Abstract
Toxicological studies use “specialty chemicals” and, thus, should assess and report both identity and degree of purity (homogeneity) of the chemicals (or toxicants) under investigation to ensure that other scientists can replicate experimental results. Although detailed reporting criteria for the synthesis and characterization of organic compounds have been established by organic chemistry journals, such criteria are inconsistently applied to the chemicals used in toxicological studies. Biologically active trace impurities may lead to incorrect conclusions about the chemical entity responsible for a biological response, which in turn may confound risk assessment. Based on our experience with the synthesis of PCBs and their metabolites, we herein propose guidelines for the “authentication” of synthetic PCBs and, by extension, other organic toxicants, and provide a checklist for documenting the authentication of toxicants reported in the peer-reviewed literature. The objective is to expand guidelines proposed for different types of biomedical and pre-clinical studies to include a thorough authentication of specialty chemicals, such as PCBs and their derivatives, with the goal of ensuring transparent and open reporting of scientific results in toxicology and the environmental health sciences.
Keywords: Authentication, congener specific analysis, gas chromatography, polychlorinated biphenyls, reproducibility, scientific rigor, purity
Introduction
Reproducibility is a well-established scientific concept that is based on the idea that the results of scientific experiments can be replicated and, thus, can be the foundation of new research. To improve the reproducibility of biomedical research, the National Institutes of Health (NIH) recently established principles and guidelines to improve the rigor and reproducibility of preclinical research (National Institutes of Health). Updated grant application and review instructions were established to ensure that only the most rigorous science is funded. Moreover, NIH established principles and guidelines for reporting preclinical research to ensure that biomedical research is reproducible, robust and transparent (Landis et al. 2012). These efforts complement discipline and study specific reporting guidelines established by scientific organizations, publishers and other organizations to ensure transparent and open reporting of scientific results in peer reviewed publications. Guidelines for the reporting of various studies are, for example, accessible through the EQUATOR Network (Enhancing the Quality and Transparency Of health Research) or have been compiled by the National Institutes of Health (U.S. National Library of Medicine). These include, for example, the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines for reporting animal pre-clinical studies; STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for observational studies; and PRISM (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews. Online tools are available to identify checklists appropriate for specific studies [e.g., the EQUATOR wizard tool, see (Penelope)].
The authentication of key chemical resources is one of the new requirements for grant applications required under the new grant application guidelines established by NIH. According to NIH key resources used in preclinical research:
“… 1) may differ from laboratory to laboratory or over time; 2) may have qualities and/or qualifications that could influence the research data; and 3) are integral to the proposed research. These include, but are not limited to, cell lines, specialty chemicals, antibodies, and other biologics.”
In vitro and in vivo toxicological studies always use “specialty chemicals” and, thus, should assess and report both identity and degree of purity (homogeneity) of the toxicants under investigation. Robust reporting criteria for the synthesis and characterization of organic compounds have been established by organic chemistry journals. However, such criteria are rarely and inconsistently applied to specialty chemicals used in toxicological studies, especially those from commercial sources, regardless of the purity designated by the supplier. Highly active minor impurities may, for example, lead to incorrect conclusions about the chemical entity responsible for a toxicological response, which in turn may confound the accuracy of applying experimental findings in risk assessment. Here, we provide a brief review of the pros and cons of different strategies employed for the synthesis of PCB derivatives and discuss two examples where impurities contributed to biological responses in toxicological studies. Based on our experience with the synthesis of PCBs and their metabolites, we subsequently propose guidelines for the “authentication” of synthetic PCBs and make recommendations for documenting authentication of chemicals reported in the peer-reviewed toxicology and environmental health journals. These recommendations not only apply to PCBs, but also extend to other organic environmental contaminants, such as environmental phenols, per- and polyfluoroalkyl substances (PFAS), polybrominated diphenyl ethers and pesticides.
Materials and Methods
Chemicals and reagents
3,3′-Dichlorobiphenyl (PCB 11) was either purchased from a commercial source (denoted hereafter as PCB 11*, batch #: 061499MT; AccuStandard, New Haven, CT, USA) or synthesized “in-house” in our laboratory from 3,3′-dichlorobenzidine by reaction with sodium nitrite and further reduction with H3PO2 (denoted hereafter as PCB 11^). The full characterization of PCB 11^ was published elsewhere (Holland et al. 2016). Methoxylated derivatives of PCB 11, including 2-methoxy-3,3′-dichlorobiphenyl, 4-methoxy-3,3′-dichlorobiphenyl, 5-methoxy-3,3′-dichlorobiphenyl and 6-methoxy-3,3′-dichlorobiphenyl, were synthesized from chlorinated benzeneboronic acids and chlorinated iodobenzenes with the Suzuki coupling reaction as described (Song et al. 2008, Zhu et al. 2013). Internal standards, 13C-2,5-dichlorobiphenyl (13C-PCB 9; chemical purity 100%; 13C12, 99%) and 13C-2,2′,3,3′,4,4′,5,5′-octachlorobiphenyl (13C-PCB 194; chemical purity ≥ 98%; 13C12, 98%) were purchased from Cambridge Isotope Laboratories (Andover, MA, USA) and used as analytical standards without further purification. Pesticide grade solvents, such as hexane, were purchased from Fisher Scientific (Pittsburg, PA, USA).
PCB purity determination using gas chromatography-mass spectrometry
In a typical experiment, 1 mg of a PCB derivative was dissolved in 1 mL of hexane. This stock solution was diluted 10-fold and analyzed on an Agilent 6890N gas chromatograph (GC) equipped with an Agilent 5975 Inert Mass Selective Detector (Agilent Technologies, CA, USA) operated in the electron ionization mode and a capillary Supelco SLB-5MS GC column (30 m column length; 250 μm inner diameter; 0.25 μm film length; Sigma-Aldrich, St. Louis, MO, USA). The temperature program was modified based on a published method (Wu et al. 2011): starting temperature 50 °C, 10 °C/min to 150 °C, then 5 °C/min to 280 °C, hold for 6 min, and 10 °C/min to 300 °C, hold for 5 min. A helium flow rate of 1.5 mL/min was used for all analyses. The injector temperature was 280 °C, and the temperatures of the transfer line, source and quadrupole were 280 °C, 230 °C and 150 °C, respectively. This GC method can also be adopted to determine the purity of synthetic PCBs with a GC equipped with a flame ionization detector (McLean et al. 1996, van den Hurk et al. 2002); however, the concentrations of the analyte solutions typically need to be higher.
Determination of PCB impurities in synthetic PCBs using congener specific analysis
To identify trace amounts of other PCB congeners present in a synthetic PCB sample, 1 mg of the PCB derivative was spiked with internal standards (13C-PCB 9 and 13C-PCB 194; 80 ng each in isooctane) and dissolved in pesticide grade hexane to give final concentrations of 1 mg/mL of the PCB derivative and 80 ng/mL of each internal standard. A calibration standard containing all 209 PCB congeners (50 ng each in isooctane; AccuStandard) and both internal standards (80 ng each in isooctane) in 250 μL of hexane was prepared in parallel. The samples were analyzed with a PCB congener specific analysis method in the select ion monitoring (SIM) mode to identify minor PCB impurities present in the synthetic PCB samples. This analysis method allows the quantification of all 209 PCB congeners as 162 peaks of individual or co-eluting peaks. The temperature program was as follows: hold at 80°C for 1 min, 2°C/min to 160°C, 1 °C/min to 170°C and hold for 15 min, 1°C/min to 180°C and hold for 15 min, 1°C/min to 245°C, then 10°C/min to 300°C and hold for 15 min (Holland et al. 2016, Hu et al. 2015). The injector and MS temperatures were the same as described above. The flow rate of helium was 1.1 mL/min. The individual and/or co-eluting peaks of PCB impurities were identified and quantified based on the calibration reference standard using the internal standard method. Additional details, including a list of ions monitored in the SIM mode, have been reported earlier (Hu et al. 2015).
RyR1 single channel recordings in planar lipid bilayer (BLM)
Analysis of single channel gating, the most direct approach for measuring the inherent potency and efficacy of compounds and mutations that modify the RyR1 channel (Pessah et al. 2010), was used to assess biological effects of both PCB 11 batches. Measurements of a single RyR1 channel were conducted by inducing junctional sarcoplasmic reticulum (JSR) vesicle fusion with BLM; composed of 30 mg/ml phosphatidylethanolamine, phosphatidylserine, phosphatidylcholine in n-decane (Avanti Polar Lipids, Inc., Alabaster, Alabama, USA). A 10:1 cis: trans chemical gradient of Cs+ was built by 500 to 50 mM of CsCl, buffered with 20 mM Hepes, pH 7.4. Channel recordings were at a holding potential of −40 mV applied to the trans side and the lumen side had 100 μM Ca2+. JSR vesicles, the respective PCB 11 sample, DMSO and defined free Ca2+ (2 μM) were introduced to the cis cytosolic channel face, which served as virtual ground. Baseline recording was acquired for 60–200 s and extended for at least 5 min after addition of vehicle or PCBs. Data acquisition was made through Digidata 1440A (Axon-Molecular Devices; Sunnyvale, CA, USA), digitized at 10 kHz after 1 kHz low-pass filter (Low-Pass Bessel Filter 8 Pole). Data analysis were performed with pClamp 10.4 (Molecular Devices; Sunnyvale, CA, USA) for channel open probability (Po) and current amplitude distribution.
Ca2+ transport measurements
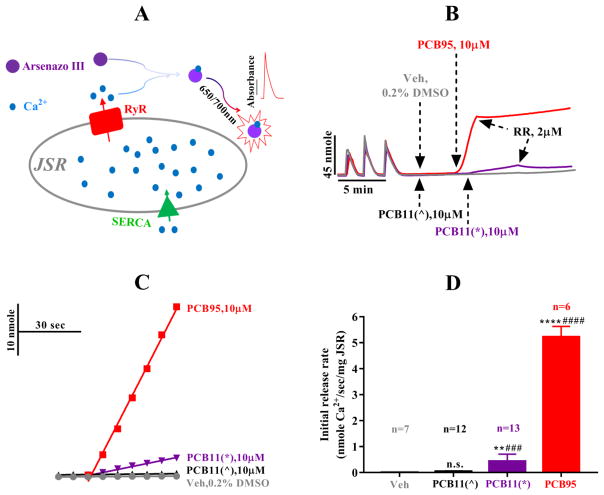
Macroscopic Ca2+ flux measurements were performed using an Agilent 8453 UV-visible Spectroscopy System (Agilent Technologies, CA, USA) to confirm the findings from the RyR1 channel gating kinetics as previously described (Wong & Pessah 1996). Briefly, Net uptake or release of Ca2+ from rabbit skeletal muscle JSR vesicles was detected with the metallochromic dye Arsenazo III at the wavelength of 650/700 nm at 35 °C. The measurement was conducted in the presence of 100 μg/mL of rabbit skeletal muscle JSR, coupling enzyme (2 mM Mg-ATP; 10 mM phosphocreatine; 20μg/mL creatine phosphokinase) and 100 mM KCl, 250 μM Arsenazo III, 20 mM MOPS and 6 mM Na4P2O7, pH 7.4. Active Ca2+ loading by sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) in the presence of coupling enzyme was done through 3 sequential additions of 45 nmol of CaCl2 into cuvettes, with a stir bar constantly mixing the buffer. About 3 minutes after the baseline recordings were established, DMSO (vehicle), PCB11^, PCB11* or PCB95 (positive control), was added into the cuvettes. RyR1 channel blocker ruthenium red (RyR1, 2 μM) was added by the end of the recording to verify the calcium release was mainly through RyR1 channel.
[3H]Ryanodine binding analysis
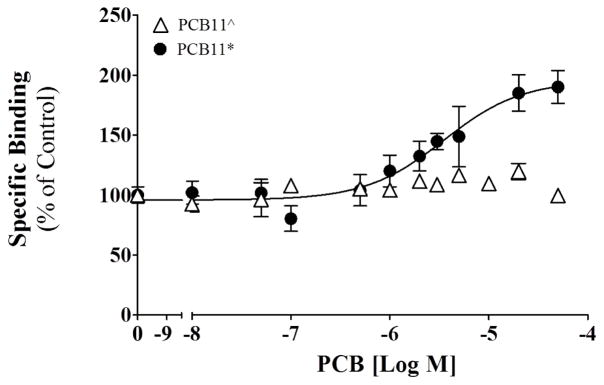
A [3H]ryanodine binding assay was used to compare the biological activity of two batches of PCB 11. The ligand ryanodine binds preferentially to the open state of the ryanodine receptor and therefore increased ligand binding signifies increased stability of the channel open state, or increased channel gating activity in a manner that increases the probability the channel will be in the open conformation over a defined period. [3H]Ryanodine ([3H]Ry; 61.8 Ci mmol−1) was purchased from Perkin Elmer (Waltham MA, USA) and binding assays were conducted following previously published protocols (Holland et al. 2016, Pessah et al. 2006). JSR protein preparations from rabbit skeletal muscle (Pessah et al. 1987) were incubated in binding buffer containing 0.01 to 10 μM of two PCB 11 batches obtained from different sources in 0.5% DMSO. All assays were run in triplicate and concentration-responses were determined in at least two different JSR preparations using PRISM (GraphPad Software, La Jolla, CA, USA).
Results and Discussion
Overview—Synthesis of PCB derivatives
Gram quantities of individual PCB congeners and other PCB derivatives for environmental and toxicological studies can be synthesized using the Suzuki coupling reaction (Bauer et al. 1995, Joshi et al. 2011, Kania-Korwel et al. 2004, Kunz et al. 2006, Lehmler & Robertson 2001b, a, Li et al. 2010, McLean et al. 1996, Song et al. 2008, Telu et al. 2010, Zhu et al. 2013). In addition, the Cadogan coupling (Espandiari et al. 2003, Kunz et al. 2006, Lin et al. 2004, Saeki et al. 1979, van den Hurk et al. 2002), Ullmann coupling (Hutzinger et al. 1971, Ma et al. 2016, Püttmann et al. 1986, Tas & Kleipool 1972, Waller et al. 1999, Webb & Mccall 1972) and Sandmeyer reaction (Lehmler & Robertson 2001a, Nakatsu et al. 1982, Rignall et al. 2013, Safe & Hutzinger 1972, Schramm et al. 1985, Telu et al. 2010, Waller et al. 1999) can provide access to pure PCB derivatives. These different synthetic approaches have advantages and disadvantages from a synthetic perspective that should be considered when preparing PCBs or their metabolites, in particular for in vitro and in vivo toxicity studies.
The Suzuki coupling reaction of a suitable (chlorinated) benzeneboronic acid with a brominated or iodinated chlorobenzene derivative has emerged as a versatile approach for the preparation of milligram to gram quantities of PCBs and their derivatives (Bauer et al. 1995, Joshi et al. 2011, Kania-Korwel et al. 2004, Kunz et al. 2006, Lehmler & Robertson 2001b, a, Li et al. 2010, McLean et al. 1996, Song et al. 2008, Telu et al. 2010, Zhu et al. 2013). Moreover, by-products of the Suzuki coupling reaction, including aryl-aryl transfer reactions from the ligand to the product (Kong & Cheng 1991, O’Keefe et al. 1992) or self-coupling reactions of the aryl boronic acid in the presence of traces of oxygen (Lehmler & Robertson 2001a), are well investigated. Suzuki coupling reactions are particularly efficient for target compounds with ≤ 2 ortho chlorine substituents, but can also be employed for the preparation of sterically hindered PCB derivatives using a highly reactive catalyst, such as Pd(dba)2/2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl (DPDB) (Joshi et al. 2011). In our hands, the synthesis of sterically hindered PCB derivatives containing three or four ortho chlorine substituents with the Pd(dba)2/DPDB catalyst allows the synthesis of large quantities of these congeners, but results in products that can be difficult to purify due to the presence of lower chlorinated PCBs and other coupling products. Although a large number of brominated/iodinated chlorobenzenes as well as chlorinated benzeneboronic acids are commercially available, access to suitable starting materials represent another limitation of this approach.
The Ullmann reaction is commonly used to synthesize symmetrical PCB congeners by coupling chloroiodobenzenes in a sealed tube with copper bronze (Hutzinger et al. 1971, Püttmann et al. 1986, Tas & Kleipool 1972, Waller et al. 1999, Waller & Mash 1997, Webb & Mccall 1972). This reaction is especially powerful for the synthesis of higher chlorinated PCB congeners with multiple ortho chlorine substituents which cannot be obtained by any other method. This reaction can also be employed for the synthesis of unsymmetrical PCB congeners by coupling two iodobenzenes with different substitution patterns; however, the resulting reaction mixtures contain three different products, which can be difficult to separate (Ma et al. 2016, Tas & Kleipool 1972, Waller et al. 1999). The synthesis of PCB metabolites with a polar functional group can be an exception. For example, the products of the Ullmann coupling of 2,3,6-trichloroiodobenzene with 2,3,5-trichloro-4-iodoanisole can be separated by column chromatography because the polarity of the three products follows the rank order 2,2′,3,3′,6,6′-hexachlorobiphenyl (PCB 136) < 4-methoxy-2,2′,3,3′,6,6′-hexachlorobiphenyl < 4,4′-dimethoxy-2,2′,3,3′,6,6′-hexachlorobiphenyl (Waller et al. 1999). The major drawback of the Ullmann coupling reaction, however, is the formation of undesired and potentially toxic byproducts, such as dibenzofurans (Moron et al. 1973) or organotin products (Shaikh et al. 2006).
The Cadogan coupling is a modification of the Gomberg-Bachmann reaction (Cadogan et al. 1962) and an important method to synthesize unsymmetrical PCB congeners and other biphenyls. In a typical reaction, a suitable chloroaniline is reacted with a large excess of a (chlorinated) benzene derivative in the presence of isoamylnitrite (Espandiari et al. 2003, Kunz et al. 2006, Lin et al. 2004, Saeki et al. 1979, van den Hurk et al. 2002). The excess of the benzene derivative is removed, and the PCB congener is isolated by column chromatography over alumina followed by crystallization from methanol. The Cadogan coupling reaction has limited chemical regioselectivity and, therefore, is particularly useful for the synthesis of PCB congeners that give a single product. For example, the isolation and purification of individual PCB congeners is greatly simplified if a chloroaniline is reacted with benzene, 1,4-dichlorobenzene, 1,3,5-trichlorobenzene, 1,2,3,4-tetrachlorobenzene, 1,2,4,5-tetrachlorobenzene, 1,2,3,5-tetrachlorobenzene or 1,2,3,4,5-pentachlorobenzene, yielding congeners with a 2,5-, 2,4,6-, 2,3,4,5-, 2,3,5,6-, 2,3,4,6- and 2,3,4,5,6-substitution pattern, respectively. The removal of the excess of the chlorobenzene, especially for higher chlorinated chlorobenzenes, represents a major challenge of these syntheses. Cadogan reactions producing a mixture of two or more PCB products also present a challenge to separate and may require extensive structural characterization to unambiguously determine the substitution pattern. For example, the Cadogan coupling of 3,4-dichloroaniline with 2,3-dichloroanisole yields a complex mixture of 2-methoxy-3,3′,4,4′-tetrachlorobiphenyl, 5-methoxy-3,3′,4,4′-tetrachlorobiphenyl and 4-methoxy-2,3,3′,4′-tetrachlorobiphenyl (van den Hurk et al. 2002). The structure of the corresponding hydroxylated PCB 77 metabolites, 2-hydroxy-3,3′,4,4′-tetrachlorobiphenyl and 5-hydroxy-3,3′,4,4′-tetrachlorobiphenyl, required 1H nuclear Overhauser effect nuclear magnetic resonance (NMR) spectroscopy experiments to unambiguously determine their chemical structure.
Last but not least, the Sandmeyer reaction is a powerful reaction to introduce chlorine groups into existing biphenyl derivatives. This approach is commonly employed for the synthesis of large quantities of PCB 77 and PCB 153 from the corresponding benzidine (Rignall et al. 2013) derivatives (Hutzinger et al. 1971, Nakatsu et al. 1982, Safe & Hutzinger 1972, Schramm et al. 1985). A modification of this method allows the facile synthesis of some PCB congeners, such as PCB 11 and PCB 52, by reducing the diazonium salts of the respective benzidines with hypophosphorus acid (Holland et al. 2016, Webb & Mccall 1972). These PCB congeners can be obtained in good yield and excellent purity using this approach. This reaction can also be employed to further modify PCB amines (Lehmler & Robertson 2001a, Telu et al. 2010, Waller et al. 1999), and has been used as a key step in the synthesis of pure atropisomers of PCB 197 (Püttmann et al. 1986).
Purification of PCB derivatives
The synthetic PCB derivatives prepared by the above mentioned methods are frequently purified by column chromatography using silica gel as stationary phase or recrystallization from methanol, or a combination of both approaches. It is also possible to separate ortho PCBs, non-ortho PCBs and/or polychlorinated dibenzodioxins (PCDDs)/polychlorinated dibenzofurans (PCDFs) using stationary phases such as alumina, charcoal or Florisil (Athanasiadou et al. 1991, Goldstein et al. 1978, Loos et al. 1997, Mannila 1992, Peterman et al. 2006, Porter & Burke 1971, Storrhansen et al. 1992). The level of activation of the respective stationary phase is critical for a successful separation (Storrhansen et al. 1992). In the case of particularly valuable synthetic products or when only small quantities are needed (e.g., as an analytical standard), PCB derivatives can also be purified by high-performance liquid chromatography with normal or reverse stationary phases (Ma et al. 2016, Püttmann et al. 1986). Charcoal treatment can be used, either during recrystallization or as part of a chromatographic clean-up step, to remove colored impurities or, as mentioned above, small amounts of PCDDs, PCDFs and dioxin-like (DL)-PCBs for toxicological studies (Danielsson et al. 2008, Kania-Korwel et al. 2007, Marsh et al. 1999, Telu et al. 2010). As discussed below, charcoal treatment has also been employed to purify an in-house synthesized batch of PCB 11, denoted PCB 11^ (Holland et al. 2016).
Biological effects of impurities in synthetic PCBs
A number of receptors have been implicated in the broad range of PCB-mediated toxicities. For example, PCB congeners differentially activate the constitutive androstane receptor (CAR), pregnane X receptor (PXR) and other nuclear receptors (Denomme et al. 1983, Parkinson et al. 1983, Schuetz et al. 1998, Wahlang et al. 2014). PCB congeners with zero or one ortho-chlorine substituent can bind to the aryl hydrocarbon receptor (AhR) (Bandiera et al. 1982), thus mimicking the action of 2,3,7,8-tetrachlorodibenzodioxin (TCDD) (Goldstein et al. 1978, Safe 1994). These DL-PCB congeners, for example PCBs 77 and 126, are of particular regulatory interest because of their mammalian and human toxicity, which appears to be mediated primarily via the AhR (van den Berg et al. 2006). Other cellular targets of individual PCB congeners include, for example, ryanodine receptors (RyR) (Niknam et al. 2013, Pessah et al. 2006, Pessah et al. 2009) and estrogen receptors (ER) (Machala et al. 2004, Matthews & Zacharewski 2000). Because the potency of PCBs and their metabolites at the level of these and other cellular targets can differ by several orders of magnitude, it is important to remove and/or characterize impurities that may be present in a synthetic PCB congener and assess if the impurities contribute or even cause PCB-mediated toxicity. To address these issues, the European Union funded the ATHON project (Assessing the Toxicity and Hazard of Non-dioxin-like PCBs present in food) to facilitate the health risk assessment of NDL-PCBs using highly pure and well characterized NDL-PCBs (see below). The following sections discuss two examples where impurities but not the PCB congener under investigation causes a biological effect.
Dioxin-like impurities in synthetic PCBs
An early study with a commercial PCB 153 sample containing traces of 2,3,7,8-tetrachlorodibenzofuran showed a mixed-type of induction of cytochrome P450 enzymes in female rats (Goldstein et al. 1978). Specifically, exposure of rats with this impure PCB 153 batch resulted in a dose-dependent increase in the activity of both CYP1A and CYP2B enzymes. In contrast, a pure batch of PCB 153 synthesized via the Ullmann coupling only slightly increased CYP1A enzyme activity, but increased CYP2B activity with similar potency as the impure compound. At the same time, pure 2,3,7,8-tetrachlorodibenzofuran was a potent inducer of CYP1A, but not CYP2B activity in this study. Taken together, the results from this study provide compelling evidence that small impurities, and not the parent PCB, can cause a toxic effect (e.g., induction of CYP1A enzymes). Because dioxin-like compounds bioaccumulate in the rodent and human liver due to binding to CYP1A2 (Chen et al. 2003, Diliberto et al. 1999) and are potent AhR agonists, even trace levels of dioxin-like impurities represent a major concern for in vivo studies, in particular sub-chronic and chronic exposure studies.
A more recent study provided an in-depth characterization of polychlorinated dibenzodioxins/furans (PCDD/Fs) and DL-PCB impurities in PCB 153 samples from several commercial suppliers (Danielsson et al. 2008). Interestingly, PCB 77 and PCB 126 were the major DL-PCB impurities in the PCB 153 samples investigated. Moreover, PCDFs, such as 2,3,7,8-tetrachlorodibenzofuran and 2,3,4,7,8-pentachlorodibenzofuran, were major impurities, whereas PCDDs were minor impurities, with TCDD below the detection limit in all PCB 153 batches. Interestingly, the profiles of the impurities differed considerably between suppliers, and the total World Health Organization Toxic Equivalent Quotient of the impurities varied 5-fold, and the concentrations of the impurities ranged from 649 pg/g to 3.1 ng/g. The purification approach used by Danielsson et al. (2008) was used as part of the ATHON project to obtain NDL-PCB congeners with a purity > 99.9999%. The results from the ATHON project have resulted in numerous studies providing toxicokinetic data; toxicity profiles; and effect biomarker information for these highly pure NDL-PCB congeners (World Health Organization 2016).
RyR-active impurities in synthetic PCBs
Activation of RyRs by PCBs has been implicated in the developmental neurotoxicity of PCBs (Pessah et al. 2010), and detailed structure activity relationships for PCB-mediated RyR sensitization have been established (Holland et al. 2016, Pessah et al. 2006, Rayne & Forest 2010). Quantitative structure-activity relationship studies predict that PCB 11, an environmentally prevalent PCB congener, does not activate RyR1. Consistent with the predictions, a highly purified batch of PCB 11, denoted as PCB 11^, lacked activity at the receptor (Holland et al. 2016). Interestingly, at 10 μM a commercial PCB 11 sample, denoted as PCB 11*, elicited a 7.4-fold (n = 2) increase in single channel open probability (Po), whereas the highly purified PCB 11^, tested under identical conditions, showed negligible effects on Po (103% of baseline activity, n = 2; Figure 1). These findings were confirmed by measures of macroscopic Ca2+ fluxes across microsomes enriched in SECRA pumps and RyR1 (Figure 2). Here, vesicles are loaded with Ca2+ by sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) activity (Ca2+ loading phase) and release of accumulated sarcoplasmic reticulum Ca2+ is monitored upon RyR1 activation. Addition of pure PCB 11^ did not release Ca2+; however, addition of the commercial PCB 11* with impurity elicited Ca2+ efflux, albeit at a much slower rate compared to highly efficacious PCB 95. Finally, these findings were confirmed in [3H]Ry binding assays where the highly pure preparation of PCB 11^ showed no activity towards the receptor, whereas PCB 11* caused an approximate 200% activation (Figure 3).
Fig. 1.
Analysis of single RyR1 channel gating behavior before and after exposure to two sources of PCB 11 on the cytoplasmic side of the channel (n=2). Right panel is changes in channel gating behavior observed in the presence of a high purity PCB 11 preparation (PCB 11^). Left panel is changes in channel gating behavior in the presence of a less pure PCB 11 preparation (PCB 11*). Data for PCB11^ have been previously published (Holland et al. 2016) and are shown with permission from Toxicological Sciences (Oxford University Press)
Fig. 2.
The less pure PCB 11* rather than the highly purified PCB 11^ sample induced the release of calcium actively loaded in microsomal vesicles. (A) A schematic system depicting the technique of calcium transport assay. (B) The representative traces of JSR vesicles through the SERCA- and RyR1-mediated Ca2+ flux events. During the loading phase, three sequential bolus of 45 nmol CaCl2 were added and actively up-taken by SERCA into JSR vesicles. Three minutes after the establishment of stable baseline, vehicle (0.2% DMSO, grey), PCB11^ (10μM, black), PCB 95 (10μM, red) or PCB11* (10μM, purple) was added to the cuvettes to test the their ability of calcium mobilization, followed by the introduction of a RyR1 channel blocker, ruthenium red (RR, 2μM), to verify that the calcium release was mainly mediated by RyR1. Dashed-lines with arrow indicate when the addition of reagent was made. (C) Representative traces of the initial 70 seconds after the addition of vehicle (0.2% DMSO, grey), PCB11^ (10 μM, black), PCB11* (10 μM, purple) or PCB 95 (10 μM, red). (D) Summary of the initial release rates of Ca2+ efflux (nmol sec−1 mg−1 JSR protein). Data presented as mean ±SD, ** p ≤ 0.01 or **** p ≤ 0.0001 vs. vehicle, ### p ≤ 0.001 or #### p ≤ 0.0001 vs. PCB11^, and “n.s.” indicates no significance compared to Veh. (one-way ANOVA followed by post hoc Tukey’s test). The total replicate numbers are denoted in the figure D, that include some data being previously published with permission from Toxicological Sciences (Oxford University Press; (Holland et al. 2016)): for PCB11* (n=9) and PCB95 (n=4) and DMSO (n=5)
Fig. 3.
Binding of [3H]Ryanodine to ryanodine receptor isoform 1 (RyR1) in the presence of PCB 11 obtained from different sources. PCB 11^ was synthesized in our laboratory and PCB 11* was purchased as a neat preparation from AccuStandard. Specific binding relative to the DMSO control (100%); mean ± SEM, n = 2 each ran in triplicate. Data for PCB11^ have been previously published (Holland et al. 2016) and are shown with permission from Toxicological Sciences (Oxford University Press)
Differences in the purity of the PCB 11 batches are a likely explanation for the observed differences in RyR activity. Indeed, an in-depth gas chromatographic (GC) analysis revealed these differences. A representative gas chromatogram of PCB 11* in total ion chromatogram mode revealed the presence of two impurities with retention times of 18.4 min and 22.5 min (Figure 4). Both impurities were not present in the PCB 11^ batch. The impurity at 18.4 min was tentatively identified as PCB 13 based on its mass spectrum, its retention time relative to PCB 11 and, ultimately, a PCB congener specific analysis (see below). The impurity at 22.5 min was an as yet unidentified, non-PCB impurity (see Figure S2 for the mass spectra of both impurities). In addition, congener specific analysis revealed an approximately 10-times higher contamination of PCB 11* than PCB 11^ with several other PCB congeners (Table 1). Based on established structure-activity relationships (Holland et al. 2016, Pessah et al. 2006, Rayne & Forest 2010) and the fact that NDL-PCBs display additivity at the RyR (Fritsch & Pessah 2013), these PCB impurities, especially those containing ortho with meta chlorine substitutions (e.g., PCB 6, 25 and 26), may in part explain the RyR activity of PCB 11*, but not PCB 11^. In addition, the unidentified, non-PCB impurity in the PCB 11* batch likely contributes to the RyR activity observed in our studies, and further studies are needed to confirm the direct RyR activator(s) in this PCB 11 batch.
Fig. 4.
Gas chromatogram of PCB 11* (AccuStandard) in total ion chromatogram (TIC) reveals the presence of two impurities at 18.4 min and 22.5 min. See the Supplementary Material for the mass spectra of both impurities (Figure S1)
Table 1.
Comparison of PCB impurities present in a commercial PCB 11* sample vs. an in-house synthesized PCB 11^ sample using a congener specific GC-MS method. See text for additional information
| Commercial PCB 11* | Synthesized PCB 11^ | |||
|---|---|---|---|---|
| PCBs | ng/mg | w% | ng/mg | w% |
| PCB 2 | 320 | 0.032 | 307 | 0.031 |
| PCB 6 | 61 | 0.006 | ND | ND |
| PCB 13 | 3,080 | 0.310 | ND | ND |
| PCB26&25 | 24 | 0.002 | ND | ND |
| PCB 35 | 17 | 0.002 | 16 | 0.002 |
| PCB 36 | 4 | < 0.001 | 6 | 0.001 |
| Total impurities | 3,500 | 0.350 | 330 | 0.034 |
ND – not detected
Recommendations for the characterization of PCB derivatives
The two examples discussed above demonstrate that even small impurities present in specialty chemicals used in toxicological studies, such as PCB derivatives, can result in false positive (or negative) outcomes in both in vitro and in vivo studies. It is therefore critical to characterize test compounds and provide adequate evidence to establish both their identity and degree of purity (homogeneity) in subsequent peer-reviewed publications. Here we outline a list of specific recommendations for the authentication of these compounds in the environmental health sciences and provide recommendations for reporting the authentication to ensure a rigorous and reproducible study design (Table 2). These recommendations also apply to other toxicants. Specific recommendations and relevant considerations for the points listed in Table 2 include:
Table 2.
Check list with recommended evidence, ranging from highly recommended (*****) to recommended (*), to establish both identity and degree of purity of synthetic PCB congeners in peer reviewed publications. See text for additional discussion.
| Source of PCB derivatives: commercial source | |
| • Source, purity and, potentially, catalog and batch numbers | ***** |
| • Purification method(s) (e.g., column chromatography on alumina, silica gel or Florisil; charcoal treatment; and recrystallization), if any | ***** |
| Source of PCB Derivatives: “in-house” synthesis | |
| • Synthesis route, including starting materials and reagents, with appropriate citation(s) or, if no published previously, a detailed description of the synthetic procedure | ***** |
| • Purification method(s) (e.g., column chromatography on alumina, silica gel or Florisil; charcoal treatment; and recrystallization) | ***** |
| Identity of PCB congeners (as supplemental data) | |
| • Description of the physical state (i.e., colorless solid, waxy solid or oil for PCB derivatives) | ***** |
| • GC-MS chromatogram with using, for example, a typical 5% phenyl/95% dimethylpolysiloxane-based stationary phase and the corresponding mass spectruma | ***** |
| • Proton and carbon NMR spectra (0–10 ppm for proton and 0–200 ppm for carbon NMR spectra, with expansions of multiplets) | **** |
| • Accurate mass determination (within 0.0003 amu of the theoretical value calculated for the expected molecular formula) | *** |
| • X-ray crystal structure determination | ** |
| • Ultraviolet–visible (UV/Vis) spectrum | * |
| Degree of purity | |
| • GC-MS chromatogram with using, for example, a typical 5% phenyl/95% dimethylpolysiloxane-based stationary phase and the corresponding mass spectrum (see above) as well as integration data, typically expressed as relative peak areaa | ***** |
| • Proton and carbon NMR spectra (0–10 ppm for proton and 0–200 ppm for carbon NMR spectra; calibrated against TMS or CDCl3) | **** |
| • Elemental analysis for carbon and hydrogen (and sulfur or nitrogen, if present) (within 0.4% of calculated values) | *** |
| • Melting point range for all solid compounds (narrow melting point range that is in close agreement with a cited literature value) | *** |
| • Purity determination by HPLC or LC-MS-based methods in certain cases (e.g., for PCB sulfates) to assess purity | ** |
| • Congener-specific impurity analysis using GC-ECD, GC-MSb or biological assays (CALUX) to assess potent impurities targeting specific receptors for studies requiring highly pure compounds, including animal studies | * |
See Materials and Methods for a general procedure for the GC-MS analysis.
For experimental details regarding the congener specific PCB impurity determination, see (Holland et al. 2016, Hu et al. 2015).
The source, purity and, potentially, catalog and batch numbers should be documented for test compounds, such as individual PCB congeners, obtained from commercial sources. The reporting of batch numbers is, for example, warranted for PCB derivatives used in animal studies. In addition, method(s) of further purification should be documented, if any. Unfortunately, information regarding the synthetic approaches and purification methods used to prepare PCB derivatives is typically not available from commercial sources; however, information about known or unknown impurities is available upon request in some cases and should be reported, if available.
For PCB derivatives synthesized “in-house”, information regarding the starting materials, synthesis routes and purification methods should be reported to provide insights into impurities present in a particular PCB derivative (for a discussion of side reactions for different routes for the synthesis of PCBs, see above) and allow other laboratories to reproduce the synthesis. An appropriate reference should be cited if the synthesis has been reported previously. For all new syntheses, a detailed description of the synthetic procedure should be provided either in the text of the manuscript or as supplementary material. The synthetic procedure should be described following standards established by organic chemistry journals, such as The Journal of Organic Chemistry.
Although most individual PCB congeners and PCB metabolites are colorless solids (Table S1), we recommend documenting and reporting their physical state to ensure a rigorous and reproducible study design. For example, several PCB congeners, such as PCB 11, are colorless oils or waxy solids, and other PCB derivatives, such as PCB quinones, are yellowish solids (Song et al. 2008). The appearance of PCB derivatives also provides some clue as to their purity. In particular some dihydroxylated PCB congeners are unstable and turn from white to grey to black over time, even if stored at −20 °C.
PCB and many of their derivatives are amenable to GC analysis. We recommend using gas chromatography-mass spectrometry (GC-MS) analyses at a minimum to assess and, ultimately, document both the identity and purity of synthetic PCB derivatives because this method uses comparatively small quantities. If instrumentation with a sufficient resolution is available, GC-MS methods are also an easy approach to determine the accurate mass of the test compound and, thus, confirm its identity. GC-MS analyses should be performed prior to any study to confirm the identity of PCB derivatives—mislabeling of vials containing PCB derivatives can happen occasionally, even for suppliers with strong quality control programs. Moreover, the original gas chromatograms and mass spectra can be easily provided as supplemental data in a peer reviewed publication. The inclusion of mass spectra in the supplemental data can, for example, facilitate a comparison of PCB metabolites used in different studies. For example, as shown in Figure 5, mass spectra of different methoxylated PCB 11 derivatives can be significantly different for ortho vs. meta and para substituted compounds, but also quite similar, as is the case for meta vs. para methoxylated PCB 11 derivatives. Last but not least, GC-MS analyses can be easily performed by core facilities at many research institutions and, thus, are easily accessible.
In addition to GC-MS, we recommend to document both the identity and purity of synthetic PCB derivatives using proton and carbon NMR spectroscopy to facilitate a comparison between different studies. One disadvantage of this approach is that, compared to GC-MS, larger quantities (ideally 50 to 100 mg) of the PCB derivatives are needed, especially for carbon NMR spectroscopy.
Additional methods, such as accurate mass determinations, X-ray crystal structure determinations and ultraviolet-visible (UV/Vis) spectra, represent other approaches that can be helpful to unambiguously identify PCB derivatives. Accurate mass determinations represent a straightforward method to verify the molecular formula of a PCB derivative. X-ray crystal structure determinations allow an unambiguous determination of substitution patterns and the determination of the absolute configuration, as we have shown recently for PCB 84 atropisomers (Li et al. 2017). However, these measurements require single crystals and, thus, do not represent a routine approach for the characterization of PCB derivatives. It is therefore not surprising that only a small number of crystal structures of individual PCB congeners and other PCB derivatives have been reported in the literature (for examples, see references cited in (Joshi et al. 2011, Vyas et al. 2006)). X-ray crystal structure determinations should be deposited with the Cambridge Structural Database. UV/Vis spectra or, more specifically, molar extinction coefficients provide limited structural information, but are useful information for experiments using UV/Vis detection (Li et al. 2010). Other analytical techniques may also provide valuable information about the identity of PCB derivatives for specific experimental applications and can be used as appropriate.
Elemental analyses are a straightforward and well accepted method to determine the purity of synthetic compounds. Since this type of analysis is typically performed by an outside laboratory, elemental analyses represent an approach to independently verify the purity of a synthetic PCB congener and verify its theoretical molecular formula. We recommend this approach for all newly synthesized PCB derivatives. Indeed, our group has used this approach routinely as part of the characterization of many PCBs and PCB metabolites used for in vitro and in vivo studies.
Melting points are a quick method to identify a synthetic PCB derivative based on its published melting point and to assess its purity using its melting point range (see Table S1 for an extensive summary of published melting points of PCBs). We would like to point out that side products of a PCB synthesis can be misidentified based on their melting point. For example, although the melting points of organotin byproducts obtained during the synthesis of PCBs via the Ullmann coupling reaction (Shaikh et al. 2006) are typically higher than the melting points of the target PCB, in some cases both products can have similar melting points (Table S2). For example, the melting points published for 3,3′,4,4′-tetrachlorobiphenyl (PCB 77) and tetrakis(3,4-dichlorophenyl)stannane are quite similar and range from 170–178 °C and 172–174 °C for PCB 77 and tetrakis(3,4-dichlorophenyl)stannane, respectively.
Although many PCB derivatives are volatile and their purity can be determined by various GC methods, some PCB metabolites, such as hydroxylated PCBs and some PCB quinones (Amaro et al. 1996, Kania-Korwel et al. 2008b, McLean et al. 1996), may require derivatization prior to GC analysis. Other PCB metabolites, such as PCB sulfate, glucuronide and glutathione conjugates as well as DNA or other adducts, are not amenable to GC analysis, and their purity needs to be determined with published liquid chromatography (LC) methods (Dhakal et al. 2013, Dhakal et al. 2012, Dhakal et al. 2014, Grimm et al. 2015, Grimm et al. 2013, Grimm et al. 2017). If instrumentation with a sufficient resolution is available, LC-MS methods can also be used to determine the accurate mass of the test compound and, thus, confirm its identity.
Some studies (e.g., chronic animal studies) may require highly pure PCB congeners, with known amounts of specific impurities. As discussed above, we have applied a GC-MS-based, PCB congener-specific analysis method to identify and quantify PCB impurities in synthetic PCB 11 samples (Holland et al. 2016). Alternatively, impurities of interest can be separated using chromatographic approaches and subsequently quantified using GC methods. An example for this approach is the identification of dioxin-like impurities in PCB 153 samples from different vendors described above (Danielsson et al. 2008).
Nineteen PCB derivatives form stable rotational isomers, or atropisomers, under physiological conditions that are non-superimposable mirror images of each other (Kania-Korwel & Lehmler 2016b, a, Lehmler et al. 2010). These chiral PCB congeners and other chiral PCB metabolites, including those derived from axially chiral PCBs (Uwimana et al. 2017), represent a special case with regards to establishing both their identity and degree of purity. To date, the absolute configuration of only a few atropisomers of PCBs and PCB metabolites has been established (Li et al. 2017, Pham-Tuan et al. 2005, Toda et al. 2012). The identity of the atropisomers of PCB is therefore typically based on published optical rotations (see (Haglund 1996b, a, Pham-Tuan et al. 2005) for optical rotations of PCBs and PCB metabolites) and the elution order on a specific enantioselective GC and LC columns (e.g., see (Kania-Korwel & Lehmler 2013)). The purity of individual atropisomers should include both the chemical and atropisomeric purity of the PCB derivative under investigation, as well as the GC or LC column used to establish the atropisomeric purity. The atropisomeric purity is typically reported using the enantiomeric fraction (Kania-Korwel et al. 2008a).
Fig. 5.
Mass spectra (electron ionization mode) of methylated derivatives of hydroxylated PCB 11 metabolites, including (a) 2-methoxy-3,3′-dichlorobiphenyl, (b) 6-methoxy-3,3′-dichlorobiphenyl, (c) 5-methoxy-3,3′-dichlorobiphenyl and (d) 4-methoxy-3,3′-dichlorobiphenyl. See Materials and Methods for additional details regarding the GC-MS analysis
Considering the limited amount of (GC-MS and NMR) data that will provide an in-depth characterization of synthetic PCBs, a deposition of raw analysis data in subject-specific repositories (e.g., ChemSpider or PubChem) or general repositories (e.g, Dryad) may not be warranted for most toxicological studies, but it certainly represents an option to ensure the availability of these data to the research community.
Conclusions
PCBs and their metabolites can be synthesized and purified using a number of different approaches, each of which has advantages and disadvantages with regards to their synthetic ease and the trace impurities present in a pure PCB derivative. Highly active impurities, for example dioxin-like or ryanodine receptor-active impurities, may lead to incorrect conclusions about the biological activity of a particular PCB or PCB metabolite, which may in turn confound the accuracy of applying experimental results in risk assessment. It is therefore critical to authenticate PCB derivatives prior to any experimental study and subsequently document their authentication in the peer-reviewed toxicology and environmental health journals. The objective of the guidelines for the “authentication” of synthetic PCBs proposed herein is to further improve the authentication of test compounds, with the goal of ensuring reproducibility in toxicological and environmental studies of environmental contaminants. Importantly, the proposed guidelines do not only apply to PCB derivatives, but should be utilized for the authentication of all organic toxicants, including other organic environmental contaminants.
Supplementary Material
Figure S1. Mass spectra of impurity 1 (18.4 min) and impurity 2 (22.5 min) from commercial PCB 11 (see Fig. 2 in the manuscript for a gas chromatogram).
Table S1. Summary of published melting points of PCBs.
Table S2. Survey of melting points of some PCBs and structural related organotin compounds.
Acknowledgments
The project described was supported by grants ES04699, ES05605, ES011269, ES013661, ES014901 and ES017425 from the National Institute of Environmental Health Sciences/National Institutes of Health. The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Environmental Health Sciences or the National Institutes of Health.
References
- Amaro AR, Oakley GG, Bauer U, Spielmann HP, Robertson LW. Metabolic activation of PCBs to quinones: Reactivity toward nitrogen and sulfur nucleophiles and influence of superoxide dismutase. Chem Res Toxicol. 1996;9:623–629. doi: 10.1021/tx950117e. [DOI] [PubMed] [Google Scholar]
- Athanasiadou M, Jensen S, Wehler EK. Preparative fractionation of a commercial PCB product. Chemosphere. 1991;23:957–970. [Google Scholar]
- Bandiera S, Safe S, Okey AB. Binding of polychlorinated biphenyls classified as either phenobarbitone, 3-methylcholanthrene or mixed-type inducers to cytosolic Ah receptor. Chem-Biol Interact. 1982;39:259–277. doi: 10.1016/0009-2797(82)90045-x. [DOI] [PubMed] [Google Scholar]
- Bauer U, Amaro AR, Robertson LW. A new strategy for the synthesis of polychlorinated biphenyl metabolites. Chem Res Toxicol. 1995;8:92–95. doi: 10.1021/tx00043a012. [DOI] [PubMed] [Google Scholar]
- Cadogan JIG, Roy DA, Smith DM. An alternative to the Sandmeyer reaction. J Chem Soc C. 1962:1249–1250. [Google Scholar]
- Chen JJ, Chen GS, Bunce NJ. Inhibition of CYP 1A2–dependent MROD activity in rat liver microsomes: An explanation of the hepatic sequestration of a limited subset of halogenated aromatic hydrocarbons. Environ Toxicol. 2003;18:115–119. doi: 10.1002/tox.10107. [DOI] [PubMed] [Google Scholar]
- Danielsson C, Harju M, Halldin K, Tysklind M, Andersson PL. Comparison of levels of PCDD/Fs and non-ortho PCBs in PCB 153 from seven different suppliers. Organohalogen Compd. 2008;70:1201–1203. [Google Scholar]
- Denomme MA, Bandiera S, Lambert I, Copp L, Safe L, Safe S. Polychlorinated biphenyls as phenobarbitone-type inducers of microsomal enzymes. Structure-activity relationships for a series of 2,4-dichloro-substituted congeners. Biochem Pharmacol. 1983;32:2955–2963. doi: 10.1016/0006-2952(83)90402-1. [DOI] [PubMed] [Google Scholar]
- Dhakal K, He X, Lehmler H-J, Teesch LM, Duffel MW, Robertson LW. Identification of sulfated metabolites of 4-chlorobiphenyl (PCB3) in the serum and urine of male rats. Chem Res Toxicol. 2012:2796–2804. doi: 10.1021/tx300416v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal K, Adamcakova-Dodd A, Lehmler HJ, Thorne PS, Robertson LW. Sulfate conjugates are urinary markers of inhalation exposure to 4-chlorobiphenyl (PCB3) Chem Res Toxicol. 2013;26:853–5. doi: 10.1021/tx4001539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal K, Uwimana E, Adamcakova-Dodd A, Thorne PS, Lehmler HJ, Robertson LW. Disposition of phenolic and sulfated metabolites after inhalation exposure to 4-chlorobiphenyl (PCB3) in female rats. Chem Res Toxicol. 2014 doi: 10.1021/tx500150h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diliberto JJ, Burgin DE, Birnbaum LS. Effects of CYP1A2 on disposition of 2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3,4,7,8-pentachlorodibenzofuran, and 2,2′,4,4′,5,5′-hexachlorobiphenyl in CYP1A2 knockout and parental (C57BL/6N and 129/Sv) strains of mice. Toxicol Appl Pharmacol. 1999;159:52–64. doi: 10.1006/taap.1999.8720. [DOI] [PubMed] [Google Scholar]
- Espandiari P, Glauert HP, Lehmler H-J, Lee EY, Srinivasan C, Robertson LW. Polychlorinated biphenyls as initiators in liver carcinogenesis: resistant hepatocyte model. Toxicol Appl Pharmacol. 2003;186:55–62. doi: 10.1016/s0041-008x(02)00018-2. [DOI] [PubMed] [Google Scholar]
- Fritsch EB, Pessah IN. Structure-activity relationship of non-coplanar polychlorinated biphenyls toward skeletal muscle ryanodine receptors in rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 2013;140–141:204–212. doi: 10.1016/j.aquatox.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JA, Hass JR, Linko P, Harvan DJ. 2,3,7,8-Tetrachlorodibenzofuran in a commercially available 99% pure polychlorinated biphenyl isomer identified as the inducer of hepatic cytochrome P-448 and aryl hydrocarbon hydroxylase in the rat. Drug Metab Dispos. 1978;6:258–264. [PubMed] [Google Scholar]
- Grimm FA, Lehmler HJ, He X, Robertson LW, Duffel MW. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin. Environ Health Perspect. 2013;121:657–662. doi: 10.1289/ehp.1206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, He X, Teesch LM, Lehmler HJ, Robertson LW, Duffel MW. Tissue distribution, metabolism, and excretion of 3,3′-dichloro-4′-sulfooxy-biphenyl in the rat. Environ Sci Technol. 2015;49:8087–8095. doi: 10.1021/acs.est.5b01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm FA, Lehmler HJ, Koh WX, DeWall J, Teesch LM, Hornbuckle KC, Thorne PS, Robertson LW, Duffel MW. Identification of a sulfate metabolite of PCB 11 in human serum. Environ Int. 2017;98:120–128. doi: 10.1016/j.envint.2016.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund P. Isolation and characterisation of polychlorinated biphenyl (PCB) atropisomers. Chemosphere. 1996a;32:2133–2140. [Google Scholar]
- Haglund P. Enantioselective separation of polychlorinated biphenyl atropisomers using chiral high-performance liquid chromatography. J Chromatogr A. 1996b;724:219–28. [Google Scholar]
- Holland EB, Feng W, Zheng J, Dong Y, Li X, Lehmler H-J, Pessah IN. An extended structure-activity relationship of non-dioxin-like PCBs evaluates and supports modeling predictions and identifies picomolar potency of PCB 202 towards ryanodine receptors. Toxicol Sci. 2016;155:170–181. doi: 10.1093/toxsci/kfw189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Adamcakova-Dodd A, Lehmler HJ, Gibson-Corley K, Thorne PS. Toxicity evaluation of exposure to an atmospheric mixture of polychlorinated biphenyls by nose-only and whole-body inhalation regimens. Environ Sci Technol. 2015;49:11875–11883. doi: 10.1021/acs.est.5b02865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzinger O, Safe S, Zitko V. Polychlorinated biphenyls - synthesis of some individual chlorobiphenyls. Bull Environ Contam Toxicol. 1971;6:209–219. doi: 10.1007/BF01539929. [DOI] [PubMed] [Google Scholar]
- Joshi SN, Vyas SM, Duffel MW, Parkin S, Lehmler H-J. Synthesis of sterically hindered polychlorinated biphenyl derivatives. Synthesis. 2011:1045–1054. doi: 10.1055/s-0030-1258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Parkin S, Robertson LW, Lehmler H-J. Synthesis of polychlorinated biphenyls and their metabolites with a modified Suzuki coupling. Chemosphere. 2004;56:735–744. doi: 10.1016/j.chemosphere.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Shaikh NS, Hornbuckle KC, Robertson LW, Lehmler H-J. Enantioselective disposition of PCB 136 (2,2′,3,3′,6,6′-hexachlorobiphenyl) in C57BL/6 mice after oral and intraperitoneal administration. Chirality. 2007;19:56–66. doi: 10.1002/chir.20342. [DOI] [PubMed] [Google Scholar]
- Kania-Korwel I, Hrycay EG, Bandiera SM, Lehmler H-J. 2,2′,3,3′,6,6′-Hexachlorobiphenyl (PCB 136) atropisomers interact enantioselectively with hepatic microsomal cytochrome P450 enzymes. Chem Res Toxicol. 2008a;21:1295–1303. doi: 10.1021/tx800059j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Zhao H, Norstrom K, Li X, Hornbuckle KC, Lehmler H-J. Simultaneous extraction and clean-up of PCBs and their metabolites from small tissue samples using pressurized liquid extraction. J Chromatogr A. 2008b;1214:37–46. doi: 10.1016/j.chroma.2008.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Lehmler H-J. Assigning atropisomer elution orders using atropisomerically enriched polychlorinated biphenyl fractions generated by microsomal metabolism. J Chromatogr A. 2013;1278:133–144. doi: 10.1016/j.chroma.2012.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Lehmler HJ. Toxicokinetics of chiral polychlorinated biphenyls across different species—a review. Environ Sci Pollut Res Int. 2016a;23:2058–2080. doi: 10.1007/s11356-015-4383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Lehmler HJ. Chiral polychlorinated biphenyls: absorption, metabolism and excretion-a review. Environ Sci Pollut Res Int. 2016b;23:2042–2057. doi: 10.1007/s11356-015-4150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KC, Cheng CH. Facile aryl-aryl exchange between the palladium center and phosphine ligands in palladium(II) complexes. J Am Chem Soc. 1991;113:6313–6315. [Google Scholar]
- Kunz S, Schwarz M, Schilling B, Paepke O, Lehmler H-J, Robertson LW, Schrenk D, Schmitz H-J. Tumor promoting potency of PCBs 28 and 101 in rat liver. Toxicol Lett. 2006;164:133–143. doi: 10.1016/j.toxlet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Landis SC, et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490:187–191. doi: 10.1038/nature11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler H-J, Robertson LW. Synthesis of polychlorinated biphenyls using the Suzuki-coupling. Chemosphere. 2001a;45:137–143. doi: 10.1016/s0045-6535(00)00546-4. [DOI] [PubMed] [Google Scholar]
- Lehmler H-J, Robertson LW. Synthesis of hydroxylated PCB metabolites with the Suzuki-coupling. Chemosphere. 2001b;45:1119–1127. doi: 10.1016/s0045-6535(01)00052-2. [DOI] [PubMed] [Google Scholar]
- Lehmler H-J, Harrad SJ, Huhnerfuss H, Kania-Korwel I, Lee CM, Lu Z, Wong CS. Chiral polychlorinated biphenyl transport, metabolism, and distribution: A review. Environ Sci Technol. 2010;44:2757–2766. doi: 10.1021/es902208u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Parkin S, Duffel MW, Robertson LW, Lehmler H-J. An efficient approach to sulfate metabolites of polychlorinated biphenyls. Environ Int. 2010;36:843–848. doi: 10.1016/j.envint.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Parkin SR, Lehmler HJ. Absolute configuration of 2,2′,3,3′,6-pentachlorinatedbiphenyl (PCB 84) atropisomers. Environ Sci Pollut Res Int. 2017 doi: 10.1007/s11356-017-9259-z. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P-H, Sangaiah R, Ranasinghe A, Ball LM, Swenberg JA, Gold A. Synthesis of chlorinated and non-chlorinated biphenyl-2,3- and 3,4-catechols and their [2H3]-isotopomers. Org Biomol Chem. 2004;2:2624–2629. doi: 10.1039/B409373A. [DOI] [PubMed] [Google Scholar]
- Loos R, Vollmuth S, Niessner R. Group separation of ortho-PCBs, coplanar non-ortho-PCBs and PCDDs/PCDFs using an alumina column as an one-step clean-up procedure. Fresenius J Anal Chem. 1997;357:1081–1087. [Google Scholar]
- Ma C, Zhai G, Wu H, Kania-Korwel I, Lehmler HJ, Schnoor JL. Identification of a novel hydroxylated metabolite of 2,2′,3,5′,6-pentachlorobiphenyl formed in whole poplar plants. Environ Sci Pollut Res Int. 2016;23:2089–2098. doi: 10.1007/s11356-015-5939-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machala M, Blaha L, Lehmler H-J, Pliskova M, Majkova Z, Kapplova P, Sovadinova I, Vondracek J, Malmberg T, Robertson LW. Toxicity of hydroxylated and quinoid PCB metabolites: Inhibition of gap junctional intercellular communication and activation of aryl hydrocarbon and estrogen receptors in hepatic and mammary cells. Chem Res Toxicol. 2004;17:340–347. doi: 10.1021/tx030034v. [DOI] [PubMed] [Google Scholar]
- Mannila E. Synthesis and structure verification of two previously uncharacterized pentachlorobiphenyls: PCB 108 and PCB 127. Chemosphere. 1992;25:271–276. [Google Scholar]
- Marsh G, Hu J, Jakobsson E, Rahm S, Bergman A. Synthesis and characterization of 32 polybrominated diphenyl ethers. Environ Sci Technol. 1999;33:3033–3037. [Google Scholar]
- Matthews J, Zacharewski T. Differential binding affinities of PCBs, HO-PCBs, and aroclors with recombinant human, rainbow trout (Onchorhynkiss mykiss), and green anole (Anolis carolinensis) estrogen receptors, using a semi-high throughput competitive binding assay. Toxicol Sci. 2000;53:326–339. doi: 10.1093/toxsci/53.2.326. [DOI] [PubMed] [Google Scholar]
- McLean MR, Bauer U, Amaro AR, Robertson LW. Identification of catechol and hydroquinone metabolites of 4-monochlorobiphenyl. Chem Res Toxicol. 1996;9:158–164. doi: 10.1021/tx950083a. [DOI] [PubMed] [Google Scholar]
- Moron M, Sundström G, Wachtmeister CA. Polychlorinated biphenyls. VI. 2,3,7,8-Tetrachlorodibenzofuran, a critical byproduct in the synthesis of 2,2′,4,4′,5,5′-hexachloro-biphenyl by the Ullmann reaction. Acta Chem Scand. 1973;27:3121–3122. doi: 10.3891/acta.chem.scand.27-3121. [DOI] [PubMed] [Google Scholar]
- Nakatsu K, Brien JF, Taub H, Racz WJ, Marks GS. Gram quantity synthesis and chromatographic assessment of 3,3′,4,4′-tetrachlorobiphenyl. J Chromatogr. 1982;239:97–106. [Google Scholar]
- National Institutes of Health. [accessed May 01, 2017];Rigor and reproducibility. https://www.nih.gov/research-training/rigor-reproducibility.
- National Institutes of Health. [accessed May 01, 2017];Updated Application Instructions to Enhance Rigor and Reproducibility. 2016 https://www.nih.gov/research-training/rigor-reproducibility/updated-application-instructions-enhance-rigor-reproducibility.
- Niknam Y, Feng W, Cherednichenko G, Dong Y, Joshi SN, Vyas SM, Lehmler H-J, Pessah IN. Structure-activity relationship of select meta- and para-hydroxylated non-dioxin-like polychlorinated biphenyls: from single RyR1 channels to muscle dysfunction. Toxicol Sci. 2013;136:500–513. doi: 10.1093/toxsci/kft202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe DF, Dannock MC, Marcuccio SM. Palladium catalysed coupling of halobenzenes with arylboronic acids: Rôle of the triphenylphosphine ligand. Tetrahedron Letters. 1992;33:6679–6680. [Google Scholar]
- World Health Organization. Safety evaluation of certain food additives and contaminants. Supplement 1: Non-dioxin-like polychlorinated biphenyls. [accessed May 01, 2017];WHO food additives series; 71-S1. 2016 apps.who.int/iris/bitstream/10665/246225/1/9789241661713-eng.pdf.
- Parkinson A, Safe SH, Robertson LW, Thomas PE, Ryan DE, Reik LM, Levin W. Immunochemical quantitation of cytochrome P-450 isozymes and epoxide hydrolase in liver microsomes from polychlorinated or polybrominated biphyenyl-treated rats. A study of structure-activity relationships. J Biol Chem. 1983;258:5967–5976. [PubMed] [Google Scholar]
- [accessed May 01, 2017];Penelope, equator-wizard. http://www.peneloperesearch.com/equator-wizard.
- Pessah IN, Stambuk RA, Casida JE. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987;31:232–238. [PubMed] [Google Scholar]
- Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW. Structure-activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1) Chem Res Toxicol. 2006;19:92–101. doi: 10.1021/tx050196m. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Lehmler H-J, Robertson LW, Perez CF, Cabrales E, Bose DD, Feng W. Enantiomeric specificity of (−)-2,2′,3,3′,6,6′-hexachlorobiphenyl toward ryanodine receptor types 1 and 2. Chem Res Toxicol. 2009;22:201–207. doi: 10.1021/tx800328u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 2010;125:260–285. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman PH, Feltz KP, Orazio CE, Echols KR. Basic alumina flash chromatographic separation of bulk ortho-PCBs from non-ortho-PCBs, PBDEs, PCDFs, PCDDs, PCDTs, OCPs, and PCTs. Organohalogen Compd. 2006;68:2458–2461. [Google Scholar]
- Pham-Tuan H, Larsson C, Hoffmann F, Bergman A, Froeba M, Huehnerfuss H. Enantioselective semipreparative HPLC separation of PCB metabolites and their absolute structure elucidation using electronic and vibrational circular dichroism. Chirality. 2005;17:266–280. doi: 10.1002/chir.20158. [DOI] [PubMed] [Google Scholar]
- Porter ML, Burke JA. Separation of three chlorodibenzon-p-dioxins from some polychlorinated biphenyls by chromatography on an aluminum oxide column. J Assoc Off Anal Chem. 1971;54:1426–1428. [PubMed] [Google Scholar]
- Püttmann M, Oesch F, Robertson LW. Characteristics of polychlorinated biphenyl (PCB) atropisomers. Chemosphere. 1986;15:2061–2064. [Google Scholar]
- Rayne S, Forest K. Quantitative structure-activity relationship (QSAR) studies for predicting activation of the ryanodine receptor type 1 channel complex (RyR1) by polychlorinated biphenyl (PCB) congeners. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2010;45:355–362. doi: 10.1080/10934520903467980. [DOI] [PubMed] [Google Scholar]
- Rignall B, Grote K, Gavrilov A, Weimer M, Kopp-Schneider A, Krause E, Appel KE, Buchmann A, Robertson LW, Lehmler HJ, Kania-Korwel I, Chahoud I, Schwarz M. Biological and tumor-promoting effects of dioxin-like and non-dioxin-like polychlorinated biphenyls in mouse liver after single or combined treatment. Toxicol Sci. 2013;133:29–41. doi: 10.1093/toxsci/kft034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki S, Yoshihara S, Uchino Y, Yoshimura H. Improved method of the synthesis of 3,4,5,3′,4′-pentachlorobiphenyl. Fukuoka Igaku Zasshi. 1979;70:85–87. [PubMed] [Google Scholar]
- Safe S, Hutzinger O. The mass spectra of polychlorinated biphenyls. J Chem Soc, Perkin Trans I. 1972:686–691. doi: 10.1039/p19720000686. [DOI] [PubMed] [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Schramm H, Robertson LW, Oesch F. Differential regulation of hepatic glutathione transferase and glutathione peroxidase activities in the rat. Biochem Pharmacol. 1985;34:3735–3739. doi: 10.1016/0006-2952(85)90239-4. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Brimer C, Schuetz JD. Environmental xenobiotics and the antihormones cyproterone acetate and spironolactone use the nuclear hormone pregnenolone X receptor to activate the CYP3A23 hormone response element. Mol Pharmacol. 1998;54:1113–1117. doi: 10.1124/mol.54.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh NS, Parkin S, Lehmler H-J. The Ullmann coupling reaction: A new approach to tetraarylstannanes. Organometallics. 2006;25:4207–4214. [Google Scholar]
- Song Y, Buettner GR, Parkin S, Wagner BA, Robertson LW, Lehmler H-J. Chlorination increases the persistence of semiquinone free radicals derived from polychlorinated biphenyl hydroquinones and quinones. J Org Chem. 2008;73:8296–8304. doi: 10.1021/jo801397g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storrhansen E, Cleemann M, Cederberg T, Jansson B. Selective retention of non-ortho substituted coplanar chlorinated biphenyl congeners on adsorbents for column chromatography. Chemosphere. 1992;24:323–333. [Google Scholar]
- Tas AC, Kleipool RJ. Characterization of components of technically polychlorinated biphenyl mixtures-II. Bull Environ Contam Toxicol. 1972;8:32–37. doi: 10.1007/BF01684501. [DOI] [PubMed] [Google Scholar]
- Telu S, Parkin S, Robertson LW, Lehmler H-J. Improved syntheses of non-dioxin-like polychlorinated biphenyls (PCBs) and some of their sulfur-containing metabolites. Environ Int. 2010;36:828–834. doi: 10.1016/j.envint.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda M, Matsumura C, Tsurukawa M, Okuno T, Nakano T, Inoue Y, Mori T. Absolute configuration of atropisomeric polychlorinated biphenyl 183 enantiomerically enriched in human samples. J Phys Chem A. 2012;116:9340–9346. doi: 10.1021/jp306363n. [DOI] [PubMed] [Google Scholar]
- U.S. National Library of Medicine. [accessed May 01, 2017];Research Reporting Guidelines and Initiatives: By Organization. https://www.nlm.nih.gov/services/research_report_guide.html.
- Uwimana E, Maiers A, Li X, Lehmler HJ. Microsomal metabolism of prochiral polychlorinated biphenyls results in the enantioselective formation of chiral metabolites. Environ Sci Technol. 2017;51:1820–1829. doi: 10.1021/acs.est.6b05387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hurk P, Kubiczak GA, Lehmler H-J, James MO. Hydroxylated polychlorinated biphenyls as inhibitors of the sulfation and glucuronidation of 3-hydroxy-benzo[a]pyrene. Environ Health Perspect. 2002;110:343–348. doi: 10.1289/ehp.02110343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas SM, Parkin S, Lehmler H-J. 2,2′,3,4,4′,5,5′-Heptachlorobiphenyl (PCB 180) Acta Cryst E. 2006;E62:o2905–o2906. [Google Scholar]
- Wahlang B, Falkner KC, Clair HB, Al-Eryani L, Prough RA, States JC, Coslo DM, Omiecinski CJ, Cave MC. Human receptor activation by Aroclor 1260, a polychlorinated biphenyl mixture. Toxicol Sci. 2014;140:283–97. doi: 10.1093/toxsci/kfu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller SC, Mash EA. Improved syntheses of 2,2′,3,3′,6,6′-hexachlorobiphenyl. Org Prep Proced Int. 1997;29:679–685. [Google Scholar]
- Waller SC, He YA, Harlow GR, He YQ, Mash EA, Halpert JR. 2,2′,3,3′,6,6′-Hexachlorobiphenyl hydroxylation by active site mutants of cytochrome P450 2B1 and 2B11. Chem Res Toxicol. 1999;12:690–699. doi: 10.1021/tx990030j. [DOI] [PubMed] [Google Scholar]
- Webb RG, Mccall AC. Identities of polychlorinated biphenyl isomers in Aroclors. J Assoc Off Anal Chem. 1972;55:746–752. [PubMed] [Google Scholar]
- Wong PW, Pessah IN. Ortho-substituted polychlorinated biphenyls alter calcium regulation by a ryanodine receptor-mediated mechanism: structural specificity toward skeletal- and cardiac-type microsomal calcium release channels. Mol Pharmacol. 1996;49:740–51. [PubMed] [Google Scholar]
- Wu X, Pramanik A, Duffel MW, Hrycay EG, Bandiera SM, Lehmler H-J, Kania-Korwel I. 2,2′,3,3′,6,6′-Hexachlorobiphenyl (PCB 136) is enantioselectively oxidized to hydroxylated metabolites by rat liver microsomes. Chem Res Toxicol. 2011;24:2249–2257. doi: 10.1021/tx200360m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Mapuskar KA, Marek RF, Xu W, Lehmler HJ, Robertson LW, Hornbuckle KC, Spitz DR, Aykin-Burns N. A new player in environmentally induced oxidative stress: polychlorinated biphenyl congener, 3,3′-dichlorobiphenyl (PCB11) Toxicol Sci. 2013;136:39–50. doi: 10.1093/toxsci/kft186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mass spectra of impurity 1 (18.4 min) and impurity 2 (22.5 min) from commercial PCB 11 (see Fig. 2 in the manuscript for a gas chromatogram).
Table S1. Summary of published melting points of PCBs.
Table S2. Survey of melting points of some PCBs and structural related organotin compounds.