Abstract
Objective
Smoking impairs wound healing, yet the underlying pathophysiological mechanisms are unclear. We evaluated tobacco-altered healing in head and neck surgery by studying the association between biomarkers and tobacco exposure, as well as cutaneous perfusion by smoking status.
Study Design
Prospective cohort study, tertiary/academic care center, 2011 to present.
Methods
Patients who required head and neck surgery were enrolled prospectively. Postsurgical drain fluid was collected 24 hours postoperatively. Biomarkers associated with postulated mechanisms of smoking-impaired healing were assayed. These included interleukin-1, -6, and -8; tumor necrosis factor- alpha; transforming growth factor-beta; epidermal growth factor (EGF); basic fibroblastic growth factor (bFGF); C-reactive protein; vascular endothelial growth factor; soluble FMS-like tyrosine kinase-1 (sFLT-1); and placental growth factor. Tobacco exposure and clinical outcomes were recorded. Two sample two-sided t tests evaluated the differences in cytokine levels by tobacco exposure. In a second cohort, cutaneous vascular assessment via indocyanine green angiography was compared by smoking status.
Results
Twenty-eight patients were enrolled with drain fluid collection. Twenty-one subjects were current/former smokers, whereas seven were never smokers. EGF was higher in never smokers than smokers in a statistically significant manner (P = 0.030). Likewise, sFLT-1 was significantly higher in never smokers (P = 0.011). Cutaneous angiography revealed nonsmokers to have significantly higher cutaneous perfusion than smokers.
Conclusion
In this head and neck surgical cohort, significantly higher EGF and sFLT-1 levels in wound fluid were associated with never smoking, suggesting that smoking has adverse effects on the inflammatory phase of wound healing. Cutaneous angiography supports the detrimental effect of smoking on skin perfusion. These findings suggest the need for further study as well as therapeutic targets for smokers undergoing surgery.
Keywords: Smoking, cytokines, wound healing
INTRODUCTION
Across most surgical disciplines, there is evidence that tobacco smoke exposure increases surgical complications, resulting in impaired local wound healing, infection, respiratory complications, delayed bone fusion, anastamotic leakage, and intensive care unit admissions.1–9 A recent U.S. Department of Veterans Affairs study of 393,794 patients demonstrated a higher rate of postoperative complications in current smokers over former and never smokers.10 Similarly, our systematic review of the head and neck surgery literature11 suggests an association between smoking and acute wound-healing failure.12 In the head and neck, where form and function are greatly intertwined, impaired healing can result in large open wounds, exposure of vital structures including the great vessels, infection, inability to take an oral diet, disfigurement, and the potential for life threatening bleeding—all requiring longer hospital stays and often costly procedures to correct.
Despite clinical evidence for smoking-related impairment in wound healing, much is unknown about the pathophysiologic mechanisms underlying this effect and the impact of smoking cessation on wound healing.13 Likewise, the structural and physiological alterations in smokers’ skin that occur prior to wounding, and that predispose these patients to poor healing, are incompletely understood. It is postulated that after injury or trauma, smoking impedes the inflammatory phase of wound healing by diminishing cellular chemotactic responsiveness, migratory function, and oxidative bacterial killing, and by creating an imbalance in protease–protease inhibitor relationships.14–16 The proliferative phase of wound healing also is potentially impaired by smoking, with diminished fibroblast proliferation and migration resulting in decreased collagen production.17–19 Increases in oxidative stress and hypoxia are other likely contributors to diminished healing in smokers.20 Although the sympathomimetic effects of smoking, which decrease cutaneous oxygen and blood flow, are thought to be transient, their long-term effect on the cutaneous microstructure is unknown.20 Also lacking are data about the contribution of changes in skin structure that go hand in hand with altered physiology in smokers to compromise wound healing.
In the field of head and neck surgery, in which many patients have a history of smoking, the detrimental effects of tobacco on wound healing are of critical importance. Thus, we sought to study the effects of tobacco exposure in a head and neck surgery cohort. To better understand the pathophysiology of smoking-impaired wound healing, we studied the cytokines in acute postsurgical wound fluid to determine whether drain fluid cytokine levels in head and neck surgery patients are associated with smoking status. In a second small cohort, we evaluated the effects of smoking status on functional perfusion via cutaneous vascular imaging.
MATERIALS AND METHODS
Institutional review board approval was obtained to evaluate this patient population. A prospective cohort study was then performed at our tertiary care center from April 2011 until the present. For our primary cohort, head and neck surgery patients who were recommended to undergo major, open surgical treatment requiring drain placement were recruited and enrolled from our academic practice. Because the aim of our study was to evaluate wound healing, both patients with benign and malignant lesions of the head and neck were entered into the cohort. Treatment decisions were made based on standard clinical criteria, including tumor conference evaluation for patients with malignant disease or complicated benign disease. Patients subsequently were treated with surgery, which in all enrolled patients included at least an 8 cm incision and accompanying dissection. At the completion of the surgery, standard 10-mm flat silicone surgical drains (Jackson-Pratt, Cardinal-Health, Dublin, OH)) were placed as clinically indicated. All subjects had at least one drain placed, running medial to lateral and superior to inferior in the lateral neck. All surgeries performed were extirpative in nature (none were completed for any other indication, such as infection or hematoma). All patients received antibiotics by a standard perioperative protocol. All wounds were fresh, surgical wounds with no evidence of wound infection at the time of drain fluid collection. Wound fluid was collected from all these surgical cases on postoperative day 1 at a protocol directed time from surgery. Once collected by a standard protocol, the fluid was stored in a −80°C.
Surgical drain fluid was evaluated for a panel of biomarkers present in the healing wound by an investigator blinded to clinical outcome endpoints. All cytokine analysis was performed in the university cytokine reference laboratory, a Clinical Laboratory Improvement Amendments of 1988-licensed facility, with considerable experience in cytokine evaluation. Biomarkers were measured by standard enzyme-linked immunosorbent assays as well as multiplex fluorescence bead-based antibody technology (Luminex; R&D Systems, Inc., Minneapolis, MN). Biomarkers were evaluated from fluid collected after the second postoperative 8-hour period (shift) on postoperative day 1. Each fluid sample was evaluated in duplicate with standards as per assay protocol.
To investigate the biochemical effects of smoking on wound healing in this cohort, surgical wound fluid was collected during the inflammatory phase of wound healing. Smoking has been postulated to alter the inflammatory stage of the wound healing cascade, and as such a panel of proinflammatory mediators was evaluated. These include interleukins-1 beta, -6, and -8, as well as tumor necrosis factor-alpha.21 In addition, C-reactive protein serves as a systemic indicator of inflammation and was assayed here. Epidermal growth factor (EGF), an important growth factor that promotes the migration of keratinocytes to allow for re-epithelialization after wounding, was measured.22 A panel of matrix metalloproteinases (MMPs) including MMP -1, -3, and -9 was evaluated in the wound fluid. These proteolytic enzymes are known to degrade extracellular matrix, which is a critical step in clearing cellular and other debris from the healing wound to allow repair to begin. They also are involved in cell growth and differentiation and cell migration.23 MMPs were measured in both their pro and active forms in the drain fluid assayed. Transforming growth factor beta serves an antiinflammatory role as well as a proangiogenic stimulus and was measured.24 Wound fluid also was evaluated for several angiogenesis-related markers. Vascular endothelial growth factor, isoform A (VEGF-A) and placental growth factor (PIGF), both protein isoforms in the VEGF family, were both measured as factors promoting wound angiogenesis. Soluble FMS-like tyrosine kinase-1 (sFLT-1), a VEGF antagonist or the soluble form of VEGF receptor-one, also was measured.25,26 Basic fibroblastic growth factor (or b FGF2) was additionally assayed, which represents a basement membrane and vessel extracellular matrix component and plays an important role in healing and angiogenesis by the regulation of cell differentiation, migration, and proliferation.24 All these factors were measured in their soluble forms in the drain fluid.
A small second cohort of patients underwent SPY (Novadaq, Burnaby, Canada) cutaneous angiography at the initiation of surgery. In this cohort, all patients underwent SPY (Novadaq) angiography as a planned part of their surgical procedure in order to direct needed reconstruction efforts. These patients were assessed with SPY (Novadaq) Imaging for angiographic evaluation of surgical flap vessel and perfusion. Prior to surgical dissection, a baseline image of the planned region of dissection was imaged angiographically. To do this, indocyanine green fluorescent dye was injected intravenously, and the skin flap was imaged using a diode laser to a depth of 3 mm to 5 mm at standard time intervals. Perforating arteries, then arterioles, and then the overall perfusion state were imaged by fluorescence intensity. Fluorescence measurements were made on a scale of 0 to 260 at the periphery and on a line bisecting the planned dissection (Fig. 1). A mean perfusion value was determined for each patient.
Fig. 1.
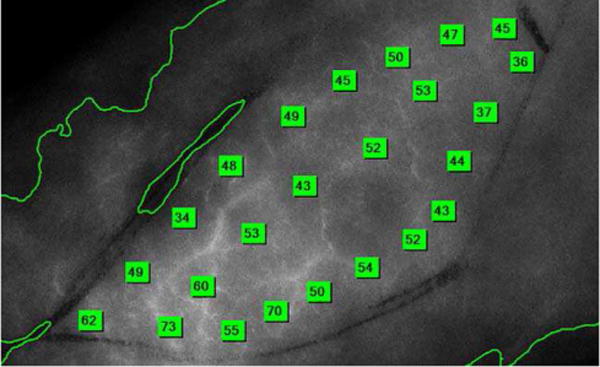
Example of SPY (Novadaq, Burnaby, Canada) Angiographic Image. Each green box represents a fluorescence measurement generated by angiography. Fluorescence measurements were made on a scale of 0–260 at the periphery and on a line bisecting the planned dissection. A mean fluorescence value was generated from the collected measures.
Clinical characteristics were recorded for all subjects in both the primary cohort and the SPY (Novadaq) cohort. Clinical wound-healing outcomes were recorded, including return to operating room after initial procedure, postoperative fever (> 101.5), postoperative wound infection, any wound complication, blood transfusion, fistula, free flap failure, need for long-term wound care (additional wound care after discharge from hospital), inpatient admission postdischarge, or not otherwise specified complication. Subjects with follow-up 1 year or greater were included.
For statistical analysis of the primary cohort, summary statistics included frequencies and percents for categorical items and median and ranges for quantitative factors. In this cohort, the two readings of each biomarker (each was measured in duplicate) were averaged for each patient. The panel of cytokines were analyzed in the logarithmic scale due to highly skewed distributions and reported as geometric means with 95% confidence intervals (CI). For comparison purposes, smoking status was defined as two groups: never smoking or former plus current smokers. Patient demographics and characteristics were compared between smoking groups using chi-square, Fisher’s exact test, and nonparametric Wilcoxon rank sum test. These same statistical procedures compared smoking status to three postoperative outcomes: mortality, length of hospital stay in days, and any occurrence of 10 preidentified complications during follow-up (see above list). Wilcoxon rank sum test also was used to compare the series of 15 cytokines between smoking groups. With regards to SPY (Novadaq) angiographic imaging cohort, statistical analysis only was completed on the primary outcome of fluorescence. A mean perfusion value was determined for each patient, and a two-sample, two-sided t test assuming equal group variances tested differences in mean fluorescence by smoking status. For both cohorts, statistical significance was defined as P values less than 0.05. All analyses were carried out using SAS version 9.3 (SAS Institute, Inc, Cary, NC).
RESULTS
Twenty-eight patients were successfully enrolled in the primary cohort and had complete specimen collection. Patient characteristics are seen in Table I. The median age of the subjects was 56 years, and gender was evenly divided between male and female. The majority of patients were non-Hispanic whites. With regard to smoking status, seven patients (25%) were never smokers; 12 patients were former smokers (42.9%); and nine patients were current smokers (32.1%). Current and former smokers were analyzed together, and median pack years was 10.0. Alcohol use also was documented, with seven patients being never drinkers, one patient being a former drinker, and 20 patients being current drinkers. Nineteen of 28 patients had benign disease (67.9%). Conditions predisposing to poor wound healing were documented, including previous radiation, previous chemotherapy, previous head and neck surgery, diabetes, and immunosuppression. Length of surgery in minutes was documented, and median was 328.5 minutes. For all patient characteristics, there was no statistically significant difference by smoking group, as indicated by the P value in the rightmost column.
TABLE I.
Table of Patient Characteristics, Primary Cohort, Overall and by Smoking Status.
| Characteristic | All Patients (N = 28) Freq (%) |
Never Smokers (N = 7) Freq (%) |
Current/Former Smokers (N = 21) Freq (%) |
P Value* |
|---|---|---|---|---|
| Smoking Status: | ||||
| Never | 7 (25.0) | |||
| Former | 12 (42.9) | |||
| Current | 9 (32.1) | |||
| Ethnicity: | NA | |||
| Non-Hispanic | 28 (100) | 7 (100) | 21 (100) | |
| Race: | 0.280 | |||
| White | 24 (96.0) | 6 (85.7) | 18 (100) | |
| Non-white | 1 (4.0) | 1 (14.3) | 0 (0) | |
| Unknown | 3 | |||
| Gender: | 0.385 | |||
| Male | 14 (50.0) | 2 (28.6) | 12 (57.1) | |
| Female | 14 (50.0) | 5 (71.4) | 9 (42.9) | |
| Alcohol: | 0.175 | |||
| Never | 7 (25.0) | 0 (0) | 7 (33.3) | |
| Former | 1 (3.6) | 0 (0) | 1 (4.8) | |
| Current | 20 (71.4) | 7 (100) | 13 (61.9) | |
| Tumor: | 0.918 | |||
| Oral cavity/oropharynx | 7 (25.0) | 2 (28.6) | 5 (23.8) | |
| Hypopharynx/larynx | 1 (3.6) | 0 (0.0) | 1 (4.8) | |
| Salivary gland | 12 (42.9) | 4 (57.1) | 8 (38.1) | |
| Cutaneous | 1 (3.6) | 0 (0.0) | 1 (4.8) | |
| Other | 7 (25.0) | 1 (14.3) | 6 (28.6) | |
| AJCC Stage: | 0.715† | |||
| 0 (benign) | 19 (67.9) | 5 (71.4) | 14 (66.7) | |
| 1 | 2 (7.1) | 1 (14.3) | 1 (4.8) | |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 3 | 1 (3.6) | 0 (0.0) | 1 (4.8) | |
| 4 | 6 (21.4) | 1 (14.3) | 5 (23.8) | |
| 1 thru 4 (malignant) | 9 (32.1) | 2 (28.6) | 7 (33.3) | |
| T Stage (malignant only): | 0.667 | |||
| 1 | 2 (22.2) | 1 (50.0) | 1 (14.3) | |
| 2 | 3 (33.3) | 0 (0.0) | 3 (42.9) | |
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 4 | 4 (44.4) | 1 (50.0) | 3 (42.9) | |
| N Stage (malignant only): | 1.00 | |||
| 0 | 6 (66.7) | 2 (100) | 4 (57.1) | |
| 1 | 1 (11.1) | 0 (0) | 1 (14.3) | |
| 2 | 2 (22.2) | 0 (0) | 2 (28.6) | |
| 3 | 0 (0.0) | 0 (0) | 0 (0.0) | |
| M Stage: | NA | |||
| 0 | 8 (100) | 2 (100) | 6 (100) | |
| Previous external beam: | 0.551 | |||
| No | 25 (89.3) | 7 (100) | 18 (85.7) | |
| Yes | 3 (10.7) | 0 (0) | 3 (14.3) | |
| Previous chemo | 1.00 | |||
| No | 26 (92.9) | 7 (100) | 19 (90.5) | |
| Yes | 2 (7.1) | 0 (0) | 2 (9.5) | |
| Previous H&N surgery: | 1.00 | |||
| No | 24 (85.7) | 6 (85.7) | 18 (85.7) | |
| Yes | 4 (14.3) | 1 (14.3) | 3 (14.3) | |
| Diabetes: | 1.00 | |||
| No | 26 (92.9) | 7 (100) | 19 (90.5) | |
| Yes | 2 (7.1) | 0 (0) | 2 (9.5) | |
| immunosuppressed: | NA | |||
| No | 28 (100) | 7 (100) | 21 (100) | |
| Median [range] | Median [range] | Median [range] | P value‡ | |
| Age in years | 56 [29/80] | 54 [33/60] | 58 [29/80] | 0.155 |
| Pack years§ | 7.1 [0/66] | NA | 10.0 [1.5/66] | NA |
| Length of surgery (min) | 328.5 [138/942] | 308.0 [265/699] | 338.0 [138/942] | 0.675 |
Note: the unknowns were not included in the comparisons between smoking status groups.
P value was derived from the Chi-square or the Fisher’s exact test.
P value reflects the comparison of benign vs. malignant between smoking status groups.
P value was based on the Wilcoxon rank sum test.
Pack years was missing for 2 patients.
AJCC = American Joint Committee on Cancer; chemo = chemotherapy; Freq = frequency; H&N = head and neck; M = metastasis; min = minutes; N = node; NA = nonapplicable; T = tumor.
We then evaluated for associations between wound fluid biomarker levels and smoking status (Table II). There was a statistically significant association between two of the cytokines and smoking status. Patients who were never smokers had higher levels of EGF, which were statistically significant (P = 0.030). Never smokers’ geometric mean of EGF was 192.6 pg/mL (72.8–509.9 95% CI), whereas former and current smokers’ geometric mean was 44.72 pg/mL (24.21–82.61 95% CI). There also was a statistically significant association between sFLT-1 levels and smoking status, with levels of the cytokine being lower in current or former smokers (P = 0.011). Geometric mean for never smokers was 3.22 pg/mL (1.20–8.63 95% CI), and geometric mean for smokers was 0.71 pg/mL (0.36–1.39 95% CI).
TABLE II.
Table of Cytokines, by Smoking Status.
| Never Smokers (N = 7)
|
Former/Current Smokers (N = 21)
|
||||
|---|---|---|---|---|---|
| Cytokine | Geo Mean | 95% CI | Geo Mean | 95% CI | P Value* |
| IL-6 | 22565 | 160369, 317529 | 20459 | 16454, 25437 | 0.633 |
| IL-8 | 7611.4 | 5003.5, 11578.6 | 5946.7 | 3966.1, 8916.4 | 0.560 |
| IL-1 beta | 63.23 | 8.59, 465.20 | 67,88 | 23.07, 199.73 | 0.853 |
| TGF_b1 | 693.1 | 307.3, 1563.3 | 234.0 | 123.5, 443.2 | 0.090 |
| TNF alpha | 6.35 | 1.52, 26.48 | 5.17 | 2.67, 10.03 | 0.652 |
| EGF | 192.6 | 72.8, 509.9 | 44.72 | 24.21, 82.61 | 0.030* |
| FGF | 999.2 | 387.1, 2579.5 | 711.7 | 393.1, 1288.9 | 0.524 |
| MMP-1 | 4564.1 | 1287.1, 16184.3 | 5837.4 | 2632.8, 12942.4 | 0.958 |
| MMP-2 | 95649 | 62071, 147391 | 100915 | 78017, 130534 | 0.874 |
| MMP-9 | 445730 | 384429, 516807 | 388525 | 345168, 437329 | 0.100 |
| VEGF-A | 7372.3 | 4810.3, 11298.8 | 7995.6 | 5445.2, 11740.5 | 0.710 |
| PLGF | 66.45 | 26.62, 165.86 | 173.42 | 85.9, 350.0 | 0.124 |
| sFLT-1 | 3.22 | 1.20, 8.63 | 0.71 | 0.36, 1.39 | 0.011* |
| CRP | 1289.6 | 160.9, 10334.9 | 3031.6 | 1391.6, 6604.6 | 0.396 |
All cytokines reported in pg/mL with the exception of CRP, reported in ng/mL.
Highlights statistical significance from the Wilcoxon rank sum test.
bFGF = basic fibroblastic growth factor; CI = confidence interval; Geo Mean = geometric mean; MMP = matrix metalloproteinases.
We performed further analysis to investigate possible associations between smoking status and clinical outcomes (Table III). Clinical outcomes documented were mortality, length of hospital stay, and wound healing outcomes, which included return to operating room after initial procedure, postoperative fever (> 101.5), postoperative wound infection, any wound complication, blood transfusion, fistula, free flap failure, need for long-term wound care (additional wound care after discharge from hospital), inpatient admission post discharge, not otherwise specified complication, and length of hospital stay. There were no statistically significant differences in clinical outcomes by smoking status.
TABLE III.
Table of Clinical Outcomes, Primary Cohort, Overall and by Smoking Status.
| Outcome | All Patients (N = 28) Freq (%) |
Never Smokers (N = 7) Freq (%) |
Current/Former Smokers (N = 21) Freq (%) |
P Value* |
|---|---|---|---|---|
| Alive | 26 (92.9) | 6 (85.7) | 20 (95.2) | 0.444 |
| Dead | 2 (7.1) | 1 (14.3) | 1 (4.8) | |
| Any complications | 0.622 | |||
| No | 22 (78.6) | 5 (71.4) | 17 (81.0) | |
| Yes | 6 (21.4) | 2 (28.6) | 4 (19.1) | |
| Median [range] | Median [range] | Median [range] | P value† | |
| Length of hospital stay (days) | 3.0 [1/20] | 3.0 [2/20] | 3.0 [1/8] | 0.420 |
Note: To maximize the number of patients, if a complication was labeled unknown, it was treated as a “no” in the calculation of any complications.
The P value was derived from the Chi-square or the Fisher’s exact test.
The P value was based on the Wilcoxon rank sum test.
Freq = frequencies.
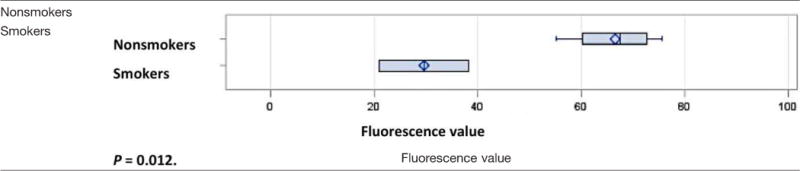
For the secondary cohort, six patients were enrolled and completed SPY (Novadaq) angiographic imaging. Patient characteristics for this cohort are seen in Table IV, and clinical outcomes are shown in Table V. Mean fluorescence values are shown for each subject in Table V and compared by smoking status. Current smokers that had used cigarettes within 24 hours of surgery were compared to former and never smokers. Two-sample t testing showed that smokers had decreased cutaneous and subcutaneous microvasculature fluorescence relative to nonsmokers (P = 0.012) (Table VI).
TABLE IV.
Table of Patient Characteristics, SPY Cohort.
| Subject | Age | Gender | Tobacco | Alcohol | Benign vs. Malignant | Previous Chemo/XRT/Head & Neck Surgery/Immunosuppression |
|---|---|---|---|---|---|---|
| 1 | 65 | M | Current | Former | Malignant | Yes |
| 2 | 70 | F | Former | Current | Malignant | No |
| 3 | 65 | M | Former | Current | Malignant | Yes |
| 4 | 50 | M | Current | Never | Benign | Yes |
| 5 | 20 | F | Never | Never | Benign | Yes |
| 6 | 61 | F | Never | Never | Malignant | Yes |
chemo = chemotherapy; XRT = radiotherapy.
SPY (Novadaq, Burnaby, Canada).
TABLE V.
Table of Clinical Outcomes, SPY Cohort.
| Subject | Mean Fluorescence | Length of Stay (days) | Any Complication | Vital Status |
|---|---|---|---|---|
| 1* | 38 | 9 | 1 | Alive |
| 2 | 70 | 10 | 0 | Dead |
| 3 | 65 | 26 | 1 | Dead |
| 4* | 21 | 10 | 4 | Alive |
| 5 | 76 | 0 | 0 | Alive |
| 6 | 55 | 12 | 3 | Alive |
Current smoker.
TABLE VI.
Mean Fluorescence Measurements Compared by Current Smoking and Nonsmoking (Never And Former) Groups. [Color table can be viewed in the online issue, which is available at www.laryngoscope.com.]
|
P = 0.012.
DISCUSSION
Approximately 21% of Americans currently smoke cigarettes, and the resultant health effects are staggering.27 Decades of clinical literature coupled with physician experience have made it clear that smoking adversely affects wound healing and perioperative outcomes. This is of critical importance in the field of head and neck surgery, in which the majority of surgical neoplasms are tobacco-related, and reconstructive procedures for benign and malignant injury must maintain the function and appearance of structures critical to a patient’s sense of self. Although tobacco-related clinical complications are well documented, the complex underlying pathophysiology is not well understood. Cigarette smoke contains thousands of toxic constituents, which potentially affect wound healing both systemically and locally through multiple mechanisms. We sought to better understand the means by which tobacco effects the head and neck surgical wound.
The healing surgical wound is an extremely complex site of well-orchestrated physiological events, which when disrupted can lead the acute wound to become chronic.28 This cellular and molecular cascade follows a well-known pattern of overlapping stages of healing. After initial injury or surgery, wound healing begins with hemostasis and clot formation, with the entry of platelets and deposition of a fibrin scaffold. This initial phase is short-lived, and the inflammatory phase follows thereafter. The inflammatory phase begins with considerable influx of monocytes, as well as neutrophils into the area of injury. Neutrophils act quickly to neutralize bacteria present in the wound via oxidative killing mechanisms. Monocytes mature to macrophages, which serve a critical role in this phase: phagocytizing dead cells and debris and releasing mediators to spur re-epithelialization and angiogenesis. The proliferative phase of wound healing follows the inflammatory phase. This phase is marked by the influx of fibroblasts, creating collagen scaffold and extracellular matrices, as well as the progression of angiogenesis. Following the proliferative phase of healing is the maturation phase, during which the wound matures and remodels over weeks and months.
In the current study, we evaluated the cytokine milieu present in postoperative head and neck wound fluid, as well as clinical outcomes by smoking status. Wound fluid was evaluated for a panel of cytokines designed to interrogate the postulated mechanisms of smoking-related impaired wound healing with attention to the inflammatory phase of healing. The inflammatory phase is a critical time for the wound. Inflammation is a necessary part of healing the acute wound; however, if inflammation does not appropriately resolve, the wound becomes chronic.29,30 Interestingly, unlike most clinical literature evaluating smoking and wound outcomes, we did not find a statistically significant association between smoking status and clinical outcomes. We did, however, find two cytokines in the postoperative wound fluid to be associated with smoking status in a statistically significant manner.
Epidermal growth factor plays a critical role in stimulating epidermal regeneration by keratinocytes, which begins during the inflammatory phase of wound healing.22 Released by platelets, fibroblasts, and macrophages, EGF stimulates keratinocyte migration and proliferation. In our study, EGF was lower in the wound fluid of current and former smokers, suggesting possible diminishment in the epithelial repair process. Both salivary and systemic EGF have been found to be diminished in cigarette smokers, which has been implicated as a factor in the development of peptic ulcer in smokers.31 Further, EGF has been found to be altered in conditions of poor wound healing, and topical EGF has been used with some success to improve wound healing.22 This therapy could potentially prove helpful for smokers undergoing surgical procedures.
Our study also found a significant association between wound fluid sFLT-1 (VEGFR-1) and smoking status. sFLT-1 is one of three members of the VEGF receptor family and binds VEGF-A, PIGF, and VEGF-B. This receptor is found on endothelial cells, as well as in a soluble form, and in this way serves as a VEGF-A antagonist in the complex feedback mechanisms underlying angiogenesis. Further, VEGFR-1 is found on monocytes and macrophages, and at this site promotes migration of these cells after VEGF stimulation in states of inflammation and wound healing.25,32,33 sFLT-1 appears to serve as a critical regulator of VEGF signaling; diminished levels support a proinflammatory phenotype with decreased endothelial cell survival, whereas elevated levels result in states such as preeclampsia.26 In our study, sFLT-1 is higher in the wound fluid of never smokers in a statistically significant manner. We postulate that the lower levels of this marker in smokers may indicate an excessively proinflammatory state in the healing wound. Similarly, a systemic state of excessive inflammation is thought to play a role in the association of cigarette smoking with cardiovascular and pulmonary disease.34
Finally, in our secondary cohort, we evaluated cutaneous perfusion using SPY (Novadaq) imaging in a small population of patients by smoking status. Although this cohort was very small, mean perfusion values were significantly lower in patients whom had smoked within 24 hours of surgery (P = 0.012). SPY (Novadaq) images reflect both cutaneous vascular anatomy as well as functional perfusion of the tissue; therefore, diminished fluorescence values in smokers suggest alteration in both.35 Because these images were collected prior to the creation of an incision, we suggest that chronic cigarette smoke exposure alters the vascular network in the skin after repeated episodes of vasoconstriction mediated by nicotine and other smoke constituents. This repeated exposure then results in diminished baseline perfusion, as seen here.
The strengths of this study are several. First, the investigation was performed with a clinical cohort of surgical patients in true-to-life clinical scenarios rather than just for investigational purposes. This allows for translation of conclusions to real clinical settings and follow-up of clinical outcomes. Other strengths include the novel use of surgical drain fluid to evaluate the healing head and neck surgical wound, as well as the use of SPY (Novadaq) angiography to evaluate the effects of smoking on healing. This study has several limitations, the greatest of which would be the small sample size in the primary cohort and the very small sample size in the SPY (Novadaq) cohort. As a result, this data must be replicated in a larger study. In addition, our population was heterogeneous, including patients with benign (68%) and malignant (32%) disease. The smoking groups were well-balanced by cancer status (P = 0.715); therefore, this was unlikely to have confounded our results. We do, however, recognize that the cytokine milieu in the wound fluid of cancer patients reflects both tumor behavior and the healing wound. We plan further study to evaluate such wound healing interactions in the head and neck cancer population. Finally, we recognize that the healing wound after head and neck surgery is extremely complex, and the associations detected here need further functional study to clarify underlying mechanisms and pathophysiology.
CONCLUSION
A significant literature supports the association between smoking and poor wound healing; however, the underlying mechanisms are not fully elucidated. We sought to evaluate the healing head and neck surgical wound via cytokines and clinical outcomes, as well as cutaneous perfusion by SPY (Novadaq) angiography in cohort of patients undergoing surgery. Our investigation showed alteration in EGF and sFLT-1 at the level of the local wound, suggesting modifications in the inflammatory phase of wound healing in current and former smokers. Further our study showed diminished cutaneous perfusion in a small group of smokers undergoing surgery. With replication, these findings will allow for improved understanding of the mechanisms of smoking-impaired wound healing to result in improved wound healing in surgical patients with tobacco exposure.
Acknowledgments
The authors gratefully acknowledge the financial support of the Triological Society for its generous funding this work. The authors further recognize the important contributions of Sarah Cooper, Rebecca Dove, Patricia Fernandes Boettner, and Meaghan House, as well as the generous participation of our patients.
Footnotes
Presented at the Triological Society Meeting, Combined Otolaryngology Spring Meetings, San Diego, California, U.S.A., April 28–29, 2017.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Contributor Information
Amy Anne D. Lassig, Department of Otolaryngology–Head and Neck Surgery, Division of Head and Neck Surgery; Department of Otolaryngology–Head and Neck Surgery, Division of Head and Neck Surgery, Hennepin County Medical Center, Minneapolis, Minnesota, U.S.A.
Joan E. Bechtold, Department of Orthopedic Surgery, Departments of Mechanical and Biomedical Engineering, Minneapolis Medical Research Foundation.
Bruce R. Lindgren, Biostatistics and Bioinformatics Core, Masonic Cancer Center, Minneapolis, Minnesota, U.S.A.
Andrew Pisansky, Department of Otolaryngology–Head and Neck Surgery, Division of Head and Neck Surgery.
Abayo Itabiyi, Department of Otolaryngology–Head and Neck Surgery, Division of Head and Neck Surgery.
Bevan Yueh, Department of Otolaryngology–Head and Neck Surgery, Division of Head and Neck Surgery.
Anne M. Joseph, Department of Medicine, Division of General Internal Medicine, Minneapolis, Minnesota, U.S.A.
BIBLIOGRAPHY
- 1.Adams CI, Keating JF, Court-Brown JM. Cigarette smoking and open tibial fractures. Injury. 2001;32:61–65. doi: 10.1016/s0020-1383(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 2.Karim A, Pandit H, Murray J, et al. Smoking and reconstruction of the anterior cruciate ligament. J Bone Joint Surg Br. 2006;88:1027–1031. doi: 10.1302/0301-620X.88B8.17189. [DOI] [PubMed] [Google Scholar]
- 3.Moller AM, Pederson T, Villebro N, et al. Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br. 2003;85:178–181. doi: 10.1302/0301-620x.85b2.13717. [DOI] [PubMed] [Google Scholar]
- 4.Soriano JC, Revuelta SM, Fuente FM, et al. Predictors of outcome after decompressive lumbar surgery. Eur Spine J. 2010;19:1841–1848. doi: 10.1007/s00586-010-1284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogliani M, Gentile P, Silvi E, et al. Smokers: risks and complications in abdominal dermolipectomy. Aesth Plast Surg. 2006;30:422–424. doi: 10.1007/s00266-006-0010-2. [DOI] [PubMed] [Google Scholar]
- 6.Araco A, Gravante G, Sorge R, et al. Wound infections in aesthetic abdominoplasties: the role of smoking. Plast Reconstr Surg. 2001;121:305e–310e. doi: 10.1097/PRS.0b013e31816b13c2. [DOI] [PubMed] [Google Scholar]
- 7.Manassa E, Herti CH, Olbrisch RR. Wound healing problems in smokers and nonsmokers after 132 abdominoplasties. Plast Reconstr Surg. 2003;111:2082–2087. doi: 10.1097/01.PRS.0000057144.62727.C8. [DOI] [PubMed] [Google Scholar]
- 8.Bartsch RH, Weiss G, Kastenbauer T, et al. Crucial aspects of smoking in wound healing after breast reduction surgery. J Plast, Reconstr Aesth Surg. 2007;60:1045–1049. doi: 10.1016/j.bjps.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Padubidri AN, Yetman R, Browne E, et al. Complications of postmastectomy breast reconstructions in smokers, ex-smokers, and nonsmokers. Plast Reconstr Surg. 2001;107:342–349. doi: 10.1097/00006534-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Hawn MT, Houston TK, Campagna EJ, et al. The attributable risk of smoking on surgical complications. Ann Surg. 2011;254:914–920. doi: 10.1097/SLA.0b013e31822d7f81. [DOI] [PubMed] [Google Scholar]
- 11.Lassig AA, et al. The effect of smoking on perioperative complications in head and neck oncologic surgery: a systematic review. Laryngoscope. 2012;122:1800–1808. doi: 10.1002/lary.23308. [DOI] [PubMed] [Google Scholar]
- 12.Marin VP, et al. Serum cotinine concentrations and wound complications in head and neck reconstruction. Plast Reconstr Surg. 2008;121:451–457. doi: 10.1097/01.prs.0000297833.53794.27. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen LT. Wound healing and infection in surgery: the pathophysiological impact of smoking, smoking cessation, and nicotine replacement therapy. Ann Surg. 2012;255:1069–1079. doi: 10.1097/SLA.0b013e31824f632d. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen LT, Toft B, Rygaard J, et al. Smoking attenuates wound inflammation and proliferation while smoking cessation restores inflammation but not proliferation. Wound Repair Regen. 2010;18:186–192. doi: 10.1111/j.1524-475X.2010.00569.x. [DOI] [PubMed] [Google Scholar]
- 15.Michaud SE, Dussault S, Groleau J, et al. Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: role of NO and reactive oxygen species. J Mol Cell Cardiol. 2006;41:275–284. doi: 10.1016/j.yjmcc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Raitio A, Tuomas H, Kokkonen N, et al. Levels of matrix metalloproteinase-2, -9, and -8 in the skin, serum, and saliva of smokers and non-smokers. Arch Dematol Res. 2005;297:242–248. doi: 10.1007/s00403-005-0597-1. [DOI] [PubMed] [Google Scholar]
- 17.Sorensen LT, Toft B, Rygaard J, et al. Effect of smoking, smoking cessation and nicotine patch on wound dimension, vitamin C, and systemic markers of collagen metabolism. Surgery. 2010;148:982–990. doi: 10.1016/j.surg.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Wong LS, Marins-Green M. Firsthand cigarette smoke alters fibroblast migration and survival: implications for impaired healing. Wound Repair Regen. 2004;12:471–484. doi: 10.1111/j.1067-1927.2004.12403.x. [DOI] [PubMed] [Google Scholar]
- 19.Knuutinen A, Kokkonen N, Risteli J, et al. Smoking affects collagen synthesis and extracellular matrix turnover in human skin. Br J Dermatol. 2002;146:588–594. doi: 10.1046/j.1365-2133.2002.04694.x. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen LT, Jorgensen S, Petersen LJ, et al. Acute effects of nicotine and smoking on blood flow, tissue oxygen, and aerobe metabolism of the skin and subcutis. J Surg Res. 2009;152:224–230. doi: 10.1016/j.jss.2008.02.066. [DOI] [PubMed] [Google Scholar]
- 21.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Investig Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 22.Shen C, Sun L, Zhu N, Qi F. Kindlin-1 contributes to EGF-induced re-epithelialization in skin wound healing. Int J Mol Med. 2017;39:949–959. doi: 10.3892/ijmm.2017.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smigiel KS, Parks WC. Matrix metalloproteinases and leukocyte activation. Prog Mol Biol Transl Sci. 2017;147:167–195. doi: 10.1016/bs.pmbts.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Vannella KM, Wynn TA. Mechanisms of organ injury and repair by macrophages. Annu Rev Physiol. 2017;79:593–617. doi: 10.1146/annurev-physiol-022516-034356. [DOI] [PubMed] [Google Scholar]
- 25.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibuya M. VEGF-VEGFR System as a target for suppressing inflammation and other diseases. Endocr Metab Immune Disord Drug Targets. 2015;15:135–144. doi: 10.2174/1871530315666150316121956. [DOI] [PubMed] [Google Scholar]
- 27.Samet JM, Wipfli HL. Globe still in grip of addiction. Nature. 2010;463:1020–1021. doi: 10.1038/4631020a. [DOI] [PubMed] [Google Scholar]
- 28.Zhao R, Liang H, Clarke E, Jackson C, Xue M. Inflammation in chronic wounds. Int J Mol Sci. 2016;17:2085. doi: 10.3390/ijms17122085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawksworth JS, et al. Inflammatory biomarkers in combat wound healing. Ann Surg. 2009;250:1002–1007. doi: 10.1097/sla.0b013e3181b248d9. [DOI] [PubMed] [Google Scholar]
- 30.Hahm G, Glaser JJ, Elster EA. Biomarkers to predict wound healing: the future of complex war wound management. Plast Reconstr Surg. 2011;127(suppl):21S–26S. doi: 10.1097/PRS.0b013e3181fbe291. [DOI] [PubMed] [Google Scholar]
- 31.Maity P. Smoking and the pathogenesis of gastroduodenal ulcer—recent mechanistic update. Mol Cell Biochem. 2003;253:329–338. doi: 10.1023/a:1026040723669. [DOI] [PubMed] [Google Scholar]
- 32.Barleon B, Sozzani S, Zhou D, et al. Migration of human monocytes in response to VEGF is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- 33.Clauss M, Weich H, Breier G, et al. The VEGF receptor Flt-1 mediates biological activities. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 34.Daloee MH, Avan A, Mirhafez SR, et al. Impact of cigarette smoking on serum pro- and anti-inflammatory cytokines and growth factors. Am J Mens Health. 2017;11:1169–1173. doi: 10.1177/1557988315601724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alander JT, Kaartinen I, Laasko A, et al. A Review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. doi: 10.1155/2012/940585. [DOI] [PMC free article] [PubMed] [Google Scholar]


