Abstract
A subcutaneous implantable cardioverter-defibrillator (S-ICD) is an alternative device for prevention of sudden cardiac death, without any leads within the heart. Patients implanted with any type of ICD may need catheter ablation of ventricular tachycardia (VT) to reduce the overall arrhythmia burden (e.g., recurrent monomorphic VT) and lower the incidence of painful shocks induced by the device. Late gadolinium enhancement (LGE) MRI is a useful pre-test for guiding VT ablation, because it can be used to map myocardial scar and produce better outcomes. Growing evidence suggests that MRI can be performed with manageable risks on patients with a cardiac implantable electronic device (CIED). Nonetheless, the diagnostic yield of cardiac MRI is still low because of severe image artifacts, regardless of MR-conditional or non-MR conditional labeling. Image artifacts in the heart induced by an S-ICD is expected to be larger than the artifacts induced by a transvenous ICD, because the former is twice as large in size and implanted closer to the heart. This is the first reported case of successful wideband LGE MRI in a patient implanted with an MR-conditional S-ICD. A 37-year-old man with ischemic cardiomyopathy was referred for a cardiac MRI at 1.5T ten months after S-ICD implantation, in order to rule out constrictive pericarditis. Clinical standard LGE MRI produced severe image artifacts, rendering it useless. In contrast, wideband LGE MRI provided unobstructed viewing of myocardial scarring. This case illustrates the usefulness of wideband LGE MRI for assessment of myocardial scarring in a patient with an MR-conditional S-ICD.
Introduction
A transvenous implantable cardioverter-defibrillator (TV-ICD) is clinically indicated in various clinical scenarios for prevention of sudden cardiac death [1]. Despite its effectiveness for primary and secondary prevention of sudden cardiac death, lead-related complications remain a significant problem [2, 3]. Recently developed subcutaneous ICD (S-ICD) is an alternative device for sensing subcutaneous electrocardiogram and delivering appropriate shocks with no leads within the heart [4].
Patients implanted with any type of ICD may need catheter ablation of ventricular tachycardia (VT) to reduce the overall arrhythmia burden (e.g., recurrent monomorphic VT) and lower the incidence of painful shocks delivered by the device [5]. Late gadolinium enhancement (LGE)[6] MRI is a useful pre-test for guiding VT ablation, because it can be used to map myocardial scar, which is known to be an arrhythmogenic substrate for inducing ventricular arrhythmia [7, 8]. Growing evidence suggests that MRI can be performed with manageable risks on patients with a cardiac implantable electronic device (CIED) using established guidelines [9–13]. Nonetheless, the diagnostic yield of cardiac MRI is still low because of severe image artifacts, regardless of MR-conditional or non-MR conditional labeling. Specifically, up to 1/3 of clinical cases produce non-diagnostic image quality, whereas the remaining 2/3 of cases produce diagnostically adequate but nonetheless suboptimal image quality [14]. Image artifacts in the heart induced by an S-ICD is expected to be larger than the artifacts induced by a transvenous ICD, because the former is twice as large in size and implanted closer to the heart.
Two research groups have recently reported the usefulness of “wideband” late gadolinium enhanced (LGE)[15, 16] pulse sequences for suppressing image artifacts induced by the generator of a CIED. Because S-ICDs are relatively new to the market, the capability of wideband LGE MRI to suppress image artifacts in a patient with an MR-conditional S-ICD is unknown. This case report seeks to bridge the aforementioned gap in knowledge.
Case Report
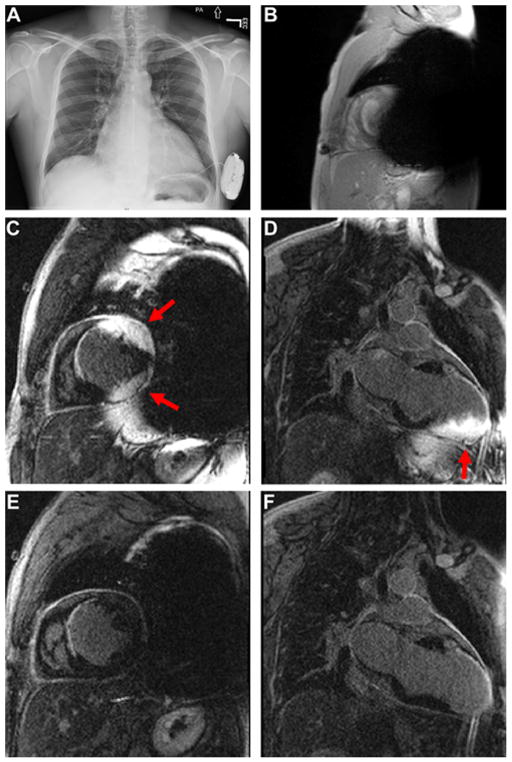
A 36-year-old man with ischemic cardiomyopathy was referred for a cardiac MRI ten months after S-ICD implantation, in order to rule out constrictive pericarditis. The patient had a past medical history of alcohol abuse, marijuana use, hypertension, dyslipidemia, smoking, left ventricle (LV) thrombus, myocardial infarction, and LV ejection fraction of 25%. As shown in Figure 1A, an MR-conditional S-ICD (Boston Scientific S-ICD, model A209 Emblem) was implanted on the patient’s left side near the apex of LV, and a Q-TRAK subcutaneous electrode lead (model Cameron Health 3400) was implanted subcutaneously from the device pocket along the rib margin to the sternum.
Figure 1.
(A) Chest X-ray of a 37-year old man with an MR-conditional S-ICD implanted on the left side. (B) Scout image in a short axis view the patient showing significant signal voids induced by the S-ICD. (C and D) Standard LGE images in short-axis and 2-chamber planes. (E and F) The corresponding wideband LGE images in short-axis and 2-chamber planes. Arrows point to image artifact induced by the S-ICD. MRI images were obtained with standard imaging parameters, including spatial resolution, inversion time, and gradient echo readout.
The patient was scanned on a whole-body 1.5T MRI scanner (Magnetom Avanto, Siemens Healthcare, Erlangen, Germany) using a recommended guideline [10], which included interrogation and programming before and after MRI by an electrophysiology staff, monitoring the patient throughout the MRI by a nurse staff, and specific absorption rate (SAR) < 2.0 W/kg. As per institutional clinical practice, after explaining the risks, a written consent was obtained from the patient by a physician. The MRI protocol included cardiac cine and LGE MRI acquisitions with standard imaging parameters [17]. For technical details such as the frequency bandwidth of the inversion pulse used in wideband LGE MRI, see reference [16]. Other than the inversion pulse, both standard and wideband LGE MRI scans used identical set of parameters, including: field of view = 360 mm x 270 mm, TE = 1.52 ms, TR = 3.9 ms, flip angle = 25°, receiver bandwidth = 501 Hz /pixel, slice thickness = 7 mm. Cardiac cine images produced significant signal voids caused by the S-ICD (Figure 1B). Standard LGE images in short-axis (Figure 1C) and long-axis (Figure 1D) planes showed severe image artifacts, which rendered them useless. In contrast, wideband LGE images in short-axis (Figure 1E) and long-axis (Figure 1F) planes showed significantly reduced image artifacts and enabled our readers to appreciate scar borders and pericardial space more clearly.
As per institutional clinical practice, the patient’s heart rhythm was closely monitored throughout the scan. The MRI scan was considered uneventful, since no rhythm disturbances were detected during MRI by an external patient monitoring device as well as by the patient’s S-ICD. Device interrogation immediately and two weeks after MRI reported to be stable and fully operational. At 2 weeks following MRI, the device had normal lead impedance measurements and 90% battery life remaining, which is within the expected range of an 11-month old device. The patient also reported as not having any problems with the device.
Discussion
This report shows, to our knowledge, first case of successful wideband LGE MRI in a patient with an MR-conditional S-ICD. Compared with standard LGE, wideband LGE produced significantly less image artifacts and enabled readers to appreciate myocardial scar borders and pericardial space. The artifact suppression level in a patient implanted with an S-ICD shown in this report is comparable with the artifact suppression level in patients implanted with TV-ICDs reported in prior wideband MRI studies [15, 16, 18–20]. In this context, the results in this report are remarkable since an S-ICD is twice as large in size and implanted closer to the heart than a TV-ICD.
The capability of wideband LGE MRI to suppress image artifacts in patients with a CIED has important clinical implications. First, non-invasive assessment of myocardial scar is clinically important for guiding VT ablation in patients with a CIED, because pre-test evaluation of myocardial scar may lead to higher procedural success rates (e.g., a lower need of RF delivery) and better outcomes (e.g., higher rates of noninducibility after ablation, VT-recurrence-free survival) [21]. Second, wideband LGE MRI might be useful for guiding cardiac resynchronization therapy (CRT) upgrade implantation in patients with a pacemaker or TV-ICD, since scar evaluation might be useful for identifying optimal sites for pacing leads [22–24]. Third, wideband LGE MRI might be useful for reevaluating the risk for sudden cardiac death in patients for whom a battery replacement for an ICD is due.
In summary, wideband LGE MRI is useful for assessing myocardial scarring in patients with an MR-conditional S-ICD.
Highlights.
Image artifacts in standard LGE MRI are severe in patients implanted with an ICD
Image artifacts induced by an S-ICD is expected to be larger than the artifacts induced by a TV-ICD.
Wideband LGE MRI provides unobstructed viewing of scar in patients with an S-ICD
Acknowledgments
Grant Support: This work was supported in part by funding from the National Institutes of Health (R01HL116895, R01HL138578, R21EB024315, R21AG055954)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography & References Cited
- 1.Kusumoto FM, Calkins H, Boehmer J, Buxton AE, Chung MK, Gold MR, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. J Am Coll Cardiol. 2014;64(11):1143–77. doi: 10.1016/j.jacc.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Atwater BD, Daubert JP. Implantable cardioverter defibrillators: risks accompany the life-saving benefits. Heart. 2012;98(10):764–72. doi: 10.1136/heartjnl-2012-301853. [DOI] [PubMed] [Google Scholar]
- 3.Yaminisharif A, Soofizadeh N, Shafiee A, Kazemisaeid A, Jalali A, Vasheghani-Farahani A. Generator and lead-related complications of implantable cardioverter defibrillators. International cardiovascular research journal. 2014;8(2):66–70. [PMC free article] [PubMed] [Google Scholar]
- 4.Gold MR, Theuns DA, Knight BP, Sturdivant JL, Sanghera R, Ellenbogen KA, et al. Head-to-head comparison of arrhythmia discrimination performance of subcutaneous and transvenous ICD arrhythmia detection algorithms: the START study. Journal of cardiovascular electrophysiology. 2012;23(4):359–66. doi: 10.1111/j.1540-8167.2011.02199.x. [DOI] [PubMed] [Google Scholar]
- 5.Aliot EM, Stevenson WG, Almendral-Garrote JM, Bogun F, Calkins CH, Delacretaz E, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart rhythm : the official journal of the Heart Rhythm Society. 2009;6(6):886–933. doi: 10.1016/j.hrthm.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100(19):1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 7.Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45(7):1104–8. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A, Azevedo CF, Cheng A, Gupta SN, Bluemke DA, Foo TK, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115(15):2006–14. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nazarian S, Halperin HR. How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices. Heart rhythm : the official journal of the Heart Rhythm Society. 2009;6(1):138–43. doi: 10.1016/j.hrthm.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Nazarian S, Hansford R, Roguin A, Goldsher D, Zviman MM, Lardo AC, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Annals of internal medicine. 2011;155(7):415–24. doi: 10.7326/0003-4819-155-7-201110040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nazarian S, Roguin A, Zviman MM, Lardo AC, Dickfeld TL, Calkins H, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1. 5 tesla. Circulation. 2006;114(12):1277–84. doi: 10.1161/CIRCULATIONAHA.105.607655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommer T, Naehle CP, Yang A, Zeijlemaker V, Hackenbroch M, Schmiedel A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1. 5 tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations. Circulation. 2006;114(12):1285–92. doi: 10.1161/CIRCULATIONAHA.105.597013. [DOI] [PubMed] [Google Scholar]
- 13.Russo RJ, Costa HS, Silva PD, Anderson JL, Arshad A, Biederman RW, et al. Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. N Engl J Med. 2017;376(8):755–64. doi: 10.1056/NEJMoa1603265. [DOI] [PubMed] [Google Scholar]
- 14.Dandamudi S, Collins JD, Carr JC, Mongkolwat P, Rahsepar AA, Tomson TT, et al. The Safety of Cardiac and Thoracic Magnetic Resonance Imaging in Patients with Cardiac Implantable Electronic Devices. Academic radiology. 2016;23(12):1498–505. doi: 10.1016/j.acra.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Rashid S, Rapacchi S, Vaseghi M, Tung R, Shivkumar K, Finn JP, et al. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology. 2014;270(1):269–74. doi: 10.1148/radiol.13130942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ranjan R, McGann CJ, Jeong EK, Hong K, Kholmovski EG, Blauer J, et al. Wideband late gadolinium enhanced magnetic resonance imaging for imaging myocardial scar without image artefacts induced by implantable cardioverter-defibrillator: a feasibility study at 3 T. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2015;17(3):483–8. doi: 10.1093/europace/euu263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. Journal of Cardiovascular Magnetic Resonance. 2013;15(1):91. doi: 10.1186/1532-429X-15-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens SM, Tung R, Rashid S, Gima J, Cote S, Pavez G, et al. Device artifact reduction for magnetic resonance imaging of patients with implantable cardioverter-defibrillators and ventricular tachycardia: late gadolinium enhancement correlation with electroanatomic mapping. Heart rhythm : the official journal of the Heart Rhythm Society. 2014;11(2):289–98. doi: 10.1016/j.hrthm.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hong K, Jeong EK, Wall TS, Drakos SG, Kim D. Wideband arrhythmia-Insensitive-rapid (AIR) pulse sequence for cardiac T1 mapping without image artifacts induced by an implantable-cardioverter-defibrillator. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2015;74(2):336–45. doi: 10.1002/mrm.25712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao J, Rashid S, Renella P, Nguyen KL, Hu P. Myocardial T1 mapping for patients with implanted cardiac devices using wideband inversion recovery spoiled gradient echo readout. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2017;77(4):1495–504. doi: 10.1002/mrm.26223. [DOI] [PubMed] [Google Scholar]
- 21.Andreu D, Penela D, Acosta J, Fernandez-Armenta J, Perea RJ, Soto-Iglesias D, et al. Cardiac magnetic resonance-aided scar dechanneling: Influence on acute and long-term outcomes. Heart rhythm : the official journal of the Heart Rhythm Society. 2017;14(8):1121–8. doi: 10.1016/j.hrthm.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Kandala J, Upadhyay GA, Altman RK, Parks KA, Orencole M, Mela T, et al. QRS morphology, left ventricular lead location, and clinical outcome in patients receiving cardiac resynchronization therapy. European heart journal. 2013;34(29):2252–62. doi: 10.1093/eurheartj/eht123. [DOI] [PubMed] [Google Scholar]
- 23.Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59(17):1509–18. doi: 10.1016/j.jacc.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 24.Khan FZ, Virdee MS, Fynn SP, Dutka DP. Left ventricular lead placement in cardiac resynchronization therapy: where and how? Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2009;11(5):554–61. doi: 10.1093/europace/eup076. [DOI] [PubMed] [Google Scholar]