Abstract
Prebiotic milk oligosaccharides (MO) are complex sugars that selectively enhance the growth of Bifidobacterium infantis (B. infantis). The current study examines the effects of bovine MO and B. infantis in preventing non-alcoholic steatohepatitis (NASH) in Western diet (WD)-fed bile acid (BA) receptor FXR (farnesoid × receptor) knockout (KO) mice. WD-fed FXR KO mice, which have cancer-prone NASH and reduced B. infantis, were supplemented with B. infantis, MO, and combination of both. Two months intervention by B. infantis and/or MO improved insulin sensitivity. In addition, all 3 treatments reduced expression of pro-inflammatory genes in the liver and ileum. Consistently, 7 months treatment reduced hepatic lymphocyte infiltration in WD-fed FXR KO mice. In addition, B. infantis, but not MO, decreased hepatic triglyceride and cholesterol. A combination of both further reduced hepatic cholesterol, the precursor of BAs, but not hepatic triglyceride. All three treatments modulated hepatic and serum BA profile by reducing deoxycholic acid, hyodeoxycholic acid and increasing chenodeoxycholic acid as well as ursodeoxycholic acid level. In addition, B. infantis and MO decreased hepatic CYP7A1 and increased the expression of Sult2a1, Sult2a2, and Sult2a3 suggesting decreased BA synthesis and increased detoxification. Furthermore, B. infantis and MO increased ileal BA membrane receptor TGR5 as well as Glp1r, PC1/3, and Nos3 suggesting increased TGR5-regulated signaling. Moreover, MO alone, but not B. infantis, could increase the abundance of butyrate-generating bacterium that has beneficial effect in NASH treatment. Together, B. infantis and MO have their unique and combined effects in reversing NASH in WD-fed FXR KO mice.
Keywords: Probiotics, prebiotics, microbiota, liver cancer, bile acid, dysbiosis, TGR5
1. Introduction
Bifidobacteria have co-evolved with humans, and it appears to be beneficial to the well-being of infants [1]. Specifically, Bifidobacterium longum subsp. infantis (B. infantis) has intestinal and extra-intestinal health benefits [2]. B. infantis modulates gut barrier functions and protects epithelial cells against cytokine or chemical-induced inflammation [3, 4]. In addition, B. longum is effective in preventing allergic pathologies, psoriasis, and chronic fatigue syndrome [2, 5]. Moreover, other Bifidobacterium spp. has anti-obesity effect and can improve alcohol-induced liver injury [6]. During evolution, B. infantis acquired an ability to metabolize human milk oligosaccharides (MO) that are complex sugar and nutrient for newborns [7, 8]. Additionally, MO enhance the growth of Bifidobacteria and Lactobacilli at the expense of potentially harmful bacteria such as Clostridia, Enterococci, Eubacteria, and Enterobacteria creating an acidic environment that is less favorable to pathogens [9–11]. Thus, the synbiotics containing probiotics B. infantis and prebiotics MO have added health benefits. For example, B. infantis plus MO are effective in inducing the expression of genes involved in integrity of barrier function (14). They also have anti-inflammatory effect in the intestinal epithelial cells [12]. Moreover, growing evidence indicate that breast milk in neonates as well as MO are beneficial to intestinal health [13]. Therefore, breast-fed infants have reduced intestinal permeability compared to formula-fed infants [14]. Because gut dysbiosis contribute to hepatic inflammation [15–18], we hypothesize that synbiotics B. infantis plus MO may have beneficial effects in protecting against the development of non-alcoholic steatohepatitis (NASH) caused by dysregulated bile acid (BA) synthesis and dysbiosis.
BAs are essential for lipid and carbohydrate metabolism and play a significant role in regulating host immunity and inflammation [18, 19]. Activation of farnesoid × receptor (FXR) by BAs, protects the distal small intestine from bacterial invasion and overgrowth in bile duct ligation in mouse models [20]. Activation of FXR also has beneficial effects against metabolic disorders [21, 22]. In addition, FXR agonists have promising effect in NASH treatment as revealed in clinical trials [23]. In metabolic disease mouse models, feeding wild type mice with a Western diet (WD) will not produce liver cancer in their lifetime. In contrast, mice lacking FXR develop steatosis, NASH, and liver cancer spontaneously, and WD-feeding facilitates the liver carcinogenesis process [24–26]. Consistently, patients with severe cirrhosis and liver cancer have reduced hepatic FXR [26, 27]. Moreover, dysregulated bile acid synthesis is frequently found in patients who have metabolic diseases including liver cancer. Together, FXR knockout (KO) mice are human relevant models that are useful to study the prevention and treatment of liver carcinogenesis.
It is known that dysregulated BA synthesis is always accompanied by dysbiosis because BAs are generated by host and bacterial enzymes [18, 28, 29]. Our published data have already uncovered specific BAs and gut microbiota that contribute to the development of NASH leading to cancer formation in FXR KO mouse models [15–17]. In the current study, we investigated a hypothesis that through modulating BA synthesis, synbiotics B. infantis plus bovine MO are effective in preventing the development of NASH. Our novel data revealed the beneficial effects of B. infantis and MO in reversing cancer-prone NASH in WD-fed FXR KO mice.
2. Materials and Methods
2.1. Bacterial culture condition
B. infantis (ATCC 15697, Manassas, VA, USA) were grown in a food grade facility and stored at −80°C [30]. The purity and viability of the bacteria were confirmed every 6 months; they were grown anaerobically at 37°C in a semisynthetic de Man, Rogosa, Sharpe broth (Becton Dickinson, Franklin Lakes, NJ, USA) supplemented with 1% (wt/vol) l-cysteine hydrochloride. After centrifugation, bacteria were suspended in saline before oral administration via gavage.
2.2 Bovine MO production and characterization
A product enriched in MO was supplied by Hilmar Ingredients (Hilmar, CA, USA). Bovine MO were concentrated and purified from commercially available whey permeate using a series of ultrafiltration and chromatographic steps. Lactose was partially removed by concentration, crystallization, and precipitation. The lactose and mineral reduced permeate was treated by an adsorption column containing 100 liters of functionalized copolymer of styrene and divinyl benzene to remove color in the stream. MO in the decolorized stream was further concentrated using a single stage ultrafiltration membranes (Molecular weight cut off of 1000 Dalton) to remove minerals and lactose in the solution. The final concentrate was freeze-dried and stored in a vacuum desiccator at room temperature. The total carbohydrate composition of MO was determined using an Agilent 6520 accurate-mass Q-TOF LC/MS with a microfluidic nano-electrospray chip according to previously published methods [31]. The MO profile of this product was consistent with a previously formulated supplement that has been used in a human clinical study [32]. The concentration of lactose and select MO was measured using a high-performance anion-exchange chromatography with pulsed amperometric detection (Thermo Scientific HPAEC-PAD ICS-5000, Sunnyvale, CA, USA). See supplemental information (Supplementary Table S1).
2.3. Mice
Specific pathogen-free C57BL/6 wild type (WT) mice (Jackson Laboratory, Sacramento, CA, USA) as well as age- and sex-matched FXR KO mice [33] were housed in steel microisolator cages at 22°C with a 12-hour light/dark cycle. They were given a WD containing 21.2% fat, 34% sucrose, and 0.2% cholesterol (Envigo, Indianapolis, IN, USA) right after weaning (3 weeks, 6–10 mice per group). For interventions, mice were given B. infantis (109 cfu per mouse, orally, once a week) in saline, bovine MO (7% in a diet that lacks 7% cellulose), or a combination of B. infantis plus MO while mice continued a WD. For short-term study, 3-month old mice were treated for 2 months and euthanized when they were 5-month old. For long-term study, 3-month old male mice were treated for 7 months and euthanized when they were 10-month old. Experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals under protocols approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
2.4. Biochemical assays and histology
Serum alanine aminotransferase (ALT; Pointe Scientific, Canton, MI, USA), serum alkaline phosphatase (ALP; Pointe Scientific), hepatic triglyceride, and hepatic cholesterol (BioAssay Systems, Hayward, CA, USA) levels were quantified according to the manufacturer’s instructions. Hematoxylin and eosin stained liver tissues were subjected to histology analysis. Steatosis score was graded on a scale of 0 (<5%), 1 (5%–33%), 2 (34%–66%), and 3 (>66%), and hepatic lymphocyte infiltration score was graded on a scale of 0 (absent), 1 (rare), 2 (mild), 3 (moderate), and 4 (severe).
2.5 Bile acid quantification
Frozen liver tissues (50 mg) were homogenized in cold methanol/internal standard solution (100 μl internal standard solution and 500 μl methanol). Serum samples (50 μl) were added to 500 μl of cold methanol and 100 μl of internal standard based on published methods [15–17, 34–36]. After centrifuge, the supernatant was dried in a Savant speedvac concentrator (Thermo Scientific, Rockford, IL, USA). The residue was then reconstituted by adding 50 μl of methanol: water (50:50, v/v) followed by centrifugation at 10,000 g for 1 min at 4°C. The supernatants were used for BA quantification. The detection of BAs was carried out on a Prominence™ UFLC system (Shimadzu, Kyoto, Japan) coupled to an API 4000 QTRAP™ mass spectrometer (ABSciex, Redwood City, CA, USA) operated in the negative ionization mode. Chromatography was performed on a Kinetex C18 column (50 mm × 2.1 mm, 2.6 μm) maintained at 40°C preceded by a high-pressure column prefilter. The mobile phase consisted of a gradient of methanol delivered at a flow rate of 0.4 ml/min. Mass Spectrometer parameters were described in our publications [35].
2.6. Mouse gene expression and quantification of bacterial genes
RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA, USA) and reverse transcribed into cDNA. qRT-PCR was performed on an ABI 7900HT Fast real-time PCR system using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Primers sequences are available in supplementary table S2. The mRNA levels were normalized to the level of Gapdh mRNA.
For bacterial gene quantification, DNA was extracted from cecal content (0.05 gram) using ZR Fecal DNA MiniPrep Kit (Zymo Research, Irvine, CA, USA), quantified by NanoDrop (Thermo Scientific, Wilmington, DE, USA), and amplified using primers (Supplementary table S2) based on the published sequences [15, 37–41]. A dissociation step was included to analyze the melting profile of amplified products. In parallel, qPCR was done using ten-fold serial diluted synthetic DNA fragments (Integrative DNA technologies, Redwood city, CA, USA) of a bacterial gene with known concentrations. Bacterial DNA concentration was calculated using standard curves of diluted synthetic DNA fragment based on published methods [42].
2.7. Protein extraction and quantification
Proteins were extracted (30 mg) by T-PER protein extraction reagent (Thermo Scientific, Rockford, IL, USA) with protease and phosphatase inhibitor cocktail (Thermo Scientific, Rockford, IL, USA) followed by protein quantification using protein bicinchoninic acid assay reagent (Thermo Scientific, Rockford, IL, USA). Hepatic protein (40 μg) was subjected to polyacrylamide gel electrophoresis under reducing conditions followed by transfer to polyvinylidene difluoride membranes. The membranes were incubated with 4% non-fat milk followed by an antibody. The following primary antibodies (catalogue number and dilutions) were used: CYP7A1 (MABD42, 1:1000, Merck, Temecula, CA, USA), CYP8B1 (TA313734, 1:1000, Origene Technologies, Rockville, MD, USA), SULT2A1 (OABF01591, 1:1000, Aviva systems, San Diego, CA, USA), and β-ACTIN (A1978, 1:10000; Sigma Chemical Co, St Louis, MO, USA).
Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies. The signals were detected using an ECL enhanced chemiluminescence system with Pierce SuperSignal West Pico chemiluminescent substrates (Thermo Fisher Scientific, Rockford, IL, USA).
2.8. Statistical analysis
Unpaired Student’s t-test and one-way analysis of variance were performed using Prism 6.0 (GraphPad, La Jolla, CA, USA). Data are expressed as mean ± SD. P-values were adjusted for multiple comparisons using false discovery rate. P < 0.05 was considered statistically significant.
3. Results
3.1. B. infantis and MO improve insulin sensitivity and reduce hepatic inflammatory signaling in WD-fed FXR KO mice after 2-months treatment
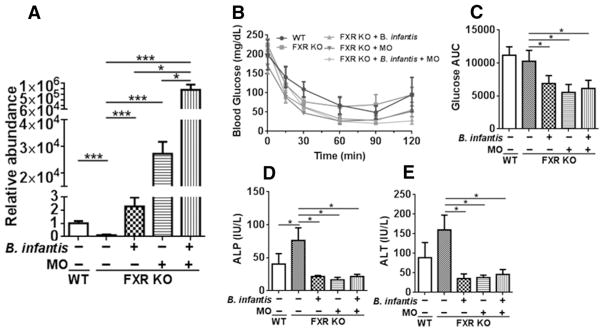
Our published data revealed that feeding FXR KO mice with a WD facilitated the development of NASH that lead to liver carcinogenesis [16]. The current study reveals for the first time that FXR KO mice had reduced B. infantis. However, supplementation of either B. infantis or MO for two months increased the abundance of B. infantis. In addition, a combination of both further increased B. infantis compared with single supplementation (Figure 1A). Although FXR regulates carbohydrate homeostasis, FXR deficiency did not affect blood glucose level post insulin injection in 5-month old WD-fed mice. However, B. infantis and/or MO improved insulin sensitivity (Figure 1B, C). In addition, FXR deficiency elevated serum ALP and ALT levels, which were reduced by B. infantis and/or MO (Figure 1D–E).
Figure 1. The effects of B. infantis, MO, and synbiotics B. infantis plus MO in WD-fed FXR KO mice.
(A) Relative abundance of B. infantis, (B) insulin sensitivity test, (C) area under curve of glucose post insulin injection, (D) serum alkaline phosphatase (ALP), and (E) serum alanine transferase (ALT) levels of WD-fed wild type (WT) mice as well as WD-fed FXR KO mice supplemented with and without B. infantis, MO, and B. infantis plus MO for 2 months. Data expressed as mean±SD. n ≥ 6 per group. *p<0.05, ***p<0.001, WT mice compared with FXR KO mice, and untreated FXR KO mice compared with treated FXR KO mice.
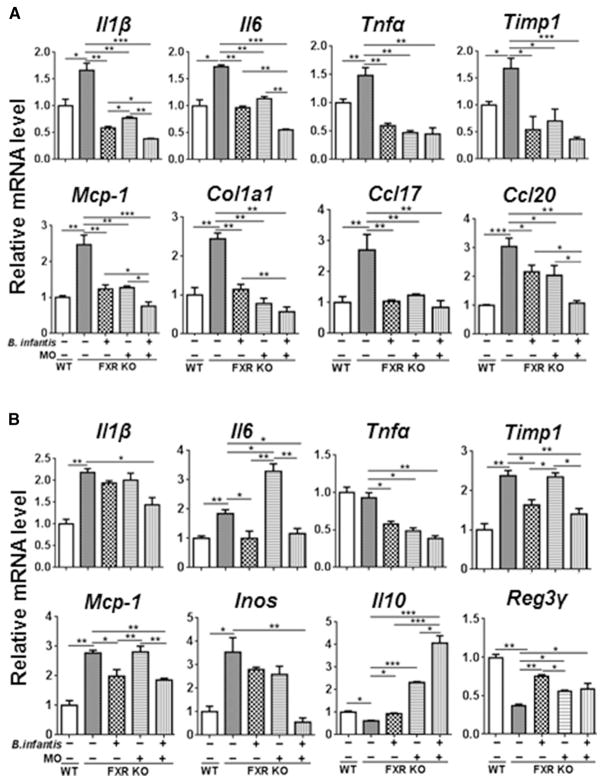
By studying the expression of hepatic pro-inflammatory and pro-fibrotic genes, the data revealed that Il1β, Il6, Tnfα, Timp1, Mcp-1, Col1a1, Ccl17, and Ccl20, which had increased expression level due to WD feeding and FXR inactivation, were reduced by 2 months of B. infantis and MO supplementation (Figure 2A).
Figure 2. The expression of inflammatory signaling genes in response to B. infantis and/or MO treatment.
The expression of hepatic genes (A) and ileal genes (B) in diet and gender-matched WT and FXR KO mice supplemented with and without B. infantis, MO, and B. infantis plus MO for 2 months. Data expressed as mean±SD. n ≥ 6 per group. *p<0.05, **p<0.01, ***p<0.001, WT mice compared with FXR KO mice, and untreated FXR KO mice compared with treated FXR KO mice.
Enterohepatic circulation of bile salts takes place in ileum, where expresses high level of FXR to regulate BA homeostasis. In addition, the liver receives 70% of its blood from the intestine and is constantly exposed to intestinal-derived metabolites. Moreover, our published data revealed that dysregulated BA homeostasis and gut dysbiosis contribute to the development of NASH [16,17]. Thus, gene expression was also studied in the ileum. Consistent with the data generated in the liver, the expression of ileal inflammatory genes, such as Il1β, Il6, Tnfα, Timp1, Mcp-1, and Inos, were also reduced with B. infantis plus MO supplementation (Figure 2B). However, B. infantis or MO alone did not reduce the expression of several pro-inflammatory genes such as ileal Il1β and Inos. Surprisingly, ileal Il6 mRNA was induced by MO alone, but reduced by B. infantis. Further, ileal Il10 and Reg3γ, which were reduced with FXR deficiency, were increased by all three treatments.
3.2. B. infantis and MO prevent NASH in WD-FXR KO mice after a long-term 7 months treatment
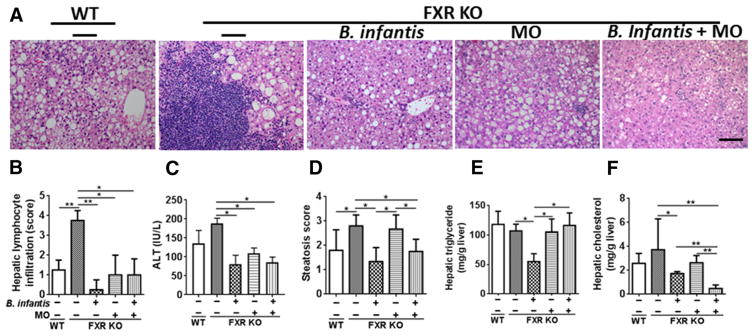
Although 5-month old FXR KO mice had increased hepatic inflammatory signaling, liver histology did not reveal apparent inflammation [15]. Hepatic lymphocyte infiltration was only detected when WD-fed FXR KO male mice were 10 months old [16]. Thus, long-term 7 months intervention was performed to analyze whether B. infantis and MO could eliminate hepatic lymphocytes induced by dysregulated BA synthesis. Consistent with previous observation, 10-month old WD-fed FXR KO mice developed severe NASH with massive hepatic lymphocyte infiltration (Figure 3A, B). B. infantis and MO eliminated hepatic lymphocytes and reduced ALT level (Figure 3A–C). Moreover, B. infantis improved fat score, as well as hepatic triglyceride and cholesterol levels. In contrast, MO had no effect in reducing hepatic triglyceride or cholesterol (Figure 3D–F). Nevertheless, hepatic cholesterol was substantially reduced and became even lower than that of B. infantis-treated group when a combination of both was used (Fig. 3F).
Figure 3. The effects of B. infantis, MO and B. infantis plus MO on liver pathology.
(A) Hematoxylin and eosin (H&E) staining of liver, (B) hepatic lymphocyte score, (C) serum ALT level, (D) steatosis score, (E) hepatic triglyceride, and (F) hepatic cholesterol levels of diet and gender-matched mice supplemented with and without B. infantis, MO, and B. infantis plus MO for 7 months. Data expressed as mean±SD. n ≥ 6 per group. *p<0.05, **p<0.01, WT mice compared with FXR KO mice, and untreated FXR KO mice compared with treated FXR KO mice.
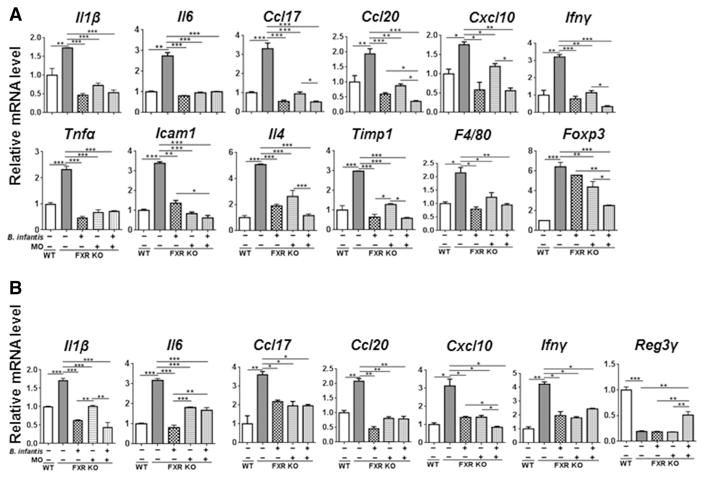
Both short and long-term experiments revealed the impressive anti-inflammatory effect of B. infantis and MO in the liver. For examples, the expressions of hepatic and Ileal genes, such as Il1β, Il6, Ccl17, Ccl20, Cxcl10, and Ifnγ, were induced in WD-fed FXR KO mice, but reduced by B. infantis and MO supplementation. In addition, WD intake and FXR KO-induced hepatic Tnfα, Icam1, Il4, Timp1, and F4/80 mRNAs were reduced by B. infantis and MO intake. However, a combination of B. infantis plus MO had better effects in reducing the expression of hepatic Foxp3 and inducing ileal Reg3γ mRNA levels (Figure 4A–B).
Figure 4. The expression of inflammatory signaling genes in response to B. infantis and/or MO treatments.
Hepatic (A) and Ileal (B) gene expression in in diet and gender-matched WT and FXR KO mice supplemented with and without B. infantis, MO, and B. infantis plus MO for 7 months. Data expressed as mean±SD. n ≥ 6 per group. **p<0.05, **p<0.01, ***p<0.001, WT mice compared with FXR KO mice, and untreated FXR KO mice compared with treated FXR KO mice.
3.3. B. infantis and MO modulate BA profile
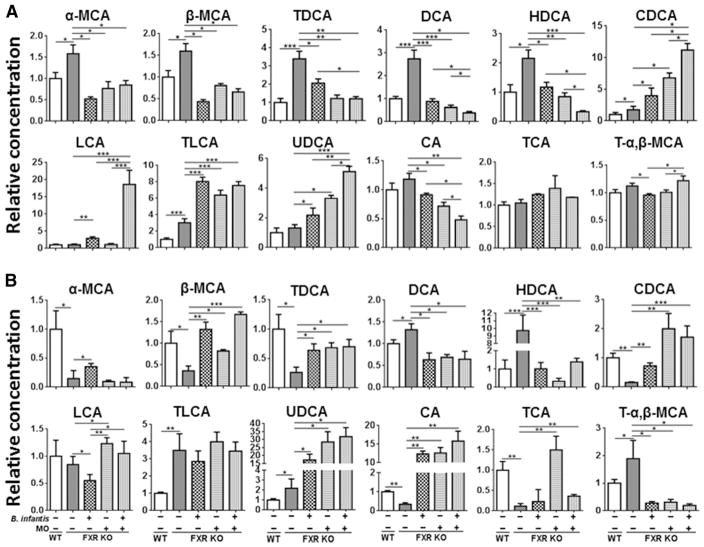
Because elevated BA leads to liver carcinogenesis in FXR KO mice [25, 43–45], we studied the effect of B. infantis and MO on shifting BA profile. The data showed that B. infantis and MO normalized the concentration of certain BAs in 10-month old WD-fed FXR KO mice. For example, FXR deficiency had opposite effects on changing hepatic and serum level of α-muricholic acid (α-MCA), β-muricholic acid (β-MCA) as well as taurodeoxycholic acid (TDCA), but B. infantis and/or MO treatments reversed those changes to a certain degree (Figure 5). In addition, increased hepatic and serum deoxycholic acid (DCA) and hyodeoxycholic acid (HDCA) found in WD-fed FXR KO mice were reduced by B. infantis and MO (Figure 5). However, B. infantis and MO increased hepatic chenodeoxycholic acid (CDCA) and taurolithocholic acid (TLCA), which are the endogenous ligands of FXR and TGR5 (Takeda G protein coupled receptor 5) (Figure 5A). Another TGR5 ligand, hepatic lithocholic acid (LCA) was increased by B. infantis and a combination of B. infantis plus MO. Moreover, B. infantis plus MO were more effective in reducing hepatic DCA, HDCA, and CA (cholic acid), as well as inducing hepatic CDCA, LCA, and UDCA (ursodeoxycholic acid) than the single treatment. Furthermore, all three treatments reduced hepatic CA, but increased serum CA suggesting the effect of B. infantis and MO in redistribution of CA and increasing TCA (taurocholic acid) de-conjugation in the gut (Figure 5). It has been shown that FXR deficiency can increase TCA pool size [46]. Our data showed TCA was reduced in serum, but not changed in liver, in FXR KO mice, suggesting TCA might be spilled from the liver to other sites in WD-fed FXR KO mice. Moreover, B. infantis and/or MO reversed the changes of TCA and T-α,β-MCA (tauro-α, β-muricholic acid) in the serum caused by FXR deficiency.
Figure 5. The effects of B. infantis and/or MO on hepatic and serum bile acid profiles in WD-fed FXR KO mice.
(A) Hepatic and (B) serum bile acid data are expressed as mean±SD. n ≥ 6 per group. *p<0.05, **p<0.001, ***p<0.001, WT mice compared with FXR KO mice, and untreated FXR KO mice compared with treated FXR KO mice.
3.4. B. infantis and MO affect BA synthesis
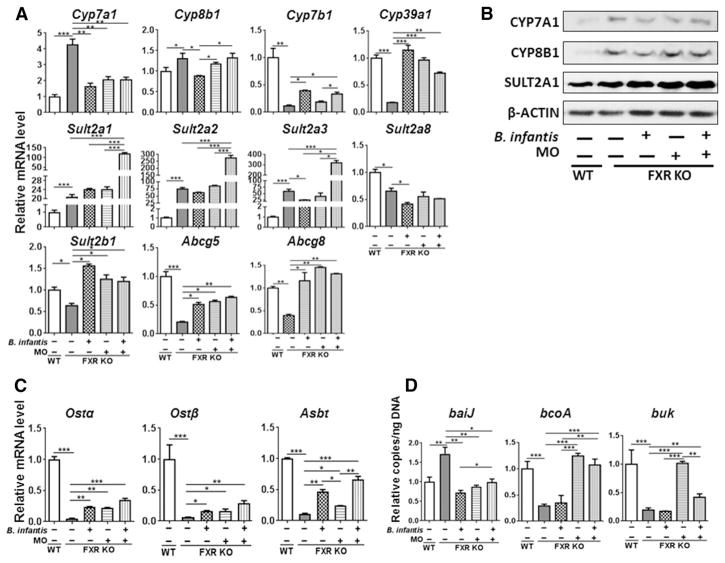
The expression of genes that regulate BA homeostasis was also shifted by B. infantis and MO treatment. For example, FXR deficiency-induced hepatic CYP7A1 was reduced by B. infantis and MO suggesting their effect in reducing hepatic CA synthesis (Fig. 6A, B). Only B. infantis reversed FXR deficiency-induced change of Cyp8b1 at mRNA and protein level. In addition, FXR deficiency-reduced hepatic Cyp7b1 and Cyp39a1 were increased by B. infantis and MO supplementation (Figure 6A). Moreover, the level of hepatic SULT2A1, which was increased with FXR deficiency, was further increased by B. infantis plus MO treatment (Figure 6A, B). Consistently, the mRNA level of Sult2a1, Sult2a2, and Sult2a3 was increased by FXR deficiency and further increased by a combination of B. infantis and MO treatment (Figure 6A). In contrast to all three isoforms of Sult2a, the hepatic mRNA level of Sult2a8, which was reduced with FXR deficiency, was further reduced by B. infantis alone (Figure 6A). Furthermore, the mRNA levels of Sult2b1, Abcg5, and Abcg8 were reduced by FXR deficiency, and all three treatments reversed such reductions (Figure 6A). In the ileum, FXR deficiency reduced mRNA level of organic solute transporters Ostα and Ostβ as well as Asbt, and all three treatments increased their levels (Figure 6C).
Figure 6. The expression of bile acid homeostasis genes in WD-fed FXR KO mice in response to B. infantis and/or MO treatment for 7 months.
(A) Hepatic gene expression, (B) Western blot analysis of indicated hepatic protein levels, (C) ileal gene expression, and (D) targeted functional quantitative PCR analyses of microbial genes. Data expressed as mean±SD. n ≥ 6 per group. *p<0.05, **p<0.001, ***p<0.001, WT mice compared with FXR KO mice, and untreated FXR KO mice compared with treated FXR KO mice.
Moreover, the abundance of cecal bacterial gene baiJ, which is responsible for secondary BA synthesis, was increased by FXR deficiency and reduced by B. infantis and MO supplementation (Figure 6D). Bacteria-generated butyrate has known anti-inflammatory effects [17, 47]. WD-fed FXR KO mice had reduced copy number of butyrate-generating genes bcoA (butyryl-CoA: acetate CoA-transferase) and buk (butyrate kinase) in the cecum. MO and B. infantis plus MO, but not B. infantis alone, were able to increase the abundance of bcoA and buk (Figure 6D).
3.5. B. infantis and MO affect Tgr5 signaling
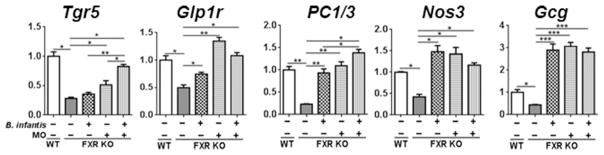
Because B. infantis and MO increased the concentration of endogenous TGR5 ligands LCA and TLCA in FXR KO mice, we hypothesize that the beneficial effect of B. infantis and MO in the digestive tract might in part be due to increased TGR5 signaling. Thus, the expression of Tgr5 and its associated pathways were studied. The data revealed that B. infantis and MO supplementation could reverse FXR deficiency-reduced mRNA levels of Tgr5, Glp1r (glucagon like peptide1 receptor), PC1/3 (proprotein convertase 1 and 3), Nos3 (nitric oxide synthase 3), and Gcg (preproglucagon) in the ileum (Figure 7). These data revealed the increased TGR5-associated GLP1 signaling in B. infantis and MO supplemented mice.
Figure 7. The effects of B. infantis and/or MO on TGR5 signaling in WD-fed FXR KO mice.
Ileal gene expression of WD-fed WT mice and WD-fed FXR KO mice supplemented with and without B. infantis and/or MO for 7 months. Data expressed as mean±SD. n ≥ 6 per group.**p<0.05, **p<0.01, ***p<0.001, WT mice compared with FXR KO mice, and untreated FXR KO mice compared with treated FXR KO mice.
4. Discussion
Bifidobacteria are inhabitants of the human intestine found in the infant gut especially in the breast-fed infants [12]. Bifidobacteria regulate immune response and can reduce intestinal inflammation [48]. The anti-inflammatory effects of Bifidobacteria have been revealed. For example, B. infantis decreases inflammation in necrotizing enterocolitis [3, 49]. B. infantis 35624 also reduces LPS-stimulated plasma TNFα and IL6 in healthy volunteers [2]. Furthermore, B. infantis DSM15159 improves dextran sodium sulfate-induced acute colitis [50]. In addition, B. adolescentis attenuates diet-induced steatohepatitis [51]. Furthermore, Bifidobacterium spp. lower gut endotoxin concentration [52] and B. pseudocatenulatum CECT 7765 reduces inflammation by reduced IL17A and TNFα [53]. The current study is the first that reveals the anti-inflammatory effect of B. infantis in cancer prone NASH caused by dysregulated BA synthesis.
MO has known effect in regulating bacterial colonization and preventing the attachment of pathogens to the intestine [54]. In addition, human MO has been tested in in vitro for their anti-inflammatory effect [55]. Human MO inhibited NF-κB activation, thereby attenuating TNFα- and pathogen-induced inflammation in human intestine [56]. Moreover, MO reduced gut permeability [57]. Our data showed that bovine and human MO had a similar effect. Additionally, a combination of B. infantis plus MO gave better outcomes. For examples, in the short-term treatment, B. infantis and MO individually reduced hepatic Il1β, Il6, Mcp-1, Col1a1, as well as Ccl20 and a combination B. infantis plus MO had higher fold reduction. A similar trend was noted for hepatic Ccl17, Ccl20, Cxcl10, Ifnγ, Icam1, Il4 as well as Timp1, and Foxp3 in the long-term treatment. All those encoded cytokines were implicated in hepatitis C virus infection, alcoholic patients, LPS-induced liver injury, and intestinal inflammation [58–60]. In addition, Icam1 and Cxcl10 were implicated in lymphocyte adhesion to hepatic sinusoids in NASH patients [61, 62]. Moreover, only B. infantis plus MO could increase ileal Reg3γ, which has an anti-microbial effect [63].
In contrast to the anti-inflammatory effect shared by B. infantis and MO, B. infantis alone, but not MO alone, were effective in reducing hepatic triglyceride as well as hepatic score. When B. infantis and MO were used together, they did not change hepatic triglyceride level, but further reduced hepatic cholesterol. These findings suggest their differential effects in regulating lipid metabolism. Previous study showed MO reduced hepatic fat score in high fat diet-fed WT mice [64]. The difference may due to the diet used. The differential effects of B. infantis and MO are also demonstrated in other outcomes. Our published data showed that FXR KO mice have reduced cecal butyrate and increased hepatic inflammation, and butyrate supplementation can treat hepatitis [17]. In the current study, our data showed that MO, but not B. infantis, increased the abundance of butyrate-generating bcoA and buk bacterial genes. These results suggested that MO have other benefits, which are independent from supporting the growth of B. infantis.
Our previous data showed that dysregulated BA and gut dysbiosis contribute to hepatic lymphocyte and neutrophil infiltration [15–17]. In this study, we found B. infantis, MO, and combination treatment could increase UDCA in the liver and serum, and UDCA is useful to treat NASH [65]. CDCA and TLCA are the endogenous ligands for TGR5 [66]. Activation of TGR5 protects intestinal barrier function, reduces inflammation, improves insulin sensitivity, and stimulates GLP-1 secretion [67, 68]. Moreover, administration of TGR5 agonists INT-777 or INT-767 reduces diet-induced steatosis and insulin resistance [69]. Therefore, B. infantis and MO treatment might activate TGR5. This scenario is supported by our findings that TGR5 and its associated signaling pathways including Glp1r, PC1/3, and Nos3 etc. were coordinately induced by B. infantis and MO. DCA is a secondary BA and has DNA damaging effects [70, 71]. In our study, hepatic DCA was increased in FXR KO mice, and B. infantis and MO reduced it. These results are consistent with the changes of bacterial baiJ gene in the gut. Moreover, B. infantis plus MO were effective in inducing hepatic LCA, which has a known effect in protecting hepatocytes from cholestatic injury [72]. In addition, LCA is a potent pregnane × receptor (PXR) agonist, and induce BA-specific sulfotransferase 2 (Sult2) family to conjugate sulfur to LCA for renal and fecal excretion [73, 74]. Furthermore, increased SULT2A1 might contribute to BA detoxification in B. infantis and MO-treated mice [28, 75]. It has been shown that increased hepatic Sult2a1 or Sult2a2 expression is an indicator of hepatic sulfonation of BA in mice [76]. Sult2a8, which is highly specific for 7α-hydroxylated bile acids/salts, is reduced due to FXR inactivation [77]. Only B. infantis modestly reduced its expression level and the significance of this finding needs to be further investigated. BA homeostasis is tightly regulated. Our data showed, the mRNA and protein level of Cyp7a1 as well as the mRNA level of Abcg5 and Abcg8 were normalized by B. infantis and MO supplementation in FXR KO mice suggesting reduced BA synthesis and increased sterols excretion. In addition, B. infantis and MO reversed reduced expression of Ostα, Ostβ, and Asbt in FXR KO mice [78, 79].
In conclusion, B. infantis and MO inhibit hepatic inflammation and reduced hepatic fat as well as liver injury in WD-fed FXR KO mice. In addition, synbiotics B. infantis and MO normalized dysregulated BA synthesis and are useful to prevent NASH in this mouse model. Moreover, MO increased butyrate-generating bacteria, which have proven useful to prevent NASH. Together, dysbiosis and dysregulated BAs jointly contribute to the development of NASH, and synbiotics can normalize some of those changes and be an effective treatment strategy.
Supplementary Material
Acknowledgments
Funding: This study is supported by grants funded by National Institutes of Health U01CA179582.
The authors thank Dr. Frank J. Gonzalez (National Cancer Institute, MD, USA) for providing FXR KO mice. We also thank Niki T. DeGeorge for manuscript preparation.
Abbreviations
- ALT
alanine transferases
- ALP
alkaline phosphatase
- BA
bile acid
- bcoA
butyryl-coenzyme-A-CoA transferase
- buk
butyrate kinase
- B. infantis
Bifidobacterium longum subsp. infantis
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- FXR
farnesoid X receptor
- HDCA
hyodeoxycholic acid
- KO
knockout
- MO
milk oligosaccharides
- NASH
nonalcoholic steatohepatitis
- SCFA
short chain fatty acid
- TGR5
Takeda G-protein coupled receptor 5
- TCA
taurocholic acid
- TLCA
taurolithocholic acid
- T-α,β-MCA
tauro-α, β-muricholic acid
- UDCA
ursodeoxycholic acid
- TCDCA
taurochenodeoxycholic acid
- TDCA
taurodeoxycholic acid
- WT
wild type
- WD
Western diet
Footnotes
Contributors: YJW designed the study; PKJ, LS, and NN performed experiments, PKJ, LS, NN, CY, DB, DAM, and YJW analyzed and interpreted the data; PKJ, LS, and YJW wrote the manuscript; all authors commented and approved the final manuscript.
Provenance and peer review: Not commissioned; externally peer reviewed.
Competing interests: The authors of this paper declare that they do not have any disclosures regarding funding or conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bode L. Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nutr. 2006;136:2127–30. doi: 10.1093/jn/136.8.2127. [DOI] [PubMed] [Google Scholar]
- 2.Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut microbes. 2013;4:325–39. doi: 10.4161/gmic.25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganguli K, Meng D, Rautava S, Lu L, Walker WA, Nanthakumar N. Probiotics prevent necrotizing enterocolitis by modulating enterocyte genes that regulate innate immune-mediated inflammation. Am J Physiol Gastrointest Liver Physiol. 2013;304:132–41. doi: 10.1152/ajpgi.00142.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyauchi E, Ogita T, Miyamoto J, Kawamoto S, Morita H, Ohno H, et al. Bifidobacterium longum alleviates dextran sulfate sodium-induced colitis by suppressing IL-17A response: involvement of intestinal epithelial costimulatory molecules. PloS one. 2013;8:e79735. doi: 10.1371/journal.pone.0079735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akay HK, Bahar Tokman H, Hatipoglu N, Hatipoglu H, Siraneci R, Demirci M, et al. The relationship between bifidobacteria and allergic asthma and/or allergic dermatitis: a prospective study of 0–3 years-old children in Turkey. Anaerobe. 2014;28:98–103. doi: 10.1016/j.anaerobe.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–82. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asakuma S, Hatakeyama E, Urashima T, Yoshida E, Katayama T, Yamamoto K, et al. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J Biol Chem. 2011;286:34583–92. doi: 10.1074/jbc.M111.248138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldredge DL, Geronimo MR, Hua S, Nwosu CC, Lebrilla CB, Barile D. Annotation and structural elucidation of bovine milk oligosaccharides and determination of novel fucosylated structures. Glycobiology. 2013;23:664–76. doi: 10.1093/glycob/cwt007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bezkorovainy A. Probiotics: determinants of survival and growth in the gut. Am J Clin Nutr. 2001;73:399–405. doi: 10.1093/ajcn/73.2.399s. [DOI] [PubMed] [Google Scholar]
- 10.Rivero-Urgell M, Santamaria-Orleans A. Oligosaccharides: application in infant food. Early Human Development. 2001;65(Supplement 2):S43–S52. doi: 10.1016/s0378-3782(01)00202-x. [DOI] [PubMed] [Google Scholar]
- 11.Newburg DS, Ruiz-Palacios GM, Morrow AL. Human milk glycans protect infants against enteric pathogens. Annual review of nutrition. 2005;25:37–58. doi: 10.1146/annurev.nutr.25.050304.092553. [DOI] [PubMed] [Google Scholar]
- 12.Wickramasinghe S, Pacheco AR, Lemay DG, Mills DA. Bifidobacteria grown on human milk oligosaccharides downregulate the expression of inflammation-related genes in Caco-2 cells. BMC Microbiol. 2015;15:172. doi: 10.1186/s12866-015-0508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton MK, Ronveaux CC, Rust BM, Newman JW, Hawley M, Barile D, et al. Prebiotic milk oligosaccharides prevent development of obese phenotype, impairment of gut permeability, and microbial dysbiosis in high fat-fed mice. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2017;312:G474–G87. doi: 10.1152/ajpgi.00427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor SN, Basile LA, Ebeling M, Wagner CL. Intestinal permeability in preterm infants by feeding type: mother’s milk versus formula. Breastfeed Med. 2009;4:11–5. doi: 10.1089/bfm.2008.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheng L, Jena PK, Liu HX, Kalanetra KM, Gonzalez FJ, French SW, et al. Gender Differences in Bile Acids and Microbiota in Relationship with Gender Dissimilarity in Steatosis Induced by Diet and FXR Inactivation. Sci Rep. 2017;7:1748. doi: 10.1038/s41598-017-01576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jena PK, Sheng L, Liu H-X, Kalanetra KM, Mirsoian A, Murphy WJ, et al. Western Diet–Induced Dysbiosis in Farnesoid X Receptor Knockout Mice Causes Persistent Hepatic Inflammation after Antibiotic Treatment. Am J Pathol. 2017;187:1800–13. doi: 10.1016/j.ajpath.2017.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheng LJ, PK, Hu Y, Liu HX, Nagar N, Kalanetra KM, French SW, French SW, Mills DA, Wan YY. Hepatic inflammation caused by dysregulated bile acid synthesis is reversible by butyrate supplementation. J Pathol. 2017;243:431–41. doi: 10.1002/path.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuei J, Chau T, Mills D, Wan YJ. Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp Biol Med. 2014;239:1489–504. doi: 10.1177/1535370214538743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu HX, Keane R, Sheng L, Wan YJ. Implications of microbiota and bile acid in liver injury and regeneration. J Hepatol. 2015;63:1502–10. doi: 10.1016/j.jhep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A. 2006;103:3920–5. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol. 2013;368:17–29. doi: 10.1016/j.mce.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seyer P, Vallois D, Poitry-Yamate C, Schutz F, Metref S, Tarussio D, et al. Hepatic glucose sensing is required to preserve beta cell glucose competence. J Clin Invest. 2013;123:1662–76. doi: 10.1172/JCI65538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015;3:5. doi: 10.3978/j.issn.2305-5839.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim I, Morimura K, Shah Y, Yang Q, Ward JM, Gonzalez FJ. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–6. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–7. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 26.Liu N, Meng Z, Lou G, Zhou W, Wang X, Zhang Y, et al. Hepatocarcinogenesis in FXR−/− mice mimics human HCC progression that operates through HNF1alpha regulation of FXR expression. Mol Endocrinol. 2012;26:775–85. doi: 10.1210/me.2011-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su H, Ma C, Liu J, Li N, Gao M, Huang A, et al. Downregulation of nuclear receptor FXR is associated with multiple malignant clinicopathological characteristics in human hepatocellular carcinoma. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1245–53. doi: 10.1152/ajpgi.00439.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang JY. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang JYL. Bile acid metabolism and signaling in liver disease and therapy. Liver Res. 2017;1:3–9. doi: 10.1016/j.livres.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Underwood MA, Kalanetra KM, Bokulich NA, Lewis ZT, Mirmiran M, Tancredi DJ, et al. A comparison of two probiotic strains of bifidobacteria in premature infants. J Pediatr. 2013;163:1585–91. e9. doi: 10.1016/j.jpeds.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H, Cuthbertson DJ, Otter DE, Barile D. Rapid Screening of Bovine Milk Oligosaccharides in a Whey Permeate Product and Domestic Animal Milks by Accurate Mass Database and Tandem Mass Spectral Library. J Agric Food Chem. 2016;64:6364–74. doi: 10.1021/acs.jafc.6b02039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smilowitz JT, Lemay DG, Kalanetra KM, Chin EL, Zivkovic AM, Breck MA, et al. Tolerability and safety of the intake of bovine milk oligosaccharides extracted from cheese whey in healthy human adults. J Nutr Sci. 2017;6:e6. doi: 10.1017/jns.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–44. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 34.Jena PK, Sheng L, Lucente JD, Jin LW, Maezawa I, Wan Y-JY. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity. The FASEB J. 2018:32. doi: 10.1096/fj.201700984RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu HX, Rocha CS, Dandekar S, Wan YJ. Functional analysis of the relationship between intestinal microbiota and the expression of hepatic genes and pathways during the course of liver regeneration. J Hepatol. 2016;64:641–50. doi: 10.1016/j.jhep.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu HX, Hu Y, Wan YJ. Microbiota and bile acid profiles in retinoic acid-primed mice that exhibit accelerated liver regeneration. Oncotarget. 2016;7:1096–106. doi: 10.18632/oncotarget.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat Commun. 2015;6:6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ridlon JM, Hylemon PB. Identification and characterization of two bile acid coenzyme A transferases from Clostridium scindens, a bile acid 7alpha-dehydroxylating intestinal bacterium. J Lipid Res. 2012;53:66–76. doi: 10.1194/jlr.M020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol. 2004;186:2099–106. doi: 10.1128/JB.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louis P, Flint HJ. Development of a semiquantitative degenerate real-time pcr-based assay for estimation of numbers of butyryl-coenzyme A (CoA) CoA transferase genes in complex bacterial samples. App Environ Microbiol. 2007;73:2009–12. doi: 10.1128/AEM.02561-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuki T, Watanabe K, Fujimoto J, Kado Y, Takada T, Matsumoto K, et al. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. App Environ Microbiol. 2004;70:167–73. doi: 10.1128/AEM.70.1.167-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. AM J Clin Nutr. 2013;98:111–20. doi: 10.3945/ajcn.112.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–22. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang F, Wang T, Lan Y, Yang L, Pan W, Zhu Y, et al. Deletion of mouse FXR gene disturbs multiple neurotransmitter systems and alters neurobehavior. Front Behav Neurosci. 2015;9:70. doi: 10.3389/fnbeh.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–72. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Micro. 2014;12:661–72. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 48.Hardy H, Harris J, Lyon E, Beal J, Foey AD. Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients. 2013;5:1869–912. doi: 10.3390/nu5061869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Underwood MA, Arriola J, Gerber CW, Kaveti A, Kalanetra KM, Kananurak A, et al. Bifidobacterium longum subsp. infantis in experimental necrotizing enterocolitis: alterations in inflammation, innate immune response, and the microbiota. Pediatr Res. 2014;76:326–33. doi: 10.1038/pr.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osman N, Adawi D, Molin G, Ahrne S, Berggren A, Jeppsson B. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 2006;6:31. doi: 10.1186/1471-230X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reichold A, Brenner SA, Spruss A, Forster-Fromme K, Bergheim I, Bischoff SC. Bifidobacterium adolescentis protects from the development of nonalcoholic steatohepatitis in a mouse model. J Nutri Biochem. 2014;25:118–25. doi: 10.1016/j.jnutbio.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 52.Griffiths EA, Duffy LC, Schanbacher FL, Qiao H, Dryja D, Leavens A, et al. In vivo effects of bifidobacteria and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig Dis Sci. 2004;49:579–89. doi: 10.1023/b:ddas.0000026302.92898.ae. [DOI] [PubMed] [Google Scholar]
- 53.Moya-Perez A, Neef A, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 Reduces Obesity-Associated Inflammation by Restoring the Lymphocyte-Macrophage Balance and Gut Microbiota Structure in High-Fat Diet-Fed Mice. PloS one. 2015;10:e0126976. doi: 10.1371/journal.pone.0126976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharon N, Ofek I. Safe as mother’s milk: carbohydrates as future anti-adhesion drugs for bacterial diseases. Glycoconj J. 2000;17:659–64. doi: 10.1023/a:1011091029973. [DOI] [PubMed] [Google Scholar]
- 55.Kunz C, Rudloff S. Potential anti-inflammatory and anti-infectious effects of human milk oligosaccharides. Adv Exp Med Biol. 2008;606:455–65. doi: 10.1007/978-0-387-74087-4_18. [DOI] [PubMed] [Google Scholar]
- 56.Newburg DS, Ko JS, Leone S, Nanthakumar NN. Human Milk Oligosaccharides and Synthetic Galactosyloligosaccharides Contain 3′-, 4-, and 6′-Galactosyllactose and Attenuate Inflammation in Human T84, NCM-460, and H4 Cells and Intestinal Tissue Ex Vivo. J Nutr. 2016;146:358–67. doi: 10.3945/jn.115.220749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boudry G, Hamilton MK, Chichlowski M, Wickramasinghe S, Barile D, Kalanetra KM, et al. Bovine milk oligosaccharides decrease gut permeability and improve inflammation and microbial dysbiosis in diet-induced obese mice. J Dairy sci. 2017;100:2471–81. doi: 10.3168/jds.2016-11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heiseke AF, Faul AC, Lehr HA, Forster I, Schmid RM, Krug AB, et al. CCL17 promotes intestinal inflammation in mice and counteracts regulatory T cell-mediated protection from colitis. Gastroenterol. 2012;142:335–45. doi: 10.1053/j.gastro.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 59.Affo S, Morales-Ibanez O, Rodrigo-Torres D, Altamirano J, Blaya D, Dapito DH, et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut. 2014;63:1782–92. doi: 10.1136/gutjnl-2013-306098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterol. 2014;147:577–94. e1. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 61.Shetty S, Lalor PF, Adams DH. Lymphocyte recruitment to the liver: molecular insights into the pathogenesis of liver injury and hepatitis. Toxicol. 2008;254:136–46. doi: 10.1016/j.tox.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bertola A, Bonnafous S, Anty R, Patouraux S, Saint-Paul MC, Iannelli A, et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PloS one. 2010;5:e13577. doi: 10.1371/journal.pone.0013577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loonen LM, Stolte EH, Jaklofsky MT, Meijerink M, Dekker J, van Baarlen P, et al. REG3gamma-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal immunol. 2014;7:939–47. doi: 10.1038/mi.2013.109. [DOI] [PubMed] [Google Scholar]
- 64.Hamilton MK, Ronveaux CC, Rust BM, Newman JW, Hawley M, Barile D, et al. Prebiotic milk oligosaccharides prevent development of obese phenotype, impairment of gut permeability, and microbial dysbiosis in high fat-fed mice. Am J Physiol Gastrointest Liver Physiol. 2017;312:G474–g87. doi: 10.1152/ajpgi.00427.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsuchida T, Shiraishi M, Ohta T, Sakai K, Ishii S. Ursodeoxycholic acid improves insulin sensitivity and hepatic steatosis by inducing the excretion of hepatic lipids in high-fat diet-fed KK-Ay mice. Metabolism-Clin Exp. 2012;61:944–53. doi: 10.1016/j.metabol.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 66.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 67.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009;10:167–77. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, et al. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endrocrinol. 2006;191:197–205. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- 69.Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem. 2017;292:11055–69. doi: 10.1074/jbc.M117.784322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernstein H, Bernstein C, Payne CM, Dvorak K. Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J Gastroenterol. 2009;15:3329–40. doi: 10.3748/wjg.15.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delzenne NM, Calderon PB, Taper HS, Roberfroid MB. Comparative hepatotoxicity of cholic acid, deoxycholic acid and lithocholic acid in the rat: in vivo and in vitro studies. Toxicol Lett. 1992;61:291–304. doi: 10.1016/0378-4274(92)90156-e. [DOI] [PubMed] [Google Scholar]
- 72.Han S, Li T, Ellis E, Strom S, Chiang JY. A novel bile acid-activated vitamin D receptor signaling in human hepatocytes. Mol Endocrinol. 2010;24:1151–64. doi: 10.1210/me.2009-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Echchgadda I, Song CS, Oh T, Ahmed M, De La Cruz IJ, Chatterjee B. The xenobiotic-sensing nuclear receptors pregnane X receptor, constitutive androstane receptor, and orphan nuclear receptor hepatocyte nuclear factor 4alpha in the regulation of human steroid-/bile acid-sulfotransferase. Mol Endocrinol. 2007;21:2099–111. doi: 10.1210/me.2007-0002. [DOI] [PubMed] [Google Scholar]
- 74.Pathak P, Cen X, Nichols RG, Ferrell JM, Boehme S, Krausz KW, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatol. 2018 doi: 10.1002/hep.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug metab Rev. 2004;36:703–22. doi: 10.1081/dmr-200033475. [DOI] [PubMed] [Google Scholar]
- 76.Alnouti Y. Bile Acid Sulfation: A Pathway of Bile Acid Elimination and Detoxification. Toxicol Sci. 2009;108:225–46. doi: 10.1093/toxsci/kfn268. [DOI] [PubMed] [Google Scholar]
- 77.Feng L, Yuen YL, Xu J, Liu X, Chan MY, Wang K, et al. Identification and characterization of a novel PPARalpha-regulated and 7alpha-hydroxyl bile acid-preferring cytosolic sulfotransferase mL-STL (Sult2a8) J Lipid Res. 2017;58:1114–31. doi: 10.1194/jlr.M074302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rao A, Haywood J, Craddock AL, Belinsky MG, Kruh GD, Dawson PA. The organic solute transporter α-β, Ostα-Ostβ, is essential for intestinal bile acid transport and homeostasis. Proc Natl Acad Sci U S A. 2008;105:3891–6. doi: 10.1073/pnas.0712328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jung D, Inagaki T, Gerard RD, Dawson PA, Kliewer SA, Mangelsdorf DJ, et al. FXR agonists and FGF15 reduce fecal bile acid excretion in a mouse model of bile acid malabsorption. J Lipid Res. 2007;48:2693–700. doi: 10.1194/jlr.M700351-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.