Abstract
Crying is the principal means by which newborn infants shape parental behavior to meet their needs. While this mechanism can be highly effective, infant crying can also be an aversive stimulus that leads to parental frustration and even abuse. Fathers have recently become more involved in direct caregiving activities in modern, developed nations, and fathers are more likely than mothers to physically abuse infants. In this study, we attempt to explain variation in the neural response to infant crying among human fathers, with the hope of identifying factors that are associated with a more or less sensitive response. We imaged brain function in 39 first-time fathers of newborn infants as they listened to both their own and a standardized unknown infant cry stimulus, as well as auditory control stimuli, and evaluated whether these neural responses were correlated with measured characteristics of fathers and infants that were hypothesized to modulate these responses. Fathers also provided subjective ratings of each cry stimulus on multiple dimensions. Fathers showed widespread activation to both own and unknown infant cries in neural systems involved in empathy and approach motivation. There was no significant difference in the neural response to the own vs. unknown infant cry, and many fathers were unable to distinguish between the two cries. Comparison of these results with previous studies in mothers revealed a high degree of similarity between first-time fathers and first-time mothers in the pattern of neural activation to newborn infant cries. Further comparisons suggested that younger infant age was associated with stronger paternal neural responses, perhaps due to hormonal or novelty effects. In our sample, older fathers found infant cries less aversive and had an attenuated response to infant crying in both the dorsal anterior cingulate cortex (dACC) and the anterior insula, suggesting that compared with younger fathers, older fathers may be better able to avoid the distress associated with empathic over-arousal in response to infant cries. A principal components analysis revealed that fathers with more negative emotional reactions to the unknown infant cry showed decreased activation in the thalamus and caudate nucleus, regions expected to promote positive parental behaviors, as well as increased activation in the hypothalamus and dorsal ACC, again suggesting that empathic over-arousal might result in negative emotional reactions to infant crying. In sum, our findings suggest that infant age, paternal age and paternal emotional reactions to infant crying all modulate the neural response of fathers to infant crying. By identifying neural correlates of variation in paternal subjective reactions to infant crying, these findings help lay the groundwork for evaluating the effectiveness of interventions designed to increase paternal sensitivity and compassion.
Keywords: infant, father, cry, fMRI
1. Introduction
Crying is the principal means by which newborn infants shape parental behavior to meet their needs. While this mechanism can be highly effective, crying, particularly when prolonged and inconsolable, can also be an aversive stimulus that leads to parental frustration and even abuse (Barr 2012). On the other hand, sensitive responding to infant distress predicts secure infant attachment, which in turn predicts positive social and emotional development (DeWolff and van Ijzendoorn 1997; Fearon and Rosiman 2017). Thus, a significant challenge that parents face is to respond to this potentially aversive stimulus with sensitivity and compassion rather than frustration.
In this paper, we investigate paternal brain responses to infant crying. We focus specifically on fathers for three reasons. First, fathers have recently become more involved in direct caregiving activities in the U.S. and other modern, developed nations (Cabrera et al. 2000; Yeung et al. 2001). Second, research on fathers lags behind research on mothers even though paternal involvement is known to be associated with multiple positive developmental outcomes (Rosenbaum and Gettler This Issue; Sarkadi et al. 2008). Third, fathers are more likely than mothers to physically abuse infants and infant crying is a known trigger for infant abuse (Barr 2012). Thus, it may be more challenging for fathers to respond sensitively to infant crying. Indeed, fathers report finding infant crying more aversive than mothers do (Zeifman 2003). While earlier studies focused on the average neural response to infant crying among parents (Lorberbaum et al. 1999; Lorberbaum et al. 2002), in this study we attempt to explain variation in the neural response to infant crying among fathers with the hope of identifying factors that are associated with a more sensitive and compassionate response.
The neurobiology of parental behavior is understood in great detail in rats. Numan (2007) argues that adult rats have separate systems motivating approach and avoidance of offspring, and that parental behavior emerges when the former exceeds the latter. The medial preoptic area (MPOA) is a critical node that both activates the mesolimbic dopamine (approach) system and inhibits an avoidance circuit that runs from the medial amygdala to the periaqueductal gray of the midbrain (PAG). In both male and nulliparous female rats, several days of habituation to pups are required to suppress the avoidance system to the point where parental behavior is exhibited. However, pregnancy-related hormones like prolactin and estrogen augment MPOA function so that maternal behavior emerges at parturition. Oxytocin (OT) also facilitates parental behavior through actions at MPOA (Pedersen et al. 1994), but also acts at each node of the mesolimbic DA system (VTA and NA) to facilitate DA release in NA (Numan and Stolzenberg 2009). Is Numan’s model of maternal care relevant to paternal care? Studies in California mice support this possibility since MPOA lesions inhibit paternal behavior, and pup exposure increases Fos-like immunoreactivity in the MPOA in new fathers (de Jong et al. 2009). Furthermore, optogenetic stimulation of MPOA galanin neurons facilitates paternal behavior in mice (Wu et al. 2014).
For human mothers, the basic pattern of brain activity in response to infant crying was fi rst described by Lorberbaum and colleagues (Lorberbaum et al. 1999; Lorberbaum et al. 2002). In this initial study, mothers listened to cries of unknown infants. Relative to a white noise control, unknown infant cries activated circuits implicated in maternal care in non-human animals. In particular, activations were observed within components of the midbrain dopamine systems, such as the substantia nigra (SN) and ventral tegmental area (VTA), that rat studies implicate in approach-related motivation towards offspring (Numan 2007). However, activation was not found in other critical nodes of this system, such as the MPOA and regions of the ventral striatum most consistently implicated in reward and motivation (e.g., NAc). Lorberbaum et al also observed activation in both anterior and posterior cingulate cortex, as well as the midline thalamic nuclei, consistent with recruitment of the thalamocingulate system as outlined by MacLean (MacLean 1990). In addition to these, prominent activations were observed in the right fronto-insular cortex as well as the dorsomedial prefrontal cortex (DMPFC), regions involved with emotional and cognitive empathy, respectively (Singer and Lamm 2009). Thus, engagement of these two systems may facilitate maternal understanding of the mental and emotional state of a crying infant. Finally, right lateralized activation in auditory cortices extending to the temporal pole was also observed. In summary, this initial study revealed engagement of the following five systems in response to unknown infant cries: 1) midbrain dopaminergic (approach motivation), 2) thalamocingulate, 3) fronto-insular (emotional empathy), 4) DMPFC (perspective-taking and theory of mind), and 5) right lateralized auditory cortex extending to the temporal pole.
A few studies have extended this approach to investigate how these neural responses might relate to real-world parenting. For example, Laurent et al (2011) related the neural response of mothers to their infant cries to their physiological stress reactivity in the Strange Situation, in which mothers were separated from their infants with the ability to see and hear their distress on a remote video monitor. Mothers who more strongly activated the lateral orbitofrontal cortex (OFC) in response to their infant’s crying exhibited less reactive hypothalamo-pituitary-adrenal (HPA) axis responses to the Strange Situation. The authors suggest that mothers who maintain prefrontal control of HPA reactivity will not be overcome by stress and will make better parenting decisions. In another study focusing specifically on high-risk mothers, stronger anterior insula responses to own-infant cries were related to more intrusive parenting (Musser et al. 2012). Given the anterior insula’s role in emotional empathy (Lockwood, 2016; Singer and Lamm, 2009), this finding might reflect empathic over-arousal in the mothers. Although empathy is essential for parental care, empathic over-arousal can lead to distress that interferes with compassionate behavior and effective parenting. The anterior insula is a visceral somatosensory cortex that is known to track autonomic arousal (Critchley et al. 2000; Critchley et al. 2004). Child-abusing parents have a more pronounced sympathetic nervous system response to infant crying compared with non-abusive parents (Frodi and Lamb 1980; Joosen et al. 2013), and might therefore be expected to have a stronger anterior insula response to infant crying. Fathers also show robust anterior insula activation in response to infant cries, and fathers with moderate anterior insula activation are the most involved in instrumental caregiving. Fathers with low and high insula activation may be less involved due to empathic under- and overarousal to cries (Mascaro et al. 2014).
The paternal neural response to infant crying is likely to be modulated by characteristics of the father. For example, paternal experience might be an important modulator if fathers with more accumulated exposure to infant crying learn to more effectively regulate their negative emotional reaction to it. Second, similar to post-partum depression in mothers (Campbell et al. 1992; Forman et al. 2007; Reissland et al. 2003), depression in fathers is expected to decrease sensitivity to infant cues. Indeed, depressed fathers exhibit decreased positive and increased negative parenting behaviors (Wilson and Durbin 2010). Sleep quality is another potentially important variable. Parents of newborn infants sometimes struggle with sleep deprivation, which is known to impair emotion regulation abilities (Beattie et al. 2015). Personality is also expected to play a role, as parents who score high on neuroticism find infant cries more aversive than do their peers with lower scores on neuroticism (Zeifman 2003). Infanticide and Abusive Head trauma (AHT; also known as Shaken Baby Syndrome (SBS))(Christian et al. 2009), which is most often triggered by infant crying, are associated with younger parental age, lower SES, the presence of recent stressful life events and a childhood history of parental abuse (Barr 2012). These variables might therefore also modulate the neural response to infant crying. Finally, hormone levels could also have an impact, since men with higher testosterone levels report less sympathy for infant crying (Fleming et al. 2002), and both testosterone and oxytocin administration have been shown to modulate the neural response to infant crying in women (Bos et al. 2010; Riem et al. 2011).
We hypothesized that greater depressive symptomology, lower quality sleep, higher levels of neuroticism, younger paternal age, lower SES, more recent stressful life events, a childhood history of parental abuse, and higher levels of testosterone would be associated with more negative and less positive emotional responses to infant cry stimuli, and accompanying weaker activation in components of the midbrain dopamine (VTA, SN, striatum) and thalamocingulate systems that are associated with positive parenting behaviors, along with stronger activation in the anterior insula that tracks sympathetic hyperarousal associated with child abuse.
In addition to characteristics of the father, characteristics of the infant are also likely to influence paternal neural responses to infant crying. Fathers with particularly fussy infants who cry often and do not sleep well may find their infant’s cry more aversive. Particular characteristics of the cry itself are also known to influence perceived aversiveness. For example, cries with higher fundamental frequencies are perceived as more aversive (Bisping et al. 1990; Gustafson and Green 1989). Other cry characteristics, such as roughness, a quality that contributes to the aversiveness of human screams and artificial alarm signals, might also play a role (Arnal et al. 2015). Roughness refers to amplitude modulations ranging from 30 to 150 Hz that typically induce unpleasant auditory percepts. The sex of the crying infant might also be an important factor. Male infants suffer higher rates of abuse than do female infants (Barr 2012), raising the possibility that some acoustic property of male cries is more aversive (although other explanations are possible). The peak incidence of Shaken Baby Syndrome closely coincides with a postnatal peak in infant male testosterone levels (Barr et al. 2006; Kuiri-Hanninen et al. 2014). Although speculative, it is possible that testosterone influences the acoustic properties of infant cries, rendering them more aversive.
We hypothesized that fathers of infants who were male, had higher testosterone levels, had difficult temperaments, and had cries high in roughness would have more negative and less positive emotional responses to those cries and accompanying weaker activation in components of the midbrain dopamine (VTA, SN, striatum) and thalamocingulate systems, along with stronger activation in the anterior insula.
The goal of this paper was to evaluate the relationship between these paternal and infant characteristics and paternal neural responses to infant crying. Furthermore, we sought to relate paternal emotional responses to the cries with neural responses. To do so, we imaged brain function in 39 first-time fathers of newborn infants as they listened to infant cry stimuli, as well as auditory control stimuli, and we evaluated whether these neural responses were correlated with measured characteristics of fathers and infants as well as paternal subjective cry ratings.
Given our focus on paternal responses to crying, we were most interested in how fathers responded to their own infant’s cries. However, each infant’s cry will necessarily differ from the others, and these idiosyncrasies will affect paternal neural responses. This will introduce noise when attempting to detect correlations between paternal characteristics and neural responses. Therefore, we also included a standardized unknown infant cry that is the same for all fathers. Despite being less directly relevant to the father, the unknown infant cry may reveal correlations that are not detectable with the own infant cries.
2. Methods
2.1 Subjects
Forty-two first-time, biological fathers of newborn infants (<4 months of age; mean=67.5 days, sd=23.0 days, n=41, 1 missing data) were recruited by posting flyers around the Emory University campus, at local parks and businesses and at daycare centers. Participants were required to be currently cohabitating with their infant and their romantic partner. Enrollment in the study required participation by the father and his infant. All enrolled fathers had female partners. The study was approved by the Emory Institutional Review Board, and all participants gave written informed consent (a parent signed on behalf of the infant). Fathers were screened and excluded for self-reported history of head trauma, seizures, or other neurological disorders, psychiatric illness, alcoholism, or any other substance abuse, serious medical illness, claustrophobia, and for ferrous metal in any part of the body. One recruited father did not meet inclusion criteria and two more had excessive motion during the MRI scan, leaving us with a final sample size for analysis of n=39. Fathers were between the age of 23 and 40 years (mean = 30.8, sd = 4.3). 36 out of 39 fathers were married to their partner. The average amount of time married fathers had been married was 3.8 years (sd = 2.3). For the 3 fathers who were not married to their partner, the average amount of time they had lived with their partner was 1.9 years (sd = 1.2). The racial/ethnic breakdown of the sample was as follows: 22 Caucasian, 10 Asian, 3 African American, 3 Hispanic, and 1 Other. 26 fathers had infant boys and 13 had infant girls.
2. 2 Study Design
The study involved one home visit and one scanning visit per participant. During the home visit, study staff recorded the infant crying using an Olympus digital voice recorder (model WS-802) at a distance of 2–3 feet. In most cases, study staff were able to record the infant cry, however in two cases the infant did not cry during the visit and the parents were asked to record the cry themselves at a later time. They were instructed to hold the recorder at arm’s length when recording the cry. A saliva sample was also collected from the infant (using a cotton swab) for subsequent measurement of infant testosterone levels. Samples were placed on dry ice and transferred to a −80 degree Celsius freezer within 3 hours of the visit. A series of questionnaires (described below) were left for the father to complete prior to the subsequent scanning visit. Fathers were instructed on how to complete the forms and asked to bring the completed forms to the subsequent scanning visit.
For the scanning visit, fathers first provided a blood sample for subsequent measurement of plasma testosterone levels. Fathers were then imaged with fMRI as they listened to their own and a standardized unknown infant cry, as well as auditory control stimuli. Afterwards, they were removed from the scanner and asked to rate their own and the unknown infant cry on several dimensions (see below). Study staff then administered either the Period of PURPLE Crying DVD, a 10-minute educational program for new parents (Barr) or a control intervention to participants after which participants were scanned a second time while listening to the same stimuli. Twenty-three of the 39 subjects then returned for a third session 2–3 months later in which they were again scanned with fMRI as they listened to the same stimuli. In this paper, we focus exclusively on the pre-intervention scans collected during the first scanning session.
2.3 Questionnaires
Fathers completed the following questionnaires prior to the scanning visit. A demographic questionnaire acquired information about the father’s age, socioeconomic status, and the infant’s age and sex [4 minutes]. The 19-item Pittsburgh Sleep Quality Index was used to assess the father’s sleep quality and quantity (Buysse et al. 1989) [4 minutes]. The 8-item Brief Infant Sleep Questionnaire (Sadeh 2004) was used to assess infant sleep patterns [4 minutes]. The 24-item Infant Characteristics Questionnaire (Bates et al. 1979) was used to assesses aspects of infant temperament such as crying, fussiness, and soothability [6 minutes]. Total score on this scale measures infant difficultness. The 21-item Beck Depression Inventory was used to assess depressive symptoms in fathers (Beck et al. 1961) [5 minutes]. The 28-item Childhood Trauma Questionnaire (CTQ-SF) was used to assess maltreatment history of fathers (Bernstein et al. 2003) [8 minutes]. The 12-item List of Threatening Experiences (LTE-Q) measures stressful life events (Brugha and Cragg 1990) [4 minutes]. This scale was used to measure the number of stressful life events in the past year. The 60 item NEO-FFI was used to measure neuroticism in fathers (Costa and McCrae 1992; McCrae et al. 1996) [12 minutes]. The 36-item Parenting Stress Index – Short Form (PSI–SF) (Abidin and Brunner 1995; Haskett et al. 2006) was used to measure the degree to which fathers agree with statements related to stress related to childcare and perceptions of their child’s self-regulatory abilities [8 minutes].
2.4 Task
The cry task included both the father’s own infant cry (OC) and a cry stimulus from a standardized unknown infant (UC). The standardized unknown infant cry stimulus (UC) was recorded previously from a 3 month old boy in his home. Cry stimuli were edited to 10 s clips using online available software (www.audacity.com). Control stimuli included pure tones that preserved the mean fundamental frequency and amplitude envelop of the cry (Con), as well as frog (F) and bird (B) calls to minimize habituation to cry sounds. Participants listened to the audio stimuli through Pro Ears Ultra 28 MRI-compatible headphones and were told prior to the task: “You will now hear a series of sounds. You do not have to do anything except listen to them”. The six different sound files (OC, UC, OCon, UCon, F and B) were presented in pseudorandom order two times per block over four blocks such that each sound is presented eight times. There was an inter-trial interval of 6 s between each stimulus. Each of the four blocks lasted 192 s and the total duration of the task was 12 min 48 s.
Immediately following the task, after exiting the scanner, fathers again listened to the own and unknown infant cry stimuli and rated on a 7 point Likert scale how grating, urgent, piercing, aversive, compelling, manipulative, and spoiled they found the cries (1 = not at all, 7 = extremely); they also rated how irritated, sympathetic, alarmed, angry, upset, compassionate, distressed, annoyed, and tender the cries made them feel (1 = not at all, 7 = extremely).
After completing the ratings following the post-intervention scan, fathers again listened to both their own infant cry and the unknown infant cry without being told the identity of either. To assess fathers’ ability to identify their own infant cry, they were asked, “Is this your baby’s cry?” after each cry was played.
To evaluate whether differences in subjective ratings between own and unknown infant cries were truly a function of whether the infant belonged to the father or not, rather than some unique properties of the particular unknown cry we selected, we also recruited a separate group of n=40 adult (over 21 years of age, mean age = 26.4 years, sd=7.86) non-fathers and asked them to rate one of the “own” cries from the fathers and the unknown cry for aversiveness.
2.5 Neuroimaging acquisition
A T1-weighted structural image and blood-oxygenation-level-dependent (BOLD) functional MRI images were collected for each subject using a 3 Tesla Siemens Trio MRI scanner (Siemens Medical System, Malvern, PA, USA).
High-resolution T1-weighted images were acquired using a 3D magnetization-prepared rapid gradient-echo (MPRAGE) sequence with a GRAPPA factor of 2. The T1 scan protocol, optimized for 3 Tesla, used the following imaging parameters: a repetition time/inversion time/echo time (TR/TI/TE) of 2600/900/3.02 ms, a flip angle of 8°, a volume of view of 256×256×176 mm3, a matrix of 256×256×176, and isotropic spatial resolution of 1.0×1.0×1.0 mm3, one average. Total T1 scan time was approximately 5 minutes.
T2*-weighted images were collected using an Echo-Planar Imaging (EPI) sequence for BOLD fMRI. EPI images were collected in an interleaved fashion with the following imaging parameters: TR=2380 ms, TE=30 ms, matrix=64 X64, FOV=192mm, slice thickness=3.0 mm, and 38 axial slices. Subjects listened to infant cry and control stimuli during scanning.
2.6 Image analysis
The analysis was conducted with the Oxford Center for Functional Magnetic Resonance Imaging of the Brain’s software library (FSL, http://www.fmrib.ox.ac.uk/fsl/). The preprocessing pipeline of the fMRI data involved (1) motion correction using the MCFLIRT (Jenkinson et al. 2002), (2) non-brain tissue removal using the Brain Extraction Tool (BET) (Smith 2002), (3) slice timing correction, (4) high-pass temporal filtering with a cut-off of 200 s, (5) spatially smoothing with a Gaussian kernel of full-width at half maximum (FWHM) of 5 mm, and (6) normalizing to MNI space via corresponding extracted T1 brain using Boundary-Based-Registration (Greve and Fischl 2009).
The preprocessed fMRI data were analyzed using the general linear model (GLM) for univariate statistical analysis. A separate GLM was defined for each subject, with regressors specified for the neural response to own infant cry (OC), unknown infant cry (UC), their respective tone controls (OCon and UCon) as well frog (F) and bird (B) calls. Regressors were convolved with a standardized model of the hemodynamic response function. Analyses were focused on the contrasts OC-OCon and UC-UCon. The individual-level GLM was implemented using FILM (FMRIB’s Improved Linear Model).
At the group level, analyses included both whole brain and region-of-interest (ROI) approaches. For the whole brain analysis, paternal and infant variables were entered as covariates into a mixed effects model that examined the contrasts OC-OCon and UC-UCon. Each covariate (10 paternal characteristics, 4 infant characteristics and 2 principal components for cry ratings) was evaluated in a separate model. The mean beta value of each covariate was compared with zero using a one-sample t-test. The resultant Z statistic (Gaussianized t) images were thresholded using clusters determined by Z>3.1 (voxel -wise 1-tailed p<0.001), and a family-wise error (FWE)-corrected cluster significance threshold of p<0.05 was applied to the suprathreshold clusters. For the ROI analysis, we defined functional ROIs from the group level contrasts OC-OCon and UC-UCon. This ROI approach allows us to focus our covariate analysis on brain areas that we know are responsive to cries in our data set. Each contrast was compared with zero at every brain voxel using a one-sample t-test. The resultant Z statistics (Gaussianized t) images was thresholded using clusters determined by Z>3.1 (voxel -wise 1-tailed p<0.001), and a family-wise error (FWE)-corrected cluster significance threshold of p<0.05 was applied to the suprathreshold clusters. These statistical images were then further thresholded at either Z>5.0 (for UC-UCon) or Z>5.5 (OC-OCon) with a 20 voxel spatial extent threshold to better highlight only the strongest activations for each contrast, and ROIs were then defined at each cluster. ROIs were centered on the voxel with peak activation and extended across all contiguous activated voxels within 10 mm in X, Y and Z directions (Poldrack 2007). The average contrast value across all ROI voxels was calculated for each subject and these values were tested for correlations with paternal and infant variables using the Pearson Product Moment Correlation (r). For each paternal and infant variable, ROI results were corrected for multiple comparisons across the number of ROIs tested.
2.7 Hormone assays
Plasma testosterone assay
Blood samples were centrifuged at 4 °C within 20 min of blood draw. Plasma was collected and frozen at −80 °C until assayed. Assays were analyzed in duplicate by the Biomarkers Core Laboratory of the Yerkes National Primate Research Center at Emory University using a coated-tube RIA kit (Coat-A-Count Total Testosterone, Cat No. TKTT1, Siemens). On the day of the assay, frozen plasma samples were thawed, centrifuged for 30 min at 3,000 revolutions per minute with a Thermo Scientific, Sorvall ST 16R rotor and centrifuge, and assayed according to the protocol provided by the manufacturer. The interassay CV% ranged from 4.05–4.37%, intra-assay CV%ranged from 2.07–2.28%, and the sensitivity of the assay was 6.0–1,667.00 ng/dL.
Salivary testosterone assay
The Yerkes Biomarkers Core Laboratory at Emory University utilized the Salimetrics Testosterone Enzyme Immunoassay Kit for the quantitative measurement of salivary Testosterone. Samples were assayed in duplicate, with the results read using the Sectramax i3× plate reader, manufactured by Molecular Devices. On the day of the assay, frozen saliva samples were thawed at 4°C, centrifuged at 1,500 × g for 15 minutes, and assayed according to the vendor-provided protocol. The reportable range of this assay is: 6.1–600 pg/mL, with an assay sensitivity of 1.0 pg/mL.
2.8 Cry roughness measurements
In order to quantify the power of temporal modulations in the roughness range (30–150 Hz), the two-dimensional Fourier transform of the spectrogram was calculated to obtain the Modulation Power Spectrum (MPS) of each sound excerpt (Elliott and Theunissen 2009). Sound waveforms were transformed into a time-frequency representation (spectrogram) using a filter-bank approach. Waveforms were filtered using 128 different linear-phase finite impulse response filters (512th order). Filters were designed to estimate critical bands (Moore and Glasberg 1981) with center frequencies logarithmically spanning the frequency space and corresponding equivalent rectangular bandwidths (Moore and Glasberg 1981). Each filter’s output was then Hilbert transformed in order to extract the analytic amplitude and log-transformed. The output of this filter-bank processing is a spectrogram that provides a time-frequency representation estimating the output of the cochlea (Moore and Glasberg 1981). Modulation power spectra were derived by applying a two-dimensional Fourier transform to the spectrogram and log-transforming the resulting spectral power density estimates. The MPS value in the roughness range was calculated for each tested sound by averaging MPS values across temporal modulations from 30 to 150 Hz, and across all spectral modulations.
2.9 Principal Components Analysis
For own infant cries, a principal component analysis of the 16 different subjective cry rat ings was done using Varimax rotations with Kaiser Normalization. The Kaiser-Meyer-Olkin measure of sampling adequacy was 0.71, above the commonly recommended value of 0.6, and Bartlett's test of sphericity was significant, (χ2 (120) = 438.02, p < .001).
For unknown infant cries, a separate principal component analysis of the 16 different subjective cry ratings was done using Varimax rotations with Kaiser Normalization. The Kaiser-Meyer-Olkin measure of sampling adequacy was 0.62, above the commonly recommended value of 0.6, and Bartlett's test of sphericity was significant, (χ2 (120) = 286.83, p < .001).
3. Results
3.1 Identification of own vs. unknown infant cries
In response to hearing their own infant cry, 36 of 39 fathers correctly answered “yes” to the question, “Is this your baby’s cry?” (recognition rate = 92.3%). However, in response to hearing the unknown infant cry, 17 of 39 incorrectly answered “yes” (false positive rate = 44%) and 3 of 39 answered “I don’t know” to the same question. Only 19 of 39 fathers (48.7%) answered both questions correctly. Answering both questions correctly was not related to either infant age (t(37)=0.33, p=0.74) or to the amount of time the father took off of work after the birth of his infant (t(37)=1.36, p=0.18).
3.2 Subjective cry ratings for own and unknown infant cries
Fathers rated the unknown infant cry as more “grating”, “urgent”, “piercing” and “aversive” than their own infant cry. They also reported feeling more irritated and alarmed when listening to the unknown vs. their own infant cry (supplementary table 1). A separate sample of n=40 non-fathers also rated the unknown infant cry as more aversive than the fathers’ own infants’ cries (unknown mean=3.76, sd=0.11; own mean=3.31, sd=0.11; t(39)=4.18, p=0.0001). Thus, it appears that some characteristic of the unknown infant cry other than it’s identity as own or unknown is driving the difference in subjective ratings.
3.3 Main effect of infant cry stimuli on paternal brain function
The contrast between fathers listening to their own infant’s cry and the associated tone control (OC-OCon) yielded widespread activation, including most prominently the medial prefrontal cortex, bilateral anterior insula and inferior frontal gyrus, bilateral striatum, bilateral thalamus, bilateral auditory cortex, bilateral posterior cingulate, and bilateral midbrain including the VTA and SN (Table 1). The contrast between listening to the unknown infant cry and its tone control (UC-UCon) yielded a very similar activation pattern (Table 1, figure 1A). A direct statistical comparison between OC-OCon and UC-UCon yielded no significant activation. This was also true when the comparison was limited to the 19 fathers that correctly identified both their own and the unknown infant cry.
Table 1.
Activations to own and unknown infant cries Voxels are 2mm isotropic.
| Brain Regions | MNI Coordination of Local Maxima (mm) |
Local Maxima Z |
Cluster Size (voxel) |
||
|---|---|---|---|---|---|
| x | y | z | |||
| OC>OCon | 68,343 | ||||
| R superior temporal gyrus, extending into supramarginal gyrus, Heschl’s gyrus, putamen | 62 | −26 | 8 | 8.14 | |
| R thalamus extending into caudate, VTA, SN and pallidum | 6 | −8 | 0 | 6.96 | |
| L Heschl’s gyrus, extending into superior temporal gyrus | −48 | −14 | 2 | 7.02 | |
| Pons | −6 | −28 | −8 | 6.99 | |
| Superior frontal gyrus extending into paracingulate gyrus | 8 | 20 | 66 | 6.35 | |
| R insula extending into frontal pole | 38 | 28 | 0 | 6.21 | |
| L insula extending into frontal orbital cortex, frontal operculum cortex, inferior frontal gyrus, fontal pole and pars triangularis | −40 | 28 | −4 | 5.91 | |
| L putamen extending into thalamus, pallidum, VTA, SN and caudate | −28 | −24 | 4 | 6.44 | |
| R posterior cingulate gyrus, extending into precuneus | 10 | −22 | 32 | 6.18 | |
| L posterior cingulate gyrus, extending into precuneus | −4 | −26 | 32 | 6.06 | |
| Paracingulate gyrus extending into superior frontal gyrus, anterior cingulate gyrus | 6 | 26 | 42 | 5.83 | |
| R frontal operculum cortex extending into inferior frontal gyrus, temporal pole | 50 | 14 | −2 | 5.93 | |
| C<OCon | |||||
| R postcentral gyrus, superior parietal lobule | 18 | −44 | 68 | 4.76 | 2,132 |
| L postcentral gyrus, supramarginal gyrus | −56 | −20 | 54 | 4.68 | 1,667 |
| R parahippocampal gyrus, temporal fusiform | 28 | −26 | −20 | 4.9 | 512 |
| R lateral occipital cortex, occipital pole | 40 | −84 | 24 | 4.4 | 479 |
| L temporal fusiform cortex, parahippocampal gyrus, lingual gyrus | −28 | −40 | −14 | 4.72 | 143 |
| R precuneus cortex, supracalcarine cortex | 22 | −60 | 18 | 4.36 | 127 |
| UC>UCon | 70,611 | ||||
| R superior temporal gyrus, extending into parietal operculum cortex, Heschl’s gyrus, putamen | 62 | −34 | 10 | 7.88 | |
| L Heschl’s gyrus, extending into planum polare, superior temporal gyrus, middle temporal gyrus, putamen | −48 | −14 | 0 | 7.43 | |
| Precuneus cortex extending into cingulate gyrus | 6 | −64 | 34 | 5.88 | |
| Superior frontal gyrus, extending into paracingulate gyrrus, cingulate gyrus | 2 | 12 | 62 | 6.23 | |
| L frontal operculum cortex, extending into inferior frontal gyrus, pars triangularis, orbitofrontal cortex | −40 | 26 | 4 | 6.71 | |
| R caudate, extending into thalamus, pallidum, putamen, VTA and SN | 14 | 4 | 20 | 5.89 | |
| R angular gyrus, extending into lateral occipital cortex | 44 | −50 | 44 | 5.98 | |
| R insula extending into temporal pole, orbitofrontal cortex, frontal pole | 30 | 16 | −12 | 5.74 | |
| L caudate extending into thalamus, pallidum, SN, VTA and putamen | −10 | −2 | 18 | 5.51 | |
| R orbitofrontal cortex extending into frontal pole | 34 | 30 | −14 | 5.75 | |
| Pons | 10 | −26 | −6 | 5.51 | |
| Anterior cingulate gyrus extending into paracingulate gyrus | −2 | 22 | 24 | 5.36 | |
| L temporal pole extending into orbitofrontal cortex, inferior frontal gyrus, frontal opercular cortex | −48 | 18 | −18 | 5.58 | |
| UC<UCon | |||||
| R lateral occipital cortex, extending into postcental gyrus, superior parietal lobule | 16 | −58 | 64 | 4.2 | 730 |
| R lateral occipital cortex | 50 | −78 | 10 | 5.29 | 393 |
| L postcentral gyrus, extending into supramarginal gyrus | −54 | −24 | 46 | 4.49 | 351 |
| L postcentral gyrus, extending into precuneus cortex and superior parietal lobule | −12 | −42 | 66 | 4.64 | 188 |
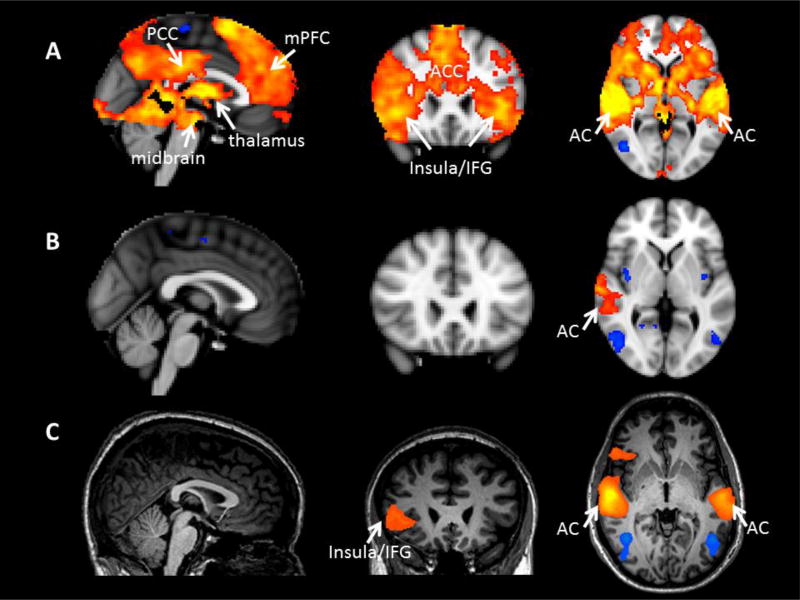
Figure 1.
Activations (orange) and deactivations (blue) for the contrast between the unknown infant cry and its control stimulus (UC-UCon) for A) the present study of fathers with newborn infants and B), C) two previous studies of fathers with toddlers. All images are similarly thresholded with clusters determined by Z>3.1 (voxel-wise 1-tailed p<0.001), and a family-wise error (FWE)-corrected cluster significance threshold of p<0.05 was applied to the suprathreshold clusters. PCC=posterior cingulate cortex, mPFC=medial prefrontal cortex, IFG=inferior frontal gyrus, AC=auditory cortex.
3.4 Relationship between paternal characteristics and paternal neural responses to infant cry stimuli
Descriptive statistics for paternal characteristics are provided in Table 2.
Table 2.
Characteristics of Fathers
| N | Minimum | Maximum | Mean | Std. Deviation |
|
|---|---|---|---|---|---|
| Age | 39 | 23.0 | 40.0 | 30.8 | 4.3 |
| Years of Education | 39 | 12.0 | 21.0 | 17.5 | 2.5 |
| Household Income ($) | 39 | 2,000 | 276,000 | 86,587 | 57,970 |
| Overall Sleep Quality* | 39 | 2.0 | 4.0 | 2.9 | 0.6 |
| Hours of sleep per night | 39 | 3.5 | 9.0 | 6.3 | 1.2 |
| BDI score | 39 | 0.0 | 18.0 | 5.7 | 3.9 |
| CTQ score | 39 | 34.0 | 57.0 | 42.8 | 5.4 |
| LTE score | 39 | 0.0 | 19.0 | 6.5 | 3.7 |
| Neuroticism** | 39 | 0.0 | 29.0 | 15.7 | 7.7 |
| PSI total score | 39 | 40.0 | 88.0 | 67.6 | 11.9 |
| PSI-Difficult Child score | 39 | 11.0 | 37.0 | 23.3 | 5.1 |
| Plasma Testosterone (pg/ml) | 39 | 473.0 | 7,318.5 | 2,474.8 | 1,601.2 |
Sleep quality is rated from 1-4, 1 = very bad, 2 = fairly bad, 3 = fairly good 4 = very good
Neuroticism score from NEO-FFI-3
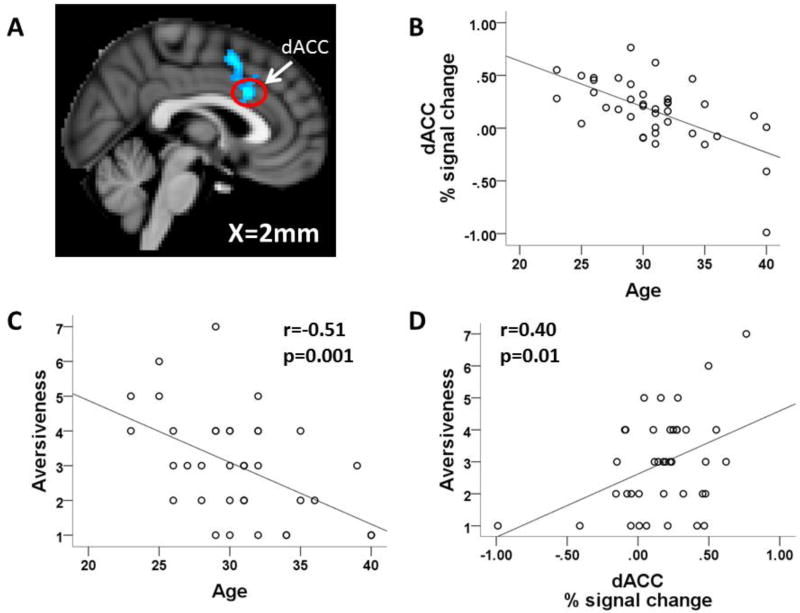
In whole brain analyses, only paternal age was significantly correlated with the neural response to infant cries. For own infant cries, paternal age was negatively correlated with the response to crying in both the dorsal anterior cingulate cortex (ACC, 325 voxels, peak z stat=4.37 at MNI coordinate 2,18,30; figure 2A and 2B) and the right postcentral gyrus (185 voxels, peak z stat = 3.83 at MNI coordinate 36,−18,66). Thus, older fathers had an attenuated response in these areas. For unknown infant cries, paternal age was also negatively correlated with the neural response to crying i n the dorsal ACC (299 voxels, peak z stat=4.58 at MNI coordinate −8,26,38), left postcentral gyrus (262 voxels, peak z stat = 4.33 at MNI coordinate −50, −12, 24), and the right angular gyrus (191 voxels, peak z stat = 4.16 at MNI coordinate 66,−54,22).
Figure 2.
Correlations between paternal age, subjective ratings and neural response to own infant cry. A) Image showing regions with significant negative correlations between paternal age and activation for OC-OCon; image is thresholded with clusters determined by Z>3.1 (voxel-wise 1-tailed p<0.001), and a family-wise error (FWE)-corrected cluster significance threshold of p<0.05 was applied to the suprathreshold clusters, B) scatterplot of paternal age vs. dACC activation for the contrast OC-OCon, C) scatterplot of paternal age vs. own infant cry aversiveness, D) scatterplot of dorsal ACC activation for OC-OCon vs. own cry aversiveness.
In ROI analyses, paternal age was negatively correlated with the response to unknown infant crying in both the right anterior insula (r=−0.45, p=0.004) and the right superior frontal gyrus (r=−0.49, p=0.001), and household income was negatively correlated with the response to unknown infant crying in the right superior frontal gyrus (r=−0.49, p=0.002) (supplementary table 2). There were no significant correlations for own infant cries (supplementary table 3).
3.5 Relationship between infant characteristics and paternal neural responses to infant cry stimuli
Descriptive statistics for infant characteristics are provided in Table 3. Infant T was negatively correlated with infant age (r=−0.46, p=0.004, n=37).
Table 3.
Characteristics of Infants
| N | Minimum | Maximum | Mean | Std. Deviation | |
|---|---|---|---|---|---|
| Infant Age (days) | 39 | 30.0 | 105.0 | 69.5 | 21.4 |
| BICQ FussyDifficult | 39 | 19.0 | 49.0 | 31.5 | 7.0 |
| BICQ Total | 39 | 56.0 | 129.0 | 90.5 | 14.4 |
| Infant Testosterone (pg/ml) | 37 | 27.4 | 164.3 | 61.2 | 30.8 |
| Do you consider your child’s sleep as aproblem?* | 39 | 1.0 | 3.0 | 1.2 | 0.5 |
| Average number of wakings per night | 39 | .0 | 10.0 | 2.5 | 1.7 |
3 = very serious problem, 2 = small problem, 1 = not a problem at all
Own infant cries were matched to tone controls on RMS amplitude and fundamental frequency. However, own infant cries had significantly higher roughness than their respective tone controls (cry = 3.8 +/− 0.38, tone control = 1.0 +/− 0.41; t(76)=30.89, p<0.001). Roughness was positively correlated with multiple negative emotion ratings of the own infant cry stimuli. However, only urgent (r=0.47, p=0.002), piercing (r=0.55, p=0.001) and spoiled (r=0.52, p=0.001) survived correction for multiple comparisons (supplementary figure 1).
In whole brain analyses, there was only one significant correlation between infant characteristics and the neural response to own infant cries. Total score on the Bates Infant Characteristics Questionnaire, a measure of infant difficultness, was positively correlated with the response to own infant crying in the right superior temporal sulcus (STS, 177 voxels, peak z stat=4.92 at MNI coordinate 64,−40,6).
In ROI analyses, there were no significant correlations between any infant characteristic and the neural response to own infant cries after correction for multiple comparisons across ROIs (supplementary table 4).
3.6 Relationship between paternal subjective ratings and neural responses to infant cry stimuli
Own Cry
A principal component analysis of the 16 different subjective cry ratings provided by fathers for their own infant revealed two components that explained a large amount of variance. PC1 explained 31% variance and loaded heavily on negative adjectives (e.g., annoyed, distressed, upset, angry, irritated and aversive). PC2 explained 16% variance and loaded heavily on positive adjectives (e.g., sympathetic, tender and compassionate).
In a whole brain analysis of the neural response to own infant cries, PC1 was positively correlated with activation in the right posterior MTG and right angular gyrus response to own infant cries (Table 4). There were no significant correlations for PC2.
Table 4.
Correlations between brain activation and principal components of infant cry ratings Voxels are 2mm isotropic.
| Brain Regions | MNI Coordination of Local Maxima (mm) |
Local Maxima Z |
Cluster Size (voxel) |
||
|---|---|---|---|---|---|
| x | y | z | |||
| OC>OCon vs. PC1 | |||||
| R angular gyrus extending into posterior MTG | 40 | −60 | 22 | 4.47 | 350 |
| UC<UCon vs. PC1 | |||||
| L caudate nucleus extending into thalamus | −20 | −12 | 20 | 4.23 | 280 |
| UC>UCon vs. PC2 | |||||
| Precentral gyrus, extending into postcentral gyrus and posterior cingulate gyrus | −2 | −26 | 66 | 5.57 | 2568 |
| L middle frontal gyrus, extending into frontal pole | −32 | 34 | 36 | 5.04 | 1128 |
| Paracingulate gyrus extending into anterior cingulate gyrus | 4 | 12 | 46 | 4.97 | 876 |
| White matter near L caudate | −24 | −22 | 26 | 5.01 | 718 |
| R caudate | 20 | −12 | 24 | 4.9 | 684 |
| Hypothalamus extending into brain stem | −2 | −12 | −18 | 4.54 | 505 |
| R inferior frontal gyrus | 58 | 22 | 2 | 4.77 | 474 |
| R thalamus | 14 | −30 | 14 | 4.34 | 435 |
| R Lingual Gyrus, extending into occipital fusiform gyrus | 12 | −70 | −12 | 4.59 | 417 |
| L lingual gyrus | −10 | −54 | −12 | 3.92 | 190 |
| R frontal pole | 24 | 46 | 10 | 4.16 | 182 |
| L inferior frontal gyrus | −58 | 10 | 2 | 4.57 | 168 |
| Superior frontal gyrus | 4 | 36 | 54 | 4.25 | 159 |
| L occipital pole | −12 | −92 | 40 | 3.88 | 157 |
| R posterior superior temporal gyrus, extending into middle temporal gyrus | 52 | −26 | −2 | 4.34 | 147 |
ROI analyses revealed no significant correlations with either PC1 or PC2 after multiple comparison correction (supplementary table 5).
Unknown Cry
A principal component analysis of the 16 different subjective cry ratings provided by fathers for the unknown infant revealed two components that explained a large amount of variance. PC1 explained 27% variance and loaded heavily on negative adjectives (e.g., irritated, upset, annoyed, angry and distressed). PC2 explained 16% variance and, in contrast to PC2 for own cries that loaded on positive adjectives, loaded heavily on a different set of negative adjectives (e.g., spoiled, manipulative, alarmed and aversive).
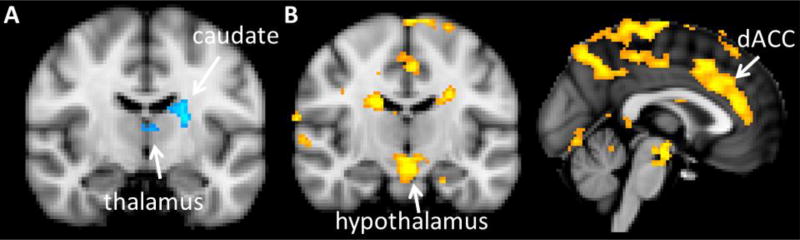
In a whole brain analysis of the neural response to unknown infant cries, PC1 was negatively correlated with activation in the left caudate nucleus and thalamus (figure 3A, Table 4). On the other hand, PC2 showed widespread positive correlations, including prominently the dorsal ACC and hypothalamus (figure 3B, Table 4).
Figure 3.
Correlations between principal components of subjective ratings and neural responses to the unknown infant cry. A) regions in which UC-UCon is negatively correlated with PC1, B) regions in which UC-Con is positively correlated with PC2. Images are thresholded with clusters determined by Z>3.1 (voxel-wise 1-tailed p<0.001), and a family-wise error (FWE)-corrected cluster significance threshold of p<0.05 was applied to the suprathreshold clusters.
In ROI analyses, PC1 was negatively correlated with the left caudate nucleus response to unknown infant cries (r=−0.49, p=0.001). None of the other correlations survived multiple comparison correction. There were no significant correlations for PC2 (supplementary table 6).
4. Discussion
The pattern of brain activation we observed in first-time fathers in response to unknown newborn infant cries is very similar to that first reported by Lorberbaum and colleagues for first-time mothers in response to unknown newborn infant cries (Lorberbaum et al. 1999; Lorberbaum et al. 2002). In their study, relative to a white noise control, listening unknown infant cries engaged five neural systems: 1) midbrain dopaminergic (approach motivation), 2) thalamocingulate (parental caregiving), 3) fronto-insular (emotional empathy), 4) DMPFC (perspective-taking and theory of mind), and 5) right lateralized auditory cortex extending to the temporal pole (auditory perception). Our own findings similarly suggest engagement of these five systems in first-time fathers of newborn infants, as we also observed prominent activation in the midbrain, thalamus, anterior and posterior cingulate, insula, DMPFC and auditory cortex. In contrast to Lorberbaum and colleagues, we also observed activation in the hypothalamus, a key node in the parental brain system of other species (Numan 2007; Wu et al. 2014). On the other hand, similar to Lorberbaum and colleagues, we did not observe activation in NAc. We did, however, observe activation throughout other components of the striatum, including the caudate nucleus and putamen. Overall, comparison of the results of the two studies reinforces the idea that maternal and paternal behavior draw on similar neural systems (Abraham and Feldman This Issue; Numan 2017; Rilling and Mascaro 2017).
This pattern of activation contrasts with the more limited activation patterns we observed in two previous studies in which fathers of 1 or 2 year old children listened to unknown infant cry stimuli (Li et al. 2017; Mascaro et al. 2014) (figure 1). In our previous studies, activation for the same contrast (UC-UCon) yielded activation only in auditory cortex (both studies, figure 1B and 1C) and the right insula/IFG (one study, figure 1C). All three studies were acquired on a Siemens 3T MRI scanner, and like the current study, the study illustrated in figure 1B was also analyzed using the FSL software package (http://www.fmrib.ox.ac.uk/fsl/) (study in figure 1C was analyzed using Brain Voyager using similar preprocessing parameters). In contrast to the current study that used an unknown cry from a 3 month old infant that we previously recorded (figure 1A), the studies shown in figures 1B and 1C used unknown infant cry stimuli from 3 and 5 month old infants that were purchased from an online audio database (www.audionetwork.com). However, there was no significant difference in the perceived aversiveness of the unknown infant cry stimulus between the current study (3.7 on 7 point scale) and our previous study (3.8 on 7 point scale; t(67)=−0.02, p=0.98) (in figure 1B). Therefore, age of the father’s infant is a possible explanation for the observed differences in activation across these three studies. Fathers with older infants may have had more exposure to infant crying and perhaps greater psychological and neural habituation to it as reflected in decreased brain activation. Additionally, fathers with newborn infants may be in a different hormonal state compared with fathers of toddlers and this may alter brain activation in response to crying. For example, a longitudinal study of testosterone levels in Filipino men showed the most pronounced decreases in testosterone in fathers with infants less than 1 month of age, with levels significantly increasing when infants reached 1 year of age (Gettler et al. 2011). Furthermore, plasma oxytocin (OT) levels have been shown to increase across the first 6 months of fatherhood (Abraham and Feldman This Issue; Gordon et al. 2010). Thus, the combination of suppressed T and elevated OT may stimulate stronger engagement of these neural systems in fathers of newborn infants. The plausibility of this idea is supported by studies showing that both exogenous T and exogenous OT treatment modulate the neural response to infant cries among nulliparous women, with OT augmenting the anterior insula and inferior frontal gyrus response (Riem et al. 2011) and T augmenting activation within the thalamo-cingulate system (Bos et al. 2010). On the other hand, a recent study in fathers found no effect of intranasal OT on paternal neural responses to infant cry stimuli, although these were fathers of 1–2 year old children rather than fathers of newborn infants as in the present study (Li et al. 2017). Although we consider cumulative paternal exposure to crying and paternal hormonal state to be likely explanations for differences between the current and our previous studies, the differences could also arise from other variables that were not held constant across studies such as the lack of own infant cry stimuli, as well as frog and bird stimuli, in our previous studies.
The neural response to own infant cries did not differ from that to the unknown infant cry. This may partially reflect the difficulty many fathers had in distinguishing between their own and the unknown infant cry. Similar to previous studies, fathers had a high recognition rate for their own infant cry, coupled with a high false positive rate for the unknown infant cry (Gustafsson et al. 2013b; Wiesenfeld et al. 1981). As suggested previously, this bias to err on the side of more false positives and fewer false negatives rather than the converse may reflect an adaptive strategy that ensured ancestral human fathers would not fail to respond to their own infant in distress (Gustafsson et al. 2013b). It might also reflect an adaptation on the part of infants to solicit aid from even unrelated adults. Even for fathers that correctly identified both the own and unknown infant cries, there was still no significant difference in the neural response to the two cry stimuli, demonstrating the ability of infants to engage parental brain function in even unrelated adults. On the other hand, a recent study of mothers showed stronger activation to their own vs. unknown infant cries within the right fronto-insular cortex (Hipwell et al. 2015). The difference with our study of fathers could possibly be attributable to hormonal or neurobiological differences between mothers and fathers and/or to differences in the amount of exposure to the infant. Indeed, fathers who spend more than four hours per day with their infant are as accurate as mothers in identifying own infant cries (Gustafsson et al. 2013a).
Own infant cries were higher in roughness than their respective tone controls, raising the possibility that roughness could partially explain the robust activations for the contrast between the two stimuli. Roughness contributes to the aversiveness of human screams and artificial alarm signals (Arnal et al. 2015). Here we show that roughness also tracks the aversive quality of infant cries, as in our sample it was positively correlated with ratings of urgent, piercing and spoiled. Nevertheless, it was not significantly correlated with the neural response to own infant cries. Thus, although our imaging data may have been sensitive to large differences in roughness between infant cry and control stimuli, they were not sensitive to subtler variation in roughness among the different own infant cries.
The unknown infant cry was rated more negatively than were the own infant cries as a group. In a follow-up study, non-fathers also judged the unknown cry to be more aversive than the own cries from the fathers. Thus, the differences appear to be explained by the particular characteristics of the unknown infant cry used in this experiment rather than the perceived identity of the cry as own or unknown by fathers. The more negative evaluation of the unknown infant cry had no detectable influence on brain activity at the statistical thresholds we adopted. However, when more liberal thresholds were adopted (p<0.05 cluster-corrected), stronger activation to unknown cries was observed in the anterior cingulate cortex, right anterior insula and right inferior frontal gyrus, regions that also tracked negative emotional reactions to the unknown infant cries (PC2, figure 3B). These findings should be regarded with caution give that this threshold does not appear to adequately protect against false positive activations (Eklund et al. 2016; Woo et al. 2014).
Paternal age was negatively correlated with the dorsal ACC response to both own and unknown infant crying. Thus, older fathers showed less activation compared with younger fathers. Dorsal ACC activation has been linked with both physical and psychological pain and the dorsal ACC has been proposed to function as a neural alarm signal (Eisenberger and Lieberman 2004; Panksepp 2003). This raises the possibility that older fathers find baby cries less aversive or alarming. To investigate this possibility, we first tested for correlations between paternal age and each of our 16 subjective rating measures. For own cry ratings, after correcting for multiple comparisons across ratings, only aversiveness was significantly correlated with age (r=−0.51, p=0.001; figure 2C). Aversiveness was also negatively correlated with age for unknown cries, though this did not survive the multiple comparison correction (r=−0.40, p=0.013 uncorrected). This suggested that the dorsal ACC response may be tracking the perceived aversiveness of infant cries. Indeed the dorsal ACC response to crying was positively correlated with aversiveness ratings for own infant cries (r=0.40, p=0.01), although this correlation did not reach significance for unknown infant cries (r=0.25, p=0.12)(figure 2D).
In addition to the dorsal ACC, age was also negatively correlated with the anterior insula response to unknown infant crying. Both the ACC and the insula are strongly implicated in emotional empathy (Lockwood, 2016; Singer and Lamm, 2009), so greater activation in these areas might be associated with more parental empathy. Indeed, one study found that more empathic mothers more strongly activate the anterior insula when viewing pictures of their children (Lenzi et al. 2009). However, there is also evidence to suggest that empathic overarousal can lead to distress that interferes with compassionate behavior (Batson and Powell 2003; Eisenberg 2000; Mascaro 2011), which could potentially interfere with sensitive caregiving. For example, in high-risk mothers, stronger anterior insula responses to own-infant cries were related to more intrusive parenting (Musser et al. 2012). In another study, fathers activated the anterior insula to infant cries, but it was fathers with moderate anterior insula activation that were most involved in instrumental caregiving (Mascaro et al. 2014). Fathers with low and high insula activation may have been less involved due to empathic under and over-arousal to cries, respectively. Thus, the relationship between insula activation to crying and sensitive caregiving may be non-linear. For our current study, we suggest that lower dorsal ACC and anterior insula activation in older first-time fathers renders them better able to avoid the distress associated with empathic over-arousal in response to infant crying. This finding is consistent with other evidence that older adults report better regulation and greater control over their emotions (Blanchard-Fields et al. 2004).
Fathers’ subjective ratings of the standardized unknown infant cry were correlated with their neural response to that cry. PC1, which loaded heavily on negative emotions such as irritated, upset, annoyed, angry and distressed, was negatively correlated with activation in the left caudate nucleus and thalamus (figure 3A). The thalamocingulate system is known to be involved in parental behavior in other mammals (MacLean 1990), and the thalamus was activated in response to infant cries in both this study of fathers as well as Lorberbaum et al’s study of mothers (Lorberbaum et al. 2002). The caudate nucleus may also have a role in parental motivation given that it receives midbrain DA projections that are involved in reward and motivation (Ikemoto et al. 2015). Furthermore, two separate studies have now reported that intranasal oxytocin, a hormone known to be important for parental motivation (Abraham and Feldman This Issue), augments caudate nucleus activation in human fathers viewing pictures of their children (Li et al. 2017; Wittfoth-Schardt et al. 2012). Thus, our data suggest that negative emotional reactions to infant crying may inhibit activation of brain regions like the caudate nucleus and thalamus that are expected to facilitate positive paternal behaviors. Alternatively, activation of these areas may inhibit negative emotional reactions to crying.
In contrast to the negative correlations found for PC1, PC2, which loaded heavily on “spoiled”, “manipulative”, “alarmed” and “aversive”, showed positive correlations with the neural response to unknown infant crying (figure 3B). These correlations were widespread, with a particular focus on the dorsal ACC and hypothalamus. Subsequent analysis of the individual component ratings showed that correlations were strongest for “spoiled” and “manipulative”. Thus, fathers who judged the cry as more spoiled or manipulative showed stronger activation in these areas. Given the well-established role of the medial preoptic area (MPOA) in promoting parental behavior, these results might initially seem counterintuitive. However, the hypothalamus is composed of multiple nuclei with a variety of different functions and some of these nuclei seem to promote offspring avoidance or even infanticide (Kohl et al. 2017; Numan 2017). As mentioned above, the dorsal ACC is implicated in empathy (Lockwood, 2016; Singer and Lamm, 2009), but also in negative affect (Shackman et al. 2011), so it may be that fathers who can feel the infant’s emotions to a greater degree when listening to the cry find the cries more aversive, alarming and manipulative.
Some of the observed correlations were specific to unknown rather than own infant cries. Each father listened to a different own infant cry, whereas each father listened to the same unknown infant cry. Therefore, when looking for effects of paternal characteristics or paternal subjective ratings on the neural response to crying, variability in the own infant cries may introduce noise that makes it more difficult to detect significant relationships. Of course, when looking for effects of the infant cry characteristics themselves, it only makes sense to examine the response to own infant cries. For these infant variables, the only significant finding was a positive correlation between total score on the Bates Infant Characteristics Questionnaire and the response to own infant crying in the right superior temporal sulcus (STS). Fathers who report having more difficult babies (fussy, unadaptable, dull, and unpredictable) have stronger activation in this region in response to their baby’s cry. Although STS is well-known for its role in processing biological motion (Allison et al. 2000), it also contains voice-selective regions (Belin et al. 2000). Our data suggest that behavioral characteristics of the infant can modulate activation in this region as fathers listen to their infant crying.
Many of the paternal and infant variables that we hypothesized would show effects on brain activation did not. These include paternal depression, sleep quality, neuroticism, recent stressful life events, childhood history of parental abuse, and testosterone levels, as well as infant sex, fussiness, testosterone levels and the roughness of infant cries. One possible explanation is that we did not have sufficient variability in these measures to detect correlations. For example, BDI scores ranged from 0–18, a range that does not include severe or even moderate depression (Beck et al. 1961). Another possibility is that the neural response fathers have to listening to infant cries while lying in the MRI scanner differs from that when they are actually in the presence of their crying infant. In the latter situation, they may have additional visual cues from the infant, it could be during the middle of the night, and they may be in a situation where they can or are expected to respond to the crying. That is, our paradigm may lack the ecological validity needed to detect many of the hypothesized associations between paternal and infant characteristics and the neural response to infant crying. Finally, recent simulations suggest the need to threshold neuroimaging data quite conservatively to adequately control the rate of any false positive activations (Eklund et al. 2016). However, adoption of these more rigorous thresholds also increases the false negative rate. That is, the conservative statistical thresholding we adopt here may have prevented us from detecting some true positive relationships. On the other hand, our false positive rate may have been increased by testing separate regression models for each of the many paternal and infant covariates.
In conclusion, we show that younger first-time fathers have a stronger neural response to newborn infant cries within brain areas that track negative emotional reactions to those cries. These findings coupled with evidence that younger fathers are at greater risk to abuse their infants and that crying is a common trigger for abuse, suggest that interventions aimed at improving emotional reactions to infant crying and decreasing infant abuse should target young, first-time fathers.
Supplementary Material
Supplementary figure 1: Correlations between roughness and subjective cry ratings for own infant cries.
Highlights.
first-time mothers and fathers show similar neural activation to newborn infant cries
older fathers have an attenuated neural response to their infant’s cry
older fathers find their infant’s cry less aversive
paternal neural responses track their emotional reactions to infant cry stimuli
Acknowledgments
We thank Erin Trifilio, Nicole Asante and Lynnet Richey for assistance with various aspects of this study.
Funding:
This work was supported by a grant from the Emory Center for Translational Social Neuroscience (CTSN) and by the National Center for Advancing Translational Sciences of the National Institutes of Health [UL1TR000454].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abidin RR, Brunner JF. Development of a parenting alliance inventory. Journal of Clinical Child Psychology. 1995;24(1):31–40. [Google Scholar]
- Abraham E, Feldman R. The Neurobiology of Human Allomaternal Care; Implications for Fathering, Coparenting, and Children's Social Development. Physiology and Behavior. doi: 10.1016/j.physbeh.2017.12.034. This Issue. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4(7):267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Arnal LH, Flinker A, Kleinschmidt A, Giraud AL, Poeppel D. Human screams occupy a privileged niche in the communication soundscape. Curr Biol. 2015;25(15):2051–2056. doi: 10.1016/j.cub.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RG. The Period of Purple Crying. Farmington, UT: The National Center on Shaken Baby Syndrome; [Google Scholar]
- Barr RG. Preventing abusive head trauma resulting from a failure of normal interaction between infants and their caregivers. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17294–17301. doi: 10.1073/pnas.1121267109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr RG, Trent RB, Cross J. Age-related incidence curve of hospitalized Shaken Baby Syndrome cases: convergent evidence for crying as a trigger to shaking. Child Abuse and Neglect. 2006;30(1):7–16. doi: 10.1016/j.chiabu.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Bates JE, Freeland CAB, Lounsbury ML. Measurement of infant difficultness. Child Development. 1979:794–803. [PubMed] [Google Scholar]
- Batson C, Powell AA. Altruism and prosocial behavior. In: Millon T, Lerner MJ, editors. Handbook of psychology: Personality and social psychology. Vol. 5. New York, NY: John Wiley & Sons, Inc.; 2003. pp. 463–484. [Google Scholar]
- Beattie L, Kyle SD, Espie CA, Biello SM. Social interactions, emotion and sleep: A systematic review and research agenda. Sleep medicine reviews. 2015;24:83–100. doi: 10.1016/j.smrv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M. An Inventory for Measuring Depression. Archives of general psychiatry. 1961;4(6) doi: 10.1001/archpsyc.1961.01710120031004. 561-&. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403(6767):309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abus Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bisping R, Steingrueber HJ, Oltmann M, Wenk C. Adults' tolerance of cries: an experimental investigation of acoustic features. Child Dev. 1990;61(4):1218–1229. [PubMed] [Google Scholar]
- Blanchard-Fields F, Stein R, Watson TL. Age differences in emotion-regulation strategies in handling everyday problems. The journals of gerontology Series B, Psychological sciences and social sciences. 2004;59(6):261–269. doi: 10.1093/geronb/59.6.p261. [DOI] [PubMed] [Google Scholar]
- Bos PA, Hermans EJ, Montoya ER, Ramsey NF, van Honk J. Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocrinology. 2010;35(1):114–121. doi: 10.1016/j.psyneuen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Brugha TS, Cragg D. The List of Threatening Experiences: the reliability and validity of a brief life events questionnaire. Acta Psychiatrica Scandinavica. 1990;82(1):77–81. doi: 10.1111/j.1600-0447.1990.tb01360.x. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cabrera NJ, Tamisk-LeMonda CS, Bradley RH, Hofferth S, Lamb ME. Fatherhood in the twentyfirst century. Child Development. 2000;71(1):127–136. doi: 10.1111/1467-8624.00126. [DOI] [PubMed] [Google Scholar]
- Campbell SB, Cohn JF, Flanagan C, Popper S, Meyers T. Course and Correlates of Postpartum Depression during the Transition to Parenthood. Development and Psychopathology. 1992;4(1):29–47. [Google Scholar]
- Christian CW, Block R, Committee on Child A, Neglect, and American Academy of P Abusive head trauma in infants and children. Pediatrics. 2009;123(5):1409–1411. doi: 10.1542/peds.2009-0408. [DOI] [PubMed] [Google Scholar]
- Costa PT, McCrae RR. "'Normal'personality inventories in clinical assessment: General requirements and the potential for using the NEO Personality Inventory". 1992 Reply. [Google Scholar]
- Critchley H, Elliot R, Mathias C, Dolan R. Neural activity relating to the generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. J Neurosci. 2000;20(8):3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- de Jong TR, Chauke M, Harris BN, Saltzman W. From here to paternity: neural correlates of the onset of paternal behavior in California mice (Peromyscus californicus) Horm Behav. 2009;56(2):220–231. doi: 10.1016/j.yhbeh.2009.05.001. [DOI] [PubMed] [Google Scholar]
- DeWolff MS, van Ijzendoorn MH. Sensitivity and attachment: A meta-analysis on parental antecedents of infant attachment. Child Development. 1997;68(4):571–591. [PubMed] [Google Scholar]
- Eisenberg N. Emotion, regulation, and moral development. Annual Review of Psychology. 2000;51:665–697. doi: 10.1146/annurev.psych.51.1.665. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8(7):294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci U S A. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott TM, Theunissen FE. The modulation transfer function for speech intelligibility. PLoS computational biology. 2009;5(3):e1000302. doi: 10.1371/journal.pcbi.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon RMP, Rosiman GI. Attachment theory: progress and future directions. Current Opinion in Psychology. 2017;15:131–136. doi: 10.1016/j.copsyc.2017.03.002. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Corter C, Stallings J, Steiner M. Testosterone and prolactin are associated with emotional responses to infant cries in new fathers. Horm Behav. 2002;42(4) doi: 10.1006/hbeh.2002.1840. pp. [DOI] [PubMed] [Google Scholar]
- Forman DR, O'Hara MW, Stuart S, Gorman LL, Larsen KE, Coy KC. Effective treatment for postpartum depression is not sufficient to improve the developing mother-child relationship. Development and Psychopathology. 2007;19(2):585–602. doi: 10.1017/S0954579407070289. [DOI] [PubMed] [Google Scholar]
- Frodi AM, Lamb ME. Child abusers' responses to infant smiles and cries. Child Dev. 1980;51(1):238–241. [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Longitudinal evidence that fatherhood decreases testosterone in human males. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(39):16194–16199. doi: 10.1073/pnas.1105403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson GE, Green JA. On the importance of fundamental frequency and other acoustic features in cry perception and infant development. Child Dev. 1989;60(4):772–780. [PubMed] [Google Scholar]
- Gustafsson E, Levrero F, Reby D, Mathevon N. Fathers are just as good as mothers at recognizing the cries of their baby. Nature communications. 2013a;4:1698. doi: 10.1038/ncomms2713. [DOI] [PubMed] [Google Scholar]
- Gustafsson E, Levrero F, Reby D, Mathevon N. Fathers are just as good as mothers at recognizing the cries of their baby. Nature communications. 2013b;4 doi: 10.1038/ncomms2713. [DOI] [PubMed] [Google Scholar]
- Haskett ME, Ahern LS, Ward CS, Allaire JC. Factor structure and validity of the parenting stress index-short form. Journal of Clinical Child and Adolescent Psychology. 2006;35(2):302–312. doi: 10.1207/s15374424jccp3502_14. [DOI] [PubMed] [Google Scholar]
- Hipwell AE, Guo CH, Phillips ML, Swain JE, Moses-Kolko EL. Right Frontoinsular Cortex and Subcortical Activity to Infant Cry Is Associated with Maternal Mental State Talk. Journal of Neuroscience. 2015;35(37):12725–12732. doi: 10.1523/JNEUROSCI.1286-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Yang C, Tan A. Basal ganglia circuit loops, dopamine and motivation: A review and enquiry. Behav Brain Res. 2015;290:17–31. doi: 10.1016/j.bbr.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Joosen KJ, Mesman J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Maternal overreactive sympathetic nervous system responses to repeated infant crying predicts risk for impulsive harsh discipline of infants. Child Maltreat. 2013;18(4):252–263. doi: 10.1177/1077559513494762. [DOI] [PubMed] [Google Scholar]
- Kohl J, Autry AE, Dulac C. The neurobiology of parenting: A neural circuit perspective. BioEssays : news and reviews in molecular, cellular and developmental biology. 2017;39(1):1–11. doi: 10.1002/bies.201600159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiri-Hanninen T, Sankilampi U, Dunkel L. Activation of the hypothalamic-pituitary-gonadal axis in infancy: minipuberty. Hormone research in paediatrics. 2014;82(2):73–80. doi: 10.1159/000362414. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Stevens A, Ablow JC. Neural correlates of hypothalamic-pituitary-adrenal regulation of mothers with their infants. Biological Psychiatry. 2011;70(9):826–832. doi: 10.1016/j.biopsych.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, Ammaniti M. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cerebral Cortex. 2009;19(5):1124–1133. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- Li T, Chen X, Mascaro J, Haroon E, Rilling JK. Intranasal oxytocin, but not vasopressin, augments neural responses to toddlers in human fathers. Horm Behav. 2017 doi: 10.1016/j.yhbeh.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, Bloomer CW, Bohning DE, Vincent D, Johnson MR, et al. Feasibility of using fMRI to study mothers responding to infant cries. Depression and Anxiety. 1999;10(3):99–104. doi: 10.1002/(sici)1520-6394(1999)10:3<99::aid-da2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biological Psychiatry. 2002;51(6):431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- MacLean PD. The triune brain in evolution: role in paleocerebral functions. New York: Plenum Press; 1990. p. 672. [DOI] [PubMed] [Google Scholar]
- Mascaro J. A Longitudinal Investigation of Empathic Behavior and Neural Activity and Their Modulation by Compassion Meditation [Dissertation] Atlanta: Emory University; 2011. p. 211. [Google Scholar]
- Mascaro JS, Hackett PD, Gouzoules H, Lori A, Rilling JK. Behavioral and genetic correlates of the neural response to infant crying among human fathers. Soc Cogn Affect Neurosci. 2014;9(11):1704–1712. doi: 10.1093/scan/nst166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Zonderman AB, Costa PT, Bond MH, Paurnonen S. Evaluating repl icebility of factors in the Revised NEO Personality inventory: confirmatory factor analysis versus procrustes rotation. Journal of Personality and Social Psychology. 1996;70:552–566. [Google Scholar]
- Moore BC, Glasberg BR. Auditory filter shapes derived in simultaneous and forward masking. The Journal of the Acoustical Society of America. 1981;70(4):1003–1014. doi: 10.1121/1.386950. [DOI] [PubMed] [Google Scholar]
- Musser ED, Kaiser-Laurent H, Ablow JC. The neural correlates of maternal sensitivity: An fMRI study. Developmental Cognitive Neuroscience. 2012 doi: 10.1016/j.dcn.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental Psychobiology. 2007;49(1):12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M. Parental Behavior. Reference Module in Neuroscience and Biobehavioral Psychology. Elsevier; 2017. [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology. 2009;30(1):46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Neuroscience. Feeling the pain of social loss. Science. 2003;302(5643):237–239. doi: 10.1126/science.1091062. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behavioral and Brain Sciences. 1994;108(6):1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2(1):67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissland N, Shepherd J, Herrera E. The pitch of maternal voice: a comparison of mothers suffering from depressed mood and non-depressed mothers reading books to their infants. J Child Psychol Psychiatry. 2003;44(2):255–261. doi: 10.1111/1469-7610.00118. [DOI] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, van Ijzendoorn MH, Rombouts SA. Oxytocin Modulates Amygdala, Insula, and Inferior Frontal Gyrus Responses to Infant Crying: A Randomized Controlled Trial. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Mascaro JS. The neurobiology of fatherhood. Current Opinion in Psychology. 2017;15:26–32. doi: 10.1016/j.copsyc.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Rosenbaum S, Gettler L. With a little help from her friends (and family) Part II: nonmaternal caregiving behavior and physiology in mammals. Physiology and Behavior. doi: 10.1016/j.physbeh.2017.12.027. This Issue. [DOI] [PubMed] [Google Scholar]
- Sadeh A. A Brief Screening Questionnaire for Infant Sleep Problems: Validation and Findings for an Internet Sample. Pediatrics. 2004;113(6):e570–e577. doi: 10.1542/peds.113.6.e570. [DOI] [PubMed] [Google Scholar]
- Sarkadi A, Kristiansson R, Oberklaid F, Bremberg S. Fathers' involvement and children's developmental outcomes: a systematic review of longitudinal studies. Acta Paediatrica. 2008;97(2):153–158. doi: 10.1111/j.1651-2227.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature reviews Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Annals of the New York Academy of Sciences. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld AR, Malatesta CZ, Deloach LL. Differential Parental Response to Familiar and Unfamiliar Infant Distress Signals. Infant Behav Dev. 1981;4(3):281–295. [Google Scholar]
- Wilson S, Durbin CE. Effects of paternal depression on fathers' parenting behaviors: a metaanalytic review. Clinical psychology review. 2010;30(2):167–180. doi: 10.1016/j.cpr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Wittfoth-Schardt D, Grunding J, Wittfoth M, Lanfermann H, Heinrichs M, Domes G, Buchheim A, Gundel H, Waller C. Oxytocin Modulates Neural Reactivity to Children's Faces as a Function of Social Salience. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Krishnan A, Wager TD. Cluster-extent based thresholding in fMRI analyses: pitfal ls and recommendations. Neuroimage. 2014;91:412–419. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Autry AE, Bergan JF, Watabe-Uchida M, Dulac CG. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509(7500):325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung WJ, Sandberg JF, Davis-Kean PE, Hofferth SL. Children's time with fathers in intact families. J Marriage Fam. 2001;63(1):136–154. [Google Scholar]
- Zeifman DM. Predicting adult responses to infant distress: Adult characteristics associated with perceptions, emotional reactions, and timing of intervention. Infant Mental Health Journal. 2003;24(6):597–612. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Correlations between roughness and subjective cry ratings for own infant cries.