Abstract
In 2016, scientists reported that human exposure to low doses of ionizing radiation (CT scans of the brain) might relieve symptoms of both Alzheimer’s disease (AD) and Parkinson disease (PD). The findings were unbelievable for those who were not familiar with neurohormesis. X-ray stimulation of the patient’s adaptive protection systems against neurodegenerative diseases was the mechanism proposed by those authors. Now, some more recent studies performed in the field of neurobiological research confirm that low levels of stress can produce protective responses against the pathogenic processes. This paper outlines possible protective consequences of LDR in preventing the pathogenesis of AD through mechanisms such as restoring the myelin sheath and preventing neurodegeneration caused by oxidative stress. Focal demyelination is frequently reported in the proximity of beta-amyloid plaques within neocortex. Extracellular accumulation of amyloid is among well-characterized pathological changes in AD. It should be noted that LDR has been shown to contribute to the regeneration and functional recovery after transverse peripheral nerve injury (through inducing increased production of VEGF and GAP-43), which advances both the axonal regeneration and myelination. Another mechanism which is possibly involved is preventing neurodegeneration caused by oxidative stress. While high doses can induce reactive oxygen species (ROS) formation, oxidative stress and neuro-inflammation, substantial evidence now indicates that LDR can mitigate tissue damage through antioxidant defenses. Although adult neurogenesis has been reported to be beneficial for the regeneration of nervous system, some studies demonstrate that neurogenesis increases in AD brains. In spite of these reports, cellular therapy is introduced as a promising strategy for AD, and hence, LDR can affect the proliferation and differentiation of neural stem cells. Although such mechanisms are not fully known yet, it is hoped that this paper would foster further investigation into the mechanisms of this phenomenon, which accordingly improves human health.
Keywords: Alzheimer’s Disease , Neurohormesis , Low Dose Radiation , Mechanism
Introduction
The size and information processing capabilities of the human brain are exceptional [1]. Alzheimer’s disease (AD) is an age-related progressive neurodegenerative disorder that causes high levels of suffering without existing or sufficient treatment [2]. Approximately 70% of dementia cases are attributed to AD [3]. As an age-related disorder, AD is characterized by progressive cognitive decline and dementia [4]. It has been predicted that by 2025, the number of people with neurodegenerative diseases in developed countries will increase by several hundred percents compared to 1980 [5]. Although AD is the most common cause of dementia, its intricate pathogenic mechanisms are not fully known. Moreover, effective treatment methods are still in progress [2].
DNA repair and other natural body processes, including the human immune system, provide a robust means to protect the body from a range of agents. These processes have the potential to facilitate the repair of the damage caused by low-dose ionizing radiation, and these same processes have potential therapeutic effects to repair the damage caused by Alzheimer’s disease. An instance of this effect is exhibited by the use of low-dose radiation to treat Alzheimer’s disease and the associated improvement in the patient’s quality of life.
The role of exposure to low-dose radiation to save the life of a patient with advanced Alzheimer’s disease has been previously reported [6,7]. The patient received 5 computed tomography brain scans (dose of about 40 mGy each) over a period of 3 months. The improvement appears to be due to radiation-induced stimulation of the adaptive protection systems. The treatments appears to have partially restored cognition, memory, speech, movement and appetite. Although considered as a limited study. The case report is another example of the positive effects of low-dose radiation in treating the disease.
Repair mechanisms triggered by LDR via adaptive response mechanism can be recast in more qualitative terms as physical or metabolic processes. Physical mechanisms include molecular repair of cellular structures including DNA; removal of the damaged cells by apoptosis, necrosis and phagocytosis; cell differentiation and senescence; and response of the immune system to facilitate removal of damaged cells. In the case Alzheimer’s disease, these mechanisms combat biological damage caused by the disease. Repair mechanisms enhanced by LDR provide a mechanism for the effects observed by Cutler et al. [6,7]. The effects appear to be sustainable with additional CT scans with a noted improvement and reversal of the effects of the disease. These observations suggest that LDR triggers a mechanism for the relief of the Alzheimer’s condition and partial repair of the associated damage. Potential mechanisms behind neurohormesis are not yet fully known; however, we hope that this paper would foster further investigation into the mechanisms of this phenomenon to improve human health.
Possible Mechanisms behind Neurohormesis
Due to the global phenomenon of population aging, the rate of age-related neurodegenerative diseases is drastically on the increase in both developed and developing countries. Among the elderly, Alzheimer’s disease is the most common neurodegenerative disorder [8]. In this light, finding effective methods for preventing the pathogenesis of AD is of great significance. The role of non-ionizing radiofrequency radiation in protecting against cognitive impairment in Alzheimer’s disease has been addressed previously [9,10]. It has been revealed that long-term mobile phone users (> 10 years) had a 30-40% lower risk of hospitalization because of AD and vascular dementia [11]. Moreover, Arendash et al. have stressed that the drugs which are currently available only treat/mask AD symptoms for a short time. Therefore, they suggested that high-frequency electromagnetic fields (EMF) could be used as a safe, non-pharmaceutical approach to treat AD [12-14]. Subsequently, although the effect on non-ionizing radiation on AD is well documented, the reports published on the effects of ionizing radiation are very scarce. In this section, we explore three possible mechanisms (see Figure 1) behind the protective role of LDR in preventing the pathogenesis of AD.
Figure1.
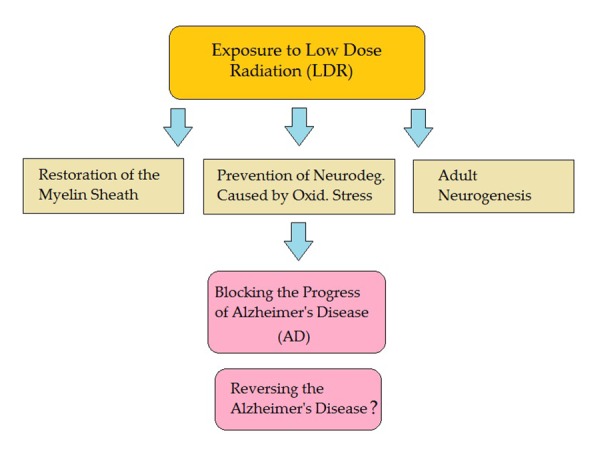
Possible mechanisms behind the protective role of LDR in preventing the pathogenesis of AD
A. Restoring Myelin Sheath
Focal demyelination is frequently reported in the proximity of beta-amyloid plaques within the neocortex [15]. Extracellular accumulation of amyloid is among well-characterized pathological changes in AD [16]. While high doses of ionizing radiation can induce demyelination (starting from demyelination of isolated nerve fibers, increasing in severity to hemorrhagic malacia)[17], exposure to low-dose radiation has been shown to contribute to the regeneration and functional recovery after transverse peripheral nerve injury (through inducing increased production of VEGF and GAP-43), which advances both the axonal regeneration and myelination [18].
B. Preventing Neurodegeneration Caused by Oxidative Stress
Another mechanism, which is possibly involved, is preventing neurodegeneration caused by oxidative stress. It should be noted that oxidative stress is a pathological hallmark of neurodegenerative tauopathic diseases (e.g. AD and PD)[19]. While high doses can induce reactive oxygen species (ROS) formation, oxidative stress and neuroinflammation, substantial evidence now indicates that LDR can mitigate tissue damage through antioxidant defenses [20].
C. Enhanced Ault Neurogenesis
Recent studies demonstrate that electromagnetic radiation (non-ionizing radiofrequency part of the electromagnetic spectrum) can alter the proliferation and differentiation of stem cells [21,22]. The effect of low doses of ionizing radiation (10-100 mGy) on neural differentiation is also studied [23]. Although adult neurogenesis has been reported to be beneficial for regeneration of the nervous system [24], some studies show neurogenesis increases in AD brains [25]. Along with this evidence, cellular therapy has been introduced as a promising strategy for AD, and hence, LDR can affect the proliferation and differentiation of neural stem cells. It is worth noting that throughout life, in the adult brain of humans and other mammals, new neurons are continuously being generated. The dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricle are two brain areas with neurogenic capacity. Reportedly, factors such as aging and neurodegenerative diseases such as AD and HD can decrease the neurogenic capacity of DG. Accordingly, it can be hypothesized that low-dose radiation can restore the neurogenic capacity of DG.
Acknowledgement
We would like to thank our colleagues who commented on earlier drafts of this paper.
Conflict of Interest:None Declared.
References
- 1.Mattson MP. Lifelong brain health is a lifelong challenge: from evolutionary principles to empirical evidence. Ageing Res Rev. 2015;20:37–45. doi: 10.1016/j.arr.2014.12.011. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardoso S, Carvalho C, Correia SC, Seica RM, Moreira PI. Alzheimer’s Disease: From Mitochondrial Perturbations to Mitochondrial Medicine. Brain Pathol. 2016;26:632–47. doi: 10.1111/bpa.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith B, Medda F, Gokhale V, Dunckley T, Hulme C. Recent advances in the design, synthesis, and biological evaluation of selective DYRK1A inhibitors: a new avenue for a disease modifying treatment of Alzheimer’s? . ACS Chem Neurosci . 2012;3:857–72. doi: 10.1021/cn300094k. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwiatkowski D, Sliwinski T. Base excision repair in Alzheimer’s disease. Postepy Hig Med Dosw (Online) 2014;68:976–86. doi: 10.5604/17322693.1114036. [DOI] [PubMed] [Google Scholar]
- 6.Cuttler JM, Moore ER, Hosfeld VD, Nadolski DL. Update on a patient with Alzheimer disease treated with CT scans. Dose-Response. 2017;15:1559325817693167. doi: 10.1177/1559325817693167. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuttler JM, Moore ER, Hosfeld VD, Nadolski DL. Treatment of Alzheimer Disease With CT Scans: A Case Report. Dose Response. 2016;14:1559325816640073. doi: 10.1177/1559325816640073. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fratiglioni L, Qiu C. Prevention of common neurodegenerative disorders in the elderly. Exp Gerontol. 2009;44:46–50. doi: 10.1016/j.exger.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Mortazavi S, Shojaei-Fard M, Haghani M, Shokrpour N, Mortazavi S. Exposure to mobile phone radiation opens new horizons in Alzheimer’s disease treatment. J Biomed Phys Eng. 2013;3:109–12. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortazavi SA, Tavakkoli-Golpayegani A, Haghani M, Mortazavi SM. Looking at the other side of the coin: the search for possible biopositive cognitive effects of the exposure to 900 MHz GSM mobile phone radiofrequency radiation. J Environ Health Sci Eng. 2014;12:75. doi: 10.1186/2052-336X-12-75. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuz J, Waldemar G, Olsen JH, Johansen C. Risks for central nervous system diseases among mobile phone subscribers: a Danish retrospective cohort study. PLoS One. 2009;4:e4389. doi: 10.1371/journal.pone.0004389. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arendash GW, Mori T, Dorsey M, Gonzalez R, Tajiri N, Borlongan C. Electromagnetic treatment to old Alzheimer’s mice reverses beta-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS One. 2012;7:e35751. doi: 10.1371/journal.pone.0035751. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arendash GW, Sanchez-Ramos J, Mori T, Mamcarz M, Lin X, Runfeldt M, et al. Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer’s disease mice. J Alzheimers Dis. 2010;19:191–210. doi: 10.3233/JAD-2010-1228. [DOI] [PubMed] [Google Scholar]
- 14.Dragicevic N, Bradshaw PC, Mamcarz M, Lin X, Wang L, Cao C, et al. Long-term electromagnetic field treatment enhances brain mitochondrial function of both Alzheimer’s transgenic mice and normal mice: a mechanism for electromagnetic field-induced cognitive benefit? . Neuroscience. 2011;185:135–49. doi: 10.1016/j.neuroscience.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Zhan X, Jickling GC, Ander BP, Stamova B, Liu D, Kao PF, et al. Myelin basic protein associates with AbetaPP, Abeta1-42, and amyloid plaques in cortex of Alzheimer’s disease brain. J Alzheimers Dis. 2015;44:1213–29. doi: 10.3233/JAD-142013. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papuc E, Rejdak K. Does Myelin Play the Leading Role in Alzheimer’s Disease Pathology. J Alzheimers Dis Parkinsonism. 2017;7:2161–0460.1000321. [Google Scholar]
- 17.Tosi R. Nuclear magnetic resonance spectroscopy in the study of neoplastic tissue. New York: Nova Publishers; 2005. [Google Scholar]
- 18.Jiang B, Zhang Y, Zhao J, She C, Zhou X, Dong Q, et al. Effects of Localized X-Ray Irradiation on Peripheral Nerve Regeneration in Transected Sciatic Nerve in Rats. Radiat Res. 2017;188:455–62. doi: 10.1667/RR14799.1. [DOI] [PubMed] [Google Scholar]
- 19.Kurian P, Obisesan TO, Craddock TJA. Oxidative species-induced excitonic transport in tubulin aromatic networks: Potential implications for neurodegenerative disease. J Photochem Photobiol B. 2017;175:109–24. doi: 10.1016/j.jphotobiol.2017.08.033. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betlazar C, Middleton RJ, Banati RB, Liu GJ. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 2016;9:144–56. doi: 10.1016/j.redox.2016.08.002. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eghlidospour M, Ghanbari A, Mortazavi SMJ, Azari H. Effects of radiofrequency exposure emitted from a GSM mobile phone on proliferation, differentiation, and apoptosis of neural stem cells. Anat Cell Biol. 2017;50:115–23. doi: 10.5115/acb.2017.50.2.115. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eghlidospour M, Mortazavi SM, Yousefi F, Mortazavi SA. New Horizons in Enhancing the Proliferation and Differentiation of Neural Stem Cells Using Stimulatory Effects of the Short Time Exposure to Radiofrequency Radiation. J Biomed Phys Eng. 2015;5:95–104. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajinskis A, Lindegren H, Johansson L, Harms-Ringdahl M, Forsby A. Low-dose/dose-rate gamma radiation depresses neural differentiation and alters protein expression profiles in neuroblastoma SH-SY5Y cells and C17. 2 neural stem cells. Radiat Res 2011;175:185–92. doi: 10.1667/rr2090.1. [DOI] [PubMed] [Google Scholar]
- 24.Taupin P. Adult neurogenesis, neural stem cells and Alzheimer’s disease: developments, limitations, problems and promises. Curr Alzheimer Res. 2009;6:461–70. doi: 10.2174/156720509790147151. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Castaneda RE, Galvez-Contreras AY, Luquin S, Gonzalez-Perez O. Neurogenesis in Alzheimer s disease: a realistic alternative to neuronal degeneration? . Curr Signal Transduct Ther. 2011;6:314–9. doi: 10.2174/157436211797483949. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]


