Abstract
Reliance on hepatitis C virus (HCV) replicon systems and protein-based screening assays has led to treatments that target HCV viral replication proteins. The model does not encompass other viral replication cycle steps such as entry, processing, assembly and secretion, or viral host factors. We previously applied a phenotypic high-throughput screening platform based on an infectious HCV system and discovered an aryloxazole-based anti-HCV hit. Structure– activity relationship studies revealed several compounds exhibiting EC50 values below 100 nM. Lead compounds showed inhibition of the HCV pseudoparticle entry, suggesting a different mode of action from existing HCV drugs. Hit 7a and lead 7ii both showed synergistic effects in combination with existing HCV drugs. In vivo pharmacokinetics studies of 7ii showed high liver distribution and long half-life without obvious hepatotoxicity. The lead compounds are promising as preclinical candidates for the treatment of HCV infection and as molecular probes to study HCV pathogenesis.
Graphical Abstract
INTRODUCTION
Hepatitis C virus (HCV) leads to chronic infection in 80% of patients, and disease progression can eventually cause liver cancer or cirrhosis following decades of asymptomatic infection.1 HCV is the major underlying cause for liver transplants and is responsible for significant healthcare costs.2 The prevalence of HCV has been estimated at around 200 million people worldwide3 and, to date, no effective vaccine has been developed.4 The historical treatment regimen for HCV involved combination therapy of ribavirin with pegylated interferon, a poorly tolerated course of treatment with only moderate success in achieving a six-month post-treatment sustained virological response (SVR), an undetectable viral RNA level six months after treatment cessation.5 The recent approval of several small-molecule direct-acting antivirals (DAAs) has dramatically improved the standard of care for HCV.5 These drugs target the viral proteins (NS3/4A protease, NS5B polymerase, and NS5A) involved in the replication stage of HCV infection. Although these treatments offer renewed hope toward curing HCV infection, the price of the medicines is prohibitively expensive for many high-risk populations such as intravenous drug users, prisoners, and those in the developing world.6 Furthermore, these agents can lead to rapid development of viral resistance if the virus is not fully eradicated during the treatment regimen.
The hegemony of replication inhibitors as treatments for HCV infection is a direct consequence of the available methods to screen for inhibitors of the virus. Current HCV inhibitors have mostly been discovered through either the replicon assay, which measures the replication of isolated viral RNA, or protein-based assays using HCV proteins involved in viral replication (e.g., NS3, NS5A, or NS5B). On the other hand, a cell-based infectious HCV assay platform can cover the complete spectrum of potentially druggable targets in all stages of the HCV replication cycle and allows for the development of inhibitors acting on different phases of the viral life cycle less prone to mutation. More importantly, targeting several key processes in the viral replication cycle may not only increase antiviral efficacy but also reduce the capacity of the virus to develop resistance to the compound. We developed such an assay platform based on an HCV infectious cell culture system.7 The assay was adapted for the high-throughput screening of a 350 000-member compound collection, affording 149 validated hit compounds.8 Herein, we describe the optimization, preliminary mode of action (MOA) studies, and detailed biopharmaceutical and pharmacokinetic (PK) properties for an aryloxazole class of compounds discovered during the screening campaign and demonstrate that the class is promising for further pharmaceutical development.
RESULTS AND DISCUSSION
Chemistry
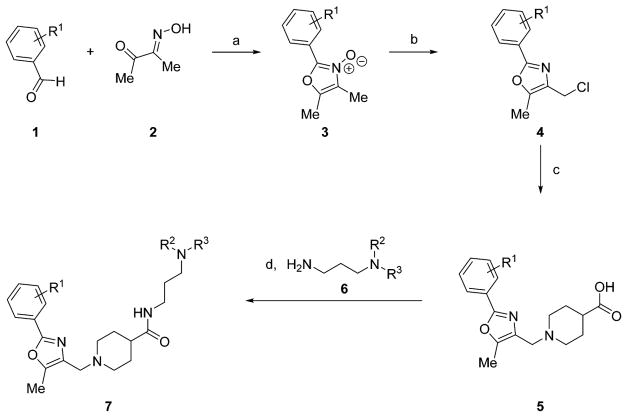
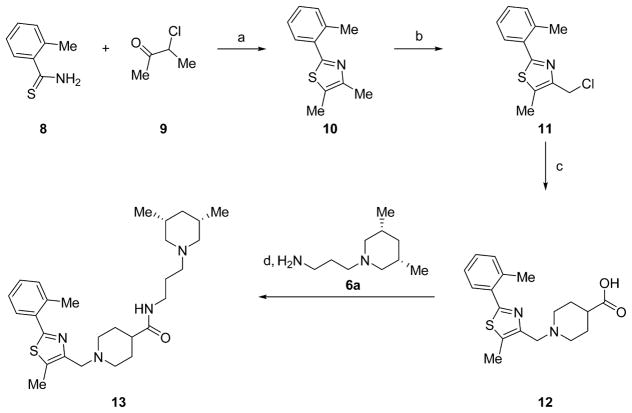
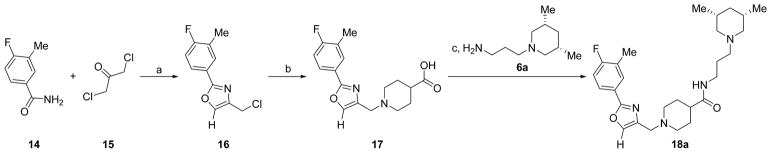
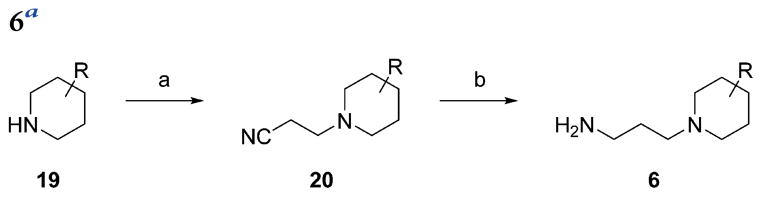
The resynthesized aryloxazole HTS hit compound and most SAR analogues were constructed according to the general route in Scheme 1. The aryloxazole cores 4 were assembled using the method of Goto et al. from commercial benzaldehydes 1 and butane-2,3-dione mono-oxime 2 via the N-oxide intermediates 3.9 Derivatization of 4 with isonipecotic acid provided the carboxylic acids 5, which were coupled with the diamine fragments 6 using diisopropylcarbodiimide and 1-hydroxybenzotriazole (HOBt) hydrate to afford the final analogues 7. The coupling reactions were conducted either at room temperature in DCM or at 100 °C using microwave irradiation in MeCN, depending on when during the SAR campaign the analogues were synthesized. Detailed synthetic protocols for individual final analogues are provided in the Experimental Section. The final compounds were purified by either flash chromatography or reverse-phase, preparative-scale, mass-directed high performance liquid chromatography (HPLC), and their purity was assessed by analytical-scale HPLC under analogous conditions. The thiazole core 10 was synthesized via condensation of 3-chlorobuta-2-one and thioamide 8 (Scheme 2). Chlorination with N-chlorosuccinimide followed by derivatization with isonipecotic acid and diamine fragment 6a as described above afforded the final analogue 13. The desmethyl oxazole core was constructed via condensation and monodehalogenation of 1,3-dichloroacetone with benzamide 14 to directly afford the choromethyloxazole scaffold 16 (Scheme 3). Derivatization with isonipecotic acid and diamine fragments 6 as described above afforded the final analogues 18. The 3-piperidinylpropylamines 6 that were not commercially available were readily synthesized by a short two-step sequence (Scheme 4). Briefly, addition of acrylonitrile to the appropriately substituted piperidine followed by Raney nickel-mediated nitrile reduction under a hydrogen atmosphere afforded the requisite diamine fragments 6. Full synthetic protocols for individual diamine fragments are provided in the Supporting Information.
Scheme 1.
General Synthetic Route to Aryloxazole Analogues 7a
aReagents and conditions: (a) HCl(g) (4 M in dioxane), AcOH, 0 °C to rt; (b) POCl3, DCE, reflux; (c) KOH, EtOH, isonipecotic acid, rt; (d) HOBt hydrate, DMAP, diisopropylcarbodiimide, DCM, rt or HOBt hydrate, diisopropylcarbodiimide, MeCN, μW irradiation, 100 °C, 10 min.
Scheme 2.
Synthetic Route to Thiazole Analogue 13a
aReagents and conditions: (a) iPrOH, μW irradiation, 120 °C, 1 h, 65% yield; (b) NCS, MeCN, 60 °C, 2 h, 82% yield; (c) KOH, EtOH, isonipecotic acid, rt, 78% yield; (d) HOBt hydrate, diisopropylcarbodiimide, MeCN, μW irradiation, 100 °C, 10 min, 45% yield.
Scheme 3.
Representative Synthetic Route to Desmethyloxazole Analogues; Synthesis of 18aa
aReagents and conditions: (a) toluene, 140 °C, 5 h, 57% yield; (b) KOH, EtOH, isonipecotic acid, rt, 93% yield; (d) HOBt hydrate, DMAP, diisopropylcarbodiimide, DCM, rt, 60% yield.
Scheme 4.
General Synthetic Route to Diamine Fragments 6a
aReagents and conditions: (a) acrylonitrile, formamide, water; (b) Raney nickel, H2(g), 200 psi, MeOH, NH3(MeOH).
Structure–Activity Relationship (SAR) Studies
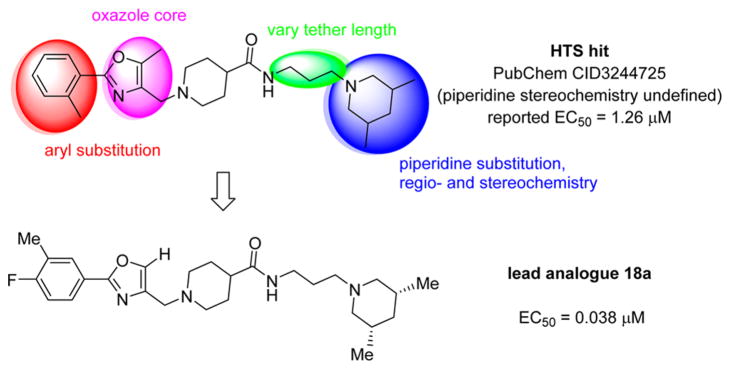
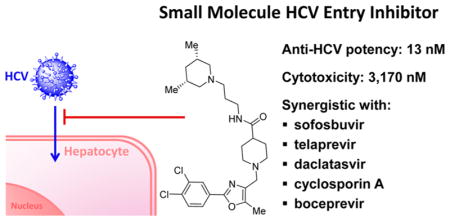
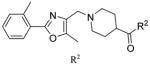
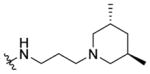
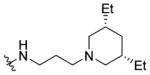
Shown in Figure 1, the structural elements that were investigated during the SAR study are highlighted in the HTS hit molecule (PubChem CID3244725). The majority of the structural analogues focused on varying either aryl-ring substitution or substitution on, or replacement of, the piperidine moiety (the molecular fragments on the left and right ends of the compound hit, respectively). All analogues were screened for inhibition of HCV infection in the cell-based HCV-Luc assay and counterscreened for cytotoxicity in Huh 7.5.1 cells (ATPlite assay). In addition, most analogues were profiled in three assays to assess in vitro biopharmaceutical properties: rat microsomal stability, cell permeability, and aqueous kinetic solubility. Tables 1–3 summarize the results of these SAR investigations. The stereochemistry on the dimethyl-substituted piperidine ring of the HTS hit (PubChem CID3244725) was undefined in the registered structure. Thus, we began our SAR investigation by synthesizing both the cis and trans stereoisomers of the hit compound, 7a and 7b, respectively. The trans stereoisomer 7b was synthesized and screened as a racemic mixture. Both isomers were equipotent in the HCV-Luc assay and showed favorable aqueous solubility; however, 7a possessed slightly lower cytotoxicity and appeared to have better stability in the rat liver microsomes. Replacing the 3,5-dimethyl substitution with bulkier 3,5-diethyl (7c) or 3,3,5,5-tetramethyl substitution (7d) also afforded potent analogues, though with slightly greater cytotoxicity. Moreover, analogues exploring substitution on the piperidine moiety revealed potency to be dependent on both the size and location of substituents on the heterocycle ring. Sufficiently bulky substitution at the 4-position afforded potent analogues (7e and 7f), while a single methyl group substituent at this position (7g) afforded less potent analogues. Consistent with this trend, the unsubstituted piperidine 7h was even less potent. While the cis-2,6-dimethyl substituted analogue 7i did not improve the potency, the 3-substituted analogues (racemic compounds 7j–7l) possessed slightly improved potency compared to that of the 3,5-disubstituted compound 7a. The cis-disubstituted compounds (e.g., 7a), however, do not possess any asymmetric centers (meso compounds) and were therefore screened as single stereoisomers rather than enantiomeric mixtures. The constrained analogue 7m did not provide any improvement in potency or cytotoxicity and added unnecessary structural complexity. In all the above cases, increased steric bulk on the piperidine also correlated with decreased microsomal stability. Decreasing or increasing the tether length by one –CH2 group in the diamine fragment (7n and 7o, respectively) did not improve the potency, and we retained the three-carbon linker for the remaining SAR investigation. Ring-opening of the piperidine to the dialkyl amine 7p afforded a slightly less potent analogue, possibly suggesting that the piperidine constraint might not be required. However, limited exploration of more structurally drastic piperidine replacements was detrimental to potency (7q–7s).
Table 1.
Effect of Varying the Diamine Fragment on Potency, Cytotoxicity, and in Vitro Biopharmaceutical Properties
| entry/cmpd | structure | cell-based assay activity | in vitro pharmacokinetic assays | ||||
|---|---|---|---|---|---|---|---|
|
potency, EC50 (μM)a | cytotoxicity, CC50 (μM)a | selectivity index (CC50/EC50) | rat liver microsome stability t1/2 (min) | PAMPA permeability (1×10−6 cm/s) | aqueous solubility ((μg/ml) | |
| 7a |

|
0.138 ± 0.134 (n=8) | 13.4 ± 2.258 | 97 | >30.0 | 489.1 | 53.9 |
| 7b |
|
0.050 ± 0.025 | 9.390 ± 0.348 | 188 | 14 | 553.7 | >69.0 |
| 7c |
|
0.181 ± 0.104 | 3.467 ± 0.266 | 19 | 5.5 | 1267 | NDb |
| 7d |
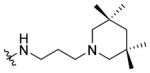
|
0.083 ± 0.046 | 4.377 ± 0.260 | 53 | 5.7 | >1397 | NDb |
| 7e |
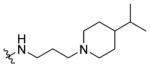
|
0.056 ± 0.015 | 4.283 ± 0.135 | 76 | 8.8 | 395.6 | >71.0 |
| 7f |
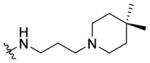
|
0.066 ± 0.029 | 10.153 ± 0.393 | 154 | >30.0 | 241.4 | >69.0 |
| 7g |
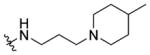
|
0.225 ± 0.160 | 12.267 ± 0.586 | 55 | >30.0 | 424.7 | >67.0 |
| 7h |
|
0.473 ± 0.300 | 18.1 ± 0.800 | 38 | >30.0 | 34.5 | >65.0 |
| 7i |
|
0.120 ± 0.023 | 21.300 ± 1.277 | 178 | >30.0 | 55.4 | >69.0 |
| 7j |
|
0.037 ± 0.006 | 15.000 ± 1.572 | 405 | >30.0 | NDb | >67.0 |
| 7k |
|
0.054 ± 0.013 | 5.823 ± 1.541 | 108 | 19.4 | NDb | >71 |
| 7l |
|
0.094 ± 0.018 | 4.640 ± 0.243 | 49 | 2.3 | 3 | 54.9 |
| 7m |
|
0.106 ± 0.034 | 10.867 ± 0.252 | 103 | 7.4 | 330.9 | >71 |
| 7n |
|
0.156 ± 0.035 | 24.437 ± 4.502 | 157 | >30.0 | 712.4 | >67.0 |
| 7o |
|
0.113 ± 0.065 | 23.133 ± 4.155 | 205 | 12 | 287.8 | >71.0 |
| 7p |
|
0.341 ± 0.142 | 30.467 ± 4.225 | 89 | >30.0 | 913.1 | >65.0 |
| 7q |
|
1.063 ± 0.727 | 65.300 ± 3.387 | 61 | >30.0 | 432.4 | >65.0 |
| 7r |
|
1.621 ± 1.426 | 19.933 ± 2.159 | 12 | >30.0 | <3.2 | >71.0 |
| 7s |
|
2.789 ± 2.800 | 27.233 ± 2.060 | 10 | >30.0 | 14 | >67.0 |
Average of three separate assays (unless otherwise noted) ± standard deviation.
ND, not determined.
Table 3.
Effect of Core Scaffold Modifications on Potency, Cytotoxicity, and in Vitro Biopharmaceutical Properties
| entry/cmpd | structure | cell-based activity and cytotoxicity | in vitro pharmacokinetic assays | ||||
|---|---|---|---|---|---|---|---|
| potency, EC50 (μM)a | cytotoxicity, CC50 (μM)a | selectivity index (CC50/EC50) | rat liver microsome stability t1/2 (min) | PAMPA permeability (1×10−6 cm/s) | aqueous solubility (μg/ml) | ||
| 13 |
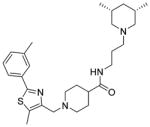
|
0.081 ± 0.041 | 7.053 ± 0.788 | 87 | 7.4 | NDb | >71 |
| 18a |
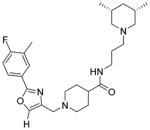
|
0.038 ± 0.037 | 12.967 ± 0.851 | 341 | 14.1 | 215.4 | >70 |
| 18b |
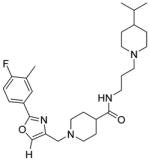
|
0.055 ± 0.020 | 6.195 ± 0.587 | 113 | 2.5 | 276.4 | >72 |
Average of three separate assays (unless otherwise noted) ± standard deviation.
ND, not determined.
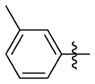
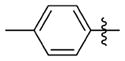
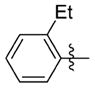
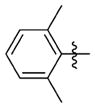
The relationship between activity and aryl ring substitution is summarized in Table 2. Changing the position of the 2-methyl substituent on the aryl ring to either the 3- or 4-position (7t and 7u, respectively) had no significant effect on potency; however, the 4-methyl analogue 7u had increased cytotoxicity. 2-Ethyl substitution (7v) or various arrangements of dimethyl substituted aryl analogues (7w–7aa) all possessed a satisfactory potency of less than 100 nM. Both 7v and the 3,5-dimethyl-substituted analogue 7w possessed reduced microsomal stability compared to those of other analogues. Further increasing the substitution to tri- and penta-methyl substituted aryl analogues (7bb and 7cc, respectively) afforded compounds less potent than those of the mono- or dimethyl substituted analogues. The activities for the monohalogen substituted analogues (7dd–7gg) were on par with the activities for the most potent 2-methyl aryl analogues. The dihalo analogues (7hh–7 mm) were generally less potent; the notable exception being the 3,4-dichloro analogue 7ii, which possessed a potency of 13 nM, ultimately the most potent analogue synthesized in this study. Other than 7ii and the 3,5-difluoro-substituted analogue 7jj, dihalo substitution reduced the microsomal stability. While either monohalogen or methyl substitution was found to afford analogues with attractive potency (<100 nM), the combination of both monohalogen and methyl substitution in a single analogue (7nn–7ss) afforded analogues of only modest potency (124–370 nM) and microsomal stability lower than that of either substitution in isolation. Methoxy substitution (7tt–7zz) was uniformly detrimental to the potency. The naphthyl analogues (7aaa and 7bbb) both possess potency below 100 nM; however, the anthracene analogue (7ccc) was significantly less potent (357 nM).
Table 2.
Effect of Varying the Aryl Substitution on Potency, Cytotoxicity, and in Vitro Biopharmaceutical Properties
| entry/ cmpd |
structure | cell-based activity and cytotoxicity | in vitro pharmacokinetic assays | |||||
|---|---|---|---|---|---|---|---|---|
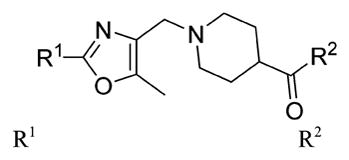
|
potency, EC50 (μM)a |
cytotoxicity, CC50 (μM)a |
selectivity index (CC50/EC50) |
rat liver microsome stability t1/2 (min) |
PAMPA permeability (1×10−6 cm/s) |
solubility (μg/ml) |
||
| 7t |
|

|
0.096 ± 0.027 | 16.483 ± 3.963 | 172 | >30.0 | 279.1 | >69.0 |
| 7u |
|

|
0.070 ± 0.038 | 4.333 ± 2.012 | 62 | >30.0 | 274.3 | >69.0 |
| 7v |
|

|
0.111 ± 0.035 | 9.323 ± 0.784 | 84 | 17 | 106.1 | 55.9 |
| 7w |
|

|
0.091 ± 0.037 | 21.033 ± 4.910 | 231 | 3.1 | 253.9 | 55.9 |
| 7x |
|

|
0.093 ± 0.022 | 6.910 ± 0.834 | 74 | >30.0 | 219.4 | >71.0 |
| 7y |
|

|
0.076 ± 0.016 | 4.113 ± 0.122 | 54 | >30.0 | 353.1 | >71.0 |
| 7z |
|

|
0.037 ± 0.031 | 4.05 ± 0.108 | 109 | <30.0 | 864 | >71.0 |
| 7aa |
|

|
0.098 ± 0.29 | 4.070 ± 0.130 | 42 | 30 | NDb | 56.1 |
| 7bb |
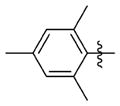
|

|
0.190 ± 0.094 | 6.573 ± 1.253 | 35 | >30 | NDb | NDb |
| 7cc |
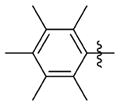
|

|
0.149 ± 0.074 | 8.183 ± 0.671 | 55 | NDb | 455.2 | NDb |
| 7dd |
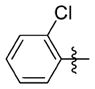
|

|
0.057 ± 0.025 | 16.500 ± 1.735 | 289 | 15 | 352.4 | >72.0 |
| 7ee |
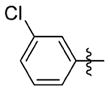
|

|
0.094 ± 0.028 | 4.210 ± 0.201 | 45 | >30.0 | 615.1 | >72.0 |
| 7ff |
|

|
0.041 ± 0.025 | 11.517 ± 6.370 | 281 | >30.0 | >635.0 | >72.0 |
| 7gg |
|

|
0.053 ± 0.029 | 12.800 ± 2.307 | 242 | 28.1 | ND | NDb |
| 7hh |
|

|
0.196 ± 0.024 | 13.233 ± 0.252 | 68 | 2.9 | NDb | >77.0 |
| 7ii |
|

|
0.013 ± 0.008 | 3.17 ± 0.635 | 244 | >30 | 522 | NDb |
| 7jj |
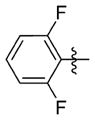
|

|
0.421 ± 0.229 | 32.667 ± 1.159 | 78 | 21 | 82.4 | >72.0 |
| 7kk |
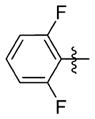
|

|
0.539 ± 0.343 | 12.633 ± 2.307 | 23 | 3.9 | 129.9 | NDb |
| 7ll |
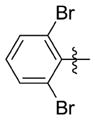
|

|
0.220 ± 0.144 | 6.497 ± 0.166 | 30 | 2.1 | <1.0 | 62.4 |
| 7mm |
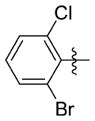
|

|
0.193 ± 0.086 | 12.200 ± 0.173 | 63 | 1.4 | NDb | >83.0 |
| 7nn |
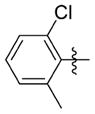
|

|
0.124 ± 0.087 (n=6) | 12.750 ± 1.569 | 103 | 2.9 | 196.3 | >74.0 |
| 7oo |

|

|
0.370 ± 0.179 (n=2) | 16.411 ± 0.501 | 44 | 5.4 | 650.6 | 52.6 |
| 7pp |

|

|
0.220 ± 0.060 | 11.700 ± 0.361 | 53 | 4.4 | NDb | >74 |
| 7qq |
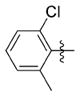
|

|
0.124 ± 0.059 | 4.907 ± 0.364 | 40 | 2 | NDb | >76 |
| 7rr |
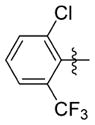
|

|
0.326 ± 0.083 | 12.033 ± 0.723 | 37 | 1.8 | NDb | 65.1 |
| 7ss |
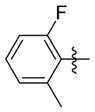
|

|
0.226 ± 0.088 | 13.000 ± 0.458 | 58 | 4.4 | 233.8 | >72.0 |
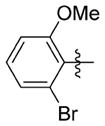
| 7tt |
|

|
0.351 ± 0.167 | 14.900 ± 0.520 | 42 | 3.5 | 245.3 | >83.0 |
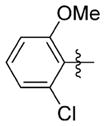
| 7uu |
|

|
0.243 ± 0.195 | 15.833 ± 0.513 | 65 | 3.3 | 51.0 | >76.0 |
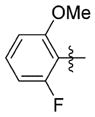
| 7vv |
|

|
0.577 ± 0.238 | 37.000 ± 2.946 | 64 | 8.6 | NDb | >74.0 |
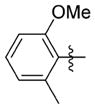
| 7ww |
|

|
0.428 ± 0.057 | 16.700 ± 0.265 | 39 | 4.6 | 197.7 | >73.0 |
| 7xx |
|

|
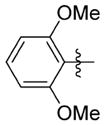
3.110 ± 0.199 | 77.133 ± 12.596 | 25 | 12 | NDb | >76.0 |
| 7yy |
|

|
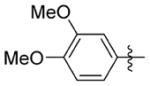
0.440 ± 0.264 | 57.233 ± 3.213 | 130 | >30.0 | 81.9 | >76.0 |
| 7zz |
|
|
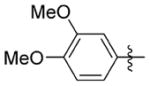
33.983 ± 24.162 | >100 | >3 | >30.0 | 10.4 | >72.0 |
| 7aaa |
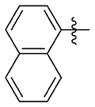
|

|
0.052 ± 0.021 | 4.307 ± 0.479 | 83 | >30.0 | NDb | >74.0 |
| 7bbb |
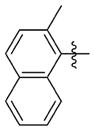
|

|
0.097 ± 0.024 | 4.057 ± 0.177 | 42 | 4.9 | 547.1 | >76.0 |
| 7ccc |
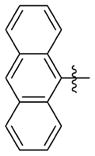
|

|
0.357 ± 0.147 | 4.277 ± 0.110 | 12 | 22 | NDb | 62.1 |
Average of three separate assays (unless otherwise noted) ± standard deviation.
ND, not determined.
We also explored limited structural alterations to the scaffold core, as summarized in Table 3. The thiazole analogue 13 displayed potency similar to that of the hit and, with no clear advantage, further thiazole analogues were not explored. The desmethyl oxazole analogues 18a and 18b were found to be among the most potent analogues, suggesting that the methyl group on the oxazole may not be essential for activity. The cis-dimethylpiperidine analogue 18a also possessed a selectivity index (SI = CC50/EC50) higher than that of either 7a or 7ii (SI = 97, 244, and 341 for 7a, 7ii, and 18a, respectively).
During this SAR study, we established a clear relationship between substitution on the terminal piperidine moiety and ontarget activity; highly potent analogues all contained apparent steric bulk at the 3- and/or 4-position of the piperidine. While a single methyl group at the 3-position gave highly potent analogues, the 4-position required more steric bulk, gem dimethyl or isopropyl substitution to provide equipotent analogues. The aryl moiety distal to the piperidine of the chemotype also allowed some flexibility in substitution. A single substituent (e.g., methyl or chloro) at the ortho position provided highly potent analogues. Disubstituted analogues either at the 3,5- or 3,4-positions also afforded highly potent compounds with these analogues also possessing improved stability in the microsomal stability assay. The limited exploration of changes to the oxazole core indicates that modest changes in this portion of the molecule are tolerated; however, more extensive investigation is needed to establish a more detailed analysis. In summary, we prepared a number of highly potent lead compounds with low cytotoxicity and promising in vitro biopharmaceutical properties.
In Vitro Profiling of Anti-HCV Activity and Selectivity
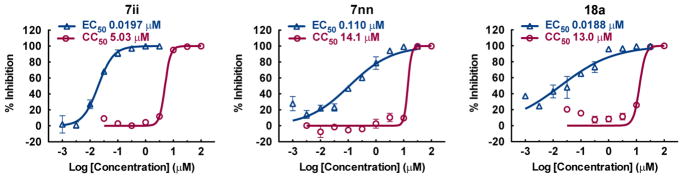
During the SAR studies, a number of promising compounds were identified, and three analogues were chosen for further biological evaluation and characterization. Figure 2 shows the representative titration curves for the anti-HCV activity and cytotoxicity of selected leads. Selectivity indices between 103 and 341 were achieved for these compounds, showing a significant therapeutic index. The analogue used for a particular profiling study switched over the course of the project from 7nn to 7ii once the latter compound emerged as the more potent and selective analogue. The intent of the profiling results is to illustrate the potential of this compound class for further development or mechanistic investigation.
To determine whether this class of compounds possessed a distinct antiviral mode of action from those of the known HCV inhibitors, the activities of the hit compound 7a and the selected lead compounds were measured at 10 μM in a series of assays focused on discrete phases of the viral replication cycle (Table 4). The HCV-Luc antiviral activity and cytotoxicity were confirmed at the same concentration for comparison. In the HCV single-cycle infection assay, single-round infectious HCV defective particles (HCVsc, genotype 2a) can infect and replicate but do not assemble new virions; thus, this assay detects compounds with inhibitory activity to HCV replication cycle events prior to assembly. The activities of 7a and the selected lead compounds 7ii, 7nn, and 18a in the HCVsc infection assay were equivalent to the values obtained in the phenotypic assay, suggesting an early stage target of the viral replication cycle such as HCV entry and/or RNA replication. Therefore, HCV subgenomic replicon assays evaluate whether compounds target viral RNA replication. All compounds showed less than 40% inhibitory effect on HCV replication in GT 1b replicon cell line except for 7ii, possibly due to cytotoxicity, as suggested in the ATPlite assay result. This would indicate that replication-stage targets are not responsible for the HCV inhibition. HCV pseuodoparticles (HCVpp and GT 1a and 1b) utilize defective retroviral particles that display HCV envelope glycoproteins. HCVpp are a well-established surrogate system that mimic the entry stage of cell-culture adapted HCV (HCVcc).10 We thus utilized the HCVpp assay to assess the effect of compound treatment on viral entry. Viral pseudoparticles from vesicular stomatitis virus (VSVpp) were used to test the effect of compounds on an unrelated virus. The hit 7a showed potent inhibitory activity (<30% RLU of control) in HCVpp GT 1a assay and moderate inhibition (~50% RLU) in VSVpp assay. The lead compounds also exhibited potent to moderate inhibition in HCVpp GT 1a and 1b assays. The combined profile for 7a and the lead compounds suggest that the chemotype is targeting an early stage of the viral replication cycle, likely by inhibiting HCV entry based on the HCVpp activity.
Table 4.
Activity of Selected Leads in a Series of HCV Lifecycle Assaysa
| entry | % RLUb | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| HCV-Luc | ATPlite | HCVscc | HCV replicon GT 1bd | HCVppe | MLVppf | VSVppg | ||
|
| ||||||||
| GT 1a | GT 1b | |||||||
| 7a | 2.16 ± 0.508 | 90.0 ± 3.0 | 1.63 ± 1.57 | 75.2 ± 4.9 | 21.9 ± 14.7 | 16.8 ± 6.5 | 48.7 ± 3.85 | 61.5 ± 5.2 |
| 7ii | 0.27 ± 0.07 | 4.88 ± 0.19 | 3.92 ± 1.51 | 9.1 ± 3.0 | 14.9 ± 11.6 | 25.3 ± 6.4 | 57.7 ± 2.4 | 28.0 ± 3.1 |
| 7nn | 0.72 ± 0.32 | 90.4 ± 1.5 | 3.29 ± 2.06 | 83.3 ± 7.0 | 76.8 ± 7.7 | 16.6 ± 10.5 | 102 ± 5 | 77.7 ± 12.0 |
| 18a | 0.59 ± 0.39 | 74.1 ± 3.0 | 4.14 ± 1.94 | 65.2 ± 3.2 | 35.1 ± 5.8 | 31.7 ± 8.5 | 111 ± 6 | 78.0 ± 4.9 |
Activity of compound (10 μM) in a series of assays to assess efficacy in different stages of the viral life cycle. Results are presented as percentage response compared to the untreated control.
Relative luminescence units.
HCV single-cycle infection assay.
HCV subgenomic replicon assay.
HCV pseudoparticle assay.
Murine leukemia virus pseudoparticle assay.
Vesicular stomatitis virus pseudoparticle assay.
In Vitro Toxicity Profiling against a Panel of 50 CNS-Relevant Targets
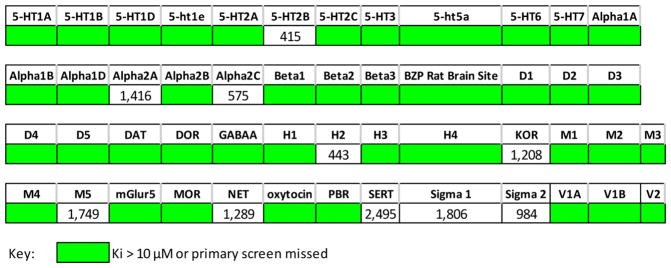
Compound 7nn was evaluated in a panel of 50 CNS-relevant receptors and neurotransmitter transporters by the Psychoactive Drug Screening Program (PDSP) at the University of North Carolina, Chapel Hill (Figure 3). The results show that 7nn has submicromolar affinity for only 4 targets across the 50-component panel tested. The highest affinity was for the serotoninergic receptor 5-HT2B (415 nM), which has been implicated in the development of myofibroblast proliferation.11,12 While any cardiac issue is of concern, the selectivity for 7nn against the other targets in the PDSP panel and the modest affinity for the 5-HT2B receptor bode well that this off-target activity could be minimized with further compound development. The receptor profiling of other compounds will be conducted following additional characterization of select lead compounds for further development.
In Vitro Combination Profiling with Different Classes of Anti-HCV Drugs
We investigated the combination of 7ii with different classes of anti-HCV drugs. The HCV-Luc and the ATPlite assays were performed in the presence of various concentrations of 7ii in combination with various concentrations of each drug (Table 5). A synergistic effect was indicated if the combination led to an HCV inhibitory effect greater than that of either of them alone in a concentrationdependent manner without a toxic effect on cell viability. Log volumes of synergy or antagonism were generated according to the Bliss independence model using the MacSynergy II program.13 The results were also analyzed with the CalcuSyn program,14 in which the combination indices were calculated from combination of 7ii and the tested drug at or near their EC50 values when tested alone. The hit compound 7a was tested following the same protocol. We found that 7ii was synergistic with ribavirin, sofosbuvir, telaprevir, daclatasvir, cyclosporin A, and boceprevir without significant cytotoxicity, supporting its potential for use in combination therapy with these drugs. Similar synergistic effects were observed with compound 7a, except for nearly additive effect in combination with sofosbuvir. The observed synergistic effects suggest that the aryloxazole analogues likely operate via a distinct mode of action from that of any one of these drugs. The mechanism of action of ribavirin is mediated through host antiviral response. Telaprevir and boceprevir are NS3/4A protease inhibitors and daclatasvir inhibits HCV NS5A. Cyclosporin A targets virus RNA replication. Together, this collection of HCV drugs covers the known modes of action for currently available therapeutics; thus this chemotype, is attractive for further development.
Table 5.
Synergistic Activity of 7a and 7ii with Selected HCV Therapeutics
| entry | program | parameter | ribavirin | sofosbuvir | telaprevir | daclatasvir | cyclosporin A | boceprevir |
|---|---|---|---|---|---|---|---|---|
| 7a | CalcuSyn | CI valuea | 0.770 ± 0.189 | 1.13 ± 0.12 | 0.775 ± 0.108 | 0.510 ± 0.082 | 0.625 ± 0.136 | ND |
| synergy volumeb | ++ | ± | ++ | +++ | +++ | ND | ||
| MacSynergy | synergy volumec | +++ | + | +++ | + | ++ | ND | |
| 7ii | CalcuSyn | CI valuea | 0.745 ± 0.084 | 0.759 ± 0.076 | 0.740 ± 0.102 | 0.831 ± 0.133 | 0.867 ± 0.115 | 0.782 ± 0.104 |
| synergy volumeb | ++ | ++ | ++ | + | + | ++ | ||
| MacSynergy | synergy volumec | ++ | +++ | +++ | +++ | ++ | ++ |
Values are mean ± SEM of combination indices (CI) obtained from combinations of the tested drug with 7a or 7ii at or near their EC50 values when tested alone (n ≥ 6).
The level of synergy is defined as the following: ± means nearly additive (0.9 ≤ CI < 1.1), + means minor synergy (0.8 ≤ CI < 0.9), ++ means moderate synergy (0.7 ≤ CI < 0.8), and +++ means strong synergy (CI < 0.7).
The levels of synergy are defined as the following: ± means nearly additive (0 ≤ log volume <2), + means minor synergy (2 ≤ log volume <5), ++ means moderate synergy (5 ≤ log volume <9), and +++ means strong synergy (log volume >9).
In Vivo Pharmacokinetic Studies in Mice at Multiple Doses
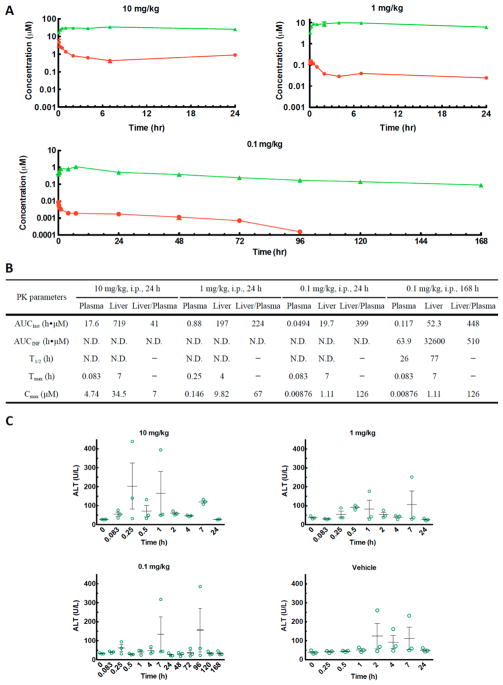
We evaluated the in vivo pharmacokinetics and tissue distribution of 7ii in a mouse model after a single dose of 10 and 1 mg/kg through the intraperitoneal (i.p.) route of administration (Figure 4). Excellent liver distribution was observed at both doses, as shown in the liver/plasma AUC0–24h ratio of 41 and 224, respectively. Slow clearance in plasma was observed at both doses, leading to half-lives longer than 24 h. When dosed at 1 mg/kg, the liver concentration of 7ii throughout 24 h post-administration (3.33–9.82 μM) was more than 100-fold of its in vitro EC50 values (0.013 μM). In light of the above results, we further evaluated the pharmacokinetics properties of 7ii at 0.1 mg/kg and elongated the study time to 168 h (7 days). 7ii retained excellent liver distribution at this dose during the first 24 h post-administration and during the total 168 h study (liver/plasma AUC0–24h and AUC0–168h = 399 and 448, respectively). A reasonably long half-life was observed in liver (t1/2 = 77 h) as well as in plasma (t1/2 = 26 h). At a dose as low as 0.1 mg/kg, the concentration of 7ii in liver reached 0.39 μM in the first 5 min and remained at least 7-fold above its EC50 values (0.013 μM) throughout the 168 h. Alanine aminotransaminase (ALT) level in the mouse serum was monitored to detect any potential hepatotoxicity effect (Figure 4C).15 Regardless of the dose, the ALT levels were around or below 80 U/L at most time points with a few exceptions of higher ALT at random time points from individual mice. These elevations are unlikely due to the effect of the compound because similar random elevations were noted in mice treated with vehicle only (Figure 4C).16 Moreover, there was not an obvious correlation between the ALT levels and the liver concentration of compound 7ii. Overall, the representative analogue 7ii exhibited high liver distribution and long half-life without obvious hepatotoxicity, indicated by ALT level in the mouse model.
In Vitro Antiviral Specificity Profiling against a Panel of 13 Viruses
To assess whether this chemotype exhibits nonspecific antiviral effects against viruses other than HCV, we carried out an antiviral screen with representative lead compound 7nn against 13 viruses utilizing the nonclinical and preclinical services program offered by the National Institute of Allergy and Infectious Diseases (https://www.niaid.nih.gov/research/vitro-assessment-antimicrobial-activityresources-niaid). The 13 types of viruses are hepatitis B virus, HCV replicon, herpes simplex virus-1, human cytomegalovirus, vaccinia virus, dengue virus, influenza A (H1N1) virus, respiratory syncytial virus, SARS coronavirus, poliovirus 3, Rift Valley fever virus, Tacaribe virus, and Venezuelan equine encephalitis virus. As shown in the Supporting Information, compound 7nn had little or no activity (selective index ≤10 and/or EC50 > 2 μM) against all the above viruses. These results suggest that this series of compounds is selectively active against HCV infection.
CONCLUSIONS
We presented the development of a new class of HCV inhibitors. The SAR study generated a number of potent analogues with low cytotoxicity and a promising preliminary PK profile in mice, the species potentially used for the efficacy evaluation. The compound class appears to act via a mode of action distinct from that of HCV inhibitors currently approved for HCV therapy. Such HCV inhibitors with a novel mechanism targeting entry could offer a lower probability of developing resistant virus strains during treatment as well as provide an additional weapon against nonresponsive cases.17 The attractiveness of HCV entry as an anti-HCV target is evidenced by multiple recent efforts in developing HCV entry inhibitors.18–20 To attain an all oral, pangenotypic HCV treatment with the shortest possible course of treatment, it would be of benefit to target multiple viral or host targets simultaneously. Studies toward determining the molecular target and validating the efficacy in vivo are underway and will be reported in due course.
EXPERIMENTAL SECTION
General Synthesis and Analysis Experimental Details
All reagents were used as received from the following suppliers: Alfa Aesar, Ark Pharm, Aldrich, and Fisher Scientific. Acetonitrile and THF were purified using the Innovative Technology PureSolv solvent purification system. The 1H and 13C spectra were recorded on a Bruker Avance 400 or 500 MHz spectrometer. Chemical shifts are reported in parts per million and were referenced to residual proton solvent signals. 13C multiplicities were determined with the aid of an APT pulse sequence, differentiating the signals for methyl (CH3) and methyne (CH) carbons as “d” from methylene (CH2) and quaternary (C) carbons as “u”. The infrared (IR) spectra were acquired as thin films using a universal ATR sampling accessory on a Thermo Scientific Nicolet iS5 FT-IR spectrometer, and the absorption frequencies are reported in cm−1. Microwave syntheses were conducted in a Biotage Initiator constant temperature microwave synthesizer. Flash column chromatography separations were performed using the Teledyne Isco CombiFlash RF using RediSep RF silica gel columns. TLC was performed on Analtech UNIPLATE silica gel GHLF plates (gypsum inorganic hard layer with fluorescence). TLC plates were developed using iodine vapor. Automated preparative RP HPLC purification was performed using an Agilent 1200 mass-directed fractionation system (Prep Pump G1361 with gradient extension, makeup pump G1311A, pH modification pump G1311A, HTS PAL autosampler, UV-DAD detection G1315D, fraction collector G1364B, and Agilent 6120 quadrapole spectrometer G6120A). The preparative chromatography conditions included a Waters X-Bridge C18 column (19 × 150 mm, 5 μm, with 19 × 10 mm guard column), elution with a water and acetonitrile gradient, which increases 20% in acetonitrile content over 4 min at a flow rate of 20 mL/min (modified to pH 9.8 through addition of NH4OH by auxiliary pump), and sample dilution in DMSO. The preparative gradient, triggering thresholds, and UV wavelength were selected according to the analytical RP HPLC analysis of each crude sample. The analytical method used an Agilent 1200 RRLC system with UV detection (Agilent 1200 DAD SL) and mass detection (Agilent 6224 TOF). The analytical method conditions included a Waters Aquity BEH C18 column (2.1 × 50 mm, 1.7 μm) and elution with a linear gradient of 5% acetonitrile in pH 9.8 buffered aqueous ammonium formate to 100% acetonitrile at 0.4 mL/min flow rate. Compound purity was measured on the basis of peak integration (area under the curve) from UV–vis absorbance (at 214 nm), and compound identity was determined on the basis of mass analysis. Compounds used for assays or biological studies have HPLC purity >95% with the exception of 7yy (purity = 94.2%). The analytical HPLC system used is a dedicated instrument for assessing compound purity and routinely detects impurities as low as 0.1% that elute within the detection window. Any compounds with a measured purity of 100% were thus conservatively assigned a purity of >99.8%. Any compounds purified by reverse-phase, preparative HPLC utilized the same solvent gradient and column material as the analytical conditions to minimize the possibility of impurities that were not detected in the analytical method. All final compounds were inspected for functional groups known to contribute PAINS liabilities, and none were found.
General Procedure A-1: Coupling of Carboxylic Acid Fragment 5 or 12 and Amine Fragment 6
To a mixture of oxazolecarboxylic acid 5 or thiazolecarboxylic acid 12, amine 6 (1.0–2.0 equiv), and HOBt (1.0 equiv) in MeCN (1.5 to 4 mL, ca. 0.1 M) was added diisopropyl carbodiimide (2.0 equiv). The microwave vial was capped and irradiated at 100 °C for 10 min. After cooling to rt, the solvent was removed, and the residue purified by silica gel chromatography (eluents 0–20% MeOH + 0–2% NH4OH(aq) in CH2Cl2) to afford the coupled product.
General Procedure A-2: Coupling of Oxazolecarboxylic Acid 5 and Amine Fragment 6
To a mixture of oxazolecarboxylic acid 5, amine 6 (1.0–2.0 equiv), and HOBt (1.0 equiv) in MeCN (1.5 to 4 mL, ca. 0.1 M) was added diisopropyl carbodiimide (2.0 equiv). The microwave vial was capped and irradiated at 100 °C for 10 min. After cooling to rt, the solvent was removed, and the residue was purified by C-18 functionalized silica chromatography (eluents 5–100% MeCN in deionized water with 0.5% NH4OH(aq)) to afford the coupled product 7.
General Procedure A-3: Coupling of Oxazolecarboxylic Acid 5 or 17 and Amine Fragment 6
To a mixture of oxazolecarboxylic acid 5 or 17, amine 6 (1.0–2.0 equiv), DMAP (0.1 equiv), and HOBt (1.0 equiv) in CH2Cl2 (1.5 to 4 mL, ca. 0.05 M) was added diisopropyl carbodiimide (5.0 equiv). The reaction was stirred at rt for 16 h; the solvent was removed, and the residue was purified by silica gel chromatography (eluents 0–20% MeOH + 0–2% NH4OH(aq) in CH2Cl2) to afford the coupled product 7.
General Procedure A-4: Coupling of Oxazolecarboxylic Acid 5 and Amine Fragment 6
To a mixture of oxazolecarboxylic acid 5, amine 6 (1.0–2.0 equiv), DMAP (0.1 equiv), and HOBt (1.0 equiv) in CH2Cl2 (1.5 to 4 mL, ca. 0.05 M) was added diisopropyl carbodiimide (5.0 equiv). The reaction was stirred at rt for 16 h; the solvent was removed, and the residue was purified by automated preparative RP HPLC purification as described in the general Experimental Section to afford the coupled product 7.
Synthesis of Aryloxazole Final Analogues
N-(3-(cis-3,5- Dimethylpiperidin-1-yl)propyl)-1-((5-methyl-2-(o-tolyl)oxazol-4-yl)- methyl)piperidine-4-carboxamide 7a
1-((5-Methyl-2-(o-tolyl)- oxazol-4-yl)methyl)piperidine-4-carboxylic acid (41 mg, 0.13 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (22 mg, 0.13 mmol) were reacted according to general procedure A-1 to afford the product as a white solid (46 mg, 0.098 mmol, 75% yield). 1H NMR (400 MHz, CDCl3) δ 0.53 (q, J = 12.3 Hz, 1H), 0.86 (d, J = 6.6 Hz, 6H), 1.39 (t, J = 11.0 Hz, 2H), 1.58–1.85 (complex, 9H), 1.97–2.14 (m, 3H), 2.39 (s, 3H), 2.42 (t, J = 6.2 Hz, 2H), 2.65 (s, 3H), 2.85–2.89 (m, 2H), 3.01–3.05 (m, 2H), 3.33 (q, J = 6.1 Hz, 2H), 3.48 (s, 2H), 7.22–7.32 (m, 3H), 7.91–7.94 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 21.8, 31.4, 43.5, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.9, 29.1, 40.0, 42.1, 53.1, 53.9, 58.3, 61.8, 126.9, 132.4, 137.0, 145.5, 159.8, 174.8; IR 1541, 1646, 2950 cm−1; HRMS calcd for C28H43N4O2 [M + H]+ 467.3381; found 467.3373; HPLC purity: >99.8%.
(±)-N-(3-(trans-3,5-Dimethylpiperidin-1-yl)propyl)-1-((5-methyl- 2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxamide 7b
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (64 mg, 0.20 mmol) and 3-(trans-3,5-dimethylpiperidin-1-yl)propan-1- amine (42 mg, 0.24 mmol) were reacted according to general procedure A-2 to afford the product as a light yellow solid (81 mg, 0.17 mmol, 85% yield). 1H NMR (400 MHz, CDCl3) δ 0.96 (d, J = 6.8 Hz, 6H), 1.30 (t, J = 5.5 Hz, 2H), 1.56–1.71 (m, 2H), 1.76–1.92 (complex, 6H), 1.99–2.11 (complex, 5H), 2.30–2.36 (m, 4H), 2.38 (s, 3H), 2.65 (s, 3H), 3.01–3.06 (m, 2H), 3.19–3.27 (m, 1H), 3.38–3.45 (m, 1H), 3.47 (s, 2H), 7.13 (t, J = 4.1 Hz, 1H), 7.21–7.29 (m, 2H), 7.91–7.93 (m, 1H); 13C NMR ((101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.3, 21.8, 27.4, 43.6, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.8, 28.9, 29.1, 38.9, 39.8, 53.1, 53.9, 58.4, 61.4, 126.9, 132.3, 137.0, 145.6, 159.7, 174.8; IR 1546, 1645, 1710, 2930 cm−1; HRMS calcd for C28H43N4O2 [M + H]+ 467.3386; found 467.3376; HPLC purity: 98.5%.
N-(3-(cis-3,5-Diethylpiperidin-1-yl)propyl)-1-((5-methyl-2-(otolyl) oxazol-4-yl)methyl)piperidine-4-carboxamide 7c
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.08 mmol) and 3-(cis-3,5-diethylpiperidin-1-yl)propan-1-amine (17 mg, 0.09 mmol) were reacted according to general procedure A-3 to afford the product as a light yellow, viscous oil (14 mg, 0.028 mmol, 35% yield). 1H NMR (400 MHz, CDCl3) δ 0.91 (t, J = 7.4 Hz, 6H), 1.18–1.29 (m, 4H), 1.41–1.48 (m, 4H), 1.66–1.72 (m, 2H),1.79–1.89 (complex, 5H), 2.00–2.09 (m, 2H), 2.12 (dt, J = 3.4, 11.2 Hz, 2H), 2.41 (s, 3H), 2.44 (t, J = 6.2 Hz, 2H), 2.67 (s, 3H), 2.96 (d, J = 6.8 Hz, 2H), 3.06 (d, J = 11.7 Hz, 2H), 3.36 (q, J = 6.2 Hz, 2H), 3.51 (s, 2H), 7.24–7.34 (m, 3H), 7.93–7.96 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 11.5, 21.8, 38.1, 43.5, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.9, 27.4, 29.1, 37.4, 40.0, 53.1, 53.9, 58.5, 60.4, 126.9, 132.3, 137.0, 145.6, 159.8, 174.9; HRMS calcd for C30H47N4O2 [M + H]+ 495.3694; found 495.3693; HPLC purity: >99.8%.
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)-N-(3-(cis-3,3,5,5-tetramethylpiperidin- 1-yl)propyl)piperidine-4-carboxamide 7d
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.08 mmol) and 3-(3,3,5,5-tetramethylpiperidin-1-yl)propan-1-amine (17 mg, 0.09 mmol) were reacted according to general procedure A-3 to afford the product as a light yellow, viscous oil (23 mg, 0.046 mmol, 58% yield). 1H NMR (400 MHz, CDCl3) δ 1.03 (s, 12H), 1.21 (s, 2H), 1.74–1.81 (m, 2H), 1.90–1.95 (complex, 4H), 2.17–2.27 (m, 3H), 2.41 (s, 3H), 2.50–2.57 (m, 2H), 2.63 (s, 4H), 2.66 (s, 3H), 3.21–3.27 (m, 2H), 3.34 (q, J = 6.2 Hz, 2H), 3.69 (s, 2H), 7.24–7.34 (m, 3H), 7.91–7.94 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 21.9, 29.5, 41.0, 125.9, 128.6, 129.7, 131.5; u (C, CH2): 24.9, 27.9, 31.4, 38.2, 50.1, 52.1, 52.8, 56.8, 66.1, 126.4, 129.6, 137.1, 147.3, 160.0, 175.1; IR 1557, 1670, 2957 cm−1; HRMS calcd for C30H47N4O2 [M + H]+ 495.3694; found 495.3692; HPLC purity: >99.8%.
N-(3-(4-Isopropylpiperidin-1-yl)propyl)-1-((5-methyl-2-(o-tolyl)-oxazol-4-yl)methyl)piperidine-4-carboxamide 7e
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (65 mg, 0.21 mmol) and 3-(4-isopropylpiperidin-1-yl)propan-1-amine (46 mg, 0.25 mmol) were reacted according to general procedure A-2 to afford the product as a colorless, viscous oil (55 mg, 0.11 mmol, 55% yield). 1H NMR (400 MHz, CDCl3) δ 0.83 (d, J = 6.8 Hz, 6H), 1.08–0.99 (m, 1H), 1.33–1.15 (m, 2H), 1.40 (dq, J = 13.3, 6.4 Hz, 1H), 1.67–1.61 (m, 4H), 1.91–1.73 (m, 6H), 2.14–1.96 (m, 3H), 2.38 (s, 3H), 2.45–2.39 (m, 2H), 2.65 (s, 3H), 3.08–2.92 (m, 4H), 3.34–3.30 (m, 2H), 3.47 (s, 2H), 7.33–7.17 (m, 3H), 7.59 (t, J = 4.6 Hz, 1H), 7.92 (dd, J = 8.0, 1.7 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 21.8, 32.4, 42.2, 43.4, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.7, 29.1, 29.4, 40.2, 53.1, 53.8, 54.5, 58.5, 126.8, 132.3, 137.0, 145.6, 159.7, 174.8; IR 1541, 1641, 2938 cm−1; HRMS (m/z): calcd for C29H45N4O2 [M + H]+ 481.3543; found 481.3537; HPLC purity: >99.8%.
N-(3-(4,4-Dimethylpiperidin-1-yl)propyl)-1-((5-methyl-2-(o-tolyl)- oxazol-4-yl)methyl)piperidine-4-carboxamide 7f
1-((5-Methyl-2-(otolyl) oxazol-4-yl)methyl)piperidine-4-carboxylic acid (45 mg, 0.14 mmol) and 3-(4,4-dimethylpiperidin-1-yl)propan-1-amine (24 mg, 0.14 mmol) were reacted according to general procedure A-2 to afford the product as a light yellow, viscous oil (47 mg, 0.10 mmol, 70% yield). 1H NMR (400 MHz, CDCl3) δ 0.90 (s, 6H), 1.23–1.28 (m, 2H), 1.39 (t, J = 5.6 Hz, 4H), 1.61–1.68 (m, 2H), 1.75–1.90 (complex, 4H), 2.00–2.13 (complex, 3H), 2.38 (s, 3H), 2.39–2.46 (m, 2H), 2.45 (t, J = 6.2 Hz, 2H), 2.64 (s, 3H), 3.05 (d, J = 11.6 Hz, 2H), 3.30–3.35 (m, 2H), 3.48 (s, 2H), 7.21–7.31 (m, 3H), 7.56 (br s, 1H), 7.90–7.93 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 21.8, 43.4, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.7, 28.5, 29.1, 38.9, 40.1, 50.3, 53.1, 53.8, 58.4, 126.9, 132.3, 137.0, 145.6, 159.7, 174.8; HRMS (m/z): calcd for C28H43N4O2 [M + H]+ 467.3381; found 467.3389; HPLC purity: >99.8%.
N-(3-(4-Methylpiperidin-1-yl)propyl)-1-((5-methyl-2-(o-tolyl)- oxazol-4-yl)methyl)piperidine-4-carboxamide 7g
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (51 mg, 0.16 mmol) and 3-(4-methylpiperidin-1-yl)propan-1-amine (25 mg, 0.16 mmol) were reacted according to general procedure A-4 to afford the product as a colorless, viscous oil (16 mg, 0.034 mmol, 21% yield). 1H NMR (400 MHz, CDCl3) δ 0.90 (d, J = 6.4 Hz, 3H), 1.18 (dq, J = 3.2, 12.2 Hz, 2H), 1.31–1.43 (m, 1H), 1.62–1.68 (complex, 4H), 1.72–1.92 (complex, 6H), 1.99–2.13 (complex, 3H), 2.38 (s, 3H), 2.42 (t, J = 6.2 Hz, 2H), 2.65 (s, 3H), 2.91 (d, J = 11.6 Hz, 2H), 3.04 (d, J = 11.6 Hz, 2H), 3.33 (q, J = 5.5 Hz, 2H), 3.48 (s, 2H), 7.21–7.31 (m, 3H), 7.49 (br s, 1H), 7.90–7.93 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 21.8, 22.0, 30.7, 43.4, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.8, 29.1, 34.5, 40.0, 53.1, 53.8, 54.1, 58.4, 126.9, 132.3, 137.0, 145.6, 159.8, 174.9; HRMS (m/z): calcd for C27H41N4O2 [M + H]+ 453.3224; found 453.3236; HPLC purity: >99.8%.
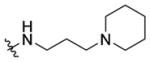
N-(3-(Piperidin-1-yl)propyl)-1-((5-methyl-2-(o-tolyl)oxazol-4-yl)-methyl)piperidine-4-carboxamide 7h
1-((5-Methyl-2-(o-tolyl)-oxazol-4-yl)methyl)piperidine-4-carboxylic acid (51 mg, 0.16 mmol) and 3-(piperidin-1-yl)propan-1-amine (23 mg, 0.16 mmol) were reacted according to general procedure A-4 to afford the product as a colorless, viscous oil (19 mg, 0.044 mmol, 27% yield). 1H NMR (400 MHz, CDCl3) δ 1.41–1.49 (m, 2H), 1.55–1.68 (complex, 6H), 1.73–1.88 (complex, 5H), 2.00–2.14 (complex, 3H), 2.39 (s, 3H), 2.39–2.42 (complex, 5H), 2.65 (s, 3H), 3.04 (d, J = 11.6 Hz, 2H), 3.33 (q, J = 6.0 Hz, 2H), 3.48 (s, 2H), 7.22–7.31 (m, 3H), 7.40 (br s, 1H), 7.90–7.94 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 21.8, 43.5, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.3, 24.7, 26.1, 29.1, 40.0, 53.1, 53.9, 54.7, 58.8, 126.9, 132.4, 137.0, 138.7, 145.6, 174.9; HRMS (m/z): calcd for C26H39N4O2 [M + H]+ 439.3068; found 439.3083; HPLC purity: >99.8%.
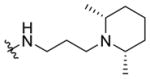
N-(3-(cis-2,6-Dimethylpiperidin-1-yl)propyl)-1-((5-methyl-2-(otolyl) oxazol-4-yl)methyl)piperidine-4-carboxamide 7i
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (78 mg, 0.25 mmol) and 3-(cis-2,6-dimethylpiperidin-1-yl)propan-1-amine (51 mg, 0.30 mmol) were reacted according to general procedure A-2 to afford the product as a white solid (81 mg, 0.17 mmol, 70% yield). 1H NMR (400 MHz, CDCl3) δ 0.53 (q, J = 12.3 Hz, 1H), 0.86 (d, J = 6.6 Hz, 6H), 1.39 (t, J = 11.0 Hz, 2H), 1.58–1.85 (complex, 9H), 1.97–2.14 (m, 3H), 2.39 (s, 3H), 2.42 (t, J = 6.2 Hz, 2H), 2.65 (s, 3H), 2.85–2.89 (m, 2H), 3.01–3.05 (m, 2H), 3.33 (q, J = 6.1 Hz, 2H), 3.48 (s, 2H), 7.22–7.32 (m, 3H), 7.91–7.94 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 21.8, 31.4, 43.5, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.9, 29.1, 40.0, 42.1, 53.1, 53.9, 58.3, 61.8, 126.9, 132.4, 137.0, 145.5, 159.8, 174.8; HRMS calcd for C28H43N4O2 [M + H]+ 467.3386; found 467.3377; HPLC purity: 98.3%.
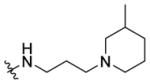
(±)-N-(3-(3-Methylpiperidin-1-yl)propyl)-1-((5-methyl-2-(o-tolyl)-oxazol-4-yl)methyl)piperidine-4-carboxamide 7j
1-((5-Methyl-2-(otolyl) oxazol-4-yl)methyl)piperidine-4-carboxylic acid (70 mg, 0.22 mmol) and 3-(3-methylpiperidin-1-yl)propan-1-amine (42 mg, 0.67 mmol) were reacted according to general procedure A-2 to afford the product as a white solid (81 mg, 0.18 mmol, 80% yield). 1H NMR (400 MHz, CDCl3) δ 0.89 (d, J = 6.5 Hz, 3H), 1.50–1.88 (complex, 13H), 2.01–2.15 (m, 3H), 2.40 (s, 3H), 2.43 (t, J = 6.2 Hz, 2H), 2.66 (s, 3H), 2.84–2.94 (m, 2H), 3.05 (d, J = 11.6 Hz, 2H), 3.35 (q, J = 6.0 Hz, 2H), 3.50 (s, 2H), 7.23–7.33 (m, 3H), 7.37 (br s, 1H), 7.92–7.95 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 19.7, 21.8, 31.3, 43.4, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.7, 25.6, 29.1, 32.9, 39.9, 53.1, 53.9, 54.1, 58.4, 62.2, 126.9, 132.4, 137.0, 145.5, 159.7, 174.9; HRMS (m/z): calcd for C27H41N4O2 [M + H]+ 453.3224; found 453.3227; HPLC purity: >99.8%.
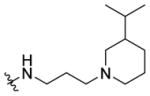
(±)-N-(3-(3-Isopropylpiperidin-1-yl)propyl)-1-((5-methyl-2-(otolyl) oxazol-4-yl)methyl)piperidine-4-carboxamide 7k
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.080 mmol) and 3-(3-isopropylpiperidin-1-yl)propan-1-amine (16 mg, 0.087 mmol) were reacted according to general procedure A-3 to afford the product as a light yellow, viscous oil (33 mg, 0.068 mmol, 85% yield). 1H NMR (400 MHz, CDCl3) δ 0.88 (dd, J = 2.5, 6.8 Hz, 6H), 0.95 (dq, J = 3.7, 11.9 Hz, 1H), 1.24–1.34 (m, 2H), 1.40–1.56 (m, 2H), 1.60–1.86 (complex, 9H), 2.00–2.14 (m, 3H), 2.40 (s, 3H), 2.43 (t, J = 6.2 Hz, 2H), 2.66 (s, 3H), 2.88–2.94 (m, 2H), 3.05 (d, J = 11.6 Hz, 2H), 3.31–3.37 (m, 2H), 3.49 (s, 2H), 7.23–7.32 (m, 3H), 7.41 (br s, 1H), 7.92–7.95 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.8, 20.2, 21.8, 31.1, 42.8, 43.5, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.8, 25.8, 27.7, 29.0, 39.9, 53.1, 53.8, 54.4, 58.4, 58.6, 126.9, 132.3, 137.0, 145.6, 159.7, 174.9; HRMS (m/z): calcd for C29H45N4O2 [M + H]+ 481.3543; found 481.3539; HPLC purity: 99.3%.
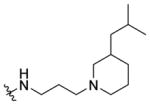
(±)-N-(3-(3-Isobutylpiperidin-1-yl)propyl)-1-((5-methyl-2-(otolyl) oxazol-4-yl)methyl)piperidine-4-carboxamide 7l
1-((5-Methyl- 2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (67 mg, 0.21 mmol) and 3-(3-isobutylpiperidin-1-yl)propan-1-amine (42 mg, 0.21 mmol) were reacted according to general procedure A-4 to afford the product as a colorless, viscous oil (22 mg, 0.044 mmol, 21% yield). 1H NMR (400 MHz, CDCl3) δ 0.88 (dd, J = 2.5, 6.8 Hz, 6H), 0.95 (dq, J = 3.7, 11.9 Hz, 1H), 1.24–1.34 (m, 2H), 1.40–1.56 (m, 2H), 1.60–1.86 (complex, 9H), 2.00–2.14 (m, 3H), 2.40 (s, 3H), 2.43(t, J = 6.2 Hz, 2H), 2.66 (s, 3H), 2.88–2.94 (m, 2H), 3.05 (d, J = 11.6 Hz, 2H), 3.31–3.37 (m, 2H), 3.49 (s, 2H), 7.23–7.32 (m, 3H), 7.41 (br s, 1H), 7.92–7.95 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.8, 20.2, 21.8, 31.1, 42.8, 43.5, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.8, 25.8, 27.7, 29.0, 39.9, 53.1, 53.8, 54.4, 58.4, 58.6, 126.9, 132.3, 137.0, 145.6, 159.7, 174.9; HRMS (m/z): calcd for C30H47N4O2 [M + H]+ 495.3694; found 495.3695; HPLC purity: >99.8%.
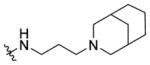
N-(3-(3-Azabicyclo[3.3.1]nonan-3-yl)propyl)-1-((5-methyl-2-(otolyl) oxazol-4-yl)methyl)piperidine-4-carboxamide 7m
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (62 mg, 0.20 mmol) and 3-(3-azabicyclo[3.3.1]nonan-3-yl)propan-1-amine (55 mg, 0.30 mmol) were reacted according to general procedure A-4 to afford the product as a colorless, viscous oil (21 mg, 0.044 mmol, 22% yield). 1H NMR (400 MHz, CDCl3) δ 1.46–1.55 (m, 3H), 1.60–1.86 (complex, 11H), 2.02–2.15 (complex, 5H), 2.22 (t, J = 6.4 Hz, 2H), 2.31–2.44 (m, 2H), 2.38 (s, 3H), 2.64 (s, 3H), 2.90 (d, J = 10.7 Hz, 2H), 3.05 (d, J = 11.8 Hz, 2H), 3.31 (q, J = 5.6 Hz, 2H), 3.48 (s, 2H), 6.06 (br s, 1H), 7.22–7.31 (m, 3H), 7.91–7.93 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 21.8, 29.5, 43.4, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 22.6, 25.8, 29.0, 31.4, 34.1, 38.4, 53.0, 53.8, 57.6, 60.1, 126.9, 132.2, 137.0, 145.6, 159.8, 175.0; HRMS (m/z): calcd for C29H43N4O2 [M + H]+ 479.3381; found 479.3379; HPLC purity: 99.8%.
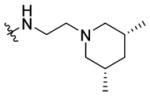
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)ethyl)-1-((5-methyl-2-(otolyl) oxazol-4-yl)methyl)piperidine-4-carboxamide 7n
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (50 mg, 0.16 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)ethan-1-amine (30 mg, 0.19 mmol) were reacted according to general procedure A-4 to afford the product as a white solid (59 mg, 0.13 mmol, 82% yield). 1H NMR (400 MHz, CDCl3) δ 0.53 (q, J = 11.9 Hz, 1H), 0.82–0.87 (m, 1H), 0.85 (d, J = 6.5 Hz, 6H), 1.46 (t, J = 11.0 Hz, 2H), 1.58–1.88 (complex, 6H), 2.06–2.15 (m, 3H), 2.38 (s, 3H), 2.42 (t, J = 5.9 Hz, 2H), 2.65 (s, 3H), 2.72–2.82 (m, 2H), 3.01–3.05 (m, 2H), 3.32 (q, J = 5.5 Hz, 2H), 3.47 (s, 2H), 6.33 (br s, 1H), 7.22–7.32 (m, 3H), 7.91–7.94 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.5, 21.8, 31.1, 43.3, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 28.9, 35.9, 42.1, 53.0, 53.7, 56.6, 61.2, 126.9, 132.2, 137.0, 145.6, 159.8, 175.0; HRMS calcd for C27H41N4O2 [M + H]+ 453.3224; found 453.3221; HPLC purity: 96.6%.
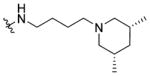
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)butyl)-1-((5-methyl-2-(otolyl) oxazol-4-yl)methyl)piperidine-4-carboxamide 7o
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (51 mg, 0.16 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)butan-1-amine (36 mg, 0.20 mmol) were reacted according to general procedure A-4 to afford the product as a white solid (24 mg, 0.050 mmol, 31% yield). 1H NMR (400 MHz, CDCl3) δ 0.52 (q, J = 11.5 Hz, 1H), 0.85 (d, J = 6.5 Hz, 6H), 1.38 (t, J = 11.1 Hz, 2H), 1.49–1.56 (complex, 4H), 1.60–1.72 (m, 3H), 1.75–1.85 (m, 3H), 2.01–2.12 (complex, 4H), 2.29 (t, J = 6.9 Hz, 2H), 2.38 (s, 3H), 2.65 (s, 3H), 2.78–2.83 (m, 2H), 3.04 (d, J = 11.8 Hz, 2H), 3.21–3.26 (m, 2H), 3.47 (s, 2H), 6.22 (br s, 1H), 7.21–7.32 (m, 3H), 7.89–7.94 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.7, 21.8, 31.1, 43.5, 125.8, 128.7, 129.5, 131.4; u (C, CH2): 24.5, 27.5, 29.0, 39.1, 42.2, 53.0, 53.7, 58.2, 61.6, 126.8, 132.2, 137.0, 145.7, 159.8, 174.9; HRMS calcd for C29H45N4O2 [M + H]+ 481.3537; found 481.3537; HPLC purity: >99.8%.
N-(2-(Dipropylamino)ethyl)-1-((5-methyl-2-(o-tolyl)oxazol-4-yl)-methyl)piperidine-4-carboxamide 7p
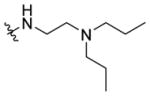
1-((5-Methyl-2-(o-tolyl)-oxazol-4-yl)methyl)piperidine-4-carboxylic acid (50 mg, 0.16 mmol) and N1,N1-dipropylethane-1,2-diamine (25 mg, 0.18 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (20 mg, 0.046 mmol, 29% yield). 1H NMR (400 MHz, CDCl3) δ 0.86–0.95 (complex, 6H), 1.44–1.71 (complex, 7H), 1.95 (dq, J = 3.0, 12.4 Hz, 2H), 2.09–2.18 (m, 4H), 2.41 (s, 3H), 2.57–2.63 (m, 2H), 2.67 (s, 3H), 2.77 (q, J = 7.0 Hz, 2H), 3.03–3.10 (m, 2H), 3.02–3.12 (m, 2H), 3.38–3.46 (m, 2H), 3.53 (s, 2H), 7.24–7.33 (m, 3H), 7.93–7.97 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 11.2, 11.7, 21.8, 39.0, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 21.0, 22.9, 23.2, 29.0, 46.2, 47.7, 48.7, 50.0, 51.8, 53.0, 53.9, 126.9, 132.3, 137.0, 145.6, 159.7, 175.4; HRMS (m/z): calcd for C26H41N4O2 [M + H]+ 441.3230; found 441.3226; HPLC purity: >99.8%.
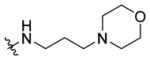
1- ( (5-Methyl - 2 - ( o - to l y l )oxazo l - 4 - y l )methy l ) -N- (3- morpholinopropyl)piperidine-4-carboxamide 7q
1-((5-Methyl-2-(otolyl) oxazol-4-yl)methyl)piperidine-4-carboxylic acid (47 mg, 0.15 mmol) and 3-morpholinopropan-1-amine (43 mg, 0.30 mmol) were reacted according to general procedure A-4 to afford the product as a viscous, light yellow oil (26 mg, 0.060 mmol, 40% yield). 1H NMR (400 MHz, CDCl3) δ 0.86–0.95 (complex, 6H), 1.64–1.70 (m, 2H), 1.72–1.88 (complex, 4H),1.97–2.14 (m, 3H), 2.39 (s, 3H), 2.42–2.50 (complex, 6H), 2.65 (s, 3H), 3.05 (d, J = 11.6 Hz, 2H), 3.34 (q, J = 5.5 Hz, 2H), 3.48 (s, 2H), 3.71 (t, J = 4.6 Hz, 4H), 6.80 (br s, 1H), 7.22–7.32 (m, 3H), 7.90–7.94 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 21.8, 43.5, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 24.9, 29.1, 39.4, 53.1, 53.78, 53.83, 58.1, 67.0, 126.9, 132.3, 137.0, 145.6, 159.8, 174.9; IR 1547, 1642, 2812, 2940 cm−1; HRMS (m/z): calcd for C25H37N4O3 [M + H]+ 441.2860; found 441.2879; HPLC purity: >99.8%.
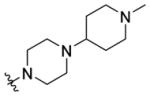
(1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidin-4-yl)(4-(1-methylpiperidin-4-yl)piperazin-1-yl)methanone 7r
1-((5-Methyl-2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (50 mg, 0.16 mmol) and 1-(1-methylpiperidin-4-yl)piperazine (29 mg, 0.16 mmol) were reacted according to general procedure A-4 to afford the product as an off-white solid (36 mg, 0.075 mmol, 47% yield). 1H NMR (400 MHz, CDCl3) δ 1.57 (dq, J = 3.4, 12.0 Hz, 2H), 1.71 (ABq, ΔδAB = 0.08, J = 12.2 Hz, 2H), 1.85–1.96 (complex, 4H), 2.13 (dt, J = 2.0, 11.6 Hz, 2H), 2.21–2.29 (m, 1H), 2.26 (s, 3H), 2.39 (s, 3H), 2.40–2.47 (m, 1H), 2.48–2.56 (m, 4H), 2.65 (s, 3H), 2.90 (d, J = 11.8 Hz, 2H), 3.05 (d, J = 11.6 Hz, 2H), 3.45–3.51 (m, 2H), 3.51 (s, 2H), 3.58–3.64 (m, 2H), 7.22–7.32 (m, 3H), 7.91–7.94 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 21.9, 38.5, 46.1, 61.6, 125.8, 128.7, 129.4, 131.4; u (C, CH2): 28.1, 28.7, 42.0, 45.8, 49.1, 49.4, 52.9, 53.8, 55.4, 126.9, 132.2, 137.0, 145.6, 159.7, 173.3; IR 1446, 1625, 2807, 2938 cm−1; HRMS (m/z): calcd for C28H42N5O2 [M + H]+ 480.3333; found 480.3349; HPLC purity: >99.8%.
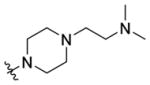
(4-(2-(Dimethylamino)ethyl)piperazin-1-yl)(1-((5-methyl-2-(otolyl) oxazol-4-yl)methyl)piperidin-4-yl)methanone 7s
1-((5-Methyl- 2-(o-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (55 mg, 0.18 mmol) and N,N-dimethyl-2-(piperazin-1-yl)ethanamine (28 mg, 0.18 mmol) were reacted according to general procedure A-4 to afford the product as an off-white solid (30 mg, 0.067 mmol, 38% yield). 1H NMR (400 MHz, CDCl3) δ 1.64–1.71 (m, 2H), 1.90 (dq, J = 2.2, 11.7 Hz, 2H), 2.13 (dt, J = 2.1, 11.6 Hz, 2H), 2.25 (s, 6H), 2.38 (s, 3H), 2.40–2.50 (complex, 5H), 2.59–2.62 (m, 4H), 2.65 (s, 3H), 3.05 (d, J = 11.6 Hz, 2H), 3.47–3.52 (m, 2H), 3.51 (s, 2H), 3.60–3.65 (m, 2H), 7.22–7.31 (m, 3H), 7.91–7.94 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 21.8, 38.5, 41.0, 45.9, 125.8, 128.6, 129.4, 131.4; u (C, CH2): 28.7, 41.5, 45.3, 52.9, 53.3, 53.8, 54.1, 56.6, 56.8, 126.9, 132.2, 137.0, 145.6, 159.7, 173.4; IR 1445, 1622, 2816, 2944 cm−1; HRMS (m/z): calcd for C26H40N5O2 [M + H]+ 454.3177; found 454.3192; HPLC purity: 99.3%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((5-methyl-2-(mtolyl) oxazol-4-yl)methyl)piperidine-4-carboxamide 7t
1-((5-Methyl-2-(m-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (30 mg, 0.095 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (18 mg, 0.10 mmol) were reacted according to general procedure A-3 to afford the product as a white solid (29 mg, 0.062 mmol, 65% yield). 1H NMR (400 MHz, CDCl3) δ 0.54 (q, J = 11.9 Hz, 1H), 0.87 (d, J = 6.5 Hz, 6H), 1.41 (t, J = 11.1 Hz, 2H), 1.57–1.88 (complex, 9H), 1.97–2.11 (m, 3H), 2.38 (s, 3H), 2.40 (s, 3H), 2.42 (t, J = 6.1 Hz, 2H), 2.85–2.89 (m, 2H), 2.96–3.05 (m, 2H), 3.33 (q, J = 6.0 Hz, 2H), 3.45 (s, 2H), 7.20–7.23 (m, 1H), 7.28–7.33 (m, 2H), 7.79 (d, J = 7.7 Hz, 1H), 7.86 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 21.3, 31.3, 43.4, 123.1, 126.6, 128.5, 130.6; u (C, CH2): 24.9, 29.0, 39.8, 42.1, 53.1, 53.9, 58.1, 61.7, 127.6, 132.6, 138.3, 145.9, 159.5, 174.8; HRMS calcd for C28H43N4O2 [M + H]+ 467.3381; found 467.3388; HPLC purity: 97.2%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((5-methyl-2-(ptolyl) oxazol-4-yl)methyl)piperidine-4-carboxamide 7u
1-((5-Methyl-2-(p-tolyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (28 mg, 0.089 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (17 mg, 0.098 mmol) were reacted according to general procedure A-3 to afford the product as a white solid (28 mg, 0.059 mmol, 66% yield). 1H NMR (400 MHz, CDCl3) δ 0.54 (q, J = 11.9 Hz, 1H), 0.87 (d, J = 6.5 Hz, 6H), 1.41 (t, J = 11.1 Hz, 2H), 1.57–1.88 (complex, 9H), 1.97–2.11 (m, 3H), 2.38 (s, 3H), 2.39 (s, 3H), 2.41 (t, J = 6.1 Hz, 2H), 2.85–2.90 (m, 2H), 2.97–3.02 (m, 2H), 3.33 (q, J = 6.1 Hz, 2H), 3.44 (s, 2H), 7.23 (d, J = 8.0 Hz, 2H), 7.31 (br s, 1H), 7.90 (d, J = 8.2 Hz, 2H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 21.5, 31.3, 43.5, 126.0, 129.3; u (C, CH2): 24.9, 29.0, 39.9, 42.1, 53.2, 54.0, 58.2, 61.8, 125.1, 132.5, 139.9, 145.6, 159.6, 174.8; HRMS calcd for C28H43N4O2 [M + H]+ 467.3381; found 467.3389; HPLC purity: 99.0%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2-ethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7v
1-((2-(2-Ethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (31 mg, 0.094 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (18 mg, 0.10 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, colorless oil (22 mg, 0.045 mmol, 48% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 11.9 Hz, 1H), 0.87 (d, J = 6.5 Hz, 6H), 1.22 (t, J = 7.5 Hz, 2H), 1.44 (t, J = 11.0 Hz, 2H), 1.59–1.90 (complex, 9H), 1.97–2.18 (m, 3H), 2.40 (s, 3H), 2.44 (t, J = 6.2 Hz, 2H), 2.87–2.92 (m, 2H), 3.01–3.14 (complex, 4H), 3.34 (q, J = 6.4 Hz, 2H), 3.50 (s, 2H), 7.21–7.39 (complex, 4H), 7.89 (dd, J = 1.4, 7.8 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 15.5, 19.6, 31.2, 43.4, 125.8, 129.1, 129.7, 129.8; u (C, CH2): 24.8, 27.4, 29.1, 39.8, 42.0, 53.0, 53.7, 58.1, 61.7, 126.4, 132.3, 143.3, 145.5, 159.5, 174.9; HRMS calcd for C29H45N4O2 [M + H]+ 481.3537; found 481.3543; HPLC purity: 98.7%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2,6-dimethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7w
1-((2-(2,6-Dimethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (52 mg, 0.16 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (27 mg, 0.16 mmol) were reacted according to general procedure A-4 to afford the product as a white solid (74 mg, 0.15 mmol, 97% yield). 1H NMR (400 MHz, CDCl3) δ 0.52 (q, J = 11.6 Hz, 1H), 0.83 (d, J = 6.4 Hz, 6H), 1.42 (t, J = 11.1 Hz, 2H), 1.60–1.84 (complex, 9H), 1.94–2.02 (m, 1H), 2.08 (dt, J = 2.8, 11.5 Hz, 2H), 2.19 (s, 6H), 2.34 (s, 3H), 2.42 (t, J = 6.1 Hz, 2H), 2.83–2.90 (m, 2H), 2.96–3.02 (m, 2H), 3.30 (q, J = 6.1 Hz, 2H), 3.48 (s, 2H), 7.04 (d, J = 7.6 Hz, 2H), 7.18 (t, J = 7.5 Hz, 1H), 7.31 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.3, 19.5, 20.2, 31.1, 43.3, 127.4, 129.5; u (C, CH2): 24.7, 29.0, 39.6, 41.9, 52.9, 53.5, 58.0, 61.5, 128.5, 131.3, 138.3, 145.7, 158.7, 174.9; HRMS calcd for C29H45N4O2 [M + H]+ 481.3537; found 481.3532; HPLC purity: 98.1%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(3,4-dimethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7x
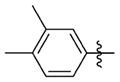
1-((2-(3,4-Dimethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.076 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (16 mg, 0.091 mmol) were reacted according to general procedure A-4 to afford the product as a white solid (34 mg, 0.071 mmol, 93% yield). 1H NMR (400 MHz, CDCl3) δ 0.54 (q, J = 11.2 Hz, 1H), 0.86 (d, J = 6.6 Hz, 6H), 1.41 (t, J = 11.1 Hz, 2H), 1.56–1.84 (complex, 9H), 1.99–2.08 (m, 3H), 2.29 (s, 3H), 2.30 (s, 3H), 2.37 (s, 3H), 2.41 (t, J = 6.2 Hz, 2H), 2.84–2.90 (m, 2H), 3.00 (d, J = 11.2 Hz, 2H), 3.32 (q, J = 6.1 Hz, 2H), 3.43 (s, 2H), 7.17 (d, J = 7.9 Hz, 1H), 7.71 (dd, J = 1.7, 7.8 Hz, 1H), 7.80 (s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 19.7, 19.8, 31.3, 43.4, 123.5, 127.2, 129.9; u (C, CH2): 24.9, 28.9, 39.8, 42.0, 53.1, 53.9, 58.1, 61.7, 125.3, 132.3, 136.9, 138.7, 145.6, 159.8, 174.9; HRMS calcd for C29H45N4O2 [M + H]+ 481.3537; found 481.3532; HPLC purity: 95.4%.
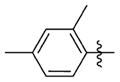
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2,4-dimethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7y
1-((2-(2,4-Dimethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.076 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (14 mg, 0.084 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (23 mg, 0.047 mmol, 62% yield). 1H NMR (400 MHz, CDCl3) δ 0.53 (q, J = 12.0 Hz, 1H), 0.85 (d, J = 6.5 Hz, 6H), 1.41 (t, J = 11.1 Hz, 2H), 1.59–1.84 (complex, 9H), 1.96–2.11 (m, 3H), 2.32 (s, 3H), 2.36 (s, 3H), 2.41 (t, J = 6.2 Hz, 2H), 2.60 (s, 3H), 2.84–2.91 (m, 2H), 3.02 (d, J = 11.7 Hz, 2H), 3.32 (q, J = 6.5 Hz, 2H), 3.46 (s, 2H), 7.03–7.06 (m, 2H), 7.31 (br s, 1H), 7.80 (d, J = 7.7 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 21.3, 21.7, 31.3, 43.5, 126.5, 128.7, 132.2; u (C, CH2): 24.8, 29.0, 39.8, 42.0, 53.1, 53.9, 58.2, 61.7, 124.2, 131.9, 136.8, 139.4, 145.2, 159.9, 174.9; HRMS calcd for C29H45N4O2 [M + H]+ 481.3537; found 481.3534; HPLC purity: 99.2%.
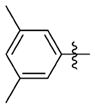
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(3,5-dimethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7z
1-((2-(3,5-Dimethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.076 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (14 mg, 0.084 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, colorless oil (19 mg, 0.040 mmol, 52% yield). 1H NMR (400 MHz, CDCl3) δ 0.54 (q, J = 11.9 Hz, 1H), 0.85 (d, J = 6.5 Hz, 6H), 1.42 (t, J = 11.1 Hz, 2H), 1.60–1.84 (complex, 9H), 1.97–2.08 (m, 3H), 2.34 (s, 6H), 2.37 (s, 3H), 2.42 (t, J = 6.1 Hz, 2H), 2.85–2.90 (m, 2H), 2.99 (d, J = 11.6 Hz, 2H), 3.32 (q, J = 6.3 Hz, 2H), 3.43 (s, 2H), 7.03 (s, 1H), 7.29 (br s, 1H), 7.73 (s, 2H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 21.2, 31.2, 43.4, 123.8, 131.5; u (C, CH2): 24.8, 29.0, 39.8, 42.0, 53.2, 54.0, 58.1, 61.7, 127.5, 132.5, 138.2, 145.8, 159.7, 174.9; HRMS calcd for C29H45N4O2 [M + H]+ 481.3537; found 481.3545; HPLC purity: 99.8%.
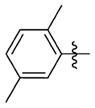
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2,5-dimethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7aa
1-((2-(2,5-Dimethylphenyl)-5-methyloxazol-4-yl)methyl)-piperidine-4-carboxylic acid (25 mg, 0.076 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (14 mg, 0.084 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (8 mg, 0.017 mmol, 22% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 11.4 Hz, 1H), 0.87 (d, J = 6.5 Hz, 6H), 1.46 (t, J = 11.0 Hz, 2H), 1.64–1.87 (complex, 9H), 1.98–2.13 (m, 3H), 2.34 (s, 3H), 2.37 (s, 3H), 2.46 (t, J = 6.0 Hz, 2H), 2.59 (s, 3H), 2.87–2.94 (m, 2H), 3.03 (d, J = 11.6 Hz, 2H), 3.33 (q, J = 6.3 Hz, 2H), 3.47 (s, 2H), 7.07–7.15 (m, 2H), 7.30 (br s, 1H), 7.75 (s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.3, 20.8, 21.3, 30.3, 43.1, 129.2, 130.3, 131.4; u (C, CH2): 24.4, 28.8, 38.5, 41.2, 52.9, 53.7, 56.8, 60.6, 126.5, 131.9, 133.9, 135.3, 145.7, 160.1, 175.3; HRMS calcd for C29H45N4O2 [M + H]+ 481.3537; found 481.3533; HPLC purity: 98.7%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-mesityl-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7bb
1-((2-Mesityl-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.073 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (14 mg, 0.080 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (19 mg, 0.039 mmol, 53% yield). 1H NMR (400 MHz, CDCl3) δ 0.54 (q, J = 11.8 Hz, 1H), 0.86 (d, J = 6.5 Hz, 6H), 1.43 (t, J = 11.1 Hz, 2H), 1.62–1.87 (complex, 9H), 1.97–2.04 (m, 1H), 2.10 (dt, J = 2.6, 11.4 Hz, 2H), 2.19 (s, 6H), 2.28 (s, 3H), 2.35 (s, 3H), 2.44 (t, J = 6.2 Hz, 2H), 2.86–2.93 (m, 2H), 3.01 (d, J = 11.6 Hz, 2H), 3.32 (q, J = 6.4 Hz, 2H), 3.50 (s, 2H), 6.88 (s, 2H), 7.33 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.3, 19.6, 20.2, 21.2, 31.1, 43.4, 128.3; u (C, CH2): 24.7, 29.1, 39.7, 42.0, 52.9, 53.6, 58.0, 61.6, 125.7, 131.3, 138.2, 139.3, 145.7, 158.9, 174.9; HRMS calcd for C30H47N4O2 [M + H]+ 495.3694; found 495.3666; HPLC purity: 96.2%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2,3,4,5,6-pentamethylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7cc
1-((2-(2,3,4,5,6-Pentamethylphenyl)-5-methyloxazol-4-yl)-methyl)piperidine-4-carboxylic acid (25 mg, 0.067 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (13 mg, 0.074 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (24 mg, 0.045 mmol, 67% yield). 1H NMR (400 MHz, CDCl3) δ 0.54 (q, J = 12.0 Hz, 1H), 0.85 (d, J = 6.5 Hz, 6H), 1.41 (t, J = 11.0 Hz, 2H), 1.58–1.87 (complex, 9H), 1.94–2.02 (m, 1H), 2.01 (s, 6H), 2.10 (dt, J = 2.6, 11.4 Hz, 2H), 2.19 (s, 6H), 2.24 (s, 3H), 2.35 (s, 3H), 2.42 (t, J = 6.1 Hz, 2H), 2.85–2.93 (m, 2H), 3.02 (d, J = 11.6 Hz, 2H), 3.32 (q, J = 5.9 Hz, 2H), 3.52 (s, 2H), 7.33 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.3, 16.3, 16.9, 17.9, 19.6, 31.2, 43.4; u (C, CH2): 24.8, 29.1, 39.9, 42.0, 52.8, 53.5, 58.2, 61.7, 126.9, 130.9, 132.5, 133.5, 136.7, 145.5, 160.4, 174.9; HRMS calcd for C32H51N4O2 [M + H]+ 523.4007; found 523.3984; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2-chlorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7dd
1-((2-(2-Chlorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (33 mg, 0.098 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (33 mg, 0.20 mmol) were reacted according to general procedure A-3 to afford the product as a white solid (7 mg, 0.014 mmol, 14% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 12.0 Hz, 1H), 0.89 (d, J = 6.5 Hz, 6H), 1.41 (t, J = 11.0 Hz, 2H), 1.57–1.90 (complex, 9H), 1.98–2.17 (m, 3H), 2.44 (s, 3H), 2.46 (t, J = 6.2 Hz, 2H), 2.86–2.90 (m, 2H), 3.03 (d, J = 11.8 Hz, 2H), 3.34 (q, J = 6.2 Hz, 2H), 3.52 (s, 2H), 7.30–7.40 (m, 2H), 7.45–7.54 (m, 1H), 7.93–8.02 (m, 1H), 8.23 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 19.6, 31.1, 43.4, 126.7, 130.6, 130.9, 131.0; u (C, CH2): 24.8, 29.0, 39.7, 41.9, 53.1, 53.8, 58.0, 61.6, 126.7, 132.2, 132.7, 146.7, 157.3, 174.9; HRMS calcd for C27H40ClN4O2 [M + H]+ 487.2834; found 487.2842; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(3-chlorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7ee
1-((2-(3-Chlorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (47 mg, 0.14 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (24 mg, 0.14 mmol) were reacted according to general procedure A-4 to afford the product as an off-white solid (52 mg, 0.11 mmol, 76% yield). 1H NMR (400 MHz, CDCl3) δ 0.54 (q, J = 12.4 Hz, 1H), 0.86 (d, J = 6.6 Hz, 6H), 1.40 (t, J = 11.1 Hz, 2H), 1.57–1.87 (complex, 9H), 2.00–2.10 (m, 3H), 2.39 (s, 3H), 2.40 (t, J = 6.2 Hz, 2H), 2.84–2.89 (m, 2H), 2.96–3.02 (m, 2H), 3.32 (q, J = 6.1 Hz, 2H), 3.43 (s, 2H), 7.29 (br s, 1H), 7.35–7.38 (m, 2H), 7.87–7.90 (m, 1H), 8.00–8.01 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 31.3, 43.4, 124.1, 126.1, 129.8, 130.0; u (C, CH2): 24.9, 28.9, 39.8, 42.1, 53.1, 53.8, 58.0, 61.7, 129.3, 133.0, 134.7, 146.7, 158.1, 174.9; HRMS calcd for C27H40ClN4O2 [M + H]+ 487.2834; found 487.2829; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(4-chlorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7ff
1-((2-(4-Chlorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (28 mg, 0.083 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (28 mg, 0.17 mmol) were reacted according to general procedure A-3 to afford the product as a white solid (27 mg, 0.055 mmol, 66% yield). 1H NMR (400 MHz, CDCl3) δ 0.55 (q, J = 11.9 Hz, 1H), 0.87 (d, J = 6.6 Hz, 6H), 1.41 (t, J = 11.1 Hz, 2H), 1.56–1.88 (complex, 9H), 1.95–2.11 (m, 3H), 2.39 (s, 3H), 2.41 (t, J = 6.1 Hz, 2H), 2.85–2.89 (m, 2H), 3.00 (d, J = 11.5 Hz, 2H), 3.33 (q, J = 6.1 Hz, 2H), 3.44 (s, 2H), 7.31 (br s, 1H), 7.38–7.42 (m, 2H), 7.92–7.97 (m, 2H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 31.4, 43.4, 127.3, 128.9; u (C, CH2): 24.9, 28.9, 39.9, 42.1, 53.2, 53.9, 58.2, 61.8, 126.2, 133.0, 135.8, 146.3, 158.5, 174.8; HRMS calcd for C27H40ClN4O2 [M + H]+ 487.2834; found 487.2841; HPLC purity: >99.8%.
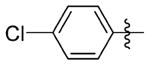
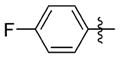
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(4-fluorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7gg
1-((2-(4-Fluorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.079 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (15 mg, 0.086 mmol) were reacted according to general procedure A-3 to afford the product as a white solid (25 mg, 0.054 mmol, 69% yield). 1H NMR (400 MHz, CDCl3) δ 0.55 (q, J = 11.9 Hz, 1H), 0.87 (d, J = 6.6 Hz, 6H), 1.41 (t, J = 11.1 Hz, 2H), 1.56–1.88 (complex, 9H), 1.95–2.11 (m, 3H), 2.39 (s, 3H), 2.41 (t, J = 6.1 Hz, 2H), 2.85–2.89 (m, 2H), 3.00 (d, J = 11.5 Hz, 2H), 3.33 (q, J = 6.1 Hz, 2H), 3.44 (s, 2H), 7.31 (br s, 1H), 7.38–7.42 (m, 2H), 7.92–7.97 (m, 2H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.4, 30.8, 43.3, 115.7 (d, J = 22.1 Hz), 128.1 (d, J = 8.5 Hz); u (C, CH2): 24.7, 28.8, 39.2, 41.7, 53.1, 53.9, 57.5, 61.2, 124.1 (d, J = 3.2 Hz), 132.6, 146.1, 158.6 (d, J = 0.4 Hz), 163.7 (d, J = 250.9 Hz), 175.0; HRMS calcd for C27H40FN4O2 [M + H]+ 471.3130; found 471.3114; HPLC purity: >99.8%.
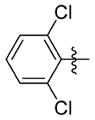
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2,6-dichlorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7hh
1-((2-(2,5-Dichlorophenyl)-5-methyloxazol-4-yl)methyl)- piperidine-4-carboxylic acid (25 mg, 0.068 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (13 mg, 0.074 mmol) were reacted according to general procedure A-4 to afford the product as a viscous, light yellow oil (21 mg, 0.041 mmol, 60% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 11.9 Hz, 1H), 0.88 (d, J = 6.5 Hz, 6H), 1.44 (t, J = 11.0 Hz, 2H), 1.64–1.91 (complex, 9H), 1.97–2.06 (m, 1H), 2.12 (dt, J = 2.6, 11.4 Hz, 2H), 2.42 (s, 3H), 2.45 (t, J = 6.1 Hz, 2H), 2.88–2.92 (m, 2H), 3.00–3.03 (m, 2H), 3.34 (q, J = 6.1 Hz, 2H), 3.56 (s, 2H), 7.30–7.43 (complex, 4H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 31.2, 43.4, 128.0, 131.6; u (C, CH2): 24.7, 29.1, 39.8, 42.0, 52.8, 53.5, 58.1, 61.6, 128.3, 132.0, 136.4, 147.1, 154.0, 174.9; HRMS calcd for C27H39Cl2N4O2 [M + H]+ 521.2445; found 521.2439; HPLC purity: >99.8%.
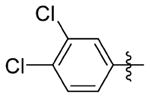
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(3,4-dichlorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7ii
1-((2-(3,4-Dichlorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.068 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (13 mg, 0.074 mmol) were reacted according to general procedure A-4 to afford the product as a viscous, light yellow oil (28 mg, 0.053 mmol, 78% yield). 1H NMR (400 MHz, CDCl3) δ 0.71 (q, J = 12.9 Hz, 1H), 0.93 (d, J = 6.1 Hz, 6H), 1.82–2.10 (complex, 11H), 2.15–2.32 (m, 3H), 2.37 (s, 3H), 2.94 (t, J = 7.3 Hz, 2H), 3.05–3.14 (m, 2H), 3.23–3.34 (m, 4H), 3.54 (s, 2H), 7.18 (br s, 1H), 7.48 (d, J = 8.4 Hz, 1H), 7.81 (dd, J = 2.0, 8.4 Hz, 1H), 8.08 (d, J = 2.0 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 18.8, 28.8, 41.0, 125.1, 127.9, 130.8; u (C, CH2): 23.9, 28.0, 28.0, 36.4, 40.0, 52.6, 53.1, 58.9, 127.4, 133.1, 134.0, 157.6, 162.5, 162.8, 175.6; IR 1541, 1641, 2922, 2947 cm−1; HRMS calcd for C27H39Cl2N4O2 [M + H]+ 521.2445; found 521.2437; HPLC purity: 99.5%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2,6-difluorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7jj
1-((2-(2,6-Difluorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.074 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (14 mg, 0.082 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (12 mg, 0.024 mmol, 33% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 12.3 Hz, 1H), 0.87 (d, J = 6.5 Hz, 6H), 1.49 (t, J = 10.4 Hz, 2H), 1.67–1.86 (complex, 9H), 1.98–2.12 (m, 3H), 2.40 (s, 3H), 2.48 (t, J = 5.4 Hz, 2H), 2.89–2.96 (m, 2H), 3.00 (d, J = 11.6 Hz, 2H), 3.33 (q, J = 6.4 Hz, 2H), 3.50 (s, 2H), 6.99 (t, J = 8.3 Hz, 2H), 7.29–7.40 (m, 2H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 19.5, 30.9, 43.3, 112.0 (d, J = 25.6 Hz), 131.4 (t, J = 10.5 Hz); u (C, CH2): 24.6, 29.0, 39.3, 41.7, 53.1, 53.8, 57.7, 61.3, 107.0 (d, J = 16.1 Hz), 132.9, 147.2, 150.5 (d, J = 3.1 Hz), 160.7 (dd, J = 5.9, 257.3 Hz), 175.0; HRMS calcd for C27H39F2N4O2 [M + H]+ 489.3036; found 489.3040; HPLC purity: 97.3%.
N-(3-(cis-3,5-Diethylpiperidin-1-yl)propyl)-1-((2-(2,6-difluorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7kk
1-((2-(2,6-Difluorophenyl)-5-methyloxazol-4-yl)methyl)- piperidine-4-carboxylic acid (25 mg, 0.074 mmol) and 3-(cis-3,5-diethylpiperidin-1-yl)propan-1-amine (16 mg, 0.082 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (19 mg, 0.036 mmol, 49% yield). 1H NMR (400 MHz, CDCl3) δ 0.49 (q, J = 12.4 Hz, 1H), 0.91 (d, J = 7.5 Hz, 6H), 1.19–1.29 (m, 4H), 1.14–1.49 (m, 4H), 1.66–1.89 (complex, 7H), 2.00–2.13 (m, 3H), 2.43 (s, 3H), 2.45 (t, J = 6.0 Hz, 2H), 2.94–2.98 (m, 2H), 2.99–3.06 (m, 2H), 3.35 (q, J = 6.3 Hz, 2H), 3.53 (s, 2H), 7.02 (t, J = 8.4 Hz, 2H), 7.34–7.43 (m, 2H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 11.4, 38.0, 43.5, 112.0 (d, J = 25.6 Hz), 131.4 (t, J = 10.5 Hz); u (C, CH2): 24.9, 27.4, 29.0, 37.3, 39.9, 53.1, 53.8, 58.4, 60.4, 106.8 (d, J = 16.3 Hz), 132.9, 147.1, 150.5, 160.7 (d, J = 257.3 Hz), 174.9; HRMS calcd for C29H43F2N4O2 [M + H]+ 517.3349; found 517.3351; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2,6-dibromophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7ll
1-((2-(2,5-Dibromophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.055 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (10 mg, 0.060 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (22 mg, 0.037 mmol, 67% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 12.2 Hz, 1H), 0.88 (d, J = 6.5 Hz, 6H), 1.43 (t, J = 11.0 Hz, 2H), 1.61–1.90 (complex, 9H), 1.98–2.02 (m, 1H), 2.14 (dt, J = 2.8, 11.5 Hz, 2H), 2.42 (s, 3H), 2.44 (t, J = 6.0 Hz, 2H), 2.87–2.93 (m, 2H), 2.99–3.05 (m, 2H), 3.34 (q, J = 6.3 Hz, 2H), 3.58 (s, 2H), 7.20 (t, J = 8.1 Hz, 1H), 7.37 (br s, 1H), 7.62 (d, J = 8.1 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 31.3, 43.4, 131.6, 132.3; u (C, CH2): 24.7, 29.1, 39.9, 42.0, 52.6, 53.3, 58.2, 61.7, 125.3, 131.7, 132.2, 146.8, 156.4, 174.9; HRMS calcd for C27H39Br2N4O2 [M + H]+ 609.1434; found 609.1430; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2-bromo-6-chlorophenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7mm
1-((2-(2-Bromo-6-chlorophenyl)-5-methyloxazol-4-yl)- methyl)piperidine-4-carboxylic acid (25 mg, 0.060 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (11 mg, 0.066 mmol) were reacted according to general procedure A-3 to afford the product as a white solid (23 mg, 0.041 mmol, 68% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 11.8 Hz, 1H), 0.88 (d, J = 6.5 Hz, 6H), 1.44 (t, J = 11.0 Hz, 2H), 1.61–1.90 (complex, 9H), 1.98–2.04 (m, 1H), 2.13 (dt, J = 2.8, 11.5 Hz, 2H), 2.42 (s, 3H), 2.45 (t, J = 6.1 Hz, 2H), 2.86–2.95 (m, 2H), 2.98–3.05 (m, 2H), 3.34 (q, J = 6.3 Hz, 2H), 3.57 (s, 2H), 7.27 (t, J = 8.1 Hz, 1H), 7.36 (br s, 1H), 7.44 (dd, J = 1.1, 8.1 Hz, 1H), 7.58 (dd, J = 1.1, 8.1 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 31.2, 43.4, 128.5, 131.1, 131.9; u (C, CH2): 24.7, 29.1, 39.8, 42.0, 52.7, 53.4, 58.2, 61.7, 125.4, 130.3, 131.8, 136.4, 147.0, 155.2, 174.9; HRMS calcd for C27H39BrClN4O2 [M + H]+ 565.1939; found 565.1939; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2-chloro-6-methylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7nn
1-((2-(2-Chloro-6-methylphenyl)-5-methyloxazol-4-yl)- methyl)piperidine-4-carboxylic acid (80 mg, 0.23 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (59 mg, 0.078 mmol) were reacted according to general procedure A-4 to afford the product as a white solid (71 mg, 0.14 mmol, 62% yield). 1H NMR (400 MHz, CDCl3) δ 0.53 (q, J = 12.0 Hz, 1H), 0.86 (d, J = 6.5 Hz, 6H), 1.39 (t, J = 11.0 Hz, 2H), 1.53–1.89 (complex, 9H), 1.96–2.04 (m, 1H), 2.11 (dt, J = 2.8, 11.5 Hz, 2H), 2.24 (s, 3H), 2.39 (s, 3H), 2.40 (t, J = 6.0 Hz, 2H), 2.84–2.88 (m, 2H), 2.99–3.04 (m, 2H), 3.32 (q, J = 6.0 Hz, 2H), 3.53 (s, 2H), 7.14–7.17 (m, 1H), 7.23–7.31 (m, 2H), 7.36 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.3, 19.6, 20.2, 31.4, 43.4, 126.9, 128.3, 130.7; u (C, CH2): 24.8, 29.1, 40.0, 42.1, 52.8, 53.5, 58.3, 61.8, 128.3, 131.7, 134.8, 140.9, 146.5, 156.2, 174.8; HRMS calcd for C28H42ClN4O2 [M + H]+ 501.2991; found 501.2995; HPLC purity: 96.1%.
(±)-1-((2-(2-Chloro-6-methylphenyl)-5-methyloxazol-4-yl)-methyl)-N-(3-(3-methylpiperidin-1-yl)propyl)piperidine-4-carboxamide 7oo
1-((2-(2-Chloro-6-methylphenyl)-5-methyloxazol-4-yl)-methyl)piperidine-4-carboxylic acid (25 mg, 0.072 mmol) and 3-(3-methylpiperidin-1-yl)propan-1-amine (12 mg, 0.079 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (20 mg, 0.041 mmol, 56% yield). 1H NMR (400 MHz, CDCl3) δ 0.80–0.91 (m, 1H), 0.85 (d, J = 6.4 Hz, 3H), 1.46–1.88 (complex, 12H), 1.96–2.04 (m, 1H), 2.09 (dt, J = 2.8, 11.5 Hz, 2H), 2.22 (s, 3H), 2.37 (s, 3H), 2.40 (t, J = 6.2 Hz, 2H), 2.81–2.90 (m, 2H), 2.97–3.03 (m, 2H), 3.30 (q, J = 6.2 Hz, 2H), 3.51 (s, 2H), 7.12–7.15 (m, 1H), 7.22–7.28 (m, 2H), 7.38 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.3, 19.7, 20.2, 31.3, 43.4, 126.9, 128.3, 130.7; u (C, CH2): 24.7, 25.6, 29.0, 32.9, 39.9, 52.8, 53.5, 54.1, 58.4, 62.1, 128.3, 131.6, 134.8, 140.9, 146.5, 156.2, 174.9; HRMS calcd for C27H40ClN4O2 [M + H]+ 487.2834; found 487.2839; HPLC purity: >99.8%.
1-((2-(2-Chloro-6-methylphenyl)-5-methyloxazol-4-yl)methyl)-N-(3-(4,4-dimethylpiperidin-1-yl)propyl)piperidine-4-carboxamide 7pp
1-((2-(2-Chloro-6-methylphenyl)-5-methyloxazol-4-yl)methyl)-piperidine-4-carboxylic acid (25 mg, 0.072 mmol) and 3-(4,4-dimethylpiperidin-1-yl)propan-1-amine (13 mg, 0.079 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (20 mg, 0.039 mmol, 55% yield). 1H NMR (400 MHz, CDCl3) 0.92 (s, 6H), 1.40 (t, J = 5.7 Hz, 4H), 1.63–1.69 (m, 2H), 1.73–1.93 (complex, 4H), 1.98–2.07 (m, 1H), 2.12 (dt, J = 2.6, 11.5 Hz, 2H), 2.25 (s, 3H), 2.39 (s, 3H), 2.39–2.44 (m, 4H), 2.46 (t, J = 6.1 Hz, 2H), 3.03 (d, J = 11.7 Hz, 2H), 3.33 (q, J = 5.92 Hz, 2H), 3.53 (s, 2H), 7.12–7.20 (m, 1H), 7.22–7.33 (m, 2H), 7.54 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.3, 20.3, 41.0, 43.4, 126.9, 128.3, 130.7; u (C, CH2): 24.7, 28.5, 29.1, 38.9, 40.1, 50.3, 52.9, 53.5, 58.3, 128.3, 131.6, 134.8, 140.9, 146.5, 156.2, 174.9; HRMS calcd for C28H42ClN4O2 [M + H]+ 501.2991; found 501.2985; HPLC purity: >99.8%.
1-((2-(2-Chloro-6-methylphenyl)-5-methyloxazol-4-yl)methyl)-N-(3-(4-isopropylpiperidin-1-yl)propyl)piperidine-4-carboxamide 7qq
1-((2-(2-Chloro-6-methylphenyl)-5-methyloxazol-4-yl)methyl)-piperidine-4-carboxylic acid (25 mg, 0.072 mmol) and 3-(4-isopropylpiperidin-1-yl)propan-1-amine (15 mg, 0.079 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (21 mg, 0.041 mmol, 57% yield). 1H NMR (400 MHz, CDCl3) δ 0.87 (d, J = 6.8 Hz, 6H), 1.02–1.09 (m, 1H), 1.38–1.49 (m, 1H), 1.61–1.71 (m, 4H), 1.73–1.93 (complex, 6H), 1.99–2.05 (m, 1H), 2.11 (dt, J = 2.6, 11.5 Hz, 2H), 2.25 (s, 3H), 2.39 (s, 3H), 2.42 (t, J = 6.0 Hz, 2H), 3.01 (t, J = 10.5 Hz, 4H), 3.34 (q, J = 6.0 Hz, 2H), 3.53 (s, 2H), 7.13–7.20 (m, 1H), 7.23–7.34 (m, 2H), 7.52 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.3, 19.7, 20.3, 32.4, 42.3, 43.4, 126.9, 128.3, 130.7; u (C, CH2): 24.8, 29.1, 29.4, 40.1, 52.9, 53.5, 54.5, 58.4, 128.3, 131.6, 134.8, 140.9, 146.5, 156.2, 174.8; HRMS calcd for C29H44ClN4O2 [M + H]+ 515.3147; found 515.3149; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2-chloro-6-(trifluoromethyl)phenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7rr
1-((2-(2-Chloro-6-(trifluoromethyl)phenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (24 mg, 0.061 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (11 mg, 0.067 mmol) were reacted according to general procedure A-4 to afford the product as a viscous, light yellow oil (20 mg, 0.035 mmol, 58% yield). 1H NMR (400 MHz, CDCl3) δ 0.55 (q, J = 11.9 Hz, 1H), 0.87 (d, J = 6.6 Hz, 6H), 1.41 (t, J = 11.0 Hz, 2H), 1.62–1.91 (complex, 9H), 1.96–2.01 (m, 1H), 2.12 (dt, J = 2.7, 11.5 Hz, 2H), 2.41 (s, 3H), 2.43 (t, J = 5.9 Hz, 2H), 2.85–2.93 (m, 2H), 2.97–3.02 (m, 2H), 3.35 (q, J = 6.4 Hz, 2H), 3.57 (s, 2H), 7.41 (br s, 1H) 7.54–7.59 (m, 1H), 7.70 (d, J = 8.4 Hz, 2H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.3, 19.6, 31.3, 43.4, 124.6 (q, J = 4.9 Hz), 131.3, 132.9; u (C, CH2): 24.7, 29.1, 40.1, 42.1, 52.6, 53.3, 58.4, 61.8, 125.5 (q, J = 286.8 Hz), 131.6, 1325 (q, J = 31.6 Hz), 137.1, 147.7, 153.1, 155.3, 175.4; HRMS calcd for C29H39ClF3N4O2 [M + H]+ 555.2708; found 555.2705; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2-fluoro-6-methylphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7ss
1-((2-(2-Fluoro-6-methylphenyl)-5-methyloxazol-4-yl)-methyl)piperidine-4-carboxylic acid (25 mg, 0.075 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (14 mg, 0.083 mmol) were reacted according to general procedure A-4 to afford the product as a viscous, light yellow oil (22 mg, 0.046 mmol, 61% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 12.3 Hz, 1H), 0.88 (d, J = 6.6 Hz, 6H), 1.41 (t, J = 11.0 Hz, 2H), 1.58–1.91 (complex, 9H), 1.96–2.06 (m, 1H), 2.11 (dt, J = 3.0, 11.4 Hz, 2H), 2.41 (s, 3H), 2.43 (s, 3H), 2.43 (t, J = 6.2 Hz, 2H), 2.86–2.91 (m, 2H), 2.99–3.09 (m, 2H), 3.35 (q, J = 6.0 Hz, 2H), 3.52 (s, 2H), 6.99 (t, J = 9.3 Hz, 1H), 7.07 (d, J = 7.7 Hz, 1H), 7.27–7.33 (m, 1H), 7.35 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 20.4, 31.4, 43.5, 113.2 (d, J = 22.1 Hz), 126.0, 130.8; u (C, CH2): 24.8, 29.1, 40.0, 42.1, 53.0, 53.7, 58.3, 61.8, 116.7 (d, J = 13.9 Hz), 132.2, 140.6 (d, J = 1.5 Hz), 146.4, 154.4, 161.1 (d, J = 252.2 Hz) 174.8; HRMS calcd for C28H42FN4O2 [M + H]+ 485.3286; found 485.3278; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2-bromo-6-methoxyphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7tt
1-((2-(2-Bromo-6-methoxyphenyl)-5-methyloxazol-4-yl)-methyl)piperidine-4-carboxylic acid (25 mg, 0.061 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (11 mg, 0.067 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (26 mg, 0.046 mmol, 76% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 11.9 Hz, 1H), 0.88 (d, J = 6.5 Hz, 6H), 1.45 (t, J = 11.0 Hz, 2H), 1.65–1.90 (complex, 9H), 1.98–2.05 (m, 1H), 2.13 (dt, J = 2.9, 11.5 Hz, 2H), 2.40 (s, 3H), 2.46 (t, J = 6.1 Hz, 2H), 2.87–2.99 (m, 2H), 3.01–3.05 (m, 2H), 3.34 (q, J = 6.3 Hz, 2H), 3.55 (s, 2H), 3.78 (s, 3H), 6.91 (dd, J = 1.3, 8.1 Hz, 1H), 7.20–7.36 (m, 3H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 31.1, 43.4, 56.2, 109.9, 124.6, 132.1; u (C, CH2): 24.8, 29.1, 39.7, 41.9, 52.8, 53.6, 58.0, 61.6, 120.2, 125.3, 131.6, 146.6, 155.2, 159.9, 174.9; HRMS calcd for C28H42BrN4O3 [M + H]+ 561.2435; found 561.2434; HPLC purity: 98.4%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2-chloro-6-methoxyphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7uu
1-((2-(2-Chloro-6-methoxyphenyl)-5-methyloxazol-4-yl)-methyl)piperidine-4-carboxylic acid (25 mg, 0.069 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (13 mg, 0.075 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (23 mg, 0.045 mmol, 66% yield). 1H NMR (400 MHz, CDCl3) δ 0.55 (q, J = 11.9 Hz, 1H), 0.87 (d, J = 6.5 Hz, 6H), 1.43 (t, J = 11.1 Hz, 2H), 1.58–1.91 (complex, 9H), 1.97–2.07 (m, 1H), 2.12 (dt, J = 2.8, 11.5 Hz, 2H), 2.40 (s, 3H), 2.43 (t, J = 6.2 Hz, 2H), 2.87–2.91 (m, 2H), 3.01–3.05 (m, 2H), 3.33 (q, J = 6.3 Hz, 2H), 3.53 (s, 2H), 3.80 (s, 3H), 6.87 (dd, J = 0.9, 8.5 Hz, 1H), 7.07 (dd, J = 0.9, 8.1 Hz, 1H), 7.32–7.37 (m, 2H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 31.2, 43.4, 56.2, 109.4, 121.6, 131.7; u (C, CH2): 24.8, 29.0, 39.8, 42.0, 52.8, 53.6, 58.1, 61.7, 118.0, 135.9, 146.8, 154.1, 159.8, 175.0; HRMS calcd for C28H42ClN4O3 [M + H]+ 517.2940; found 517.2939; HPLC purity: 99.6%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2-fluoro-6-methoxyphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7vv
1-((2-(2-Fluoro-6-methoxyphenyl)-5-methyloxazol-4-yl)-methyl)piperidine-4-carboxylic acid (25 mg, 0.072 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (13 mg, 0.079 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (21 mg, 0.041 mmol, 58% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 11.9 Hz, 1H), 0.88 (d, J = 6.5 Hz, 6H), 1.44 (t, J = 11.0 Hz, 2H), 1.64–1.91 (complex, 9H), 1.98–2.06 (m, 1H), 2.10 (dt, J = 2.8, 11.4 Hz, 2H), 2.40 (s, 3H), 2.43 (t, J = 6.1 Hz, 2H), 2.86–2.95 (m, 2H), 3.02–3.06 (m, 2H), 3.35 (q, J = 6.3 Hz, 2H), 3.51 (s, 2H), 3.85 (s, 3H), 6.73–6.83 (m, 2H), 7.29–7.42 (m, 2H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 19.6, 31.2, 43.4, 56.4, 106.8, 108.2 (d, J = 22.2 Hz), 131.7 (d, J = 10.8 Hz); u (C, CH2): 24.8, 29.0, 39.8, 42.0, 53.1, 53.8, 58.1, 61.7, 132.2, 146.7, 152.1, 159.4 (d, J = 5.8 Hz), 161.7 (d, J = 252.4 Hz), 174.9; HRMS calcd for C28H42FN4O3 [M + H]+ 501.3235; found 501.3228; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(2-chloro-6-methoxyphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7ww
1-((2-(2-Methoxy-6-methylphenyl)-5-methyloxazol-4-yl)- methyl)piperidine-4-carboxylic acid (25 mg, 0.073 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (14 mg, 0.080 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (25 mg, 0.050 mmol, 69% yield). 1H NMR (400 MHz, CDCl3) δ 0.55 (q, J = 11.9 Hz, 1H), 0.87 (d, J = 6.5 Hz, 6H), 1.40 (t, J = 11.0 Hz, 2H), 1.58–1.90 (complex, 9H), 1.95–2.05 (m, 1H), 2.10 (dt, J = 2.8, 11.4 Hz, 2H), 2.22 (s, 3H), 2.38 (s, 3H), 2.42 (t, J = 6.1 Hz, 2H), 2.85–2.90 (m, 2H), 3.02–3.06 (m, 2H), 3.34 (q, J = 5.6 Hz, 2H), 3.52 (s, 2H), 3.77 (s, 3H), 6.79 (d, J = 8.3 Hz, 1H), 6.86 (d, J = 7.7 Hz, 1H), 7.24–7.31 (m, 1H), 7.34 (br s, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.4, 19.6, 31.4, 41.0, 43.5, 55.9, 108.4, 122.4, 130.7; u (C, CH2): 24.9, 29.1, 40.0, 42.1, 53.0, 53.7, 58.4, 61.8, 118.1, 131.5, 140.2, 146.0, 156.5, 158.7, 174.8; HRMS calcd for C29H45N4O3 [M + H]+ 497.3486; found 497.3483; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2,6-dimethoxyphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7xx
1-((2-(2,6-Dimethoxyphenyl)-5-methyloxazol-4-yl)methyl)-piperidine-4-carboxylic acid (25 mg, 0.069 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (13 mg, 0.076 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (22 mg, 0.042 mmol, 61% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 12.0 Hz, 1H), 0.88 (d, J = 6.5 Hz, 6H), 1.43 (t, J = 11.0 Hz, 2H), 1.60–1.91 (complex, 9H), 1.97–2.06 (m, 1H), 2.10 (dt, J = 2.7, 11.5 Hz, 2H), 2.39 (s, 3H), 2.43 (t, J = 6.1 Hz, 2H), 2.87–2.91 (m, 2H), 3.03–3.07 (m, 2H), 3.34 (q, J = 6.2 Hz, 2H), 3.51 (s, 2H), 3.78 (s, 6H), 6.59 (d, J = 8.4 Hz, 2H), 7.29 (br s, 1H), 7.35 (t, J = 8.4 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 19.6, 31.3, 43.5, 56.1, 103.8, 131.7; u (C, CH2): 24.9, 29.1, 39.8, 42.0, 53.1, 53.9, 58.2, 61.7, 107.2, 131.7, 146.3, 154.2, 159.7, 175.0; HRMS calcd for C29H45N4O4 [M + H]+ 513.3435; found 513.3431; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((3,4-dimethoxyphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7yy
1-((2-(3,4-Dimethoxyphenyl)-5-methyloxazol-4-yl)methyl)-piperidine-4-carboxylic acid (57 mg, 0.16 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (81 mg, 0.47 mmol) were reacted according to general procedure A-1 to afford the product as a white solid (46 mg, 0.090 mmol, 56% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 12.0 Hz, 1H), 0.89 (d, J = 6.5 Hz, 6H), 1.43 (t, J = 11.1 Hz, 2H), 1.60–1.88 (complex, 9H), 2.01–2.12 (m, 3H), 2.40 (s, 3H), 2.43 (t, J = 6.1 Hz, 2H), 2.86–2.93 (m, 2H), 3.00–3.06 (m, 2H), 3.35 (q, J = 5.8 Hz, 2H), 3.45 (s, 2H), 3.94 (s, 3H), 3.98 (s, 3H), 6.93 (d, J = 8.4 Hz, 1H), 7.37 (br s, 1H), 7.55–7.57 (m, 1H), 7.61 (dd, J = 1.8, 8.4 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.3, 19.5, 31.2, 43.3, 55.9, 56.0, 108.9, 110.8, 119.1; u (C, CH2): 24.9, 28.8, 39.7, 42.0, 53.1, 53.9, 58.0, 61.6, 120.7, 132.3, 145.4, 148.9, 150.4, 159.3, 174.8; IR 1502, 1644, 2949 cm−1; HRMS calcd for C29H45N4O4 [M + H]+ 513.3435; found 513.3458; HPLC purity: 94.2%.
N-(4-(Diethylamino)butyl)-1-((2-(3,4-dimethoxyphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxamide 7zz
1-((2-(3,4-Dimethoxyphenyl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (30 mg, 0.091 mmol) and N1,N1-diethylbutane-1,4-diamine (16 mg, 0.11 mmol) were reacted according to general procedure A-1 to afford the product as a white solid (26 mg, 0.053 mmol, 59% yield). 1H NMR (400 MHz, CDCl3) δ 1.10 (t, J = 7.2 Hz, 6H), 1.51–1.64 (complex, 4H), 1.77–1.85 (m, 3H), 1.99–2.14 (m, 3H), 2.37 (s, 3H), 2.56 (t, J = 7.2 Hz, 2H), 2.67 (q, J = 7.2 Hz, 4H), 2.98–3.04 (m, 2H), 3.26 (q, J = 5.8 Hz, 2H), 3.42 (s, 2H), 3.59–3.65 (m, 1H), 3.93 (s, 3H), 3.96 (s, 3H), 6.33 (br s, 1H), 6.90 (d, J = 8.4 Hz, 1H), 7.54 (d, J = 1.9 Hz, 1H), 7.58 (dd, J = 2.08.4 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 8.1, 10.4, 10.6, 43.4, 55.9, 109.0, 110.9, 119.2; u (C, CH2): 23.7, 27.4, 28.8, 38.7, 46.5, 52.1, 53.1, 53.9, 120.8, 132.3, 145.6, 149.0, 150.5, 159.5, 175.1; HRMS calcd for C27H43N4O4 [M + H]+ 487.3279; found 487.3286; HPLC purity: 95.7%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((5-methyl-2-(naphthalen-1-yl)oxazol-4-yl)methyl)piperidine-4-carboxamide 7aaa
1-((5-Methyl-2-(naphthalen-1-yl)oxazol-4-yl)methyl)-piperidine-4-carboxylic acid (28 mg, 0.080 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (15 mg, 0.088 mmol) were reacted according to general procedure A-3 to afford the product as a white solid (21 mg, 0.041 mmol, 51% yield). 1H NMR (400 MHz, CDCl3) δ 0.57 (q, J = 11.5 Hz, 1H), 0.88 (d, J = 6.4 Hz, 6H), 1.46 (t, J = 11.0 Hz, 2H), 1.69–1.93 (complex, 9H), 2.01–2.10 (m, 1H), 2.16 (dt, J = 3.0, 11.2 Hz, 2H), 2.46 (t, J = 6.2 Hz, 2H), 2.48 (s, 3H), 2.87–2.97 (m, 2H), 3.09–3.12 (m, 2H), 3.36 (q, J = 6.4 Hz, 2H), 3.59 (s, 2H), 7.34 (br s, 1H), 7.51–7.59 (m, 2H), 7.62–7.66 (m, 1H), 7.89–7.95 (m, 2H), 8.17 (dd, J = 1.2, 7.3 Hz, 1H), 9.24 (d, J = 8.7 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 19.6, 31.2, 43.5, 124.9, 126.1, 126.4, 127.3, 127.4, 128.4, 130.6; u (C, CH2): 24.8, 29.1, 39.7, 41.9, 53.1, 54.0, 58.0, 61.6, 124.4, 130.1, 132.8, 133.9, 145.9, 159.2, 174.9; HRMS calcd for C31H43N4O2 [M + H]+ 503.3381; found 503.3386; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((5-methyl-2-(2-methylnaphthalen-1-yl)oxazol-4-yl)methyl)piperidine-4-carboxamide 7bbb
1-((5-Methyl-2-(2-methylnaphthalen-1-yl)oxazol-4-yl)-methyl)piperidine-4-carboxylic acid (25 mg, 0.069 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (13 mg, 0.075 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (20 mg, 0.038 mmol, 55% yield). 1H NMR (400 MHz, CDCl3) δ 0.56 (q, J = 11.8 Hz, 1H), 0.88 (d, J = 6.5 Hz, 6H), 1.44 (t, J = 11.0 Hz, 2H), 1.60–1.95 (complex, 9H), 2.01–2.10 (m, 1H), 2.17 (dt, J = 2.9, 11.4 Hz, 2H), 2.45 (t, J = 6.2 Hz, 2H), 2.461 (s, 3H), 2.464 (s, 3H), 2.88–2.92 (m, 2H), 3.08–3.13 (m, 2H), 3.36 (q, J = 6.3 Hz, 2H), 3.60 (s, 2H), 7.35 (br s, 1H), 7.40 (d, J = 8.4 Hz, 1H), 7.42–7.51 (m, 2H), 7.72–7.75 (m, 1H), 7.79–7.90 (m, 2H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.5, 19.6, 20.6, 31.3, 43.4, 125.1, 125.3, 126.9, 127.9, 128.4, 129.9; u (C, CH2): 24.8, 29.1, 39.8, 42.0, 53.1, 53.7, 58.2, 61.7, 124.7, 131.79, 131.83, 132.8, 137.0, 146.3, 158.1, 174.9; HRMS calcd for C32H45N4O2 [M + H]+ 517.3537; found 517.3535; HPLC purity: >99.8%.
1-((2-(Anthracen-9-yl)-5-methyloxazol-4-yl)methyl)-N-(3-(cis-3,5-dimethylpiperidin-1-yl)propyl)piperidine-4-carboxamide 7ccc
1-((2-(Anthracen-9-yl)-5-methyloxazol-4-yl)methyl)piperidine-4-carboxylic acid (25 mg, 0.062 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (12 mg, 0.069 mmol) were reacted according to general procedure A-3 to afford the product as a viscous, light yellow oil (16 mg, 0.029 mmol, 47% yield). 1H NMR (400 MHz, CDCl3) δ 0.57 (q, J = 11.2 Hz, 1H), 0.88 (d, J = 6.4 Hz, 6H), 1.51 (t, J = 11.1 Hz, 2H), 1.69–1.98 (complex, 9H), 2.05–2.16 (m, 1H), 2.23 (dt, J = 3.0, 11.1 Hz, 2H), 2.50 (t, J = 6.2 Hz, 2H), 2.53 (s, 3H), 2.91–3.02 (m, 2H), 3.14–3.19 (m, 2H), 3.36 (q, J = 6.4 Hz, 2H), 3.68 (s, 2H), 7.36 (br s, 1H), 7.46–7.58 (complex, 4H), 7.95–8.03 (m, 2H), 8.03–8.11 (m, 2H), 8.59 (s, 3H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 10.6, 19.5, 30.9, 43.3, 125.4, 125.7, 126.9, 128.5, 129.8; u (C, CH2): 24.7, 29.0, 39.3, 41.7, 53.1, 53.9, 57.6, 61.3, 122.2, 131.1, 131.3, 132.3, 147.0, 157.8, 175.0; HRMS calcd for C35H45N4O2 [M + H]+ 553.3537; found 553.3533; HPLC purity: >99.8%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((5-methyl-2-(otolyl) thiazol-4-yl)methyl)piperidine-4-carboxamide 13
1-((5-Methyl-2-(o-tolyl)thiazol-4-yl)methyl)piperidine-4-carboxylic acid (56 mg, 0.17 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (29 mg, 0.17 mmol) were reacted according to general procedure A-1 to afford the product as a white solid (37 mg, 0.077 mmol, 45% yield). 1H NMR (400 MHz, CDCl3) δ 0.57 (q, J = 12.3 Hz, 1H), 0.87 (d, J = 6.4 Hz, 6H), 1.51 (t, J = 11.0 Hz, 2H), 1.66–1.85 (complex, 9H), 1.98–2.00 (m, 1H), 2.13 (dt, J = 3.2, 11.1 Hz, 2H), 2.47–2.56 (m, 2H), 2.49 (s, 3H), 2.53 (s, 3H), 2.92–2.99 (m, 2H), 2.99–3.06 (m, 2H), 3.32 (q, J = 6.6 Hz, 2H), 3.68 (s, 2H), 7.20–7.30 (complex, 4H), 7.62 (d, J = 7.5 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 11.4, 19.5, 21.3, 30.9, 43.4, 125.9, 129.0, 129.8, 131.2; u (C, CH2): 24.7, 29.0, 39.2, 41.7, 53.0, 56.0, 57.5, 61.2, 131.1, 133.3, 136.3, 148.9, 163.0, 175.1; IR 1653, 2928, 2960 cm−1; HRMS calcd for C28H43N4OS [M + H]+ 483.3152; found 483.3154; HPLC purity: 97.1%.
N-(3-(cis-3,5-Dimethylpiperidin-1-yl)propyl)-1-((2-(4-fluoro-3-methylphenyl)oxazol-4-yl)methyl)piperidine-4-carboxamide 18a
1-((2-(4-Fluoro-3-methylphenyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (5.95 g, 18.69 mmol) and 3-(cis-3,5-dimethylpiperidin-1-yl)propan-1-amine (3.82 g, 22.43 mmol) were reacted according to general procedure A-3 to afford the product as a white solid (5.30 g, 11.26 mmol, 60% yield). 1H NMR (400 MHz, CDCl3) δ 0.52 (q, J = 12.1 Hz, 1H), 0.84 (d, J = 6.5 Hz, 6H), 1.38 (t, J = 11.1 Hz, 2H), 1.57–1.84 (complex, 9H), 1.96–2.08 (m, 3H), 2.29 (s, 3H), 2.39 (t, J = 6.1 Hz, 2H), 2.82–2.88 (m, 2H), 2.98–3.05 (m, 2H), 3.30 (q, J = 6.0 Hz, 2H), 3.48 (s, 2H), 7.03 (t, J = 8.9 Hz, 1H), 7.31 (br s, 1H), 7.52 (s, 1H), 7.77–7.82 (m, 1H), 7.86–7.90 (m, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 14.4 (d, J = 3.5 Hz), 19.6, 31.3, 43.3, 115.4 (d, J = 23.3 Hz), 125.7 (d, J = 8.7 Hz), 129.8 (d, J = 5.7 Hz), 136.1; u (C, CH2): 24.8, 28.9, 39.8, 42.0, 53.2, 54.2, 58.1, 61.7, 123.5 (d, J = 3.6 Hz), 125.5 (d, J = 18.2 Hz), 139.0, 161.0 (d, J = 1.1 Hz), 162.6 (d, J = 250.8 Hz), 174.7; 19F NMR 376 MHz, CDCl3) δ –114.1; IR 1549, 1630, 2944 cm−1; HRMS calcd for C27H40FN4O2 [M + H]+ 471.3130; found 471.3137; HPLC purity: 99.3%.
1-((2-(4-Fluoro-3-methylphenyl)oxazol-4-yl)methyl)-N-(3-(4-isopropylpiperidin-1-yl)propyl)piperidine-4-carboxamide 18b
1-((2-(4-Fluoro-3-methylphenyl)oxazol-4-yl)methyl)piperidine-4-carboxylic acid (40 mg, 0.13 mmol) and 3-(4-isopropylpiperidin-1-yl)propan-1-amine (46 mg, 0.25 mmol) were reacted according to general procedure A-3 to afford the product as a viscous light yellow oil (14 mg, 0.028 mmol, 22% yield). 1H NMR (400 MHz, CDCl3) δ 0.80 (d, J = 6.8 Hz, 6H), 0.96–1.06 (m, 1H), 1.24 (dq, J = 2.9, 12.2 Hz, 2H), 1.57–1.84 (complex, 9H), 1.96–2.08 (m, 3H), 2.29 (s, 3H), 2.39 (t, J = 6.1 Hz, 2H), 1.38 (sextet, J = 6.6 Hz, 1H), 1.59–1.68 (complex, 4H), 1.69–1.87 (complex, 5H), 1.97–2.08 (m, 3H), 2.28 (s, 3H), 2.41 (t, J = 6.0 Hz, 2H), 2.94–3.04 (m, 4H), 3.30 (q, J = 5.7 Hz, 2H), 3.47 (s, 2H), 7.02 (t, J = 8.9 Hz, 1H), 7.51 (s, 1H), 7.55 (br s, 1H), 7.75–7.81 (m, 1H), 7.87 (d, J = 7.1 Hz, 1H); 13C NMR (101 MHz, CDCl3, APT pulse sequence) δ d (CH, CH3): 14.4 (d, J = 3.5 Hz), 19.6, 32.3, 42.1, 43.3, 115.4 (d, J = 23.3 Hz), 125.7 (d, J = 8.7 Hz), 129.8 (d, J = 5.8 Hz), 136.1; u (C, CH2): 24.5, 29.0, 29.1, 39.9, 53.3, 54.2, 54.4, 58.2, 123.5 (d, J = 3.5 Hz), 125.5 (d, J = 18.2 Hz), 138.9, 161.0 (d, J = 1.0 Hz), 162.5 (d, J = 250.7 Hz), 174.8; 19F NMR 376 MHz, CDCl3) δ −114.1; HRMS calcd for C28H42FN4O2 [M + H]+ 485.3286; found 485.3263; HPLC purity: 96.5%.
Cells and Viruses
All the cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies, Grand Island, NY, United States) with 10% fetal bovine serum (FBS) (Life Technologies) and antibiotics in 5% CO2 at 37 °C. HCV-Luc was made through insertion of luciferase reporter gene in the HCV JFH-1 strain. Single-round infectious defective HCV particles (HCVsc) and pseudotyped viruses (HCVpp-1a, HCVpp-1b, and VSV-Gpp) were produced as reported before.7,10
HCV-Luc Infection and ATPlite Cytotoxicity Assays
Huh7.5.1 cells seeded in 96-well plates (104 cells/well) were cultured overnight. HCV-Luc virus was used to infect the cells in the presence of titration of the compound of interest. Viral inhibition was evaluated using Renilla Luciferase assay system (Promega, Madison, WI, United States) 48 h post treatment. Cytotoxicity of each compound was measured in parallel using ATPlite assay kit (PerkinElmer, Waltham, MA, United States). EC50 and CC50 values were calculated by nonlinear regression equation in GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, CA, United States).
HCV Replication Cycle Assays
In HCV single-cycle infection assay, Huh7.5.1 cells seeded in 96-well plates (104 cells/well) were cultured overnight. The cells were inoculated with the infectious HCVsc together with the tested compounds. Luciferase activity of the cells was measured 48 h after the compound treatment. In transient replicon assay, Huh7.5.1 cells seeded in 96-well plates (104 cells/well) were cultured overnight. Then, the cells were transiently transfected with the replicon RNA transcript with DMRIE-C for 4 h. After the transfection reagent was removed, the cells were incubated with DMEM culture medium containing 10 μM of each compound for 48 h. Luciferase activity was measured. In HCV subgenomic replicon assay with HCV replicon (GT 2a) cells, cells were plated into 96-well plate (104 cells/well) and incubated overnight. The cells were treated with tested compounds. Luciferase activity was measured 48 h after the compound treatment. In HCVpp assays, Huh7.5.1 cells were seeded in 96-well plates (104 cells/well) and cultured overnight. Then, the cells were treated with 10 μM of the compounds together with infection of HCVpp GT 1a or VSVpp for 4 h. The cells were then washed and cultured for 48 h followed by a luciferase assay to detect the HCV entry. The results shown are the means of five replicates ± SEM.
Compound Binding Affinity in CNS-Relevant Receptor Panel
Radioligand binding assays using cloned GPCRs, ion channels, and transporters were performed from transiently transfected or stable cell lines as previously detailed21 through the resources of the National Institute of Mental Health Psychoactive Drug Screening Program. Detailed protocols (including cell handling, buffer composition, assay conditions, etc.) for all assays are available online (http://pdspdb.unc.edu/pdspWeb/content/PDSP%20Protocols%20II%202013-03-28.pdf). Initial screening assays were performed using 10 μM (final concentration) of test compound, and the percent inhibition of specific binding by the test compound was determined. When the test compound inhibited >50% of radioligand specific binding, Ki determinations were performed by measuring the inhibition of radioligand binding by various concentrations of test compound (11 concentrations spanning 6 orders of magnitude). Radioligand binding isotherms were regressed using the One Site competition binding function built into GraphPad Prism 4.0 to estimate compound IC50 values. Affinity constants (Ki values) were calculated from IC50 values using the Cheng–Prusoff approximation.
In Vitro Combination Test
HCV-Luc infection and ATPlite assays were carried out in 96-well plates in the presences of the compound of interest titrated in vertical and the known antiviral drug titrated in horizontal.13,22 The known antiviral drugs include ribavirin (Sigma-Aldrich), sofosbuvir (Advanced Chemblocks), telaprevir (Selleckchem), daclatasvir (Selleckchem), cyclosporin A (Sigma-Aldrich), and boceprevir (ChemScene). Two independent mathematical models, the Bliss independence model and the Loewe additivity model, were used to predict the theoretical additive, synergistic, or antagonistic effects. By the Bliss independence model, log volumes of synergistic or antagonistic effect were calculated with the MacSynergy program. By the Loewe additivity model, combination indices were calculated at or near the EC50 values of the compound and the antiviral drug when tested alone with CalcuSyn program (Biosoft).
Antiviral Screen
The antiviral activity of compound of interest was screened against 13 viruses, including hepatitis B virus, HCV replicon, herpes simplex virus-1, human cytomegalovirus, vaccinia virus, dengue virus, influenza A (H1N1) virus, respiratory syncytial virus, SARS coronavirus, poliovirus 3, Rift Valley fever virus, Tacaribe virus, and Venezuelan equine encephalitis virus, using the nonclinical and preclinical services program offered by the National Institute of Allergy and Infectious Diseases (NIAID) (https://www.niaid.nih.gov/research/vitro-assessment-antimicrobial-activity-resources-niaid).
In Vitro Biopharmaceutical and in Vivo Pharmacokinetic Properties
In vitro biopharmaceutical properties were measured with a fully automated system for sample preparation, sample analysis, and data processing. The microsomal stability of compounds was estimated in rat liver microsomes at 37 °C in the presence of the cofactor NADPH. Concentration of a test article was measured by LC–MS/ MS, and half-life (t1/2) was calculated as described before.23 The solubility of compounds was determined in phosphate buffer (pH 7.4) using μSOL Evolution (Pion Inc., Billerica, MA, United States). The permeability of compounds was estimated via passive diffusion using stirring double-sink parallel artificial membrane permeability assay (PAMPA) (Pion Inc.). The in vivo pharmacokinetics properties of compounds of interest were evaluated in male CD-1 mice after single intraperitoneal administration. Adult male CD-1 mice (25–39 g, n = 3/sampling time point) were obtained from Charles River Laboratories (Wilmington, MA). Mice were housed at the centralized animal facilities at the NIH (Bethesda, MD) with a 12 h light-dark cycle. The housing temperature and relative humidity were controlled at 22 °C and 55%, respectively. The animals had free access to water and food. All experimental procedures were approved by the Animal Care and Use Committee (ACUC) of the NIH Division of Veterinary Resources (DVR). A single dose of 0.1, 1, and 10 mg/kg was administered through i.p. route of administration. The concentration of compounds in the plasma and liver samples was measured by the ultraperformance liquid chromatography–mass spectrometry analysis (UPLC–MS/MS).
Supplementary Material
Figure 1.
Summary of structural modifications explored in optimizing the aryloxazole hit and structure of the selected lead analogue 18a.
Figure 2.
Titration curves for anti-HCV activity (EC50, triangle in blue) and cytotoxicity (CC50, circle in red) of selected leads. Cell-culture adapted HCV (HCVcc) harboring a luciferase reporter gene (HCV-Luc, genotype 2a) was used to infect Huh7.5.1 cells in the presence of increasing concentrations of test compound. Viral infection and replication were measured by luciferase signal 48 h after treatment. Cytotoxicity was evaluated in parallel with the ATP-based cell viability assay (ATPlite). The results are mean from three replicates ± SEM. The EC50 and CC50 values were calculated with GraphPad Prism 5.0 software using nonlinear regression. Curves and values for each compound were from single representative experiments.
Figure 3.
Profile of representative analogue 7nn against a panel of 50 CNS-relevant targets. Values are Ki determinations (nM) of radioligand binding in a displacement assay. Primary screen assays are single-point experiments to determine percent radioligand displacement at 10 μM of test compound. The threshold for hit or miss in the primary screen was 50% displacement.
Figure 4.
Pharmacokinetics studies of compound 7ii in the mouse model. (A) Mean liver (green triangle) and plasma (red dot) concentration–time profiles of compound 7ii after administration of a single i.p. dose at 10, 1, and 0.1 mg/kg at indicated time points. Compound concentration of 7ii was measured by UPLC-MS/MS methods and is shown in means ± SEM (n = 3 per time point). (B) Pharmacokinetic parameters of compound 7ii. AUClast = AUC0–24h or AUC0–168h depending on the sample collection interval. N.D., not determined. (C) ALT levels of the mouse serum samples collected during the pharmacokinetics study. Results for each mouse are shown with scatter plots, and error bars show means ± SEM.
Acknowledgments
We thank S. Michael and M. Balcom for assistance in robotic operation in qHTS; P. Shinn, M. Itkin, and D. van Leer for help with compound management; B. Zhang for technical assistance; Benjamin Neuenswander for compound purification and analysis; and Patrick Porubsky for compound management. Receptor binding profile was generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract HHSN-271-2013-00017-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth MD, Ph.D at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscoll at NIMH, Bethesda, MD, United States. This research was funded by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, the Intramural Research Program of the National Center for Advancing Translational Sciences, and Molecular Libraries Initiative funding to the University of Kansas Specialized Chemistry Center (U54HG005031).
ABBREVIATIONS USED
- HCV
hepatitis C virus
- IFN-α
interferon α
- RBV
ribavirin
- DAAs
direct-acting antivirals
- HTAs
host-targeting agents
- qHTS
high-throughput screening
- SAR
structure–activity relationship
- DMSO
dimethyl sulfoxide
- HCV-Luc
HCV JFH-1 strain with insertion of the luciferase reporter gene
- EC50
the concentration of compound that inhibited 50% of virus level of DMSO
- CC50
the concentration of compound that exhibited 50% of cytotoxicity of DMSO
- HCVsc
singleround infectious defective HCV particles
- HCVpp
HCV pseudoparticle
- RLU
relative luminescence units
- ADME
absorption, distribution, metabolism, and excretion
- i.p
intraperitoneal
- DMF
N,N-dimethylformamide
- LC–MS
liquid chromatography–mass spectrometry
- TOF
time-of-flight
- TFA
trifluoroacetic acid
- NCS
N-chlorosuccinimide
- SFC
supercritical fluid chromatography
- THF
tetrahydrofuran
- DMEM
Dulbecco’s modified Eagle’s medium
- FBS
fetal bovine serum
- NIAID
National Institute of Allergy and Infectious Diseases
Footnotes
Notes
The authors declare the following competing financial interest(s): T.J.L., S.H., X.H., Z.H., J.J.M., N.S., J.X., M.F., W.Z., K.J.F., K.L., and F.J.S. are named as inventors on U.S. provisional patent application (no. 62/011,462; Heterocyclic Compounds and Methods of Use Thereof) and international patent application (no. WO 2015192077A1; Preparation of Heterocyclic Compounds Useful in the Treatment of Diseases) related to this work. Other authors declare no competing interests.
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem. 7b00561.
Tables showing all synthetic intermediates, synthetic procedures, compound characterization for synthetic intermediates, and NIAID antiviral screen data (PDF) Molecular formula strings (SMILES) for final analogues (CSV)
References
- 1.Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14:1–21. vii. doi: 10.1016/j.cld.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Ferenci P. Treatment of hepatitis C in difficult-to-treat patients. Nat Rev Gastroenterol Hepatol. 2015;12:284–292. doi: 10.1038/nrgastro.2015.53. [DOI] [PubMed] [Google Scholar]
- 3.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 4.Liang TJ. Current progress in development of hepatitis C virus vaccines. Nat Med. 2013;19:869–878. doi: 10.1038/nm.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907–1917. doi: 10.1056/NEJMra1213651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callaway E. Hepatitis C drugs not reaching poor. Nature. 2014;508:295–296. doi: 10.1038/508295a. [DOI] [PubMed] [Google Scholar]
- 7.Hu Z, Lan KH, He S, Swaroop M, Hu X, Southall N, Zheng W, Liang TJ. Novel cell-based hepatitis C virus infection assay for quantitative high-throughput screening of anti-hepatitis C virus compounds. Antimicrob Agents Chemother. 2014;58:995–1004. doi: 10.1128/AAC.02094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Z, Hu X, He S, Yim HJ, Xiao J, Swaroop M, Tanega C, Zhang YQ, Yi G, Kao CC, Marugan J, Ferrer M, Zheng W, Southall N, Liang TJ. Identification of novel anti-hepatitis C virus agents by a quantitative high throughput screen in a cell-based infection assay. Antiviral Res. 2015;124:20–29. doi: 10.1016/j.antiviral.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goto YY, Hamana M. Studies on azole compounds. III. Reactions of oxazole N-oxides with phosphoryl chloride and acetic anhydride. Chem Pharm Bull. 1971;19:2050–2057. [Google Scholar]
- 10.Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci U S A. 2003;100:7271–7276. doi: 10.1073/pnas.0832180100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. 2000;57:75–81. [PubMed] [Google Scholar]
- 12.Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 13.Prichard MN, Shipman C., Jr A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 1990;14:181–205. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- 14.Bassit L, Grier J, Bennett M, Schinazi RF. Combinations of 2′-C-methylcytidine analogues with interferon-alpha2b and triple combination with ribavirin in the hepatitis C virus replicon system. Antiviral Chem Chemother. 2008;19:25–31. doi: 10.1177/095632020801900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amacher DE. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul Toxicol Pharmacol. 1998;27:119–130. doi: 10.1006/rtph.1998.1201. [DOI] [PubMed] [Google Scholar]
- 16.He S, Xiao J, Dulcey AE, Lin B, Rolt A, Hu Z, Hu X, Wang AQ, Xu X, Southall N, Ferrer M, Zheng W, Liang TJ, Marugan JJ. Discovery, optimization, and characterization of novel chlorcyclizine derivatives for the treatment of hepatitis C virus infection. J Med Chem. 2016;59:841–853. doi: 10.1021/acs.jmedchem.5b00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schweitzer CJ, Liang TJ. Border control in hepatitis C virus infection: inhibiting viral entry. ACS Infect Dis. 2015;1:416–419. doi: 10.1021/acsinfecdis.5b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Wang S, Cheng L, Chen L, Wang Y, Qing J, Huang S, Wang Y, Lei X, Wu Y, Ma Z. Discovery of imidazo [1, 2-α][1, 8] naphthyridine derivatives as potential HCV entry inhibitor. ACS Med Chem Lett. 2015;6:977–981. doi: 10.1021/acsmedchemlett.5b00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittapalli GK, Jackson A, Zhao F, Lee H, Chow S, McKelvy J, Wong-Staal F, Macdonald JE. Discovery of highly potent small molecule hepatitis C virus entry inhibitors. Bioorg Med Chem Lett. 2011;21:6852–6855. doi: 10.1016/j.bmcl.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Baldick CJ, Wichroski MJ, Pendri A, Walsh AW, Fang J, Mazzucco CE, Pokornowski KA, Rose RE, Eggers BJ, Hsu M, Zhai W. A novel small molecule inhibitor of hepatitis C virus entry. PLoS Pathog. 2010;6:e1001086. doi: 10.1371/journal.ppat.1001086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, Norval S, Sassano MF, Shin AI, Webster LA, Simeons FR, Stojanovski L, Prat A, Seidah NG, Constam DB, Bickerton GR, Read KD, Wetsel WC, Gilbert IH, Roth BL, Hopkins AL. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin B, He S, Yim HJ, Liang TJ, Hu Z. Evaluation of antiviral drug synergy in an infectious HCV system. Antiviral Ther. 2016;21:595–603. doi: 10.3851/IMP3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27:1350–1359. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.