1.00 | ||
AUC indicates area under the receiver operating characteristic curve; CI, confidence interval; CKD, chronic kidney disease; NIHSS, National Institute of Health Stroke Scale; OR, odds ratio.
Discussion
Current randomized‐controlled clinical trials and international recommendations acknowledge the importance of reducing SBP in acute ICH but focus on specific SBP targets without considering systemic organ autoregulation17 or providing recommendations for patients who present with SBPs >220 mm Hg.5 The ATACH 2 trial complicated the benefit of intensive SBP reduction in acute ICH by demonstrating a significant increase in adverse renal events in patients randomized to intensive antihypertensive therapy.6 We investigated this association and found that aggressive SBP reduction may play a role in the development of in‐hospital AKI. This became clinically relevant because AKI was an independent predictor of in‐hospital mortality in patients with normal prehospital renal function.
AKI Risk and Autoregulation
This study revealed that a threshold “maximum SBP reduction” of 90 mm Hg significantly predicted AKI regardless of preexisting CKD. Although other trials, such as SPRINT (Systolic Blood Pressure Intervention Trial), have also found significant increases in AKI on the basis of intensive SBP reduction7 and secondary analysis of the ATACH trial18 and supplementary analysis of the ATACH 2 trial6 showed greater incidence of AKI in patients treated with intensive SBP control, this is the first trial to define a threshold value for SBP reduction (ie, maximum SBP reduction) in ICH that is associated with in‐hospital AKI.
Pathological changes in renal and cerebral autoregulation may explain our findings. A rightward shift in cerebral autoregulation may occur because of both chronic hypertension19 and stroke.20 Although the results of INTERACT 2 appeared to contradict the long‐standing belief that intensive SBP control would exacerbate perihematomal ischemia,4 cautious and stepwise blood pressure reduction has long been recommended in the management of malignant hypertension21, 22 and hypertensive encephalopathy.8, 10, 23 The current American Heart Association/American Stroke Association guidelines do not offer recommendations on SBP reduction in patients presenting with SBPs >220 mm Hg. Our findings suggest that this may be appropriate and that patients who present with SBPs >230 mm Hg may actually be at risk for AKI if intensive SBP reduction is initiated.
The negative effects of aberrant autoregulation likely extend beyond the brain. The most prevalent risk factor for ICH is chronic hypertension,24, 25 which also causes a rightward shift in the renal autoregulation curve26 and increases the threshold value of mean arterial pressure needed to maintain renal perfusion.27, 28 Drastic and rapid lowering of blood pressure may also shift the renal autoregulation curve rightward9 and create a new less robust limit, where even nonhypotensive or normotensive blood pressures decrease renal blood flow and exacerbate renal injury.
The extent of autoregulatory shifts may correlate with the severity of hypertension,29 but they are unpredictable, making absolute blood pressure targets potentially inappropriate. Using a threshold SBP range as a guide may offer the adjustment needed when treating patients with different, possibly unknown, baseline blood pressures. For example, pursuing the current suggested goal of <140 mm Hg may harm a patient whose autoregulation threshold has adjusted rightward, but a goal range allows variability in proportion to presentation, regardless of chronic hypertension or presenting SBPs >220 mm Hg.
Impact of CKD on AKI Risk
We also sought to evaluate the role of baseline renal function on AKI risk and clinical outcome. CKD severity and eGFR are known risk factors for AKI,13, 14 and AKI that occurs with preexisting CKD has been shown to increase the risk of end‐stage renal disease and death.13 Nevertheless, we did not find baseline renal function to be an independent predictor for in‐hospital AKI or death. The absence of CKD as a determinant in AKI is consistent with current treatment guidelines that target hypertensive emergencies in the absence of ICH, such as hypertensive encephalopathy, and indicates that antihypertensive therapy does benefit patients with CKD.30 Similarly, secondary analysis using the INTERACT 2 cohort demonstrated that the effects of blood pressure reduction were comparable across different eGFRs, despite the association between decreased renal function and higher mortality.12
AKI and Risk of Mortality
Our study supported the increased risk of mortality in ICH associated with patients with AKI found in other recent ICH studies.12, 31 The pathophysiological characteristics linking this relationship between AKI and poor outcomes in patients with ICH have proved difficult to define because of other confounding variables, such as older age,32 and numerous complications caused by AKI, including uremia, acid‐base disturbance, fluid overload, and electrolyte imbalance.33 The global decreased perfusion leading to AKI may also lead to systemic end organ damage, but studies have shown that the association between AKI and mortality persisted even after adjustment for these comorbidities.34
However, although we found that AKI increased mortality risk in patients with normal prehospital renal function, this association was not maintained in patients with CKD. We surmise that patients with CKD may be preconditioned to have greater resilience against mortality associated with AKI because of their diminished renal function. In addition, although CKD is associated with higher mortality, this mortality has been found to increase exponentially with more severe CKD stages 4 and 5,35 which only pertained to 17.1% of our patients with CKD.
Limitations
This study has several limitations. The assumptions necessary to differentiate between CKD and prerenal AKI in patients presenting with elevated SCr precipitated our primary methodological shortcomings. First, we defined CKD by estimating GFR using the Modification of Diet in Renal Disease formula, which relies heavily on SCr. In addition to these values being estimations, this also impedes differentiating prerenal AKI from intrarenal AKI superimposed on CKD. Formulas for calculating eGFR from SCr are limited in accuracy, classifying ≈63% to 69% of patients correctly, with the Modification of Diet in Renal Disease equation being biased toward age but exhibiting the lowest mean bias of all formulas.36 By using eGFR rather than observed GFR, we may have underestimated GFR and overestimated the number of patients with CKD.37 Obtaining eGFR data throughout length of stay would be more ideal and should be explored in future trials. Second, we were unable to include the time component (duration ≥3 months) that defines CKD, because of lack of available data. However, the ease of calculating eGFR over observed GFR is not unique to this study and offers some standardization compared with potentially unreliable chart documentation.
Our definition of AKI and AKI resolution also had limitations. Admission SCr values were always present. However, subsequent SCr (on days 2 and 3) and discharge SCr were less consistently available and made it impossible for us to analyze these patients for AKI or AKI resolution. Therefore, although our AKIs are likely true‐positive values, the true incidence of AKI may have been underestimated.
Methodological differences between our retrospective analysis and the larger gold standard trials, INTERACT 24 and ATACH 2,6 also may limit our conclusions and applications. Our sample size was modest, retrospectively evaluated, and nonrandomized, with a significant number of patients removed because of inadequate renal monitoring. This may have introduced bias. However, the data were collected prospectively, with unbiased standardized treatments. Our onset time for initiating intensive SBP treatment (within 24 hours) also differed when compared with INTERACT 2 (6 hours) and ATACH 2 trial (4.5 hours). Although this may have improved the clinical efficacy of intensive SBP reduction by reducing the probability of hematoma expansion, the role of delayed intensive SBP reduction on renal function is unclear, with no clear guidelines.5 In addition, the duration of review for blood pressure measurements was shortened in our study. However, this is likely less consequential because as most patients are tightly monitored for antihypertensive medications or placed on continuous infusions, blood pressure variability and elevations would likely be controlled by the twelfth hour of hospitalization. Finally, in our study, functional outcome and mortality were evaluated at hospital discharge instead of 3 months. However, our primary clinical outcome noted was in‐hospital mortality, where patients would have the largest probability of death.38
Finally, certain variables, including blood pressure recordings and use of nephrotoxic agents, were documented retrospectively, as we have previously described,39 and the nephrotoxicity of our antihypertensive regimen was not independently evaluated. The lack of randomization and limitation of this study to a single center may have introduced bias, which was apparent in the nonheterogeneous distribution of nephrotoxic agents: patients with AKI were more likely to be given piperacillin/tazobactam, vancomycin, fluoroquinolone, and ampicillin and less likely to be given an ACEI or undergo contrast from computed tomographic angiogram. However, none of these agents, with the exception of fluoroquinolones and ACEIs for patients with normal renal function, proved to be an independent predictor for AKI, and all variables were collected by investigators blinded to study outcomes.
Conclusion
Although antihypertensive therapy is one of the mainstays of ICH treatment, it comes with risks. Our results demonstrate that a maximum SBP reduction threshold of >90 mm Hg may increase the likelihood of in‐hospital AKI, regardless of baseline renal function. Such findings may support the use of a targeted stepwise reduction in SBP rather than a targeted absolute value for patients who present with SBPs >230 mm Hg. Although antihypertensive treatment may interfere with hemodynamic autoregulation, future study is necessary to fully characterize the pathophysiological characteristics behind intensive SBP reduction and AKI. Our results also indicate that AKI is predictive of in‐hospital mortality in patients with normal renal function. Rethinking current antihypertensive management in the context of these risks could reduce morbidity and mortality in patients with ICH.
Disclosures
None.
Supporting information
Table S1. Nephrotoxic Agents and Interventions in AKI and Non‐AKI Subjects
Table S2. Blood Pressure Parameters in Patients Who Developed In‐Hospital Acute Kidlney Injury
(J Am Heart Assoc. 2018;7:e008439 DOI: 10.1161/JAHA.117.008439.)29654207
References
- 1. Ohwaki K, Yano E, Nagashima H, Hirata M, Nakagomi T, Tamura A. Blood pressure management in acute intracerebral hemorrhage. Stroke. 2004;35:1364–1367. [DOI] [PubMed] [Google Scholar]
- 2. Anderson CS, Huang Y, Wang JG, Arima H, Neal B, Peng B, Heeley E, Skulina C, Parsons MW, Kim JS, Tao QL, Li YC, Jiang JD, Tai LW, Zhang JL, Xu E, Cheng Y, Heritier S, Morgenstern LB, Chalmers J; INTERACT Investigators . Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol. 2008;7:391–399. [DOI] [PubMed] [Google Scholar]
- 3. Tsivgoulis G, Katsanos AH, Behrouz R, Hafeez S, Mutgi SA. Author Response: Tsivgoulis G, Katsanos AH. Intensive blood pressure reduction in acute intracerebral hemorrhage: A meta‐analysis. Neurology. 2015;84:2464–2465. [DOI] [PubMed] [Google Scholar]
- 4. Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, Lindley R, Robinson T, Lavados P, Neal B, Hata J, Arima H, Parsons M, Li Y, Wang J, Heritier S, Li Q, Woodward M, Simes RJ, Davis SM, Chalmers J; INTERACT2 Investigators . Rapid blood‐pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–2365. [DOI] [PubMed] [Google Scholar]
- 5. Hemphill JC, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology . Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
- 6. Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, Moy CS, Silbergleit R, Steiner T, Suarex JI, Toyoda K, Wang Y, Yamamoto H, Yoon BW; ATACH‐2 Trial Investigators and Neurological Emergency Treatment Trials Network . Intensive blood‐pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375:1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. SPRINT Research Group , Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356:411–417. [DOI] [PubMed] [Google Scholar]
- 9. Varon J, Marik PE. Clinical review: the management of hypertensive crises. Crit Care. 2003;7:374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia JY Jr, Vidt DG. Current management of hypertensive emergencies. Drugs. 1987;34:263–278. [DOI] [PubMed] [Google Scholar]
- 11. Jansen PAF, Schulte BPM, Meyboom RHB, Gribnau FWJ. Antihypertensive treatment as a possible cause of stroke in the elderly. Age Ageing. 1986;15:129–138. [DOI] [PubMed] [Google Scholar]
- 12. Zheng D, Sato S, Arima H, Heeley E, Delcourt C, Cao Y, Chalmers J, Anderson CS; INTERACT2 Investigators . Estimated GFR and the effect of intensive blood pressure lowering after acute intracerebral hemorrhage. Am J Kidney Dis. 2016;68:94–102. [DOI] [PubMed] [Google Scholar]
- 13. Hsu CY, Ordoñez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iseki K, Kinjo K, Iseki C, Takishita S. Relationship between predicted creatinine clearance and proteinuria and the risk of developing ESRD in Okinawa, Japan. Am J Kidney Dis. 2004;44:806–814. [PubMed] [Google Scholar]
- 15. Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J. 2013;6:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G; National Kidney Foundation . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 17. de Oliveira Manoel AL, Goffi A, Zampieri FG, Turkel‐Parrella D, Duggal A, Marotta TR, Macdonald RL, Abrahamson S. The critical care management of spontaneous intracranial hemorrhage: a contemporary review. Crit Care. 2016;20:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qureshi AI, Palesch YY, Martin R, Novitzke J, Cruz Flores S, Ehtisham A, Goldstein JN, Kirmani JF, Hussein HM, Suri MF, Tariq N; Antihypertensive Treatment of Acute Cerebral Hemorrhage Investigators . Systolic blood pressure reduction and risk of acute renal injury in patients with intracerebral hemorrhage. Am J Med. 2012;125:718.e711–718.e716. [DOI] [PubMed] [Google Scholar]
- 19. Strandgaard S, Olesen J, Skinhøj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. BMJ. 1973;1:507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer JS, Shimazu K, Fukuuchi Y, Ohuchi T, Okamoto S, Koto A. Impaired neurogenic cerebrovascular control and dysautoregulation after stroke. Stroke. 1973;4:169–186. [DOI] [PubMed] [Google Scholar]
- 21. Kitiyakara C, Guzman N. Malignant hypertension and hypertensive emergencies. J Am Soc Nephrol. 1998;9:133–142. [DOI] [PubMed] [Google Scholar]
- 22. Woods JW, Blythe WB, Huffines WD. Management of malignant hypertension complicated by renal insufficiency. N Engl J Med. 1974;291:10–14. [DOI] [PubMed] [Google Scholar]
- 23. Varon J, Fromm RE. Handbook of Practical Critical Care Medicine. New York, NY: Springer; 2001. [Google Scholar]
- 24. Brott T, Thalinger K, Hertzberg V. Hypertension as a risk factor for spontaneous intracerebral hemorrhage. Stroke. 1986;17:1078–1083. [DOI] [PubMed] [Google Scholar]
- 25. Rasool AHG, Rahman ARA, Choudhury SR, Singh RB. Blood pressure in acute intracerebral haemorrhage. J Hum Hypertens. 2004;18:187–192. [DOI] [PubMed] [Google Scholar]
- 26. Kato R, Pinsky MR. Personalizing blood pressure management in septic shock. Ann Intensive Care. 2015;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dünser MW, Takala J, Ulmer H, Mayr VD, Luckner G, Jochberger S, Daudel F, Lepper P, Hasibeder WR, Jakob SM. Arterial blood pressure during early sepsis and outcome. Intensive Care Med. 2009;35:1225–1233. [DOI] [PubMed] [Google Scholar]
- 28. Poukkanen M, Wilkman E, Vaara ST, Pettilä V, Kaukonen K‐M, Korhonen A‐M, Uusara A, Hovilehto S, Inkinen O, Laru‐Sompa R, Hautamaki R, Kuitunen A, Karlsson S; FINNAKI Study Group . Hemodynamic variables and progression of acute kidney injury in critically ill patients with severe sepsis: data from the prospective observational FINNAKI study. Crit Care. 2013;17:R295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strandgaard S. Autoregulation of cerebral blood flow in hypertensive patients: the modifying influence of prolonged antihypertensive treatment on the tolerance to acute, drug‐induced hypotension. Circulation. 1976;53:720–727. [DOI] [PubMed] [Google Scholar]
- 30. Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg. 2004;107:1–16. [DOI] [PubMed] [Google Scholar]
- 31. Snarska K, Kapica‐Topczewska K, Bachórzewska‐Gajewska H, Małyszko J. Renal function predicts outcomes in patients with ischaemic stroke and haemorrhagic stroke. Kidney Blood Press Res. 2016;41:424–433. [DOI] [PubMed] [Google Scholar]
- 32. Rådholm K, Arima H, Lindley RI, Wang J, Tzourio C, Robinson T, Heeley E, Anderson CS, Chalmers J; INTERACT2 Investigators . Older age is a strong predictor for poor outcome in intracerebral haemorrhage: the INTERACT2 study. Age Ageing. 2015;44:422–427. [DOI] [PubMed] [Google Scholar]
- 33. Doyle JF, Forni LG. Acute kidney injury: short‐term and long‐term effects. Crit Care. 2016;20:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. [DOI] [PubMed] [Google Scholar]
- 35. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 36. Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft‐Gault, MDRD, and new CKD‐EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bagshaw SM, Uchino S, Cruz D, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans‐van Straaten HM, Ronco C, Kellum JA; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators . A comparison of observed versus estimated baseline creatinine for determination of RIFLE class in patients with acute kidney injury. Nephrol Dial Transplant. 2009;24:2744. [DOI] [PubMed] [Google Scholar]
- 38. Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. [DOI] [PubMed] [Google Scholar]
- 39. Chang JJ, Khorchid Y, Dillard K, Kerro A, Burgess LG, Cherkassky G, Goyal N, Chapple K, Alexandrov AW, Buechner D, Alexandrov AV, Tsivgoulis G. Elevated pulse pressure levels are associated with increased in‐hospital mortality in acute spontaneous intracerebral hemorrhage. Am J Hypertens. 2017;30:719–727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Nephrotoxic Agents and Interventions in AKI and Non‐AKI Subjects
Table S2. Blood Pressure Parameters in Patients Who Developed In‐Hospital Acute Kidlney Injury


