Abstract
Transforming growth factor-β (TGF-β) is a central player in fibrotic disease. Clinical trials with global inhibitors of TGF-β have been disappointing, suggesting that a more targeted approach is warranted. Conversion of the latent precursor to the biologically active form of TGF-β represents a novel approach to selectively modulating TGF-β in disease, as mechanisms employed to activate latent TGF-β are typically cell, tissue, and/or disease specific. In this review, we will discuss the role of the matricellular protein, thrombospondin 1 (TSP-1), in regulation of latent TGF-β activation and the use an antagonist of TSP-1 mediated TGF-β activation in a number of diverse fibrotic diseases. In particular, we will discuss the TSP-1/TGF-β pathway in fibrotic complications of diabetes, liver fibrosis, and in multiple myeloma. We will also discuss emerging evidence for a role for TSP-1 in arterial remodeling, biomechanical modulation of TGF-β activity, and in immune dysfunction. As TSP-1 expression is upregulated by factors induced in fibrotic disease, targeting the TSP-1/TGF-β pathway potentially represents a more selective approach to controlling TGF-β activity in disease.
Keywords: thrombospondin-1, TGF-β, fibrosis, anti-fibrotic, latent TGF-β, diabetic nephropathy
Introduction
Transforming growth factor-β (TGF-β) is central to diseases involving elaboration of a fibrotic extracellular matrix (ECM), epithelial to mesenchymal transition and tumor metastasis, and immune dysregulation [1, 2]. However, TGF-β is essential for tissue homeostasis and control of ligand expression, its multiple receptors, and complex downstream signaling mediators is critical for maintaining appropriate contextual action. Another regulatory checkpoint occurs through mechanisms that convert the latent precursor of TGF-β to its biologically active form, a process called activation [3, 4]. Non-covalent binding of the N-terminal latency associated peptide (LAP) to the C-terminal mature domain prevents TGF-β receptor binding and this interaction must be disrupted for TGF-β to be active. Only a small fraction of total TGF-β is biologically active. Latent TGF-β can be activated through proteolysis (including plasmin and matrix metalloproteinases); binding to integrins βvβ1, βvβ3, βvβ5, βvβ8, or βvβ6 in concert with mechanical forces due to ECM stiffness and/or cytoskeletal forces, or in the case of βvβ3, and βvβ8, through localizing matrix metalloproteinases to the latent complex; through viral or oxidative modifications of the latent complex; or by binding to the secreted and extracellular matrix (ECM) protein thrombospondin 1 (TSP-1) [4–13]. The primary mechanism of latent TGF-β activation varies with cell type, tissue, and disease milieu. Blockade of a major disease-related activation mechanism typically attenuates the effects of disease-induced TGF-β activity and thus represents a novel therapeutic strategy to attack disease-induced activity and spare homeostatic TGF-β.
Integrin and TSP-1 dependent activation have been the most widely studied mechanisms of latent TGF-β activation. This review will focus on TSP-1 control of latent activation as a regulatory mechanism and discuss relevant diseases in which this mechanism is operative, with an emphasis on TGF-β in fibrotic complications of diabetes in the kidney and in the heart. We will also discuss the involvement of TSP-1 in controlling TGF-β activity in various models of liver fibrosis and in the hematologic cancer, multiple myeloma. Finally, we will briefly address emerging evidence for the role of the TSP-1/TGF-β axis in other disease indications and approaches to therapeutic targeting of the TSP-1/TGF-β pathway.
Thrombospondin 1 (TSP-1) activates latent TGF-β
TSP-1 is a multi-functional matricellular ECM and secreted protein, abundant in platelet β-granules, with widely upregulated expression in tissue injury and repair [14, 15]. TSP-1 has structural domains with multiple specific receptors/binding partners and cellular functions [16]. TSP-1 is a major regulator of TGF-β activation, but also has TGF-β-independent functions in hemostasis, cell adhesion, migration, growth factor (EGF, VEGF, FGF) regulation, and inhibition of angiogenesis and nitric oxide signaling [14].
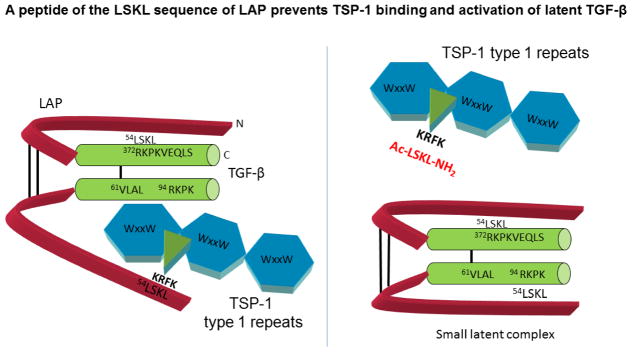
A number of years ago we observed that preparations of human TSP-1 purified from pools of thrombin-stimulated platelets contained biologically active TGF-β1 that co-migrated with TSP-1 in gel permeation columns, suggesting direct binding between the two proteins [17]. In more interesting further studies, we showed that purified TSP-1, as well as certain proteolytic or recombinant fragments of the protein can bind to either the small or large form of the purified latent TGF-β complex and to latent TGF-β secreted by cultured cells. TSP-1 binding to the latent complex converts latent TGF-β to its biologically active form through a non-proteolytic mechanism [18, 19]. Subsequent studies identified the KRFK sequence in the TSP-1 type 1 repeats as critical for latent TGF-β activation [20, 21]. The KRFK sequence in the 2nd TSP-1 type 1 repeat binds to a conserved sequence, LSKL, in the LAP, which disrupts LAP-mature domain interactions to expose the receptor binding sequences, rendering TGF-β capable of signaling (Figure 1) [22, 23]. TSP-2 lacks the KRFK sequence and does not activate TGF-β and in fact, can act as a competitive antagonist of TSP-1 mediated TGF-β activation [21, 24]. We identified the LSKL sequence in the LAP as a critical determinant of latency, a result that has been confirmed biochemically and by the crystal structure of the latent complex [22, 25, 26]. In addition, we identified sites of interaction between the tryptophan-rich motifs in the type 1 repeats of TSPs 1 and 2 and the latent complex, which are important for binding but not sufficient for activation [27]. TSP-1 binds to latent TGF-β to activate TGF-β in solution, at the cell surface, or in the extracellular milieu in a manner independent of proteases [7]. The molecular nuances of TSP-1-latent TGF-β interactions and the activation process were discussed extensively in a previous review and the reader is encouraged to consult this article for additional details [7].
Figure 1. Latent TGF-β activation by TSP-1 and antagonism by peptides of the LAP LSKL sequence.
TSP-1 binds to the TGF-β small latent complex comprised of the N-terminal latency associated peptide (LAP) and the C-terminal mature domain through its type 1 repeats. The C-terminus of the mature TGF-β homodimer binds to TGF-β signaling receptors. The small latent complex covalently binds to the fibrillin-like latent TGF-β binding protein (LTBP) at cysteine 33 of the LAP to form the large latent complex (not shown). Binding of the LSKL sequence at the N-terminus of the LAP to the RKPKVEQLS sequence in the receptor-binding region of the mature domain is necessary to confer latency [22, 25, 26]. The KRFK sequence of TSP1 located at the N-terminal portion of the second type 1 repeat of TSP-1 recognizes the LSKL sequence in the LAP and competitively disrupts LSKL-RKPKVEQLS binding to displace LAP from the mature domain. This results in access of the mature domain for TGF-β receptor binding and signaling as noted by changes in the secondary structure of the latent complex (data not shown). The tryptophan-rich motifs (WSxW) in each of the three type 1 repeats of TSP-1 also bind to the VLAL sequences in the mature domain and the LAP, although these interactions are not sufficient for activation. LSKL as a peptide can competitively block TSP-1 binding to the latent complex, thus preventing TSP-1 binding and activation of latent TGF-β. Using recombinant latent TGF-β1 and purified TSP-1, LSKL is an effective antagonist at picomolar concentrations (see supplemental data in [114].
The LSKL peptide is a competitive antagonist that inhibits TSP-1-TGF-β activation by preventing the interaction of TSP-1’s KRFK sequence with the LAP of latent TGF-β (Figure 1) [23]. This antagonist tetrapeptide has been widely used by our lab and others, along with thbs1 (TSP-1) null animals, to establish TSP-1 as a primary regulator of TGF-β bioactivity in numerous fibrotic diseases, including diabetic nephropathy and cardiomyopathy [7, 28–31]. A summary of animal studies from numerous labs in which LSKL has been used to antagonize TSP-1-dependent TGF-β activation is found in TABLE 1. In addition, there are diverse indications for TSP-1 involvement in latent TGF-β activation based on either in vitro studies, models using thbs1 deficient animals, or blocking antibody or other antagonist peptide studies as presented in [7]. Some more recent indications for involvement of the TSP-1/TGF-β axis in disease will be discussed.
Table 1.
Disease models in which the TSP-1 antagonist LSKL impacts TGF-β activity and disease pathogenesis
| Disease | Organ | In vivo model | TSP1 antagonist | Reference |
|---|---|---|---|---|
| Diabetes | Kidney | Mouse, Akita | LSKL | [28] |
| Diabetes/Hypertension | Heart | Rats, STZ with aortic coarctation | LSKL | [29] |
| Endomyocardial fibrosis | Heart | Id1/3 DKO adult mice | LSKL | [118] |
| Mesangial Proliferative glomerulonephritis | Kidney | Rats, Anti-Thy-1 antibody injury | LSKL, | [180] |
| Chronic Kidney Disease | Kidney | Rat, UUO | LSKL | [174] |
| Liver fibrosis | liver | Rat, DMN-induced | LSKL | [102] |
| Enhanced liver regeneration | liver | Mouse, partial hepatectomy | LSKL | [106] |
| Autoimmune | MS/Brain inflammation | EAE model of MS | LSKL, candesartan | [181] |
| Pulmonary arterial hypertension | Pulmonary artery | hypoxia-induced pulmonary arterial hypertension | LSKL | [116] |
| Arteriosclerosis | Carotid artery | Disturbed flow | LSKL | [117] |
| Subarachnoid fibrosis | brain | Rat, model of subarachnoid hemorrhage with hydrocephalus | LSKL | [182] |
| Multiple myeloma | Bone marrow | Heparanase expressing human myeloma/SCID mouse 5TGM1 syngeneic model | LSKL, SRI31277 | [114] |
TSP-1 activation of TGF-β isoforms
The LSKL sequence is conserved in the LAP regions of all three mammalian isoforms of latent TGF-β, suggesting that TSP-1 can activate all three isoforms [23]. TGF-β1 is considered the primary isoform driving fibrosis and much of the focus on TSP-1 control of TGF-β1 activation has been centered on investigation of its role in various fibrotic diseases. Indeed, there is ample evidence for TSP-1 activation of latent TGF-β1 as discussed here and elsewhere [7]. Initial evidence for a physiologic role for TSP-1 in regulating activation of latent TGF-β1 resulted from observations that thbs1 null mice partially phenocopies the TGF-β1 null mice, albeit with some distinctions and a less severe phenotype than the TGF-β1 null mice [30]. These studies demonstrated that treatment of neonatal mice with a TSP-1 peptide that activates latent TGF-β (KRFK) could partially rescue the TGF-β1 null phenotype and the LSKL blocking peptide could partially replicate this phenotype in wildtype neonatal mice [30]. With respect to the other mammalian isoforms, little is known about mechanisms involved in activating latent TGF-β3 in vivo, although in vitro studies indicate that MMPs and integrin βvβ6 can activate TGF-β3: furthermore, TGF-β3 and βvβ6 and βvβ8 knockout mice all have cleft palate, suggesting a role for integrin-dependent activation [13, 32–34]. The ability of TSP-1 to activate latent TGF-β3 has not been directly tested and nor has its co-expression in various tissues been examined. In contrast, there is evidence that TSP-1 can activate latent TGF-β2 and that activation of latent TGF-β2 can be blocked by LSKL both using recombinant latent TGF-β2 and in hypoxia-stimulated endothelial cells [23, 35]. Furthermore, murine antigen presenting cells use TSP-1 to activate latent TGF-β2, a process that is important for induction of Foxp3+ T regs [36]. In this system, TSP-1 binding to CD36 was required for TGF-β activation and the authors suggest that TSP-1 represents a mechanism for controlling TGF-beta activation at mucosal surfaces [36]. Interestingly, unlike in the antigen presenting cells, TSP-1 regulates expression of latent TGF-β2 at the protein level in lactate-treated glioma cells with TSP-1 knockdown reducing both TGF-β2 protein and Smad phosphorylation [37]. This is similar to the observation that LSKL reduces TGF-β2 mRNA in hypoxic endothelial cells and likely reflects auto-stimulatory effects of active TGF-β2 on its own expression [35]. Similarly, TSP-1 is known to induce TGF-β2 expression in vascular smooth muscle cells [38]. As the LAP of TGF-β2 lacks the integrin binding RGD sequence and this isoform is not activated by integrins [33], together these data suggest that TSP-1 is a major regulator of activation of the latent TGF-β2 isoform. The fact that there is little overlap in phenotypes between thbs1 null and TGF-β2 null mice suggests that TSP-1 control of latent TGF-β2 activation would occur primarily under post-natal conditions, rather than during development [30, 39]. For clarity, “TGF-β” will be used to designate either TGF-β1 in disease models in which this isoform predominates or which have not rigorously addressed isoform specificity. If the TGF-β isoform is known, it will be specifically designated.
TGF-β is a therapeutic target in Diabetic Nephropathy
TGF-β is central to development of diabetic nephropathy. TGF-β1 is a key mediator of progressive diabetic nephropathy by inducing glomerular hypertrophy with mesangial matrix expansion, renal tubular injury and interstitial fibrosis, and alterations in podocyte slit barrier function, leading to proteinuria [40–42] [43]. TGF-β inhibits epithelial repair by blocking growth, promoting apoptosis, and by stimulating epithelial plasticity [44]. The diabetic milieu (hyperglycemia, the renin-angiotensin system, and increased reactive oxygen species) stimulates increased TGF-β activity. In addition, increased expression of TGF-β in glomeruli and tubules is documented in both diabetic patients and animal models [45]. Thus, it is well-established that TGF-β is a therapeutic target for diabetic nephropathy [40, 46–51].
TSP-1 regulates latent TGF-β activation in diabetic complications
There are multiple lines of evidence from clinical specimens, cell culture, and animal models supporting the premise that TSP-1-mediated latent TGF-β activation is a critical factor in the development of diabetic nephropathy. Factors associated with increased TGF-β activity in diabetes stimulate TSP-1 expression. Oxidative stress under high glucose conditions increases TSP-1 expression by mesangial cells due to a decrease of nitric oxide-protein kinase G-mediated transcriptional repression and increased expression of the transcription factor USF2 [52, 53]. Glucose also increases podocyte TSP-1 expression [54]. Angiotensin II increases TSP-1 expression by mesangial cells and cardiac fibroblasts in vitro, which results in increased TGF-β activity [53, 55, 56]. TSP-1 protein is increased in the glomeruli of patients with both types 1 and 2 diabetic nephropathy and its expression correlates with increased TGF-β activity [57, 58] and with proteinuria in patients with type 2 diabetes [57]. Thbs1 gene expression is increased in mesangial cells isolated from db/db mice with type 2 diabetic nephropathy [59]. TSP-1 was identified as a biological marker of obesity and metabolic syndrome in human adipose tissues and serum [60]. Glucose-induced TGF-β activity in rat mesangial cells is blocked by LSKL and WSHW peptide antagonists and by an anti-TSP1 antibody [55, 61]. Glycated albumin stimulates increased TSP-1 expression and tubular hypertrophy: antibodies to TSP-1 block the increase in TGF-β activity and tubular hypertrophy [62]. The increased TGF-β activity in angiotensin II stimulated mesangial cells is blocked by TSP-1-TGF-β antagonist peptides [56]. Moreover, streptozotocin-treated thbs1 null mice do not develop glomerulosclerosis or podocyte loss [31] and conversely, mice with induced expression of the transcription factor USF2 have increased TSP-1 expression, TGF-β activity, and renal fibrosis [63].
TSP1 and obesity-related diabetic complications
Type 2 diabetes and hypertension are the two leading causes of end stage renal disease (reviewed in [64]). Obesity is a major risk factor for type 2. There are overlapping mechanisms underlying kidney disease in diabetes and obesity, which include hyperfiltration, the influence of inflammatory cytokines released from adipose tissue, and activation of the renin-angiotensin system [64–66]. TGF-β is elevated in patients with type 2 diabetes and in at least two mouse models of diabetic nephropathy (db/db, KK-Ay) [67–69]. Anti-TGF-β antibody reduces glomerulosclerosis and reverses glomerular basement membrane thickening in the db/db model of type 2 diabetic nephropathy [67, 70]. TSP-1 protein is increased in type 2 diabetic nephropathy and its expression correlates with increased TGF-β activity and with proteinuria in patients with type 2 diabetes [57, 58]. TSP-1 was the most highly upregulated protein in mesangial cells from db/db mice [59]. TSP-1 is also implicated in renal dysfunction and fibrosis in a mouse model of high fat diet induced obesity downstream of leptin signaling [71].
Interestingly, leptin stimulates TSP-1 expression, and TSP-1 deficient mice on a high fat diet are protected from renal fibrosis and albuminuria and have reduced TGF-β signaling [71]. In vitro leptin treatment of TSP-1-deficient mesangial cells failed to stimulate increased TGF-β activity or fibronectin and type IV collagen expression [71]. This is paradoxical to models of type 2 diabetic nephropathy in which either leptin or its receptors are genetically deleted (ob/ob, db/db, respectively).
Both TGF-β and TSP-1 have been shown to play causal roles in insulin resistance and obesity-related renal fibrosis, although there is evidence for both TGF-β-dependent and independent roles of TSP-1 [72–75]. It remains to be determined whether blocking TSP-1-TGF-β activation would lower insulin resistance.
TSP-1-TGF-β antagonists in diabetic complications
In Akita C57BL/6J-Ins2Akita mice, we showed that antagonism of TSP-1-dependent TGF-β activation by intraperitoneal injection of LSKL peptide (30 mg/kg, 3x/week for 15 weeks) decreased proteinuria and tubulointerstitial fibronectin expression, increased nephrin expression, and reduced TGF-β activity in the kidney and urine [28]. It is notable that LSKL reduced both fibrosis and proteinuria, since studies with a monoclonal antibody to TGF-β or knockout of Smad 3 reduced fibrosis without attenuating proteinuria [67, 76]. Data suggest TGF-β signaling through the ALK1 receptor, Smad 1/5 pathway might be critical for TGF-β-induced proteinuria [77]. It is possible that the TSP-1 antagonist by blocking latent TGF-β activation could impact both the classical and alternate Smad pathways. In addition to blocking the development of fibrosis and improving renal function in the diabetic kidney, LSKL peptide reversed myocardial fibrosis and improved left ventricular function through reducing TGF-β activity in hypertensive diabetic rats when treatment was initiated 6 weeks after disease induction [29]. This is an important finding since antagonist peptide treatment began at a time when fibrotic changes were already detectable in the left ventricle, suggesting that blocking the TSP-1-TGF-β pathway might be an effective anti-fibrotic strategy in patients with early diabetic cardiomyopathy.
TSP-1 antagonists and the renin-angiotensin system
Antagonists of the renin-angiotensin system are the standard of care for diabetic nephropathy. However, 20% of patients progress to end stage renal disease despite treatment (reviewed in [78]). Although the renin-angiotensin system drives diabetic nephropathy through multiple mechanisms, it directly impacts both TSP-1 expression and TGF-β expression and activity. We showed that angiotensin II stimulates TSP-1 expression by rat mesangial cells and that losartan blocks angiotensin II stimulation of TSP-1 [56]. The TSP-1 antagonist LSKL reduces TGF-β activity, but not TGF-β protein expression, in rat mesangial cells stimulated by angiotensin II [56]. Valsartan attenuates TSP-1 and TGF-β expression and TGF-β protein and Smad signaling in aortas of diabetic rats [79]. Despite some overlap in their targets, synergy between TGF-β antagonists and renin-angiotensin inhibitors has been observed in animal models of diabetic nephropathy [80, 81] [82]. It remains to be determined experimentally as to whether combinations of TSP-1-TGF-β antagonists with renin-angiotensin antagonists would provide additional benefit.
Other TGF-β activation mechanisms in diabetic complications
Integrin-dependent TGF-β activation also represents a potential target for diabetic nephropathy [83–85]. There is a recently completed phase 2 trial (NCT02251067) for diabetic nephropathy using an antibody (VPI-2690B) that blocks ligand binding to the βvβ3 integrin and integrin activation: this antibody also reduces TSP-1 and TGF-β expression [86, 87], although it is not known whether this antibody directly blocks TGF-β activation. Another integrin antagonist, the cyclic peptide that targets βvβ3 and βvβ5, Cilengitide, recently failed in trials of aggressive glioblastoma and importantly, there was no correlation of p-Smad 2 levels with Cilengitide treatment in this disease for reasons that are not clear [88]. There is a small molecule inhibitor of βvβ1 (C8) which blocks fibrosis in several organs including the renal unilateral ureteral obstruction (UUO) model, although the relevance of this integrin to diabetic nephropathy has not yet been established [89, 90].
The role of TGF-β in liver fibrosis
TGF-β is a central regulator of chronic liver disease through induction of fibrogenic responses [91–95]. Although infiltrating macrophages are a source of TGF-β, hepatic stellate cells are a significant source of TGF-β in liver fibrosis. TGF-β stimulates induction of myofibroblast-like properties of hepatic stellate cells to produce extracellular matrix, leading to fibrosis. Although TGF-β inhibits hepatocyte proliferation under basal conditions, it has pro-oncogenic properties during malignant progression through stimulating epithelial to mesenchymal transition, cell survival and migration, and reduced immune surveillance.
TSP-1 control of latent TGF-β activation in liver fibrosis
TSP-1 expression is increased in human liver disease with the THBS1 gene identified as part of a characteristic gene signature of chronic liver disease, including alcoholic and NASH cirrhosis, in humans and in mouse models of liver fibrosis induced by carbon tetrachloride or DDC [96]. In vitro studies show that bile acids increase expression of TSP-1 by hepatocytes, resulting in increased TGF-β signaling in co-cultured hepatic stellate cells [97]. Both TSP-1 and TGF-β are increased in congenital hepatic fibrosis [98]. TSP-1 regulated TGF-β activation prevented hepatocyte proliferation and liver regeneration after partial hepatectomy in thbs1 deficient mice [99] and TSP-1 induction by obstructed portal flow in mice is thought to lead to TGF-β-dependent liver atrophy [100]. TSP-1 has been shown to regulate latent TGF-β activation in animal models of liver fibrosis and in cell culture models (reviewed in [101]). Treatment of rats with the TSP-1 antagonist peptide LSKL prevented TGF-β activation and reduced liver fibrosis in the dimethylnitrosamine model [102]. TSP-1 is required for TGF-β signaling in both cultured hepatocytes and hepatic stellate cells, which is blocked by LSKL peptide [103, 104]. Interestingly, TSP-1-dependent latent TGF-β activation might play a role in hepatitis C induced fibrosis and carcinogenesis as the hepatitis C virus core protein induces TSP-1 expression by hepatocytes to increase active TGF-β and LSKL peptide blocks hepatitis C core protein activation of TGF-β [105]. Administration of LSKL peptide immediately after injury also accelerated liver regeneration in mice following partial hepatectomy through blocking TGF-β activation and signaling [106]. Both the TGF-β1 and the TGF-β2 isoforms are upregulated in mouse models and in human tissues with liver fibrosis and also hepatocellular carcinoma [107]. This is interesting since TSP-1 can activate both the TGF-β1 and β2 isoforms of latent TGF-β, whereas TGF-β2 is not activated by integrin-dependent mechanisms.
Other diseases with a role for TSP-1-dependent latent TGF-β activation
Since our discovery of the role of TSP-1 in mediating latent TGF-β activation, identification of the LAP sequence LSKL as an antagonist of binding and activation and demonstration of its activity in vivo, studies from multiple labs using LSKL in a diverse array of diseases have established the involvement of TSP-1 in a particular disease process [18, 23, 30]. Many of these in vitro and in vivo studies were summarized in a previous review [7]. Here we will discuss some more recent findings regarding disease models in which TSP-1 appears to be important for controlling latent TGF-β activation.
Multiple Myeloma
Multiple myeloma (MM) is a systemic cancer of malignant plasma cells. TGF-β has important roles in mediating myeloma cell-bone marrow stromal cell adhesion, activation of osteolytic pathways, and immune dysfunction [108–111]. TSP-1 expression is increased in the bone marrow plasma of MM patients [112, 113]. In a mouse xenograft model using heparanase expressing human MM cells, treatment with either LSKL or a novel compound based on LSKL, SRI31277, reduced tumor burden, TGF-β signaling in the bone marrow, host IL-6 production, and osteolytic bone disease [114]. Interestingly, SRI31277 both reduced osteoclasts and increased osteoblasts on marrow bone surfaces, consistent with previous in vitro observations that TSP1 blocks osteogenic differentiation of mesenchymal stem cells and that LSKL can reverse this blockade to promote osteogenic differentiation [115]. Cocktails of various drugs are the standard of care in myeloma. Our studies showed that SRI31227 has added benefit when combined with the proteasome inhibitor, bortezomib, but there was no additional benefit when SRI31277 was combined with dexamethasone [114].
Pulmonary arterial hypertension, flow-mediated arterial stiffening, cardiomyopathy
Both TSP-1 and TGF-β are upregulated in pulmonary arterial hypertension due to chronic hypoxia, Schistosomiasis, and in scleroderma: recent studies show that thbs1 knockout or treatment with the peptide antagonist LSKL protected against development of pulmonary hypertension due to hypoxia or Schistosome infection and also reduced active TGF-β [116]. In models of disturbed arterial flow leading to arterial stiffness in aged mice, TSP-1 and markers of pro-fibrotic TGF-β pathways were upregulated: in thbs1 knockout mice or mice treated with LSKL via intraperitoneal administration and subjected to disturbed flow, there was reduced arterial collagen, connective tissue growth factor, and stiffening [117]. Other studies show that LSKL blocks the development of endomyocardial fibrosis in mice with genetic deletion of inhibitor of DNA binding 1 and 3 (Id1 and Id3), a suppressor of TSP-1 expression [118].
Fibrotic diseases with a biomechanical component
Although mechanotransduction-dependent activation of latent TGF-β is considered to occur through integrin and cytoskeletal-dependent mechanisms that induce sufficient force to unfold the straightjacket domain of LAP [9–11, 119], TSP-1 expression is increased by mechanical forces in a number of tissues as described here, raising the possibility that that mechanical forces also play a role in stimulating TSP-1-mediated latent TGF-β activation. An early observation showed that mechanical stretch of neonatal sheep lungs increased TSP-1 expression and TGF-β activity [120]. TSP-1 was also shown to be important for shear-stress activation of TGF-β induced by stirring of platelets [121]. In scleroderma, TSP-1 is increased in the plasma and dermis of patients [122, 123] and both hypoxia and TGF-β induce TSP-1 expression [124]. Furthermore, TSP-1 mRNA levels are one of a 4-gene signature biomarker that is predictive for disease activity in patients with diffuse cutaneous systemic sclerosis [125]. Stiffening of the skin is a characteristic of scleroderma and in vitro studies showed that force-loaded skin fibroblasts increase TSP-1 expression [126]. In addition, pre-treatment of fibroblasts with LSKL and then with active TGF-β reduced TGF-β-dependent fibroblast contractility, presumably by preventing autocrine TGF-β activation by TSP-1, with the effects of LSKL more pronounced in skin fibroblasts derived from scleroderma patients than from normal controls [126]. Although there is no direct evidence for TSP-1 control of TGF-β activation in scleroderma obtained through the use of specific TSP-1 antagonists in animal models, recent evidence obtained in the bleomycin model of systemic sclerosis using the anti-fibrotic glycyrrhizin shows that TSP-1 levels, TGF-β signaling, and fibrosis were reduced [127].
Glaucoma is another disease in which biomechanical forces due to increased intraocular pressure and possibly intrinsic tissue responses are thought to contribute to disease progression [128, 129]. Increased intraocular pressure in glaucoma affects ECM remodeling in the trabecular meshwork with increased resistance to outflow and also remodeling of the lamina cribrosa, the collagenous tissue at the optic nerve head, which leads to optic neuropathy [130]. TSP-1 is increased by mechanical strain of cells isolated from the lamina cribrosa, and TSP-1 is also increased in the trabecular meshwork of eyes from patients with glaucoma [131–135]. Both TGF-βs 1 and 2 are increased in glaucoma and are important for the pathologic remodeling of these ocular tissues under elevated intraocular pressure [136, 137]. Interestingly, the TGF-β2 isoform, which is not activated by integrins, is widely expressed in glaucoma, suggesting either TSP-1 or other non-integrin dependent activation mechanisms could be important in glaucoma.
For both scleroderma and glaucoma—fibrotic diseases in which biomechanical alterations are a key characteristic, additional studies are needed to determine whether increased TSP-1 expression in response to mechanical strain contributes to latent TGF-β activation directly. It also remains unknown how mechanical forces might affect TSP-1 folding, presentation at the cell surface, or release from the ECM to favor binding to and activation of the latent TGF-β complex.
Immunotherapy modulators
TGF-β is immunosuppressive through induction of T regs and M2 macrophages and impairment of dendritic cell maturation and NK cell activity [138–140]. Based on these data, there is interest in using TGF-β antagonists to enhance anti-tumor immunotherapies and to augment bone marrow reconstitution following stem cell transplant [141–143]. Integrin βvβ8 expressed on dendritic cells is known to activate latent TGF-β and stimulate Th17 and T reg development, thereby influencing autoimmune disease pathogenesis [144–147]. Expression of βvβ8 on T regs also regulates TGF-β activity [148]. Despite the importance of integrins, there are also multiple lines of evidence showing that TSP-1 plays a role in TGF-β activation in the context of immune cells. TSP-1 null mice have reduced Th 17 cells and IL-17 levels due to decreased TGF-β activity [8, 149]. TSP-1 is expressed by T cells and accumulates on the T cell surface with latent TGF-β. In addition, TSP-1 activates latent TGF-β2 expressed by antigen-presenting cells to control T reg development in ocular tissues [36]. There is also evidence that B cell-derived TSP-1 regulates latent TGF-β activation on dendritic cells [150]. TSP-1 can also inhibit early NK proliferation in a manner blocked by anti-TGF-β neutralizing antibodies, suggesting a role for TSP-1 in latent TGF-β activation by human NK cells [151]. Moreover, adipose-derived stem cells reduced autoimmune colitis by inducing T regs through TSP-1 dependent TGF-β activation, but independent of integrin βv [152]. Studies also suggest that TSP-1 regulated latent TGF-β activation, which can be blocked by LSKL, plays a role in T cell activation following renal transplant [153]; in immune tolerance in glioma [154]; in pigmented epithelial cell generation of T regs in ocular tissues [155]; and in cell senescence [156]. Clearly, additional studies are needed to better understand the roles of integrin and TSP-1-dependent TGF-β activation mechanisms in different immune contexts. Nonetheless, these data suggest that TSP-1 antagonists might have potential therapeutic uses to optimize immunotherapy treatments through controlling TGF-β action.
Possible roles of TSP-1 receptors
It is possible that in some diseases, multiple activation mechanisms can control latent TGF-β activation by differing cell types and at different stages of disease progression. Alternately, there can be co-operative mechanisms that employ aspects of multiple activation mechanisms. For example, some integrins and MMPs can cooperate to regulate latent TGF-β activation [169]. There is also evidence that CD36-bound TSP-1 on bleomycin activated alveolar macrophages or on antigen presenting cells can activate latent TGF-β, but plasmin activity is required, at least in the former case [36, 170]. TSP-1 also binds to a number of different integrins and we observed that an antibody to the βvβ3 integrin blocks TSP-1 activation of latent TGF-β in breast cancer cells and others showed that VP1-2690B antibody to integrin subunit β3 decreases TSP-1 expression [86, 171]. TSP-1 binding to integrins or other TSP-1 receptors, as in the case of macrophage or antigen presenting cell CD36, might serve to localize TSP-1 and the latent complex at the cell membrane to enhance activation in the vicinity of TGF-β receptors. Although purified TSP-1 can activate recombinant latent TGF-β in a test tube in the absence of cells [114], in the cellular context, other stabilizing or localizing factors such as TSP-1 binding receptors or other cell surface or soluble molecules might be involved that could either favor or hinder TSP-1 availability for binding to the latent complex. Interestingly, in the absence of the TSP-1 receptor, CD47, there are elevated levels of both TSP-1 and active TGF-β following thermal injury, raising the possibility that signaling downstream of CD47 antagonizes the TSP-1/TGF-β pathway or possibly TGF-β signaling itself [172]. However, antibodies to integrin-associated protein (CD47) blocked activation of TGF-β in estrogen-depleted breast cancer cells in a system in which TSP-1 associated with the cell surface is increased and in which TGF-β activity is reduced by a blocking antibody (Mab133) and a blocking peptide [171]. Clearly, additional studies are needed to elucidate the role of TSP-1’s complex interactions with cells on its ability to control latent TGF-β activation.
Relationship to integrin dependent activation
It is unknown whether integrin antagonists with anti-fibrotic activity also affect TSP-1 dependent TGF-β activation or whether combining TSP-1 and integrin antagonists would have additional therapeutic benefit. Double knockout mice that lack both thbs1 and the integrin β6 subunit have a more severe inflammatory phenotype than either single knockout mouse. Also, the β6 single knockout mice have increased inflammation and tumorigenesis as compared to the thbs1 knockout mice [173]. Curiously, the double knockout mice had increased staining of phosphorylated Smad2/3 in hyperplastic lung epithelium as compared to wild type mice for reasons that are not clear. Single knockout mice were not examined in these studies, although single knockout mice would be predicted to have reduced TGF-β signaling based on previous studies [6, 30, 173]. It is not definitively known if both TSP-1 and integrin-dependent activation can be operative in the same tissue and disease milieu. Interestingly, there are several disease indications in which both integrin antagonists and TSP-1/TGF-β antagonists have been shown to reduce TGF-β activity and fibrotic disease: examples include the unilateral ureteral obstruction model of renal fibrosis, liver fibrosis, pulmonary fibrosis, and T cell regulation [6, 89, 102, 144, 146, 148, 149, 151, 152, 170, 174–177]. It has largely been assumed that blockade of a particular pathway with resultant attenuation of disease indicates that only that one process is critical for disease pathogenesis. However, it is possible that two independent pathways could control latent TGF-β activation in a particular organ or disease. For example, each pathway might differentially regulate activation by distinct cell populations or at different stages of disease progression. Both or multiple pathways could be required for full disease progression, given the cross-talk between cell populations in fibrosis, so that attenuation of one pathway is sufficient to attenuate disease. However, both integrin and TSP-1/TGF-β antagonists can affect similar cell populations such as myofibroblasts to reduce TGF-β signaling, so other explanations are likely and this remains an important question for the fibrosis field. In contrast to diseases in which the TGF-β1 isoform predominates, it is likely that TSP-1 is a major factor in TGF-β2-mediated diseases as integrins do not activate this isoform.
The lack of a complete phenocopy of thbs1 null mice with TGF-β1–3 null mice has been used as an argument to suggest that TSP-1 is not a primary regulator of latent TGF-β activation. It should be noted that these phenotypes reflect developmental alterations and cannot predict the roles of these activation mechanisms in diseases processes that occur post-natally or in the adult. TSP-1 is a matricellular extracellular matrix protein and one of the defining characteristics of matricellular proteins is that knockout phenotypes at birth are mild with more robust phenotypes appearing as the animal ages or is exposed to stresses or in disease [15, 178]. This concept is consistent with multiple lines of evidence showing a role for TSP-1 in controlling latent TGF-β in various diseases in which TSP-1 expression is upregulated, but with only mild developmental phenotypes. Furthermore, TSP-1 binds multiple integrins and other receptors to regulate diverse biologic function such as cell adhesion, migration, apoptosis, VEGFR and nitric oxide signaling, angiogenesis, vascular barrier function, and inflammation in addition to regulation of TGF-β activation [179]. Thus, any thbs1 knockout phenotype likely reflects a complex interplay of pathways altered by the loss of TSP-1 function. Nonetheless, more direct assessment of possible co-activity between integrin and TSP-1 mediated control of latent TGF-β activation through use of specific antagonists rather than via genetic approaches is needed.
Approaches to therapeutic control of TGF-β in disease and potential role for TSP-1/TGF-β antagonists
Although TGF-β is a significant player in fibrotic diseases, metastatic progression, and immune dysfunction, it is nonetheless critical for homeostasis. Genetic ablation of TGF-β, its receptors, or its signaling mediators can result in developmental defects, unremitting inflammation, and an increased incidence of carcinomas [157, 158]. Current anti-TGF-β antagonists target the molecule, receptors, or signaling mediators and provide no mechanism for distinguishing homeostatic from disease-induced TGF-β activity. This raises the potential for adverse effects with the use of global inhibitors such as monoclonal antibodies to ligand or receptor kinase inhibitors as seen in long-term animal studies and more critically, in clinical trials [159–161]. In diseases such as diabetes, chronic treatment will be required and thus a targeted approach to reducing disease-specific TGF-β activity would potentially increase therapeutic benefit [1, 2, 138, 162]. To date, the use of the TSP1-TGF-β antagonists in animal models has not induced adverse events such as inflammation or tumorigenesis. Mice treated intermittently with LSKL (15 weeks, 3x/week i.p.) or continuously over 4 weeks with SRI31277 did not show inflammation or unrelated tumors [29, 114]. These findings are consistent with studies showing increased GI tumors and inflammation in mice with dual mutations that reduce basal TGF-β activity and prevent activation by integrin β8, but no increase in tumors was observed in dual mutant mice with thbs1 knockout [163]. Although TSP-1 deficient mice have modest inflammation, this could be due to TGF-β independent functions of TSP-1and it is not replicated by LSKL or SRI31277 [30, 164]. Importantly, systemic administration of LSKL peptide did not impair wound healing in diabetic db/db mice [28]. In addition, SRI31277 displayed no off-target activity in CEREP receptor binding assays (Suto M.J., Murphy-Ullrich, unpublished data). Although these multiple animal studies suggest that chronic use of TSP-1-TGF-β antagonists would be safe, there might be situations in which TSP-1-TGF-β activation is beneficial and in which blockade could lead to adverse events, as in a mouse model of pre-formed aneurysms [165].
In addition to the risks of globally attenuating TGF-β activity, results from clinical trials with global inhibitors of TGF-β have failed to show efficacy. Despite abundant evidence for the importance of TGF-β in diabetic nephropathy, clinical trials with monoclonal antibodies to TGF-β1 have not demonstrated therapeutic benefit. TGF-β antagonists (monoclonal antibodies GC-1008 and LY2382770) are no longer in clinical trials for diabetic nephropathy due to corporate decisions or lack of phase 2 efficacy, respectively [51]. A recent phase 2 study using a humanized anti-TGF-β1 antibody in combination with renin-angiotensin inhibitors was stopped early for lack of efficacy [161]. The anti-TGF-β antibody, 1D11, when tested in a mouse model of pulmonary fibrosis, o failed to reduce pulmonary fibrosis, but exacerbated inflammation [166]. There is an emerging consensus that global inhibition of TGF-β, particularly through use of ligand-targeted monoclonal antibodies, is not effective. The reasons are not clear, although observations of reduced efficacy with higher doses of antibody, coupled with adverse effects in monkeys, limit dosing intensity in chronic diseases [161, 167, 168]. It is possible that molecules as large as antibodies do not have sufficient tissue penetrance and access to the ligand, especially as latent TGF-β can be sequestered in the ECM or at the cell surface.
Conclusion
In light of clinical failures with global TGF-β antagonists, newer, more targeted approaches to controlling disease-related TGF-β activity are needed. Given the relative disease-restricted utilization of TGF-β activation mechanisms, antagonists of disease-specific TGF-β activation pathways potentially represent a more therapeutically effective means of targeting TGF-β in fibrotic diseases. Ideally, the antagonist should target only the TGF-β activating function of the inducing molecule. As we have described, antagonists of the TSP-1/TGF-β pathway could be suitable drug candidates for fibrotic and other diseases involving pathologic TGF-β activation.
Supplementary Material
Highlights.
TGF-β is a central mediator of fibrotic diseases
Targeting the latent TGF-β activation mechanism is a selective approach to controlling TGF-β in disease
The matricellular protein Thrombospondin-1 binds and activates latent TGF-β
TSP-1 plays an important role in controlling TGF-β activation in fibrotic diseases, particularly in diabetic complications and in liver fibrosis
Antagonists of the TSP-1/TGF-β axis represent a targeted approach to modulating TGF-β in disease
Acknowledgments
The author wishes to thank the many superb trainees and colleagues who have contributed to these studies over the years.
Funding:
The most recent studies (myeloma, drug development) described in this review are supported by grants from Alabama Drug Development Alliance and the UAB Comprehensive Cancer Center pilot grant program, the American Society for Hematology Bridge Grant with supporting funds from the UAB Department of Pathology, and NIH grant 1R01CA175012.
Abbreviations
- ECM
extracellular matrix
- LAP
latency associated peptide
- MM
Multiple Myeloma
- NK
Natural Killer
- TSP-1
thrombospondin 1
- TGF-β
transforming growth factor-β
- UUO
unilateral ureteral obstruction
Footnotes
Conflicts of interest:
Drs. Murphy-Ullrich and Suto are holders of US patent US2014/0336115 A1 “Compounds, compositions, and methods for the treatment of diseases through inhibiting TGF-β activity.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prud’homme GJ. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest. 2007;87(11):1077–91. doi: 10.1038/labinvest.3700669. [DOI] [PubMed] [Google Scholar]
- 2.Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. TGF-beta - an excellent servant but a bad master. J Transl Med. 2012;10:183. doi: 10.1186/1479-5876-10-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int. 1997;51(5):1376–82. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 4.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116(Pt 2):217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 5.Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, Taylor SE, Ledbetter S, Lawrence CM, Rifkin DB, Barcellos-Hoff MH. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat Res. 2006;166(6):839–48. doi: 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- 6.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–28. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 7.Sweetwyne MT, Murphy-Ullrich JE. Thrombospondin1 in tissue repair and fibrosis: TGF-beta-dependent and independent mechanisms. Matrix Biol. 2012;31(3):178–86. doi: 10.1016/j.matbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bige N, Shweke N, Benhassine S, Jouanneau C, Vandermeersch S, Dussaule JC, Chatziantoniou C, Ronco P, Boffa JJ. Thrombospondin-1 plays a profibrotic and pro-inflammatory role during ureteric obstruction. Kidney Int. 2012;81(12):1226–38. doi: 10.1038/ki.2012.21. [DOI] [PubMed] [Google Scholar]
- 9.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol. 2007;179(6):1311–23. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinz B. The extracellular matrix and transforming growth factor-beta1: Tale of a strained relationship. Matrix Biol. 2015 doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87(8–9):601–15. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Schultz-Cherry S, Hinshaw VS. Influenza virus neuraminidase activates latent transforming growth factor beta. J Virol. 1996;70(12):8624–9. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson IB, Rifkin DB. Regulation of the Bioavailability of TGF-beta and TGF-beta-Related Proteins. Cold Spring Harbor perspectives in biology. 2016;8(6) doi: 10.1101/cshperspect.a021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3(10):a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy-Ullrich JE, Iozzo RV. Thrombospondins in physiology and disease: new tricks for old dogs. Matrix Biol. 2012;31(3):152–4. doi: 10.1016/j.matbio.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy-Ullrich JE, Schultz-Cherry S, Hook M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992;3(2):181–8. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122(4):923–32. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich JE. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-beta in a chemically defined system. J Biol Chem. 1994;269(43):26775–82. [PubMed] [Google Scholar]
- 20.Schultz-Cherry S, Lawler J, Murphy-Ullrich JE. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-beta. J Biol Chem. 1994;269(43):26783–8. [PubMed] [Google Scholar]
- 21.Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270(13):7304–10. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- 22.Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-beta. J Biol Chem. 2004;279(36):38032–9. doi: 10.1074/jbc.M405658200. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro SM, Poczatek M, Schultz-Cherry S, Villain M, Murphy-Ullrich JE. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-beta. J Biol Chem. 1999;274(19):13586–93. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- 24.Daniel C, Wagner A, Hohenstein B, Hugo C. Thrombospondin-2 therapy ameliorates experimental glomerulonephritis via inhibition of cell proliferation, inflammation, and TGF-beta activation. Am J Physiol Renal Physiol. 2009;297(5):F1299–309. doi: 10.1152/ajprenal.00254.2009. [DOI] [PubMed] [Google Scholar]
- 25.Walton KL, Makanji Y, Chen J, Wilce MC, Chan KL, Robertson DM, Harrison CA. Two distinct regions of latency-associated peptide coordinate stability of the latent transforming growth factor-beta1 complex. J Biol Chem. 2010;285(22):17029–37. doi: 10.1074/jbc.M110.110288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, Springer TA. Latent TGF-beta structure and activation. Nature. 2011;474(7351):343–9. doi: 10.1038/nature10152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young GD, Murphy-Ullrich JE. The tryptophan-rich motifs of the thrombospondin type 1 repeats bind VLAL motifs in the latent transforming growth factor-beta complex. J Biol Chem. 2004;279(46):47633–42. doi: 10.1074/jbc.M404918200. [DOI] [PubMed] [Google Scholar]
- 28.Lu A, Miao M, Schoeb TR, Agarwal A, Murphy-Ullrich JE. Blockade of TSP1-dependent TGF-beta activity reduces renal injury and proteinuria in a murine model of diabetic nephropathy. Am J Pathol. 2011;178(6):2573–86. doi: 10.1016/j.ajpath.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell’italia L, Murphy-Ullrich JE, Berecek KH. A thrombospondin-1 antagonist of transforming growth factor-beta activation blocks cardiomyopathy in rats with diabetes and elevated angiotensin II. Am J Pathol. 2007;171(3):777–89. doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93(7):1159–70. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 31.Daniel C, Schaub K, Amann K, Lawler J, Hugo C. Thrombospondin-1 is an endogenous activator of TGF-beta in experimental diabetic nephropathy in vivo. Diabetes. 2007;56(12):2982–9. doi: 10.2337/db07-0551. [DOI] [PubMed] [Google Scholar]
- 32.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14(2):163–76. [PMC free article] [PubMed] [Google Scholar]
- 33.Annes JP, Rifkin DB, Munger JS. The integrin alphaVbeta6 binds and activates latent TGFbeta3. FEBS Lett. 2002;511(1–3):65–8. doi: 10.1016/s0014-5793(01)03280-x. [DOI] [PubMed] [Google Scholar]
- 34.Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J Cell Sci. 2009;122(Pt 2):227–32. doi: 10.1242/jcs.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Akman HO, Smith EL, Zhao J, Murphy-Ullrich JE, Batuman OA. Cellular response to hypoxia involves signaling via Smad proteins. Blood. 2003;101(6):2253–60. doi: 10.1182/blood-2002-02-0629. [DOI] [PubMed] [Google Scholar]
- 36.Mir FA, Contreras-Ruiz L, Masli S. Thrombospondin-1-dependent immune regulation by transforming growth factor-beta2-exposed antigen-presenting cells. Immunology. 2015;146(4):547–56. doi: 10.1111/imm.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seliger C, Leukel P, Moeckel S, Jachnik B, Lottaz C, Kreutz M, Brawanski A, Proescholdt M, Bogdahn U, Bosserhoff AK, Vollmann-Zwerenz A, Hau P. Lactate-modulated induction of THBS-1 activates transforming growth factor (TGF)-beta2 and migration of glioma cells in vitro. PLoS One. 2013;8(11):e78935. doi: 10.1371/journal.pone.0078935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maier KG, Han X, Sadowitz B, Gentile KL, Middleton FA, Gahtan V. Thrombospondin-1: a proatherosclerotic protein augmented by hyperglycemia. J Vasc Surg. 2010;51(5):1238–47. doi: 10.1016/j.jvs.2009.11.073. [DOI] [PubMed] [Google Scholar]
- 39.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124(13):2659–70. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol. 2004;15(Suppl 1):S55–7. doi: 10.1097/01.asn.0000093460.24823.5b. [DOI] [PubMed] [Google Scholar]
- 41.Ziyadeh FN, Wolf G. Pathogenesis of the podocytopathy and proteinuria in diabetic glomerulopathy. Curr Diabetes Rev. 2008;4(1):39–45. doi: 10.2174/157339908783502370. [DOI] [PubMed] [Google Scholar]
- 42.Hathaway CK, Gasim AM, Grant R, Chang AS, Kim HS, Madden VJ, Bagnell CR, Jr, Jennette JC, Smithies O, Kakoki M. Low TGFbeta1 expression prevents and high expression exacerbates diabetic nephropathy in mice. Proc Natl Acad Sci U S A. 2015;112(18):5815–20. doi: 10.1073/pnas.1504777112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffman BB, Sharma K, Zhu Y, Ziyadeh FN. Transcriptional activation of transforming growth factor-beta1 in mesangial cell culture by high glucose concentration. Kidney Int. 1998;54(4):1107–16. doi: 10.1046/j.1523-1755.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- 44.Noble P. Idiopathic pulmonary fibrosis: new insights into classification and pathogenesis usher in a new era of therapeutic approaches. Am J Respir Cell Mol Biol. 2003;29:S27–S31. [PubMed] [Google Scholar]
- 45.Sharma K, Ziyadeh FN, Alzahabi B, McGowan TA, Kapoor S, Kurnik BR, Kurnik PB, Weisberg LS. Increased renal production of transforming growth factor-beta1 in patients with type II diabetes. Diabetes. 1997;46(5):854–9. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 46.Shah IM, Mackay SP, McKay GA. Therapeutic strategies in the treatment of diabetic nephropathy - a translational medicine approach. Curr Med Chem. 2009;16(8):997–1016. doi: 10.2174/092986709787581897. [DOI] [PubMed] [Google Scholar]
- 47.Diamond-Stanic MK, You YH, Sharma K. Sugar, sex, and TGF-beta in diabetic nephropathy. Semin Nephrol. 2012;32(3):261–8. doi: 10.1016/j.semnephrol.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang AS, Hathaway CK, Smithies O, Kakoki M. Transforming growth factor beta1 and diabetic nephropathy. Am J Physiol Renal Physiol. 2015 doi: 10.1152/ajprenal.00502.2015. ajprenal 00502 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tampe D, Zeisberg M. Potential approaches to reverse or repair renal fibrosis. Nature reviews. Nephrology. 2014;10(4):226–37. doi: 10.1038/nrneph.2014.14. [DOI] [PubMed] [Google Scholar]
- 50.Munoz-Felix JM, Gonzalez-Nunez M, Martinez-Salgado C, Lopez-Novoa JM. TGF-beta/BMP proteins as therapeutic targets in renal fibrosis. Where have we arrived after 25 years of trials and tribulations? Pharmacology & therapeutics. 2015;156:44–58. doi: 10.1016/j.pharmthera.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Sureshbabu A, Muhsin SA, Choi ME. TGF-beta Signaling in the Kidney: Pro-fibrotic and Protective Effects. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00365.2015. ajprenal 00365 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang S, Shiva S, Poczatek MH, Darley-Usmar V, Murphy-Ullrich JE. Nitric oxide and cGMP-dependent protein kinase regulation of glucose-mediated thrombospondin 1-dependent transforming growth factor-beta activation in mesangial cells. J Biol Chem. 2002;277(12):9880–8. doi: 10.1074/jbc.M108360200. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Skorczewski J, Feng X, Mei L, Murphy-Ullrich JE. Glucose up-regulates thrombospondin 1 gene transcription and transforming growth factor-beta activity through antagonism of cGMP-dependent protein kinase repression via upstream stimulatory factor 2. J Biol Chem. 2004;279(33):34311–22. doi: 10.1074/jbc.M401629200. [DOI] [PubMed] [Google Scholar]
- 54.Han SH, Yang S, Jung DS, Li JJ, Kim JJ, Kwak SJ, Kim DK, Moon SJ, Lee JE, Han DS, Kang SW. Gene expression patterns in glucose-stimulated podocytes. Biochem Biophys Res Commun. 2008;370(3):514–8. doi: 10.1016/j.bbrc.2008.03.121. [DOI] [PubMed] [Google Scholar]
- 55.Poczatek MH, Hugo C, Darley-Usmar V, Murphy-Ullrich JE. Glucose stimulation of transforming growth factor-beta bioactivity in mesangial cells is mediated by thrombospondin-1. Am J Pathol. 2000;157(4):1353–63. doi: 10.1016/s0002-9440(10)64649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou Y, Poczatek MH, Berecek KH, Murphy-Ullrich JE. Thrombospondin 1 mediates angiotensin II induction of TGF-beta activation by cardiac and renal cells under both high and low glucose conditions. Biochem Biophys Res Commun. 2006;339(2):633–41. doi: 10.1016/j.bbrc.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 57.Hohenstein B, Daniel C, Hausknecht B, Boehmer K, Riess R, Amann KU, Hugo CP. Correlation of enhanced thrombospondin-1 expression, TGF-beta signalling and proteinuria in human type-2 diabetic nephropathy. Nephrol Dial Transplant. 2008;23(12):3880–7. doi: 10.1093/ndt/gfn399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wahab NA, Schaefer L, Weston BS, Yiannikouris O, Wright A, Babelova A, Schaefer R, Mason RM. Glomerular expression of thrombospondin-1, transforming growth factor beta and connective tissue growth factor at different stages of diabetic nephropathy and their interdependent roles in mesangial response to diabetic stimuli. Diabetologia. 2005;48(12):2650–60. doi: 10.1007/s00125-005-0006-5. [DOI] [PubMed] [Google Scholar]
- 59.Brunskill EW, Potter SS. Changes in the gene expression programs of renal mesangial cells during diabetic nephropathy. BMC Nephrol. 2012;13:70. doi: 10.1186/1471-2369-13-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuo Y, Tanaka M, Yamakage H, Sasaki Y, Muranaka K, Hata H, Ikai I, Shimatsu A, Inoue M, Chun TH, Satoh-Asahara N. Thrombospondin 1 as a novel biological marker of obesity and metabolic syndrome. Metabolism. 2015;64(11):1490–9. doi: 10.1016/j.metabol.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yevdokimova N, Wahab NA, Mason RM. Thrombospondin-1 is the key activator of TGF-beta1 in human mesangial cells exposed to high glucose. J Am Soc Nephrol. 2001;12(4):703–12. doi: 10.1681/ASN.V124703. [DOI] [PubMed] [Google Scholar]
- 62.Yang YL, Chuang LY, Guh JY, Liu SF, Hung MY, Liao TN, Huang YL. Thrombospondin-1 mediates distal tubule hypertrophy induced by glycated albumin. Biochem J. 2004;379(Pt 1):89–97. doi: 10.1042/BJ20031730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu S, Shi L, Wang S. Overexpression of upstream stimulatory factor 2 accelerates diabetic kidney injury. Am J Physiol Renal Physiol. 2007;293(5):F1727–35. doi: 10.1152/ajprenal.00316.2007. [DOI] [PubMed] [Google Scholar]
- 64.Maric-Bilkan C. Obesity and diabetic kidney disease. Med Clin North Am. 2013;97(1):59–74. doi: 10.1016/j.mcna.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mascali A, Franzese O, Nistico S, Campia U, Lauro D, Cardillo C, Di Daniele N, Tesauro M. Obesity and kidney disease: Beyond the hyperfiltration. International journal of immunopathology and pharmacology. 2016 doi: 10.1177/0394632016643550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bayliss G, Weinrauch LA, D’Elia JA. Pathophysiology of obesity-related renal dysfunction contributes to diabetic nephropathy. Curr Diab Rep. 2012;12(4):440–6. doi: 10.1007/s11892-012-0288-1. [DOI] [PubMed] [Google Scholar]
- 67.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci U S A. 2000;97(14):8015–20. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Brien SP, Smith M, Ling H, Phillips L, Weber W, Lydon J, Maloney C, Ledbetter S, Arbeeny C, Wawersik S. Glomerulopathy in the KK.Cg-A(y)/J mouse reflects the pathology of diabetic nephropathy. J Diabetes Res. 2013;2013:498925. doi: 10.1155/2013/498925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pfeiffer A, Middelberg-Bisping K, Drewes C, Schatz H. Elevated plasma levels of transforming growth factor-beta 1 in NIDDM. Diabetes Care. 1996;19(10):1113–7. doi: 10.2337/diacare.19.10.1113. [DOI] [PubMed] [Google Scholar]
- 70.Chen S, Iglesias-de la Cruz MC, Jim B, Hong SW, Isono M, Ziyadeh FN. Reversibility of established diabetic glomerulopathy by anti-TGF-beta antibodies in db/db mice. Biochem Biophys Res Commun. 2003;300(1):16–22. doi: 10.1016/s0006-291x(02)02708-0. [DOI] [PubMed] [Google Scholar]
- 71.Cui W, Maimaitiyiming H, Qi X, Norman H, Wang S. Thrombospondin 1 mediates renal dysfunction in a mouse model of high-fat diet-induced obesity. Am J Physiol Renal Physiol. 2013;305(6):F871–80. doi: 10.1152/ajprenal.00209.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner AE, Finkel T, Rane SG. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14(1):67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Tong X, Rumala C, Clemons K, Wang S. Thrombospondin1 deficiency reduces obesity-associated inflammation and improves insulin sensitivity in a diet-induced obese mouse model. PLoS One. 2011;6(10):e26656. doi: 10.1371/journal.pone.0026656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maimaitiyiming H, Clemons K, Zhou Q, Norman H, Wang S. Thrombospondin1 deficiency attenuates obesity-associated microvascular complications in ApoE−/− mice. PLoS One. 2015;10(3):e0121403. doi: 10.1371/journal.pone.0121403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cui W, Maimaitiyiming H, Zhou Q, Norman H, Zhou C, Wang S. Interaction of thrombospondin1 and CD36 contributes to obesity-associated podocytopathy. Biochim Biophys Acta. 2015;1852(7):1323–33. doi: 10.1016/j.bbadis.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang A, Ziyadeh FN, Lee EY, Pyagay PE, Sung SH, Sheardown SA, Laping NJ, Chen S. Interference with TGF-beta signaling by Smad3-knockout in mice limits diabetic glomerulosclerosis without affecting albuminuria. Am J Physiol Renal Physiol. 2007;293(5):F1657–65. doi: 10.1152/ajprenal.00274.2007. [DOI] [PubMed] [Google Scholar]
- 77.Fan Y, Li X, Xiao W, Fu J, Harris RC, Lindenmeyer M, Cohen CD, Guillot N, Baron MH, Wang N, Lee K, He JC, Schlondorff D, Chuang PY. BAMBI elimination enhances alternative TGF-beta signaling and glomerular dysfunction in diabetic mice. Diabetes. 2015;64(6):2220–33. doi: 10.2337/db14-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson SA, Spurney RF. Twenty years after ACEIs and ARBs: emerging treatment strategies for diabetic nephropathy. Am J Physiol Renal Physiol. 2015;309(10):F807–20. doi: 10.1152/ajprenal.00266.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun H, Zhao Y, Bi X, Li S, Su G, Miao Y, Ma X, Zhang Y, Zhang W, Zhong M. Valsartan blocks thrombospondin/transforming growth factor/Smads to inhibit aortic remodeling in diabetic rats. Diagn Pathol. 2015;10:18. doi: 10.1186/s13000-015-0246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sugaru E, Nakagawa T, Ono-Kishino M, Nagamine J, Tokunaga T, Kitoh M, Hume WE, Nagata R, Taiji M. Enhanced effect of combined treatment with SMP-534 (antifibrotic agent) and losartan in diabetic nephropathy. Am J Nephrol. 2006;26(1):50–8. doi: 10.1159/000091786. [DOI] [PubMed] [Google Scholar]
- 81.Sugaru E, Nakagawa T, Ono-Kishino M, Nagamine J, Tokunaga T, Kitoh M, Hume WE, Nagata R, Taiji M. Amelioration of established diabetic nephropathy by combined treatment with SMP-534 (antifibrotic agent) and losartan in db/db mice. Nephron Exp Nephrol. 2007;105(2):e45–52. doi: 10.1159/000097603. [DOI] [PubMed] [Google Scholar]
- 82.Benigni A, Zoja C, Corna D, Zatelli C, Conti S, Campana M, Gagliardini E, Rottoli D, Zanchi C, Abbate M, Ledbetter S, Remuzzi G. Add-on anti-TGF-beta antibody to ACE inhibitor arrests progressive diabetic nephropathy in the rat. J Am Soc Nephrol. 2003;14(7):1816–24. doi: 10.1097/01.asn.0000074238.61967.b7. [DOI] [PubMed] [Google Scholar]
- 83.Lakhe-Reddy S, Li V, Arnold TD, Khan S, Schelling JR. Mesangial cell alphavbeta8-integrin regulates glomerular capillary integrity and repair. Am J Physiol Renal Physiol. 2014;306(12):F1400–9. doi: 10.1152/ajprenal.00624.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jin DK, Fish AJ, Wayner EA, Mauer M, Setty S, Tsilibary E, Kim Y. Distribution of integrin subunits in human diabetic kidneys. J Am Soc Nephrol. 1996;7(12):2636–45. doi: 10.1681/ASN.V7122636. [DOI] [PubMed] [Google Scholar]
- 85.Hafdi Z, Lesavre P, Nejjari M, Halbwachs-Mecarelli L, Droz D, Noel LH. Distribution of alphavbeta3, alphavbeta5 integrins and the integrin associated protein--IAP (CD47) in human glomerular diseases. Cell Adhes Commun. 2000;7(6):441–51. doi: 10.3109/15419060009040302. [DOI] [PubMed] [Google Scholar]
- 86.Maile LA, Gollahon K, Wai C, Dunbar P, Busby W, Clemmons D. Blocking alphaVbeta3 integrin ligand occupancy inhibits the progression of albuminuria in diabetic rats. J Diabetes Res. 2014;2014:421827. doi: 10.1155/2014/421827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lacava V, Pellicano V, Ferrajolo C, Cernaro V, Visconti L, Conti G, Buemi M, Santoro D. Novel avenues for treating diabetic nephropathy: new investigational drugs. Expert opinion on investigational drugs. 2017;26(4):445–462. doi: 10.1080/13543784.2017.1293039. [DOI] [PubMed] [Google Scholar]
- 88.Weller M, Nabors LB, Gorlia T, Leske H, Rushing E, Bady P, Hicking C, Perry J, Hong YK, Roth P, Wick W, Goodman SL, Hegi ME, Picard M, Moch H, Straub J, Stupp R. Cilengitide in newly diagnosed glioblastoma: biomarker expression and outcome. Oncotarget. 2016;7(12):15018–32. doi: 10.18632/oncotarget.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang Y, Lau WL, Jo H, Tsujino K, Gewin L, Reed NI, Atakilit A, Nunes AC, DeGrado WF, Sheppard D. Pharmacologic Blockade of alphavbeta1 Integrin Ameliorates Renal Failure and Fibrosis In Vivo. J Am Soc Nephrol. 2017 doi: 10.1681/ASN.2015050585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reed NI, Tang YZ, McIntosh J, Wu Y, Molnar KS, Civitavecchia A, Sheppard D, DeGrado WF, Jo H. Exploring N-Arylsulfonyl-l-proline Scaffold as a Platform for Potent and Selective alphavbeta1 Integrin Inhibitors. ACS Med Chem Lett. 2016;7(10):902–907. doi: 10.1021/acsmedchemlett.6b00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weiskirchen R, Tacke F. Liver Fibrosis: From Pathogenesis to Novel Therapies. Dig Dis. 2016;34(4):410–22. doi: 10.1159/000444556. [DOI] [PubMed] [Google Scholar]
- 92.Katz LH, Likhter M, Jogunoori W, Belkin M, Ohshiro K, Mishra L. TGF-beta signaling in liver and gastrointestinal cancers. Cancer letters. 2016;379(2):166–72. doi: 10.1016/j.canlet.2016.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yoshida K, Murata M, Yamaguchi T, Matsuzaki K. TGF-beta/Smad signaling during hepatic fibro-carcinogenesis (review) Int J Oncol. 2014;45(4):1363–71. doi: 10.3892/ijo.2014.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fabregat I, Moreno-Caceres J, Sanchez A, Dooley S, Dewidar B, Giannelli G, Ten Dijke P I.-L. Consortium. TGF-beta signalling and liver disease. The FEBS journal. 2016;283(12):2219–32. doi: 10.1111/febs.13665. [DOI] [PubMed] [Google Scholar]
- 95.Xu F, Liu C, Zhou D, Zhang L. TGF-beta/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J Histochem Cytochem. 2016;64(3):157–67. doi: 10.1369/0022155415627681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smalling RL, Delker DA, Zhang Y, Nieto N, McGuiness MS, Liu S, Friedman SL, Hagedorn CH, Wang L. Genome-wide transcriptome analysis identifies novel gene signatures implicated in human chronic liver disease. Am J Physiol Gastrointest Liver Physiol. 2013;305(5):G364–74. doi: 10.1152/ajpgi.00077.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Myung SJ, Yoon JH, Gwak GY, Kim W, Yang JI, Lee SH, Jang JJ, Lee HS. Bile acid-mediated thrombospondin-1 induction in hepatocytes leads to transforming growth factor-beta-dependent hepatic stellate cell activation. Biochem Biophys Res Commun. 2007;353(4):1091–6. doi: 10.1016/j.bbrc.2006.12.157. [DOI] [PubMed] [Google Scholar]
- 98.El-Youssef M, Mu Y, Huang L, Stellmach V, Crawford SE. Increased expression of transforming growth factor-beta1 and thrombospondin-1 in congenital hepatic fibrosis: possible role of the hepatic stellate cell. Journal of pediatric gastroenterology and nutrition. 1999;28(4):386–92. doi: 10.1097/00005176-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 99.Hayashi H, Sakai K, Baba H, Sakai T. Thrombospondin-1 is a novel negative regulator of liver regeneration after partial hepatectomy through transforming growth factor-beta1 activation in mice. Hepatology. 2012;55(5):1562–73. doi: 10.1002/hep.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hayashi H, Kuroki H, Higashi T, Takeyama H, Yokoyama N, Okabe H, Nitta H, Beppu T, Takamori H, Baba H. Thrombospondin-1 expression may be implicated in liver atrophic mechanism due to obstructed portal venous flow. Hepatol Res. 2016 doi: 10.1111/hepr.12792. [DOI] [PubMed] [Google Scholar]
- 101.Li Y, Turpin CP, Wang S. Role of thrombospondin 1 in liver diseases. Hepatol Res. 2016 doi: 10.1111/hepr.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kondou H, Mushiake S, Etani Y, Miyoshi Y, Michigami T, Ozono K. A blocking peptide for transforming growth factor-beta1 activation prevents hepatic fibrosis in vivo. J Hepatol. 2003;39(5):742–8. doi: 10.1016/s0168-8278(03)00377-5. [DOI] [PubMed] [Google Scholar]
- 103.Breitkopf K, Sawitza I, Westhoff JH, Wickert L, Dooley S, Gressner AM. Thrombospondin 1 acts as a strong promoter of transforming growth factor beta effects via two distinct mechanisms in hepatic stellate cells. Gut. 2005;54(5):673–81. doi: 10.1136/gut.2004.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Narmada BC, Chia SM, Tucker-Kellogg L, Yu H. HGF regulates the activation of TGF-beta1 in rat hepatocytes and hepatic stellate cells. J Cell Physiol. 2013;228(2):393–401. doi: 10.1002/jcp.24143. [DOI] [PubMed] [Google Scholar]
- 105.Benzoubir N, Lejamtel C, Battaglia S, Testoni B, Benassi B, Gondeau C, Perrin-Cocon L, Desterke C, Thiers V, Samuel D, Levrero M, Brechot C, Bourgeade MF. HCV core-mediated activation of latent TGF-beta via thrombospondin drives the crosstalk between hepatocytes and stromal environment. J Hepatol. 2013;59(6):1160–8. doi: 10.1016/j.jhep.2013.07.036. [DOI] [PubMed] [Google Scholar]
- 106.Kuroki H, Hayashi H, Nakagawa S, Sakamoto K, Higashi T, Nitta H, Hashimoto D, Chikamoto A, Beppu T, Baba H. Effect of LSKL peptide on thrombospondin 1-mediated transforming growth factor beta signal activation and liver regeneration after hepatectomy in an experimental model. Br J Surg. 2015;102(7):813–25. doi: 10.1002/bjs.9765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dropmann A, Dediulia T, Breitkopf-Heinlein K, Korhonen H, Janicot M, Weber SN, Thomas M, Piiper A, Bertran E, Fabregat I, Abshagen K, Hess J, Angel P, Coulouarn C, Dooley S, Meindl-Beinker NM. TGF-beta1 and TGF-beta2 abundance in liver diseases of mice and men. Oncotarget. 2016;7(15):19499–518. doi: 10.18632/oncotarget.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Urashima M, Ogata A, Chauhan D, Hatziyanni M, Vidriales MB, Dedera DA, Schlossman RL, Anderson KC. Transforming growth factor-beta1: differential effects on multiple myeloma versus normal B cells. Blood. 1996;87(5):1928–38. [PubMed] [Google Scholar]
- 109.Takeuchi K, Abe M, Hiasa M, Oda A, Amou H, Kido S, Harada T, Tanaka O, Miki H, Nakamura S, Nakano A, Kagawa K, Yata K, Ozaki S, Matsumoto T. TGF-Beta inhibition restores terminal osteoblast differentiation to suppress myeloma growth. PLoS One. 2010;5(3):e9870. doi: 10.1371/journal.pone.0009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hayashi T, Hideshima T, Nguyen AN, Munoz O, Podar K, Hamasaki M, Ishitsuka K, Yasui H, Richardson P, Chakravarty S, Murphy A, Chauhan D, Higgins LS, Anderson KC. Transforming growth factor beta receptor I kinase inhibitor down-regulates cytokine secretion and multiple myeloma cell growth in the bone marrow microenvironment. Clin Cancer Res. 2004;10(22):7540–6. doi: 10.1158/1078-0432.CCR-04-0632. [DOI] [PubMed] [Google Scholar]
- 111.Matsumoto T, Abe M. TGF-beta-related mechanisms of bone destruction in multiple myeloma. Bone. 2011;48(1):129–34. doi: 10.1016/j.bone.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 112.Pour L, Svachova H, Adam Z, Almasi M, Buresova L, Buchler T, Kovarova L, Nemec P, Penka M, Vorlicek J, Hajek R. Levels of angiogenic factors in patients with multiple myeloma correlate with treatment response. Ann Hematol. 2010;89(4):385–9. doi: 10.1007/s00277-009-0834-3. [DOI] [PubMed] [Google Scholar]
- 113.Rendtlew Danielsen JM, Knudsen LM, Dahl IM, Lodahl M, Rasmussen T. Dysregulation of CD47 and the ligands thrombospondin 1 and 2 in multiple myeloma. Br J Haematol. 2007;138(6):756–60. doi: 10.1111/j.1365-2141.2007.06729.x. [DOI] [PubMed] [Google Scholar]
- 114.Lu A, Pallero MA, Lei W, Hong H, Yang Y, Suto MJ, Murphy-Ullrich JE. Inhibition of Transforming Growth Factor-beta Activation Diminishes Tumor Progression and Osteolytic Bone Disease in Mouse Models of Multiple Myeloma. Am J Pathol. 2016;186(3):678–90. doi: 10.1016/j.ajpath.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bailey Dubose K, Zayzafoon M, Murphy-Ullrich JE. Thrombospondin-1 inhibits osteogenic differentiation of human mesenchymal stem cells through latent TGF-beta activation. Biochem Biophys Res Commun. 2012;422(3):488–93. doi: 10.1016/j.bbrc.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, Sanders L, Barthel L, Meadows C, Fox D, Irwin D, Li M, McKeon BA, Riddle S, Dale Brown R, Morgan LE, Evans CM, Hernandez-Saavedra D, Bandeira A, Maloney JP, Bull TM, Janssen WJ, Stenmark KR, Tuder RM, Graham BB. TGF-beta activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun. 2017;8:15494. doi: 10.1038/ncomms15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim CW, Pokutta-Paskaleva A, Kumar S, Timmins LH, Morris AD, Kang DW, Dalal S, Chadid T, Kuo KM, Raykin J, Li H, Yanagisawa H, Gleason RL, Jr, Jo H, Brewster LP. Disturbed Flow Promotes Arterial Stiffening Through Thrombospondin-1. Circulation. 2017;136(13):1217–1232. doi: 10.1161/CIRCULATIONAHA.116.026361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chang C, Zhao Q, Gonzalez JP, Kim JH, Alzahrani K, Del Re D, Fraidenraich D. Hematopoietic Id Deletion Triggers Endomyocardial Fibrotic and Vascular Defects in the Adult Heart. Sci Rep. 2017;7(1):3079. doi: 10.1038/s41598-017-03160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. The single-molecule mechanics of the latent TGF-beta1 complex. Curr Biol. 2011;21(24):2046–54. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 120.Warburton D, Kaartinen V. When the lung is stretched, could it be thrombospondin via TGFbeta1 peptide activation? J Physiol. 2007;584(Pt 2):365. doi: 10.1113/jphysiol.2007.144394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahamed J, Janczak CA, Wittkowski KM, Coller BS. In vitro and in vivo evidence that thrombospondin-1 (TSP-1) contributes to stirring- and shear-dependent activation of platelet-derived TGF-beta1. PLoS One. 2009;4(8):e6608. doi: 10.1371/journal.pone.0006608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Macko RF, Gelber AC, Young BA, Lowitt MH, White B, Wigley FM, Goldblum SE. Increased circulating concentrations of the counteradhesive proteins SPARC and thrombospondin-1 in systemic sclerosis (scleroderma). Relationship to platelet and endothelial cell activation. J Rheumatol. 2002;29(12):2565–70. [PubMed] [Google Scholar]
- 123.Avouac J, Clemessy M, Distler JH, Gasc JM, Ruiz B, Vacher-Lavenu MC, Wipff J, Kahan A, Boileau C, Corvol P, Allanore Y. Enhanced expression of ephrins and thrombospondins in the dermis of patients with early diffuse systemic sclerosis: potential contribution to perturbed angiogenesis and fibrosis. Rheumatology (Oxford) 2011;50(8):1494–504. doi: 10.1093/rheumatology/keq448. [DOI] [PubMed] [Google Scholar]
- 124.Distler JH, Jungel A, Pileckyte M, Zwerina J, Michel BA, Gay RE, Kowal-Bielecka O, Matucci-Cerinic M, Schett G, Marti HH, Gay S, Distler O. Hypoxia-induced increase in the production of extracellular matrix proteins in systemic sclerosis. Arthritis Rheum. 2007;56(12):4203–15. doi: 10.1002/art.23074. [DOI] [PubMed] [Google Scholar]
- 125.Farina G, Lafyatis D, Lemaire R, Lafyatis R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 2010;62(2):580–8. doi: 10.1002/art.27220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen Y, Leask A, Abraham DJ, Kennedy L, Shi-Wen X, Denton CP, Black CM, Verjee LS, Eastwood M. Thrombospondin 1 is a key mediator of transforming growth factor beta-mediated cell contractility in systemic sclerosis via a mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK)-dependent mechanism. Fibrogenesis Tissue Repair. 2011;4(1):9. doi: 10.1186/1755-1536-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yamashita T, Asano Y, Taniguchi T, Nakamura K, Saigusa R, Miura S, Toyama T, Takahashi T, Ichimura Y, Yoshizaki A, Trojanowska M, Sato S. Glycyrrhizin Ameliorates Fibrosis, Vasculopathy, and Inflammation in Animal Models of Systemic Sclerosis. The Journal of investigative dermatology. 2017;137(3):631–640. doi: 10.1016/j.jid.2016.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grytz R, Girkin CA, Libertiaux V, Downs JC. Perspectives on biomechanical growth and remodeling mechanisms in glaucoma() Mechanics research communications. 2012;42:92–106. doi: 10.1016/j.mechrescom.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roberts MD, Sigal IA, Liang Y, Burgoyne CF, Downs JC. Changes in the biomechanical response of the optic nerve head in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51(11):5675–84. doi: 10.1167/iovs.10-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–77. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 131.Murphy-Ullrich JE, Downs JC. The Thrombospondin1-TGF-beta Pathway and Glaucoma. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2015;31(7):371–5. doi: 10.1089/jop.2015.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wallace DM, Murphy-Ullrich JE, Downs JC, O’Brien CJ. The role of matricellular proteins in glaucoma. Matrix Biol. 2014;37:174–82. doi: 10.1016/j.matbio.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 133.Kirwan RP, Wordinger RJ, Clark AF, O’Brien CJ. Differential global and extra-cellular matrix focused gene expression patterns between normal and glaucomatous human lamina cribrosa cells. Molecular vision. 2009;15:76–88. [PMC free article] [PubMed] [Google Scholar]
- 134.Kirwan RP, Fenerty CH, Crean J, Wordinger RJ, Clark AF, O’Brien CJ. Influence of cyclical mechanical strain on extracellular matrix gene expression in human lamina cribrosa cells in vitro. Molecular vision. 2005;11:798–810. [PubMed] [Google Scholar]
- 135.Flugel-Koch C, Ohlmann A, Fuchshofer R, Welge-Lussen U, Tamm ER. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Experimental eye research. 2004;79(5):649–63. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 136.Fuchshofer R, Tamm ER. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell and tissue research. 2012;347(1):279–90. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- 137.Prendes MA, Harris A, Wirostko BM, Gerber AL, Siesky B. The role of transforming growth factor beta in glaucoma and the therapeutic implications. The British journal of ophthalmology. 2013;97(6):680–6. doi: 10.1136/bjophthalmol-2011-301132. [DOI] [PubMed] [Google Scholar]
- 138.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nature reviews. Immunology. 2010;10(8):554–67. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31(6):220–7. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Esebanmen GE, Langridge WHR. The role of TGF-beta signaling in dendritic cell tolerance. Immunol Res. 2017 doi: 10.1007/s12026-017-8944-9. [DOI] [PubMed] [Google Scholar]
- 141.Chen X, Yang Y, Zhou Q, Weiss JM, Howard OZ, McPherson JM, Wakefield LM, Oppenheim JJ. Effective chemoimmunotherapy with anti-TGFbeta antibody and cyclophosphamide in a mouse model of breast cancer. PLoS One. 2014;9(1):e85398. doi: 10.1371/journal.pone.0085398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ruscetti FW, Bartelmez SH. Transforming growth factor beta, pleiotropic regulator of hematopoietic stem cells: potential physiological and clinical relevance. Int J Hematol. 2001;74(1):18–25. doi: 10.1007/BF02982545. [DOI] [PubMed] [Google Scholar]
- 143.Ruscetti FW, Akel S, Bartelmez SH. Autocrine transforming growth factor-beta regulation of hematopoiesis: many outcomes that depend on the context. Oncogene. 2005;24(37):5751–63. doi: 10.1038/sj.onc.1208921. [DOI] [PubMed] [Google Scholar]
- 144.Fenton TM, Kelly A, Shuttleworth EE, Smedley C, Atakilit A, Powrie F, Campbell S, Nishimura SL, Sheppard D, Levison S, Worthington JJ, Lehtinen MJ, Travis MA. Inflammatory cues enhance TGFbeta activation by distinct subsets of human intestinal dendritic cells via integrin alphavbeta8. Mucosal Immunol. 2017;10(3):624–634. doi: 10.1038/mi.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Melton AC, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of alphavbeta8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest. 2010;120(12):4436–44. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449(7160):361–5. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Worthington JJ, Fenton TM, Czajkowska BI, Klementowicz JE, Travis MA. Regulation of TGFbeta in the immune system: an emerging role for integrins and dendritic cells. Immunobiology. 2012;217(12):1259–65. doi: 10.1016/j.imbio.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Worthington JJ, Kelly A, Smedley C, Bauche D, Campbell S, Marie JC, Travis MA. Integrin alphavbeta8-Mediated TGF-beta Activation by Effector Regulatory T Cells Is Essential for Suppression of T-Cell-Mediated Inflammation. Immunity. 2015;42(5):903–15. doi: 10.1016/j.immuni.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang K, Vega JL, Hadzipasic M, Schatzmann Peron JP, Zhu B, Carrier Y, Masli S, Rizzo LV, Weiner HL. Deficiency of thrombospondin-1 reduces Th17 differentiation and attenuates experimental autoimmune encephalomyelitis. Journal of autoimmunity. 2009;32(2):94–103. doi: 10.1016/j.jaut.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang G, Geng XR, Liu ZQ, Liu JQ, Liu XY, Xu LZ, Zhang HP, Sun YX, Liu ZG, Yang PC. Thrombospondin-1 (TSP1)-producing B cells restore antigen (Ag)-specific immune tolerance in an allergic environment. J Biol Chem. 2015;290(20):12858–67. doi: 10.1074/jbc.M114.623421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pierson BA, Gupta K, Hu WS, Miller JS. Human natural killer cell expansion is regulated by thrombospondin-mediated activation of transforming growth factor-beta 1 and independent accessory cell-derived contact and soluble factors. Blood. 1996;87(1):180–9. [PubMed] [Google Scholar]
- 152.Takeyama H, Mizushima T, Uemura M, Haraguchi N, Nishimura J, Hata T, Matsuda C, Takemasa I, Ikenaga M, Murata K, Yamamoto H, Doki Y, Mori M. Adipose-Derived Stem Cells Ameliorate Experimental Murine Colitis via TSP-1-Dependent Activation of Latent TGF-beta. Dig Dis Sci. 2017;62(8):1963–1974. doi: 10.1007/s10620-017-4578-y. [DOI] [PubMed] [Google Scholar]
- 153.Willet JD, Pichitsiri W, Jenkinson SE, Brain JG, Wood K, Alhasan AA, Spielhofer J, Robertson H, Ali S, Kirby JA. Kidney transplantation: analysis of the expression and T cell-mediated activation of latent TGF-beta. J Leukoc Biol. 2013;93(4):471–8. doi: 10.1189/jlb.0712324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ma Y, Qu B, Xia X, Yang L, Kuang Y, Yang T, Cheng J, Sun H, Fan K, Gu J. Glioma-derived thrombospondin-1 modulates cd14+ cell tolerogenic properties. Cancer investigation. 2015;33(4):152–7. doi: 10.3109/07357907.2015.1010089. [DOI] [PubMed] [Google Scholar]
- 155.Futagami Y, Sugita S, Vega J, Ishida K, Takase H, Maruyama K, Aburatani H, Mochizuki M. Role of thrombospondin-1 in T cell response to ocular pigment epithelial cells. J Immunol. 2007;178(11):6994–7005. doi: 10.4049/jimmunol.178.11.6994. [DOI] [PubMed] [Google Scholar]
- 156.Mikula-Pietrasik J, Sosinska P, Janus J, Rubis B, Brewinska-Olchowik M, Piwocka K, Ksiazek K. Bystander senescence in human peritoneal mesothelium and fibroblasts is related to thrombospondin-1-dependent activation of transforming growth factor-beta1. Int J Biochem Cell Biol. 2013;45(9):2087–96. doi: 10.1016/j.biocel.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 157.Markowitz SD, Roberts AB. Tumor suppressor activity of the TGF-beta pathway in human cancers. Cytokine Growth Factor Rev. 1996;7(1):93–102. doi: 10.1016/1359-6101(96)00001-9. [DOI] [PubMed] [Google Scholar]
- 158.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–61. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 159.Connolly EC, Saunier EF, Quigley D, Luu MT, De Sapio A, Hann B, Yingling JM, Akhurst RJ. Outgrowth of drug-resistant carcinomas expressing markers of tumor aggression after long-term TbetaRI/II kinase inhibition with LY2109761. Cancer Res. 2011;71(6):2339–49. doi: 10.1158/0008-5472.CAN-10-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Denton CP, Merkel PA, Furst DE, Khanna D, Emery P, Hsu VM, Silliman N, Streisand J, Powell J, Akesson A, Coppock J, Hoogen F, Herrick A, Mayes MD, Veale D, Haas J, Ledbetter S, Korn JH, Black CM, Seibold JR G. Cat-192 Study, C. Scleroderma Clinical Trials. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007;56(1):323–33. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 161.Voelker J, Berg PH, Sheetz M, Duffin K, Shen T, Moser B, Greene T, Blumenthal SS, Rychlik I, Yagil Y, Zaoui P, Lewis JB. Anti-TGF-beta1 Antibody Therapy in Patients with Diabetic Nephropathy. J Am Soc Nephrol. 2017;28(3):953–962. doi: 10.1681/ASN.2015111230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Connolly EC, Freimuth J, Akhurst RJ. Complexities of TGF-beta targeted cancer therapy. Int J Biol Sci. 2012;8(7):964–78. doi: 10.7150/ijbs.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shibahara K, Ota M, Horiguchi M, Yoshinaga K, Melamed J, Rifkin DB. Production of gastrointestinal tumors in mice by modulating latent TGF-beta1 activation. Cancer Res. 2013;73(1):459–68. doi: 10.1158/0008-5472.CAN-12-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zeisberg M, Tampe B, LeBleu V, Tampe D, Zeisberg EM, Kalluri R. Thrombospondin-1 deficiency causes a shift from fibroproliferative to inflammatory kidney disease and delays onset of renal failure. Am J Pathol. 2014;184(10):2687–98. doi: 10.1016/j.ajpath.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Krishna SM, Seto SW, Jose RJ, Biros E, Moran CS, Wang Y, Clancy P, Golledge J. A peptide antagonist of thrombospondin-1 promotes abdominal aortic aneurysm progression in the angiotensin II-infused apolipoprotein-E-deficient mouse. Arterioscler Thromb Vasc Biol. 2015;35(2):389–98. doi: 10.1161/ATVBAHA.114.304732. [DOI] [PubMed] [Google Scholar]
- 166.Tsujino K, Reed NI, Atakilit A, Ren X, Sheppard D. Transforming growth factor-beta plays divergent roles in modulating vascular remodeling, inflammation, and pulmonary fibrosis in a murine model of scleroderma. Am J Physiol Lung Cell Mol Physiol. 2017;312(1):L22–L31. doi: 10.1152/ajplung.00428.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma LJ, Jha S, Ling H, Pozzi A, Ledbetter S, Fogo AB. Divergent effects of low versus high dose anti-TGF-beta antibody in puromycin aminonucleoside nephropathy in rats. Kidney Int. 2004;65(1):106–15. doi: 10.1111/j.1523-1755.2004.00381.x. [DOI] [PubMed] [Google Scholar]
- 168.Khanna AK, Plummer MS, Hilton G, Pieper GM, Ledbetter S. Anti-transforming growth factor antibody at low but not high doses limits cyclosporine-mediated nephrotoxicity without altering rat cardiac allograft survival: potential of therapeutic applications. Circulation. 2004;110(25):3822–9. doi: 10.1161/01.CIR.0000150400.15354.7D. [DOI] [PubMed] [Google Scholar]
- 169.Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H, Sheppard D, Broaddus VC, Nishimura SL. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157(3):493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yehualaeshet T, O’Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol. 1999;155(3):841–51. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Harpel JG, Schultz-Cherry S, Murphy-Ullrich JE, Rifkin DB. Tamoxifen and estrogen effects on TGF-beta formation: role of thrombospondin-1, alphavbeta3, and integrin-associated protein. Biochem Biophys Res Commun. 2001;284(1):11–4. doi: 10.1006/bbrc.2001.4922. [DOI] [PubMed] [Google Scholar]
- 172.Soto-Pantoja DR, Shih HB, Maxhimer JB, Cook KL, Ghosh A, Isenberg JS, Roberts DD. Thrombospondin-1 and CD47 signaling regulate healing of thermal injury in mice. Matrix Biol. 2014;37:25–34. doi: 10.1016/j.matbio.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ludlow A, Yee KO, Lipman R, Bronson R, Weinreb P, Huang X, Sheppard D, Lawler J. Characterization of integrin beta6 and thrombospondin-1 double-null mice. J Cell Mol Med. 2005;9(2):421–37. doi: 10.1111/j.1582-4934.2005.tb00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xie XS, Li FY, Liu HC, Deng Y, Li Z, Fan JM. LSKL, a peptide antagonist of thrombospondin-1, attenuates renal interstitial fibrosis in rats with unilateral ureteral obstruction. Arch Pharm Res. 2010;33(2):275–84. doi: 10.1007/s12272-010-0213-6. [DOI] [PubMed] [Google Scholar]
- 175.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(−/−) mice. Am J Pathol. 2003;163(4):1261–73. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, Pellicoro A, Raschperger E, Betsholtz C, Ruminski PG, Griggs DW, Prinsen MJ, Maher JJ, Iredale JP, Lacy-Hulbert A, Adams RH, Sheppard D. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nature medicine. 2013;19(12):1617–24. doi: 10.1038/nm.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yehualaeshet T, O’Connor R, Begleiter A, Murphy-Ullrich JE, Silverstein R, Khalil N. A CD36 synthetic peptide inhibits bleomycin-induced pulmonary inflammation and connective tissue synthesis in the rat. Am J Respir Cell Mol Biol. 2000;23(2):204–12. doi: 10.1165/ajrcmb.23.2.4089. [DOI] [PubMed] [Google Scholar]
- 178.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130(3):503–6. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Adams JC, Lawler J. The thrombospondins. Cold Spring Harbor perspectives in biology. 2011;3(10):a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin-1 is a major activator of TGF-beta in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65(2):459–68. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 181.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T, Steinman L. Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest. 2010;120(8):2782–94. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liao F, Li G, Yuan W, Chen Y, Zuo Y, Rashid K, Zhang JH, Feng H, Liu F. LSKL peptide alleviates subarachnoid fibrosis and hydrocephalus by inhibiting TSP1-mediated TGF-beta1 signaling activity following subarachnoid hemorrhage in rats. Exp Ther Med. 2016;12(4):2537–2543. doi: 10.3892/etm.2016.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.