Abstract
Glucocorticoid receptor (GR) function may have aetiopathogenic significance in chronic fatigue syndrome (CFS), via its essential role in mediating inflammatory responses as well as in hypothalamic-pituitary-adrenal axis regulation. GR function can be estimated ex vivo by measuring dexamethasone (dex) modulation of cytokine response to lipopolysaccharide (LPS), and in vivo using the impact of dex on cortisol levels. This study aimed to compare the GR function between CFS (n = 48), primary Sjögren's syndrome (a disease group control) (n = 27), and sedentary healthy controls (HCs) (n = 20), and to investigate its relationship with clinical measures. In the GR ex vivo response assay, whole blood was diluted and incubated with LPS (to stimulate cytokine production), with or without 10 or 100 nanomolar concentrations of dex. Cytometric bead array (CBA) and flow cytometry enabled quantification of cytokine levels (TNFα, interleukin- (IL-) 6, and IL-10) in the supernatants. In the in vivo response assay, five plasma samples were taken for determination of total cortisol concentration using ELISA at half-hourly intervals on two consecutive mornings separated by ingestion of 0.5 mg of dex at 11 pm. The association of the data from the in vivo and ex vivo analyses with reported childhood adversity was also examined. CFS patients had reduced LPS-induced IL-6 and TNFα production compared to both control groups and reduced suppression of TNFα by the higher dose of dex compared to HCs. Cortisol levels, before or after dex, did not differ between CFS and HCs. Cortisol levels were more variable in CFS than HCs. In the combined group (CFS plus HC), cortisol concentrations positively and ex vivo GR function (determined by dex-mediated suppression of IL-10) negatively correlated with childhood adversity score. The results do not support the hypothesis that GR dysregulation is aetiopathogenic in CFS and suggest that current and future endocrine cross-sectional studies in CFS may be vulnerable to the confounding influence of childhood trauma which is likely increased by comorbid depression.
1. Introduction
Chronic fatigue syndrome (CFS) has a prevalence of 2% in the UK [1, 2]. It is defined by profound, persistent, medically unexplained fatigue lasting at least 6 months, which is not caused by ongoing exertion, not significantly eased by rest, and is severe enough to cause considerable loss of function [3–5]. Alongside this are symptoms of inflammation, pain, cognitive deficits, and psychiatric and bowel problems [4]. Often, biological tests and physical examinations are unremarkable. CFS affects all ages and the peak age of onset is 20–40. Full recovery is rare [2, 6] and comorbidity with depression is common.
Many putative causes of CFS have been investigated but the absence of an agreed pathogenesis impacts the development of effective diagnostics and treatments. It is likely that multiple factors contribute which involve a number of interacting biological, environmental, and psychosocial factors [1, 2, 5, 7–11].
The recognised temporal relationship between stressors and the onset and course of CFS suggests an aetiopathogenic role for systems controlling the stress response including the sympathetic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis [4, 8, 12–17]. The functionality of the glucocorticoid receptor (GR), determined by its sensitivity, affinity, and density and by its interaction with transcription factors [18], is arguably the defining factor in HPA axis regulation [19] and responsible, in a large part, for basal concentrations of cortisol throughout the day. In HPA axis downregulation in response to the GR agonist, dexamethasone (dex) is the most commonly used in vivo method of determining GR function. An ex vivo technique dependent on the inhibitory effect of GR activation on cytokine release is also utilized [20, 21]. Cross-sectional studies in CFS tend to show basal hypocortisolaemia [22, 23], attenuated diurnal variation [4, 24], an attenuated response to activation by CRH or ACTH [16, 23, 25–28], an enhanced suppression by dex [10, 17, 28–30], and an enhanced dex-induced suppression of IL-6, TNFα, IL-10, and IL-4 synthesis [21, 31, 32] and of peripheral blood mononuclear cell proliferation [18]. Genetic studies in CFS have shown the salience of functional single nuclear polymorphisms in NR3C1 [31, 32], which codes for GR, and have also shown hypomethylation of the 1F promotor region of this gene [33–35]. The endocrine findings however are not consistent, and elevated [36] or normal [18, 27, 25, 37] basal cortisol levels have also been reported, as has a normal cortisol response to wakening [25] and to psychosocial stress [21, 38]. There is also some evidence of an association of HPA axis dysregulation with symptom severity and poorer prognosis [15, 39] but only speculation about the mechanism through which HPA axis abnormalities may result in the symptoms of CFS; glucose supply [40], hypotension with associated reduced cerebral perfusion [41], and CRH-induced appetite and sleep disturbance have been considered [16].
The HPA axis interacts with many other systems, notably the immune system [42]. Glucocorticoids (GCs) modulate immune responses by altering gene expression, transcription, translation, and protein secretion [42], either directly, by decreasing transcription of the genes which code for cytokines, or indirectly, by inhibiting proinflammatory transcription factors [42]. GCs inhibit, with different sensitivities, cytokines such as IL-6, IL-1, and TNF (TNF the most, IL-6 the least) [42, 43]. Immune activation, with an increase in proinflammatory cytokine concentrations, including IL-6, TNFα, and IL-1 [2, 44, 45], has been rather inconsistently, for example [32], demonstrated in CFS, may be secondary to insufficient glucocorticoid signaling [7], and may result in pain, fatigue, cognitive deficits, and other symptoms which are characteristic of CFS [12, 16, 38, 46].
In order to examine the nature, extent, and impact of HPA axis dysregulation in CFS, we sought to compare GR function using both in vivo and ex vivo assessment. We therefore examined the HPA axis and immune system function in a sample of patients with CFS and in healthy comparators and in participants with the systemic autoimmune condition, primary Sjögren's syndrome (pSS), who acted as disease group comparators.
2. Methods
2.1. Participants
Three groups were recruited. The study was carried out in accordance with the Declaration of Helsinki. The study design was approved by the Newcastle and North Tyneside Ethics Committee. All participants provided written informed consent. Participants were aged 22–68 years old. Exclusion criteria consisted of age < 18 years, a current or past axis I psychiatric diagnosis confirmed using the Structured Clinical Interview for DSM-IV [47, 48], and use, in the 72 hours prior to enrolment, of antihypertensives, antidepressants, or analgesics. Samples were collected as part of an MRC-funded cohort study (MRC MR/J002712/1). 48 participants with CFS (13 males (mean age 52.2) and 35 females (mean age = 44.9)) were recruited via the local CFS clinical service, all fulfilled the Fukuda diagnostic criteria, and had a mean FIS of 88 and CTQ of 32. Twenty healthy comparators (HC; 7 males (mean age = 43.1) and 13 females (mean age 44.9)) were recruited from a HC database, word of mouth, social media, and advertisement in the hospital (mean FIS = 4, CTQ = 29). HCs were age and sex matched to the patients, and attempts were made to match on activity levels using the Mean International Physical Activity Questionnaire (IPAQ) although the mean CFS IPAQ rating was “low” and the HC rating was “medium.” Primary Sjögren's syndrome (pSS) patients (n = 27) fulfilled the American European Consensus Group classification [49] and were recruited from the United Kingdom pSS Registry [50].
2.2. Symptom Assessment Tools
The CFS participants completed the Fatigue Impact Scale (FIS) [51] and the Childhood Trauma Questionnaire-Short Form (CTQ-SF) [52]. The FIS quantifies individual perception of the impact that fatigue has on daily functioning (Fisk et al.) [53]. There are 40 items, each scored on a 5-point Likert scale providing a continuous scale of 0–160. It comprises three subscales looking at the impact that fatigue has on physical (10 items: motivation, effort, stamina, and coordination), psychosocial (20 items: isolation, emotions, coping, and workload), and cognitive (10 items: concentration, memory, and thinking) functioning. A higher score indicates greater fatigue. The CTQ is a 28 item self-report scale which measures the frequency and severity of childhood adversity. It consists of five factors: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect, and possesses good psychometric properties [52]. Items are scored on a Likert scale, with responses ranging from 1 (“never true”) to 5 (“very often true”).
2.3. In Vivo Assessment of HPA Axis Function by Measurement of Cortisol Levels in Response to Low-Dose dex in CFS Compared to Healthy Controls
In the 48 CFS patients and the first 10 healthy controls (HCs), plasma samples were taken in lithium-heparin vacutainers at 30-minute intervals between 10 am and noon on two consecutive days (day 1 and day 2). At 11 pm on day 1, participants took oral dex (0.5 mg). Practical consideration meant that only the baseline blood (10 am day 1 sample) were taken for the other 10 HCs. Within one hour of collection, the blood was spun at 1600 g for 10 minutes at room temperature. Aliquots of plasma were extracted and stored at −80°C until analysis. Plasma cortisol concentrations were quantified using 15 lot-matched cortisol ELISA kits, supplied by Abcam and used according to the manufacturer's protocol. The lower limit of cortisol detection was 2.44 ng/ml.
2.4. Ex Vivo Measurement of GR Function: The Glucocorticoid Receptor Response Assay
The GR response assay [20] utilised lithium-heparin-treated blood taken at 10 am on day 1. It was set up in sterile 48-well plates within 3 hours of collection. Blood was diluted 1/10 with room temperature RPMI 1640 containing penicillin-streptomycin and L-glutamine, mixed thoroughly by inversion, and then incubated for 24 hours at 37°C in a humidified atmosphere of 95% air and 5% CO2. There were four conditions, “null” (medium alone), “LPS” in which 50 μl of a 200 μg/ml LPS solution was added to the 400 μl of diluted blood (to stimulate cytokine production from cells), “dex10” in which 50 μl of a 100 nanomolar (nM) dex solution was also added, and “dex100” in which a tenfold stronger dex solution was added. After incubation, the assay plates were spun in a 4°C centrifuge for 10 minutes at 1000 RPM. 300 μl supernatant samples were then harvested for each condition, transferred to 0.6 ml microcentrifuge tubes, and stored at −20°C. Cytokine concentrations (TNFα, IL-6, and IL-10) were determined using Cytometric Bead Array (CBA) and flow cytometry according to the manufacturer's instruction (BD Bioscience). Percentage suppression on dex 10 nM (% dex10) and on dex 100 nM (% dex100) was calculated using the following equation:
| (1) |
2.5. Baseline Cytokine Levels
Gel-based specimen tubes were used to collect a further serum sample at 10 am on day 1 for baseline measurement of a range of inflammatory markers. These were spun within 3 hours at 1600 RPM for 10 minutes at room temperature. 2 × 1 ml of serum was extracted and stored at −80°C until analysis which utilized the method described above, that is, CBA and flow cytometry. Two different dilutions of serum samples were required for the measurement of different cytokines; this was therefore conducted in 2 batches for CFS (n = 45), HC (n = 19), and pSS (n = 9) participants.
2.6. Data and Statistical Analysis
Statistical tests were carried out using SPSS version 23 and the “R” statistical package. Graphs were produced using GraphPad Prism version 5.01 and MATLAB. p values are two-tailed with significance set at p < 0.05.
Repeated measures ANOVA was conducted with time (5) as within and group (2) as between factors to examine cortisol concentrations on each of the two days. Area under the curve (AUC) was also calculated using trapezoid integration, both for day 1 and day 2. Specifically, AUC with respect to ground (AUCg), considered to be a measure of overall cortisol output including baseline activity, and AUC increase (AUCi), a putative measure of the sensitivity of GR to modulation, were calculated [54]. The difference between AUCg on the 2 days was also calculated (delta AUCg). Shapiro-Wilk and QQ plots (data not shown) revealed that even after Box-Cox transformation, neither AUC nor cytokine data met the assumptions required for ANOVA; thus, nonparametric comparisons were used. Spearman correlations were conducted to examine the relationship between childhood adversity and endocrine parameters. All data, shown or not shown, is available for scrutiny upon request.
3. Results
3.1. In Vivo Assessment of HPA Axis Function by Measurement of Cortisol Levels in Response to Low-Dose dex in CFS Compared to Healthy Controls
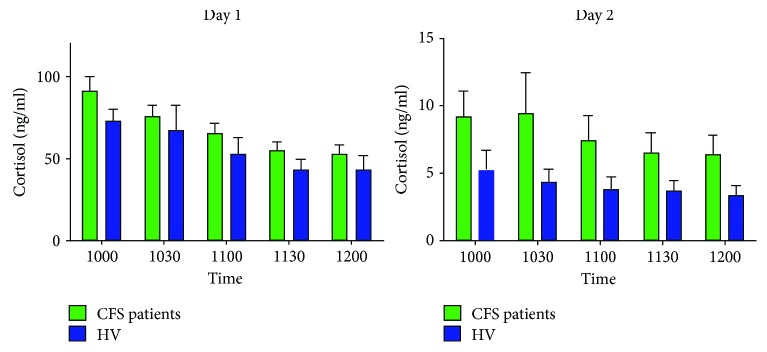
There was a significant effect of time on day one (F = 16.61, df = 4, 56, p < 0.0005) but not on day two (F = 0.87, df = 4, 56, p = 0.418). No effect of group on either day (day one, F = 0.73, df = 1, 56, p = 0.398; day 2, F = 0.79, df = 1, 56, p = 0.378) (see Figure 1). Cortisol AUCs (g or i, day one, day two, or delta) did not differ (p > 0.2).
Figure 1.
Total cortisol values at five time points over two days in participants with CFS and healthy controls. Bar chart showing cortisol concentrations in CFS patients and healthy controls at five morning time points over two consecutive days. Participants administered oral dex at 11 pm on day 1. Data are shown as mean plus standard error of the mean.
3.2. Ex Vivo Assessment of HPA Axis Function: The Glucocorticoid Receptor (GR) Response Assay
GR response assay blood was not taken for two CFS patients. One HC was removed due to an abnormally high null value and one CFS patient removed due to not stimulating sufficiently on LPS. Analysis was therefore conducted on samples taken from CFS (n = 40), HC (n = 19), and pSS (n = 27) participants.
In the null sample, pSS participants had higher IL-10 levels than the CFS or HC participants but there were no differences between groups for IL-6 or TNFα. LPS induced a robust cytokine response, and after LPS, group differences were evident such that, for the positive cytokines IL-6 and TNFα, pSS participants had higher levels than CFS participants, who had higher levels than HCs. For IL-10, the difference was between pSS (higher) and HCs. Median cytokine levels were, on the whole, lower in the dex 10 nM samples and invariably in the dex 100 nM samples than the LPS alone samples. Percentage suppression was greater for samples incubated with dex 100 nM than those incubated with dex 10 nM. Percentage suppression with dex 10 nM or dex 100 nM was not different between the groups, except for a greater suppression in the dex 100 nM condition determined using TNFα (see Table 1).
Table 1.
Standard deviations for endocrine data between chronic fatigue and healthy participants.
| CFS | Healthy controls | Difference | p value | |
|---|---|---|---|---|
| Day 1 AUCg | 79.4 (63.1, 97.5) | 72.1 (38.3, 113.2) | 7.32 (−38.8, 48.4) | 0.389 |
| Day 1 AUCi | 66.3 (52.9, 81.0) | 27.5 (14.9, 43.4) | 38.75 (16.9, 60.3) | 0.014 |
| Day 2 AUCg | 25.4 (20.0, 31.4) | 6.8 (3.7, 10.8) | 18.61 (11.6, 25.8) | <0.0005 |
| Day 2 AUCi | 16.1 (12.7, 19.7) | 5.3 (2.8, 8.4) | 10.75 (5.9, 15.5) | <0.0005 |
| % suppression TNFα | 31.2 (24.3, 39.0) | 15.6 (10.5, 21.3) | 15.6 (6.1, 25.1) | 0.001 |
| % suppression IL-6 | 18.3 (14.2, 22.9) | 13.0 (8.9, 17.9) | 5.3 (−1.2, 11.8) | 0.054 |
| % suppression IL-10 | 47.4 (36.5, 59.3) | 56.4 (38.2, 78.0) | −9.1 (−33.8, 13.9) | 0.774 |
AUCg: area under the curve with respect to ground; AUCi: area under the curve with respect to increase. The % suppressions refer to the percentage difference in cytokine concentration in the dexamethasone 10 nM condition compared with the LPS condition. Values presented are the mean values of the posterior distribution of standard deviations with 95% credible intervals in brackets. Differences are significant if the credible interval does not include zero. p values refer to the proportion of the posterior mean standard deviation difference plot which is less than or equal to zero.
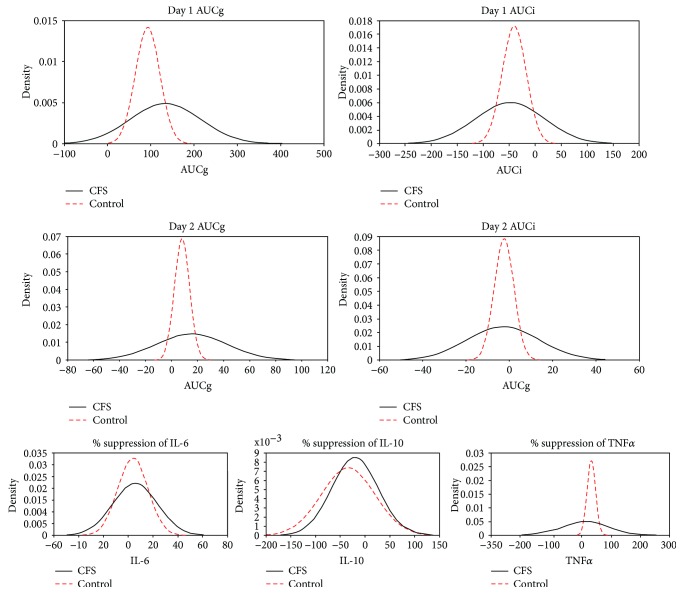
Individual AUC and cytokine values were visualized using frequency density plots. Visual inspection suggested greater variability in patients compared to HCs with some patients showing less suppression (see Figure 2). In order to investigate this, we performed post hoc t-tests of standard deviations derived from Bayesian hierarchical models of outcome measures under Gaussian (normal) priors. The advantage of this approach lies in its treatment of parameters as sampling variables rather than population attributes which allows us to formally compare modelled estimates of their values. Significance was assessed by comparing the 95% credible intervals of the posterior distributions. Vague priors were used for the mean and standard deviation, and analysis was carried out using the BEST package in the R statistical environment [55]. Results are displayed in Table 1. There was no difference in standard deviation of day 1 AUCg distributions. There was a significant difference in standard deviations for day 1 AUCi (p = .014), day 2 AUCg (p < .0005), and day 2 AUCi (p < .0005). There was also a significant difference in standard deviations of TNF distributions (p = .001). The difference in IL-6 was marginal (p = .054) though there was no significant difference in IL-10 distributions (p = .774).
Figure 2.
Frequency density graphs for cortisol area under the curve data and for the suppression of cytokine release by 10 nM solution of dexamethasone. Frequency density graphs for participants with chronic fatigue syndrome and healthy controls for area under the curve (AUC) with respect to ground (g) and increase (i) and for % suppression of TNFα, IL-6, and IL-10 by dex10.
3.3. The Relationship between Reported Adversity and HPA Axis Function in CFS and Controls
Correlation coefficients, in CFS participants, reveal a negative relationship between the CTQ score (and the emotional subscores) and cortisol AUC but no significant relationship with % suppression (after incubation with 10 nm dex). In HCs, there was no significant relationship between CTQ scores and cortisol AUC, but there was a positive relationship with percentage IL-6 suppression (see Table 2). In the combined sample, of CFS and HC (see Table 3), the significant correlations were between the CTQ total score and AUCg (positive) and IL-10 (negative).
Table 2.
Ex vivo glucocorticoid receptor response assay data.
| HC (n = 19) | CFS (n = 40) | PSS (n = 27) | KW | CFS versus HC | CFS versus PSS | PSS versus HC | ||
|---|---|---|---|---|---|---|---|---|
| IL-6 | Null | 1.3 (0.4 to 2.3) | 1.6 (0.7–5.5) | 1.3 (0.8 to1.7) | 0.193 | 0.115 | 0.158 | 0.841 |
| LPS | 14515.2 (12521.4 to 15196.4) | 9517.8 (6994.4 to 12705.7) | 16407.6 (12750.4 to 21912.2) | <0.0005 | 0.001 | <0.0005 | 0.111 | |
| dex10 | 13070.1 (10768 to 15175.2) | 9013.1 (6268.4 to 12568.1) | 15517.0 (12445.0 to 20557.3) | <0.0005 | 0.007 | <0.0005 | 0.096 | |
| dex100 | 7035.9 (5553.0 to 10618.0) | 5606.2 (3394.6 to 7914.2) | 10757.4 (7879.5 to 12655.4) | <0.0005 | 0.037 | <0.0005 | 0.014 | |
| % suppression dex10 | 3.0 (−4.5 to 12.1) | 7.1 (−3.6 to 15.2) | 4.3 (−1.1 to 11.8) | 0.806 | 0.626 | 0.561 | 0.938 | |
| % suppression dex100 | 47.1 (24.4 to 54.1) | 40.5 (28.4 to 54.8) | 36.2 (24.7 to 48.9) | 0.235 | 0.697 | 0.172 | 0.113 | |
| LPS versus dex10 | 0.171 | 0.019 | 0.008 | |||||
| LPS versus dex100 | <0.0005 | <0.0005 | <0.0005 | |||||
| % suppression dex10 versus dex100 | <0.0005 | <0.0005 | <0.0005 | |||||
|
| ||||||||
| IL-10 | Null | 0.0 (0.0 to 0.0) | 0.0 (0.0 to 0.5) | 0.4 (0.0 to 1.0) | 0.007 | 0.615 | 0.003 | 0.031 |
| LPS | 121.7 (66.6 to 260.0) | 95.8 (58.3 to 149.5) | 149.7 (80.3 to 240.6) | 0.032 | 0.114 | 0.011 | 0.585 | |
| dex10 | 179.3 (100 to 264.3) | 111.9 (76.7 to 151.8) | 157.7 (98.0 to 249.0) | 0.029 | 0.020 | 0.040 | 0.772 | |
| dex100 | 248.1 (144.7 to 314.6) | 135.7 (81.4 to 214.0) | 197.6 (115.2 to 250.9) | 0.004 | 0.003 | 0.014 | 0.346 | |
| % suppression dex10 | −17.5 (−59.4 to −1.0) | −5.3 (−31.6 to 6.4) | −12.2 (−23.6 to 9.8) | 0.375 | 0.249 | 0.779 | 0.177 | |
| % suppression dex100 | −56.1 (−110.4 to −24.5) | −29.2 (−95.2 to 1.9) | −33.7 (−69.6 to 4.1) | 0.321 | 0.168 | 1.000 | 0.183 | |
| LPS versus dex10 | 0.027 | 0.010 | 0.091 | |||||
| LPS versus dex100 | 0.002 | 0.002 | 0.006 | |||||
| % suppression dex10 versus dex100 | 0.001 | 0.007 | 0.003 | |||||
|
| ||||||||
| TNFα | Null | 0.6 (0.0 to 1.2) | 0.5 (0.0 to 0.9) | 0.7 (0.0 to 1.0) | 0.547 | 0.596 | 0.264 | 0.778 |
| LPS | 1682.6 (895.0 to 2055.8) | 937.5 (636.4 to 1262.9) | 1872.4 (1275.1 to 2410.9) | <0.0005 | 0.008 | <0.0005 | 0.403 | |
| dex10 | 1072.0 (659.0 to 1473.4) | 743.5 (500.0 to 900.2) | 1428.2 (797.5 to 1942.8) | 0.001 | 0.019 | 0.001 | 0.170 | |
| dex100 | 261.4 (129.3 to 500.6) | 246.2 (152.2 to 245.4) | 389.2 (242.3 to 611.9) | 0.026 | 0.511 | 0.006 | 0.129 | |
| % suppression dex10 | 29.7 (25.0 to 37.2) | 21.3 (10.6 to 37.5) | 28.9 (12.7 to 39.9) | 0.443 | 0.183 | 0.645 | 0.496 | |
| % suppression dex100 | 82.7 (77.9 to 87.0) | 74.9 (62.6 to 83.6) | 76.9 (67.6 to 84.5) | 0.080 | 0.034 | 0.818 | 0.060 | |
| LPS versus dex10 | <0.0005 | <0.0005 | 0.001 | |||||
| LPS versus dex100 | <0.0005 | <0.0005 | <0.0005 | |||||
| % suppression dex10 versus dex100 | <0.0005 | <0.0005 | <0.0005 | |||||
Cytokine concentrations (in pg/ml) and percent cytokine suppression on 10 nm dexamethasone and 100 nm dexamethasone in healthy controls (HCs), participants with chronic fatigue syndrome (CFS), and primary Sjögren's syndrome (PSS). Comparisons are the p values for the independent samples Kruskal-Wallis (KW) comparison of the medians across the 3 groups (HC, CFS and PSS) and the Mann–Whitney U comparisons between two groups (e.g. HC versus CFS). The significance levels for the comparison of cytokine levels in the samples treated with LPS and those treated in addition with dexamethasone, using Related Samples Wilcoxon Signed Rank Test, are also shown for the three groups.
Table 3.
Correlation coefficients for the relationship between childhood trauma and endocrine variables.
| CFS | HC | Combined | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUCg day 1 | IL-6% suppression dex10 | IL-10% suppression dex10 | TNFα % suppression dex10 | AUCg day 1 | IL-6% suppression dex10 | IL-10% suppression dex10 | TNFα % suppression dex10 | AUCg day 1 | IL-6% suppression dex10 | IL-10% suppression dex10 | TNFα % suppression dex10 | |
| CTQ total score | −0.40 ∗∗ | 0.03 | 0.24 | −0.23 | 0.15 | 0.64 ∗∗ | 0.44 | 0.02 | −0.32∗ | 0.17 | 0.29 ∗ | −0.19 |
| CTQ emotional neglect | −0.33∗ | 0.07 | 0.29 | −0.22 | 0.20 | 0.72 ∗∗ | 0.53 ∗ | −0.08 | −0.21 | 0.22 | 0.37 ∗∗ | −0.17 |
Spearman's rho values with significance level indicated by asterisk (p∗∗ < 0.005, ∗ < 0.05) for relationship between childhood adversity and endocrine data in participants with CFS, healthy controls and in a combined group. Data analyses for each group are in the 4 columns below CFS, HC and Combined.
3.4. Baseline Cytokine Levels
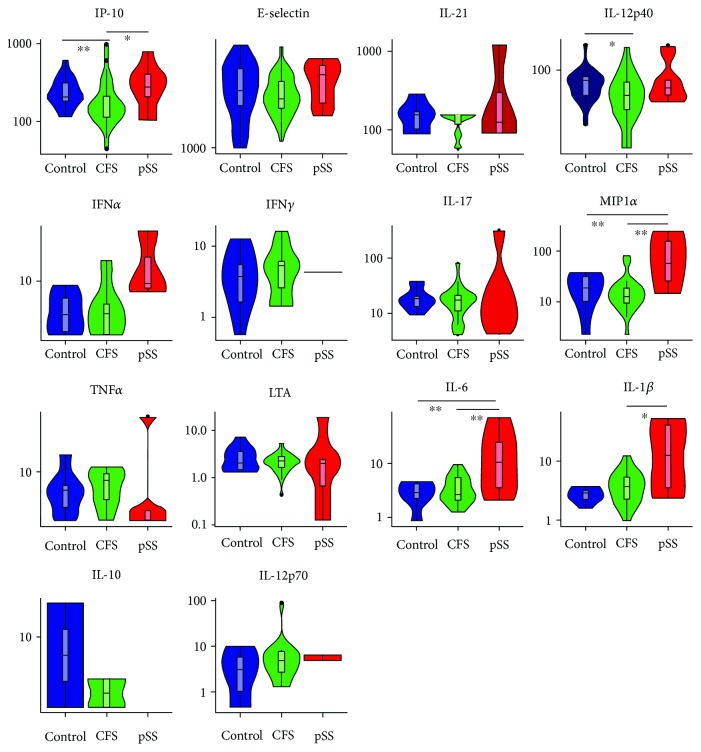
CFS patients showed reduced production of IP-10 and IL-12/23p40 compared to HC and of IP-10, MIP1α, IL-6, and IL-1β compared to pSS participants. pSS participants showed increased production of MIP1α and IL-6 compared to HV. A violin plot was designed post hoc using “R” statistical software to visualize variance between populations and determine whether subpopulations were present and is displayed in Figure 3.
Figure 3.
Violin plot showing baseline cytokine and chemokine concentrations. Baseline cytokine and chemokine concentrations (in pg/ml); asterisks refer to Mann–Whitney U comparisons ∗∗p < 0.01, ∗p < 0.05.
4. Discussion
We did not demonstrate a difference in cortisol levels between participants with CFS and healthy volunteers. This differs from the majority of cross-sectional HPA axis studies in this population (see “Introduction” and [10] for review). This difference may be related to the population; the sample here, for instance, was rigorously screened for comorbid depression, and Papadopoulos et al. [36] have previously demonstrated that dex-induced cortisol suppression differed only in CFS patients with comorbid depression or it may be a type II error consequent on the small sample size combined with the marked variation in cortisol levels in CFS as highlighted by the frequency density graph and the significantly greater cortisol variability in patients with CFS. The aetiopathogenic relevance of this variability is unknown but it suggests a lack of precision in cortisol regulation [56]. The heterogeneity in cortisol concentrations may suggest clinical heterogeneity within the diagnostic grouping of CFS and emphasizes the impact of disparate and competing factors on GR function including current and previous stressors, the common use of antidepressants [20, 54] (even in those who have never met criteria for major depressive disorder) [20, 57], and the impact of a primary dysregulation of proinflammatory cytokines [58].
The baseline cytokine data emphasized the status of pSS as an inflammatory disorder. The ex vivo data revealed a reduced capacity for a proinflammatory cytokine response to LPS in CFS compared with HCs (and an increased responsivity compared to the pSS participants). It further revealed that (independent of group) incubation with dex, in a dose-dependent manner, as expected, suppressed cytokine release. The percent suppression of LPS-induced TNFα release by 100 nM solution of dex was less in CFS patients than HCs. This may be suggestive of reduced GR function in CFS but any such interpretation must be made with caution as the impact of 10 nM dex did not significantly differ between CFS and HCs, neither was a significant effect seen when IL-6 or IL-10 was used as the output variable. That TNFα was most sensitive to suppression by dex accords with the existing literature [42], is congruent with the theory that GCs may preferentially inhibit Th1 over Th2 cells [59], and suggests that TNFα may be the most appropriate cytokine for GR response assay studies in CFS. The variability in percentage suppression of cytokine levels by dex is also greater in CFS than healthy or pSS controls.
In CFS participants, there was a relationship between the score on the childhood trauma questionnaire and cortisol AUCs such that higher reported levels of early adversity correlated negatively with cortisol. Interestingly, a different pattern was seen in healthy volunteers in whom reported childhood adversity associated positively with dex-induced IL-6 suppression in the absence of an effect of cortisol concentrations. When the groups were combined to maximise power, a negative relationship between reported adversity and cortisol levels and a positive relationship with GR function (here shown using IL-10 not IL-6) are revealed.
The variability in cortisol has implications for the interpretation of existing and future endocrine cohort studies in CFS because of the associated risk of type I and type II errors; our data, for instance, would suggest that the proportion of participants in a sample who experienced childhood adversity will be expected to determine the likelihood that basal hypocortisolaemia will be shown. In addition to the CTQ total score, we report here also the emotional neglect subscale, having previously argued that the pervasive nature of emotional neglect ensures that it enacts the greater sustained impact on behavioural and endocrine function [60, 61].
There can be few who argue with the notion that, in the general population, early adversity, acting for instance through methylation or other epigenetic mechanisms, impacts GR function and so GR mediated negative feedback on the HPA axis and thus cortisol synthesis [62, 63] and, further, that this has relevance for understanding the pathophysiology of mood disorders [64]. It is of interest, here, to consider the implication that this has for our understanding of the pathophysiology of CFS and for the interpretation of endocrine studies in this population. We have previously postulated that childhood adversity is not a risk factor for CFS per se, but it can appear to be because of the impact of comorbid or misdiagnosed depression [65]. It has been further conjectured that comorbid depression may commonly confound CFS studies [36] and, just as this may lead to erroneous finding of increased rates of childhood adversity in CFS, similarly, it may explain the methylation pattern [66] including in the NR3C1-1F promoter region [33], the increased GR function (shown using the DST, the dex/CRH test [28], or ex vivo measures), and the basal hypocortisolaemia which have been (inconsistently [10]) shown in previous CFS studies. In this current study, CTQ scores were not greater in the CFS participants than HCs, and it is interesting to note that the basal cortisol or GR function as determined by post-dex cortisol or dex-induced suppression of cytokine synthesis was not convincingly different.
Despite our rigorous exclusion of those who met the diagnostic criteria for depression and the lack of difference in childhood adversity reported by CFS patients compared with HCs, there was a signal that HPA axis regulation was different in CFS; the variability of pre- and post-dex cortisol levels and of dex-induced cytokine suppression was increased in CFS, the proinflammatory cytokine response to LPS was attenuated, and the TNFα suppression by the larger dex dose was greater, and, whilst we do not want to make too much of this, the graph suggested (but the stats did not back up) the possibility that post-dex cortisol was lower in CFS than HCs. Further research is needed to understand the cause and significance of this data; this will need large, well-characterised groups and will need consideration to be given to the interacting networks of biological, psychological, and social factors.
Acknowledgments
This study was funded by the Medical Research Council (MR/J002712/1), by ME Research UK, and by Action for ME. Thanks are due to the participants and to Heather Slater for the help with the standard deviation graphs.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Newton J. L., Okonkwo O., Sutcliffe K., Seth A., Shin J., Jones D. E. J. Symptoms of autonomic dysfunction in chronic fatigue syndrome. QJM: An International Journal of Medicine. 2007;100(8):519–526. doi: 10.1093/qjmed/hcm057. [DOI] [PubMed] [Google Scholar]
- 2.Lorusso L., Mikhaylova S. V., Capelli E., Ferrari D., Ngonga G. K., Ricevuti G. Immunological aspects of chronic fatigue syndrome. Autoimmunity Reviews. 2009;8(4):287–291. doi: 10.1016/j.autrev.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda K., Straus S. E., Hickie I., Sharpe M. C., Dobbins J. G., Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Annals of Internal Medicine. 1994;121(12):953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Papadopoulos A. S., Cleare A. J. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nature Reviews Endocrinology. 2012;8(1):22–32. doi: 10.1038/nrendo.2011.153. [DOI] [PubMed] [Google Scholar]
- 5.Afari N., Buchwald D. Chronic fatigue syndrome: a review. American Journal of Psychiatry. 2003;160(2):221–236. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 6.Cairns R., Hotopf M. A systematic review describing the prognosis of chronic fatigue syndrome. Occupational Medicine. 2005;55(1):20–31. doi: 10.1093/occmed/kqi013. [DOI] [PubMed] [Google Scholar]
- 7.Straus S. E., Komaroff A. L., Wedner H. J. Chronic fatigue syndrome: point and counterpoint. Journal of Infectious Diseases. 1994;170(1):1–6. doi: 10.1093/infdis/170.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Parker A. J., Wessely S., Cleare A. J. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychological Medicine. 2001;31(8):1331–1345. doi: 10.1017/s0033291701004664. [DOI] [PubMed] [Google Scholar]
- 9.Klimas N. G., Salvato F. R., Morgan R., Fletcher M. A. Immunologic abnormalities in chronic fatigue syndrome. Journal of Clinical Microbiology. 1990;28(6):1403–1410. doi: 10.1128/jcm.28.6.1403-1410.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomas C., Newton J., Watson S. A review of hypothalamic-pituitary-adrenal axis function in chronic fatigue syndrome. ISRN Neuroscience. 2013;2013:8. doi: 10.1155/2013/784520.784520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardcastle S. L., Brenu E. W., Johnston S., et al. Serum immune proteins in moderate and severe chronic fatigue syndrome/myalgic encephalomyelitis patients. International Journal of Medical Sciences. 2015;12(10):764–772. doi: 10.7150/ijms.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Houdenhove B., Van Den Eede F., Luyten P. Does hypothalamic-pituitary-adrenal axis hypofunction in chronic fatigue syndrome reflect a ‘crash’ in the stress system? Medical Hypotheses. 2009;72(6):701–705. doi: 10.1016/j.mehy.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Theorell T., Blomkvist V., Lindh G., Evengard B. Critical life events, infections, and symptoms during the year preceding chronic fatigue syndrome (CFS): an examination of CFS patients and subjects with a nonspecific life crisis. Psychosomatic Medicine. 1999;61(3):304–310. doi: 10.1097/00006842-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Lutgendorf S. K., Antoni M. H., Ironson G., et al. Physical symptoms of chronic fatigue syndrome are exacerbated by the stress of Hurricane Andrew. Psychosomatic Medicine. 1995;57(4):310–323. doi: 10.1097/00006842-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Tak L. M., Rosmalen J. G. M. Dysfunction of stress responsive systems as a risk factor for functional somatic syndromes. Journal of Psychosomatic Research. 2010;68(5):461–468. doi: 10.1016/j.jpsychores.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Raison C. L., Miller A. H. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. American Journal of Psychiatry. 2003;160(9):1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 17.Cleare A. J. The neuroendocrinology of chronic fatigue syndrome. Endocrine Reviews. 2003;24(2):236–252. doi: 10.1210/er.2002-0014. [DOI] [PubMed] [Google Scholar]
- 18.Visser J., Lentjes E., Haspels I., et al. Increased sensitivity to glucocorticoids in peripheral blood mononuclear cells of chronic fatigue syndrome patients, without evidence for altered density or affinity of glucocorticoid receptors. Journal of Investigative Medicine. 2001;49(2):195–204. doi: 10.2310/6650.2001.34047. [DOI] [PubMed] [Google Scholar]
- 19.Pariante C. M., Lightman S. L. The HPA axis in major depression: classical theories and new developments. Trends in Neurosciences. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Carvalho L. A., Juruena M. F., Papadopoulos A. S., et al. Clomipramine in vitro reduces glucocorticoid receptor function in healthy subjects but not in patients with major depression. Neuropsychopharmacology. 2008;33(13):3182–3189. doi: 10.1038/npp.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaab J., Rohleder N., Heitz V., et al. Enhanced glucocorticoid sensitivity in patients with chronic fatigue syndrome. Acta Neuropsychiatrica. 2003;15(4):184–191. doi: 10.1034/j.1601-5215.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 22.Tak L. M., Cleare A. J., Ormel J., et al. Meta-analysis and meta-regression of hypothalamic-pituitary-adrenal axis activity in functional somatic disorders. Biological Psychology. 2011;87(2):183–194. doi: 10.1016/j.biopsycho.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Demitrack M. A., Dale J. K., Straus S. E., et al. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. The Journal of Clinical Endocrinology & Metabolism. 1991;73(6):1224–1234. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- 24.Nater U. M., Maloney E., Boneva R. S., et al. Attenuated morning salivary cortisol concentrations in a population-based study of persons with chronic fatigue syndrome and well controls. The Journal of Clinical Endocrinology & Metabolism. 2008;93(3):703–709. doi: 10.1210/jc.2007-1747. [DOI] [PubMed] [Google Scholar]
- 25.Gaab J., Hüster D., Peisen R., et al. Hypothalamic-pituitary-adrenal axis reactivity in chronic fatigue syndrome and health under psychological, physiological and pharmacological stimulation. Psychosomatic Medicine. 2002;64(6):951–962. doi: 10.1097/01.psy.0000038937.67401.61. [DOI] [PubMed] [Google Scholar]
- 26.Roberts A. D. L., Wessely S., Chalder T., Papadopoulos A., Cleare A. J. Salivary cortisol response to awakening in chronic fatigue syndrome. British Journal of Psychiatry. 2004;184(2):136–141. doi: 10.1192/bjp.184.2.136. [DOI] [PubMed] [Google Scholar]
- 27.Scott L. V., Medbak S., Dinan T. G. Blunted adrenocorticotropin and cortisol responses to corticotropin-releasing hormone stimulation in chronic fatigue syndrome. Acta Psychiatrica Scandinavica. 1998;97(6):450–457. doi: 10.1111/j.1600-0447.1998.tb10030.x. [DOI] [PubMed] [Google Scholar]
- 28.Eede F. v. d., Moorkens G., Hulstijn W., et al. Combined dexamethasone/corticotropin-releasing factor test in chronic fatigue syndrome. Psychological Medicine. 2008;38(7) doi: 10.1017/S0033291707001444. [DOI] [PubMed] [Google Scholar]
- 29.Gaab J., Hüster D., Peisen R., et al. Low-dose dexamethasone suppression test in chronic fatigue syndrome and health. Psychosomatic Medicine. 2002;64(2):311–318. doi: 10.1097/00006842-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 30.Jerjes W. K., Taylor N. F., Wood P. J., Cleare A. J. Enhanced feedback sensitivity to prednisolone in chronic fatigue syndrome. Psychoneuroendocrinology. 2007;32(2):192–198. doi: 10.1016/j.psyneuen.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 31.Visser J., Graffelman W., Blauw B., et al. LPS-induced IL-10 production in whole blood cultures from chronic fatigue syndrome patients is increased but supersensitive to inhibition by dexamethasone. Journal of Neuroimmunology. 2001;119(2):343–349. doi: 10.1016/S0165-5728(01)00400-3. [DOI] [PubMed] [Google Scholar]
- 32.Visser J., Blauw B., Hinloopen B., et al. CD4 T lymphocytes from patients with chronic fatigue syndrome have decreased interferon-gamma production and increased sensitivity to dexamethasone. The Journal of Infectious Diseases. 1998;177(2):451–454. doi: 10.1086/517373. [DOI] [PubMed] [Google Scholar]
- 33.Vangeel E., Van Den Eede F., Hompes T., et al. Chronic fatigue syndrome and DNA hypomethylation of the glucocorticoid receptor gene promoter 1F region: associations with HPA axis hypofunction and childhood trauma. Psychosomatic Medicine. 2015;77(8):853–862. doi: 10.1097/PSY.0000000000000224. [DOI] [PubMed] [Google Scholar]
- 34.Goertzel B. N., Pennachin C., de Souza Coelho L., Gurbaxani B., Maloney E. M., Jones J. F. Combinations of single nucleotide polymorphisms in neuroendocrine effector and receptor genes predict chronic fatigue syndrome. Pharmacogenomics. 2006;7(3):475–483. doi: 10.2217/14622416.7.3.475. [DOI] [PubMed] [Google Scholar]
- 35.Rajeevan M. S., Smith A. K., Dimulescu I., et al. Glucocorticoid receptor polymorphisms and haplotypes associated with chronic fatigue syndrome. Genes, Brain and Behavior. 2007;6(2):167–176. doi: 10.1111/j.1601-183X.2006.00244.x. [DOI] [PubMed] [Google Scholar]
- 36.Papadopoulos A., Ebrecht M., Roberts A. D. L., Poon L., Rohleder N., Cleare A. J. Glucocorticoid receptor mediated negative feedback in chronic fatigue syndrome using the low dose (0.5 mg) dexamethasone suppression test. Journal of Affective Disorders. 2009;112(1-3):289–294. doi: 10.1016/j.jad.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 37.Scott L. V., Medbak S., Dinan T. G. The low dose ACTH test in chronic fatigue syndrome and in health. Clinical Endocrinology. 1998;48(6):733–737. doi: 10.1046/j.1365-2265.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 38.Gaab J., Rohleder N., Heitz V., et al. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30(2):188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Torres-Harding S., Sorenson M., Jason L., et al. The associations between basal salivary cortisol and illness symptomatology in chronic fatigue syndrome. Journal of Applied Biobehavioral Research. 2008;13(3):157–180. doi: 10.1111/j.1751-9861.2008.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heim C., Nater U. M., Maloney E., Boneva R., Jones J. F., Reeves W. C. Childhood trauma and risk for chronic fatigue syndrome: association with neuroendocrine dysfunction. Archives of General Psychiatry. 2009;66(1):72–80. doi: 10.1001/archgenpsychiatry.2008.508. [DOI] [PubMed] [Google Scholar]
- 41.Thayer J. F., Sternberg E. Beyond heart rate variability: vagal regulation of allostatic systems. Annals of the New York Academy of Sciences. 2006;1088(1):361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor T. M., O'Halloran D. J., Shanahan F. The stress response and the hypothalamic-pituitary-adrenal axis: from molecule to melancholia. QJM: An International Journal of Medicine. 2000;93(6):323–333. doi: 10.1093/qjmed/93.6.323. [DOI] [PubMed] [Google Scholar]
- 43.Buckingham J. C., Loxley H. D., Christian H. C., Philip J. G. Activation of the HPA axis by immune insults: roles and interactions of cytokines, eicosanoids, and glucocorticoids. Pharmacology, Biochemistry, and Behavior. 1996;54(1):285–298. doi: 10.1016/0091-3057(95)02127-2. [DOI] [PubMed] [Google Scholar]
- 44.Cannon J. G., Angel J. B., Ball R. W., Abad L. W., Fagioli L., Komaroff A. L. Acute phase responses and cytokine secretion in chronic fatigue syndrome. Journal of Clinical Immunology. 1999;19(6):414–421. doi: 10.1023/A:1020558917955. [DOI] [PubMed] [Google Scholar]
- 45.Gupta S., Aggarwal S., See D., Starr A. Cytokine production by adherent and non-adherent mononuclear cells in chronic fatigue syndrome. Journal of Psychiatric Research. 1997;31(1):149–156. doi: 10.1016/S0022-3956(96)00063-5. [DOI] [PubMed] [Google Scholar]
- 46.Houdenhove B. v., Luyten P. Customizing treatment of chronic fatigue syndrome and fibromyalgia: the role of perpetuating factors. Psychosomatics. 2008;49(6):470–477. doi: 10.1176/appi.psy.49.6.470. [DOI] [PubMed] [Google Scholar]
- 47.First M. B., Spitzer R. L., Gibbon M., Williams J. B. W. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: Research Version. Biometrics Research; 1997. [Google Scholar]
- 48.First M. B., Spitzer R. L., Gibbon M., JBW W. SCID-I/P, Version 2.0. New York, NY, USA: Biometrics Research Department; 1995. Structured Clinical Interview for DSM-IV Axis I disorders-Patient Edition. [Google Scholar]
- 49.Vitali C., Bombardieri S., Jonsson R., et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Annals of the Rheumatic Diseases. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng W.-F., Bowman S. J., Griffiths B., on behalf of the UKPSSR study group United Kingdom Primary Sjogren’s Syndrome Registry--a united effort to tackle an orphan rheumatic disease. Rheumatology. 2010;50(1):32–39. doi: 10.1093/rheumatology/keq240. [DOI] [PubMed] [Google Scholar]
- 51.Frith J., Newton J. Fatigue Impact Scale. Occupational Medicine. 2010;60(2):p. 159. doi: 10.1093/occmed/kqp180. [DOI] [PubMed] [Google Scholar]
- 52.Bernstein D. P., Stein J. A., Newcomb M. D., et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27(2):169–190. doi: 10.1016/S0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 53.Fisk J. D., Ritvo P. G., Ross L., Haase D. A., Marrie T. J., Schlech W. F. Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clinical Infectious Diseases. 1994;18(Supplement_1):S79–S83. doi: 10.1093/clinids/18.Supplement_1.S79. [DOI] [PubMed] [Google Scholar]
- 54.Pruessner J. C., Kirschbaum C., Meinlschmid G., Hellhammer D. H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 55.Kruschke J. K. Bayesian estimation supersedes the t test. Journal of Experimental Psychology: General. 2013;142(2):573–603. doi: 10.1037/a0029146. [DOI] [PubMed] [Google Scholar]
- 56.Pezzulo G., Rigoli F., Friston K. Active inference, homeostatic regulation and adaptive behavioural control. Progress in Neurobiology. 2015;134(Supplement C):17–35. doi: 10.1016/j.pneurobio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carvalho L. A., Garner B. A., Dew T., Fazakerley H., Pariante C. M. Antidepressants, but not antipsychotics, modulate GR function in human whole blood: an insight into molecular mechanisms. European Neuropsychopharmacology. 2010;20(6):379–387. doi: 10.1016/j.euroneuro.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pariante C. M., Miller A. H. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biological Psychiatry. 2001;49(5):391–404. doi: 10.1016/S0006-3223(00)01088-X. [DOI] [PubMed] [Google Scholar]
- 59.Visser J. T., De Kloet E. R., Nagelkerken L. Altered glucocorticoid regulation of the immune response in the chronic fatigue syndrome. Annals of the New York Academy of Sciences. 2000;917:868–875. doi: 10.1111/j.1749-6632.2000.tb05453.x. [DOI] [PubMed] [Google Scholar]
- 60.Watson S., Owen B. M., Gallagher P., Hearn A. J., Young A. H., Ferrier I. N. Family history, early adversity and the hypothalamic-pituitary-adrenal (HPA) axis: mediation of the vulnerability to mood disorders. Neuropsychiatric Disease and Treatment. 2007;3(5):647–653. [PMC free article] [PubMed] [Google Scholar]
- 61.Watson S., Gallagher P., Dougall D., et al. Childhood trauma in bipolar disorder. Australian & New Zealand Journal of Psychiatry. 2014;48(6):564–570. doi: 10.1177/0004867413516681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weaver I. C. G., Cervoni N., Champagne F. A., et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7(8):847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 63.McGowan P. O., Sasaki A., D'Alessio A. C., et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krishnan V., Nestler E. J. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clark J. E., Davidson S. L., Maclachlan L., Newton J. L., Watson S. Rethinking childhood adversity in chronic fatigue syndrome. Fatigue: Biomedicine, Health & Behavior. 2017;6(1):20–29. doi: 10.1080/21641846.2018.1384095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Vega W. C., Herrera S., Vernon S. D., McGowan P. O. Epigenetic modifications and glucocorticoid sensitivity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) BMC Medical Genomics. 2017;10(1):p. 11. doi: 10.1186/s12920-017-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.