Abstract
Merkel cell carcinoma (MCC) is a highly aggressive, often lethal neuroendocrine cancer. Its carcinogenesis may be either caused by the clonal integration of the Merkel cell polyomavirus into the host genome or by UV-induced mutations. Notably, virally-encoded oncoproteins and UV-induced mutations affect comparable signaling pathways such as RB restriction of cell cycle progression or p53 inactivation. Despite its low incidence, MCC recently received much attention based on its exquisite immunogenicity and the resulting major success of immune modulating therapies. Here, we summarize current knowledge on epidemiology, biology and therapy of MCC as conclusion of the project ‘Immune Modulating strategies for treatment of Merkel Cell Carcinoma’, which was funded over a 5-year period by the European Commission to investigate innovative immunotherapies for MCC.
Keywords: Merkel cell carcinoma, Epidemiology, Cell of origin, Merkel cell polyomavirus, Immunotherapy, IMMOMEC
Introduction
Merkel cell carcinoma (MCC) is a highly aggressive, often lethal neuroendocrine cancer of the skin. The majority of cases is associated with the recently discovered common Merkel cell polyomavirus (MCPyV) while the remaining are triggered by UV-mediated mutations [1]. With an incidence of 0.6 per 100,000 per year in the US in 2009 (US Standard Population) MCC is a very rare cancer [1]. Although MCC is 40 times less common than malignant melanoma, MCC has a dramatically lower survival probability than melanoma, rendering MCC the most lethal skin cancer. Indeed, epidemiologic data suggest that there are approximately 2500 new MCC cases per year within the European Union (EU), and approximately 1000 of these patients will die from their disease [2]. This high mortality rate is largely due to the fact that until recently none of the standard therapeutic interventions was able to improve overall survival of patients suffering from metastatic disease. Since several lines of evidence indicate the outstanding immunogenicity of MCC, immune modulating treatment strategies are particularly attractive.
IMMOMEC was a 5-year project to establish and investigate an innovative immunotherapy for MCC which is based on the targeted delivery of interleukin-2 to the tumor microenvironment. However, fortunately for the patients, but unfortunately for the IMMOMEC trial, the advent of immune checkpoint-inhibiting antibodies [specifically the programmed death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) blocking antibodies, pembrolizumab and avelumab, respectively] revolutionized the treatment of advanced MCC [3, 4]. Indeed, these agents achieve durable objective response rates of up to 50% of the treated patients. Thus, the bar for any new therapeutic, particularly immunotherapeutic, to be tested in advanced MCC had been raised high [5]. As a consequence, the initial recruitment into the IMMOMEC trial was substantially slowed by the fully sponsored competing trials using checkpoint inhibitors, and once their strong clinical efficacy had been reported the recruitment rate virtually ceased. However, IMMOMEC was not only testing a new therapeutic option for MCC patients, but also aimed at establishing and validating new tools to monitor patients receiving immunotherapies as well as compile prognostic and predictive biomarkers to individualize immune modulating therapies [6–15].
The epidemiology of Merkel cell carcinoma
A comprehensive analysis of time trends of the population-based incidence of MCC is complicated, considering that MCC was first described in 1972 and had many synonyms. In addition, in its first edition the International Classification of Diseases for Oncology (ICD-O) (1976–1989) did not contain specific morphology codes for MCC. The second edition of ICD-O, released in 1990, introduced the codes M8246/3 (neuroendocrine carcinoma) and M8247/3 (MCC) [16]. Hence, reliable time trends should have starting times after 1990. Analyses of more than 100 regional or national population-based cancer registry data sets on MCC identified more than 19,000 cases for the period from 2003 until 2007, and more than 12,000 cases for the time trend analysis of the years 1990 through 2007 (A. Stang, unpublished data). Several cancer registries demonstrated incidence rates of MCC in the early 1990s (1990–1994) that were close to zero, indicating unreliable incidence registration. Importantly, since the mid of the 1990s, the registries of US SEER White, Canada, Australia, The Netherlands, and Italy revealed an increase of MCC incidence. Age-standardized incidence rates (World Standard Population) of the period from 2003 until 2007 were highest in Australia (men: 5.2 per million person years; women: 2.2 per million person years).
Cell of origin of Merkel cell carcinoma
The cellular origin of MCC is still controversial. Initially, the favored theory was that MCC originates from Merkel cells. However, because normal Merkel cells are terminally differentiated and do not undergo cell division, they probably are not the cell of origin for MCC. Thus, this notion was followed by the hypothesis that a Merkel cell precursor, for example, epidermal or dermal stem cells, is the possible cell of origin of MCCs [17]. Still it is important to note that the majority of MCCs develop in a spatially distant Merkel cell-free micro-anatomic compartment of the skin. Based on the finding of concomitant expression of paired box 5 (PAX-5), Terminal deoxynucleotidyl transferase (TdT) and diverse immunoglobulins, including monoclonal IgH and Igk rearrangement in MCC cells, which is normally restricted to early B cells, it was postulated that pre-/pro-B cells might constitute the cellular origin of MCCs [18]. The suggested interpretation of MCC as a cutaneous manifestation of a highly malignant pre-/pro-B cell neoplasia would allow a different understanding of the diverse pathological and clinical features of MCC [19, 20]. However, to date none of these cells could be transformed by the expression of the MCPyV-encoded T antigens in vitro. Notably, human dermal fibroblasts support productive MCPyV infection [21]. Induction of genes encoding matrix metalloproteinases by the WNT/β-catenin signaling pathway stimulated MCPyV infection, suggesting that UV radiation and aging (that is, well established risk factors for MCC), which are known to stimulate WNT signaling and matrix metalloproteinases expression, could promote MCPyV infection of fibroblasts and, therefore, drive MCC development [21]. Identification of the cell of origin together with an improved understanding of the mechanism of viral carcinogenesis might also enable the identification of susceptibility factors for MCPyV-driven MCC carcinogenesis.
Elucidating the role of MCPyV-encoded oncoproteins in transformation
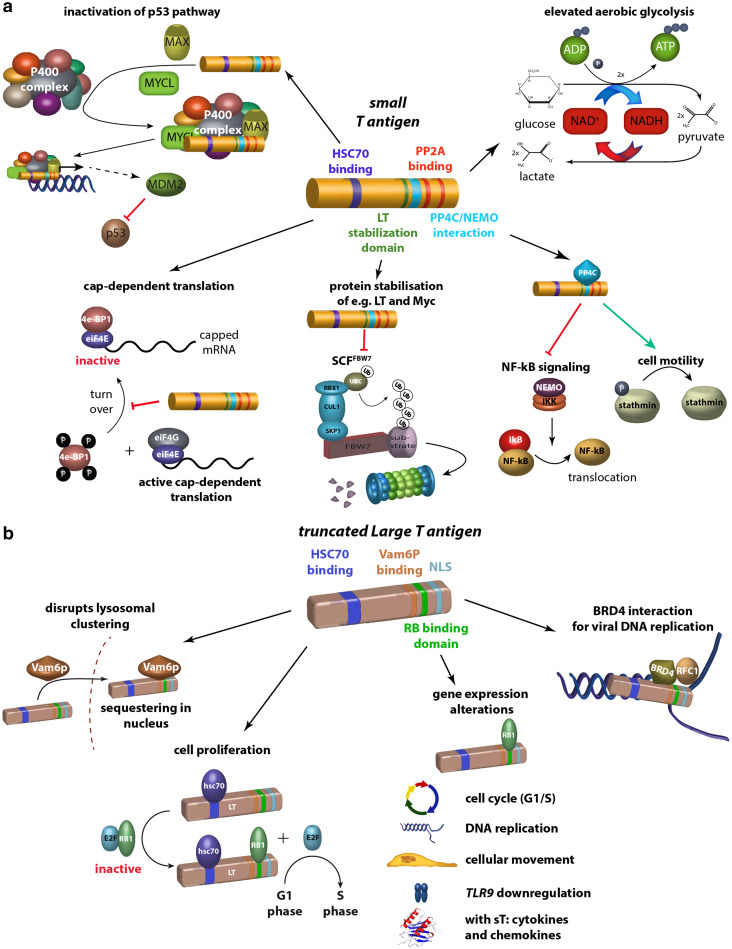
The human tumor virus MCPyV establishes lifelong persistent infections in ~ 90% of the healthy human population, and is the leading cause of MCC [22]. A number of observations suggest that the virus is causally linked to MCC tumorigenesis. For example, viral sequences are monoclonally integrated into the tumor cell genome, i.e. in MCPyV-positive cases the virus has the identical integration sites in the primary tumor and the respective metastases. Furthermore, expression of the early viral proteins, i.e. the T antigens consisting of small T-Antigen (sT) and large T-Antigen (LT), can be detected in tumor tissues [23–26]. While both sT and LT are necessary for maintaining the oncogenic phenotype of MCPyV+ MCC cell lines, sT appears to be more potent in transforming model cell lines such as rodent fibroblast cell lines [27–29]. It should be further noted that all LT sequences recovered from primary MCC tumors or MCC cell lines harbour signature mutations resulting in the expression of shortened LTs. These truncation products always retain the retinoblastoma protein (RB)-binding motif, but lack the C-terminal region that is required for viral replication and—as deducted from SV40 LT—supposedly binding of p53. Recently, it was also demonstrated that truncated LT proteins not only exhibit an increased binding affinity for RB, but are also able to partially re-localize RB to the cytoplasm. This notion suggest additional properties of the tumor-derived truncated LT compared to the wild-type protein and a major importance role of its RB-binding capacity in MCC tumorigenesis [30] (Fig. 1).
Fig. 1.
Domains and functions of MCPyV T antigens. a sT can (i) preserve 4E-BP1 hyperphosphorylation resulting in dysregulated cap-dependent translation [31], (ii) through inhibition of SCF Fbx7 ubiquitin ligase stabilize their cellular targets and MCPyV LT [32], (iii) interact with NEMO thereby inhibiting NF-κB-mediated transcription [33–35], (iv) lead to a motile and migratory phenotype by promoting microtubule destabilization [36], (v) elevate aerobic glycolysis by regulating MCT-1 levels [37] and (vi) inactivate p53 pathway through recruitment of MAX and MYCL and binding to P400 complex resulting in increased expression of the p53 inhibitor MDM2. b In tumor cells, the C-terminal domains of LT containing several crucial elements required for viral replication are consistently truncated by tumor-associated mutations. The truncated LT can (i) mediate cell proliferation by binding RB1 [38], (ii) lead to gene expression alterations most of which require an intact RB binding site [39], (iii) disrupt lysosomal clustering by binding hVam6p and translocating it into the nucleus [40] and (iv) interact with Brd4 to RFC to the viral replication sites facilitating replication [41]. The latter two functions seem to be relevant for the normal life cycle of the virus. 4E-BP1 = eukaryotic translation initiation factor 4E-binding protein 1; SCF = the Skp1-Cul1-F-box protein; FBx7 = F-box protein 7; NEMO = NF-κB essential modulator; MCT-1 = monocarboxylat-Transporter 1; hVamp6 = human VAM-encoded protein; 6Brd4 = bromodomain protein 4; RFC = recruit replication factor C
To understand early events of viral integration and cellular transformation, an in vitro MCPyV replication model was developed which will allow to mimic these early events of virus-induced transformation [23, 26]. To understand MCC pathogenesis and in particular MCC metastasis formation, a spontaneous metastasis xenograft mouse model was used. Applying genome-wide transcriptome analyses of the xenograft tumors arising from transplanted MCC cell lines, it was demonstrated that several MCC cell lines are well suited to study metastasis formation of MCC tumors in a xenograft (severe combined immunodeficiency) SCID mouse model system [42]. This model will significantly contribute to elucidate the virally mediated mechanisms in metastasis formation.
Functions of large T antigen in MCC cells
MCC cells harboring MCPyV are dependent on the expression of the viral T antigens, i.e. sT and LT. Previous studies demonstrated that knockdown of T antigens in MCC cells impairs their growth [25]. This effect can be rescued by ectopic expression of an shRNA-insensitive LT. Consequently, modifying this ectopically expressed LT allows identifying domains or regions required for the growth-promoting function of LT. To this end, experiments with mutated LTs demonstrated, that the HSC70 as well as pocket protein (PP)-binding domains are required for the growth-promoting functions of LT [43]. In addition, for proper function of LT the protein has to enter the nucleus, which, however, is independent of the nuclear localization sequence (NLS) reported to be at position 177–180. Phosphorylation of serine at position 220 was identified as a post-translational modification required for the oncogenic function of truncated LT [38]. A recent study aiming at the identification of the cellular interaction partner(s) for the PP-binding domain of LT identified the three PP family members RB1, p107 and p130 as the main candidates. Knockdown of these individually demonstrated that knockdown of RB1 alone was sufficient to rescue the effect of LT knockdown-mediated cellular growth impairment [44] (Fig. 1). Thus, in contrast to other polyomavirus LTs, which interact with all three PP family members, for MCPyV’s LT RB1 is the predominant cellular partner.
Does sunlight and MCPyV target the same pathways in MCC?
Virus-positive MCC tumors express MCPyV sT and a truncated MCPyV LT, which both bind to multiple proteins thereby altering their functions. A comprehensive, yet not exhausting overview of these interactions are depicted in Fig. 1. It should be noted that full length LT—as deducted from SV40 LT—supposedly binds to p53, whereas the truncated form expressed in MCC does not. These functions, however, are not sufficient to promote oncogenesis [45]. In contrast, virus-negative MCCs are characterized by high frequencies of UV-induced DNA damage with mutations that inactivate the RB1 and p53 genes [46]. It was recently demonstrated that MCPyV sT specifically recruits l-myc-1 proto-oncogene protein (MYCL) and myc-associated factor X (MAX) to the 15-component p400 complex. sT, MYCL and the p400 complex bind specifically to transcription start sites and promote gene expression [37]. Wild-type sT, but not a p400 binding mutant, can cooperate with MYCL to transform human fibroblasts. sT-MYCL-p400 can increase expression of Mouse double minute 2 homolog (MDM2), which contributes to the inactivation of the p53 pathway (Fig. 1). These results demonstrate that MCPyV sT recruitment of MYCL to the p400 complex contributes to MCC oncogenesis and provides insights into the function of MYC in malignant transformation.
Genomic and immune heterogeneity in non-viral Merkel cell carcinoma
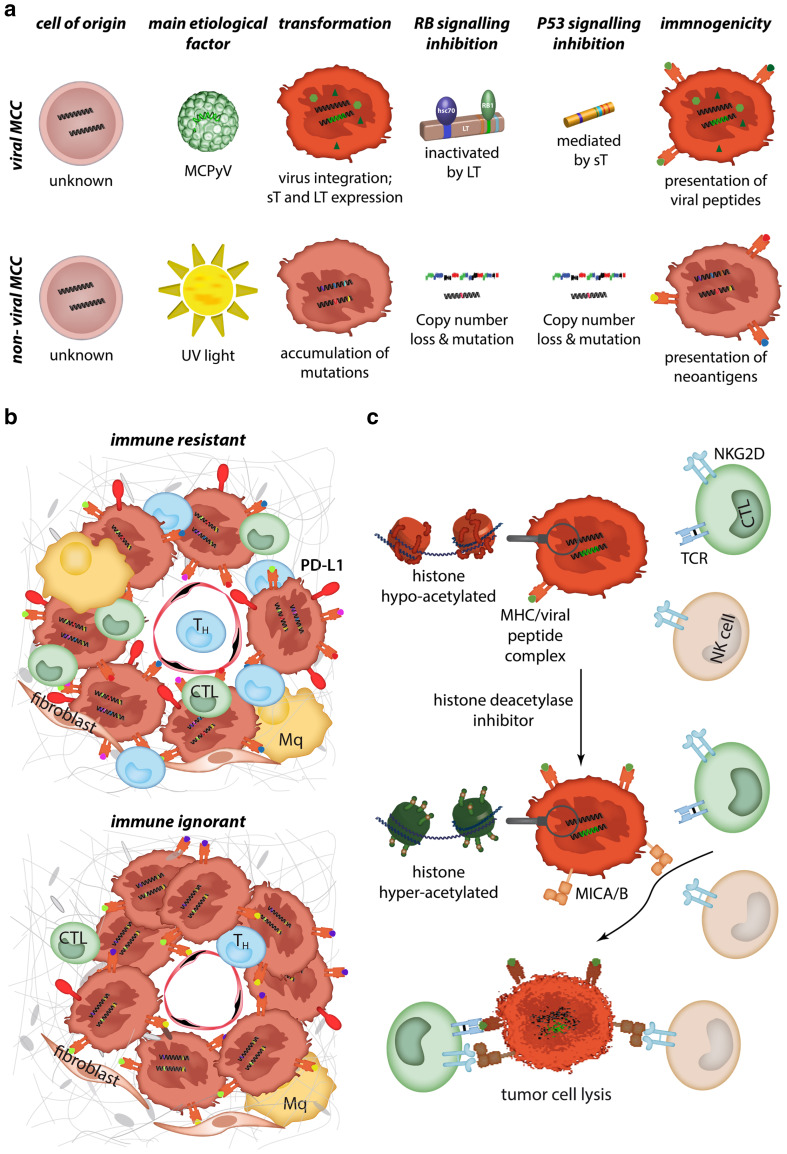
While MCPyV can be detected in up to 80% of MCC cases in the northern hemisphere, its presence is much lower in other geographic regions such as Australia (~ 30%) [1]. MCPyV-negative, aka non-viral, MCC appears to be a distinct entity with hallmarks of a high mutation load caused by UV-damage [47]. Excessive sun-exposure is, therefore, the predominant risk factor for the development of non-viral MCC (Fig. 2a). Despite clear genetic differences between viral and non-viral MCC, immune escape is likely to be important for both groups, as viral-antigen or mutation-associated neoantigens are potent immunogenic stimuli. Importantly, immune checkpoint therapies involving the PD-1/PD-L1 axis have recently been shown to be effective in both viral and non-viral subtypes of MCC, thereby offering a promising treatment strategy for advanced disease [3, 4]. Considering that checkpoint-based immunotherapy will not be effective for all MCC patients, it is important to have a better understanding of cellular and genomic determinants of the immune response. By characterizing non-viral MCC based on a framework of immune reactivity within the tumor microenvironment, MCC tumors can be classified into two groups based on the frequency of tumorinfiltrating lymphocytes (TIL) and PD-L1 expression, i.e. type 1 “immune resistant” (high TIL/high PD-L1) and type 2 “immune ignorant” (low TIL/low PD-L1). Mutation burden is higher in type 1 non-viral MCC, consistent with the notion that a higher neoantigen load exists within more densely infiltrated tumors [47–53] (Fig. 2b). However, there are also individual exceptions to this rule, suggesting that other factors can influence the immune response. An additional level of complexity is added by the intra-patient heterogeneity between metastatic lesions. Such a heterogeneity not only explains the potential difficulty to identify predictive biomarkers from a single biopsy, but also that heterogeneous responses to immune checkpoint blockade have to be expected.
Fig. 2.
Immunogenicity of MCC. a MCCs can be divided upon their association with MCPyV. For both, viral and non-viral MCCs, the cell of origin has not yet been identified and, consequently, it is currently not known if they originate from the same cell. Although transformation is caused by the virus or by UV-induced mutations, they both lead to RB and p53 pathway inactivation. Immunogenicity of both tumors is high due to presentation of viral peptides or neoantigens, respectively [47]. b Based on programmed death-ligand 1 (PD-L1) expression and immune infiltrate, non-viral MCC cases can be divided into immune-resistant or immune-ignorant tumors. The latter demonstrate a lower mutagenic burden [47]. c MCC tumors present with a low/absent expression of the stress molecules MHC class I polypeptide-related sequence A and B (MICA/B). Treatment of MCC cells with histone deacetylases leads to induction/increased expression of MICA/B [8]. These natural killer group 2D (NKG2D) ligands act co-stimulatory on T cells and activate on NK cells. Consequently, the lysis of tumor cells by immune cells is increased after histone acetylase inhibition
Novel tools to gain insights into T-cell responses to MCC
Discovery of CD8+ T-cell epitopes in MCC is important for understanding the immune recognition of cancer cells. This cellular component plays a major role in mediating tumor cell eradication, and can be utilized in T cell-based immunotherapeutic strategies. To this end, current state-of-the-art strategies use combinatorial encoding of fluorescence-labeled peptide–MHC multimers. With this strategy, 56 T-cell recognitions towards 35 different MCPyV-derived peptides were detected in a cohort of 38 MCC patients. T-cell responses towards LT and sT antigens were solely detected in cancer patients compared to healthy donors [11]. However, a major limitation to this strategy is the maximum number of peptide specificities (< 36) to be screened for in a single sample. Accordingly, screening for a large peptide library will thus require a large amount of patient material. To address this problem, a novel technology with DNA barcode-labeled peptide-MHC multimers to increase the possibly covered complexity has recently been introduced [54]. This technology uses an oligonucleotide to form a barcode tag that is associated to the identity of a given peptide–MHC complex, allowing the mixing of > 1000 T-cell specificities. After selection of MHC multimer-binding T cells, the specificity can be revealed by sequencing the attached barcode. A correlation with the current state-of-the-art strategies revealed a similar sensitivity coupled with a significantly better detection of low-avidity T-cell populations. Using this novel technology when screening for cancer-specific T cells, a high-throughput platform for epitope mapping with less demand of patient material has been created.
Loss of non-classical MHC molecules is an immune escape mechanism of MCC
Viral infection as well as malignant transformation are well-known inducers of MHC class I chain-related protein (MIC) A and B expression. These ligands signal stress to immune cells, resulting in the elimination of target cells via a natural killer group 2D (NKG2D) receptor-dependent mechanism [55]. Interestingly, despite the malignant nature of MCC cells, the viral infection with the Merkel cell polyomavirus (MCPyV) and the permanent expression of virally encoded genes, MICs are not expressed in the majority of MCC tumours in situ, and are completely lacking on MCC cell lines in vitro [8]. This absence of MIC expression in MCC is mediated by epigenetic silencing of gene transcription in the MIC promoter region via histone hypo-acetylation. However, MIC expression is re-inducible by inhibition of histone deacetylases (HDACs) with an FDA (Food and Drug Administration)-approved HDAC inhibitor in vitro, as well as in vivo. Moreover, the re-induction of MICs on MCC cells results in an increased sensitivity towards immune cell-mediated lysis [8] (Fig. 2c). These findings demonstrate that epigenetic silencing of MICs is an essential immune escape strategy of MCCs, and that epigenetic priming with HDAC inhibitors could consequently increase the effectiveness of immunotherapeutic approaches towards MCC. It should be noted that polyomavirus infection may also use other epigenetic mechanisms to downregulate NKG2D ligands, e.g. a viral miRNA identical in sequence between John Cunningham virus (JCV) and B.K. Virus (BKV) targets the NKG2D ligand UL16 binding protein 3 (ULBP3) [56].
Progress and problems on the road to immunotherapy for Merkel cell carcinoma
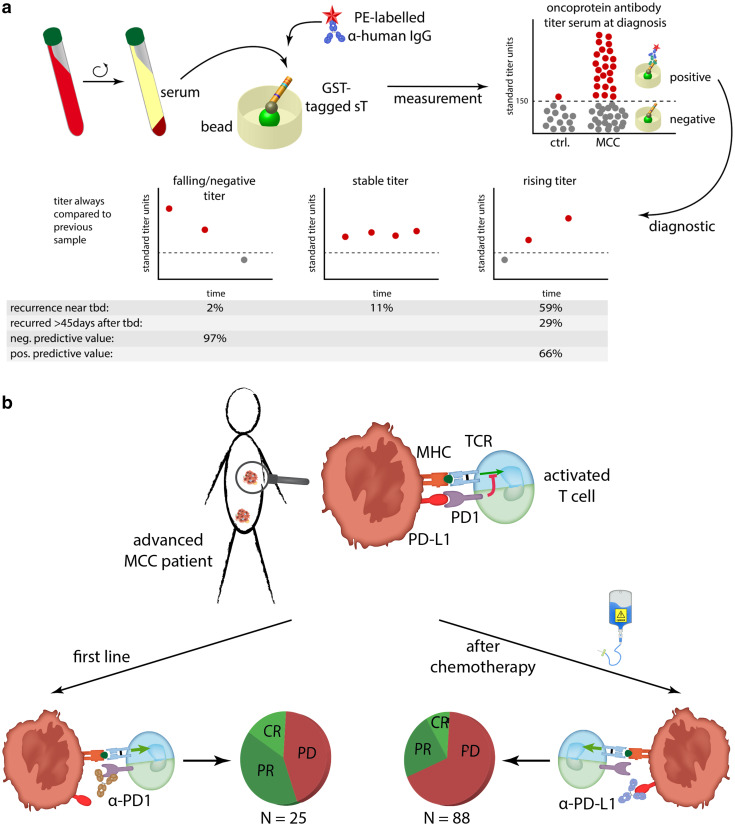
The observation that antibodies to the MCPyV oncoprotein fluctuate with disease burden is the basis for a blood test to detect early recurrence [57]. This test has recently been validated in a prospective cohort of several hundred patients. It appears to be useful for all MCC patients because it separates them into two distinct groups. The first group consists of patients not producing these antibodies, which need to be followed-up closely with imaging studies as they are at a 42% higher risk of recurrence than antibody-positive patients. The second group of patients who produce these antibodies can be followed-up with this blood test as dropping antibody titers correlate with an improved prognosis and increasing titers indicate reoccurrence of the disease [57] (Fig. 3a).
Fig. 3.
Blood-based biomarkers and immunotherapy. a Determination of antibody reactivity against sT in serum of MCC patients. Seropositivity at the time point of diagnosis is not only associated with a reduced risk of recurrence, but can be used diagnostically. To this end, a falling (< 20% to last sample) or negative titer has a negative predictive value of 97%, while rising titers (> 20%) has a positive predictive value of 66% for the occurrence of recurrence [57]. b Interfering with the programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) signaling pathway by inhibitory antibodies achieve response rate of > 50% in the first line setting and about 30% in patients previously treated with chemotherapy [3, 4]
Recent clinical trials indicate that PD-1 blockade leads to clinical responses in > 50% of patients with advanced MCC when treated in first line (no prior chemotherapy) [4]. For patients who previously received chemotherapy, the response rate is lower, but still significant with about 30% [3] (Fig. 3b). Diverse efforts are ongoing to determine predictors of whether a patient will respond to PD-1 pathway blockade or not, as well as how to treat patients who do not respond [5]. In addition to the properties of the tumor cells itself, the tumor microenvironment strongly modulates immune responses to solid cancers and thus contributes to a possible immune escape [58].
MCC research needs a multidisciplinary clinical and translational research program
The inception of a multidisciplinary MCC program at the University of Michigan in 2006 attracts approximately 80 new patients with all stages of MCC annually, i.e. accounting for about 5% of all patients diagnosed with MCC in the US. This allowed enrollment of more than 600 MCC patients into a prospective clinical database, establishment of an extensive tumor repository and development of a robust translational research program [59]. Clinical data and expertise from this large MCC center have contributed to the development of the new 8th edition American Joint Committee on Cancer (AJCC) MCC staging system [60]. This revised staging system will include a separate stage group for patients with metastatic MCC with unknown primary tumor, who carry a more favorable prognosis compared to patients presenting with concurrent primary and nodal metastatic disease. Consistent clinical management according to a previously established treatment algorithm in addition to multidisciplinary care through tumor board consensus has demonstrated the importance of sentinel lymph node biopsy in patients with stage I and II MCC. It also allowed to identify low-risk primary tumors for which adjuvant radiation therapy to the primary tumor bed can safely be omitted [61].
Similarly, there is a prospective biobank for MCC patients comprising tumor cell lines, tumor tissue, peripheral blood lymphocytes, plasma and serum, as well as an online clinical data bank on MCC patients from German speaking countries. The latter is hosted by the German Dermato-Oncology Cooperative Group (DeCOG/ADO; http://www.ado-homepage.de/merkelzellkarzinom-register.html). Operation of the biobank, with respect to establishing and characterizing tumor cell lines, benefits from experience with ESTDAB (European Searchable Tumour Cell and Data Bank) and OISTER (Outcome and Impact of Specific Treatment in European Research on Melanoma) two previous projects funded by the European Commission (https://www.tati-group.de/tati-projects/estdab/).
Conclusion
The increased understanding of the epidemiology, biology and immunology of MCC, which at least in part was spurred by the IMMOMEC project funded by the European Commission, allowed a more personalized therapy of MCC improving the lifespan and quality of life for the concerned patients and their relatives. Albeit, the IMMOMEC clinical trial was not as successful as hoped for, the IMMOMEC project and its consortium paved the way for future collaborative research within and well beyond the borders of Europe. Moreover, aided by the preparatory work within the IMMOMEC Project, it was possible to launch the first prospectively randomized adjuvant trial for MCC, which of course is based on an immune modulating intervention (checkpoint inhibition versus observation; EudraCT 2013-000043-78; ADMEC).
Abbreviations
- HDAC
Histone deacetylase
- ICD-O
International Classification of Diseases for Oncology
- IMMOMEC
Immune Modulating Strategies for Treatment of Merkel Cell Carcinoma
- LT
Large T-antigen
- MAX
Myc-associated factor X
- MCC
Merkel cell carcinoma
- MCPyV
Merkel cell polyomavirus
- MDM2
Mouse double minute 2 homolog
- MHC
Major histocompatibility complex
- MIC
MHC class I chain-related protein
- MYCL
l-Myc-1 proto-oncogene protein
- NKG2D
Natural killer group 2D
- PD-1
Programmed death protein 1
- PD-L1
Programmed death-ligand 1
- PP
Pocket protein
- RB
Retinoblastoma protein
- sT
Small T-Antigen
- TIL
Tumor-infiltrating lymphocytes
Compliance with ethical standards
Funding
J. C. Becker is funded by the European Commission Grant Agreement #277,775/IMMOMEC, the BMBF 03VP01062/CTCelect, the Hiege Stiftung, and the German Cancer Consortium (DKTK). A. Stang receives a Grant from the German Federal Ministry of Education and Science (BMBF), Grant Number 01ER1305. J. A. DeCaprio was supported in part by US Public Health Service Grants R01CA63113, R01CA173023, P01CA050661, the DFCI Helen Pappas Merkel Cell Research Fund and the Claudia Adams Barr Program in Cancer Research. P. Nghiem was supported in part by US Public Health Service grants K24-CA139052, RO1-CA176841, and the UW MCC Gift Fund.
Conflict of interest
J. C. Becker has received speaker honoraria from Amgen, MerckSerono, and Pfizer, advisory board honoraria from Amgen, CureVac, eTheRNA, Lytix, MerckSerono, Novartis, Rigontec, and Takeda as well as research funding from Boehringer Ingelheim, BMS and MerckSerono; the activities with BMS, MerckSerono and Pfizer are related to the submitted report (therapy of advanced MCC). A research project in J. A. DeCaprio’s laboratory is supported by Constellation Pharmaceuticals. P. Nghiem has served as a consultant for EMD Serono and Pfizer and has received research support to his institution from Bristol-Myers Squibb. The other authors declare that they have no conflict of interest.
References
- 1.Schadendorf D, Lebbé C, Hausen zur A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69. doi: 10.1016/j.ejca.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Eisemann N, Jansen L, Castro FA, et al. Survival with nonmelanoma skin cancer in Germany. Br J Dermatol. 2016;174:778–785. doi: 10.1111/bjd.14352. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman HL, Russell J, Hamid O, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17:1374–1385. doi: 10.1016/S1470-2045(16)30364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nghiem PT, Bhatia S, Lipson EJ, et al. PD-1 blockade with pembrolizumab in advanced Merkel-cell carcinoma. N Engl J Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terheyden P, Becker JC. New developments in the biology and the treatment of metastatic Merkel cell carcinoma. Curr Opin Oncol. 2017;29:221–226. doi: 10.1097/CCO.0000000000000363. [DOI] [PubMed] [Google Scholar]
- 6.Gambichler T, Wieland U, Silling S, et al. Left-sided laterality of Merkel cell carcinoma in a German population: more than just sun exposure. J Cancer Res Clin Oncol. 2017;143:347–350. doi: 10.1007/s00432-016-2293-2. [DOI] [PubMed] [Google Scholar]
- 7.Gambichler T, Mohtezebsade S, Wieland U, et al. Prognostic relevance of high atonal homolog-1 expression in Merkel cell carcinoma. J Cancer Res Clin Oncol. 2017;143:43–49. doi: 10.1007/s00432-016-2257-6. [DOI] [PubMed] [Google Scholar]
- 8.Ritter C, Fan K, Paulson KG, et al. Reversal of epigenetic silencing of MHC class I chain-related protein A and B improves immune recognition of Merkel cell carcinoma. Sci Rep. 2016;6:21678. doi: 10.1038/srep21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulson KG, Tegeder A, Willmes C, et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol Res. 2014;2(11):1071–1079. doi: 10.1158/2326-6066.CIR-14-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buder K, Lapa C, Kreissl MC, et al. Somatostatin receptor expression in Merkel cell carcinoma as target for molecular imaging. BMC Cancer. 2014;14:268. doi: 10.1186/1471-2407-14-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyngaa R, Pedersen NW, Schrama D, et al. T-cell responses to oncogenic Merkel cell polyomavirus proteins distinguish patients with Merkel cell carcinoma from healthy donors. Clin Cancer Res. 2014;20:1768–1778. doi: 10.1158/1078-0432.CCR-13-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loader DE, Feldmann R, Baumgartner M, et al. Clinical remission of Merkel cell carcinoma after treatment with imatinib. J Am Acad Dermatol. 2013;69:e181–e183. doi: 10.1016/j.jaad.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 13.Schrama D, Hesbacher S, Becker JC, Houben R. Survivin downregulation is not required for T antigen knockdown mediated cell growth inhibition in MCV infected merkel cell carcinoma cells. Int J Cancer. 2013;132:2980–2982. doi: 10.1002/ijc.27962. [DOI] [PubMed] [Google Scholar]
- 14.Vlahova L, Doerflinger Y, Houben R, et al. P-cadherin expression in Merkel cell carcinomas is associated with prolonged recurrence-free survival. Br J Dermatol. 2012;166:1043–1052. doi: 10.1111/j.1365-2133.2012.10853.x. [DOI] [PubMed] [Google Scholar]
- 15.Willmes C, Adam C, Alb M, et al. Type I and II IFNs inhibit Merkel cell carcinoma via modulation of the Merkel cell polyomavirus T antigens. Cancer Res. 2012;72:2120–2128. doi: 10.1158/0008-5472.CAN-11-2651. [DOI] [PubMed] [Google Scholar]
- 16.Schadendorf D, Lebbé C, zur Hausen A, et al. Merkel cell carcinoma: epidemiology, prognosis, therapy and unmet medical needs. Eur J Cancer. 2017;71:53–69. doi: 10.1016/j.ejca.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Tilling T, Wladykowski E, Failla AV, et al. Immunohistochemical analyses point to epidermal origin of human Merkel cells. Histochem Cell Biol. 2013;141:407–421. doi: 10.1007/s00418-013-1168-8. [DOI] [PubMed] [Google Scholar]
- 18.Sauer CM, Chteinberg E, Rennspiess D, et al. Merkel cell carcinoma: cutaneous manifestation of a highly malignant pre-/pro-B cell neoplasia? Hautarzt. 2017;68:204–210. doi: 10.1007/s00105-017-3945-0. [DOI] [PubMed] [Google Scholar]
- 19.Becker JC, zur Hausen A. Cells of origin in skin cancer. J Invest Dermatol. 2014;134:2491–2493. doi: 10.1038/jid.2014.233. [DOI] [PubMed] [Google Scholar]
- 20.zur Hausen A, Rennspiess D, Winnepenninckx V. Early B-cell differentiation in Merkel cell carcinomas: clues to cellular ancestry. Cancer Res. 2013;73:4982–4987. doi: 10.1158/0008-5472.CAN-13-0616. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Yang R, Payne AS, et al. Identifying the target cells and mechanisms of Merkel Cell polyomavirus infection. Cell Host Microbe. 2016;19:775–787. doi: 10.1016/j.chom.2016.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grundhoff A, Fischer N. Merkel cell polyomavirus, a highly prevalent virus with tumorigenic potential. Curr Opin Virol. 2015;14:129–137. doi: 10.1016/j.coviro.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Verhaegen ME, Mangelberger D, Harms PW, et al. Merkel cell polyomavirus small T antigen is oncogenic in transgenic mice. J Invest Dermatol. 2014;135:1415–1424. doi: 10.1038/jid.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shuda M, Chang Y, Moore PS. Merkel cell polyomavirus-positive Merkel cell carcinoma requires viral small T-antigen for cell proliferation. J Invest Dermatol. 2014;134:1479–1481. doi: 10.1038/jid.2013.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Houben R, Shuda M, Weinkam R, et al. Merkel cell Polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore PS, Chang Y. The conundrum of causality in tumor virology: the cases of KSHV and MCV. Semin Cancer Biol. 2014;26:4–12. doi: 10.1016/j.semcancer.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shuda M, Kwun HJ, Feng H, et al. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest. 2011;121:3623–3634. doi: 10.1172/JCI46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards KF, Gustafierro A, Shuda M, Toptan T, Moore PS, Chang Y. Merkel cell polyomavirus T antigens promote cell proliferation and inflammatory cytokine gene expression. J Gen Virol. 2015;96(12):3532–3544. doi: 10.1099/jgv.0.000287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shuda M, Guastafierro A, Geng X, et al. Merkel Cell polyomavirus small T antigen induces cancer and embryonic Merkel cell proliferation in a transgenic mouse model. PLoS One. 2015;10:e0142329–e0142320. doi: 10.1371/journal.pone.0142329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borchert S, Czech-Sioli M, Neumann F, et al. High-affinity Rb binding, p53 inhibition, subcellular localization, and transformation by wild-type or tumor-derived shortened merkel cell polyomavirus large T antigens. J Virol. 2014;88:3144–3160. doi: 10.1128/JVI.02916-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shuda M, Kwun HJ, Feng H, et al. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J Clin Invest. 2011;121:3623–3634. doi: 10.1172/JCI46323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwun HJ, Shuda M, Feng H, et al. Merkel cell polyomavirus small T antigen controls viral replication and oncoprotein expression by targeting the cellular ubiquitin ligase SCFFbw7. Cell Host Microbe. 2013;14:125–135. doi: 10.1016/j.chom.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths DA, Abdul-Sada H, Knight LM, et al. Merkel cell polyomavirus small T antigen targets the NEMO adaptor protein to disrupt inflammatory signaling. J Virol. 2013;87:13853–13867. doi: 10.1128/JVI.02159-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdul-Sada H, Müller M, Mehta R, et al. The PP4R1 sub-unit of protein phosphatase PP4 is essential for inhibition of NF-κB by merkel polyomavirus small tumour antigen. Oncotarget. 2017;8:25418–25432. doi: 10.18632/oncotarget.15836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wendzicki JA, Moore PS, Chang Y. Large T and small T antigens of Merkel cell polyomavirus. Curr Opin Virol. 2015;11:38–43. doi: 10.1016/j.coviro.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knight LM, Stakaityte G, Wood JJ, et al. Merkel cell polyomavirus small T antigen mediates microtubule destabilisation to promote cell motility and migration. J Virol. 2015;89(1):35–47. doi: 10.1128/JVI.02317-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berrios C, Padi M, Keibler MA, et al. Merkel cell polyomavirus small T antigen promotes pro-glycolytic metabolic perturbations required for transformation. PLoS Pathog. 2016;12:e1006020–e1006021. doi: 10.1371/journal.ppat.1006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hesbacher S, Pfitzer L, Wiedorfer K, et al. RB1 is the crucial target of the Merkel cell polyomavirus Large T antigen in Merkel cell carcinoma cells. Oncotarget. 2016;7:32956–32968. doi: 10.18632/oncotarget.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arora R, Shuda M, Guastafierro A, et al. Survivin is a therapeutic target in Merkel cell carcinoma. Sci Transl Med. 2012;4(133):133ra56. doi: 10.1126/scitranslmed.3003713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Hein J, Richardson SCW, et al. Merkel cell polyomavirus large T antigen disrupts lysosome clustering by translocating human Vam6p from the cytoplasm to the nucleus. J Biol Chem. 2011;286:17079–17090. doi: 10.1074/jbc.M110.192856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Li J, Schowalter RM, et al. Bromodomain protein Brd4 plays a key role in Merkel cell polyomavirus DNA replication. PLoS pathog. 2012;8:e1003021–e1003016. doi: 10.1371/journal.ppat.1003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knips J, Czech-Sioli M, Spohn M, et al. Spontaneous lung metastasis formation of human Merkel cell carcinoma cell lines transplanted into scid mice. Int J Cancer. 2017;141:160–171. doi: 10.1002/ijc.30723. [DOI] [PubMed] [Google Scholar]
- 43.Schrama D, Hesbacher S, Angermeyer S, et al. Serine 220 phosphorylation of the Merkel cell polyomavirus large T antigen crucially supports growth of Merkel cell carcinoma cells. Int J Cancer. 2015;138:1153–1162. doi: 10.1002/ijc.29862. [DOI] [PubMed] [Google Scholar]
- 44.Houben R, Angermeyer S, Haferkamp S, et al. Characterization of functional domains in the Merkel cell polyoma virus Large T antigen. Int J Cancer. 2014;136:E290–E300. doi: 10.1002/ijc.29200. [DOI] [PubMed] [Google Scholar]
- 45.Cheng J, Rozenblatt-Rosen O, Paulson KG, et al. Merkel cell polyomavirus large T antigen has growth-promoting and inhibitory activities. J Virol. 2013;87:6118–6126. doi: 10.1128/JVI.00385-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Starrett GJ, Marcelus C, Cantalupo PG, et al. Merkel cell polyomavirus exhibits dominant control of the tumor genome and transcriptome in virus-associated merkel cell carcinoma. MBio. 2017;8(1):e02079–e02016. doi: 10.1128/mBio.02079-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong SQ, Waldeck K, Vergara IA, et al. UV-associated mutations underlie the etiology of MCV-negative Merkel cell carcinomas. Cancer Res. 2015;75:5228–5234. doi: 10.1158/0008-5472.CAN-15-1877. [DOI] [PubMed] [Google Scholar]
- 48.Erstad DJ, Cusack JC., Jr Mutational analysis of Merkel cell carcinoma. Cancers. 2014;6:2116–2136. doi: 10.3390/cancers6042116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harms PW, Vats P, Verhaegen ME, et al. The distinctive mutational spectra of polyomavirus-negative Merkel cell carcinoma. Cancer Res. 2015;75:3720–3727. doi: 10.1158/0008-5472.CAN-15-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veija T, Sarhadi VK, Koljonen V, et al. Hotspot mutations in polyomavirus positive and negative Merkel cell carcinomas. Cancer Genet. 2016;209:30–35. doi: 10.1016/j.cancergen.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Goh G, Walradt T, Markarov V, et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2016;7:3403–3415. doi: 10.18632/oncotarget.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen PR, Tomson BN, Elkin SK, et al. Genomic portfolio of Merkel cell carcinoma as determined by comprehensive genomic profiling: implications for targeted therapeutics. Oncotarget. 2016;7:23454–23467. doi: 10.18632/oncotarget.8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harms KL, de la LazoVega L, Hovelson DH, et al. Molecular profiling of multiple primary Merkel cell carcinoma to distinguish genetically distinct tumors from clonally related metastases. JAMA Dermatol. 2017;153:505–508. doi: 10.1001/jamadermatol.2017.0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bentzen AK, Marquard AM, Lyngaa R, et al. Large-scale detection of antigen-specific T cells using peptide-MHC-I multimers labeled with DNA barcodes. Nat Biotechnol. 2016;34:1037–1045. doi: 10.1038/nbt.3662. [DOI] [PubMed] [Google Scholar]
- 55.López-Soto A, Huergo-Zapico L, Acebes-Huerta A, et al. NKG2D signaling in cancer immunosurveillance. Int J Cancer. 2014;136:1741–1750. doi: 10.1002/ijc.28775. [DOI] [PubMed] [Google Scholar]
- 56.Bauman Y, Nachmani D, Vitenshtein A, et al. An identical miRNA of the human JC and BK polyoma viruses targets the stress-induced ligand ULBP3 to escape immune elimination. Cell Host Microbe. 2011;9:93–102. doi: 10.1016/j.chom.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 57.Paulson KG, Lewis CW, Redman MW, et al. Viral oncoprotein antibodies as a marker for recurrence of Merkel cell carcinoma: a prospective validation study. Cancer. 2016;123:1464–1474. doi: 10.1002/cncr.30475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Becker JC, Andersen MH, Schrama D, thor Straten P. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother. 2013;62:1137–1148. doi: 10.1007/s00262-013-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Frohm ML, Griffith KA, Harms KL, et al. Recurrence and survival in patients with Merkel cell carcinoma undergoing surgery without adjuvant radiation therapy to the primary site. JAMA Dermatol. 2016;152:1001–1007. doi: 10.1001/jamadermatol.2016.1428. [DOI] [PubMed] [Google Scholar]
- 60.Harms KL, Healy MA, Nghiem P, et al. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23:3564–3571. doi: 10.1245/s10434-016-5266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Becker JC, Stang A, DeCaprio JA, et al. Merkel cell carcinoma. Nat Rev Dis Primers. 2017;3:17077. doi: 10.1038/nrdp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]