SUMMARY
Selective neuronal loss is the hallmark of neurodegenerative diseases. In patients with amyotrophic lateral sclerosis (ALS), most motor neurons die but those innervating extraocular, pelvic sphincter and slow limb muscles exhibit selective resistance. We identified 18 genes that show >10-fold differential expression between resistant and vulnerable motor neurons. One of these, matrix metalloproteinase-9 (MMP-9), is expressed only by fast motor neurons, which are selectively vulnerable. In ALS model mice expressing mutant SOD1, reduction of MMP-9 function using gene ablation, viral gene therapy or pharmacological inhibition significantly delayed muscle denervation. In the presence of mutant SOD1, MMP-9 expressed by fast motor neurons themselves enhances activation of ER stress and is sufficient to trigger axonal die-back. These findings define MMP-9 as a candidate therapeutic target for ALS. The molecular basis of neuronal diversity thus provides novel insights into mechanisms of selective vulnerability to neurodegeneration.
INTRODUCTION
All neurodegenerative diseases are characterized by neuronal dysfunction and cell death, which lead to incurable and often fatal functional deficits (Saxena and Caroni, 2011). However, the neuronal populations that degenerate are specific to each disease and it is the selective loss of the corresponding neuronal function that defines the clinical phenotype. This selective vulnerability manifests itself at multiple levels. Typically in a given disease a single neuronal class is principally affected. Additionally, however, within this class different regional subpopulations or functional subtypes degenerate to widely varying degrees. The fact that most disease-triggering mutant proteins are expressed ubiquitously suggests that the specificity of degeneration reflects intrinsic differences between different neuronal subpopulations. Identifying the molecular basis of selective vulnerability has the potential to identify novel therapeutic targets, which are currently lacking for most neurodegenerative diseases.
The uniformly fatal disease amyotrophic lateral sclerosis (ALS) provides examples of neuronal specificity at every level. First, motor neurons in the spinal cord, brainstem, and cortex are selectively lost as compared to other neuronal classes (Cleveland and Rothstein, 2001). Second, individual motor pools - groups of motor neurons innervating single muscles - show widely varying degrees of disease resistance: in the final stages of ALS, nearly all voluntary movement is lost but eye movement and eliminative and sexual functions remain relatively unimpaired (reviewed in Kanning et al., 2010). These functions are controlled by motor neurons of the oculomotor (III), trochlear (IV) and abducens (VI) nuclei in the midbrain and brainstem, and by Onuf’s nucleus in the lumbosacral spinal cord, respectively. Correspondingly, in ALS autopsies the oculomotor and Onuf’s nuclei are almost completely preserved (Iwata and Hirano, 1978; Mannen et al., 1977; Schrøder and Reske-Nielsen, 1984). A third level of specificity is that, even within a given motor pool, certain functional subtypes of motor neuron are more vulnerable than others. Depending on their contractile properties, motor units are classified as fast fatigable (FF), fast fatigue-resistant (FR) or slow (S). FF motor neurons are the largest and are involved in short-lasting bouts of forceful contraction, whereas S motor neurons are smaller and fire regularly to control postural muscles (Kanning et al., 2010). In ALS, S motor neurons are more resistant and undergo axonal degeneration only at later stages, whereas FF motor neurons are the most vulnerable (Fischer et al., 2004; Frey et al., 2000; Pun et al., 2006). This is at least in part due to the low excitability of FF motor neurons (Saxena et al., 2013). However, the molecular basis of the selective vulnerability of fast motor neurons with respect to slow spinal motor neurons or those in the oculomotor and Onuf’s nuclei remains to be determined.
Many studies of ALS disease mechanisms have used transgenic mouse models which express mutant forms of superoxide dismutase (SOD1) that cause familial ALS in humans. In these mSOD1 mice as in human patients with sporadic forms of the disease, selective resistance of S motor units and preservation of extraocular muscle innervation are observed (Pun et al., 2006; Valdez et al., 2012). This is despite ubiquitous expression of mutant SOD1 at high levels in multiple cell types known to be involved in disease onset and progression, including astrocytes, microglia and motor neurons themselves (Ilieva et al., 2009). Multiple mechanisms of motor neuron degeneration triggered by mutant SOD1 have been proposed. Among these, early activation of ER stress in FF motor neurons correlates with their axonal degeneration, and inhibition of ER stress using salubrinal, which prevents phosphorylation of EIF2α, delays muscle denervation and leads to an increase in lifespan (Saxena et al., 2009). The preferential activation of the ER stress response in FF motor neurons seems to be triggered as a result of their reduced excitability (Saxena et al., 2013) but the molecular links between these functional characteristics and ER stress are not well understood. More generally, although some molecular differences between oculomotor and other motor neurons, or between large and small spinal motor neurons, have been reported (Alexianu et al., 1994; Aronica et al., 2001; Brockington et al., 2013; Hedlund et al., 2010; Laslo et al., 2000; Sasaki et al., 2006; Van Hoecke et al., 2012), the molecular basis of ALS resistance in vivo remains to be determined.
There are therefore at least two aspects of differential motor neuron vulnerability in ALS: a pool-specific resistance typified by oculomotor and Onuf’s nuclei, and a subtype-specific vulnerability of FF as compared to S motor units. We focused first on the former and used microarray profiling of isolated wildtype motor neurons to identify genes whose expression was characteristic of both oculomotor and Onuf’s nuclei but not of vulnerable lumbar spinal neurons, or vice versa. One such gene, matrix metalloproteinase-9 (MMP-9), while strongly expressed by a majority of cranial and spinal motor neurons, was absent from oculomotor and Onuf’s nuclei. Strikingly, it was also undetectable in S motor neurons in the spinal cord. This identifies MMP-9 as a prospective marker for all ALS-vulnerable motor neurons, in which it is first detected immediately prior to the earliest known functional changes in the neonatal spinal cord. Functionally, even partial reduction of MMP-9 levels in mutant SOD1 mice leads to pronounced delays in muscle denervation and a significant extension of lifespan. Moreover, MMP-9 acts early in the disease process, upstream of subtype-specific ER stress. Our data therefore identify MMP-9 as a strong candidate therapeutic target for ALS and substantiate this general approach for other neurodegenerative diseases in which loss of specific neuronal subpopulations is observed.
RESULTS
Identification of candidate ALS resistance and susceptibility genes
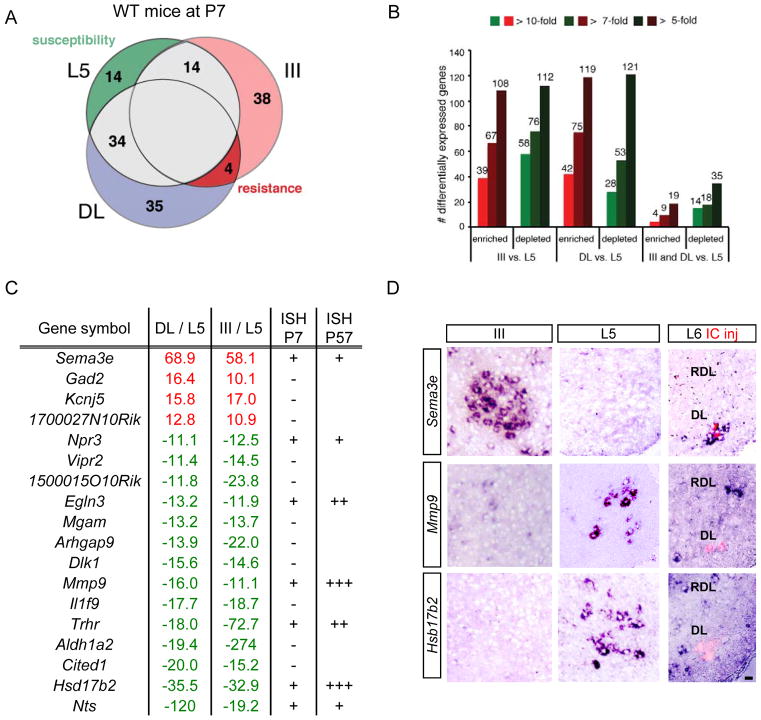
In order to identify molecular characteristics that contribute to selective vulnerability in ALS, we focused first on pool-specific resistance as typified by oculomotor and Onuf’s (referred to as DL in mouse) nuclei (Figure S1A). We performed microarray profiling of motor neurons lasercaptured from sections of wildtype mice at P7 (Figures 1A and S1B). Wildtype mice were used because we wished to identify intrinsic properties of resistance rather than disease responses, and P7 corresponds to a stage at which developmental cell death is complete and the first functional abnormalities in the spinal cord of mSOD1 mice are observed (Bories et al., 2007). Pairwise sample comparisons among the three motor neuron subpopulations revealed 66–131 genes that exhibited >10-fold differences in expression levels (Figure 1A). Lowering the threshold to 5-fold identified >200 differentially expressed genes (Figures 1B, S1F and S1G). This molecular heterogeneity of postnatal motor neurons likely reflects their widely divergent functions.
Figure 1. Identification of candidate ALS resistance and susceptibility genes by gene profiling of laser-captured motor neurons.
(A) Gene expression profiles of mouse lumbar motor neurons (L5), oculomotor nucleus (III, pink) and Onuf’s nucleus (DL, blue) overlap but also reveal significant differences between motor neuron populations. Figures indicate number of genes expressed at >10-fold higher levels in each subset (P<0.05). ALS resistance genes are more likely to be found among genes enriched in both III and DL (red), while susceptibility genes (dark green) are predicted to be enriched in L5.
(B) Numbers of genes showing significant differences (P<0.05 by one-way ANOVA) among the three populations at different fold ratios.
(C) Identities of 18 genes enriched (red) or depleted (green) in the two resistant populations showing fold changes for 3 replicates. + signs in the P7 ISH column indicate levels of in situ hybridization (ISH) in motor neurons, - signs indicate no detectable expression. For P57, +++ differential expression maintained at comparable levels to P7, ++ more restricted expression, and + low levels.
(D) Sample validation of microarray data by in situ hybridization for indicated genes in P7 midbrain and lumbar spinal cord. At L6, DL (Onuf’s) nucleus motor neurons were identified by injection of CTB (red) into the IC muscle at P5. Resistant motor neurons do not express Mmp9 or Hsd17b2. Scale bar, 20 μm.
We considered that the 4 genes expressed at >10-fold higher levels in both resistant populations than in vulnerable L5 motor neurons had a higher chance of representing ALS resistance genes (Figure 1A, red) whereas the 14 genes expressed only in vulnerable motor neurons might be ALS susceptibility genes (Figure 1A, green). For nine of them (asterisks in Table S1), we validated the microarray data by in situ hybridization (ISH) on sections of P7 hindbrain and spinal cord, using retrograde labeling from the IC muscle to identify the DL nucleus (examples in Figure 1D). These 18 genes have known functions that are highly diverse but have in most cases never been linked to ALS or neurodegenerative disease (Table S1).
This gene list represents a valuable resource for future studies of selective neuronal resistance. To prioritize genes for initial study, we considered that the most interesting candidates for therapeutic intervention in this late-onset disease would show strong, motor neuron-specific expression throughout postnatal and adult life. Only Hsd17b2 and Mmp9 fit these criteria (Fig. 1C). Since Mmp9 did not rank above #24 on either pairwise comparison, we reasoned it had the greatest chance of being linked to ALS rather than to specific properties of either pool. Moreover, given early interest from the cancer field (Swarnakar et al., 2012), and the fact that potential - though controversial - links between MMP-9 and ALS had been reported (Dewil et al., 2005; He et al., 2013; Kiaei et al., 2007; Lorenzl et al., 2003; Lorenzl et al., 2006; Swarnakar et al., 2012), there was a wide range of reagents available.
Matrix metalloproteinase-9 (MMP-9) is selectively expressed by vulnerable motor neurons
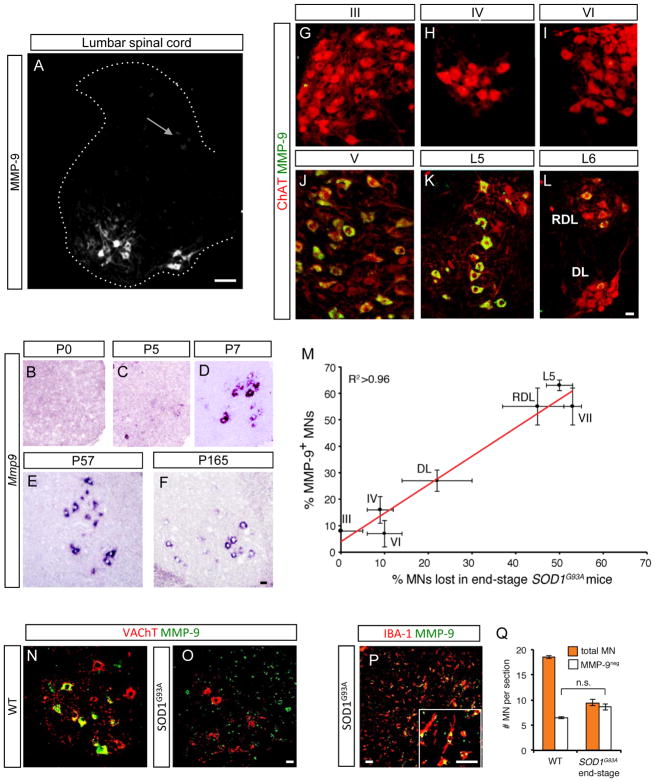
Levels of Mmp9 mRNA were 16-fold lower in oculomotor than L5 motor neurons, and 11-fold lower in DL (Figures 1C and 1D). In the spinal cord, strong MMP-9 expression is specific to motor neurons (Figure 2A). It is first detected only postnatally at P5 but subsequently remains expressed at high levels until the latest time point studied, P165 (Figures 2B to 2F). At the protein level, using an MMP-9 antibody that gave no signal in Mmp9 knockout mice (Figure S4H), MMP-9 was not detected in any of the three cranial nuclei (III, IV and VI) that innervate extraocular muscles, nor in the ventrolateral region of the DL nucleus in the L6 spinal cord that innervates the external urethral sphincter (Figures 2G to 2I, and 2L). In contrast, motor neurons expressing high levels of MMP-9 were abundant in trigeminal and facial nuclei as well as in L5-L6 spinal motor neurons (Figures 2J and 2K and not shown). Therefore, MMP-9 is absent from ALS-resistant populations in midbrain and spinal cord but present in many other motor neurons.
Figure 2. Matrix metalloproteinase-9 (MMP-9) is a prospective marker for motor neurons lost at end-stage in ALS model mice.
(A) In P25 SOD1G93A mouse lumbar spinal cord, intense MMP-9 immunostaining restricted to motor neurons, though some dorsal neurons stain weakly (arrow). Scale bar 100 μm.
(B–F) MMP-9 is first detected by ISH in non-transgenic mouse lumbar spinal cord postnatally at P5 (C), and remains at high levels until the latest time point studied, P165 (F).
(G–L) Immunostaining for MMP-9 (green) on P30 spinal cord of ChAT-Cre; Rosa-TdTomato (MN, red) reporter mice shows many motor neurons expressing high levels of MMP-9 in the vulnerable trigeminal nucleus (J), L5 spinal cord (K) and RDL in L6 (L). In contrast, resistant oculomotor (G), trochlear (H), abducens (I), and DL (L) nuclei show no or very little MMP-9 expression. Scale bars, 20 μm.
(M) Tight correlation between MMP-9 expression in wildtype motor pools and their vulnerability to ALS. Absolute values fit a linear regression y = 1.07x + 3.99, R2 >0.96. Values are mean ± s.e.m. (n=3).
(N, O and Q) By end-stage at L5 in SOD1G93A mice, 50% of motor neurons are lost (Q). Of those surviving, all are MMP-9-negative (compare O with P40 non-transgenic control in N). Scale bars, 20 μm.
(P) All MMP-9 expressed in spinal cord at end-stage co-localizes with microglial marker IBA-1. Scale bars, 20 μm.
See also Figure S2.
Even within motor neuron populations that expressed MMP-9, labeled cells were intermingled with MMP-9-negative neurons (Figures 2J to 2L, S3B) suggesting that MMP-9 might be a finer-grained marker of vulnerability. For a series of motor neuron subpopulations we therefore compared the percentage of motor neurons lost at end-stage in SOD1G93A mice (our data and Ferrucci et al., 2010) to the fraction that expressed MMP-9 in wildtype mice (Figure 2M). Absolute values in the two datasets showed remarkably strong positive correlation (R2 >0.96), suggesting that motor neurons that express MMP-9 are those subsequently lost in ALS.
To test this prediction, we examined MMP-9 staining in SOD1G93A mice. At presymptomatic stages up to P40, levels and localization of MMP-9 were indistinguishable from wildtype (Figures S2A and S2C). Mutant SOD1 does not therefore itself trigger misexpression of the enzyme. At end-stage, in striking contrast, although MMP-9 could be detected in microglia (Figure 2P), all ~50% of motor neurons that remained were MMP-9-negative (compare Figures 2N and 2O). This suggested that MMP-9-expressing motor neurons are selectively lost during the ALS disease process. An alternative explanation was that motor neurons downregulate MMP-9 prior to their death, and we indeed detected a small but significant increase in the numbers of MMP-9-negative motor neurons at intermediate stages (Figure S2N). However, the absolute number of surviving motor neurons in L5 spinal cord at end-stage was indistinguishable from the number of MMP-9-negative cells initially present (Figure 2Q; p=0.62, t-test). Thus, MMP-9 is the first prospective marker for vulnerable motor neurons in a mouse model of ALS.
MMP-9 is selectively expressed by fast motor neurons
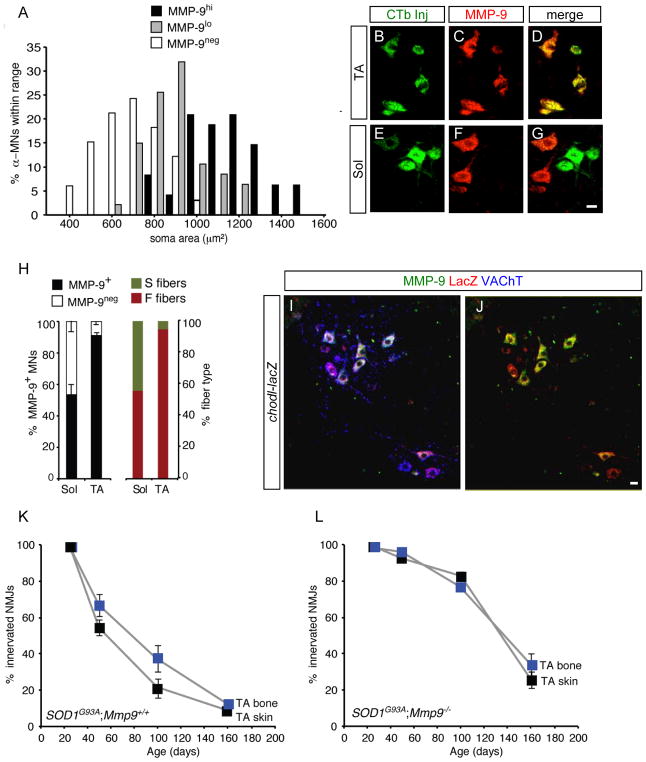
Since die-back of axons of FF motor units precedes that of FR and S (Pun et al., 2006), we asked whether the MMP-9-expressing neurons might be fast motor neurons. A first characteristic of FF/FR motor neurons is that they are larger than S motor neurons (Cullheim et al., 1987). Limiting our analysis to α-motor neurons by co-staining for NeuN (Friese et al., 2009; Shneider et al., 2009), we observed that motor neurons with the highest intensity of staining for MMP-9 were the largest (Figures 3A; Figure S3). As a second criterion, we identified motor neurons of the slow Sol and fast TA pools by injection of retrograde tracer into each muscle in adult mice (Figures 3B to 3G). The absolute values for MMP-9+ and MMP-9neg abundance among labeled motor neurons closely matched the percentage of fast and slow fiber types previously reported in soleus (53% MMP-9+; 54% FF+FR) and TA (90% MMP-9+; 93% FF+FR) muscles (Figure 3H; Hegedus et al., 2007). Lastly, we compared the expression of MMP-9 with that of chondrolectin (Chodl), reported to be a marker of fast motor neurons (Enjin et al., 2010), using a Chodl-LacZ mouse that selectively labels a subset of motor neurons (Figure 3I). Of all MMP-9+ motor neurons, 96% also expressed the Chodl-LacZ/+ reporter, and only 29% of LacZ+ cells lacked MMP-9 (Figure 3I and 3J). Overall therefore, MMP-9 is selectively expressed in fast α-motor neurons and these are the neurons destined to die in ALS mice. No markers to distinguish FF and FR motor neurons exist in rodents. However, we hypothesize that the population that shows intermediate size and lower levels of MMP-9 (Figure 3A) corresponds to FR motor neurons.
Figure 3. MMP-9 is a marker for fast motor neurons.
(A) α-MNs (ChAT+/NeuN+) expressing MMP-9 are larger, consistent with a fast identity. MMP-9hi and MMP-9lo potentially reflect FF and FR, respectively. Staining intensity was determined as in Figure S3.
(B to G) Many motor neurons of the fast TA pool, identified by retrograde labeling with CTB488 (green), are MMP-9-positive (red) whereas Sol motor neurons tend to be negative. Scale bar, 20 μm.
(H) MMP-9 staining in the TA and Sol motor pools (mean ± s.e.m., n=3) (left) compared to fiber type distribution in the corresponding muscles replotted from Hegedus et al (2007) (right).
Values for fast fibers are very close to those for MMP-9+.
(I and J) Immunostaining for VAChT (blue), MMP-9 (green), and β-galactosidase (red) on spinal cord of an adult Chodl-lacZ mouse. There is strong overlap between MMP-9 and the Chodl reporter.
(K) In SOD1G93A;Mmp9+/+ mice, denervation of the superficial, skin-facing compartment (black) precedes that of the deep, bone-facing fibers (blue) by >20 days (P = 0.039).
(L) The two subcompartments of the TA become denervated at an identical rate in SOD1G93A;Mmp9−/− mice (P = 0.560).
See also Figure S3.
Motor neurons innervating extraocular muscles and lumbar sphincters are also resistant in ALS model mice
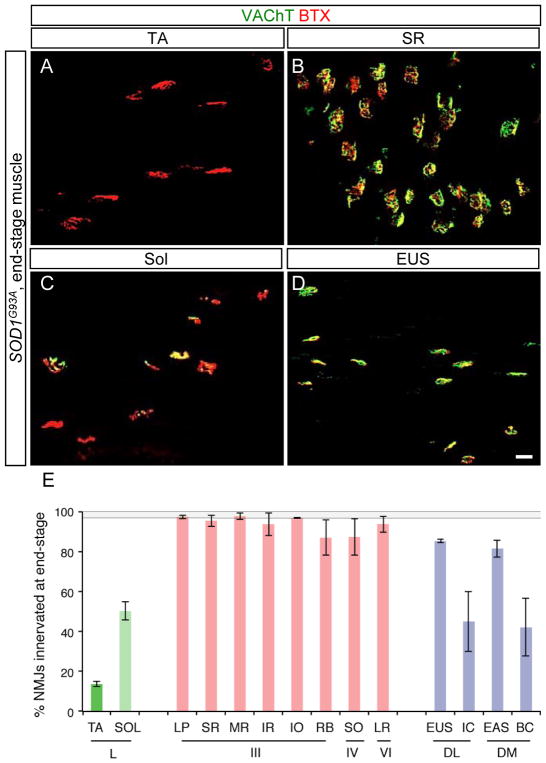
As a condition for testing the functional contribution of MMP-9 to degeneration in ALS, we needed to ascertain the pattern of selective resistance in SOD1G93A mice at end-stage. As shown by Pun et al. (2006), the fast tibialis anterior (TA) muscle showed nearly complete denervation whereas the slow soleus (Sol) was significantly more (Figures 4A, 4C and 4E). The extraocular muscles were recently reported to be collectively resistant (Valdez et al., 2012) so we examined them individually (Figure 4E). All eight extraocular muscles showed nearly complete resistance, as did the pelvic sphincters EUS (external urethral sphincter) and EAS (external anal sphincter). This remarkable overlap between the selectivity of muscle denervation in the SOD1G93A mouse and that in human patients with sporadic ALS strongly suggests the existence of shared mechanisms of vulnerability.
Figure 4. Extraocular and pelvic sphincter motor units show selective resistance in ALS model mice.
(A–D) Overlap of VAChT-positive motor terminals (green) with acetylcholine receptors stained using α-bungarotoxin (BTX, red) as an indicator of innervated motor endplates in end-stage SOD1G93A mice. (A) The fast tibialis anterior (TA) muscle exhibits marked denervation, whereas the slow soleus (Sol) remains partially innervated (C). (B) The extraocular muscle superior rectus (SR) and (D) the external urethral sphincter (EUS) show nearly complete preservation of motor units. Scale bar, 20 μm.
(E) Percentage of intact neuromuscular junctions in muscles of end-stage SOD1G93A mice (mean ± s.e.m from 3 animals). Control, non-transgenic values are indicated by the grey-shaded region. Muscles (top row): levator palpebrae (LP), medial rectus (MR), inferior rectus (IR), inferior oblique (IO), retractor bulbi (RB), superior oblique (SO), lateral rectus (LR), ischiocavernosus (IC), external anal sphincter (EAS), bulbocavernosus (BC). Motor neurons (bottom row): lumbar spinal cord (L), oculomotor nucleus (III), trochlear nucleus (IV), abducens nucleus (VI), dorsolateral and dorsomedial components of Onuf’s nucleus (DL and DM).
MMP-9 is required for muscle denervation during the ALS disease process
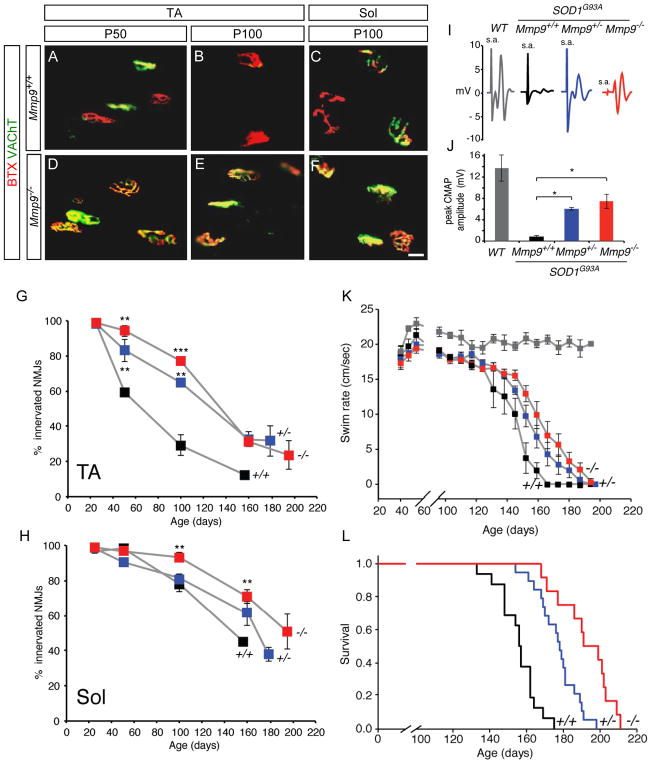
We therefore bred Mmp9−/− mice to SOD1G93A mice, both on a pure C57BL/6 background. MMP-9 knockout mice are healthy and have a normal lifespan (Vu et al., 1998). Although they have been reported to show mild cognitive deficits, we found no muscle denervation or motor behavioral phenotype at any stage as compared to wildtype controls (not shown). We first confirmed that axonal die-back occurred much earlier in the fast TA muscle (Figures 5A, 5B and 5G) than in the slow Sol muscle (Figures 5C and 5H; Pun et al., 2006).
Figure 5. Genetic ablation of MMP-9 markedly delays muscle denervation and loss of motor function in SOD1G93A mice.
(A to F) Mmp9 deletion in SOD1G93A delays loss of muscle innervation in both TA and Sol muscles. Scale bars, 20 μm.
(G to L) Morphological and functional evaluation of four different genotypes, color-coded in the same way in all panels: SOD1G93A;Mmp9+/+ (black; +/+), SOD1G93A;Mmp9+/− (blue; +/−), SOD1G93A;Mmp9−/− (red; −/−), and non-transgenic (gray, WT). (G) Denervation of tibialis anterior muscle is delayed by ~80 days by removal of either one or both alleles of Mmp9. (H) Denervation of slow soleus muscle later than TA, but is further delayed by 50 days in the absence of MMP-9. For both G and H, values are mean ± s.e.m of one muscle from each of 3-4 animals per genotype (***p<0.001, **p< 0.01).
(I and J) MMP-9 ablation significantly rescues muscle function. Evoked compound muscle action potentials (CMAPs) in the TA muscle after stimulation of the sciatic nerve; s.a., stimulus artifact.
(J) CMAP measurements from groups of the indicated genotypes aged between P105–130.
Means ± s.e.m. (n= 3–4 animals per genotype); * p<0.05.
(K) Mmp9 deletion significantly delays motor impairment in a swimming task that reflects hindlimb function. (n=10, p= 0.005).
(L) Lifespan is prolonged by 14% following deletion of one Mmp9 allele and 25% following deletion of both. Kaplan–Meier plot showing the cumulative probability of survival of indicated genotypes (n=12–19 animals per genotype; log-rank test= 49.2, p<0.001).
We next analyzed muscle denervation in SOD1G93A mice lacking MMP-9 Remarkably, at P50 in the TA muscle of SOD1G93A; Mmp9−/− mice, no denervation was detected and even at P100 more than 75% of motor endplates remained innervated, representing a 2.5-fold improvement over mutant SOD1 controls (Figures 5D, 5E and 5G). Significant protection was also observed in the slow Sol muscle (Figures 5F and 5H). These strong protective effects did not reflect altered mSOD1 levels, since neither transgene copy number nor protein levels were changed by the removal of MMP-9 (Figures S4B and S4I to S4K). Therefore, genetic ablation of a single constituent of vulnerable motor neurons led to a delay of ~80 days in loss of innervation of fast muscles. This >2.5-month retardation in a mouse mutant that lives for only 6 months is the strongest beneficial effect so far reported in ALS model mice, other than by removing the disease gene itself (reviewed in Turner and Talbot, 2008).
Strikingly, inactivation of even a single allele of Mmp9 also conferred significant benefit. MMP-9 levels in heterozygous SOD1G93A; Mmp9+/− mice were ~50% of those in wildtype mice (Figures S4A, and S4C to S4K) but this incomplete reduction of MMP-9 was sufficient to afford ~70% of the protection provided by complete ablation up to P100, and equivalent protection at end-stage (Figures 5G and 5H).
To confirm that the preserved synapses were functional, we measured the compound muscle action potential (CMAP; Towne et al., 2011). By P105-P130, the CMAP in SOD1G93A mice was reduced to 10% of control values but deletion of one or two copies of Mmp9 rescued up to 50% of normal values (Figures 5I and 5J). Similar improvement in other functional tests that measure late changes in the mSOD1 mice, such as the rotarod (Figure S4O) and swim (Figure 5K) tests was also observed. Motor neuron numbers in the spinal cord initially decreased more slowly in the absence of Mmp-9 (Figure S4L to S4N). This protection was transient, however, and by P160 motor neuron numbers were comparable between the two genotypes. Since by this stage many SOD1G93A; Mmp9+/+ mice had died whereas all SOD1G93A; Mmp9−/− were still alive, MMP-9 deletion likely also promotes axonal sprouting and an increase in the average motor unit size.
Lifespan in mSOD1 mice is determined by the stage at which mice can no longer right themselves in 30 seconds. Median survival of SOD1G93A mice in our C57BL/6 colony was 156 days. This was increased to 178 days by removal of a single allele of Mmp9 and to 195 days by complete ablation, representing a 25% increase in lifespan (Figure 5L). Our survival data are comparable to those reported by Kiaei et al. (2007; see Discussion) on a mixed background. The systemic benefit of MMP-9 deletion is clearly apparent from visual inspection of three male SOD1G93A littermate mice bearing 2, 1 and 0 copies of Mmp9 at P150 (Movie S1): even Mmp9 heterozygotes show major improvements in their ability to grasp bars and right themselves.
MMP-9 acts enhances activation of ER stress in ALS motor neurons
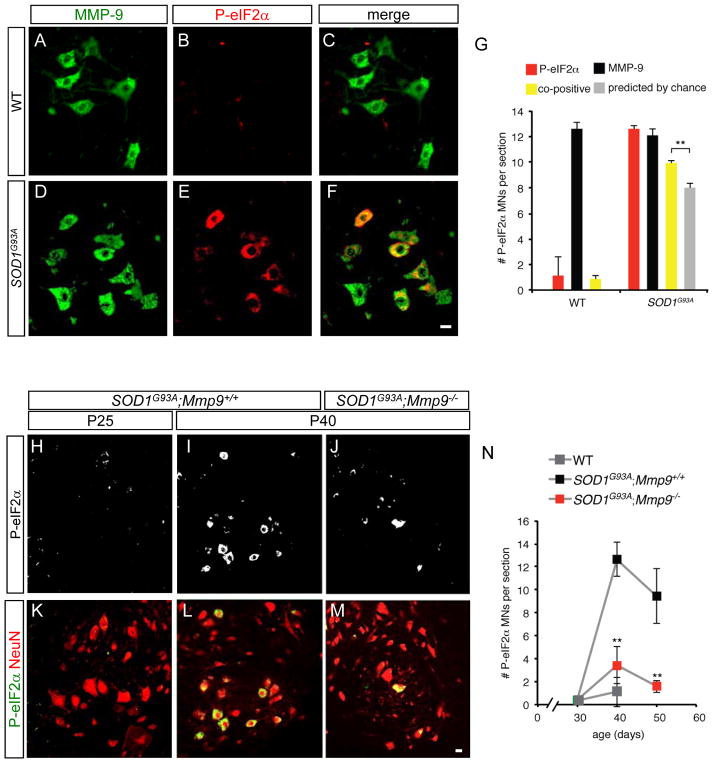
To determine the interactions between MMP-9 and the disease pathway as currently understood, we focused on the unfolded protein response (UPR), one of the earliest molecular events in SOD1G93A mice (Saxena et al., 2009), as detected by phosphorylation of eukaryotic initiation factor-2α (P-EIF2α). When we quantified the overlap of P-EIF2α and MMP-9 (Figures 6A to 6G), the number of double-labeled cells was significantly greater than expected by chance (p<0.01, t-test). Therefore ER stress and UPR are selectively induced in MMP-9+ motor neurons, in agreement with their FF/FR identity. Strikingly, at P40 in SOD1G93A mice that were null for Mmp9, only <25% of the normal number of motor neurons stained for P-EIF2α, and this fraction was even lower at P50 (Figures 6H to 6N). At later stages, P-EIF2α levels increased even in SOD1G93A ;Mmp9−/− mice (not shown), suggesting that Mmp9 deletion delays, but does not indefinitely prevent, the onset of ER stress. Thus MMP-9 is necessary for normal activation of the earliest known biochemical event triggered by mutant SOD1.
Figure 6. MMP-9 is required for early activation of ER stress in fast SOD1G93A motor neurons.
(A to F) Immunostaining for MMP-9 (green) and ER stress marker phospho-EIF2α (red) in P40 spinal cord of non-transgenic (WT) and SOD1G93A mice. P-EIF2α is restricted to mSOD1 mice (compare E with B) and co-localizes with MMP-9 (F). Scale bars, 20 μm.
(G) MMP-9-expressing motor neurons preferentially activate ER stress. There are significantly more SOD1G93A motor neurons co-positive for P-EIF2α and MMP-9 (yellow) than would be predicted by chance (grey bar).
(H to N) The normal abrupt appearance of P-EIF2α at P40 in SOD1G93A motor neurons is severely damped in the absence of MMP-9. Scale bars, 20 μm. **p<0.01 compared to P-EIF2α-positive cells in normal mSOD1 mice.
Later onset of ER stress is a characteristic of resistant S motor neurons (Saxena et al., 2009), so we asked whether ablating MMP-9 leads motor neurons to adopt a more S-like phenotype. In SOD1G93A mice, denervation of the “skin-facing” compartment of the TA muscle precedes that of the “bone-facing” fibers by >20 days, indicating that the compartments have FF and FR character, respectively (Figure 3K; Pun et al., 2006). In contrast, in TA muscle of SOD1G93A;Mmp9−/− mice the two compartments became denervated at an identical rate (Figure 3L). Moreover, the rate of muscle denervation by the “fast” motor neurons of the TA pool in SOD1G93A;Mmp9−/− mice (Figure 5G, red points) was remarkably similar to that for the mostly slow motor neurons of the neighboring Sol pool in SOD1G93A mice that had normal levels of MMP-9 (Figure 5H, black points; Supplementary Fig. S4P). Therefore, FF and FR motor neurons lacking MMP-9 both adopt an axonal die-back phenotype comparable to that of S motor neurons.
MMP-9 is sufficient to induce degeneration in fast motor neurons of ALS mice
Since MMP-9 is necessary for normal onset of muscle denervation, we asked whether it was alone sufficient to trigger axonal die-back in ALS-resistant motor units. An AAV6 vector expressing full-length Mmp9 cDNA was injected at P4 into the intraorbital space of SOD1G93A mice, resulting in stable expression of MMP-9 in a significant fraction of oculomotor neurons throughout adulthood, at levels comparable to those in FF motor neurons (Figure 7A–D). However we detected no increase in denervation of extraocular muscles even at P150 (Figure 7E) and similar results were observed in the slow Sol muscle (Figure 7C and 7E). Thus, simple co-expression of MMP-9 and mutant SOD1 in a neuron is not sufficient to trigger its degeneration, suggesting that other elements of the pathway also need to be present.
Figure 7. MMP-9 is sufficient to induce degeneration in fast motor neurons of ALS mice.
(A) (Top) AAV6 expressing full-length mouse MMP-9 under CMV promoter. (Bottom) Injections into TA or soleus muscles or intraorbitally were performed at P4 (n=3–4 animals per genotype; control AAV6-GFP injection on contralateral side).
(B) Immunostaining for MMP-9 (red) of the oculomotor nuclei (ChAT+, green) of a P150 SOD1G93A mouse following intraorbital (EOM) injection of AAV6-MMP9.
(C, D) High levels of MMP-9 (white) in soleus (C; P100) or TA (D; P50) motor pools following injection of AAV6-MMP9.
(E) Viral expression of MMP-9 is not sufficient to induce denervation in resistant motor pools - soleus (Sol) or superior rectus (SR) - of SOD1G93A mice but does accelerate denervation in a susceptible pool (TA). **p<0.01
(F) Immunostaining for MMP-9 (white) on a transverse spinal cord section of a P50 SOD1G93A;Mmp9−/− mouse injected at P4 with AAV6-MMP9 into its left TA muscle.
(G) Mmp9 significantly restores vulnerability to the TA muscle (red bar), compared to the control contralateral side (black bar). **p<0.01
Accordingly, when MMP-9 was overexpressed in the fast TA motor pool of SOD1G93A mice, denervation was further accelerated (Figure 7D and 7E). Moreover, MMP-9 was sufficient to restore vulnerability to TA motor neurons rendered resistant by Mmp9 deletion. AAV6-MMP-9 was injected into one TA muscle of a neonatal SOD1G93A;Mmp9−/− mouse, while either no virus (n=2) or AAV6-GFP (n=2) was injected into the contralateral TA as a control (Figure 7F). By P50, the control muscle remained fully innervated whereas ~22% of the NMJs became denervated in the injected muscle (Figure 7G). Overall, therefore, within the cellular context of fast motor neurons expressing mutant SOD1, the rate of degeneration is proportional to the level of MMP-9. However, activation of the pathway for axonal die-back requires neuronal subtype-specific elements in addition to MMP-9 and SOD1.
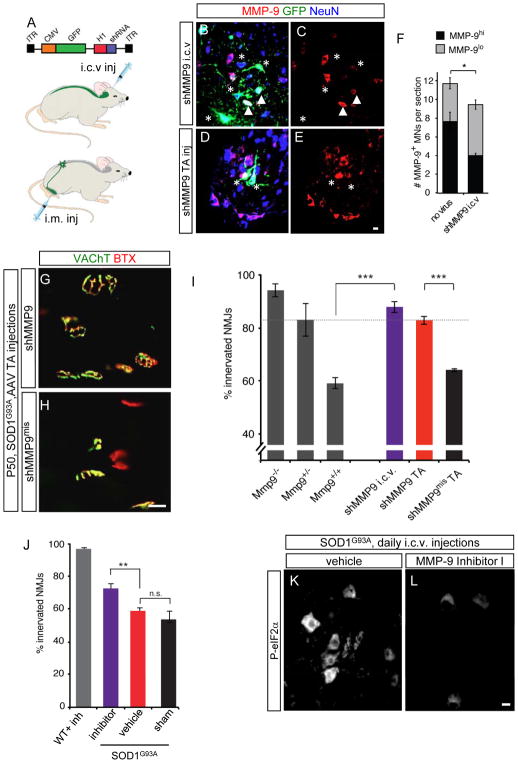
MMP-9 acts centrally in motor neurons to drive degeneration
To identify the cell type in which MMP-9 is acting to promote neurodegeneration, we took advantage of the specific targeting properties of the AAV6 serotype of adeno-associated viral vectors (Snyder et al., 2011; Towne et al., 2008). When administered into the cerebral ventricles (i.c.v.) in neonatal mice, AAV6 infected ~60% of motor neurons throughout the spinal cord (Figures S5A to S5D), as well as proprioceptive sensory neurons (not shown). In contrast, injection into a single skeletal muscle (i.m.) labels only the muscle and corresponding motor pool and proprioceptive neurons (Towne et al., 2011). Therefore the only two cell types infected in common by the i.c.v. and i.m. routes are motor neurons and proprioceptive neurons. Administration of AAV6 vectors expressing shRNA to Mmp9 led to partial (20%; p=0.03) reduction in numbers of MMP-9+ motor neurons and a stronger (50%; p=0.0003) reduction in the MMP-9hi population (Figure 8B to 8F). We observed strong protective effects of postnatal knockdown of MMP-9 by either route, but not injection of a mismatch control into the contralateral TA of the same animal, comparable to the protection afforded by heterozygote deletion of Mmp-9 (Figures 8G to 8I). Since both i.c.v. and i.m. routes conferred protection, and since proprioceptive neurons do not express MMP-9 even in ALS mice (Figures S6B to S6E), our results identify mSOD1 motor neurons as the relevant source of MMP-9 for motor neuron degeneration.
Figure 8. Gene silencing or central pharmacological inhibition of MMP-9 in motor neurons delays muscle denervation.
(A) AAV6 expressing H1:shMmp9 and CMV:GFP was injected i.c.v. at P1 or into TA muscle at P4.
(B to E) Reduction of MMP-9 at the single-cell level in P50 WT mice following AAV6:shMmp9 administration by each route. Transduced α-MNs, visualized by colocalization of GFP (green) and NeuN immunostaining (blue), stained for MMP-9 (red). Asterisks: GFP+ α-MNs negative for MMP-9; arrowheads: less frequent GFP+ α-MNs that retain MMP-9 immunoreactivity. Scale bars, 20 μm.
(F) AAV6:shMMP9 (i.c.v) leads to a 19% reduction in the total number of MMP9+ motor neurons (*p=0.03), and a 47% reduction in the numbers of MMP-9hi (p=0.0003).
(G and H) MMP-9 silencing selectively delays muscle denervation in TA muscle.
(I) AAV6-shMmp9 confers similar protection as the heterozygote knockout (dotted line), whether administered i.c.v. or i.m. TA innervation at P50 in SOD1G93A mice following genetic reduction in MMP-9 levels (gray bars; data from Figure 5G) or viral silencing performed i.c.v. (purple) or i.m. (red). Means ± s.e.m (n=3–4).
(J) i.c.v. administration of MMP-9 Inhibitor I daily from P55 to P75 led to increased innervation in SOD1G93A mice (purple bar) compared to controls (red bar, n=5–6 per treatment).
(K and L) Levels of P-EIF2α (white) at P75 in SOD1G93A motor neurons are significantly reduced by treatment with MMP-9 Inhibitor I.
See also Figure S5.
The genetic approaches used thus far did not distinguish the relative contributions of the proteolytic enzyme activity of MMP-9 and the non-enzymatic activity of its hemopexin domain (Van den Steen et al., 2006; Vandooren et al., 2013). Moreover, they left open the question of whether MMP-9 acts within the spinal cord or at the neuromuscular junction. A previous study using a chemical inhibitor of MMPs including MMP-9 found only modest benefit in mSOD1 mice (Lorenzl et al., 2006), but this may have reflected insufficient CNS exposure. A commercially available inhibitor (MMP inhibitor I), which is among the most potent and specific for MMP-9, was therefore administered centrally (2 μg i.c.v., daily) to mSOD1 mice from P55 (the earliest time point at which stereotactic administration is feasible) to P75. The inhibitor caused a significant delay in TA muscle denervation as compared to controls (Figure 8J). Moreover, it also reduced levels of ER stress in the ventral horn of SOD1G93A animals at P75, compared to vehicle-treated controls (Figure 8K and 8L). The fact that protection was incomplete may reflect partial enzyme inhibition in situ and/or a contribution of the non-enzymatic actions of MMP-9. These results show that MMP-9 inhibition initiated even after the start of the degenerative process can confer significant protection. Moreover, although we cannot formally exclude leakage of inhibitor to the periphery, some critical actions of MMP-9 likely occur at the level of the spinal cord, either directly on fast motor neurons or on neighboring cells and synapses.
Overall, these data show that MMP-9 acts in fast motor neurons to trigger their degeneration, and that the disease process depends at least in part on the enzymatic activity of MMP-9 within the spinal cord. Moreover, modulation of MMP-9 levels or activity even at postnatal stages using clinically-relevant modes of intervention can significantly delay muscle denervation.
DISCUSSION
Our data show that MMP-9 plays a major role in motor neuron degeneration in ALS model mice. Its expression prior to disease onset defines the subset of motor neurons that are destined to die: the fast motor neurons that are also most vulnerable in human patients. Subsequently MMP-9 acts in motor neurons themselves to trigger degeneration and plays an important role in enabling activation of ER stress, the earliest biochemical event thus far identified in the disease pathway downstream of mutant SOD1. Genetic, viral or pharmacological inactivation of MMP-9 each significantly delays the defining cellular event in the onset of paralysis - denervation of muscle endplates – and genetic ablation confers significant functional benefit. Our analysis of molecular diversity within a defined neuronal class has therefore identified a potential therapeutic target of general significance for this currently incurable disease.
The strong protection conferred by removal of MMP-9 raises many questions as to its mode of action, the relation of MMP-9 expression to motor neuron subtype and the potential significance of this approach for other neurodegenerative diseases.
MMP-9 as a candidate therapeutic target
The current lack of effective therapies in ALS reflects the paucity of therapeutic targets validated in vivo, other than the mutant SOD1 gene itself (Przedborski, 2004). Our findings designate MMP-9 as a promising candidate for several reasons. First, the ~80-day delay in fast muscle denervation is the strongest effect yet reported in SOD1G93A mice (Turner and Talbot, 2008). Denervation directly affects muscle strength and occurs with similar specificity in ALS mice and human patients, therefore constituting a clinically relevant endpoint. Second, removal of MMP-9 leads to a 25% increase in lifespan, an effect only surpassed by knockdown of mutant SOD1 itself (Xia et al., 2006) or transgenic over-expression of heavy neurofilament subunits, whose mechanism is not understood (Couillard-Després et al., 1998). Third, MMP-9 appears to act early in the disease pathway triggered by mutant SOD1. Fourth, reducing MMP-9 levels appears to be safe, since small-molecule inhibitors of MMP-9 were tolerated in humans and MMP-9 null mice show only mild cognitive phenotypes and defects in wound healing (Hattori et al., 2009; Nagy et al., 2006). A final argument for the viability of MMP-9 as a target is that even partial reduction in expression levels is sufficient to confer significant benefit.
These data radically modify our understanding of the role of MMP-9 in ALS as compared to earlier reports. The first relevant study found a mild negative effect of MMP-9 deletion on mSOD1 lifespan using the same C57BL/6 background as here (Dewil et al., 2005), perhaps because of the use of a different Mmp9 knockout strain. This was soon contested by Kiaei et al. (2007) who, using a mixed genetic background, found survival effects comparable to those we describe; no other behavioral data were reported. However, Kiaei et al. (2007) focused on the MMP-9 expressed late in disease by activated microglia and deduced that MMP-9 contributes to cytokine-mediated pathology in mSOD1 mice. They found that MMP-9 is not expressed significantly by healthy motor neurons but upregulated later in response to mSOD1. In contrast, we find that MMP-9 expression in motor neurons precedes all known phases of the disease and that increased MMP-9 levels at late stages are largely in microglia, not neurons. Our data define MMP-9 as a major early determinant of ALS pathogenesis, not simply an actor in the massive degeneration that occurs in the final stages of the disease.
Although mSOD1 mice lacking MMP-9 do eventually die, indicating that as expected other mechanisms also contribute to degeneration, the strong effect of deletion of a single gene was striking. MMP-9 is part of a large family of extracellular proteases that include MMP-2, which digests essentially identical substrates. In other contexts, the effects of MMP-9 inhibition have been damped or concealed by compensation by other MMPs (Krüger et al., 2010). The unique expression pattern of MMP-9 provides several potential explanations for its potency in ALS mice. First, of the 19 family members represented on our microarrays, MMP-9 was the only secreted MMP expressed at high levels in motor neurons (Figure S6A). Second, only motor neurons and a small population of dorsal interneurons express MMP-9 in the spinal cord, and few other CNS populations show strong expression (Figure 2A, and S6B to S6E). Third, though MMP-2 is expressed by spinal motor neurons, its expression is downregulated at the end of embryogenesis before the start of MMP-9 expression at P5 (Fig 2C) and is not upregulated postnatally in SOD1G93A mice even in the absence of MMP-9 (Figures S6J to S6M). These results may explain why the role of MMP-9 in motor neuron degeneration is non-redundant, a significant advantage for any candidate therapeutic target.
MMP-9 as a determinant of selective neurodegeneration
Our findings point to an active contribution of MMP-9 to the ALS disease process. However, MMP-9 is not alone sufficient to trigger neurodegeneration since it is expressed in many wildtype motor neurons. Moreover, viral overexpression of MMP-9 in resistant motor neurons did not lead them to degenerate even in the presence of mutant SOD1, whereas its expression in vulnerable fast motor neurons accelerated their degeneration. MMP-9 therefore seems to selectively condition fast motor neurons to become vulnerable to mutant SOD1. It will be interesting to determine the molecular basis of this cell type-specific function and whether the presence of MMP-9 is linked to the low excitability of FF motor neurons (Saxena et al., 2013).
Two questions about the selectivity and timing of disease onset are common to all neurodegenerative disease. The regional selectivity of neuronal loss might potentially have been explained by localized expression of the disease triggers themselves. Instead, however, disease gene expression is widespread: mutant SOD1 in the SOD1G93A mouse is expressed in all cells at levels comparable to those of actin. However, the tightly restricted expression of MMP-9 in fast motor neurons effectively gates the onset of disease to this population. Another enigma for all neurodegenerative diseases is to understand why their symptoms start so late. In ALS mice, although mutant SOD1 is expressed from early embryonic stages, the first functional and protein misfolding changes in vivo are detected only around P7 (Kanning et al., 2010; Saxena et al., 2013). Strikingly, MMP-9 is not expressed by embryonic motor neurons and is first detected in the spinal cord around P5. Therefore MMP-9 by its developmental regulation and highly localized expression may determine both the timing and specificity of the disease phenotype.
MMP-9 and motor neuron diversity
Three distinct levels of selectivity of the ALS phenotype had been identified by earlier studies in mouse and human: preferential loss of motor neurons as compared to other neuronal classes, marked resistance of oculomotor and Onuf’s nuclei with respect to spinal motor pools, and selective vulnerability of FF motor neurons over other subtypes. It was a priori possible that there were different determinants for each level. It is particularly striking that MMP-9 appears to be relevant to all three distinctions. It is expressed selectively in motor neurons in the spinal cord, excluded from oculomotor and Onuf’s nuclei, and absent from the slow, ALS-resistant subtype in spinal motor pools. MMP-9 therefore provides a unifying hypothesis for class-, pool- and subtype-specific aspects of selective motor neuron resistance to ALS.
Relevance to non-SOD1-linked neurodegeneration
It will be important to determine whether MMP-9 is relevant to the sporadic cases of ALS which make up ~90% of all patients. We identified MMP-9 based on selective resistance that is common to mouse models and sporadic ALS patients. Moreover, our data and others indicate that mSOD1 mice mirror to a remarkable degree the precise pattern of selective neurodegeneration – both in terms of motor pool and subtype - seen in patients with sporadic disease. Recently, Brockington et al. (2013), using laser capture microdissection from neurologically normal human postmortem samples, showed that MMP-9 levels are ~2.5-fold lower in oculomotor nucleus than in lumbar motor neurons. Encouragingly, a recent human population study showed that a polymorphism leading to higher Mmp9 promoter activity is significantly associated with an increased risk of sporadic ALS (He et al., 2013).
Our approach to ALS is of potential significance to neurodegenerative diseases affecting other neuronal classes. All such diseases show a marked specificity that is reflected in the clinical outcome. For example, in Parkinson’s disease dopaminergic neurons in the substantia nigra are most strongly affected. Here too, selective resistance is observed: mesostriatal neurons degenerate whereas mesolimbic and mesocortical neurons are more resistant (Damier et al., 1999; Liss and Roeper, 2008). Gene expression differences detected between these populations have been shown to protect against the effects of exogenous stressors (Chung et al., 2010). Blocking selective vulnerability may therefore be a viable strategy in multiple therapeutic contexts.
Overall our study demonstrates how a precise understanding of the biological diversity of neuronal subpopulations can lead to significant new insights into the mechanisms involved in neurological disease. Our molecular analysis further emphasizes the remarkable extent of the differences and similarities between different motor pools and subtypes. Importantly, it also allowed us to manipulate the molecular make-up of FF motor neurons postnatally so that they more closely resemble resistant S motor neurons. The strong protection thereby conferred provides hope that not only MMP-9 but also other genes with subtype-specific expression may provide a basis for rational therapies in ALS and other neurodegenerative diseases.
EXPERIMENTAL PROCEDURES
Transgenic/Knockout Mice
For expression studies, ChAT-Cre [B6;129S6-Chattm1(cre)Lowl/J] and ROSA-TdTomato reporter [B6;129S6-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J] mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and crossed to generate offspring whose motor neurons selectively express TdTomato. Chodl-LacZ mice were obtained from Lexicon Pharmaceuticals. For functional studies, mice heterozygous for the mutant human SOD1G93A transgene (B6.Cg-Tg(SOD1*G93A)1Gur/J) were crossed with mice heterozygous for an MMP9 gene disruption (B6.FVB(Cg)-Mmp9tm1Tvu/J), both on a C57BL/6J background. For all experiments, SOD1G93A; Mmp9−/− mice were always compared with their SOD1G93A; Mmp9+/+ or SOD1G93A; Mmp9+/− littermates. End-stage of disease was defined as the age at which mutant SOD1 mice could no longer right themselves for 30 seconds after being put on their side. All animal work was performed in compliance with Columbia University IACUC protocols.
Microarray analysis
Lumbosacral spinal cord and midbrain from three wild-type P7 male animals were cryosectioned, mounted on RNAse-free glass slides (Zeiss), fixed and stained with 1% cresyl violet, and dehydrated prior to LCM using the PALM Microbeam system (Zeiss). From each animal, ~200 DL, L5, and oculomotor motor neurons were collected directly into lysis buffer. At least 1.5 ng of purified RNA was amplified in the WT-Ovation Pico RNA Amplification System (Nugen, San Carlos, CA) and hybridized to Affymetrix (Santa Clara, CA) Mouse Genome 430 2.0 Arrays. Gene ontology and pathway analysis was performed using DAVID Bioinformatics Resources 6.7 (National Institute of Allergy and Infectious Diseases, NIH, Bethesda, MD).
Immunohistochemistry and morphological analysis
The following primary antibodies were used: goat anti-ChAT 1:100 (Millipore, Billerica, MA), rabbit anti-MMP-9 1:4000 (Abcam, Cambridge, MA), goat anti MMP-9 1:2000 (Sigma-Aldrich, St. Louis, MI), rabbit anti-GFP 1:1000 (Invitrogen, Carlsbad, CA), mouse anti-NeuN 1:600 (Chemicon, Billerica, MA), chicken anti beta-galactosidase 1:2000 (Abcam), and rabbit anti-phosphorylated EIF2α (Ser51) 1:150 (Cell Signaling Technology, Inc. Beverly, MA). To quantify muscle denervation, consecutive longitudinal 40 μm cryosections of muscles were stained for VAChT (Sigma) and BTX (Invitrogen). % NMJ innervation was determined by dividing the total number of areas of overlap between VAChT and BTX signals (total number innervated endplates) by the number of areas of BTX signal (total number of endplates).
Retrograde labeling
Cholera toxin subunit B (CTB) conjugated to Alexa Fluor 488 or 555 was injected into the Sol, TA, or IC muscles of WT mice at P5 or P40 or Mmp9−/− mice at P40. Animals were perfused and spinal cords collected 48–96 hrs later. At that time, the injected muscle and surrounding muscles were checked for fluorescence to assure specificity of injections.
Electrophysiological measurements
Mice were anaesthetized and two electrodes were placed on either side of the sciatic nerve at a paraspinal site. The skin overlaying the tibialis anterior was cut and bipolar electrodes were inserted into the muscle. A controlled stimulation was applied to the nerve to evoke contractions from the tibialis anterior muscle in 10 to 50 μA increments from 50 μA.
AAV6 viral vectors
For intracerebroventricular (i.c.v) injections, P1 pups were anesthetized and using a 31 Gauge 1/2 cc Insulin syringe (BD Ultra-Fine* II Short Needle Insulin Syringe, VWR) 2 μl of virus was injected into the third ventricle. For intramuscular (i.m.) injections, P4 pups were anesthetized, the skin overlaying the TA muscles was opened and 2 μl of the AAV6.shMMP9 virus was injected using an insulin syringe. For the AAV6.MMP9 i.m. administration, P4 pups were anesthetized and then 2 μl of viral vector was injected into their TA, soleus, or extraocular muscles.
Pharmacological Inhibition
WT or SOD1G93A mice (18–22 g) were anesthetized with isoflurane at P50 and placed in a stereotaxic instrument and a cannula was placed 1 mm lateral and 1 mm caudal to bregma, at a depth of 2.5 mm. The animals were allowed to recover for 5 days and then given daily injections into their cannulae of MMP-9 Inhibitor I (2 μg per day dissolved in 10% DMSO, Calbiochem) or a vehicle. The volume of all i.c.v. injections was 10 μl, using a 25 μl syringe (Hamilton Co., Reno, NV, USA). Animals were perfused after 20 days of injections, at P75.
Supplementary Material
HIGHLIGHTS.
Motor neuron subpopulations show highly divergent patterns of gene expression
Matrix metalloproteinase-9 (MMP-9) is a marker for vulnerable fast motor neurons
Reductions in MMP-9 delay muscle denervation and prolong survival in ALS model mice
MMP-9 enhances ER stress in vulnerable motor neurons and triggers degeneration
Acknowledgments
We are extremely grateful to colleagues in the Henderson lab and the Motor Neuron Center for helpful discussions and insights, and in particular to Tom Jessell, Serge Przedborski, Neil Shneider and Lloyd Greene for their input throughout the project. We thank Tom Jessell, Alan Tenney, Justin Lee, Serge Przedborski, Andrew Williams and Manish Raisinghani for constructive comments on the manuscript. We are also grateful to Elizabeth Dirren and Bernard Schneider (EPFL) for help with virus production and Paolo Guarnieri (Columbia Genome Center) for help with analysis of the microarray data. We thank Kara Spiller for assistance with filming and video editing. This work received invaluable support from P2ALS, the Tow Foundation, the SMA Foundation and NINDS for R01-NS05642201A1 and the MD-PhD program. A.K. was recipient of a Kirschstein NRSA award and K.J.S. was supported by an NSF Graduate Research Fellowship DGE-07-07425.
Footnotes
Contributions:
A.K., K.J.S. and C.E.H. conceived the study and wrote the article. A.K. and K.J.S. performed all the mouse experiments with the help of K.C.K. for expression studies, G.T.C. for behavioral analysis, A.G. for morphological outcomes and T.A. for electromyography. C.T. and P.A. generated the AAV vectors and provided critical guidance for the viral experiments. C.E.H. was responsible for oversight of the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexianu ME, Ho BK, Mohamed AH, La Bella V, Smith RG, Appel SH. The role of calcium-binding proteins in selective motoneuron vulnerability in amyotrophic lateral sclerosis. Ann Neurol. 1994;36:846–858. doi: 10.1002/ana.410360608. [DOI] [PubMed] [Google Scholar]
- Aronica E, Catania MV, Geurts J, Yankaya B, Troost D. Immunohistochemical localization of group I and II metabotropic glutamate receptors in control and amyotrophic lateral sclerosis human spinal cord: upregulation in reactive astrocytes. Neuroscience. 2001;105:509–520. doi: 10.1016/s0306-4522(01)00181-6. [DOI] [PubMed] [Google Scholar]
- Brockington A, Ning K, Heath PR, Wood E, Kirby J, Fusi N, Lawrence N, Wharton SB, Ince PG, Shaw PJ. Unravelling the enigma of selective vulnerability in neurodegeneration: motor neurons resistant to degeneration in ALS show distinct gene expression characteristics and decreased susceptibility to excitotoxicity. Acta Neuropathol. 2013;125:95–109. doi: 10.1007/s00401-012-1058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Licznerski P, Alavian KN, Simeone A, Lin Z, Martin E, Vance J, Isacson O. The transcription factor orthodenticle homeobox 2 influences axonal projections and vulnerability of midbrain dopaminergic neurons. Brain. 2010;133:2022–2031. doi: 10.1093/brain/awq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Couillard-Després S, Zhu Q, Wong PC, Price DL, Cleveland DW, Julien JP. Protective effect of neurofilament heavy gene overexpression in motor neuron disease induced by mutant superoxide dismutase. Proc Natl Acad Sci U S A. 1998;95:9626–9630. doi: 10.1073/pnas.95.16.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullheim S, Fleshman JW, Glenn LL, Burke RE. Membrane area and dendritic structure in type-identified triceps surae alpha motoneurons. J Comp Neurol. 1987;255:68–81. doi: 10.1002/cne.902550106. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122( Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Dewil M, Schurmans C, Starckx S, Opdenakker G, Van Den Bosch L, Robberecht W. Role of matrix metalloproteinase-9 in a mouse model for amyotrophic lateral sclerosis. Neuroreport. 2005;16:321–324. doi: 10.1097/00001756-200503150-00003. [DOI] [PubMed] [Google Scholar]
- Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Memic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, et al. Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as markers for fast motor neurons and partition cells. J Comp Neurol. 2010;518:2284–2304. doi: 10.1002/cne.22332. [DOI] [PubMed] [Google Scholar]
- Ferrucci M, Spalloni A, Bartalucci A, Cantafora E, Fulceri F, Nutini M, Longone P, Paparelli A, Fornai F. A systematic study of brainstem motor nuclei in a mouse model of ALS, the effects of lithium. Neurobiol Dis. 2010;37:370–383. doi: 10.1016/j.nbd.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese A, Kaltschmidt JA, Ladle DR, Sigrist M, Jessell TM, Arber S. Gamma and alpha motor neurons distinguished by expression of transcription factor Err3. Proc Natl Acad Sci U S A. 2009;106:13588–13593. doi: 10.1073/pnas.0906809106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D’Armiento J, Okada Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol. 2009;175:533–546. doi: 10.2353/ajpath.2009.081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Zhang L, Yao X, Hu J, Yu L, Jia H, An R, Liu Z, Xu Y. Association studies of mmp-9 in Parkinson’s disease and amyotrophic lateral sclerosis. PLoS One. 2013;8:e73777. doi: 10.1371/journal.pone.0073777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund E, Karlsson M, Osborn T, Ludwig W, Isacson O. Global gene expression profiling of somatic motor neuron populations with different vulnerability identify molecules and pathways of degeneration and protection. Brain. 2010;133:2313–2330. doi: 10.1093/brain/awq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28:154–164. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirano A. Sparing of the Onufrowicz nucleus in sacral anterior horn lesions. Ann Neurol. 1978;4:245–249. doi: 10.1002/ana.410040309. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci. 2010;33:409–440. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Calingasan NY, Wille E, Chen J, Heissig B, Rafii S, Lorenzl S, Beal MF. Matrix metalloproteinase-9 regulates TNF-alpha and FasL expression in neuronal, glial cells and its absence extends life in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2007;205:74–81. doi: 10.1016/j.expneurol.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Krüger A, Kates RE, Edwards DR. Avoiding spam in the proteolytic internet: future strategies for anti-metastatic MMP inhibition. Biochim Biophys Acta. 2010;1803:95–102. doi: 10.1016/j.bbamcr.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Laslo P, Lipski J, Nicholson LF, Miles GB, Funk GD. Calcium binding proteins in motoneurons at low and high risk for degeneration in ALS. Neuroreport. 2000;11:3305–3308. doi: 10.1097/00001756-200010200-00009. [DOI] [PubMed] [Google Scholar]
- Liss B, Roeper J. Individual dopamine midbrain neurons: functional diversity and flexibility in health and disease. Brain Res Rev. 2008;58:314–321. doi: 10.1016/j.brainresrev.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Lorenzl S, Albers DS, LeWitt PA, Chirichigno JW, Hilgenberg SL, Cudkowicz ME, Beal MF. Tissue inhibitors of matrix metalloproteinases are elevated in cerebrospinal fluid of neurodegenerative diseases. J Neurol Sci. 2003;207:71–76. doi: 10.1016/s0022-510x(02)00398-2. [DOI] [PubMed] [Google Scholar]
- Lorenzl S, Narr S, Angele B, Krell HW, Gregorio J, Kiaei M, Pfister HW, Beal MF. The matrix metalloproteinases inhibitor Ro 28-2653 [correction of Ro 26-2853] extends survival in transgenic ALS mice. Exp Neurol. 2006;200:166–171. doi: 10.1016/j.expneurol.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Mannen T, Iwata M, Toyokura Y, Nagashima K. Preservation of a certain motoneurone group of the sacral cord in amyotrophic lateral sclerosis: its clinical significance. J Neurol Neurosurg Psychiatry. 1977;40:464–469. doi: 10.1136/jnnp.40.5.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S. Molecular targets for neuroprotection. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(Suppl 1):14–18. doi: 10.1080/1734470410019780. [DOI] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Warita H, Komori T, Murakami T, Abe K, Iwata M. Parvalbumin and calbindin D-28k immunoreactivity in transgenic mice with a G93A mutant SOD1 gene. Brain Res. 2006;1083:196–203. doi: 10.1016/j.brainres.2006.01.129. [DOI] [PubMed] [Google Scholar]
- Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12:627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- Saxena S, Caroni P. Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron. 2011;71:35–48. doi: 10.1016/j.neuron.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Saxena S, Roselli F, Singh K, Leptien K, Julien JP, Gros-Louis F, Caroni P. Neuroprotection through Excitability and mTOR Required in ALS Motoneurons to Delay Disease and Extend Survival. Neuron. 2013;80:80–96. doi: 10.1016/j.neuron.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Schrøder HD, Reske-Nielsen E. Preservation of the nucleus X-pelvic floor motosystem in amyotrophic lateral sclerosis. Clin Neuropathol. 1984;3:210–216. [PubMed] [Google Scholar]
- Shneider NA, Brown MN, Smith CA, Pickel J, Alvarez FJ. Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival. Neural Dev. 2009;4:42. doi: 10.1186/1749-8104-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder BR, Gray SJ, Quach ET, Huang JW, Leung CH, Samulski RJ, Boulis NM, Federici T. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Hum Gene Ther. 2011;22:1129–1135. doi: 10.1089/hum.2011.008. [DOI] [PubMed] [Google Scholar]
- Swarnakar S, Mishra A, Chaudhuri SR. The gelatinases and their inhibitors: the structureactivity relationships. EXS. 2012;103:57–82. [PubMed] [Google Scholar]
- Towne C, Raoul C, Schneider BL, Aebischer P. Systemic AAV6 delivery mediating RNA interference against SOD1: neuromuscular transduction does not alter disease progression in fALS mice. Mol Ther. 2008;16:1018–1025. doi: 10.1038/mt.2008.73. [DOI] [PubMed] [Google Scholar]
- Towne C, Setola V, Schneider BL, Aebischer P. Neuroprotection by gene therapy targeting mutant SOD1 in individual pools of motor neurons does not translate into therapeutic benefit in fALS mice. Mol Ther. 2011;19:274–283. doi: 10.1038/mt.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 2008;85:94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One. 2012;7:e34640. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Steen PE, Van Aelst I, Hvidberg V, Piccard H, Fiten P, Jacobsen C, Moestrup SK, Fry S, Royle L, Wormald MR, et al. The hemopexin and O-glycosylated domains tune gelatinase B/MMP-9 bioavailability via inhibition and binding to cargo receptors. J Biol Chem. 2006;281:18626–18637. doi: 10.1074/jbc.M512308200. [DOI] [PubMed] [Google Scholar]
- Van Hoecke A, Schoonaert L, Lemmens R, Timmers M, Staats KA, Laird AS, Peeters E, Philips T, Goris A, Dubois B, et al. EPHA4 is a disease modifier of amyotrophic lateral sclerosis in animal models and in humans. Nat Med. 2012 doi: 10.1038/nm.2901. [DOI] [PubMed] [Google Scholar]
- Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. 2013;48:222–272. doi: 10.3109/10409238.2013.770819. [DOI] [PubMed] [Google Scholar]
- Vu TH, Shipley JM, Bergers G, Berger JE, Helms JA, Hanahan D, Shapiro SD, Senior RM, Werb Z. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411–422. doi: 10.1016/s0092-8674(00)81169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Zhou H, Huang Y, Xu Z. Allele-specific RNAi selectively silences mutant SOD1 and achieves significant therapeutic benefit in vivo. Neurobiol Dis. 2006;23:578–586. doi: 10.1016/j.nbd.2006.04.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.