Abstract
Background
Intensive task-oriented training such as constraint-induced movement therapy (CIMT) is thought to engage motor learning and decision-making processes, including anticipatory action planning.
Objective
To identify the effects of CIMT on anticipatory hand posture selection and movement time for task-specific reach-to-grasp performance.
Methods
Subacute and chronic poststroke participants were recruited into CIMT (n = 10) or non-CIMT (n = 10) groups. Arm and hand functions were assessed before and after 2 weeks with the Wolf Motor Function Test (WMFT), Motor Activity Log (MAL), and a unique skilled reach-to-grasp task designed to test anticipatory hand posture selection. The reach-to-grasp tasks included power and precision grasping in 2 conditions achieved optimally with either a pronated (low difficulty) or supinated (high difficulty) hand posture. Outcome measures included success rate, frequency of optimal strategy selection, and movement time.
Results
Between-group comparisons revealed a significant treatment effect for WMFT and MAL scores. The CIMT group showed larger gains in success rate, optimal posture selection (precision grasp only), and faster movement times for the supinated conditions.
Conclusion
Together, a faster movement time and greater frequency of optimal hand posture selection in the more difficult task condition highlights a set of novel findings. These results provide evidence for training-induced improvements in upper-extremity function that support neurobehavioral recovery more than compensation. Although these findings are preliminary in view of the small sample size, the authors suggest that they may be useful to design and power larger-scale studies to further the understanding of the fundamental mechanisms induced by task-oriented training interventions in neurorehabilitation.
Keywords: motor learning, motor planning, posture selection, manual dexterity
Introduction
During the past decade, there has been mounting evidence for the efficacy of forced-use and constraint-induced movement therapy (CIMT) protocols to address the suboptimal recovery of upper-extremity function after stroke.1–11 CIMT was designed to lift “learned nonuse,” incurred early after the stroke when attempted use of the paretic limb is met with failure and negative reinforcement.12 The reversal of this learned nonuse and increased paretic limb use in everyday activities has been shown to result from a 2-week protocol of training in the laboratory for 6 h/d, 5 d/wk combined with restraint of the nonparetic limb during 90% of waking hours.11,13
CIMT is also considered a subgroup of a larger category of interventions termed task-oriented training (TOT), which has emerged as the dominant approach for the restoration of motor function after stroke.14 Though individual protocols vary, TOT interventions are designed to enhance functional, goal-directed behavior through the application of principles derived from behavioral neuroscience, motor control, and motor learning.15,16 An important guiding principle for TOT is that motor learning is an integral mechanism for motor recovery. Shadmehr and Wise17 have argued convincingly that motor learning involves skill acquisition, motor adaptation, and decision-making processes, including the ability to select the correct movement in the proper context. With this logic, one might expect that an intense, focused, and progressive CIMT task practice protocol would lead to the development of specific motor skills, including associated anticipatory planning.
Anticipatory planning of reach-to-grasp actions has been studied using a novel approach18,19 that allows the performer to choose a movement strategy to solve the movement problem and thereby provides the investigator with insight about anticipatory planning and action selection. For example, to pick up and fill an inverted wine glass, a common observation reveals a seemingly awkward initial grasp posture, but when the glass is righted, the forearm unwinds and assumes a neutral and comfortable end posture.19 Rosenbaum and colleagues19 describe this phenomenon as the “end-stage comfort” effect. Through a series of experiments using various reach-to-grasp tasks, they provided strong evidence that movements are planned to account for these end-posture effects. We chose the same paradigm to compare anticipatory planning of reach-to-grasp actions in participants post-stroke with those who are nondisabled and with those poststroke who participated in 2 weeks of CIMT.
Though there is a paucity of studies investigating the effects of CIMT and, in general, other TOT protocols on decision making and anticipatory planning, quantitative measures of the kinetic20 and kinematic changes21–26 induced through a focused and intense bout of CIMT are beginning to appear in the literature. This development is consistent with the recommendations in a recent editorial, which argues strongly for the inclusion of measures of impairment and function and especially EMG and kinematics necessary to understand compensatory and/or recovery movement strategies.27 In fact, a recent article follows this recommendation and shows gains in upper-extremity function, kinematics, and EMG using a reach-to-grasp task similar to the one we use here.28 In addition, there is evidence for a significant change in the kinematics of reaching control22–25 and gross reach-to-grasp.23 Improvement in the speed of movement and measures related to the capacity for coordination21 as well as the kinetic behaviors, including force and torque generation of a key turning task,20 have also been reported. These findings are not particularly surprising, especially if viewed in the context of task-specific practice for functional upper-limb behaviors.
The purpose of this study, therefore, is to identify changes in anticipatory hand posture selection and task-specific movement time of reach-to-grasp actions induced by the signature CIMT protocol and in conjunction with the standard CIMT outcome measures. Our hypothesis is that in addition to increased paretic limb use, another benefit of CIMT will be improved anticipatory planning and task-specific movement time in the performance of reach-to-grasp tasks.
Methods
Participants
A total of 20 (5–120 months duration from stroke) participants poststroke were enrolled in the study. Patient inclusion criteria were as follows: (1) at least 3 months poststroke, (2) ability to perform at least 1 trial of the reach-to-grasp tasks, and (3) ability to achieve a minimum of 10° of voluntary wrist and finger extension. In addition, 6 nondisabled adults were recruited from the local community for a 1-time assessment of the preferred hand posture selection for each reach-to-grasp task to serve as a comparison group. Each prospective participant was provided with a written informed consent form and a HIPAA Authorization approved by the institutional review board of the University of Southern California. All participants read and gave written consent prior to study enrollment.
Experimental Design and Assessments
This pilot study was a between-group repeated-measures design with 4 independent variables: group (CIMT, non-CIMT) and reach-to-grasp task variables object type (card, dowel), task condition (same, different), and time (baseline, post). Participants were pseudorandomly assigned to receive CIMT (n = 10) or not (non-CIMT, n = 10). Participants in the CIMT group underwent 2 weeks of CIMT as defined for the EXCITE trial,11 and the non-CIMT group received 2 weeks of usual and customary care. Functional tests and task-specific measures using the reach-to-grasp task were acquired from all participants at baseline and after the 2-week intervention period.
Reach-to-Grasp Tasks
The reach-to-grasp tasks and apparatus were designed to systematically examine the anticipatory planning behavior and kinematic characteristics of reach-to-grasp actions and to characterize treatment-related changes. The goal of the reach-to-grasp task was to reach for an object, place it in a target hole, and return the hand to the home position.
Apparatus
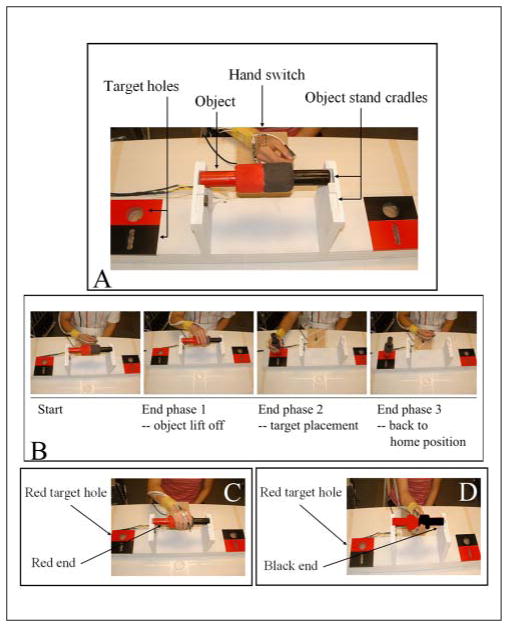
A custom-made, task-specific work board (73 × 14 × 20 cm3) provided a standard support for 2 objects (dowel or card) with corresponding goal target holes (Figure 1A). Both objects were of equal length (25 cm); the dowel was 6 cm in diameter, and the card was 0.8 cm thick and 3 cm wide. Both the objects and the target holes were painted red on one half and black on the other. Each object was horizontally positioned in the cradle stand of the work board, with the ends pointed to the target holes of the same color. This orientation afforded 2 task conditions: (1) same, putting the same colored end of the object into the hole, and (2) different, putting the different colored end of the object into the hole. The dowel and card afforded 2 different grasp types: power for dowel and 3-finger precision for card. To change object type, the work board was simply rotated, so that the desired object was directly in front of the participant, 15 cm from the front edge of the table. To trigger movement initiation, a 2-color LED signal mounted 25 cm above the table surface was positioned within view of the participant and was controlled externally by the experimenter.
Figure 1.
Reach-to-grasp task: A. Task apparatus: the task-specific work board and close up of start position shown with the dowel (card not shown). B. Procedure: example of a successful trial; movement times were captured by electronic switches at the home position, object cradle, and target hole. C. Optimal posture for same condition—pronated hand posture to place the red end in the red hole. D. Optimal posture for different condition—supinated hand posture to place the black end in the red hole (color figure available online at nnr.sagepub.com).
Testing procedure
There were 7 trials in each testing situation (4 situations: 2 object types × 2 task conditions) for a total of 28 testing trials for each participant. Trials were blocked by object type. The presentation order of object blocks was randomized across participants.
Participants sat in a straight-backed chair with the torso secured to the chair by a belt. For each trial, participants used the affected hand and assumed a neutral start position, with thumb and index finger closed and the hand resting on the hand switch (Figure 1A). At the signal, participants grasped the object, placed it in the target hole on the same side as the arm used, released the object, and returned to the start position (Figure 1B). All participants were given instructions to move as quickly as possible once they saw the LED signal. The task condition was randomly indicated by the LED color: green for same and red for different.
Reaction time, which refers to the time from LED onset to hand switch onset, was used to confirm the reliability of each trial. If the reaction time was greater than 1000 ms, the trial was deemed invalid, and another trial was collected. If the object was dropped during the trial, that trial was repeated to ensure a full complement of 7 trials for each condition. If participants were unable to complete 7 trials for each condition because of fatigue or inadequate motor control, the number of completed trials was recorded.
Dependent Measures
Functional Measures
The Wolf Motor Function test (WMFT-15) and Motor Activity Log (MAL), were used as standard functional outcome measures.29,30 The WMFT-15 consists of 15 function-based upper-extremity items, using time to completion as the primary outcome measure. In addition, a subset of 6 items from the original 15, the WMFT-6, was derived to capture fine-motor hand function (ie, lift a soda can, turn a key in the lock, pick up a pencil, stack checkers, turn over 3 cards, pick up a paperclip). The MAL is a semistructured interview in which participants are asked to rate the amount of use (AOU) and quality of movement of their more affected arm for 30 activities of daily living over a specified period (eg, in the past 3 days).
Reach-to-Grasp Task–Specific Measures
Behavioral measures
Behavioral measures in the reach-to-grasp tasks included success rate and optimal posture selection. Success rate was defined as the number of completed trials in each testing situation divided by 7. Overall success rate was defined as the total number of completed trials in all situations (4 situations = 28 trials) divided by 28. Overall optimal posture selection was defined by the total number of optimal hand postures chosen within the completed trials in each of the 4 situations. Frequency of optimal posture selection was defined as the number of participants whose optimal posture selections either increased, decreased, or did not change between baseline and postassessment. Here, an optimal hand posture was defined as a pronated grasp hand position in the same condition (Figure 1C) and a supinated grasp hand position in the different condition (Figure 1D).
Kinematic measures
Total movement time (TMT) was used as the kinematic measure for the reach-to-grasp tasks. Missing data for TMT from incomplete trials were imputed with 120 s as a maximum value.
Data Analysis
Demographic data and group mean WMFT and mean MAL scores were examined using an unpaired t test to compare characteristics between the 2 stroke groups at baseline. Baseline and postassessment mean WMFT and mean MAL scores were compared within each group using a paired t test to determine the effect of treatment. For all analyses, the significance level was set at P < .05. The novel and preliminary nature of the reach-to-grasp task–specific measures in addition to the small sample size precluded formal between-stroke-group statistical comparisons. Thus, only descriptive statistics (means and standard errors [SE]) were computed for success rate and TMT. Success rate and TMT change scores were derived by subtracting the baseline score from the postassessment score for success rate—[(Post − Baseline)/Baseline] × 100—and as baseline score minus postassessment score for TMT—[(Baseline − Post)/Baseline] × 100—so that a positive change score is consistent with improved performance. Missing data for success rate were imputed with a zero score. The number of participants who chose an optimal posture at postassessment compared with baseline is reflected by a frequency count for each of the 4 object/condition situations. Finally, an unpaired t test was used to compare baseline success rate and optimal posture selection between the stroke and nondisabled groups to determine the effect of stroke on reach-to-grasp task performance. For this analysis, optimal posture selection was calculated as a stroke group mean (n = 20) of the percentage optimal posture selection divided by 28 (7 trials × 4 object-condition situations). We computed this for the nondisabled group (n = 6) as well.
Results
Stroke Group Demographics
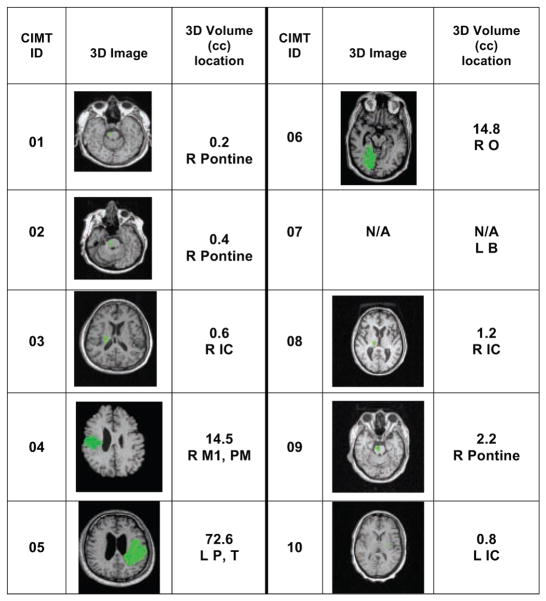
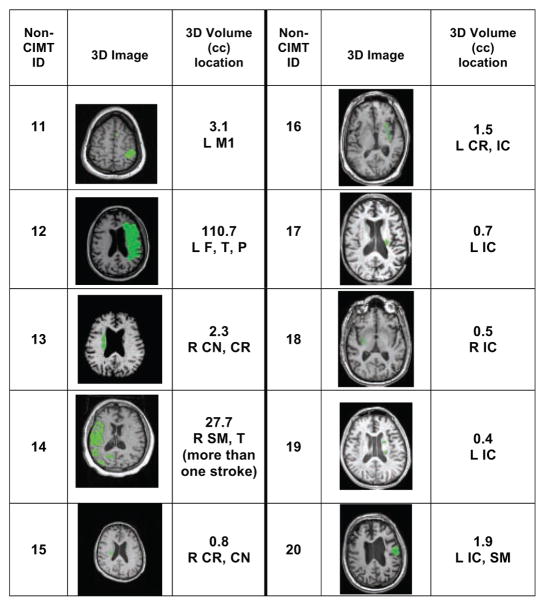
A total of 20 participants were enrolled in the study, and 10 participants (3 women) whose poststroke duration ranged from 5.2 to 10.5 months were assigned to the CIMT group. The other 10 participants (5 women) whose poststroke duration ranged from 8.0 to 119.9 months were assigned to the non-CIMT group. Table 1 summarizes demographic information for each group. Both stroke groups were equivalent in age (59.7 [CIMT] vs 58.2 years [non-CIMT]) and initial impairment severity (Fugl-Meyer score: 48.1 [CIMT] vs 47.9 [non-CIMT]). The non-CIMT group had a significantly longer mean poststroke duration (39.7 months) than the CIMT group (7.6 months). Figures 2 (CIMT) and 3 (non-CIMT) present lesion data for each participant in the 2 groups derived from MRI image analysis. 3D image sections display the section with the largest lesion size. Lesion size and location varied considerably within each group. There were no pronounced differences between groups, except that 4 out of 10 lesions in the CIMT group were located in the brainstem, and there were no brainstem lesions in the non-CIMT group. In contrast, in the non-CIMT group, 5 out of 10 lesions were located in the internal capsule, whereas there were only 3 out of 10 internal capsule lesions in the CIMT group.
Table 1.
Stroke Group Demographics
| Groups | ID | Age, (y) | Sex | Dominant Hand | Affected Hand | Time From Onset (months) | Initial Fugl-Meyer Motor Score (maximum = 66) |
|---|---|---|---|---|---|---|---|
| CIMT | 01 | 57 | M | R | L | 10.5 | 47 |
| 02 | 61 | F | R | L | 9.4 | 33 | |
| 03 | 58 | F | L | L | 5.2 | 45 | |
| 04 | 38 | M | R | L | 6.1 | 51 | |
| 05 | 57 | M | R | R | 8.1 | 50 | |
| 06 | 63 | M | R | L | 5.8 | 57 | |
| 07 | 55 | M | R | R | 9.0 | 41 | |
| 08 | 80 | M | R | L | 5.7 | 51 | |
| 09 | 73 | M | R | L | 7.0 | 53 | |
| 10 | 55 | F | R | R | 9.2 | 53 | |
| Average/Count (SEM) | n = 10 | 59.7 (3.54) | 7M/3F | 9R/1L | 3R/7L | 7.6 (0.60) | 48.1 (2.21) |
| Non-CIMT | 11 | 26 | F | R | R | 11.8 | 63 |
| 12 | 60 | F | L | R | 8.0 | 45 | |
| 13 | 69 | F | R | L | 8.6 | 49 | |
| 14 | 51 | F | R | L | 10.8 | 44 | |
| 15 | 68 | M | R | L | 11.4 | 51 | |
| 16 | 51 | M | R | R | 11.4 | 40 | |
| 17 | 62 | M | R | L | 59.1 | 41 | |
| 18 | 66 | F | R | R | 84.3 | 47 | |
| 19 | 57 | M | R | R | 72.0 | 50 | |
| 20 | 72 | M | R | R | 119.9 | 49 | |
| Average/Count (SEM) | n = 10 | 58.2 (4.25) | 5M/5F | 9R/1L | 6R/4L | 39.7a (12.92) | 47.9 (2.05) |
Abbreviations: CIMT, constraint-induced movement therapy; SEM, standard error of the mean.
P < .05, between-group comparison using an unpaired t test.
Figure 2.
Lesion Information for CIMT Groupa
Abbreviations: CIMT, constraint-induced movement therapy; R, Right; L, Left; B, brainstem; F, Frontal area; T, Temporal area; P, Parietal area; O, Occipital area; M1, primary motor area; SM, Sensorimotor area; CR, Corona radiate; CN, Caudate nucleus; IC, Internal capsule; N/A, not available.
aThe green area in the 3D image shows the section with the largest lesion size.
Figure 3.
Lesion Information for Non-CIMT Groupa
Abbreviations: CIMT, constraint-induced movement therapy; R, right; L, left; B, brainstem; F, frontal area; T, temporal area; P, parietal area; O, occipital area; M1, primary motor area; SM, sensorimotor area; CR, corona radiate; CN, caudate nucleus; IC, internal capsule; N/A, not available.
aThe green area in the 3D image shows the section with the largest lesion size.
Treatment Effects on Functional Outcomes
There were no between-group differences at baseline for the WMFT-15, WMFT-6, MAL AOU, and MAL quality of movement (Table 2). The within-group changes (baseline - postassessment) revealed a pronounced group effect, with the CIMT group showing improvement in 3 of the 4 functional outcome measures. The CIMT group had significantly improved WMFT scores (15-item: P = .04; 6-item: P = .04), but this was not the case for the non-CIMT group. The MAL AOU significantly improved for the CIMT group (CIMT: P = .01) but not for the non-CIMT group (Table 2).
Table 2.
Effect of Treatment on Functional Measuresa
| Participant ID | WMFT-15 (s)
|
WMFT-6 (s)
|
MAL
|
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre-AOU | Post-AOU | Pre-QOM | Post-QOM | |
| CIMT (n = 10) | ||||||||
| 01 | 3.96 | 3.15 | 6.21 | 4.53 | 2.20 | 2.11 | 3.92 | 4.21 |
| 02 | 7.77 | 6.07 | 11.96 | 11.06 | 0.11 | 1.59 | 1.00 | 2.81 |
| 03 | 33.052 | 8.85 | 71.802 | 17.09 | 1.38 | 3.68 | 2.29 | 3.28 |
| 04 | 30.693 | 22.542 | 70.433 | 52.712 | 0.67 | 1.25 | 2.33 | 2.18 |
| 05 | 23.221 | 7.18 | 49.661 | 13.10 | 3.00 | 4.38 | 3.31 | 4.12 |
| 06 | 16.42 | 12.77 | 22.50 | 11.18 | 2.55 | 3.35 | 3.55 | 3.60 |
| 07 | 7.92 | 4.04 | 13.25 | 5.42 | 2.70 | 2.42 | 3.52 | 2.71 |
| 08 | 7.28 | 4.91 | 11.10 | 7.99 | 4.17 | 4.37 | 3.10 | 3.85 |
| 09 | 3.83 | 2.55 | 6.03 | 4.36 | 3.58 | 4.05 | 3.44 | 3.91 |
| 10 | 1.75 | 2.42 | 8.87 | 4.44 | 2.47 | 4.20 | 3.52 | 4.10 |
| Mean (SEM) | 13.59 (4.30) | 7.45b (1.96) | 27.18 (8.36) | 13.19b (4.60) | 2.28 (0.40) | 3.14b (0.38) | 3.00 (0.28) | 3.48 (0.22) |
| Non-CIMT (n = 10) | ||||||||
| 11 | 3.71 | 2.94 | 7.54 | 5.34 | 1.71 | 2.90 | 3.23 | 4.09 |
| 12 | 25.021 | 16.391 | 51.401 | 31.201 | 0.98 | 1.62 | 3.83 | 4.24 |
| 13 | 10.22 | 4.65 | 4.55 | 4.06 | 2.22 | 2.24 | 2.95 | 3.19 |
| 14 | 46.735 | 50.895 | 101.975 | 118.505 | 1.04 | 2.86 | 0.67 | 2.03 |
| 15 | 24.541 | 7.73 | 36.01 | 14.60 | 0.74 | 0.79 | 1.17 | 1.21 |
| 16 | 11.66 | 7.37 | 24.09 | 14.43 | 2.25 | 1.95 | 2.71 | 2.13 |
| 17 | 18.031 | 18.661 | 36.111 | 16.89 | 1.44 | 1.60 | 1.56 | 1.48 |
| 18 | 3.97 | 3.59 | 5.38 | 4.91 | 2.50 | 2.23 | 2.80 | 2.17 |
| 19 | 3.87 | 2.74 | 6.68 | 4.11 | 1.53 | 1.53 | 1.27 | 1.63 |
| 20 | 12.671 | 7.30 | 28.291 | 15.82 | 2.29 | 2.27 | 2.16 | 2.21 |
| Mean (SEM) | 16.04 (4.23) | 12.23 (4.63) | 30.20 (9.46) | 22.99 (10.95) | 1.67 (0.20) | 1.99 (0.20) | 2.24 (0.33) | 2.44 (0.33) |
Abbreviations: CIMT, constraint-induced movement therapy; WMFT, Wolf Motor Function test mean score; MAL, Motor Activity Log mean score; AOU, amount of use; QOM, quality of movement; SEM, standard error.
Number of incomplete items (ie, those that could not be completed in 120 s) in the WMFT are indicated by an italic superscript above the WMFT mean time score. For the MAL AOU and QOM, a score of 5 (ie, same as before the stroke) is the maximum score. An improvement in upper-extremity performance is indicated by an increase in the average score for the MAL and a decrease in the average time for the WMFT. The CIMT group showed a significantly improved WMFT-15 and WMFT-6 (faster mean WMFT time) and MAL AOU score postassessment. No significant changes (improvements or decrements) were observed for the non-CIMT group.
Indicates that the within-group comparisons between baseline and postassessment were significant (P < .05).
Reach-to-Grasp Task Performance
Effects of stroke-specific impairment
In all, 6 nondisabled participants (25 to 59 years; 43.8 ± 5 years) performed the reach-to-grasp tasks in each of the testing situations for comparison with baseline performance of the stroke group. Participants in the stroke groups (n = 20, combined CIMT and non-CIMT groups) were able to complete an average of 23 trials out of the 28 trials (success rate = 81.48%, ±6.7%), whereas participants in the nondisabled group achieved a 100% success rate. There was a statistically significant difference between the stroke and nondisabled groups in overall success rate at baseline (P < .000).
For optimal posture selection at baseline, the stroke group (n = 20, combined CIMT and non-CIMT group) chose an optimal hand posture for most trials (group mean and SE = 67.9%, ±6.2%) but less frequently than the nondisabled group (group mean and SE = 91.07%, ±5.1%; P < .05).
Effects of CIMT on task-specific reach-to-grasp measures
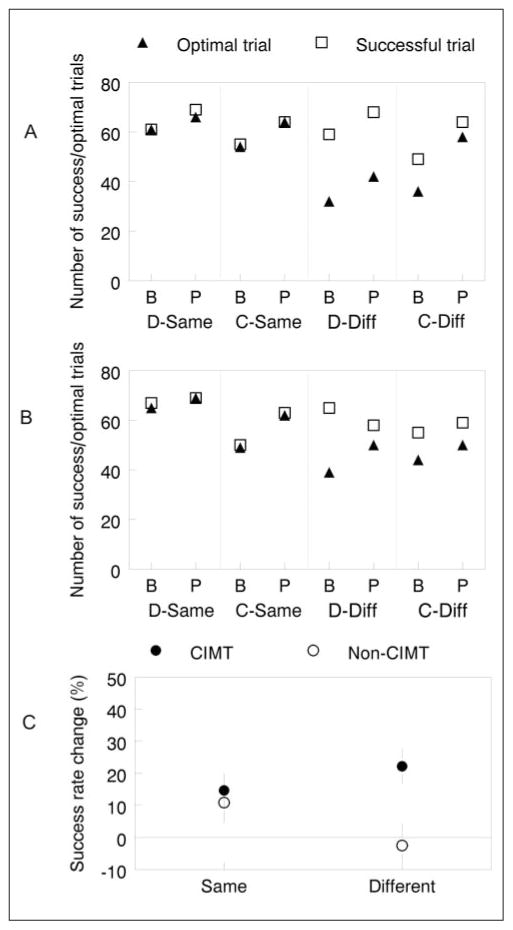
Figure 4 shows the total number of successful and optimal posture trials for each of the 2 stroke groups (n = 10/group) by situation at baseline and postassessment. If all 7 trials in each situation are performed successfully and all trials showed selection of an optimal posture, then the maximum number for all 10 participants per group in each situation would be 70 (10 × 7). For the CIMT group (Figure 4A), the number of successful trials increased in all situations but especially in the card-different situation (49/70 at baseline; 64/70 at postassessment). For the non-CIMT group (Figure 4B), the number of successful trials also improved in each situation, except in the dowel-different situation, where it decreased from 65 out of 70 at baseline to 58 out of 70 at postassessment.
Figure 4.
Optimal posture selection and success rate change: A. CIMT group. B. Non-CIMT group showing the total number of successful (open square) and optimal (closed triangle) trials. The 4 object/posture situations are arranged on the X axis in pairs, beginning with baseline (label B) followed by postassessment (label P). C. Percentage success rate change is collapsed across object type for the CIMT (closed circle) and non-CIMT (open circle) groups. The zero line represents no change between baseline and postassessment. Abbreviations: CIMT, constraint-induced movement therapy; D-Same, dowel/pronated; C-Same, card/pronated; D-Diff, dowel/supinated; C-Diff, card/supinated.
Figure 4C shows the percentage change in success rate—that is, [(Post - Baseline)/Baseline] × 100—for each stroke group by condition collapsed across object type. There was no difference between groups in the same condition (CIMT: 14.7% ± 5.4%; non-CIMT: 10.9% ± 6.4%), but for the different condition, only the CIMT group improved (CIMT: 22.2% ± 5.6%; non-CIMT: -2.5% ± 6.9%).
The number of optimal posture trials increased in each situation, for each stroke group, at postassessment. However, the number of optimal posture selections shows the largest gain in the card-different situation (36/70 baseline; 58/70 postassessment) for the CIMT group compared with a much smaller gain for the non-CIMT group (44/70 baseline; 50/70 postassessment).
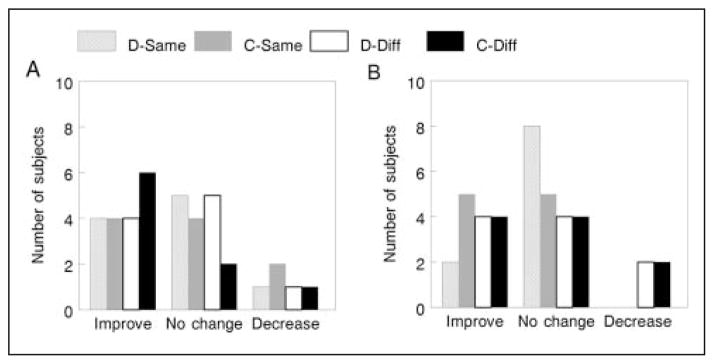
Figure 5 displays frequency plots for participants in the CIMT (Figure 5A) and non-CIMT (Figure 5B) groups whose number of optimal posture selections either increased, decreased, or did not change between baseline and postassessment in each situation. Overall, for both groups, the number of individuals who either improved or made no change was greater than the number who showed a decrease in the frequency of optimal posture selections. There were no notable differences between groups in the “improve” category for optimal posture selection except in the card-different and dowel-same situations. Specifically, there were 6 participants in the CIMT group in the card-different situation who improved, in comparison to only 4 participants in the non-CIMT group who improved. For the dowel-same situation, there were 4 participants in the CIMT group who improved, in comparison to only 2 participants in the non-CIMT group who improved.
Figure 5.
Optimal posture change: frequency plots for participants in the CIMT (A) and non-CIMT (B) groups (n = 10/group) whose number of optimal posture selections either increased, decreased, or did not change between baseline and postassessment in each object/posture situation. Abbreviations: CIMT, constraint-induced movement therapy; D-Same, dowel/pronated; C-Same, card/pronated; D-Diff, dowel/supinated; C-Diff, card/supinated.
Effects of CIMT on reach-to-grasp task TMT
For the same conditions, percentage TMT change was not different between groups (CIMT: 52.8% ± 5.9%; non-CIMT: 49.4% ± 6.3%); however, for the different conditions, only the CIMT group showed an improvement (CIMT: 58.9% ± 6.3%; non-CIMT: -14.7% ± 6.8%).
Discussion
To our knowledge, this is the first study to demonstrate the effects of CIMT on anticipatory motor behaviors engaged for task-specific reach-to-grasp actions. Our findings suggest that CIMT results in improved anticipatory planning of hand posture selection, particularly in situations that require precision grasping actions with forearm supination. Furthermore, compared with the non-CIMT group, the CIMT group not only demonstrated increased limb use but also improved success rate and movement speed of the reach-to-grasp tasks, especially in the more complex (different) task conditions.
Changes in Motor Skill After CIMT
Previous studies have demonstrated the effectiveness of CIMT for improving paretic upper-limb function and use in individuals with subacute and chronic stroke.4,13,30–35 The results from the WMFT and MAL are similar to those of the larger EXCITE trial and, for the most part, are consistent with previous findings,13 particularly considering the limitations of the MAL.36
Within the broader class of TOT protocols, some investigators have suggested that in addition to the reversal of learned nonuse, CIMT promotes the development of skill in functional motor behaviors, particularly those concerned with reach and grasp.37 This hypothesis was supported by the significant improvement of functional movements (WMFT-15) observed only for the CIMT group. Furthermore, the fine-motor tasks included in the WMFT-6 significantly improved in the CIMT group, suggesting a benefit to fine-motor skills that require digit individuation and manual dexterity. However, the WFMT does not directly capture anticipatory planning of voluntary actions. Specific to the aims of this research, we developed and used a set of task-specific measures with our instrumented reach-to-grasp task to uncover anticipatory planning strategies for these functional actions.
Effect of CIMT on Reach-to-Grasp and Anticipatory Posture Selection
Success rate
Overall success rate can be used to capture the difficulty level of the task for the stroke group compared with a nondisabled group. Most participants with stroke (82.3%) were able to accomplish the tasks at baseline; however, the overall success rate was significantly lower than for the nondisabled group. This difference between stroke and nondisabled groups showed that the grasping tasks developed for this investigation would be sensitive enough to capture improvement but challenging enough to engage motor learning with practice. In fact, after intervention, the CIMT group demonstrated a higher success rate (94.6%) than the non-CIMT group (88.9%); however, the success rate of both groups was still below that for the nondisabled group (100%), further supporting the notion that the reach-to-grasp tasks continued to present a meaningful and challenging motor problem.
The 4 object–hand-posture tasks can be thought to represent a continuum of task difficulty. The card task required a 3-finger precision grasp—a more difficult grasp compared with the dowel task that required a power grasp. Both the object-grasp (precision vs power) and optimal posture (supinated vs pronated) configurations contribute to task difficulty. This hypothesis gathers empirical support from the baseline data. There is a progressive drop in success rate, from most successful with the dowel conditions (same: 91.4%; different: 88.6%) to least successful with the card conditions (same: 75%; different: 74.2%). For both objects, the different/forearm supination condition had a slightly lower success rate than the same/forearm pronation condition. Thus, success rate provides empirical support for a progression in task difficulty across our 4 object–hand-posture tasks. Furthermore, there is considerable neurophysiological evidence that precision grasp requires greater motor cortical activation than does a power grasp,38 reflecting the unique role of direct corticomotoneuronal connections for individuation of finger movements. Finally, the anticipatory posture selection for the different trials elicits a movement strategy that is counter to the more primitive movement synergy that is biased toward a pronated forearm with shoulder flexion and elbow extension for reaching movements.39–41 Therefore, in our design, the different condition generally required more selective motor control and therefore would be considered more challenging for participants poststroke than the same condition trials.
Regardless of the type of grasp, and compared with the non-CIMT group, the CIMT group showed a greater improvement in success rate for those tasks that required forearm supination. This result indicates that the effect of CIMT on reach-to-grasp capability is more robust in situations that require more selective motor control for grasping. The increased success rate is also indicative of the stability and repeatability of the action. A reasonable concern that may have affected this result is the possibility of a ceiling effect. However, success rate for the stroke group was 91.8%, compared with 100% for the nondisabled group, still below the ceiling for the reach-to grasp tasks.
Optimal posture selection and anticipatory planning
In addition to adding a level of task difficulty, hand posture selection across objects served to identify whether CIMT would influence anticipatory planning, an important component of the development of motor skill. The end-stage comfort effect described by Rosenbaum and colleagues42 provides strong evidence for the idea that most goal-directed actions are planned in advance. For most grasping tasks in those studies, participants consistently chose a less-comfortable initial grip posture to minimize discomfort in the final position. The frequency of optimal posture selection and optimal posture change captures the degree to which our participants fit the performance predicted by the end-stage comfort hypothesis. At baseline, the stroke groups exhibited fewer optimal hand posture selections compared with the nondisabled group. These findings may represent an impairment in motor planning alone43 but are more likely the combined effects of biomechanical constraints and planning deficits.19 Previous studies have identified that deficits in active range of motion44,45 influence the choice of movement solution in grasping tasks. Because supination in conjunction with elbow extension is a common impairment in adults with stroke,39 we reasoned that the influence of challenging practice (CIMT) on the choice of hand posture would be most evident in movements that required forearm supination (ie, the different conditions). Furthermore, we expected that the practice effect would be greatest with the card task, given that precision has been shown to influence the end-state comfort effect in movement planning.46 Our findings supported this reasoning in 2 ways: (1) At baseline, and compared with the nondisabled group, the stroke groups exhibited a lower incidence of optimal hand posture selection, particularly when anticipatory supination was required. In contrast to the nondisabled group, the stroke group had a tendency to plan not only for end-stage comfort but to compensate for biomechanical constraints, which minimized forearm rotation.44 (2) After intervention, the CIMT group showed the greatest gain in the number of optimal posture selections and a higher frequency of participants who increased their optimal posture in the most difficult task situation (card-different), suggesting improved anticipatory movement planning that was not evident for those in the non-CIMT group. We speculate further that CIMT may have a task-specific practice effect on reach-to-grasp movements through motor learning, whereby more normal motor planning, that is, end-stage comfort, is elicited for optimal posture selection, especially in the most biomechanically and cognitively demanding situation.
In the card-different situation, there were 2 participants in the non-CIMT group who chose an optimal posture for all 7 trials at baseline but regressed at the postassessment by selecting a nonoptimal posture 3 out of 7 and 1 out of 7 times, respectively. This suggests that the strategy selected at baseline was neither reliable nor stable. To some degree, this supports our speculation regarding a task-specific practice effect with CIMT. The important point is that the non-CIMT group did make some improvements in reach-to-grasp over the 2-week interval, but the degree and nature of that improvement was smaller than and different from that for the CIMT group.
Reach-to-grasp task TMT
The CIMT group showed a reduction in TMT for the reach-to-grasp tasks, a finding that is consistent with other studies of the effects of CIMT on the kinematics of movement.21–26 The CIMT group showed a superior percentage TMT improvement in the different conditions, whereas the group differences were similar in the same conditions. One interpretation of the improved movement time is a more efficient feed-forward controller and less dependence on slower on-line feedback.21–23,26 Such efficiency can also be interpreted to reflect better movement planning after CIMT. However, the readers should be cautioned not to overinterpret the TMT findings. The method of imputing unsuccessful reach-to-grasp task trials with 120 s (the upper limit for functional performance borrowed from the WMFT procedure) may have biased the results. In retrospect, we chose this strategy to account for the few cases in which there were few successful trials that alone did not adequately represent an average over 7 trials. We adopted the imputation strategy as a means to effectively equalize the number of trials available for comparison of TMT, and from this, we were able to extract a more meaningful signal than the signal that would have been obtained without imputation.
Conclusion
Consistent with the EXCITE trial findings, our companion study showed that 2 weeks of CIMT resulted in greater task-specific functional improvements of the upper extremity than for the non-CIMT comparison group. More important, and central to our research aim, improvements in anticipatory planning behavior of grasping actions were observed through a greater increase in grasping task success rate, optimal hand posture selection, and a faster movement time for the CIMT group than for the non-CIMT group. The combined results of a faster movement time and higher frequency of optimal posture selection in the most difficult task conditions highlights a set of novel results never before reported in association with CIMT. Furthermore, the reach-to-grasp task paradigm used here shows promise for the assessment of functional improvements that are distinguishable between nonoptimal compensatory strategies and a more optimal movement strategy that reflects true recovery.28,47 The small sample size used in this companion study precludes a definitive conclusion. In addition, the poststroke duration was not matched between stroke groups. This, however, is not important for our research question and therefore has little bearing on the interpretation of our results. Therefore, we suggest that the research aim and findings can be used to design and power larger-scale studies in neurorehabilitation focused on understanding the mechanisms for effectiveness induced by the broad class of TOT interventions.
Acknowledgments
We acknowledge the NIH Behavioral and Brain Correlates of Arm Recovery (BCAR) grant NS045485 to CJW. We thank Yun Dong, MD, PhD, and Matthew Konersman, DPT, for lesion analysis.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Page SJ, Sisto S, Levine P, McGrath RE. Efficacy of modified constraint-induced movement therapy in chronic stroke: a single-blinded randomized controlled trial. Arch Phys Med Rehabil. 2004;85:14–18. doi: 10.1016/s0003-9993(03)00481-7. [DOI] [PubMed] [Google Scholar]
- 2.Wu C-Y, Chuang L-L, Lin K-C, Chen H-C, Tsay P-K. Randomized trial of distributed CIMT versus bilateral arm training for the rehabilitation of upper-limb motor control and function after stroke. Neurorehabil Neural Repair. 2011;25:130–139. doi: 10.1177/1545968310380686. [DOI] [PubMed] [Google Scholar]
- 3.Page SJ, Levine P, Leonard AC. Modified constraint-induced therapy in acute stroke: a randomized controlled pilot study. Neurorehabil Neural Repair. 2005;19:27–32. doi: 10.1177/1545968304272701. [DOI] [PubMed] [Google Scholar]
- 4.van der Lee JH, Wagenaar RC, Lankhorst GJ, Vogelaar TW, Deville WL, Bouter LM. Forced use of the upper extremity in chronic stroke patients: results from a single-blind randomized clinical trial. Stroke. 1999;30:2369–2375. doi: 10.1161/01.str.30.11.2369. [DOI] [PubMed] [Google Scholar]
- 5.Dromerick AW, Edwards DF, Hahn M. Does the application of constraint-induced movement therapy during acute rehabilitation reduce arm impairment after ischemic stroke? Stroke. 2000;31:2984–2988. doi: 10.1161/01.str.31.12.2984. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y-H, Wu C-Y, Hsieh Y-W, Lin K-C. Predictors for change in quality of life after distributed constraint-induced therapy in patients with chronic stroke. Neurorehabil Neural Repair. 2010;24:559–566. doi: 10.1177/1545968309358074. [DOI] [PubMed] [Google Scholar]
- 7.Roberts PS, Vegher JA, Gilewski M, Bender A, Riggs RV. Client-centered occupational therapy using constraint-induced therapy. J Stroke Cerebrovasc Dis. 2005;14:115. doi: 10.1016/j.jstrokecerebrovasdis.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–132. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 9.Ploughman M, Corbett D. Can forced-use therapy be clinically applied after stroke? An exploratory randomized controlled trial. Arch Phys Med Rehabil. 2004;85:1417–1423. doi: 10.1016/j.apmr.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Pierce SR, Gallagher KG, Schaumburg SW, Gershkoff AM, Gaughan JP, Shutter L. Home forced use in an outpatient rehabilitation program for adults with hemiplegia: a pilot study. Neurorehabil Neural Repair. 2003;17:214–219. doi: 10.1177/0888439003259424. [DOI] [PubMed] [Google Scholar]
- 11.Winstein CJ, Miller JP, Blanton S, et al. Methods for a multisite randomized trial to investigate the effect of constraint-induced movement therapy in improving upper extremity function among adults recovering from a cerebrovascular stroke. Neurorehabil Neural Repair. 2003;17:137–152. doi: 10.1177/0888439003255511. [DOI] [PubMed] [Google Scholar]
- 12.Taub E, Uswatte G, Elbert T. New treatments in neurorehabilitation founded on basic research. Nat Rev Neurosci. 2002;3:228–236. doi: 10.1038/nrn754. [DOI] [PubMed] [Google Scholar]
- 13.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 14.Timmermans AA, Spooren AIF, Kingma H, Seelen HAM. Influence of task-oriented training content on skilled arm-hand performance in stroke: a systematic review. Neurorehabil Neural Repair. 2010;24:858–870. doi: 10.1177/1545968310368963. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian SK, Massie C, Malcolm MP, Levin MF. Does provision of extrinsic feedback result in improved learning in the upper limb poststroke? A systematic review. Neurorehabil Neural Repair. 2010;24:113–124. doi: 10.1177/1545968309349941. [DOI] [PubMed] [Google Scholar]
- 16.Winstein CJ, Rose DK, Tan SM, Lewthwaite R, Chui HC, Azen SP. A randomized controlled comparison of upper-extremity rehabilitation strategies in acute stroke: A pilot study of immediate and long-term outcomes. Arch Phys Med Rehabil. 2004;85:620–628. doi: 10.1016/j.apmr.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 17.Shadmehr R, Wise S. The Computational Neurobiology of Reaching and Pointing: A Foundation for Motor Learning. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- 18.Elsinger CL, Rosenbaum DA. End posture selection in manual positioning: evidence for feedforward modeling based on a movement choice method. Exp Brain Res. 2003;152:499–509. doi: 10.1007/s00221-003-1573-7. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum DA, van Heugten CM, Caldwell GE. From cognition to biomechanics and back: the end-state comfort effect and the middle-is-faster effect. Acta Psychol (Amst) 1996;94:59–85. doi: 10.1016/0001-6918(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 20.Alberts JL, Butler AJ, Wolf SL. The effects of constraint-induced therapy on precision grip: a preliminary study. Neurorehabil Neural Repair. 2004;18:250–258. doi: 10.1177/1545968304271370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caimmi M, Carda S, Giovanzana C, et al. Using kinematic analysis to evaluate constraint-induced movement therapy in chronic stroke patients. Neurorehabil Neural Repair. 2008;22:31–39. doi: 10.1177/1545968307302923. [DOI] [PubMed] [Google Scholar]
- 22.Wu CY, Chen CL, Tang SF, Lin KC, Huang YY. Kinematic and clinical analyses of upper-extremity movements after constraint-induced movement therapy in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2007;88:964–970. doi: 10.1016/j.apmr.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Lin KC, Wu CY, Wei TH, Lee CY, Liu JS. Effects of modified constraint-induced movement therapy on reach-to-grasp movements and functional performance after chronic stroke: a randomized controlled study. Clin Rehabil. 2007;21:1075–1086. doi: 10.1177/0269215507079843. [DOI] [PubMed] [Google Scholar]
- 24.Wu CY, Chen CL, Tsai WC, Lin KC, Chou SH. A randomized controlled trial of modified constraint-induced movement therapy for elderly stroke survivors: changes in motor impairment, daily functioning, and quality of life. Arch Phys Med Rehabil. 2007;88:273–278. doi: 10.1016/j.apmr.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Massie C, Malcolm MP, Greene D, Thaut M. The effects of constraint-induced therapy on kinematic outcomes and compensatory movement patterns: an exploratory study. Arch Phys Med Rehabil. 2009;90:571–579. doi: 10.1016/j.apmr.2008.09.574. [DOI] [PubMed] [Google Scholar]
- 26.Wu CY, Lin KC, Chen HC, Chen IH, Hong WH. Effects of modified constraint-induced movement therapy on movement kinematics and daily function in patients with stroke: a kinematic study of motor control mechanisms. Neurorehabil Neural Repair. 2007;21:460–466. doi: 10.1177/1545968307303411. [DOI] [PubMed] [Google Scholar]
- 27.Kwakkel G. Towards integrative neurorehabilitation science. Physiother Res Int. 2009;14:137–146. doi: 10.1002/pri.446. [DOI] [PubMed] [Google Scholar]
- 28.Lum PS, Mulroy S, Amdur RL, Requejo P, Prilutsky BI, Dromerick AW. Gains in upper extremity function after stroke via recovery or compensation: potential differential effects on amount of real-world limb use. Top Stroke Rehabil. 2009;16:237–253. doi: 10.1310/tsr1604-237. [DOI] [PubMed] [Google Scholar]
- 29.Woodbury M, Velozo CA, Thompson PA, et al. Measurement structure of the Wolf Motor Function Test: implications for motor control theory. Neurorehabil Neural Repair. 2010;24:791–801. doi: 10.1177/1545968310370749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 31.Butefisch C, Hummelsheim H, Denzler P, Mauritz KH. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J Neurol Sci. 1995;130:59–68. doi: 10.1016/0022-510x(95)00003-k. [DOI] [PubMed] [Google Scholar]
- 32.Kunkel A, Kopp B, Muller G, et al. Constraint-induced movement therapy for motor recovery in chronic stroke patients. Arch Phys Med Rehabil. 1999;80:624–628. doi: 10.1016/s0003-9993(99)90163-6. [DOI] [PubMed] [Google Scholar]
- 33.Blanton S, Wolf SL. An application of upper-extremity constraint-induced movement therapy in a patient with subacute stroke. Phys Ther. 1999;79:847–853. [PubMed] [Google Scholar]
- 34.Wittenberg GF, Chen R, Ishii K, et al. Constraint-therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 35.Page SJ, Sisto S, Johnston MV, Levine P. Modified constraint-induced therapy after subacute stroke: a preliminary study. Neurorehabil Neural Repair. 2002;16:290–295. doi: 10.1177/154596830201600307. [DOI] [PubMed] [Google Scholar]
- 36.Park SW, Wolf SL, Blanton S, Winstein C, Nicholas-Larsen DS. The EXCITE trial: predicting a clinically meaningful motor activity log outcome. Neurorehabil Neural Repair. 2008;22:486–493. doi: 10.1177/1545968308316906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winstein C, Wolf S. Task-oriented training to promote upper extremity recovery. In: Stein J, Harvey RL, Macko R, Winstein C, Zorowitz R, editors. Stroke Recovery and Rehabilitation. New York, NY: Demos Medical; 2008. pp. 267–290. [Google Scholar]
- 38.Muir RB, Lemon RN. Corticospinal neurons with a special role in precision grip. Brain Res. 1983;261:312–316. doi: 10.1016/0006-8993(83)90635-2. [DOI] [PubMed] [Google Scholar]
- 39.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient: 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 40.Bensmail D, Robertson J, Fermanian C, Roby-Brami A. Botulinum toxin to treat upper-limb spasticity in hemiparetic patients: grasp strategies and kinematics of reach-to-grasp movements. Neurorehabil Neural Repair. 2010;24:141–151. doi: 10.1177/1545968309347683. [DOI] [PubMed] [Google Scholar]
- 41.Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target- dependent differences between free and constrained arm movements in chronic hemiparesis. Exp Brain Res. 2004;156:458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbaum DA, Engelbrecht SE, Bushe MM, Loukopoulos LD. Knowledge model for selecting and producing reaching movements. J Mot Behav. 1993;25:217–227. doi: 10.1080/00222895.1993.9942051. [DOI] [PubMed] [Google Scholar]
- 43.Fisher BE, Winstein CJ, Velicki MR. Deficits in compensatory trajectory adjustments after unilateral sensorimotor stroke. Exp Brain Res. 2000;132:328–344. doi: 10.1007/s002219900316. [DOI] [PubMed] [Google Scholar]
- 44.Steenbergen B, Hulstijn W, Dortmans S. Constraints on grip selection in cerebral palsy: minimising discomfort. Exp Brain Res. 2000;134:385–397. doi: 10.1007/s002210000458. [DOI] [PubMed] [Google Scholar]
- 45.Steenbergen B, Meulenbroek RG, Rosenbaum DA. Constraints on grip selection in hemiparetic cerebral palsy: effects of lesional side, end-point accuracy, and context. Brain Res Cogn Brain Res. 2004;19:145–159. doi: 10.1016/j.cogbrainres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 46.Short MW, Cauraugh JH. Precision hypothesis and the end-state comfort effect. Acta Psychol (Amst) 1999;100:243–252. doi: 10.1016/s0001-6918(98)00020-1. [DOI] [PubMed] [Google Scholar]
- 47.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23:313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]