Abstract
As patients with chronic kidney disease (CKD) transition from pediatric nephrology care to adult care, their kidney function is clinically assessed by estimated glomerular filtration rate (eGFR) using both pediatric and adult equations, which may not be congruent. Here we evaluated commonly used eGFR equations and directly measured iohexol GFR (iGFR) among participants between ages 18 and 26 with a diagnosis of pediatric CKD in the Chronic Kidney Disease in Children (CKiD) cohort. The bedside serum creatinine (SCr) only equation (CKiDSCr), the SCr-only CKD-EPI (CKD-EPISCr), the cystatin C (Cys)-only CKD-EPI (CKD-EPICys) and the combined SCr and Cys CKD-EPI (CKD-EPISCr-Cys) were compared with a) 279 measured iGFRs obtained from 187 participants and b) 548 eGFRs from the SCr and Cys-based CKiD equation (CKiDSCr-Cys) obtained from 219 participants. Among emerging adults with a median iGFR of 49 ml/min|1.73m2, the CKiDSCr-Cys equation had low bias (+1.5 ml/min|1.73m2) and high correlation (0.94), while CKiDSCr underestimated iGFR and CKiDSCr-Cys (-5.6 and −7.4 ml/min|1.73m2, respectively) and CKD-EPISCr had an overestimation bias (+8.2 and +6.1 ml/min|1.73m2, respectively). However, the CKD-EPICys and CKD-EPISCr-Cys exhibited strong agreement with both iGFR and CKiDSCr-Cys. GFR may also be validly estimated in this population by taking the simple average of CKiDSCr and CKD-EPISCr (average bias +1.3 compared to iGFR and −0.6 compared to CKiDSCr-Cys). Clinicians should be aware that individually the pediatric and adult SCr-based estimates of GFR had large discrepancies among emerging adults with pediatric CKD. Thus, when cystatin C is not available, we recommend the average of pediatric and adult SCr-based eGFR as a valid tool for clinical use.
Keywords: Chronic kidney disease, pediatric nephrology, glomerular filtration rate, clinical nephrology, young adults
Introduction
The most commonly used clinical equations to estimate glomerular filtration rate (eGFR) may be categorized as either pediatric1,2 - or adult3,4-specific. While these population-specific equations are considered valid for their respective target populations, they have not been formally compared among emerging adults with a history of pediatric CKD.
As clinical management of pediatric chronic kidney diseases (CKD) improves, a higher proportion of children with CKD now reach adulthood prior to end stage renal disease (ESRD) and transition to adult nephrology and urology care5–9. One clinical challenge of characterizing CKD progression is interpreting serial measurements of eGFR from different equations during the transition from pediatric to adult specialty care. A study by Selistre et al10 examined these estimations of GFR in children and young adults (10 to 25 years of age) and they demonstrated that among those over 18, the adult formulas overestimated measured GFR, and pediatric formulas underestimated measured GFR. Given the fundamental differences between the populations used to develop the pediatric and adult equations and the paucity of data in this unique age range, we sought to compare and contrast these equations among emerging adults with a history of pediatric CKD enrolled in the Chronic Kidney Disease in Children (CKiD) study.
For pediatric patients, the CKiD study published a simple bedside estimating equation utilizing only height and serum creatinine (SCr) in 20091, which we refer to as CKiDSCr (and has been previously denoted as eGFRCKiDbed11). An updated equation using CKiD data was published in 20122 utilizing SCr, cystatin C (Cys), blood urea nitrogen (BUN), height and sex, which we denote as CKiDSCr-Cys (and has been previously denoted as eGFRCKiDfull11). Similarly, for adults (i.e., age ≥ 18 years) the CKD-EPI consortium published an equation using only SCr in 2009 (CKD-EPISCr3), and in 2012 published two equations using Cys only (CKD-EPICys4) and combined SCr and Cys (CKD-EPISCr-Cys4). The CKiDSCr and CKiDSCr-Cys equations were developed in children less than 18 years of age (mean age = 11 years; standard deviation [SD] = 4) with mild to moderate CKD (mean GFR = 45 ml/min|1.73m2; SD= 16). In contrast, the CKD-EPI equations were developed in adults (mean age = 47 years, SD= 15), with a higher GFR (mean GFR = 68 ml/min|1.73m2; SD= 39).
Our objective was to describe the similarities and differences in these commonly used clinical tools using directly measured GFR by iohexol plasma disappearance as the reference, and in turn, with the CKiDSCr-Cys equation. Given the good agreement metrics for equations based on SCr and cystatin C previously reported in children11–18 and adults19–21, we expected the equations using both to be preferable to SCr alone. However, the most common current clinical scenario is having only SCr available. Therefore, we explored a simple method to validly estimate GFR when cystatin C is not available in this older adolescent/young adult population with a pediatric diagnosis of CKD.
Results
A total of 219 CKiD participants contributed 548 annual study visits at which they were at least 18 years of age with data to estimate GFR from pediatric and adult equations. Of these, 187 individuals contributed 279 person-visits with directly measured iGFR, corresponding to the original CKiD study design with iGFR measured every other year. Table 1 presents the demographic and clinical characteristics of the first available visit after age 18 years among those contributing estimated GFR data and the subset who contributed iGFR data. The average body size was typical for mature adults (median height= 1.69 m and median weight= 68 kg). Approximately half of the participants had CKD onset at birth and 27% had CKD onset after 10 years of age; 63% had an underlying non-glomerular form of CKD characterized as structural abnormalities of the kidney and urinary tract. Of the person-visits, 95% of the observations were between 18 and 23 years of age, with the maximum age being 26 years. Participant characteristics were similar between the 219 contributing eGFR data and the subset of 187 who contributed iGFR data.
Table 1.
Demographic and clinical characteristics of young adults with a history of pediatric chronic kidney disease at time of first GFR after age 18 years. Median [interquartile range] or % (frequency).
| Participants contributing estimated GFR n= 219 |
Participants contributing iohexol GFR n= 187 |
|
|---|---|---|
| Age | 18.5 [18.2, 18.9] | 18.7 [18.3, 19.3] |
| Female sex | 41% (89) | 42% (78) |
| African American race | 21% (46) | 19% (36) |
| Body size | ||
| Height, m | 1.69 [1.61, 1.77] | 1.69 [1.61, 1.77] |
| Weight, kg | 68 [57, 84] | 67 [57, 84] |
| Body mass index, kg/m2 | 24 [20, 29] | 23 [20, 29] |
| Body surface area, m2 | 1.8 [1.6, 2.0] | 1.8 [1.6, 2.0] |
| CKD characteristics | ||
| Non - glomerular form of CKD | 63% (137) | 61% (115) |
| CKD onset at birth | 51% (111) | 51% (93) |
| CKD onset > 0 and ≤ 5 years old | 12% (26) | 13% (23) |
| CKD onset > 5 and ≤ 10 years old | 10% (21) | 11% (20) |
| CKD onset > 10 and < 16 years old | 27% (58) | 26% (48) |
| Urine protein:creatinine, mg/mg Cr | 0.7 [0.2, 1.6] | 0.7 [0.2, 1.6] |
| Nephrotic range proteinuria | 19% (40) | 16% (29) |
| (> 2 mg/mg Cr) | ||
| Biomarkers of kidney function | ||
| Serum creatinine, mg/dL | 1.6 [1.2, 2.2] | 1.6 [1.2, 2.1] |
| Height/Serum creatinine, m/mg/dL | 1.0 [0.8, 1.4] | 1.1 [0.8, 1.4] |
| Cystatin c, mg/L (IFCC calibrated) | 1.6 [1.2, 2.3] | 1.6 [1.2, 2.2] |
| Blood urea nitrogen, mg/dL | 23 [17, 33] | 24 [17, 33] |
| Number of GFR observationsa | ||
| One observation | 32% (71) | 63% (118) |
| Two observations | 26% (56) | 26% (48) |
| Three or more observations | 42% (92) | 11% (21) |
| Total number of observations | 548 | 279 |
By study design, estimated GFR was measured annually and iohexol-based GFR was measured every two years.
Figure 1 displays and Table 2 quantifies the agreement between CKiDSCr-Cys (mean level= 50.7 ml/min|1.73m2; SD= 21.1) and directly measured iGFR (mean level= 49.2 ml/min|1.73m2, SD= 22.5) among 279 person-visits. The CKiDSCr-Cys had minimal overall bias (+1.5 ml/min|1.73m2), similar dispersion (ratio of standard deviations= 0.94), and a high correlation with iGFR (r = 0.94). The proportion of CKiDSCr-Cys within 30% of iGFR was 90%, which was essentially the same as the results from the validation dataset (91%) from the original equation development that did not include any of these person-visits2. Since we were primarily interested in discrepancies in commonly used clinical instruments, we additionally used CKiDSCr-Cys as the reference for the subsequent analyses in Table 3.
Figure 1.
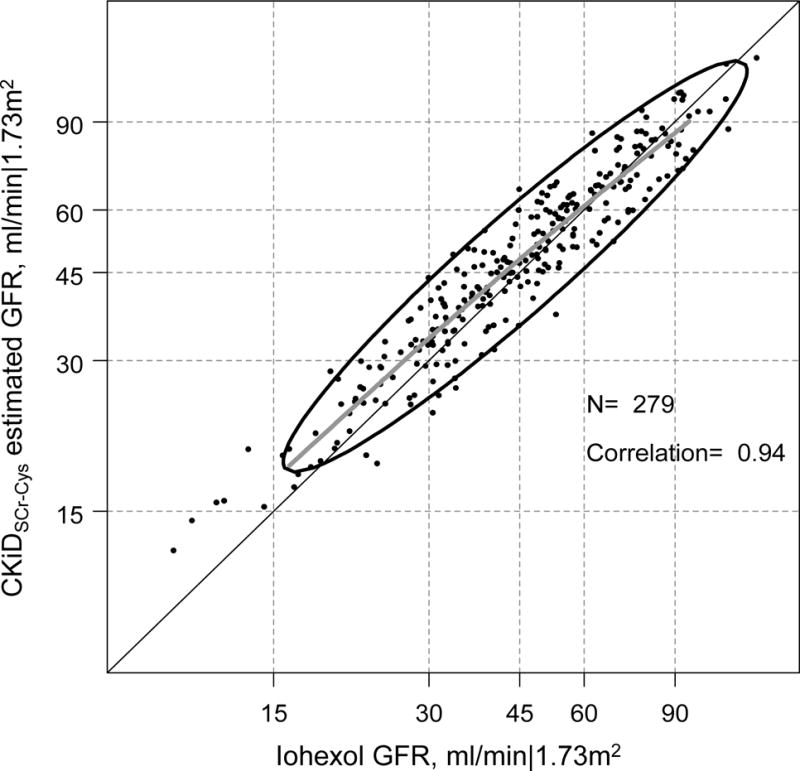
Agreement plot of CKiD eGFR based on serum creatinine and cystatin C (CKiDSCr-Cys) and directly measured iohexol GFR (iGFR) in the log scale among 279 person-visits from 187 young adults with a history of pediatric chronic kidney disease. The bolded grey line is a non-parametric spline based on 95% of the data; the ellipse region depicts the 90% confidence region of the data based on a bivariate normal distribution.
Table 2.
Measures of agreement for CKiD and CKD-EPI estimating GFR equations with iohexol GFR as the reference for 279 person-visits contributed by 187 young adults with a history of pediatric CKD.
| iGFR | CKiDSCr-Cys | CKiDSCr | CKD-EPISCr | CKD-EPICys | CKD-EPISCr-Cys | |
|---|---|---|---|---|---|---|
| Mean, ml/min|1.73m2 | 49.2 | 50.7 | 43.6 | 57.4 | 51.8 | 52.4 |
| Standard deviation | 22.5 | 21.1 | 20.2 | 30.8 | 29.4 | 29.2 |
| Average bias, ml/min|1.73m2 (95%CI) |
0 |
+1.5 (+0.6, +2.5) |
−5.6 (−7.1, −4.2) |
+8.2 (+6.7, +9.7) |
+2.7 (+1.3, +4.0) |
+3.3 (+2.1, +4.4) |
| Ratio of SDs (95%CI) |
1 |
0.94 (0.89, 0.99) |
0.89 (0.81, 0.97) |
1.39 (1.28, 1.50) |
1.32 (1.24, 1.40) |
1.31 (1.24, 1.38) |
| Correlation | 1 | 0.94 | 0.89 | 0.91 | 0.90 | 0.94 |
| RMSE, ml/min|1.73m2 | 0 | 7.1 | 9.2 | 13.0 | 12.3 | 10.0 |
| % within 30% of iGFR | Reference | 90% | 71% | 66% | 74% | 76% |
Abbreviations: Iohexol GFR (iGFR); CKiD eGFR equation based on serum creatinine and cystatin C (CKiDSCr-Cys); CKiD eGFR equation based on serum creatinine only (CKiDSCr); CKD-EPI eGFR equation based on serum creatinine only (CKD-EPISCr), cystatin C only (CKD-EPICys), and serum creatinine and cystatin C (CKD-EPISCr-Cys); standard deviations (SDs); root mean square error (RMSE).
Table 3.
Measures of agreement for CKiDSCr, CKD-EPISCr, CKD-EPICys, and CKD-EPISCr-Cys estimating equations with CKiDSCr-Cys as the reference for 548 person-visits contributed by 219 young adults with a history of pediatric CKD.
| CKiDSCr-Cys | CKiDSCr | CKD-EPISCr | CKD-EPICys | CKD-EPISCr-Cys | |
|---|---|---|---|---|---|
| Mean, ml/min|1.73m2 | 50.2 | 42.9 | 56.3 | 51.4 | 51.8 |
| Standard deviation | 20.4 | 19.4 | 29.9 | 28.9 | 28.3 |
| Average bias, ml/min|1.73m2 (95% Confidence Interval) | 0 | −7.4 (−8.1, −6.7) |
+6.1 (+5.3, +7.0) |
+1.2 (+0.3, +2.0) |
+1.5 (+1.0, +2.0) |
| Ratio of SDs (95% Confidence Interval) | 1 |
0.95 (0.91, 0.99) |
1.47 (1.40, 1.54) |
1.41 (1.35, 1.47) |
1.39 (1.35, 1.43) |
| Correlation | 1 | 0.96 | 0.96 | 0.95 | 0.99 |
| RMSE, ml/min|1.73m2 | 0 | 5.3 | 8.2 | 8.7 | 4.5 |
| % within 30% of CKiDSCr-Cys | Reference | 76% | 80% | 81% | 86% |
Abbreviations: CKiD eGFR equation based on serum creatinine and cystatin C (CKiDSCr-Cys); CKiD eGFR equation based on serum creatinine only (CKiDSCr); CKD-EPI eGFR equation based on serum creatinine only (CKD-EPISCr), cystatin C only (CKD-EPICys), and serum creatinine and cystatin C (CKD-EPISCr-Cys); standard deviations (SDs); root mean square error (RMSE).
Table 2 presents the agreement metrics for various GFR estimating equations using iGFR as the reference, among 279 person-visits. CKiDSCr substantially underestimated iGFR (average bias= −5.6 ml/min|1.73m2) and had smaller dispersion (ratio of SDs= 0.89) with a correlation of 0.89. In contrast, CKD-EPISCr substantially overestimated iGFR (average bias= +8.2 ml/min|1.73m2) and had more variability (ratio of SDs= 1.39), with a correlation of 0.91. Both CKiDSCr and CKD-EPISCr had relatively low proportions within 30% of iGFR, at 71% and 66%, respectively. Agreement was substantially better for CKD-EPI equations that included cystatin C. On average, there was less overestimation of CKiDSCr-Cys by CKD-EPICys and CKD-EPISCr-Cys (average biases= +2.7 and +3.3, respectively). Both CKD-EPICys and CKD-EPISCr-Cys equations had larger variability than iGFR (ratios of SDs= 1.32 and 1.31, respectively). The CKD-EPICys equation had good correlation (r= 0.90) and accuracy (74% within 30% of CKiDSCr-Cys). However, the CKD-EPISCr-Cys had excellent correlation (r= 0.94) and similar accuracy (76% were within 30% of iGFR) and had the best metrics compared to the other CKD-EPI equations. This similar agreement of CKiDSCr-Cys and CKD-EPISCr-Cys with iGFR was likely explained by both equations utilizing the same primary biomarkers of kidney function (i.e., SCr and Cys).
Table 3 describes the agreement when using CKiDSCr-Cys as the reference based on 548 person-visits. Similar to Table 2, biases of similar magnitude but opposite directions, were observed for both CKiDSCr (−7.4 ml/min|1.73m2) and CKD-EPISCr (+6.1 ml/min|1.73m2), and the best agreement with CKiDSCr-Cys was the CKD-EPISCr-Cys equation, including minimal bias (+1.5 ml/min|1.73m2), high correlation (r= 0.99) and highest accuracy (86% within 30% of CKiDSCr-Cys). It should be noted that CKD-EPISCr-Cys had the same phenomenon of greater dispersion common to all CKD-EPI equations. Accuracy for the CKiDSCr and CKD-EPI equations was higher than in the agreement analysis utilizing iGFR as the reference ranging from 76% to 86% within 30% of CKiDSCr-Cys. Lastly, minimal bias was observed for CKD-EPI equations including cystatin c (+1.2 and +1.5 ml/min|1.73m2 for CKD-EPICys and CKD-EPISCr-Cys respectively). While these results were statistically significant, the overall biases were clinically negligible (≈ 1 ml/min|1.73m2).
Comparing creatinine only based equations demonstrated a substantial difference between CKiDSCr and CKD-EPISCr: the CKD-EPISCr equation was on average 14 ml/min|1.73m2 higher than the CKiDSCr equation. Compared to the CKiDSCr-Cys and iGFR, the CKiDSCr was lower and the CKD-EPISCr was higher (Table 2 and Table 3). To address this large discrepancy, and provide an easy tool for clinical use, we evaluated the agreement between the simple average of CKiDSCr and CKD-EPISCr, with iGFR and CKiDSCr-Cys as references. Table 4 presents the agreement between iGFR and the average of CKiDSCr and CKD-EPISCr showing minimal average bias (+1.3 ml/min|1.73m2, 95%CI: −0.1, +2.7), a ratio of SDs of 1.13 (95%CI: 1.04, 1.22), high correlation (r= 0.91) and a root mean square error (RMSE) of 5.8 ml/min|1.73m2. The accuracy for this blended estimate was also high with 80% within 30% of iGFR, which was substantially better compared to CKiDSCr (71%) and CKD-EPISCr-Cys equations (66%) alone. Figure 2 presents the full agreement between the average of the SCr-based equations and iGFR. We observed that the average of the SCr-based equations slightly underestimated CKiDSCr-Cys at lower levels of average GFR (i.e., < 30 min/min|1.73m2). Similar results were observed with CKiDSCr-Cys as the reference: the average of the two SCr equations had minimal and non-significant bias (−0.6 ml/min|1.73m2, 95%CI: −1.3, +0.004), slightly increased variance (ratio of SDs= 1.20, 95%CI: 1.15, 1.25), very high correlation (r= 0.97), RMSE of 5.8 ml/min|1.73m2, and 88% were within 30% of iGFR which was higher than CKiDSCr (76%) and CKD-EPISCr (86%) alone. Figure 3 displays the excellent agreement with the average and CKiDSCr-Cys and shows a similar underestimation at GFR < 30 ml/min|1.73m2, but excellent agreement levels higher than this threshold.
Table 4.
Measures of agreement for the average of CKiDSCr and CKD-EPISCr estimating GFR equations with iohexol GFR (n= 279 observations from 187 individuals) and CKiDSCr-Cys estimated GFR (n= 548 observations from 219 individuals) as the reference instruments among young adults with a history of pediatric CKD.
| iGFR | Average of CKiDSCr and CKD-EPIScr | CKiDSCr-Cys | Average of CKiDSCr and CKD-EPIScr | |
|---|---|---|---|---|
| Mean, ml/min|1.73m2 | 49.2 | 50.5 | 50.2 | 49.6 |
| Standard deviation | 22.5 | 25.2 | 20.4 | 24.4 |
| Average bias, ml/min|1.73m2 (95%CI) |
0 | +1.3 (−0.1, +2.7) |
0 | −0.6 (−1.3, +0.004) |
| Ratio of SDs (95%CI) | 1 |
1.13 (1.04, 1.22) |
1 |
1.20 (1.15, 1.25) |
| Correlation | 1 | 0.91 | 1 | 0.97 |
| RMSE, ml/min|1.73m2 | 0 | 10.5 | 0 | 5.8 |
| % within 30% of referent | Reference | 80% | Reference | 88% |
Abbreviations: Iohexol GFR (iGFR); CKiD eGFR equation based on serum creatinine and cystatin C (CKiDSCr-Cys); CKiD eGFR equation based on serum creatinine only (CKiDSCr); CKD-EPI eGFR equation based on serum creatinine only (CKD-EPISCr); standard deviations (SDs); root mean square error (RMSE).
Figure 2.
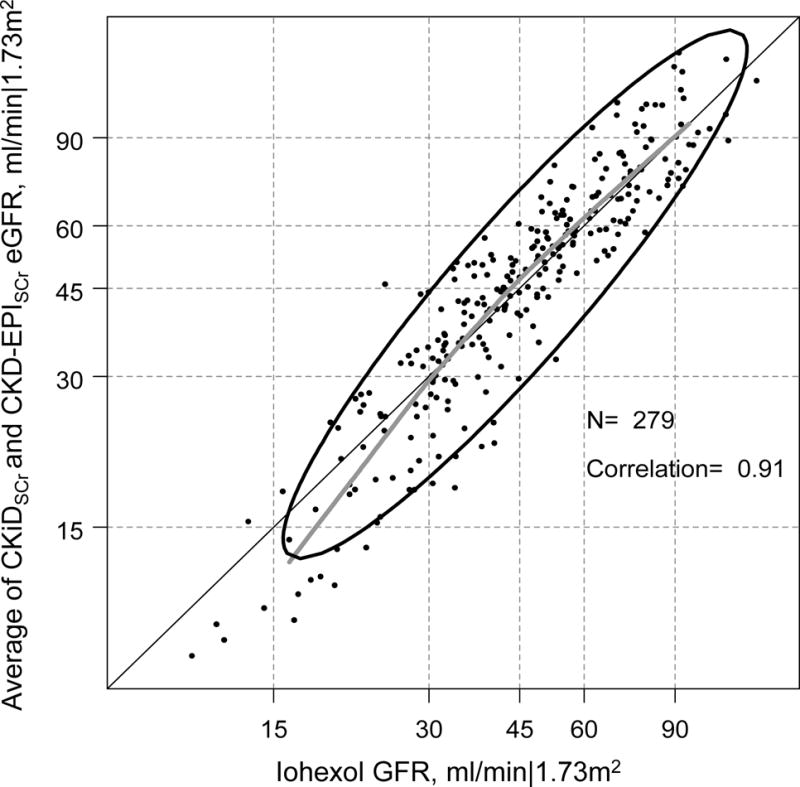
Agreement plot of the average of CKiD eGFR and CKD-EPI eGFR based on serum creatinine-only (CKiDSCr and CKD-EPISCr eGFR) and directly measured iohexol GFR (iGFR) in the log scale among 279 person-visits from 187 young adults with a history of pediatric CKD. The bolded grey line is a nonparametric spline based on 95% of the data; the ellipse region depicts the 90% confidence region of the data based on a bivariate normal distribution.
Figure 3.
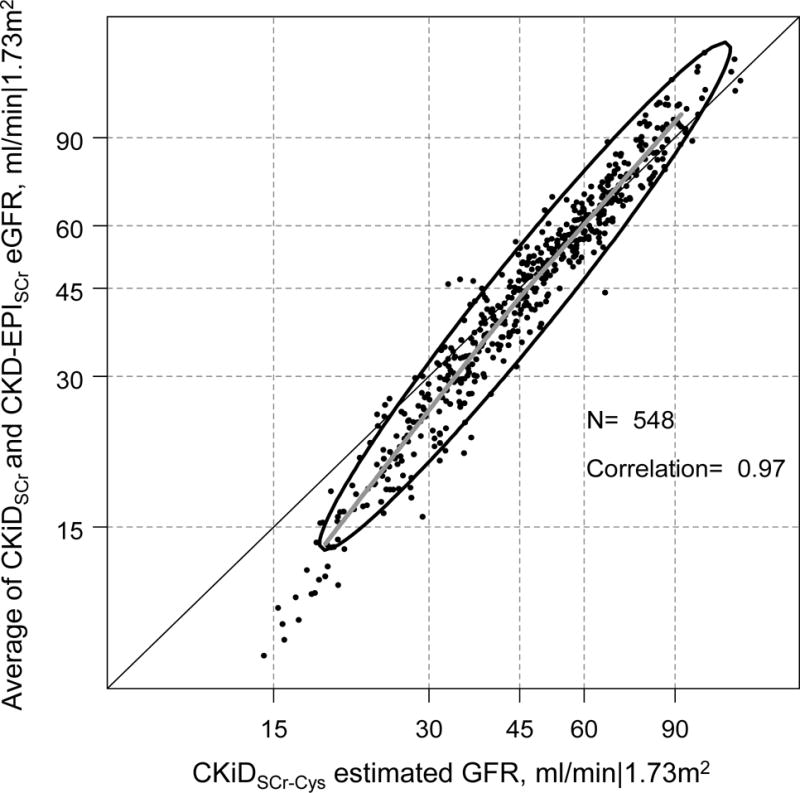
Agreement plot of the average of CKiD eGFR and CKD-EPI eGFR based on serum creatinine-only (CKiDSCr and CKD-EPISCr eGFR) and CKiDSCr-Cys in the log scale among 548 person-visits from 219 young adults with a history of pediatric CKD. The bolded grey line is a non-parametric spline based on 95% of the data; the ellipse region depicts the 90% confidence region of the data based on a bivariate normal distribution.
Discussion
In this population of emerging adults with CKD, the present study documented the clinical observation that estimates of GFR using creatinine-based estimating equations appear to “increase” when pediatric patients transition to adult care and equations developed in adult populations are used for CKD management (mean CKiDSCr = 43.6 vs. mean CKD-EPISCr= 57.4 ml/min|1.73m2). Importantly, our analysis suggested that both SCr-based equations were biased, in contrast to a report recommending the use of the CKiDSCr equation for a similar population with normal kidney function10. We provide a simple method to address this measurement problem and demonstrate that the average of the two can provide an overall unbiased estimate of GFR, when GFR is greater than 30 ml/min|1.73m2 and will likely underestimate GFR when this average is less than 30 ml/min|1.73m2. This analysis also demonstrated excellent agreement between the CKiD 2012 equation (CKiDSCr-Cys) developed for pediatric populations and the CKD-EPI eGFR equations including cystatin C that were developed for adult populations. For general clinical use, physicians can expect congruence between these equations when cystatin C is available.
When only SCr is available, the pediatric CKiD bedside equation underestimated GFR and the adult CKD-EPI equation overestimated measured and cystatin C-based estimated kidney function and this was consistent with an external emerging young adult population with relatively normal GFR10. The discrepancies were often large and systematic and present a clinical challenge in discerning GFR for this high risk population, particularly since assessment of SCr only is the most common clinical scenario as it can be measured quickly at low cost. In contrast, cystatin C is often not readily available clinically due to high expense and long processing times. We demonstrate that the mean of the creatinine-based CKiD and CKD-EPI eGFR values was unbiased on average and had excellent correlation with the CKiDSCr-Cys using both SCr and cystatin C, as well as directly measured GFR. It should be noted that while such an approach is viable in the absence of cystatin C in the clinical setting, it should not be seen as a complete replacement for cystatin C measurement or directly measured GFR. However, given the cost and potential delays in determining cystatin C, taking the average of the CKiDSCr and CKD-EPIScr is a reasonable alternative and facilitates prompt clinical decision-making in this special population of emerging adults with CKD. Figures 2 and 3 demonstrate that the average may underestimate when GFR is less than 30 ml/min|1.73m2. In those instances of lower GFR, we recommend additional measurement of cystatin C to estimate GFR using the CKiDSCr-Cys equation. The specificity of our study population suggests the need to explore these phenomena in more diverse populations.
While the pediatric and adult eGFR equations that use cystatin C demonstrated excellent agreement, the agreement was less strong when compared with directly measured iohexol GFR, especially the measure of accuracy (i.e., the proportion of eGFR observations within 30% of iGFR), with the exception of CKiDSCr-Cys. The accuracy of CKiDSCr-Cys compared to iGFR in this population replicated the results from the equation validation (90% vs. 91%). However, the range of proportions within 30% of iGFR for the other estimating equations was less robust (between 66% and 76%). In the validation of the CKD-EPISCr-Cys using iothalamate-based measured GFR as the reference, the accuracy metric was 91.5%. The lower accuracy may be due to this special clinical population being a minimal subset of the population used to develop the CKD-EPI equations, and being entirely absent in the development of the CKiD bedside equation. Despite the differences in accuracy, it is reassuring that there was excellent overall agreement, particularly correlation, between the adult estimating equations utilizing cystatin C, as well as the average of SCr-based equations, with the pediatric equation using SCr and cystatin C (CKiDSCr-Cys).
Serum creatinine concentration is influenced by kidney function and muscle mass, and the pediatric and adult equations account for the latter indirectly by height and age, respectively. Our data demonstrate that the transition from using pediatric to adult equations based on SCr (without cystatin C) in this young adult population with diminished GFR is disjointed and that both equations are biased in opposite directions on average. This may be due to a non-linear increase in pubertal muscle mass that is not captured by the height parameter in the CKiD equation, but has been proposed in equations for Japanese adolescents22–24. Similarly, age in the CKD-EPISCr equation appears not to capture the heterogeneity in muscle mass in our population, despite additional variables of sex and race. While our data are limited since muscle mass was not measured to fully investigate this relationship, we hypothesize that heterogeneity in muscle mass is lower in younger children and older adults and thus, the equations work well overall for the populations in which they were predominantly developed. The discrepancies observed here are largely due to this young adult population with a history of pediatric CKD not being well represented in the CKD-EPI equation development and being entirely absent in the CKiD equation development. The average of the pediatric and adult equations may share information based on age and height that is not present when only one is used and this could account for the improved agreement. Since cystatin C is invariant to muscle mass, it was expected that the pediatric CKiD and adult CKD-EPI equations that included cystatin C had excellent agreement. This suggests that muscle mass likely explains differences in the pediatric and adult SCr-based equations in this population.
Higher variability was consistently observed for the CKD-EPI equations compared to CKiDSCr-Cys, despite the presence of essentially the same kidney-related biomarkers. Since age and height were relatively homogenous in this population, it was apparent that biomarkers and not these variables were more influential in determining GFR level. A comparison of the coefficients of SCr and cystatin C in each equation revealed that the CKD-EPI equation had stronger associations (i.e., steeper slopes equating the larger coefficients) compared to the CKiDSCr-Cys equation. Specifically, within the CKD-EPI equations, the parameters for SCr and cystatin C were further away from 0 (i.e., away from the null) compared to the CKiDSCr-Cys. Despite these differences in parameters, there was a preservation of rank and corresponding high correlation.
Principles of regression calibration explain how these differences may influence epidemiologic inference when different eGFR measures are used as independent variables in regression models25. Specifically, the CKD-EPISCr-Cys and CKD-EPICys instruments will have attenuated associations relative to CKiDSCr-Cys. However, the high correlation between these instruments mean that the inferences will be identical, as well as the statistical significance, and this gives reassurance that either instrument, as long as cystatin C is included, will provide the same epidemiologic information. While the correlation by itself is not a sufficient metric of agreement, it is a necessary feature to evaluate these instruments that measure the same construct on the same scale.
There were several limitations to this analysis. First, this population was unique and best described as emerging adults between 18 and 23 years of age with a history of childhood or early onset CKD. We believe our results will apply to this specific population, but further studies are needed for validation. Despite this specificity, these patients represent a particularly challenging and high risk group at a vulnerable time during transitions in care and few other studies have characterized this population24. Additionally, our study population was independent from the development of the equations (i.e., no data here were used to derive or validate the CKiD equations), but was not completely external as 94 and 129 participants in our sample provided pediatric data earlier in life to develop the CKiDSCr and CKiDSCr-Cys equations, respectively. External validation to replicate the excellent agreement observed in the average of SCr-based equations in a completely separate, but similar, population is desirable. Third, we did not evaluate newly proposed universal equations26,27 as the primary aim was to investigate current clinical practice in North America, but our analysis may be a foundation for further explorations towards a unified equation across age groups. Lastly, we did not explore physical maturity in which similar dynamics may be expected, specifically among those less than 18 years, but who are physically mature.
In conclusion, we demonstrated that pediatric and adult serum creatinine-only equations are often widely discrepant and may contribute to problems in clinical assessment and decision making for emerging adults with CKD. Ideally, GFR estimating equations that use SCr and cystatin should be used for this population, with the expectation that adult equations will provide lower GFR estimates than the pediatric CKiDSCr-Cys equation at levels < 30 ml/min|1.73m2, and higher estimates at levels greater than 30 ml/min|1.73m2. The high correlation and overall low bias make any of these cystatin-based equations preferable for research purposes as well. When SCr is present but cystatin C is not, which we recognize as the most common clinical situation, the average of CKiDSCr and CKD-EPISCr provides an overall unbiased estimate of GFR, especially at levels of GFR above 30 ml/min|1.73m2, and maintains high correlation but some underestimation (conservative) bias at lower levels. While the blending of these two has many appealing properties and provides a good initial assessment of kidney function for immediate clinical use, cystatin C-based equations play a critical confirmatory role for determining kidney function, particularly at lower levels of kidney function.
Methods
The Chronic Kidney Disease in Children (CKiD) Cohort
The CKiD study is a multicenter longitudinal cohort study of 891 children diagnosed with CKD and enrolled in the study between the ages of 1 and 16 years and with a GFR < 90 ml/min|1.73m2. Study enrollment began in 2005 with 56 contributing sites in the US and Canada and study conduct consists of annual visits at which kidney function, cardiovascular health, growth and neurocognitive function are measured. Details of the study design have been previously described28. Since the primary aim of this analysis was to compare GFR by different methods and equations in older adolescents and young adults, our study population comprised 219 participants who contributed data after the age of 18.
Measurement of GFR
Direct measurement of GFR (iGFR) was determined by plasma disappearance of iohexol every two years in this study population. A two-compartment model was used from 2005 to 201129,30, and a one compartment model was used subsequently with correction based on a universal equation31. As part of a multi-laboratory assay recalibration, iohexol concentrations measured between November 2006 and March 2016 were divided by 0.89 (i.e., iGFR decreased by 1/0.89, or approximately 12%)32.
Kidney function biomarkers SCr, Cys, and BUN used to estimate GFR were measured at all annual CKiD study visits at the Central Biochemistry Laboratory. All SCr measurements were calibrated to IFCC standards. Cystatin C measurements were also calibrated to IFCC standards for calculation of CKD-EPI-based estimated GFR. Two pediatric estimated GFR equations were used: the CKiDSCr equation based on height and SCr1 and the full CKiD CKiDSCr-Cys based on SCr, Cys, BUN, height and sex2. These equations were of the form:
where height is in meters, SCr is in mg/dL, Cys is in mg/L and BUN is in mg/dL.
Three adult estimated GFR equations evaluated were based on the CKD-EPI collaboration3,4:
female: κ = .7, α = −0.329.
Male: κ = 0.9, α = −0.411
female: κ = 0.7, α = −0.248.
Male: κ = 0.9, α = −0.207.
where SCr is measured in mg/dL, and Cys is measured in mg/L for all equations. All equations were standardized to body surface area (BSA) equal to 1.73 m2 and the units of measurement are ml/min|1.73m2.
Since SCr is often the only biomarker of kidney function clinically available, we also investigated a combination of the CKiDSCr and a CKD-EPISCr by taking a simple average of the two.
Statistical methods
To describe the agreement between two measures of GFR, standard agreement plots and extensions of Bland-Altman methods were applied29. Specifically, the difference between the comparator and the reference was regressed on the average of the comparator and reference (centered at the population mean of this average). In the presence of normally distributed data, the intercept of the regression model is interpreted as the average bias, the antilog of the slope of the regression model approximates the ratio of standard deviations and the root mean square error of the regression model is directly proportional to the difference of the square of the correlation from 1 (i.e., will be zero if correlation = 1). These three components summarize the overall differences between comparator and reference values (bias), amount of variability (ratio of standard deviations) and preservation of ranking (correlation) in a single model and allow for the calculation of confidence intervals. The ratio of standard deviations is a metric of the relative difference in variability which may reflect removal of non-informative noise or informative variability (in the case of this ratio being less than 1) or the addition of non-informative noise (in the case of this ratio being greater than 1). Since our analytic sample included repeated measurements within individuals, generalized estimating equations (GEE) were used in these regression models to account for within-person correlation of observations31,33. To estimate accuracy, we additionally calculated the proportion of observations within 30% of the reference and determined the root mean square error (RMSE) based on linear regression models.
The analysis assessed the agreement between all estimating equations (i.e., CKiDSCr-Cys, CKiDSCr, CKD-EPISCr, CKD-EPICys, CKD-EPISCr-Cys) using iGFR as the reference in this population of young adults with a history of pediatric CKD. The second analysis compared the same equations with CKiDSCr-Cys as the reference to maximize available data using the best clinical pediatric eGFR instrument currently available (i.e., CKiDSCr-Cys). Lastly, we compared the simple average of CKiDSCr and CKD-EPISCr to iGFR and CKiDSCr-Cys as references and formally assessed the statistical agreement properties and provided graphical depictions of the data. For each agreement plot, an ellipsoid was derived from the bivariate normal distribution (in the log scale) to display the region that is expected to encompass 90% of the data. Thus, each ellipsoid is a function of the means, standard deviations and correlation of the two variables.
Acknowledgments
Data in this manuscript were collected by the Chronic Kidney Disease in children prospective cohort study (CKiD) with clinical coordinating centers (Principal Investigators) at Children’s Mercy Hospital and the University of Missouri - Kansas City (Bradley Warady, MD) and Children’s Hospital of Philadelphia (Susan Furth, MD, PhD), Central Biochemistry Laboratory (George Schwartz, MD) at the University of Rochester Medical Center, and data coordinating center (Alvaro Muñoz, PhD) at the Johns Hopkins Bloomberg School of Public Health. The CKiD Study is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Heart, Lung, and Blood Institute (U01-DK-66143, U01-DK-66174, U01-DK-082194, U01-DK-66116). The CKiD website is located at http://www.statepi.jhsph.edu/ckid. The authors acknowledge Drs. Alvaro Muñoz and Josef Coresh for critical input in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Competing Financial Interests
None declared.
References
- 1.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol JASN. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz GJ, Schneider MF, Maier PS, et al. Improved equations estimating GFR in children with chronic kidney disease using an immunonephelometric determination of cystatin C. Kidney Int. 2012;82:445–453. doi: 10.1038/ki.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neild GH. What do we know about chronic renal failure in young adults? I. Primary renal disease. Pediatr Nephrol Berl Ger. 2009;24:1913–1919. doi: 10.1007/s00467-008-1108-3. [DOI] [PubMed] [Google Scholar]
- 6.Neild GH. What do we know about chronic renal failure in young adults? II. Adult outcome of pediatric renal disease. Pediatr Nephrol Berl Ger. 2009;24:1921–1928. doi: 10.1007/s00467-008-1107-4. [DOI] [PubMed] [Google Scholar]
- 7.Harambat J, van Stralen KJ, Kim JJ, et al. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363–373. doi: 10.1007/s00467-011-1939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodhouse CRJ, Neild GH, Yu RN, et al. Adult care of children from pediatric urology. J Urol. 2012;187:1164–1171. doi: 10.1016/j.juro.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Wühl E, van Stralen KJ, Verrina E, et al. Timing and outcome of renal replacement therapy in patients with congenital malformations of the kidney and urinary tract. Clin J Am Soc Nephrol CJASN. 2013;8:67–74. doi: 10.2215/CJN.03310412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selistre L, De Souza V, Cochat P, et al. GFR estimation in adolescents and young adults. J Am Soc Nephrol JASN. 2012;23:989–996. doi: 10.1681/ASN.2011070705. [DOI] [PubMed] [Google Scholar]
- 11.Ng DK, Schwartz GJ, Warady BA, et al. Relationships of Measured Iohexol GFR and Estimated GFR With CKD-Related Biomarkers in Children and Adolescents. Am J Kidney Dis Off J Natl Kidney Found. 2017 doi: 10.1053/j.ajkd.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma AP, Yasin A, Garg AX, et al. Diagnostic accuracy of cystatin C-based eGFR equations at different GFR levels in children. Clin J Am Soc Nephrol CJASN. 2011;6:1599–1608. doi: 10.2215/CJN.10161110. [DOI] [PubMed] [Google Scholar]
- 13.Laskin BL, Nehus E, Goebel J, et al. Cystatin C-estimated glomerular filtration rate in pediatric autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2012;18:1745–1752. doi: 10.1016/j.bbmt.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Chehade H, Cachat F, Jannot A-S, et al. Combined serum creatinine and cystatin C Schwartz formula predicts kidney function better than the combined CKD-EPI formula in children. Am J Nephrol. 2013;38:300–306. doi: 10.1159/000354920. [DOI] [PubMed] [Google Scholar]
- 15.Nehus EJ, Laskin BL, Kathman TI, et al. Performance of cystatin C-based equations in a pediatric cohort at high risk of kidney injury. Pediatr Nephrol Berl Ger. 2013;28:453–461. doi: 10.1007/s00467-012-2341-3. [DOI] [PubMed] [Google Scholar]
- 16.Chehade H, Cachat F, Jannot A-S, et al. New combined serum creatinine and cystatin C quadratic formula for GFR assessment in children. Clin J Am Soc Nephrol CJASN. 2014;9:54–63. doi: 10.2215/CJN.00940113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siddique K, Leonard D, Borders L, et al. Validation of the CKiD formulae to estimate GFR in children post renal transplant. Pediatr Nephrol Berl Ger. 2014;29:445–451. doi: 10.1007/s00467-013-2660-z. [DOI] [PubMed] [Google Scholar]
- 18.de Souza V, Cochat P, Rabilloud M, et al. Accuracy of different equations in estimating GFR in pediatric kidney transplant recipients. Clin J Am Soc Nephrol CJASN. 2015;10:463–470. doi: 10.2215/CJN.06300614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR Using Serum Cystatin C Alone and in Combination With Serum Creatinine: A Pooled Analysis of 3,418 Individuals With CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu C, Propert K, Xie D, et al. Measured GFR does not outperform estimated GFR in predicting CKD-related complications. J Am Soc Nephrol JASN. 2011;22:1931–1937. doi: 10.1681/ASN.2010101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shlipak MG, Matsushita K, Ärnlöv J, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med. 2013;369:932–943. doi: 10.1056/NEJMoa1214234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uemura O, Honda M, Matsuyama T, et al. Is the new Schwartz equation derived from serum creatinine and body length suitable for evaluation of renal function in Japanese children? Eur J Pediatr. 2012;171:1401–1404. doi: 10.1007/s00431-012-1772-y. [DOI] [PubMed] [Google Scholar]
- 23.Uemura O, Nagai T, Ishikura K, et al. Creatinine-based equation to estimate the glomerular filtration rate in Japanese children and adolescents with chronic kidney disease. Clin Exp Nephrol. 2014;18:626–633. doi: 10.1007/s10157-013-0856-y. [DOI] [PubMed] [Google Scholar]
- 24.Uemura O, Yokoyama H, Ishikura K, et al. Performance in adolescents of the two Japanese serum creatinine based estimated glomerular filtration rate equations, for adults and paediatric patients: A study of the Japan Renal Biopsy Registry and Japan Kidney Disease Registry from 2007 to 2013. Nephrol Carlton Vic. 2017;22:494–497. doi: 10.1111/nep.12982. [DOI] [PubMed] [Google Scholar]
- 25.Rosner B, Willett WC, Spiegelman D. Correction of logistic regression relative risk estimates and confidence intervals for systematic within-person measurement error. Stat Med. 1989;8:1051–1069. doi: 10.1002/sim.4780080905. [DOI] [PubMed] [Google Scholar]
- 26.Hoste L, Dubourg L, Selistre L, et al. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant. 2014;29:1082–1091. doi: 10.1093/ndt/gft277. [DOI] [PubMed] [Google Scholar]
- 27.Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2017;32:497–507. doi: 10.1093/ndt/gfw425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furth SL, Cole SR, Moxey-Mims M, et al. Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol CJASN. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Furth S, Cole SR, et al. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz GJ, Abraham AG, Furth SL, et al. Optimizing iohexol plasma disappearance curves to measure the glomerular filtration rate in children with chronic kidney disease. Kidney Int. 2010;77:65–71. doi: 10.1038/ki.2009.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng DKS, Schwartz GJ, Jacobson LP, et al. Universal GFR determination based on two time points during plasma iohexol disappearance. Kidney Int. 2011;80:423–430. doi: 10.1038/ki.2011.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz G, Wang H, Erway B, et al. Multicenter Laboratory Comparison of Iohexol Measurement. J Appl Lab Med. doi: 10.1373/jalm.2017.024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]


