Abstract
Intra-articular (IA) injections directly deliver high concentrations of therapeutics to the joint space and are routinely used in various musculoskeletal conditions such as osteoarthritis (OA) and rheumatoid arthritis (RA). However, current IA-injected drugs are rapidly cleared and do not significantly affect the course of joint disease. In this review, we highlight recent developments in IA therapy, with a special emphasis on current and emerging therapeutic carriers and their potential to deliver disease-modifying treatment modalities for arthritis. Recent IA approaches concentrate on platforms that are safe with efficient tissue penetration, and readily translatable for controlled and sustained delivery of therapeutic agents. Gene therapy delivered by viral or non-viral vectors and cell-based therapy for cartilage preservation and regeneration are being intensively explored.
Introduction
Intra-articular (IA) drug delivery presents many advantages as it offers direct access to the joint space, thus increasing the bioavailability of therapeutic agents at the affected site while reducing systemic exposure, potential side effects and overall cost. Although IA injections are generally considered safe, their therapeutic effectiveness remains severely limited due to rapid clearance of the drugs. IA injections are routinely used for various rheumatic diseases, especially osteoarthritis (OA), the most common form of arthritis that usually affects a few large joints but can result in severe disability, often requiring costly joint replacement [1].
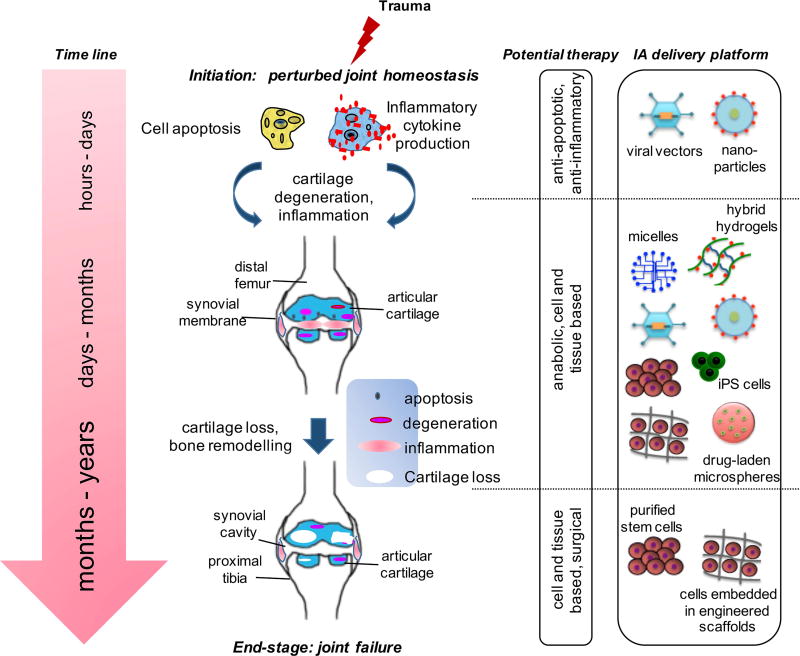
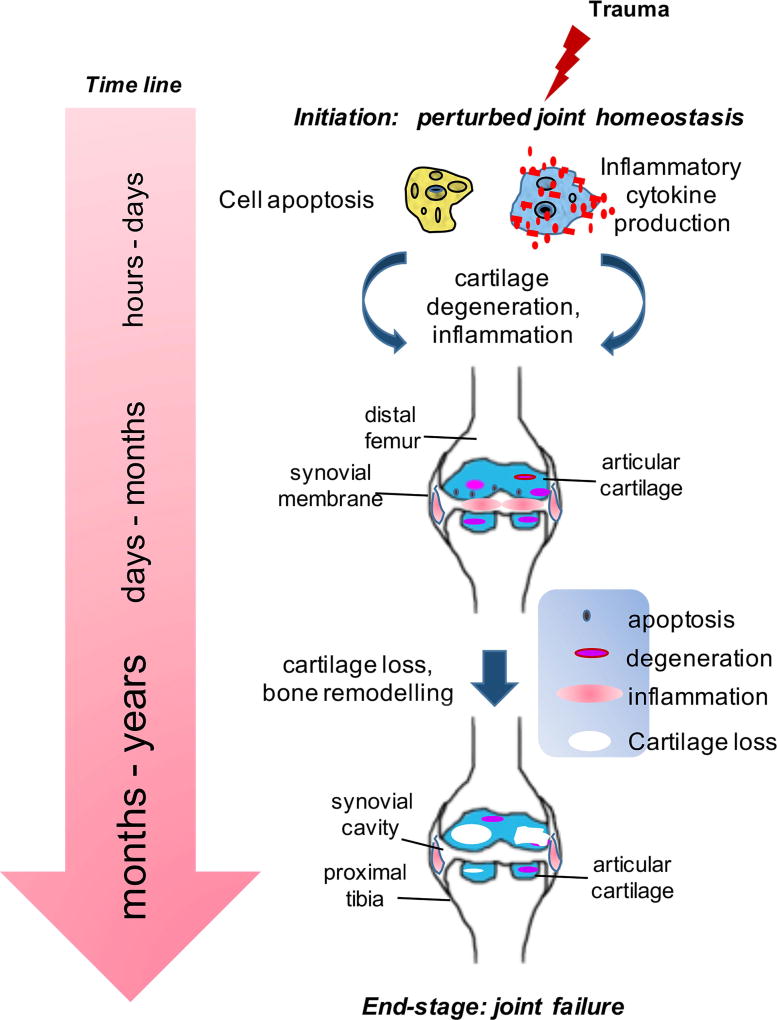
The focus of current research is to move OA from a disease requiring joint replacement to one that can be managed with early detection and medical intervention. While the pathogenesis of OA remains poorly understood, post-traumatic OA (PTOA) offers a model to study early changes and provides an opportunity for intervention as the time and nature of the initial trauma are generally known [2,3]. As depicted in Figure 1, a joint trauma can set off a series of molecular-level events beginning immediately with disturbance in joint homeostasis [4,5] and, over time, leading to end-stage OA characterized by structural changes [6]. Arthroscopic strategies for meniscus and/or ligament repair do not alter the course of disease [7]. Currently, pain management and physical therapy offer short-term benefits, but they cannot prevent surgical joint replacement [8,9]. Unless, new therapeutic interventions targeting pre-OA at the onset of disease become available, OA will remain a non-curable disease resulting in higher number of joint replacement surgeries at younger age.
Figure 1.
Stages of OA after initial trauma. At the molecular level, a joint trauma can set off a series of events immediately beginning with disturbance in joint homeostasis and, over time, leading to end-stage disease. The focus of research is shifting, albeit slowly, from end-stage disease, where total joint replacement is the only solution, to pre-OA stage where early molecular markers can predict the likelihood of clinical disease. At each stage following trauma, a distinct set of biochemical changes occur.
Currently few options exist for IA treatment. Corticosteroids are often administered IA to treat pain and resolve the joint effusion associated with rheumatoid arthritis (RA) and OA. Their effect, however, is short-lived and does not modify disease progression [10,11]. Likewise, hyaluronic acid (HA), a viscosupplementation approved by the U.S. Food & Drug Administration (FDA), is commonly used to treat OA. However, there is no conclusive evidence that HA, in its original formulation, delays or prevents the need for joint replacement [12,13]. Ideal IA drug delivery platforms should offer controlled release of the therapeutic agent with extended bioavailability and joint retention, have no or minimal safety concerns, promise a disease-modifying effect and/or cartilage regeneration, and be readily translatable. Despite recent advances, no single IA drug delivery platform fulfills all these properties.
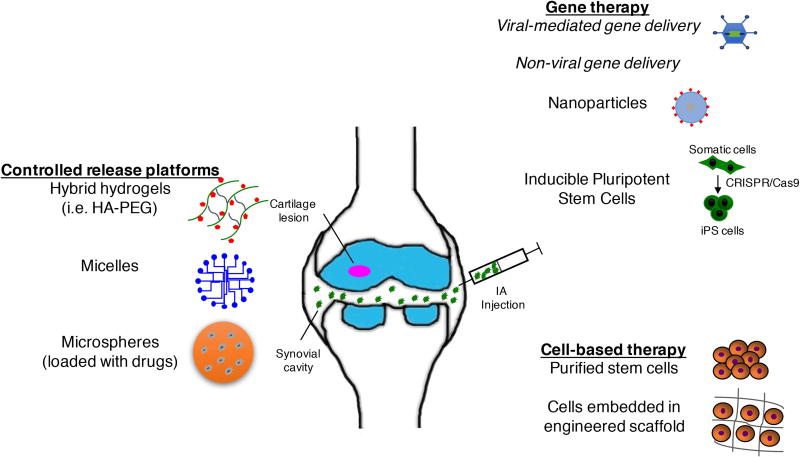
In this review, we have summarized recent developments in IA therapy (Figure 2), with a discussion on how therapeutic delivery systems are being developed to meet the above criteria.
Figure 2.
Overview of IA delivery platforms. IA injections deliver therapeutics to the joint space to treat joint disorders. The emerging trends focus is on IA delivery of disease-modifying therapeutics via: 1) sustained and controlled drug delivery platforms, 2) gene therapy using viral-mediated vectors or non-viral platforms, including nanoparticles or induced pluripotent stem cells, and 3) stem cell-based tissue-engineered products without or with scaffolds. HA-PEG = hyaluronic acid – poly(ethylene glycol).
Synthetic, controlled release drug delivery platforms
Rapid clearance of drugs from the joint limits the efficacy of many IA therapeutics such as corticosteroids and HA (reviewed in [14]), prompting the search for safe formulations that, offer sustained and extended drug availability. To this end, numerous natural and synthetic (bio)materials have been employed to achieve ideal properties such as increased articular dwell time with slow and steady (controlled) drug release and safe biodegradation of delivery vehicle. Each type of biomaterials has advantages and disadvantages as summarized in in Box I.
Highlights.
Focus on disease-modifying approaches for intra-articular drug delivery
Polymeric particles as platforms for sustained and controlled drug release
In vivo delivery of nucleic acids for cartilage preservation and regeneration
Stem cell-based tissue-engineered products for cartilage regeneration
Polymeric micelles are the most studied platforms for IA drug delivery. These nanoscale carriers are composed of amphiphilic polymers that self-assemble into nanostructures [15]. They provide several inherent properties that allow the encapsulation of a wide range of therapeutics, including poorly soluble compounds, for controlled and sustained release as well as protection of the encapsulated drugs from in vivo degradation and clearance [16]. These properties make polymeric micelles ideally suited for IA drug delivery to extend drug exposure time and to prevent rapid clearance by synovial phagocytes. Several applications of micelles have been explored for OA and RA treatment. Various hydrophobic, small molecule drugs (e.g., indomethacin, dexamethasone) have been incorporated into micelles and administered either IA or systemically [17]. Polymeric micelles have favorable toxicity profiles and could serve as extended drug delivery platforms [18].
Hydrogels represent another promising mode of IA drug delivery. It is known that HA has a short half-life (1–2 days in the tissue) and the use of unmodified HA is severely limited by high degradation rate, poor mechanical properties, and rapid clearance. To produce mechanically and chemically stable HA while retaining its biocompatibility, aqueous solutions of HA can be cross-linked to form hydrogels, increasing its retention time in the joint space. Combining HA with synthetic materials such as poly(ethylene glycol) (PEG) to form hybrid hydrogels is an alternative approach [19]. PEG is the most prevalent synthetic biomaterial used for developing hydrogels. However, PEG does not support cartilage-specific extracellular matrix synthesis to the same extent as natural biomaterials such as HA. Hybrid hydrogels promise to improve PEG bioactivity while simultaneously enhancing HA stability in the joint space [20]. The potency of HA hydrogels can be further enhanced by integrating kartogenin (KGN) into PEG as these PEG/KGN HA biodegradable hydrogels provide better chondroprotective and cartilage regenerative outcomes than HA hydrogels in experimental OA [21].
Another way to achieve sustained drug release, in the joint, is to combine natural or synthetic hydrogel materials with injectable drug delivery microspheres. Incorporation of therapeutic agents into the polymeric matrix during microsphere synthesis enables more precise and controlled drug release. For example, the homogeneous nanoporous structure of PEG microgels and the ability to precisely control pore size during microsphere synthesis leads to enhanced drug loading and more sustained release [20].
Synthetic polymeric particles, such as poly(lactic-co-glycolic acid) (PLGA), are other popular delivery platforms due to their unique characteristics such as tunable physiochemical and mechanical properties, and lack of immunogenicity [17]. In fact, they are the most widely used synthetic polymers that are obtained through reproducible industrial processes and the only FDA-approved IA delivery system since they degrade into naturally existing metabolites that are then fully resorbed. PLGA microspheres have been successfully used for IA sustained delivery of therapeutic agents to relieve pain and inflammation [22] but are less successful in targeting chondrocytes unless extremely high doses are used [23]. Nonetheless, the FDA has recently approved ZILRETTA™, a formulation that is composed of triamcinolone acetonide (TCA) embedded in a PLGA hydrogel. This extended-release corticosteroid formulation is specifically indicated for IA injection to manage OA knee pain [24]. Whether ZILRETTA™ has disease-modifying effects on OA is still being determined.
Gene therapy for cartilage preservation
Viral-based gene therapy
Adenovirus-mediated gene therapy has long been used and applied in OA (reviewed in [25,26]). One of the main impediments to gene therapy in OA is inefficient gene transduction. However, new methods such as the use of α-10 integrin antibody to conjugate the capsid of helper-dependent adenoviral-vector leads to efficient chondrocyte infection while de-targeting other cell types [27]. Likewise, gene transfer of rat Il1ra (interleukin 1 receptor antagonist) using an adeno-associated viral-vector holds promise for OA treatment, as the vector genome persists locally in the rat knee for up to a year with limited uptake by tissues outside the knee [28]. Viral-vectors, however, have several potential limitations such as immunogenicity, insertional mutagenesis, persistence and sustainability of transgene expression, and in many cases, lack of tissue and cell specificity [25,26]. To overcome the issues with cell and tissue specificity, incorporation of certain molecular adapters allows modification of adenoviruses for greater tropism toward targeted tissues. For example, the discovery of unconventional immunoglobulins derived from serum of camels and alpacas provides compatibility with the cytosolic biosynthesis of adenovirus capsid proteins, thus allowing for target cell specificity and ultimately making possible their use for directing adenovirus-mediated gene therapy to a particular tissue in the joint. Similarly, to overcome the broad negative effects of preexisting immunity to common human serotypes of adenoviruses, researchers have developed vectors based on chimpanzee-derived adenoviruses for gene therapy application (reviewed in [25]). Last year, the world’s first gene therapy product Invosaa (TissueGene) was approved for arthritis in South Korea, and Phase III studies are starting soon in the United States. The therapy targets OA by IA injection of human allogeneic chondrocytes transduced with a retrovirus encoding transforming growth factor-β1 (TGF-β1) [29]. This raises the expectation that approved genetic medicines may become a reality for arthritis in particular and musculoskeletal regenerative medicine in general.
Non-viral gene therapy
Given the shortcomings of adeno-associated viral-vectors, biocompatible and biodegradable nanomaterials are being increasingly explored for IA drug delivery since incorporation of the drug into a nanoplatform enhances its bioavailability and solubility while affording protection from biodegradation. Particle-based technologies for OA therapy have been reviewed extensively elsewhere [17,30,31]. Drug delivery into sub-compartments of cartilage, however, remains a challenging task since the avascular cartilage renders the chondrocytes inaccessible and the dense collagen-matrix prevents effective drug penetration.
Delivery systems for siRNAs and microRNAs
RNA interference (RNAi) by small interfering RNA (siRNA) is a way of ablating gene expression in cells. siRNA silences a specific gene by binding to and degrading target mRNA. Chemically modified siRNAs require no transfection reagents and can be applied to cells in vitro using relatively straightforward methods. However, critical barriers to siRNA delivery in vivo, including molecular instability and inefficient transfection of the target cells, hinder the wide application of this gene silencing approach (reviewed in [32]). Our laboratory has recently shown that modifications of the native melittin led to the formulation of a self-assembling, ~55 nm peptide-siRNA nanocomplex that deeply penetrates cartilage to specifically silence nuclear-factor kappa B (NF-κB), a signaling pathway that controls the expression of several matrix-degrading enzymes involved in the remodeling of cartilage matrix [33]. The nanocomplex persists in human cartilage explants, thus providing a clinically relevant and promising approach to overcoming the obstacles of siRNA delivery. Other key features that will likely prove advantageous for cartilage preservation include generic formulation for short nucleotide structures allowing siRNA multiplexing (i.e. targeting 3 or more pathways simultaneously) or swapping of any unmodified RNA (i.e., no need for backbone or end-piece alterations). The peptide-siRNA nanocomplex provides a favorable toxicity profile, showing no innate or adaptive immune responses to the agent after multiple IA administrations. Lastly, a simple 10-minute mixing procedure yields GMP (good manufacturing practice)-ready siRNA and peptide components for rapid self-assembly and immediate injection and thus avoiding sophisticated (i.e. expensive) processing and purification steps [33]. siRNA may also be delivered to cartilage through a chondrocyte-homing nanoparticle [34]. Along these lines, this technology has been used for delivery of many therapeutic agents such as TGF beta-activated kinase 1 (TAK1) [35], matrix metalloproteinase 13 (MMP-13) and a disintegrin and metalloproteinase with thrombospondin motif 5 (ADAMTS-5) [36] to inhibit early cartilage degeneration.
Recently, the catabolic and anabolic effects of microRNAs (miRNAs) on OA cartilage have become increasingly evident, prompting research into their IA delivery. Some emerging options include conjugation of miRNAs with lipid nanoparticles for active transportation across the adipocyte membrane and the use of liposomes. Liposomes are closed spherical vesicles composed of phospholipids that have been proposed as efficient carriers for controlled drug delivery [37]. Derived from natural, biodegradable and nontoxic lipids, liposomes can entrap a variety of hydrophilic and hydrophobic drugs and are therefore good candidates for local targeting of therapeutic agents to the site of interest [38]. However, conventional liposomal formulations are prone to rapid elimination from the bloodstream, therefore limiting therapeutic efficacy [37]. Exosomes are cell-derived vesicles that have been shown to exhibit multiple roles in inter-cellular signaling as well as transport of proteins and miRNAs. These vesicles are emerging targets for drug delivery, since they have the ability to evade the immune system and since they can directly fuse with the plasma membrane of target cell(s) for efficient delivery of therapeutic molecule directly into cytoplasm and bypassing the endosomes. While efforts to develop synthetic carriers for miRNAs are ongoing, recent studies indicate that the ideal delivery platform for miRNAs into the synovial cavity is lentivirus-mediated IA injections [39–41]. Thus, more research is still needed to develop an effective but safe miRNA delivery systems.
Inducible gene delivery systems
Transgenes can also be delivered by non-viral vectors using progenitor or differentiated cells. This cutting-edge technology stems from the concept of using inducible promoters to replace viral sequencing to drive the expression of therapeutic genes as and when needed [42,43]. While the presence of viral genes in viral vectors could potentially lower the stable expression of a transgene in the transduced cell, cellular promoters are less susceptible to promoter silencing and in fact support long-term expression in the joint. CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR associated protein 9) genome engineering systems are now being used successfully to generate cells that antagonize inflammatory signals in an autoregulated, feedback-controlled manner. These genome-engineering systems rewire endogenous cell circuits to allow for prescribed input/output relationships between inflammatory mediators and their targets, thus providing the foundation for a rapidly responsive cell-based drug delivery system [44,45].
Delivery systems for cell-based cartilage repair
In recent years, advances in knowledge about stem cells and induced pluripotent stem cells (iPSCs) have led to promising cell–based therapies for articular cartilage repair and an apparent alternative for traditional chondrocyte implantation. Cell therapy has led to short-term improvement in symptoms and may reduce or delay arthroplasty; however, long-term data are still lacking [46]. In addition, IA injection of mesenchymal stem cells (MSCs) results in pain relief, better quality of life, and significantly improved cartilage quality with no need for hospitalization or surgery. Thus, cell transplantation appears to be a reliable alternative treatment for chronic knee OA [47,48]. However, there exist significant logistic and operational problems associated with appropriate handling, expansion, storage and delivery of stem cells to the synovial joint.
Accruing evidence suggests that the direct injection of MSCs to the joint via a syringe or through an arthroscopic port can limit cartilage damage and improve its repair, perhaps due to their anti-inflammatory function, especially if delivered at early stages in the disease process [49]. However, direct injection has some limitations, including short-term retention in the joint, and lacks a high level of evidence on the restoration of the original hyaline cartilage. Therefore, continued development of tissue engineering strategies has sought to combine cells with scaffolds with and without biological signals to boost repair response in the knee. In search of the holy grail of regenerative medicine, a number of matrix-assisted techniques have been developed and numerous natural and synthetic scaffolds have been designed [49,50]. The characteristics of an ideal scaffold include (1) functional and mechanical properties resembling the desired final tissue-engineered product, (2) enhanced viability, retention and engraftment of cells in the joint, (3) ability to withstand and function in a hostile inflammatory environment within the synovial joint and (4) absence of immunogenicity and toxicity. Research in this area is on the rise and in near future we anticipate some groundbreaking developments for viable cell delivery products.
Conclusion
In summary, the ideal properties of an IA drug delivery system depend on the nature of the delivery platforms. Hydrogels, for example, need to possess good biodegradability, superior biocompatibility, low immunogenicity and flexibility in their structure to allow for optimal loading and controlled release of a drug. For gene therapy, replication deficient vectors, non-viral vectors and vectors with tunable properties are desirable. Both for cell and gene therapy vehicles, the common characteristics are safety, efficiency, specificity and sustained presence in the tissue for cartilage preservation and repair. Ideal properties of scaffolds for cell delivery include but not limited to unique functional and mechanical attributes, cell retention potential, ability to withstand the aggressive inflammatory milieu within joints, non-immunogenicity, and toxicity.
The emerging trends indicate that new IA delivery approaches have a number of distinct characteristics. The focus on sustained and controlled drug delivery platforms is rising. Gene therapy, either by direct vector administration or cell implantation with and without biomaterials is receiving great attention. Finally, localized tissue engineering is replacing generalized systemic delivery systems. However, translation of these modalities to the clinic awaits further studies to fully assess the risks and benefits of these delivery platforms and agents.
Box I. Advantages and disadvantages of sustained-release delivery platforms.
| Material type | Advantage | Disadvantage | Reference |
|---|---|---|---|
| Polymeric micelles |
|
|
[15,16,18] |
|
| |||
| Poly(ethylene glycol) (PEG) |
|
|
[19–21] |
|
| |||
| Poly lactic-co-glycolic acid (PLGA) |
|
|
[17,22–24] |
Review criteria.
Articles to include in this Review were selected after searching PubMed database for articles published within last two years for the following terms: “osteoarthritis AND therapy” and “intra-articular AND injection”. Only English-language original publications and review articles were selected on the basis of their relevance for inclusion in the bibliography. Reference lists of the publications identified were also screened for additional relevant material.
Acknowledgments
Funding sources
Work from authors’ laboratories reviewed in this paper was partially supported by R01 AR067491 (Pham) and R00 AR064837 (Rai) from the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the NIAMS.
List of abbreviations
- ADAMTS-5
A disintegrin and metalloproteinase with thrombospondin motif 5
- CRISPR/Cas9
Clustered regularly interspaced short palindromic repeats/ CRISPR associated protein 9
- FDA
The U.S. Food & Drug administration
- GMP
Good manufacturing practice
- HA
Hyaluronic acid, hyaluronan
- IA
Intra-articular
- IL-1RA/Il1ra
Interleukin 1 receptor antagonist
- iPSCs
Induced pluripotent stem cells
- KGN
Kartogenin
- miRNA
MicroRNA
- MMP-13
Matrix metallopeptidase 13
- MSCs
Mesenchymal stem cells
- NF-κB
Nuclear-factor kappa B
- OA
Osteoarthritis
- PEG
Poly(ethylene glycol)
- PLGA
Poly(lactic-co-glycolic acid)
- PTOA
Post-traumatic osteoarthritis
- RA
Rheumatoid arthritis
- RNAi
RNA interference
- siRNA
Small interfering RNA
- TAK1
Transforming growth factor beta activated kinase 1
- TCA
Triamcinolone acetonide
- TGF-β1
Transforming growth factor beta 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.March L, Smith EU, Hoy DG, Cross MJ, Sanchez-Riera L, Blyth F, Buchbinder R, Vos T, Woolf AD. Burden of disability due to musculoskeletal (MSK) disorders. Best Pract Res Clin Rheumatol. 2014;28:353–366. doi: 10.1016/j.berh.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Rai MF, Duan X, Quirk JD, Holguin N, Schmidt EJ, Chinzei N, Silva MJ, Sandell LJ. Post-Traumatic Osteoarthritis in Mice Following Mechanical Injury to the Synovial Joint. Sci Rep. 2017;7:45223. doi: 10.1038/srep45223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan X, Rai MF, Holguin N, Silva MJ, Patra D, Liao W, Sandell LJ. Early changes in the knee of healer and non-healer mice following non-invasive mechanical injury. J Orthop Res. 2017;35:524–536. doi: 10.1002/jor.23413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brophy RH, Zhang B, Cai L, Wright RW, Sandell LJ, Rai MF. Transcriptome comparison of meniscus from patients with and without osteoarthritis. Osteoarthritis Cartilage. 2017 doi: 10.1016/j.joca.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rai MF, Sandell LJ, Zhang B, Wright RW, Brophy RH. RNA Microarray Analysis of Macroscopically Normal Articular Cartilage from Knees Undergoing Partial Medial Meniscectomy: Potential Prediction of the Risk for Developing Osteoarthritis. PLoS One. 2016;11:e0155373. doi: 10.1371/journal.pone.0155373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35:1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 7.Nordenvall R, Bahmanyar S, Adami J, Mattila VM, Fellander-Tsai L. Cruciate ligament reconstruction and risk of knee osteoarthritis: the association between cruciate ligament injury and post-traumatic osteoarthritis. a population based nationwide study in Sweden, 1987–2009. PLoS One. 2014;9:e104681. doi: 10.1371/journal.pone.0104681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wellsandt E, Golightly Y. Exercise in the management of knee and hip osteoarthritis. Curr Opin Rheumatol. 2018;30:151–159. doi: 10.1097/BOR.0000000000000478. [DOI] [PubMed] [Google Scholar]
- 9.Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatology (Oxford) 2017 doi: 10.1093/rheumatology/kex419. [DOI] [PubMed] [Google Scholar]
- 10.Juni P, Hari R, Rutjes AW, Fischer R, Silletta MG, Reichenbach S, da Costa BR. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev. 2015:CD005328. doi: 10.1002/14651858.CD005328.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen C, Lefevre-Colau MM, Poiraudeau S, Rannou F. Evidence and recommendations for use of intra-articular injections for knee osteoarthritis. Ann Phys Rehabil Med. 2016;59:184–189. doi: 10.1016/j.rehab.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290:3115–3121. doi: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- 13.Newberry SJ, Fitzgerald JD, Maglione MA, O'Hanlon CE, Booth M, Motala A, Timmer M, Shanman R, Shekelle PG. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee. AHRQ Technology Assessments; 2015. [PubMed] [Google Scholar]
- 14.O'Mary H, Del Rincomicronn I, Cui Z. Nanomedicine for Intra-Articular Drug Delivery in Rheumatoid Arthritis. Curr Med Chem. 2016;23:2490–2506. doi: 10.2174/0929867323666160530144445. [DOI] [PubMed] [Google Scholar]
- 15.Deshmukh AS, Chauhan PN, Noolvi MN, Chaturvedi K, Ganguly K, Shukla SS, Nadagouda MN, Aminabhavi TM. Polymeric micelles: Basic research to clinical practice. Int J Pharm. 2017;532:249–268. doi: 10.1016/j.ijpharm.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Cagel M, Tesan FC, Bernabeu E, Salgueiro MJ, Zubillaga MB, Moretton MA, Chiappetta DA. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. Eur J Pharm Biopharm. 2017;113:211–228. doi: 10.1016/j.ejpb.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 17.Kavanaugh TE, Werfel TA, Cho H, Hasty KA, Duvall CL. Particle-based technologies for osteoarthritis detection and therapy. Drug Deliv Transl Res. 2016;6:132–147. doi: 10.1007/s13346-015-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Binkhathlan Z, Qamar W, Ali R, Kfoury H, Alghonaim M. Toxicity evaluation of methoxy poly(ethylene oxide)-block-poly(epsilon-caprolactone) polymeric micelles following multiple oral and intraperitoneal administration to rats. Saudi Pharm J. 2017;25:944–953. doi: 10.1016/j.jsps.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qayyum AS, Jain E, Kolar G, Kim Y, Sell SA, Zustiak SP. Design of electrohydrodynamic sprayed polyethylene glycol hydrogel microspheres for cell encapsulation. Biofabrication. 2017;9:025019. doi: 10.1088/1758-5090/aa703c. [DOI] [PubMed] [Google Scholar]
- 20.He Z, Wang B, Hu C, Zhao J. An overview of hydrogel-based intra-articular drug delivery for the treatment of osteoarthritis. Colloids Surf B Biointerfaces. 2017;154:33–39. doi: 10.1016/j.colsurfb.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 21*.Kang ML, Jeong SY, Im GI. Hyaluronic Acid Hydrogel Functionalized with Self-Assembled Micelles of Amphiphilic PEGylated Kartogenin for the Treatment of Osteoarthritis. Tissue Eng Part A. 2017;23:630–639. doi: 10.1089/ten.tea.2016.0524. This study prepared PEG/KGN micelles by covalently crosslinking OH-PEG-NH2 and 3-hydroxypropanoic acid-grafted KGN using carbodiimide chemistry, and demonstrated that HA/PEG/KGN hydrogels significantly suppressed OA progression in rats compared with free-HA hydrogels. [DOI] [PubMed] [Google Scholar]
- 22.Kimmerling KA, Furman BD, Mangiapani DS, Moverman MA, Sinclair SM, Huebner JL, Chilkoti A, Kraus VB, Setton LA, Guilak F, et al. Sustained intra-articular delivery of IL-1RA from a thermally-responsive elastin-like polypeptide as a therapy for post-traumatic arthritis. Eur Cell Mater. 2015;29:124–139. doi: 10.22203/ecm.v029a10. discussion 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajpayee AG, Grodzinsky AJ. Cartilage-targeting drug delivery: can electrostatic interactions help? Nat Rev Rheumatol. 2017;13:183–193. doi: 10.1038/nrrheum.2016.210. [DOI] [PubMed] [Google Scholar]
- 24**.Bodick N, Lufkin J, Willwerth C, Kumar A, Bolognese J, Schoonmaker C, Ballal R, Hunter D, Clayman M. An intra-articular, extended-release formulation of triamcinolone acetonide prolongs and amplifies analgesic effect in patients with osteoarthritis of the knee: a randomized clinical trial. J Bone Joint Surg Am. 2015;97:877–888. doi: 10.2106/JBJS.N.00918. This study reported that IA injection of FX006, an extended-release formulation of triamcinolone acetonide, provided a clinically relevant improvement in pain relief in patients with knee OA relative to immediate-release triamcinolone acetonide, the current standard of care. [DOI] [PubMed] [Google Scholar]
- 25.Rai MF, Sandell LJ. Emerging Concepts in Gene Therapy for Osteoarthritis. J Am Acad Orthop Surg. 2015;23:e56–57. doi: 10.5435/JAAOS-D-15-00339. [DOI] [PubMed] [Google Scholar]
- 26**.Evans CH, Ghivizzani SC, Robbins PD. Gene Delivery to Joints by Intra-articular Injection. Hum Gene Ther. 2018;29:2–14. doi: 10.1089/hum.2017.181. This comprehenisive review focuses on recent trends and, in particular, translation, clinical trials and the development of clinical gene therapy products for improving the treatment of the arthritides. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan MZ, Cerullo V, Cela R, Clarke C, Lundgren-Akerlund E, Barry MA, Lee BH. Treatment of osteoarthritis using a helper-dependent adenoviral vector retargeted to chondrocytes. Mol Ther Methods Clin Dev. 2016;3:16008. doi: 10.1038/mtm.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28*.Wang G, Evans CH, Benson JM, Hutt JA, Seagrave J, Wilder JA, Grieger JC, Samulski RJ, Terse PS. Safety and biodistribution assessment of sc-rAAV2.5IL-1Ra administered via intra-articular injection in a mono-iodoacetate-induced osteoarthritis rat model. Mol Ther Methods Clin Dev. 2016;3:15052. doi: 10.1038/mtm.2015.52. This preclinical safety and biodistribution study evaluated a self-complementary adeno-associated viral vector carrying rat IL-1Ra transgene in Wistar rats with mono-iodoacetate–induced osteoarthritis. The authors observed a favorable safety profile up to one year. The vector genome persisted in the right knees up to a year post-injection, but outside the knees, vector genomes were too low to measure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Evans CH, Ghivizzani SC, Robbins PD. Arthritis Gene Therapy Approved in Korea. J Am Acad Orthop Surg. 2018;26:e36–e38. doi: 10.5435/JAAOS-D-17-00695. This article is an overview of the current status of gene therapy products approved or near approval and on gene therapy clincial trials across the globe in musculoskeletal diseases and beyond. [DOI] [PubMed] [Google Scholar]
- 30.Bottini M, Bhattacharya K, Fadeel B, Magrini A, Bottini N, Rosato N. Nanodrugs to target articular cartilage: An emerging platform for osteoarthritis therapy. Nanomedicine. 2016;12:255–268. doi: 10.1016/j.nano.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Bottini M, Magrini A, Fadeel B, Rosato N. Tackling chondrocyte hypertrophy with multifunctional nanoparticles. Gene Ther. 2016;23:560–564. doi: 10.1038/gt.2016.33. [DOI] [PubMed] [Google Scholar]
- 32.Lolli A, Penolazzi L, Narcisi R, van Osch G, Piva R. Emerging potential of gene silencing approaches targeting anti-chondrogenic factors for cell-based cartilage repair. Cell Mol Life Sci. 2017 doi: 10.1007/s00018-017-2531-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Yan H, Duan X, Pan H, Holguin N, Rai MF, Akk A, Springer LE, Wickline SA, Sandell LJ, Pham CT. Suppression of NF-kappaB activity via nanoparticle-based siRNA delivery alters early cartilage responses to injury. Proc Natl Acad Sci U S A. 2016;113:E6199–E6208. doi: 10.1073/pnas.1608245113. This study generated and delivered peptidic nanoparticles complexed to NF-kB siRNA as a therapeutic approach to mediate chondroprotective effects by preserving cartilage homeostasis in chondrocytes from patients with OA via NF-kB suppression and in a mouse model of PTOA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pi Y, Zhang X, Shao Z, Zhao F, Hu X, Ao Y. Intra-articular delivery of anti-Hif-2alpha siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther. 2015;22:439–448. doi: 10.1038/gt.2015.16. [DOI] [PubMed] [Google Scholar]
- 35.Luo X, Chen Y, Lv G, Zhou Z, Chen J, Mo X, Xie J. Adenovirus-Mediated Small Interfering RNA Targeting TAK1 Ameliorates Joint Inflammation with Collagen-Induced Arthritis in Mice. Inflammation. 2017;40:894–903. doi: 10.1007/s10753-017-0534-4. [DOI] [PubMed] [Google Scholar]
- 36.Hoshi H, Akagi R, Yamaguchi S, Muramatsu Y, Akatsu Y, Yamamoto Y, Sasaki T, Takahashi K, Sasho T. Effect of inhibiting MMP13 and ADAMTS5 by intra-articular injection of small interfering RNA in a surgically induced osteoarthritis model of mice. Cell Tissue Res. 2017;368:379–387. doi: 10.1007/s00441-016-2563-y. [DOI] [PubMed] [Google Scholar]
- 37.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kapoor B, Singh SK, Gulati M, Gupta R, Vaidya Y. Application of liposomes in treatment of rheumatoid arthritis: quo vadis. ScientificWorldJournal. 2014;2014:978351. doi: 10.1155/2014/978351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng JS, Chen SY, Wu CL, Chong HE, Ding YC, Shiau AL, Wang CR. Amelioration of Experimental Autoimmune Arthritis Through Targeting of Synovial Fibroblasts by Intraarticular Delivery of MicroRNAs 140-3p and 140-5p. Arthritis Rheumatol. 2016;68:370–381. doi: 10.1002/art.39446. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Zhang H, Sun Q, Wang Y, Yang J, Yang J, Zhang T, Luo S, Wang L, Jiang Y, et al. Intra-articular Delivery of Antago-miR-483-5p Inhibits Osteoarthritis by Modulating Matrilin 3 and Tissue Inhibitor of Metalloproteinase 2. Mol Ther. 2017;25:715–727. doi: 10.1016/j.ymthe.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu G, Zhao X, Wang C, Geng Y, Zhao J, Xu J, Zuo B, Zhao C, Wang C, Zhang X. MicroRNA-145 attenuates TNF-alpha-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017;8:e3140. doi: 10.1038/cddis.2017.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garaulet G, Alfranca A, Torrente M, Escolano A, Lopez-Fontal R, Hortelano S, Redondo JM, Rodriguez A. IL10 released by a new inflammation-regulated lentiviral system efficiently attenuates zymosan-induced arthritis. Mol Ther. 2013;21:119–130. doi: 10.1038/mt.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rachakonda PS, Rai MF, Schmidt MF. Application of inflammation-responsive promoter for an in vitro arthritis model. Arthritis Rheum. 2008;58:2088–2097. doi: 10.1002/art.23598. [DOI] [PubMed] [Google Scholar]
- 44**.Brunger JM, Zutshi A, Willard VP, Gersbach CA, Guilak F. Genome Engineering of Stem Cells for Autonomously Regulated, Closed-Loop Delivery of Biologic Drugs. Stem Cell Reports. 2017;8:1202–1213. doi: 10.1016/j.stemcr.2017.03.022. In this study, authors created stem cells that antagonize IL-1- or TNF-α-mediated inflammation in an autoregulated, feedback-controlled manner using the CRISPR/Cas9 genome-engineering system. Data revealed that genome engineering can be used successfully to rewire endogenous cell circuits to allow for prescribed input/output relationships between inflammatory mediators and their antagonists, providing a foundation for cell-based drug delivery or cell-based vaccines via a rapidly responsive, autoregulated system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adkar SS, Brunger JM, Willard VP, Wu CL, Gersbach CA, Guilak F. Genome Engineering for Personalized Arthritis Therapeutics. Trends Mol Med. 2017;23:917–931. doi: 10.1016/j.molmed.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mistry H, Connock M, Pink J, Shyangdan D, Clar C, Royle P, Court R, Biant LC, Metcalfe A, Waugh N. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol Assess. 2017;21:1–294. doi: 10.3310/hta21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehrabani D, Mojtahed Jaberi F, Zakerinia M, Hadianfard MJ, Jalli R, Tanideh N, Zare S. The Healing Effect of Bone Marrow-Derived Stem Cells in Knee Osteoarthritis: A Case Report. World J Plast Surg. 2016;5:168–174. [PMC free article] [PubMed] [Google Scholar]
- 48.Davatchi F, Sadeghi Abdollahi B, Mohyeddin M, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis. 2016;19:219–225. doi: 10.1111/1756-185X.12670. [DOI] [PubMed] [Google Scholar]
- 49.Diekman BO, Guilak F. Stem cell-based therapies for osteoarthritis: challenges and opportunities. Curr Opin Rheumatol. 2013;25:119–126. doi: 10.1097/BOR.0b013e32835aa28d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Loebel C, Burdick JA. Engineering Stem and Stromal Cell Therapies for Musculoskeletal Tissue Repair. Cell Stem Cell. 2018 doi: 10.1016/j.stem.2018.01.014. [Epub ahead of print] This review presents recent clinical advances in stem cell and stromal cell delivery for musculoskeletal tissue repair, including strategies to improve cell viability and retention. [DOI] [PMC free article] [PubMed] [Google Scholar]