Abstract
Background
At present, a constant progress in pathophysiology understanding and treatment of the chronic heart failure (CHF) is arising. Meanwhile, hyperhomocysteinemia (HHcy) has been linked to impaired left ventricular function and clinical class in patients with CHF. Atorvastatin therapy can reduce the incidence of sudden cardiac death in patients with advanced CHF. Folic acid could enhance endothelial function in vascular disease states. The present study aims to investigate the effect of atorvastatin and folic acid combined on the cardiac function and ventricular remodeling in CHF patients with HHcy.
Material/Methods
Elderly CHF patients with HHcy were divided into four groups: routine, routine + atorvastatin, routine + folic acid, and routine + atorvastatin + folic acid groups. Serum homocysteine (Hcy) level was detected using enzymatic cycling methods, and N-terminal pro brain natriuretic peptide (NT-proBNP) level by ELISA. The cardiac function indexes and left ventricular early diastolic peak flow velocity/atrial systolic peak flow velocity (E/A) ratio were evaluated. The six-minute walk test was performed to measure the six-minute walk distance (6MWD).
Results
6MWD increased, the serum Hcy and NT-proBNP levels decreased, and cardiac function was improved compared with before treatment, which was the most significant in the routine + atorvastatin + folic acid group, followed by the routine + atorvastatin group, then the routine + folic acid group, and lastly, the routine group.
Conclusions
The results indicated that the combination of atorvastatin and folic acid improved the cardiac function and inhibited ventricular remodeling of elderly CHF patients with HHcy.
MeSH Keywords: Folic Acid, Heart Failure, Hyperhomocysteinemia
Background
Heart failure (HF) is a chronic, progressive disease with increasing prevalence, and patients with HF are subjected to a high symptom burden [1]. Symptoms of chronic heart failure (CHF) generally refer to ankle swelling, fatigue, and breathlessness, presenting signals such as pulmonary crackles, displaced apex beat, and elevated jugular venous pressure [2]. The prevalence of CHF rises with the advance of life spans, the lifetime risks which accounts for approximately 20% of patients older than 40 years of age [3]. Moreover, CHF can decrease the quality of life for patients with an increase in the lifetime risk [4]. An aging population and the westernization of dietary habits account for the increasing prevalence of CHF [5]. Hyperhomocysteinemia (HHcy) has been widely considered as the major determining factor of various cardiovascular diseases [6]. The effects of HHcy on endothelial dysfunction [7], oxidative damage [8], and inflammation [9] have been proven to be involved in cardiovascular morbidity and mortality; high level of homocysteine (Hcy) is the major cause for cardiovascular diseases [10].
Atorvastatin is a powerful new synthetic HMG-CoA reductase inhibitor affecting viral infection and inhibiting inflammation [11]. Atorvastatin treatment has also been shown to suppresses adventitial neovascularization as well as plaque development in ApoE deficient mice by regulating chemokines and chemokine receptors, peroxisome proliferator-activated receptors (PPAR) and nuclear factor kappa B (NF-κB) [12,13]. In addition, the safety and efficacy of atorvastatin was proven positive in a study by Langslet et al. [14]. Folic acid deficiency may facilitate several different age-related diseases, including coronary artery disease [15] and stroke [16]. HHcy is a consequence of folic acid deficiency that contributes to the pathogenesis of cardiovascular disease and ischemic stroke [17–19]. Elevation of Hcy is the risk factor of cardiovascular diseases such as atherosclerosis, stroke, thrombosis, and peripheral arterial occlusive disease [20]. Folic acid had the dominant blood homocysteine lowering effect, and a daily dose of folic acid would produce a proportional reduction in blood homocysteine [21]. In this study, considering the efficacy of atorvastatin and folic acid that has been proven in previous studies, we aimed to further discuss the combined effects of these two agents on cardiac function and ventricular remodeling in CHF patients with HHcy.
Material and Methods
Study patients
A total of 248 elderly CHF patients with HHcy were diagnosed in the Department of Geriatrics at the Hunan Provincial People’s Hospital, the First Affiliated Hospital of Hunan Normal University during April 2015 and February 2017, including 156 male patients and 92 female patients, with a mean age of 69.8±6.5 years. According to the New York Heart Association (NYHA) functional classification, the cardiac function of the enrolled patients was graded as class II–IV. The study inclusion criteria were: 1) based on the history of disease and in accordance with the Framingham risk score [22], patients were diagnosed with CHF by physical examination, x-ray, and echocardiography; 2) patients with Hcy level higher than 15 μmol/L; 3) patients aged ≥65 years. Exclusion criteria were: 1) patients with liver or kidney disease; 2) patients suffering from blood diseases, rheumatism, or digestive system diseases; 3) patients with malignancies; 4) patients suffering from malnutrition; 5) patients with peripheral arterial occlusive disease, sick sinus syndrome, II–III degree atrioventricular heart block, bronchial asthma, systolic pressure <90 mm Hg, or acute pulmonary edema [23]. This research was approved and supervised by the ethics committee of Hunan Provincial People’s Hospital, the First Affiliated Hospital of Hunan Normal University. All study patients signed the informed consent.
Treatments and clinical efficacy evaluation
The patients were grouped into four groups according to the random number table method, with 62 cases in each group: 1) the routine group received routine medical therapy (angiotensin-converting-enzyme inhibitor, angiotensin-receptor antagonist, β blockers, or diuretics), without any statin therapy or folic acid treatment; 2) the routine + atorvastatin group received in addition to routine medical therapy, atorvastatin 20 mg qd (Pfizer Ireland Pharmaceuticals, Dublin, Ireland); 3) the routine + folic acid group received in addition to routine medical therapy, folic acid 5 mg qd (Tianjin Li Sheng Pharmaceutical Co., Ltd., Tianjin, China; 5 mg ×100 pieces/box); 4) the routine + atorvastatin + folic acid group received in addition to routine medical therapy, atorvastatin 20 mg qd and folic acid 5 mg qd, once daily at bedtime. All patients underwent four-week treatment. The evaluation criteria of clinical response were as follows [24]: significant response (the CHF was controlled or ameliorated to class I); effective response (the CHF was reduced by one class but not to the class I); ineffective response: the CHF was reduced by near one class but not enough, and neither improvement nor deterioration can be perceived in patients. Overall response rate=(cases with significant response+cases with effective response)/the number of total cases ×100%.
Serum index test
Fasting peripheral venous blood (5 mL) was extracted from all patients in the morning before and after treatment, collected into anticoagulant tube and centrifuged within one hour after extraction. As the serum was isolated from blood samples, an enzymatic cycling method was applied to detect the Hcy level, and enzyme-linked immunosorbent assay (ELISA) was used to detect the N-terminal pro brain natriuretic peptide (NT-proBNP) level. The routine blood indexes were detected using automatic biochemical analyzer, including low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), cholesterol (CHOL), and triglyceride (TG).
Cardiac function assessment
A color Doppler ultrasound diagnostic system (Philips IU ELITE; Philips, Andover, MA, USA) was used to evaluate the cardiac function of all patients before and after four weeks of treatment. Routine echocardiography was performed by two experienced physicians with S3 ultrasound probe at a frequency of 2.5 MH. Patients were positioned in the left-lateral position. The cardiac function indexes included left ventricular posterior wall thickness (LVPWT), left ventricular end-diastolic dimension (LVEDD), interventricular septal thickness (IVST), left ventricular ejection fraction (LVEF), and left ventricular early diastolic peak flow velocity/atrial systolic peak flow velocity (E/A) ratio.
Six-minute walk test (6MWT)
The 6MWT was performed during 8 a.m. to 9 a.m. Enrolled patients were all informed of the test procedures. The test started after 10 minutes of sitting still. Patients were told to walk as fast as they could in the straight corridor. Proper encouragement was provided by a monitor but no accompany walking. After six minutes of walking, the stopping site was marked, and the final walking distance was determined as the six-minute walk distance (6MWD).
Statistical analysis
The data were analyzed using SPSS version 21.0 (IBM Corp. Armonk, NY, USA). Measurement data were expressed as mean ± standard deviation. Paired t-test was used for comparison before and after treatment, and independent sample t-test was used for comparisons between two groups of measurement data in normal distribution. Comparisons among multi-groups were performed using one-way analysis of variance (ANOVA), while Student Newman-Keuls (SNK) test was employed to compare the data between the two groups. The levels of HDL-C and TG are fitted in normal distribution, Kolmogorov-Smirnov test was for performed on two independent samples with abnormal distribution, and multiple independent samples were tested by Kruskal-Wallis test. Enumeration data was expressed in the form of percentage or rate, and the chi square test was performed to analyze it. The p value was determined by a two-tailed test, and when the p value was less than 0.05, the differences were considered to be of statistical significance.
Results
Baseline characteristics of CHF patients with HHcy in the four groups
There were 42 males and 20 females in the routine group, with a mean age of 69.4±1.5 years, and 38 males and 24 females in the routine + atorvastatin group, with a mean age of 69.9±1.6 years. There were 41 males and 21 females in the routine + folic acid group, with a mean age of 69.8±1.6 years, and 35 males and 27 females in the routine + atorvastatin + folic acid group, with a mean age of 69.5±1.8 years. No significant difference was found in mean age, gender, cardiac functional grade, LDL-C, HDL-C, CHOL, or TG among the four groups (all p>0.05) (Table 1).
Table 1.
Baseline characteristics of CHF patients with HHcy among the routine, routine + atorvastatin, routine + folic acid, and routine + atorvastatin + folic acid groups.
| Baseline characteristic | Routine group (n=62) | Routine + atorvastatin group (n=62) | Routine + folic acid group (n=62) | Routine + atorvastatin + folic acid group (n=62) | P |
|---|---|---|---|---|---|
| Mean age (years) | 69.4±1.5 | 69.9±1.6 | 69.8±1.6 | 69.5±1.8 | 0.267 |
| Gender | 0.557 | ||||
| Male | 42 | 38 | 41 | 35 | |
| Female | 20 | 24 | 21 | 27 | |
| Cardiac functional grade | 0.963 | ||||
| Class II | 23 | 25 | 26 | 22 | |
| Class III | 30 | 26 | 28 | 31 | |
| Class IV | 9 | 11 | 8 | 9 | |
| LDL-C (mmol/L) | 2.83±0.39 | 2.94±0.52 | 2.88±0.48 | 2.98±0.67 | 0.348 |
| HDL-C (mmol/L) | 0.65±0.16 | 0.68±0.19 | 0.62±0.15 | 0.69±0.21 | 0.125 |
| CHOL (mmol/L) | 4.96±0.75 | 4.87±0.71 | 4.77±0.64 | 4.86±0.69 | 0.514 |
| TG (mmol/L) | 1.84±0.49 | 1.89±0.51 | 1.78±0.54 | 1.82±0.48 | 0.678 |
CHF – chronic heart failure; HHcy – hyperhomocysteinemia; LDL-C – low density lipoprotein cholesterol; HDL-C – high density lipoprotein cholesterol; CHOL – cholesterol; TG – triglyceride.
Combined treatment of atorvastatin and folic acid could inhibit Hcy level
Before treatment for four weeks, the Hcy level in the routine group decreased without significance (p>0.05). In the routine + atorvastatin group, the routine + folic acid group and the routine + atorvastatin + folic acid group, the post-treatment Hcy level was evidently reduced than before treatment (all p<0.05). The Hcy level in the routine + atorvastatin + folic acid group was significantly lower than that in the routine, routine + atorvastatin and routine + folic acid groups (all p<0.05). The Hcy level in the routine + atorvastatin group was lower than that in the routine + folic acid and routine groups (all p<0.05), and the Hcy level in the routine + folic acid group was lower than that in the routine group (Figure 1).
Figure 1.
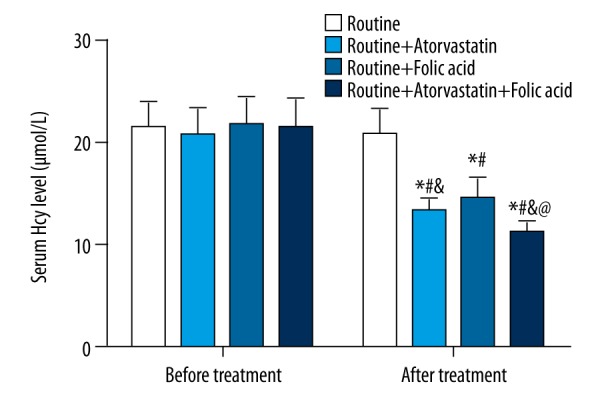
Serum Hcy level among the four groups before and after treatment. Hcy – homocysteine; * p<0.05 compared with before treatment in the same group; # p<0.05 compared with the routine group after treatment; & p<0.05 compared with the routine + folic acid group after treatment; @ p<0.05 compared with the routine + atorvastatin group after treatment.
Combined treatment of atorvastatin and folic acid can improve 6MWD and suppress serum NT-proBNP level
There was no significant difference in the 6MWD and serum NT-proBNP level among the routine, routine + atorvastatin, routine + folic acid and routine + atorvastatin + folic acid groups before treatment (all p>0.05). After treatment, the 6MWD level in the four groups was notably increased while the serum NT-proBNP levels were downregulated (all p<0.05) than before treatment. After treatment, the 6WMD in the routine + atorvastatin + folic acid group was markedly increased than that in the other three groups and the serum NT-proBNP level was lower (all p<0.05). After treatment, in the routine + atorvastatin group, the 6MWD was increased, while the serum NT-proBNP level was decreased compared with the routine + folic acid and routine groups. In the routine + folic acid group, the 6MWD was higher, and the NT-proBNP level was lower than that in the routine group (all p<0.05) (Table 2).
Table 2.
Comparisons of the 6MWD and serum NT-proBNP level in patients among the routine, routine + atorvastatin, routine + folic acid, and routine + atorvastatin + folic acid groups.
| Item | Routine group (n=62) | Routine + atorvastatin group (n=62) | Routine + folic acid group (n=62) | Routine + atorvastatin + folic acid group (n=62) |
|---|---|---|---|---|
| 6MWD (m) | ||||
| Before treatment | 219.42±34.14 | 223.77±35.26 | 217.37±34.66 | 224.37±33.20 |
| After treatment | 342.68±54.36* | 427.60±52.22*#& | 408.34±49.66*# | 497.81±56.13*#&@ |
| NT-proBNP (pg/ml) | ||||
| Before treatment | 6253.41±203.67 | 6241.56±211.35 | 6268.74±201.58 | 6247.85±206.94 |
| After treatment | 3219.65±208.73* | 3008.51±118.62*#& | 3054.68±119.35*# | 2818.76±104.65*#&@ |
6MWD – 6-minute walk distance; NT-proBNP – N-Terminal pro Brain Natriuretic Peptide;
P<0.05 compared with before treatment;
P<0.05 compared with the routine group before treatment;
P<0.05 compared with the routine + folic acid group before treatment;
P<0.05 compared with the routine + atorvastatin group before treatment.
Combined treatment of atorvastatin and folic acid treatment may improve clinical response
After four weeks of treatment, the overall response rate of the routine group, the routine + atorvastatin group, the routine + folic acid group, and the routine + atorvastatin + folic acid group was 38.3%, 64.7%, 59.5%, and 88.6%, respectively. According to the statistical analysis, the clinical response in the routine + atorvastatin + folic acid group was better than that in the routine, routine + atorvastatin, and routine + folic acid groups (all p<0.05). The clinical response of the routine + atorvastatin group and the routine + folic acid group was better than that of the routine group (both p<0.05). Besides, the clinical response of the routine + atorvastatin group was slightly better than that of the routine + folic acid group (p>0.05) (Table 3).
Table 3.
Comparison of clinical response in patients among the routine, routine + atorvastatin, routine + folic acid, and routine + atorvastatin + folic acid groups after treatment.
| Clinical response | Routine group (n=62) | Routine + atorvastatin group (n=62) | Routine + folic acid group (n=62) | Routine + atorvastatin + folic acid group (n=62) |
|---|---|---|---|---|
| Ineffective | 32 (51.6%) | 22 (35.5%) | 25 (40.3%) | 7 (11.3%) |
| Effective | 16 (25.8%) | 25 (40.3%) | 22 (35.5%) | 31 (50.0%) |
| Significant | 14 (22.6%) | 15 (24.2%) | 15 (24.2%) | 24 (38.7%) |
| Overall response rate | 30 (48.4%) | 40 (64.5%)# | 37 (59.7%)# | 55 (88.7%)#&@ |
P<0.05 compared with the routine group;
P<0.05 compared with the routine + folic acid group;
P<0.05 compared with the routine + atorvastatin group.
Combined treatment of atorvastatin and folic acid treatment can enhance cardiac function and inhibit ventricular remodeling
The cardiac function indexes exhibited no significant difference among the routine, routine + atorvastatin, routine + folic acid and routine + atorvastatin + folic acid groups before treatment (all p>0.05). After treatment, the routine + atorvastatin + folic acid group presented a significant improvement in cardiac function than the routine, routine + atorvastatin, and routine + folic acid groups (all p<0.05). The cardiac function in the routine + atorvastatin and routine + folic acid groups was evidently better than that in the routine group (all p<0.05). And the cardiac function between the routine + atorvastatin group and the routine + folic acid group showed no evident difference (p>0.05). The results above suggest that the combination of atorvastatin and folic acid contributes to inhibiting the ventricular remodeling of CHF patients with HHcy and improve the cardiac function (Table 4).
Table 4.
Comparison of cardiac function indexes of patients among the routine, routine + atorvastatin, routine + folic acid, and routine + atorvastatin + folic acid groups.
| Index | Routine group (n=62) | Routine + atorvastatin group (n=62) | Routine + folic acid group (n=62) | Routine + atorvastatin + folic acid group (n=62) |
|---|---|---|---|---|
| LVEDD (mm) | ||||
| Before treatment | 54.37±4.18 | 55.21±4.24 | 54.93±3.98 | 53.57±4.09 |
| After treatment | 50.34±3.26* | 45.68±3.17*# | 46.83±3.04*# | 42.15±2.95*#&@ |
| LVPWT (mm) | ||||
| Before treatment | 7.58±1.12 | 7.21±1.03 | 7.74±1.35 | 7.81±1.44 |
| After treatment | 6.82±0.97 | 4.98±0.65*# | 5.01±0.72*# | 3.21±0.46*#&@ |
| IVST (mm) | ||||
| Before treatment | 7.16±1.57 | 7.48±1.27 | 7.88±1.51 | 7.54±1.36 |
| After treatment | 7.98±1.69 | 9.74±1.78*# | 9.86±1.72*# | 11.33±1.84*#&@ |
| E/A (%) | ||||
| Before treatment | 0.74±0.21 | 0.78±0.19 | 0.81±0.16 | 0.79±0.17 |
| After treatment | 0.92±0.23 | 1.34±0.26*# | 1.29±0.22*# | 1.67±0.31*#&@ |
| LVEF (%) | ||||
| Before treatment | 43.56±4.62 | 44.18±4.37 | 44.26±4.18 | 43.93±4.05 |
| After treatment | 47.58±4.81* | 50.27±4.96*# | 48.87±5.01*# | 53.16±5.24*#&@ |
LVEDD – left ventricular end-diastolic dimension; LVPWT – left ventricular posterior wall thickness; IVST – interventricular septal thickness; E/A – early diastolic peak flow velocity/atrial systolic peak flow velocity; LVEF – left ventricular ejection fraction;
P<0.05 compared with before treatment;
P<0.05 compared with the routine group after treatment;
P<0.05 compared with the routine + folic acid group after treatment;
P<0.05 compared with the routine + atorvastatin group after treatment.
Discussion
HF is a cardiovascular disease manifested with ventricular dysfunction, specifically abnormality in left ventricular ejection [25], which is associated with high rates of morbidity and mortality and a burden to the healthcare system [26]. Despite advanced therapeutic drugs maintaining and stabilizing limited functional abilities, and improvement in the comfort of the patients for remaining life-span, CHF still exerts a poor prognosis [27]. Based on this, we conducted our study and from the results concluded that atorvastatin and folic acid synergistically improve the cardiac function and attenuate ventricular remodeling, and suppress the deterioration of CHF patients with HHcy.
In this study, it was found that combined treatment of atorvastatin and folic acid inhibited the Hcy level of elderly CHF patients with HHcy, and thus improved the cardiac function of patients. Statin therapy is a recognized lipid-lowering intervention to decrease the risk of acute events in patients with cardiovascular diseases [28]; as a member of statin family, atorvastatin is proved to suppress Hcy accumulation in the blood [29]. Li et al. provided evidence in a previous study that the endothelial function can be protected by simvastatin [30]. Additionally, atorvastatin is a reductase inhibitor and acts as antioxidant and anti-inflammatory independent of its lipid-lowering abilities [31]. It can also function as a hepatoprotective and hypocholesterolemic agent so as to improve the cardiovascular function through regulating oxidative stress, nitric oxide (NO) and Hcy [32]. Folic acid is a synthetic form of folate, which is a water-soluble B vitamin, and it is a promising approach for improving endothelial function in patients with HHcy [33]. HHcy is an independent putative risk factor for cardiovascular diseases [34]. Folic acid deficiency is implicated in the remethylation pathway of Hcy metabolism [35]. Hcy is supposed to induce oxidative stress and endothelial dysfunction [36]. The effect of folic acid on Hcy-lowering has been seen in clinical trials [37,38]. In addition, elevated serum levels of Hcy have various mechanisms affecting the cardiovascular system, such as endothelial dysfunction, inflammation, and oxidative stress [39,40]. In this study, our results showed that the combined treatment of folic acid and atorvastatin could improve cardiac function through downregulating Hcy level.
In addition, atorvastatin and folic acid were also found to be effective in inhibiting the NT-proBNP level, which is a biomarker for cardiac function. NT-proBNP is used as a diagnostic marker for diastolic dysfunction [41], and for the severity and prognosis of CHF [42]. Furthermore, an increased risk for 60-day HF-associated events is implicated in patients with increasing NT-proBNP [43]. In a previous study, atorvastatin has been proved to improve cardiac function through the modulation of NT-proBNP releasement [25]. Atorvastatin was indicated to be effective in improving the left ventricular systolic function with increased LVEF and decreased LVEDD [44]. Besides, in our study, the LVPWT and LVEDD of all patients were reduced while the IVST, E/A ratio and LVEF were increased after treatment. And the routine + atorvastatin + folic acid group exhibited a significant cardiac function improvement, suggesting the combination of atorvastatin and folic acid contributes to improve the cardiac function of CHF patients with HHcy through inhibiting the NT-proBNP releasement.
We also found that atorvastatin and folic acid work together to inhibit ventricular remodeling. The statin class of drugs is now recognized to have therapeutic properties beyond cholesterol lowering, including anti-oxidation, anti-inflammation, upregulation of nitric oxide synthesis [45], and prevention of cardiovascular diseases [46]. In a former study on ventricular remodeling of spontaneously hypertensive rats, atorvastatin was proved to upregulate the p27 protein level and induce cell apoptosis, by which way the ventricular remodeling was frustrated [47]. Additionally, folic acid seems to affect bone remodeling [48] and renal remodeling [49], indicating a potential role of folic acid on ventricular remodeling. The present study exhibited that the ventricular remodeling of CHF patients was inhibited in the routine + atorvastatin + folic acid group. Therefore, the ventricular remodeling can be frustrated by the combination of folic acid and atorvastatin.
Conclusions
To sum up, atorvastatin and folic acid are effective in the improvement of cardiac function and suppression of ventricular remodeling. This study provides evidence for the therapeutic effect of atorvastatin and folic acid on CHF with HHcy and more research on the specific mechanism involving the pathogenesis of CHF with HHcy are needed in the future.
Footnotes
Conflicts of interests
None.
Source of support: This study was supported by Hunan Province Science and Technology Foundation (No. 2015SK20456)
References
- 1.Stewart D, McPherson ML. Symptom management challenges in heart failure: Pharmacotherapy considerations. Heart Fail Rev. 2017;22(5):525–34. doi: 10.1007/s10741-017-9632-5. [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, et al. [ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012]. Turk Kardiyol Dern Ars. 2012;40(Suppl 3):77–137. [in Turkish] [PubMed] [Google Scholar]
- 3.Ramani GV, Uber PA, Mehra MR. Chronic heart failure: Contemporary diagnosis and management. Mayo Clin Proc. 2010;85:180–95. doi: 10.4065/mcp.2009.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cubbon RM, Gale CP, Kearney LC, et al. Changing characteristics and mode of death associated with chronic heart failure caused by left ventricular systolic dysfunction: A study across therapeutic eras. Circ Heart Fail. 2011;4:396–403. doi: 10.1161/CIRCHEARTFAILURE.110.959882. [DOI] [PubMed] [Google Scholar]
- 5.Shiba N, Shimokawa H. Chronic heart failure in Japan: implications of the CHART studies. Vasc Health Risk Manag. 2008;4:103–13. doi: 10.2147/vhrm.2008.04.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahalle N, Kulkarni MV, Garg MK, Naik SS. Vitamin B12 deficiency and hyperhomocysteinemia as correlates of cardiovascular risk factors in Indian subjects with coronary artery disease. J Cardiol. 2013;61:289–94. doi: 10.1016/j.jjcc.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Z, Jiang X, Pansuria M, et al. Hyperhomocysteinemia and hyperglycemia induce and potentiate endothelial dysfunction via mu-calpain activation. Diabetes. 2015;64:947–59. doi: 10.2337/db14-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timkova V, Tatarkova Z, Lehotsky J, et al. Effects of mild hyperhomocysteinemia on electron transport chain complexes, oxidative stress, and protein expression in rat cardiac mitochondria. Mol Cell Biochem. 2016;411:261–70. doi: 10.1007/s11010-015-2588-7. [DOI] [PubMed] [Google Scholar]
- 9.Qi X, Zhang B, Zhao Y, et al. Hyperhomocysteinemia promotes insulin resistance and adipose tissue inflammation in PCOS mice through modulating M2 macrophage polarization via estrogen suppression. Endocrinology. 2017;158(5):1181–93. doi: 10.1210/en.2017-00039. [DOI] [PubMed] [Google Scholar]
- 10.Heinz J, Kropf S, Luley C, Dierkes J. Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: A meta-analysis. Am J Kidney Dis. 2009;54:478–89. doi: 10.1053/j.ajkd.2009.01.266. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Ma T, Ouyang H, et al. Effect of atovastatin treatment on porcine circovirus 2 infection in BALB/c mice. Clin Exp Pharmacol Physiol. 2015;42:817–21. doi: 10.1111/1440-1681.12434. [DOI] [PubMed] [Google Scholar]
- 12.Bot I, Jukema JW, Lankhuizen IM, et al. Atorvastatin inhibits plaque development and adventitial neovascularization in ApoE deficient mice independent of plasma cholesterol levels. Atherosclerosis. 2011;214:295–300. doi: 10.1016/j.atherosclerosis.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Nie P, Li D, Hu L, et al. Atorvastatin improves plaque stability in ApoE-knockout mice by regulating chemokines and chemokine receptors. PLoS One. 2014;9:e97009. doi: 10.1371/journal.pone.0097009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langslet G, Breazna A, Drogari E. A 3-year study of atorvastatin in children and adolescents with heterozygous familial hypercholesterolemia. J Clin Lipidol. 2016;10:1153–62e3. doi: 10.1016/j.jacl.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Yi X, Zhou Y, Jiang D, et al. Efficacy of folic acid supplementation on endothelial function and plasma homocysteine concentration in coronary artery disease: A meta-analysis of randomized controlled trials. Exp Ther Med. 2014;7:1100–10. doi: 10.3892/etm.2014.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang B, Chen Y, Yao G, et al. Effects of differences in serum total homocysteine, folate, and vitamin B12 on cognitive impairment in stroke patients. BMC Neurol. 2014;14:217. doi: 10.1186/s12883-014-0217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Symons JD, Zaid UB, Athanassious CN, et al. Influence of folate on arterial permeability and stiffness in the absence or presence of hyperhomocysteinemia. Arterioscler Thromb Vasc Biol. 2006;26:814–18. doi: 10.1161/01.ATV.0000204408.01416.16. [DOI] [PubMed] [Google Scholar]
- 18.Baggott JE, Tamura T. Homocysteine, iron and cardiovascular disease: A hypothesis. Nutrients. 2015;7:1108–18. doi: 10.3390/nu7021108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forti P, Maioli F, Arnone G, et al. Homocysteinemia and early outcome of acute ischemic stroke in elderly patients. Brain Behav. 2016;6:e00460. doi: 10.1002/brb3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medina MA. Hyperhomocysteinemia and occlusive vascular disease: An emergent role for fibroblast growth factor 2. Circ Res. 2008;102:869–70. doi: 10.1161/CIRCRESAHA.108.175588. [DOI] [PubMed] [Google Scholar]
- 21.Baszczuk A, Thielemann A, Musialik K, et al. The impact of supplementation with folic acid on homocysteine concentration and selected lipoprotein parameters in patients with primary hypertension. J Nutr Sci Vitaminol (Tokyo) 2017;63:96–103. doi: 10.3177/jnsv.63.96. [DOI] [PubMed] [Google Scholar]
- 22.Wolf PA, Kannel WB, McNamara PM. Occult impaired cardiac function, congestive heart failure, and risk of thrombotic stroke: The Framingham Study. Neurology. 1970;20:373. [PubMed] [Google Scholar]
- 23.Volante M, Sperone P, Bollito E, et al. Matrix metalloproteinase type 2 expression in malignant adrenocortical tumors: Diagnostic and prognostic significance in a series of 50 adrenocortical carcinomas. Mod Pathol. 2006;19:1563–69. doi: 10.1038/modpathol.3800683. [DOI] [PubMed] [Google Scholar]
- 24.Chen ZS, Dong YR, Hu WY. [Clinical observation of the curative effect of Qiangxin Mixture on congestive heart failure]. Zhong Xi Yi Jie He Xue Bao. 2003;1:25–29. doi: 10.3736/jcim20030111. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 25.Duan HY, Liu DM, Qian P, et al. Effect of atorvastatin on plasma NT-proBNP and inflammatory cytokine expression in patients with heart failure. Genet Mol Res. 2015;14:15739–48. doi: 10.4238/2015.December.1.25. [DOI] [PubMed] [Google Scholar]
- 26.Choi EY, Allen K, McDonnough M, et al. A plant-based diet and heart failure: Case report and literature review. J Geriatr Cardiol. 2017;14:375–78. doi: 10.11909/j.issn.1671-5411.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sane R, Aklujkar A, Patil A, Mandole R. Effect of heart failure reversal treatment as add-on therapy in patients with chronic heart failure: A randomized, open-label study. Indian Heart J. 2017;69:299–304. doi: 10.1016/j.ihj.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruckert E, Ferrieres J. Evidence supporting primary prevention of cardiovascular diseases with statins: Gaps between updated clinical results and actual practice. Arch Cardiovasc Dis. 2014;107:188–200. doi: 10.1016/j.acvd.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Bhandari U, Pathan RA, Kumar V, Khanna N. Ameliorative role of atorvastatin on methionine-induced hyperhomocysteinemia and hematological changes in albino rats. Indian J Exp Biol. 2011;49:132–39. [PubMed] [Google Scholar]
- 30.Li L, Jia Z, Xu L, et al. Expression profile of neuro-endocrine-immune network in rats with vascular endothelial dysfunction. Korean J Physiol Pharmacol. 2014;18:177–82. doi: 10.4196/kjpp.2014.18.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia F, Wu C, Chen Z, et al. Atorvastatin attenuates atherosclerotic plaque destabilization by inhibiting endoplasmic reticulum stress in hyperhomocysteinemic mice. Mol Med Rep. 2016;13:3574–80. doi: 10.3892/mmr.2016.4975. [DOI] [PubMed] [Google Scholar]
- 32.Amin KA, Abd El-Twab TM. Oxidative markers, nitric oxide and homocysteine alteration in hypercholesterolimic rats: Role of atorvastatine and cinnamon. Int J Clin Exp Med. 2009;2:254–65. [PMC free article] [PubMed] [Google Scholar]
- 33.Woo CW, Prathapasinghe GA, Siow YL, O K. Hyperhomocysteinemia induces liver injury in rat: Protective effect of folic acid supplementation. Biochim Biophys Acta. 2006;1762:656–65. doi: 10.1016/j.bbadis.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Liu K, Xuekelati S, Zhang Y, et al. Expression levels of atherosclerosis-associated miR-143 and miR-145 in the plasma of patients with hyperhomocysteinaemia. BMC Cardiovasc Disord. 2017;17:163. doi: 10.1186/s12872-017-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taheraghdam AA, Dalirakbari N, Khalili M, et al. Hyperhomocysteinemia, low vitamin B12, and low folic acid: Are risk factors of cerebral vascular thrombosis in northwest Iran? J Res Med Sci. 2016;21:16. doi: 10.4103/1735-1995.178755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung SB, Zhang H, Lau CW, et al. Salidroside improves homocysteine-induced endothelial dysfunction by reducing oxidative stress. Evid Based Complement Alternat Med. 2013;2013:679635. doi: 10.1155/2013/679635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beigi AA, Hoghoughi MA, Eshaghian A, et al. The role of folic acid on the hyperhomocysteinemia in the Buerger’s disease (Thromboangiitis Obliterans) J Res Med Sci. 2014;19:1034–37. [PMC free article] [PubMed] [Google Scholar]
- 38.Keser I, Ilich JZ, Vrkic N, et al. Folic acid and vitamin B(12) supplementation lowers plasma homocysteine but has no effect on serum bone turnover markers in elderly women: A randomized, double-blind, placebo-controlled trial. Nutr Res. 2013;33:211–19. doi: 10.1016/j.nutres.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Mendes RH, Mostarda C, Candido GO, et al. Moderate hyperhomocysteinemia provokes dysfunction of cardiovascular autonomic system and liver oxidative stress in rats. Auton Neurosci. 2014;180:43–47. doi: 10.1016/j.autneu.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Wang R, Wang Y, Mu N, et al. Activation of NLRP3 inflammasomes contributes to hyperhomocysteinemia-aggravated inflammation and atherosclerosis in apoE-deficient mice. Lab Invest. 2017;97:922–34. doi: 10.1038/labinvest.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coats CJ, Parisi V, Ramos M, et al. Role of serum N-terminal pro-brain natriuretic peptide measurement in diagnosis of cardiac involvement in patients with anderson-fabry disease. Am J Cardiol. 2013;111:111–17. doi: 10.1016/j.amjcard.2012.08.055. [DOI] [PubMed] [Google Scholar]
- 42.Obaid FA, Maskon O, Abdolwahid F. Systolic function and intraventricular mechanical dyssynchrony assessed by advanced speckle tracking imaging with N-terminal prohormone of brain natriuretic peptide for outcome prediction in chronic heart failure patients. Sultan Qaboos Univ Med J. 2013;13:551–59. doi: 10.12816/0003315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng H, Fan WZ, Wang SC, et al. Prognostic utility of combination of NT-proBNP with high sensitive cTn I in patients with heart failure: Results from retrospective study in an emergency department. Scand J Clin Lab Invest. 2016;76:361–67. doi: 10.1080/00365513.2016.1183166. [DOI] [PubMed] [Google Scholar]
- 44.Sola S, Mir MQ, Lerakis S, et al. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol. 2006;47:332–37. doi: 10.1016/j.jacc.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 45.Hsu CP, Zhao JF, Lin SJ, et al. Asymmetric dimethylarginine limits the efficacy of simvastatin activating endothelial nitric oxide synthase. J Am Heart Assoc. 2016;5:e003327. doi: 10.1161/JAHA.116.003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fung CSC, Wan EYF, Chan AKC, Lam CLK. Statin use reduces cardiovascular events and all-cause mortality amongst Chinese patients with type 2 diabetes mellitus: A 5-year cohort study. BMC Cardiovasc Disord. 2017;17:166. doi: 10.1186/s12872-017-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang L, Ge CJ, Hu SJ. Beneficial effect of atorvastatin on left ventricular remodeling in spontaneously hypertensive rats. Pharmacology. 2007;80:120–26. doi: 10.1159/000103251. [DOI] [PubMed] [Google Scholar]
- 48.Tyagi N, Kandel M, Munjal C, et al. Homocysteine mediated decrease in bone blood flow and remodeling: role of folic acid. J Orthop Res. 2011;29:1511–16. doi: 10.1002/jor.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pushpakumar SB, Kundu S, Metreveli N, Sen U. Folic acid mitigates angiotensin-II-induced blood pressure and renal remodeling. PLoS One. 2013;8:e83813. doi: 10.1371/journal.pone.0083813. [DOI] [PMC free article] [PubMed] [Google Scholar]


