Abstract
Aging is characterized by functional decline in homeostatic regulation and vital cellular events. This process can be linked with the development of cardiovascular diseases (CVDs). In this review, we discussed aging-induced biological alterations that are associated with CVDs through the following aspects: (i) structural, biochemical, and functional modifications; (ii) autonomic nervous system (ANS) dysregulation; (iii) epigenetic alterations; and (iv) atherosclerosis and stroke development. Aging-mediated structural and biochemical modifications coupled with gradual loss of ANS regulation, vascular stiffening, and deposition of collagen and calcium often disrupt cardiovascular system homeostasis. The structural and biochemical adjustments have been consistently implicated in the progressive increase in mechanical burden and functional breakdown of the heart and vessels. In addition, cardiomyocyte loss in this process often reduces adaptive capacity and cardiovascular function. The accumulation of epigenetic changes also plays important roles in the development of CVDs. In summary, the understanding of the aging-mediated changes remains promising towards effective diagnosis, discovery of new drug targets, and development of new therapies for the treatment of CVDs.
1. Introduction
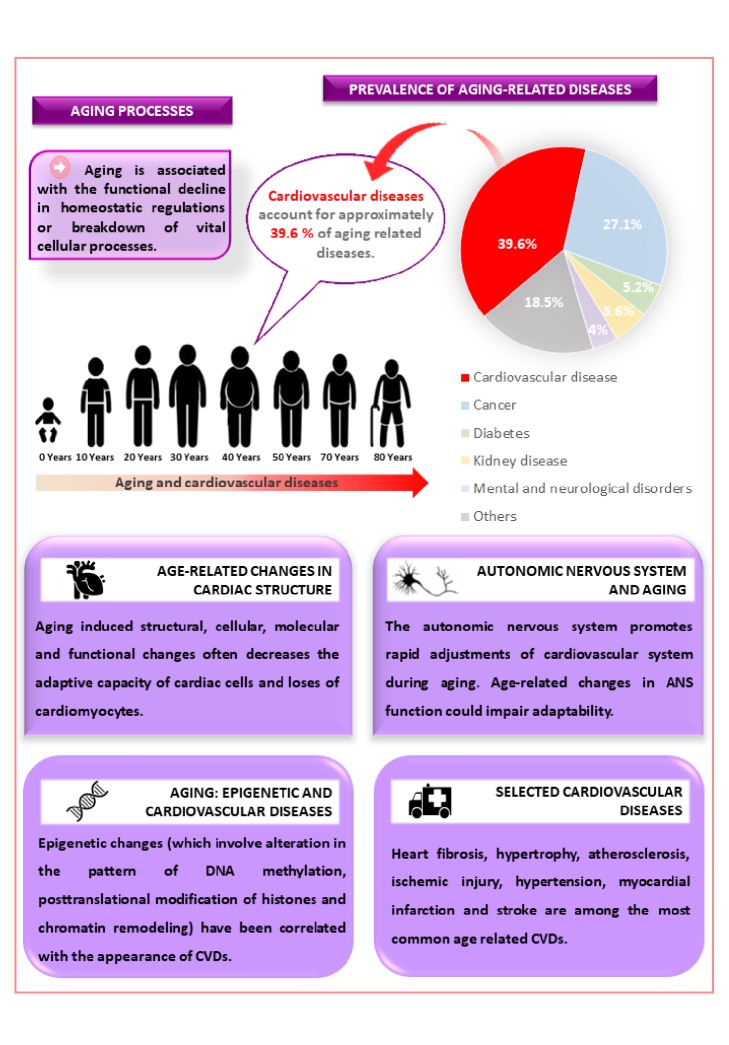
Cardiovascular diseases (CVDs) which are responsible for over 4 million annual deaths in Europe remain one of the leading causes of death worldwide [1]. Currently, there is an increase in annual cases of CVDs such as heart fibrosis, hypertrophy, atherosclerosis, ischemic injury, hypertension, myocardial infarction, and stroke which, put together, account for approximately 39.6% of age-related diseases (Figure 1). Aging reduces the efficiency of homeostatic regulation thereby promoting an increase in tissue damage, rate of morbidity, and mortality [2–4].
Figure 1.
Aging processes, prevalence of aging-related diseases, and some selected cardiovascular diseases.
Aging involves changes in the complex regulatory interplay among cells, organs, and systems [5]. Cardiac and smooth muscle cells participate in involuntary control of heart and vascular functions. The integrity, excitability, conductivity, contractility, and elasticity of these cells are important to cardiovascular control. Progressive loss of physiological function of cardiomyocytes and vascular smooth muscle cells have been associated with cellular aging [6].
Cells undergoing aging processes have a way of communicating their internal status to adjacent cells [7–9]. At the cellular level, aging could disrupt trophic and metabolic signaling pathways prior to the loss of cardiac and vascular functions. In addition, autonomic nervous system (ANS) dysregulation and complex epigenetic changes also induced CVDs [10, 11].
Recently, Steenman and Lande (2017) [12] correlated aging population and increasing prevalence of CVDs. According to these authors, human cardiac aging establishes common pathways with heart disease. Cardiac aging is often characterized by functional, structural, cellular, and molecular changes. The understanding of cardiac aging may unravel heart pathophysiology and promotes effective treatment of CVDs. The detailed reviews on the neurohormonal signaling and morphofunctional changes in diastolic, systolic, and electrical functions induced by aging have been extensively reported [12]. In the present review, we focused on the cardiac aging and autonomic and epigenetic changes underlining some selected CVDs.
2. Age-Related Changes in Cardiac Structure
Aging-related structural modifications in the cardiovascular system often interfere with the functional and adaptive capacity of the heart and vessels [13]. In humans, cardiac aging is associated with left ventricle hypertrophy, fibrosis, and diastolic dysfunction, resulting in reduction of diastolic filling and cardiac output ejection fraction [14, 15]. Although the underlining mechanisms are yet to be fully unravelled, studies suggest that cardiomyocyte apoptosis and vascular stiffness were associated with aging-induced structural and functional modifications [16, 17].
The cardiac hypertrophy in response to aging and other physiological stimuli maintained functional demands of the heart in a compensatory manner. However, excessive heart demands could make hypertrophy a pathological condition [18]. Older hearts were characterized by the thickening of left ventricular wall due to an increase in cardiomyocyte size [19], asymmetric growth of the interventricular septum, and change in the heart shape [20]. These alterations could reduce or increase contractile efficiency of the heart.
Clinical and preclinical reports have associated biochemical changes with cardiac hypertrophy. The extracellular signal-regulated kinase 1/2 (ERK1/2) played a pivotal role in the development of cardiac hypertrophy through Ras/Raf/MEK/ERK signaling pathways [21, 22]. Moreover, the protein kinase B, Akt, or mTOR among other downstream regulators have also been implicated in cardiac hypertrophy [22, 23]. Manne et al. (2014) [22] earlier reported an association between the activation of ERK1/2 and Akt signaling pathways to cardiomyocyte hypertrophy. It thus appears that these signaling pathways regulate the aging-related cardiac hypertrophy.
At the microscopic level, cardiac hypertrophy is associated with a high loss of myocytes. Earlier studies indicate that the number of ventricular myocytes was reduced with aging as a result of apoptosis [24–28]. Olivetti et al. (1995) [24] reported a loss of about 45 million myocytes per year in the left ventricle of aging men. Although aging is accompanied by cardiomyocytes loss, studies have shown an increase in the ventricular myocyte volume [19, 24]. Thus, it was hypothesized that the age-related myocyte loss can increase the mechanical load on the remaining myocytes, resulting in compensatory hypertrophy. In addition to the reduction in the cardiomyocytes number, the peripheral vascular stiffening may contribute to progressive hypertrophy in aged hearts. The loss of aortic elasticity increased the mechanical load on the heart and accelerated heart failure. The hemodynamic overload, caused by aging-related arterial stiffening, contributed to the left ventricle myocyte hypertrophy [17] and increase of collagen deposition in the cardiac tissue. Therefore, hypertrophy appears to be an adaptive mechanism to maintain cardiac function in response to aging-induced structural changes in the cardiovascular system.
Other aging-induced structural modification included epicardial adipose tissue deposition [29, 30] and calcification of the aortic valve leaflets that were associated with atherosclerosis and heart failure [31]. Additionally, the aging heart was characterized by the proliferation of cardiac fibroblasts [32]. This proliferation may result in the accumulation of collagen prior to atrial and ventricular fibrosis in the elderly [33, 34]. Over the years, aging-induced myocardial fibrosis has been studied in human [35, 36], rodent [37, 38], dog [39], and sheep [40].
Aging increased elements of the cardiac extracellular matrix such as glycoproteins, proteoglycans, glycosaminoglycans, integrins, and collagen [41]. It has been reported that collagen content in the left ventricle area of mice also increased from 1-2% to 2-4% [38]. Autopsies of elderly subjects showed an increase in collagen type I and a decrease in collagen type III when compared with younger subjects [42]. Collagen type I had higher tensile strength, whereas collagen type III was more distensible. For that reason, a higher ratio of collagen type I may contribute to left ventricle stiffness and impair cardiac biomechanical functions [35, 41, 42]. In addition, these changes in the extracellular matrix around myocytes or myofibrillar bundles inhibited the propagation of electrical signals, resulting in arrhythmias [7, 43].
The increases in collagen with aging involved posttranscriptional events [41]. Synthesis and breakdown of the extracellular matrix determined high collagen levels. In aged hearts, the stimulation of cardiac fibroblasts by fibrogenic growth factors (such as TGF-β) induced synthesis of matrix proteins and protease inhibitors, leading to fibrotic remodeling and, consequently, diastolic and systolic dysfunction [44, 45]. In addition, reduction in the evolutionarily conserved intracellular autophagy pathway in the heart with aging often triggers structural and functional cardiovascular dysfunctions [46].
Autophagy is an important intracellular process that controls lysosomal degradation of pathogens, aged or damaged proteins, and organelles to protect cells [47]. In the heart, autophagy played an essential role against structural and functional dysfunction during basal state and hemodynamic stress [48]. However, the autophagy machinery becomes susceptible during the aging, resulting in an inadequate performance of cardiac activity [23]. Therefore, the reduction of autophagy with advanced age can affect the capacity to recycle and degrade damaged intracellular components, thereby leading to structural and functional alterations [49]. The serine/threonine protein kinase negatively regulates autophagy through mTOR complex 1 [50]. Aging reduced cardiac autophagy has been associated with overactivation of Akt [23].
The autophagy inhibition shortens lifespan and exacerbates aging-associated cardiomyopathies [46]. The rapamycin-induced autophagy (rapamycin as an inhibitor of mTOR signaling) extended longevity [51] and promoted cardiac performance such as improvement of ejection fraction and reduction in ventricular hypertrophy in aged mice [52].
3. Autonomic Nervous System (ANS) and Aging
The control and integration of the cardiovascular system is characterized by a complex interaction among the heart, kidney, brain, vasculature, and endocrine systems through intrinsic and extrinsic mechanisms. The extrinsic mechanisms are dependent on the ANS and the endocrine system that facilitate rapid adjustments of the cardiovascular function [53–55]. Age-related changes in ANS function could impair adaptability of an elderly individual to the environment [56]. Normally, aging increases plasma catecholamine concentrations and sympathetic nerve activity [57, 58]. Recent studies have suggested that aging-induced increase in sympathetic tone to skeletal muscle vasculature is devoid of decline in respiratory-sympathetic coupling [59]. Hence, the elevation of muscle sympathetic nerve activity in an aged individual could result in endothelial malfunction and arterial stiffness [60]. Aging has been implicated in the impairment of α-adrenergic receptor sensitivity and vascular responsiveness [61]. A higher decrease in mean arterial pressure of older women in response to autonomic blockade as compared to younger women demonstrates the importance of the ANS in maintaining blood pressure in elderly individuals [62]. Reports have shown that age-related alterations in autonomic nerve activity reduced blood pressure, cerebral blood flow, bladder function, and heart rate variability (HRV) [54, 63, 64].
HRV is an indicator of arrhythmic complications and strong predictor of mortality and sudden death [65]. The analysis of HRV provides vital information on the contributions of the ANS to the consecutive oscillations of heart rate [63, 66]. Nocturnal reduction in cardiac parasympathetic activity in elderly individuals elicits a decline in cardiovagal control [65]. Aging could disrupt ANS through reduction and increase in the input of parasympathetic and sympathetic nervous systems, respectively [67–69]. The parasympathetic and sympathetic imbalance decreases HRV [70, 71] which in turn promotes the incidence of cardiovascular events [67]. The high frequency index of parasympathetic modulation indicated a relationship between aging and decline in HRV. The low frequency index of HRV has been associated with an increase in sympathetic modulation. The increase in heart rate combined with the HRV reduction contributes to the degeneration of cardiac autonomic function during aging [72].
Reports have linked CVDs to morbidity and mortality among postmenopausal and obese women (40-55 years) around the world [73–77]. Some studies have reported higher values of HRV in premenopausal women as compared to postmenopausal women of the same age group. The cardioprotective contributions of sex hormones in such women have been reported [78, 79]. According to Davy et al. (1998) [80], young women had higher HRV in comparison with menopausal women. Studies have shown higher prevalence age-dependent HRV reduction among men [72].
Earlier reports on epigenetic relevance in aging-induced CVDs suggested that an active lifestyle is important to the health of the elderly. Elderly people were considered to be prone to slower cardiac, metabolic, and autonomic response as compared to younger ones due to deceleration in vagal reactivation and impairment of cardiac autonomic modulation [81]. The deleterious effects of advanced age on autonomic regulation could be minimized by intensive exercise [82, 83]. Intensive exercise could increase muscle mass and strength without changes in cardiovascular function [82]. A physical training regime could improve physiological adaptations and autonomic function [84]. Although intensive exercise seems to be beneficial to older individuals, there are needs for further research that could lead to health-enhancing exercise programs designed.
The ANS abnormalities were thought to be a common underlying pathophysiology of CVDs such as hypertension and heart failure [85]. In this regard, the atrial fibrillation (AF) is the most frequent arrhythmia that was associated with the imbalance of sympathetic and parasympathetic drive to the heart. Patients with AF had reduction in cardiac performance due to the loss of the atrial contraction and ventricular disorder. The incidence of AF increases dramatically during aging [86, 87]. The surgical ablation which involves various degrees of denervation of ANS has been shown to be efficient against AF [88, 89]. The experimental procedures that target autonomic imbalance in animal models and human studies of AF have been developed [90]. Studies using a high frequency for sympathetic-mimicking atrial stimulation [91] and radiofrequency ablation of the cardiac autonomic ganglion plexus were acclaimed to be a good procedure for the abolishment of AF [92]. Nevertheless, in human patients, studies of combined ganglion plexus destruction and pulmonary vein isolation by radiofrequency ablation promoted reasonable results in preventing AF [93, 94]. On the other hand, pulmonary vein isolation plus renal denervation improved the lifespan by 1 year when compared to pulmonary vein isolation alone [95].
4. Relevance of Epigenetics and Advanced Age in the Evolution of Cardiovascular Diseases
Advanced biological age is often characterized by the accumulation of epigenetic changes that can be correlated with the appearance of CVDs [96]. Chronic stress is one of the major environmental factors responsible for epigenetic changes that affect the cardiovascular system. Evidence has shown that chronic stress promotes modification in the hypothalamic pituitary adrenal pathway [97, 98].
Previous work of Natt et al. (2015) [98] showed repetitive stress-induced decrease in DNA methylation. Kim and Stansfield (2017) [99] inferred that changes in the patterns of acetylation and methylation of genes encoding MMPs were associated with the development of aorta aneurysms. Galán et al. (2016) [100] reported an increase in the expression of histone deacetylase (HDAC) during aortic aneurysm and that administration of class 1 and 2 HDAC inhibitors resulted in a reduction of aortic aneurysm in mice. These results support epigenetic mechanisms of aortic aneurysm.
In addition to aortic aneurysm, cardiomyopathy, a pathology marked by intrinsic myocardial weakness, contractile dysfunction, and congestive heart failure, is one of the main CVDs induced by poor nutrition and a sedentary lifestyle. The development of cardiomyopathy has been associated with the appearance of mitochondrial cardiac polymerase dysfunction and an increase in the methylation of the cardiac DNA. Koczor et al. (2016) [96] correlated cardiomyopathy to the mitochondrial DNA depletion. The authors reported different methylation patterns between the hearts of old and young rats.
The synthesis and excessive accumulation of extracellular matrix proteins towards healing of lesions in the heart muscle have been identified as one of the causes of heart failure. Ghosh et al. (2016) [101] identified microRNAs as important biomarkers for the development of this pathophysiological state. MicroRNAs participate in the regulation of various genetic and epigenetic mechanisms. Structural or epigenetic modifications in these microRNAs have been linked with aging [102].
5. Atherosclerosis
Cardiovascular system disorder such as atherosclerosis is common among aged patients [103]. Atherosclerosis is a multifactorial and progressive disease. Its etiology involves the accumulation of lipid, inflammatory cells, fibrosis elements, and plaque formation and deposition in the arterial walls [104–106]. The aging process could accelerate structural and compositional modifications observed in atherosclerosis. Spatial increase in the vessels and intimal and medial layers thickening are among such modifications. In addition, the accumulation of an extracellular matrix rich in glycosaminoglycans, collagen, and elastin fibers in the vasculature has been attributed to aging [107].
As reported in literature, endothelial cell injury and atherosclerosis clearly suggest the susceptibility of aged vessels to lesion [108–110]. Previous experiments that compared old and young rabbits subjected to long period of hyperlipidemic diet showed that old rabbit arteries constantly develop fibroatheromatous plaques [109]. The formation of plaques, cholesterol deposits (atheroma) with a fibrous cap (sclerosis), characterized the inflammatory process of atherosclerosis. The infiltration of subendothelial spaces of arteries by oxidized lipoprotein often initiated atherosclerosis [111]. Kolodgie et al. (2007) [112] correlated atheroma to pathological thickening of the intima, loss of vascular smooth muscle cells, lipid deposition, and macrophage infiltration. The vascular remodeling reinforces the characterization of aging vessels by thickening and loss of elasticity [113]. The cellular changes in atherosclerosis disease could reduce the number of medial vascular smooth muscle cells and increase collagen deposition [114, 115].
Some authors have addressed the implications of cellular senescence in the atherosclerosis process [116–118]. The cellular senescence could occur in two forms: (i) replicative and (ii) stress-induced premature senescence. The replicative one arises from DNA damage-induced telomere shortening. This damage could result from the high content of reactive oxygen species (ROS), oncogenes, and telomere [117]. Biomarker such as senescence-associated β galactosidase (SAβG) was found during senescence of human cells [119]. A high amount of SAβG-positive in atherosclerotic lesions and old vessels reaffirmed the link between atherosclerosis and senescence [120]. In addition, the pathogenesis of atherosclerosis also involves the recruitment of immune cells. At the site of a lesion with abnormal functioning of endothelium, leukocytes, vascular smooth muscles, and platelets constitute the atheroma. As lipid peroxidation occurs, many molecules that control cell proliferation are released. Moreover, the endothelial cells recruit monocytes and macrophages through the release of colony-stimulating factors [121]. The monocytes and macrophages scavenge potentially harmful compounds. However, the inflammatory factor released by these cells promotes extracellular matrix protein deposition and changes of vascular smooth muscle cells proliferation and migration [122, 123].
Some experimental models of atherosclerosis have associated nutrition to age-induced vascular changes [124]. In these experiments, a modifiable diet that has beneficial effects on old vessels was used to target caloric restriction (CR) [125]. CR has been reported as a dietary intervention for promoting longevity and delaying age-related diseases, including atherosclerosis [126]. Previous study had implicated nicotinamide adenine dinucleotide- (NAD-) dependent deacetylases and adenosine diphosphate-ribosyltransferases (e.g., SIRT1) in the beneficial effect of CR [117]. According to Kitada et al. (2016) [126], CR-induced SIRT1 has an antiaging property. Hence, this molecule could be an important pharmacological target against atherosclerosis.
A possible mechanism by which CR exerts such beneficial effect could involve the actions of sirtuins, particularly SIRT1. SIRT1 has been regarded as a longevity gene that protects cells against oxidative and genotoxic stress [127]. The activation of SIRT1 could exert many physiological effects, including reduced apoptosis, enhanced mitochondrial biogenesis, the inhibition of inflammation, the regulation of glucose and lipid metabolism, and adaptations to cellular stresses such as hypoxia, endoplasmic reticulum (ER) stress, and oxidative stress [126]. Thus, SIRT1 may exert protective effects against vascular aging and atherosclerosis.
Recent studies have shown that SIRT1 could be a regulatory target of multiple miRNAs, such as miR-33, miR-34a, miR-221/222, miR-217, miR-132, and let-7g. Among these miRs, miR-34a has been implicated in the expression of SIRT1 in vascular endothelial cells. Aging endothelial cells expressed high levels of miR-34a and low levels of SIRT1. In addition, earlier reports also indicated an overexpression of miR-34a and increased acetylated p53 levels in endothelial cells. Taken together, these results suggest that miR-34a regulates endothelial senescence in part through decreases of SIRT1 [128, 129].
Earlier report showed that miR-33a/b family played an important role in posttranscriptional repression of the ATP-binding cassette transporter A1 (ABCA1). This role is essential for the biogenesis of high-density lipoprotein (HDL) and reversal of cholesterol transport from peripheral tissues to the liver. The genetic deletion or anti-miR-mediated inhibition of miR-33 in mice has led to the depression of hepatic ABCA1 and up to 40% increase in circulating HDL [130]. This result suggested that miR-33 silencing could be a useful therapeutic strategy against atherosclerosis [131]. Ouimet et. al. (2017) [132] described miR-33 regulation of autophagy in atherosclerosis. The authors reported that the treatment of atherosclerotic Ldlr −/− mice with anti-miR-33 restored defective autophagy in macrophage foam cells and plaques and promoted apoptotic cell clearance to reduce plaque necrosis.
Recent advances in cellular research have suggested new possibilities for the pharmacological treatment of atherosclerosis [118, 133]. The use of antigens as vaccines [134], immunosuppressors (cyclosporine and sirolimus), and anti-inflammatory drugs has been proposed for the treatment of atherosclerosis. However, anti-inflammatory drugs such as Rofecoxib (cyclooxygenase-2 inhibitor), which may trigger cardiovascular problems [135], require a cautious application in patients with CVDs [136]. Hence, better therapies are still needed for atherosclerosis treatment. New anti-inflammatory agents are under development and it is hoped that they will be available in the future [137].
6. Stroke
Stroke is the second most frequent cause of death after ischemic heart in the developed countries [138] and commonly occurs in individuals over 65 years [139, 140]. Stroke may be caused by brain cell damage and brain tissue ischemia due to the disruption of blood supply by a thrombus [141]. It has been reported that the incidence of stroke increases with age [142].
At midlife, neuronal atrophic and glial cell changes begin at the same time. These changes result in the degeneration of white matter. Aging-induced modifications of white matter can influence the susceptibility of axons to ischemia [113]. The astrocytic, microglial hyperactivity and subsequent development of leukoaraiosis have been reported. According to Koton et al. (2009) [143], leukoaraiosis could occur in up to 44 % of stroke patients. As observed in the brains of old rats, a reduction of Na+–K+-ATPase performance leads to damage of the integrity of brain cell membranes and causes white matter vulnerability to ischemia [144]. The increase in glutamate concentration can trigger an influx of calcium ions, excitotoxicity, and cell death [145].
The brain microvasculature also suffers from the influence of the aging process. The structural and functional degeneration of the blood-brain barrier (BBB) during aging could disrupt local perfusion [146, 147]. The changes in pH, water content, and the accumulation of glutamate and lactate in brain interstitial fluid are associated with the reduction in cerebral blood flow (CBF) [148–150]. Cerebral hypoperfusion can induce microcirculation disturbance and oligemia as well as cerebral endothelium loss devoid of ischemic injury [149, 151]. Some experimental data have suggested that the modifications of white matter brain microvasculature could lead to leukoaraiosis [152–154].
Despite the fact that BBB has the ability to adjust to slight aging-related alterations, its permeability often increases [155, 156]. Cerebral vessel alterations during aging may decrease cerebrovascular reservoirs and make the brain more prone to ischemic injury and vascular insufficiency [151]. All these changes could result in an ischemic stroke and vascular cognitive deficiency in the elderly [142]. Age-related alterations that lead to a dysfunctional phenotype [157] suggest the primary effect of aging in the development of CVDs [158]. The dysfunctional endothelial phenotype could induce hemodynamic changes in humans, nonhuman primates, and rodents [157, 159, 160]. The arterial endothelium participates in vital autocrine and paracrine functions that regulate fluid state, blood-tissue exchange of molecules, vascularization, immune system response, and vascular resistance. The resistance arteries [161] maintain a healthy vascular endothelium state through chemical mediators like pro- and antioxidants, vasodilators, vasoconstrictors, and pro- and anti-inflammatory molecules, among others.
Aging can be associated with endothelial dysfunction or impaired endothelial-dependent dilation (EDD). Earlier, Widlansky et al. (2003) [162] demonstrated that increased age was the strongest independent correlate of EDD [163]. Thus, it could be assumed that vascular aging is critical to the development of age-related CVDs. Findings from studies involving vascular aging models have also demonstrated impaired EDD, permeability, angiogenesis, and fibrinolysis [164–166]. Risk factors, such as elevated blood pressure, LDL cholesterol, blood glucose, and sodium intake [167–171], can modulate the severity of endothelial dysfunction. The clearance of high postprandial glucose and lipids decreases with age [172, 173]. In addition, studies that involved endothelial culture which reported physiological elevations in glucose and lipids corroborate the vascular aging phenotype [174, 175].
7. General Discussion and Perspective
Although aging and CVDs fall into a broad field of research with several publications, it is very difficult to cover all the relevant literature on their probable mechanistic connections. The established link between aging and biological changes has greatly advanced our knowledge about diseases such as heart fibrosis, hypertrophy, atherosclerosis, ischemic injury, hypertension, myocardial infarction, and stroke. The current review was narrowed down to the alterations at the organ (heart or cardiac tissues), cellular, molecular, and ANS levels in an attempt to connect the complexity of aging processes to the development of CVDs. Interestingly, some aging-related CVDs have been discussed and published in recent times [176–178].
According to Wang et al. (2010) [179], cardiac remodeling which is an adaptive process often leads to electrical instability, ventricular arrhythmia, cardiac dysfunction, and the death of cardiomyocytes. The incidence of cardiac dysfunction, arrhythmogenesis, and myocardial infarction has been linked to an increase of sympathetic excitation during the remodeling of ANS function as seen in the starred ganglia and dorsal root ganglia [180]. The ganglionic compromise of the autonomic plexuses during adolescence increases susceptibility to atrial fibrillation, micro- and macrostructural changes in the atrial myocardium, intracellular damage (myocyte degeneration, apoptosis), and extracellular fibrotic proliferation [181].
The ANS is predominantly an efferent system that transmits impulses from the central nervous system (CNS) to the periphery. Its physiological roles among others include control of heart rate (HR), force of heart contraction, constriction, and dilation of blood vessels. The functions of autonomic nerves are mediated through the release of neurotransmitters that bind to specific cardiac and vascular receptors. The evolution of CVDs has been associated with alterations in ANS control mechanisms. Advanced age is often characterized by structural, biochemical, and functional changes in the arterial system. In the previous report, some of these changes were associated with epigenetic modifications through DNA methylation, histone modifications, and anomalous gene regulation [99]. These changes could gradually lead to abnormal cardiac and vascular structures prior to the development of CVDs.
The ANS controls cardiac functions by activating its efferent sympathetic nerves to increase heart rate and cardiac contractility [182]. In addition, the parasympathetic nerves exert control over heart functions through direct vagal mediated bradycardia [183, 184]. Recordings from sympathetic and parasympathetic nerves suggest that aging might enhance basal norepinephrine and decrease acetylcholine levels, resulting in depression of heart rate variability [63, 64, 185]. Taken together, these evidences indicated that the imbalance of sympathetic and parasympathetic drive to the heart could promote a direct cardiac dysfunction during aging. On the other hand, cardiac marker such as left ventricular hypertrophy (LVH), an adaptive process that occurs in response to peripheral hemodynamic load, was reported as a major independent risk factor for cardiovascular morbidity and mortality in aging population [186]. In fact, the LVH in hypertensive patients correlated to higher risk of developing systolic heart failure [187]. Thus, it is difficult to determine whether age-induced cardiac dysfunction is closely associated with direct effects or through peripheral hemodynamic load on the heart. Therefore, the combination of these two factors is likely to result in morphofunctional changes in aging heart.
According to Zhang et al., 2014 [188], the evaluation of human longevity correlated lifespan and ALDH2 gene mutation. Recent work showed that ALDH2 regulated autophagic activity and played important roles in cardiac aging. For instance, the overexpression of ALDH2 suppressed autophagy and induced myocardial dysfunction in the previous study [189]. According to Wu et al. (2016) [190], the ablation of ALDH2 may lead to cardiac aging. The authors argued that aged hearts showed a significant decrease in ALDH2 activity. It was suggested that a decrease in the activity of this enzyme caused an accumulation of 4-HNE and carbonyl proteins which in turn compromises autophagic processes and cardiac function.
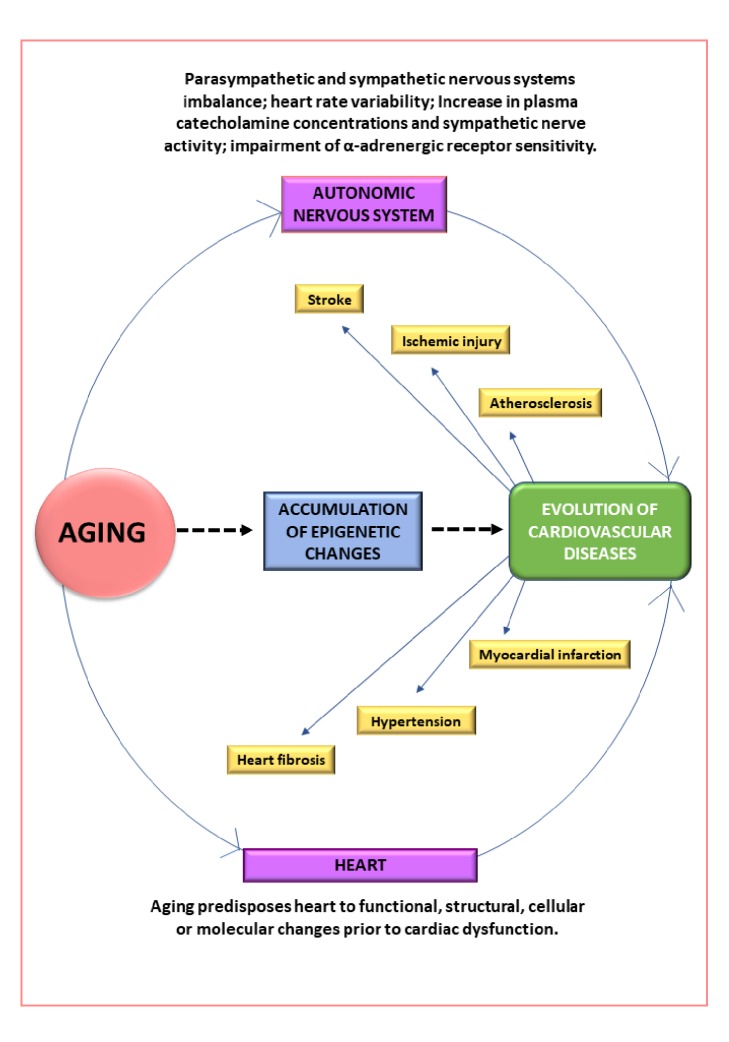
In order to further the discussion of aging-induced CVDs, atherosclerosis and stroke were selected in this review. The understanding of atherosclerosis's pathogenesis requires extensive investigation of chemical mediators, cell-cell interactions, and subsequent formation of plaques. For instance, in the elderly, atherosclerotic plaques tend to be larger with increased vascular stenosis. The progressive accumulation of lipids, collagen, and calcification often occur in the plaques of the elderly as compared with younger people. Like atherosclerosis, cellular and vascular alterations are also critical to the evolution of stroke. Aging-induced endothelial dysfunction and impaired EDD have been linked to the etiology of stroke in elderly patients. Aging-induced modifications of brain microvasculature and white matter often facilitate ischemic brain damage. The alterations in neuronal conductivity by axolemma and white matter dysfunction could increase vulnerability to stroke. As shown in Figure 2, this review summarizes complex alterations that link aging to CVDs.
Figure 2.
Summary of the complex alterations in aging-induced cardiovascular diseases.
In perspective, the course of normal aging could be altered by physiological conditioning or pharmacological intervention. Some of the aging biomarkers, cellular and molecular targets that were identified in this review, could facilitate the diagnosis of CVDs in patients and stimulate the development of new drugs. It is possible that a given CVD and treatment pair could interfere with aging processes and promote longevity. Considering genetic and epigenetic contribution to aging processes, there may be a necessity for an individualized medicine to overcome varying responses among patients. In summary, interdisciplinary research in different aspects of aging and CVDs is crucial to new drug discovery and the promotion of knowledge-based treatment in the future.
Acknowledgments
This work was supported through the financial support and research fellowship from CAPES, CNPQ, and FAPEG. The authors thank Dr. Attah Francis Alfred for reviewing this paper constructively.
Conflicts of Interest
The authors of this article declare that they have no conflicts of interest.
Authors' Contributions
James Oluwagbamigbe Fajemiroye and Gustavo Rodrigues Pedrino conceived and designed the study. Aline Andrade Mourão, Elaine Fernanda da Silva, Nabofa Enivwenaye Egide Williams, James Oluwagbamigbe Fajemiroye, José Luis Rodrigues Martins, Ana Cristina Silva Rebelo, Roberto Saavedra-Rodríguez, and Gustavo Rodrigues Pedrino wrote the manuscript. Marcos Luiz Ferreira-Neto, Angela Adamsk da Silva Reis, Karla Lima Rodrigues, Lara Marques Naves, Luiz Carlos da Cunha, Romes Bittencourt Sousa, and Rodrigo da Silva Santos proofread and contributed to reorganization of the manuscript.
References
- 1.Townsend N., Wilson L., Bhatnagar P., Wickramasinghe K., Rayner M., Nichols M. Cardiovascular disease in Europe: Epidemiological update 2016. European Heart Journal. 2016;37(42):3232–3245. doi: 10.1093/eurheartj/ehw334. [DOI] [PubMed] [Google Scholar]
- 2.Hill K. M., Bara A.-C., Davidson S., House A. O. Preventive cardiovascular care for older people: Fundamental for healthy ageing? Age and Ageing. 2013;42(6):675–676. doi: 10.1093/ageing/aft147.aft147 [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft A. J., Boirie Y., Cederholm T. Issues concerning sarcopenia in ageing adults. Age and Ageing. 2015;44(2):343–344. doi: 10.1093/ageing/afu208. [DOI] [PubMed] [Google Scholar]
- 4.Kochanek K. D., Murphy S. L., Xu J., Tejada-Vera B. Deaths: Final Data for 2014. National Vital Statistics Reports. 2016;65(4):1–122. [PubMed] [Google Scholar]
- 5.DiLoreto R., Murphy C. T. The cell biology of aging. Molecular Biology of the Cell. 2015;26(25):4524–4531. doi: 10.1091/mbc.e14-06-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett T. H., IV, Li H., Mangrum J. M., et al. Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation. 2000;102(12):1454–1460. doi: 10.1161/01.CIR.102.12.1454. [DOI] [PubMed] [Google Scholar]
- 8.Kuilman T., Michaloglou C., Mooi W. J., Peeper D. S. The essence of senescence. Genes & Development. 2010;24(22):2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walther D. M., Kasturi P., Zheng M., et al. Widespread proteome remodeling and aggregation in aging C. elegans. Cell. 2015;161(4):919–932. doi: 10.1016/j.cell.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraga M. F., Esteller M. Epigenetics and aging: the targets and the marks. Trends in Genetics. 2007;23(8):413–418. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Han S., Brunet A. Histone methylation makes its mark on longevity. Trends in Cell Biology. 2012;22(1):42–49. doi: 10.1016/j.tcb.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steenman M., Lande G. Cardiac aging and heart disease in humans. Biophysical Reviews. 2017;9(2):131–137. doi: 10.1007/s12551-017-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juhaszova M., Rabuel C., Zorov D. B., Lakatta E. G., Sollott S. J. Protection in the aged heart: Preventing the heart-break of old age? Cardiovascular Research. 2005;66(2):233–244. doi: 10.1016/j.cardiores.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 14.Mirza M., Strunets A., Shen W.-K., Jahangir A. Mechanisms of arrhythmias and conduction disorders in older adults. Clinics in Geriatric Medicine. 2012;28(4):555–573. doi: 10.1016/j.cger.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strait J. B., Lakatta E. G. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Failure Clinics. 2012;8(1):143–164. doi: 10.1016/j.hfc.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakarla S. K., Fannin J. C., Keshavarzian S., et al. Chronic acetaminophen attenuates age-associated increases in cardiac ROS and apoptosis in the Fischer Brown Norway rat. Basic Research in Cardiology. 2010;105(4):535–544. doi: 10.1007/s00395-010-0094-3. [DOI] [PubMed] [Google Scholar]
- 17.AlGhatrif M., Lakatta E. G. The conundrum of arterial stiffness, elevated blood pressure, and aging. Current Hypertension Reports. 2015;17(2) doi: 10.1007/s11906-014-0523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F., Li N., Long B., et al. Cardiac hypertrophy is negatively regulated by miR-541. Cell Death & Disease. 2014;5(4) doi: 10.1038/cddis.2014.141.e1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olivetti G., Melissari M., Capasso J. M., Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circulation Research. 1991;68(6):1560–1568. doi: 10.1161/01.RES.68.6.1560. [DOI] [PubMed] [Google Scholar]
- 20.Hees P. S., Fleg J. L., Lakatta E. G., Shapiro E. P. Left ventricular remodeling with age in normal men versus women: Novel insights using three-dimensional magnetic resonance imaging. American Journal of Cardiology. 2002;90(11):1231–1236. doi: 10.1016/S0002-9149(02)02840-0. [DOI] [PubMed] [Google Scholar]
- 21.Yamazaki T., Tobe K., Hoh E., et al. Mechanical loading activates mitogen-activated protein kinase and S6 peptide kinase in cultured rat cardiac myocytes. The Journal of Biological Chemistry. 1993;268(16):12069–12076. [PubMed] [Google Scholar]
- 22.Manne D., Kakarla S. K., Ravikumar A., Rice K. M., Blough E. R. Molecular mechanisms of age-related cardiac hypertrophy in the F344XBN rat model. Journal of Clinical & Experimental Cardiology. 2014;5(12) doi: 10.4172/2155-9880.1000353. [DOI] [Google Scholar]
- 23.Hua Y., Zhang Y., Ceylan-Isik A. F., Wold L. E., Nunn J. M., Ren J. Chronic akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: Role of autophagy. Basic Research in Cardiology. 2011;106(6):1173–1191. doi: 10.1007/s00395-011-0222-8. [DOI] [PubMed] [Google Scholar]
- 24.Olivetti G., Giordano G., Corradi D., et al. Gender differences and aging: effects on the human heart. Journal of the American College of Cardiology. 1995;26(4):1068–1079. doi: 10.1016/0735-1097(95)00282-8. [DOI] [PubMed] [Google Scholar]
- 25.Kajstura J., Cheng W., Sarangarajan R., et al. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. American Journal of Physiology-Heart and Circulatory Physiology. 1996;271(3):H1215–H1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- 26.Anna A., Riordon D. R., Boheler K. Molecular mechanisms of cardiomyocyte aging. Clinical Science. 2011;121(8):315–329. doi: 10.1042/cs20110115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai D.-F., Chen T., Johnson S. C., Szeto H., Rabinovitch P. S. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxidants & Redox Signaling. 2012;16(12):1492–1536. doi: 10.1089/ars.2011.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leon L. J., Gustafsson Å. B. Staying young at heart: autophagy and adaptation to cardiac aging. Journal of Molecular and Cellular Cardiology. 2016;95:78–85. doi: 10.1016/j.yjmcc.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silaghi A., Piercecchi-Marti M.-D., Grino M., et al. Epicardial adipose tissue extent: Relationship with age, body fat distribution, and coronaropathy. Obesity. 2008;16(11):2424–2430. doi: 10.1038/oby.2008.379. [DOI] [PubMed] [Google Scholar]
- 30.Sacks H. S., Fain J. N. Human epicardial fat: what is new and what is missing? Clinical and Experimental Pharmacology and Physiology. 2011;38(12):879–887. doi: 10.1111/j.1440-1681.2011.05601.x. [DOI] [PubMed] [Google Scholar]
- 31.Milin A. C., Vorobiof G., Aksoy O., Ardehali R. Insights into aortic sclerosis and its relationship with coronary artery disease. Journal of the American Heart Association. 2014;3(5) doi: 10.1161/JAHA.114.001111.e001111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horn M. A., Trafford A. W. Aging and the cardiac collagen matrix: Novel mediators of fibrotic remodelling. Journal of Molecular and Cellular Cardiology. 2016;93:175–185. doi: 10.1016/j.yjmcc.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biernacka A., Frangogiannis N. G. Aging and cardiac fibrosis. Aging and Disease (A&D) 2011;2(2):158–173. [PMC free article] [PubMed] [Google Scholar]
- 34.Dzeshka M. S., Lip G. Y. H., Snezhitskiy V., Shantsila E. Cardiac fibrosis in patients with atrial fibrillation: mechanisms and clinical implications. Journal of the American College of Cardiology. 2015;66(8):943–959. doi: 10.1016/j.jacc.2015.06.1313. [DOI] [PubMed] [Google Scholar]
- 35.Gazoti Debessa C. R., Mesiano Maifrino L. B., Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mechanisms of Ageing and Development. 2001;122(10):1049–1058. doi: 10.1016/S0047-6374(01)00238-X. [DOI] [PubMed] [Google Scholar]
- 36.Burkauskiene A. Age-related changes in the structure of myocardial collagen network of auricle of the right atrium in healthy persons and ischemic heart disease patients. Medicina (Kaunas) 2005;41(2):145–154. [PubMed] [Google Scholar]
- 37.Eghbali M., Robinson T. F., Seifter S., Blumenfeld O. O. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovascular Research. 1989;23(8):p. 723. doi: 10.1093/cvr/23.8.723. [DOI] [PubMed] [Google Scholar]
- 38.Chiao Y. A., Ramirez T. A., Zamilpa R., et al. Matrix metalloproteinase-9 deletion attenuates myocardial fibrosis and diastolic dysfunction in ageing mice. Cardiovascular Research. 2012;96(3):444–455. doi: 10.1093/cvr/cvs275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J., Masurekar M. R., Vatner D. E., et al. Glycation end-product cross-link breaker reduces collagen and improves cardiac function in aging diabetic heart. American Journal of Physiology-Heart and Circulatory Physiology. 2003;285(6):H2587–H2591. doi: 10.1152/ajpheart.00516.2003. [DOI] [PubMed] [Google Scholar]
- 40.Horn M. A., Graham H. K., Richards M. A., et al. Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: Collagen accumulation in the young and loss in the aged. Journal of Molecular and Cellular Cardiology. 2012;53(1):82–90. doi: 10.1016/j.yjmcc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen N. T., Yabluchanskiy A., De Castro Brás L. E., Jin Y.-F., Lindsey M. L. Aging-related changes in extracellular matrix: Implications for ventricular remodeling following myocardial infarction. Aging and Heart Failure: Mechanisms and Management. 2014:377–389. [Google Scholar]
- 42.Mendes A. B. L., Ferro M., Rodrigues B., de Souza M. R., Araujo R. C., de Souza R. R. Quantification of left ventricular myocardial collagen system in children, young adults, and the elderly. Medicina (Argentina) 2012;72(3):216–220. [PubMed] [Google Scholar]
- 43.Burstein B., Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. Journal of the American College of Cardiology. 2008;51(8):802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 44.Janicki J. S., Brower G. L. The role of myocardial fibrillar collagen in ventricular remodeling and function. Journal of Cardiac Failure. 2002;8(6):S319–S325. doi: 10.1054/jcaf.2002.129260. [DOI] [PubMed] [Google Scholar]
- 45.Burlew B. S. Diastolic dysfunction in the elderly-the interstitial issue. American Journal of Geriatric Cardiology. 2004;13(1):29–38. doi: 10.1111/j.1076-7460.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 46.Taneike M., Yamaguchi O., Nakai A., et al. Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy. 2010;6(5):600–606. doi: 10.4161/auto.6.5.11947. [DOI] [PubMed] [Google Scholar]
- 47.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 48.Nakai A., Yamaguchi O., Takeda T., et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nature Medicine. 2007;13(5):619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 49.Cuervo A. M., Bergamini E., Brunk U. T., Dröge W., Ffrench M., Terman A. Autophagy and aging: the importance of maintaining ‘clean’ cells. Autophagy. 2005;1(3):131–140. doi: 10.4161/auto.1.3.2017. [DOI] [PubMed] [Google Scholar]
- 50.Yang Z., Klionsky D. J. Eaten alive: a history of macroautophagy. Nature Cell Biology. 2010;12(9):814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrison D. E., Strong R., Sharp Z. D., et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flynn J. M., O'Leary M. N., Zambataro C. A., et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12:851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hainsworth R. Clinical Guide to Cardiac Autonomic Tests. 1998. [Google Scholar]
- 54.Hotta H., Uchida S. Aging of the autonomic nervous system and possible improvements in autonomic activity using somatic afferent stimulation. Geriatrics & Gerontology International. 2010;10(1):S127–S136. doi: 10.1111/j.1447-0594.2010.00592.x. [DOI] [PubMed] [Google Scholar]
- 55.Ferreira M. J., Zanesco A. Heart rate variability as important approach for assessment autonomic modulation. Motriz: Revista de Educação Física. 2016;22(2):3–8. doi: 10.1590/S1980-65742016000200001. [DOI] [Google Scholar]
- 56.Pal R., Singh S. N., Chatterjee A., Saha M. Age-related changes in cardiovascular system, autonomic functions, and levels of BDNF of healthy active males: Role of yogic practice. AGE. 2014;36(4, article no. 9683) doi: 10.1007/s11357-014-9683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sundlöf G., Wallin B. G. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. The Journal of Physiology. 1978;274(1):621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seals D. R., Esler M. D. Human ageing and the sympathoadrenal system. The Journal of Physiology. 2000;528(3):407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shantsila A., Mcintyre D. B., Lip G. Y. H., et al. Influence of age on respiratory modulation of muscle sympathetic nerve activity, blood pressure and baroreflex function in humans. Experimental Physiology. 2015;100(9):1039–1051. doi: 10.1113/EP085071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thijssen D. H. J., De Groot P., Kooijman M., Smits P., Hopman M. T. E. Sympathetic nervous system contributes to the age-related impairment of flow-mediated dilation of the superficial femoral artery. American Journal of Physiology-Heart and Circulatory Physiology. 2006;291(6):H3122–H3129. doi: 10.1152/ajpheart.00240.2006. [DOI] [PubMed] [Google Scholar]
- 61.Dinenno F. A., Masuki S., Joyner M. J. Impaired modulation of sympathetic α-adrenergic vasoconstriction in contracting forearm muscle of ageing men. The Journal of Physiology. 2005;567(1):311–321. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnes J. N., Hart E. C., Curry T. B., et al. Aging enhances autonomic support of blood pressure in women. Hypertension. 2014;63(2):303–308. doi: 10.1161/HYPERTENSIONAHA.113.02393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reardon M., Malik M. Changes in heart rate variability with age. Pacing and Clinical Electrophysiology. 1996;19(11):1863–1866. doi: 10.1111/j.1540-8159.1996.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 64.Shimazu T., Tamura N., Shimazu K. Aging of the autonomic nervous system. Nippon Rinsho. Japanese Journal of Clinical Medicine. 2005;63(6):973–977. [PubMed] [Google Scholar]
- 65.Bonnemeier H., Wiegand U. K. H., Brandes A., et al. Circadian profile of cardiac autonomic nervous modulation in healthy subjects: differing effects of aging and gender on heart rate variability. Journal of Cardiovascular Electrophysiology. 2003;14(8):791–799. doi: 10.1046/j.1540-8167.2003.03078.x. [DOI] [PubMed] [Google Scholar]
- 66.McCraty R., Shaffer F. Heart Rate Variability: new perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. GAHM. 2015;4(1):46–61. doi: 10.7453/gahmj.2014.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lipsitz L. A., Mietus J., Moody G. B., Goldberger A. L. Spectral characteristics of heart rate variability before and during postural tilt. Relations to aging and risk of syncope. Circulation. 1990;81(6):1803–1810. doi: 10.1161/01.CIR.81.6.1803. [DOI] [PubMed] [Google Scholar]
- 68.Longo M. J., Ferreira D., Correia M. J. Variabilidade da freqüência cardíaca. Revista Portuguesa de Cardiologia. 1995;14:241–262. [PubMed] [Google Scholar]
- 69.Akselrod S. Components of heart rate variability: basic studies. In: Malik M., Camm A., Futura A., editors. Heart Rate Variability. 1995. pp. 147–163. [Google Scholar]
- 70.Catai A. M., Chacon-Mikahil M. P. T., Martinelli F. S., et al. Effects of aerobic exercise training on heart rate variability during wakefulness and sleep and cardiorespiratory responses of young and middle-aged healthy men. Brazilian Journal of Medical and Biological Research. 2002;35(6):741–752. doi: 10.1590/S0100-879X2002000600016. [DOI] [PubMed] [Google Scholar]
- 71.Melo R. C., Quitério R. J., Takahashi A. C. M., Silva E., Martins L. E. B., Catai A. M. High eccentric strength training reduces heart rate variability in healthy older men. British Journal of Sports Medicine. 2008;42(1):59–63. doi: 10.1136/bjsm.2007.035246. [DOI] [PubMed] [Google Scholar]
- 72.Britton A., Shipley M., Malik M., Hnatkova K., Hemingway H., Marmot M. Changes in Heart Rate and Heart Rate Variability Over Time in Middle-Aged Men and Women in the General Population (from the Whitehall II Cohort Study) American Journal of Cardiology. 2007;100(3):524–527. doi: 10.1016/j.amjcard.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mosca L., Manson J. E., Sutherland S. E., Langer R. D., Manolio T., Barrett-Connor E. Cardiovascular disease in women: a statement for healthcare professionals from the American Heart Association. Writing Group. Circulation. 1997;96(7):2468–2482. doi: 10.1161/01.CIR.96.7.2468. [DOI] [PubMed] [Google Scholar]
- 74.Greendale G. A., Lee N. P., Arriola E. R. The menopause. The Lancet. 1999;353(9152):571–580. doi: 10.1016/S0140-6736(98)05352-5. [DOI] [PubMed] [Google Scholar]
- 75.Dosi R., Bhatt N., Shah P., Patell R. Cardiovascular disease and menopause. Journal of Clinical and Diagnostic Research. 2014;8(2):62–64. doi: 10.7860/JCDR/2014/6457.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gensini G. F., Micheli S., Prisco D., Abbate R. Menopause and risk of cardiovascular disease. Thrombosis Research. 1996;84(1):1–19. doi: 10.1016/0049-3848(96)00143-0. [DOI] [PubMed] [Google Scholar]
- 77.Mendelsohn M. E. Protective effects of estrogen on the cardiovascular system. American Journal of Cardiology. 2002;89(12):12–17. doi: 10.1016/S0002-9149(02)02405-0. [DOI] [PubMed] [Google Scholar]
- 78.Liu C. C., Kuo T. B., Yang C. C. Effects of estrogen on gender-related autonomic differences in humans. American Journal of Physiology-Heart and Circulatory Physiology. 2003;285(5):H2188–H2193. doi: 10.1152/ajpheart.00256.2003. [DOI] [PubMed] [Google Scholar]
- 79.Rebelo A. C., Tamburús N., Salviati M., et al. Influence of third-generation oral contraceptives on the complexity analysis and symbolic dynamics of heart rate variability. The European Journal of Contraception and Reproductive Health Care. 2011;16(4):289–297. doi: 10.3109/13625187.2011.591217. [DOI] [PubMed] [Google Scholar]
- 80.Davy K. P., Desouza C. A., Jones P. P., Seals D. R. Elevated heart rate variability in physically active young and older adult women. Clinical Science. 1998;94(6):579–584. doi: 10.1042/cs0940579. [DOI] [PubMed] [Google Scholar]
- 81.Simões R. P., Bonjorno J. C., Jr., Beltrame T., Catai A. M., Arena R., Borghi-Silva A. Slower heart rate and oxygen consumption kinetic responses in the on- and off-transient during a discontinuous incremental exercise: Effects of aging. Brazilian Journal of Physical Therapy. 2013;17(1):69–76. doi: 10.1590/S1413-35552012005000056. [DOI] [PubMed] [Google Scholar]
- 82.Kanegusuku H., Queiroz A. C. C., Silva V. J. D., De Mello M. T., Ugrinowitsch C., Forjaz C. L. M. High-intensity progressive resistance training increases strength with no change in cardiovascular function and autonomic neural regulation in older adults. Journal of Aging and Physical Activity. 2015;23(3):339–345. doi: 10.1123/japa.2012-0324. [DOI] [PubMed] [Google Scholar]
- 83.DiPietro L. Interval walking training for older people. Exercise and Sport Sciences Reviews. 2017;45(3):p. 126. doi: 10.1249/JES.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 84.Sakabe D. I. Efeitos do treinamento físico sobre a modulação autonômica da freqüencia cardíaca e a capacidade aeróbia de mulheres pós-menopausa sem o uso e em uso de terapia hormonal. http://www.teses.usp.br/teses/disponiveis/17/17145/tde-21052008-135005/, 2007.
- 85.Masuda M., Yamada T., Mizuno H., et al. Impact of atrial fibrillation ablation on cardiac sympathetic nervous system in patients with and without heart failure. International Journal of Cardiology. 2015;199:65–70. doi: 10.1016/j.ijcard.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 86.Menezes A. R., Lavie C. J., DiNicolantonio J. J., et al. Atrial fibrillation in the 21st century: A current understanding of risk factors and primary prevention strategies. Mayo Clinic Proceedings. 2013;88(4):394–409. doi: 10.1016/j.mayocp.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 87.Furberg C. D., Psaty B. M., Manolio T. A., et al. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study) American Journal of Cardiology. 1994;74(3):236–241. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 88.Stavrakis S., Nakagawa H., Po S. S., Scherlag B. J., Lazzara R., Jackman W. M. The role of the autonomic ganglia in atrial fibrillation. JACC: Clinical Electrophysiology. 2015;1(1-2):1–13. doi: 10.1016/j.jacep.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lemery R., Cleland M., Bernick J., Wells G. A. Contact force mapping and voltage thresholds during high-frequency stimulation of human cardiac ganglionated plexuses. Europace. 2015;17(4):552–558. doi: 10.1093/europace/euu336. [DOI] [PubMed] [Google Scholar]
- 90.Czick M. E., Shapter C. L., Silverman D. I. Atrial fibrillation: The science behind its defiance. Aging and Disease (A&D) 2016;7(5):635–656. doi: 10.14336/AD.2016.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Po S. S., Li S., Scherlag B. J., et al. Low-level vagosympathetic stimulation a paradox and potential new modality for the treatment of focal atrial fibrillation. Circulation: Arrhythmia and Electrophysiology. 2009;2(6):645–651. doi: 10.1161/CIRCEP.109.868331. [DOI] [PubMed] [Google Scholar]
- 92.Katritsis D. G. Autonomic denervation for the treatment of atrial fibrillation. Indian Pacing and Electrophysiology Journal. 2011;11(6):161–166. [PMC free article] [PubMed] [Google Scholar]
- 93.Lemery R., Birnie D., Tang A. S. L., Green M., Gollob M. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm. 2006;3(4):387–389. doi: 10.1016/j.hrthm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 94.Scherlag B. J., Nakagawa H., Jackman W. M., et al. Electrical stimulation to identify neural elements on the heart: Their role in atrial fibrillation. Journal of Interventional Cardiac Electrophysiology. 2005;13(1):37–42. doi: 10.1007/s10840-005-2492-2. [DOI] [PubMed] [Google Scholar]
- 95.Pokushalov E., Romanov A., Corbucci G., et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. Journal of the American College of Cardiology. 2012;60(13):1163–1170. doi: 10.1016/j.jacc.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 96.Koczor C. A., Ludlow I., Fields E., et al. Mitochondrial polymerase gamma dysfunction and aging cause cardiac nuclear DNA methylation changes. Physiological Genomics. 2016;48(4):274–280. doi: 10.1152/physiolgenomics.00099.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shalev I., Entringer S., Wadhwa P. D., et al. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013;38(9):1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nätt D., Johansson I., Faresjö T., Ludvigsson J., Thorsell A. High cortisol in 5-year-old children causes loss of DNA methylation in SINE retrotransposons: a possible role for ZNF263 in stress-related diseases. Clinical Epigenetics. 2015;7(1) doi: 10.1186/s13148-015-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kim H. W., Stansfield B. K. Genetic and epigenetic regulation of aortic aneurysms. BioMed Research International. 2017;2017:12. doi: 10.1155/2017/7268521.7268521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galán M., Varona S., Orriols M., et al. Induction of histone deacetylases (HDACs) in human abdominal aortic aneurysm: therapeutic potential of HDAC inhibitors. Disease Models & Mechanisms. 2016;9(5):541–552. doi: 10.1242/dmm.024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghosh A. K., Rai R., Flevaris P., Vaughan D. E. Epigenetics in Reactive and Reparative Cardiac Fibrogenesis: The Promise of Epigenetic Therapy. Journal of Cellular Physiology. 2017;232(8):1941–1956. doi: 10.1002/jcp.25699. [DOI] [PubMed] [Google Scholar]
- 102.Pourrajab F., Vakili Zarch A., Hekmatimoghaddam S., Zare-Khormizi M. R. The master switchers in the aging of cardiovascular system, reverse senescence by microRNA signatures; as highly conserved molecules. Progress in Biophysics and Molecular Biology. 2015;119(2):111–128. doi: 10.1016/j.pbiomolbio.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 103.Sniderman A. D., Furberg C. D. Age as a modifiable risk factor for cardiovascular disease. The Lancet. 2008;371(9623):1547–1549. doi: 10.1016/S0140-6736(08)60313-X. [DOI] [PubMed] [Google Scholar]
- 104.Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 105.Hackam D. G., Anand S. S. Emerging Risk Factors for Atherosclerotic Vascular Disease. Journal of the American Medical Association. 2003;290(7):p. 932. doi: 10.1001/jama.290.7.932. [DOI] [PubMed] [Google Scholar]
- 106.Camaré C., Pucelle M., Nègre-Salvayre A., Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox Biology. 2017;12:18–34. doi: 10.1016/j.redox.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bilato C., Crow M. T. Atherosclerosis and the vascular biology of aging. Aging. 1996;8(4):221–234. doi: 10.1007/BF03339572. [DOI] [PubMed] [Google Scholar]
- 108.Weingand K. W., Clarkson T. B., Adams M. R., Bostrom A. D. Effects of age and/or puberty on coronary artery atherosclerosis in cynomolgus monkeys. Atherosclerosis. 1986;62(2):137–144. doi: 10.1016/0021-9150(86)90059-6. [DOI] [PubMed] [Google Scholar]
- 109.Spagnoli L. G., Orlandi A., Mauriello A., et al. Aging and atherosclerosis in the rabbit, 1: distribution, prevalence and mosphology of atherosclerotic lesions. Atherosclerosis. 1991;89(1):11–24. doi: 10.1016/0021-9150(91)90003-L. [DOI] [PubMed] [Google Scholar]
- 110.Song Y., Shen H., Schenten D., Shan P., Lee P. J., Goldstein D. R. Aging enhances the basal production of IL-6 and CCL2 in vascular smooth muscle cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(1):103–109. doi: 10.1161/ATVBAHA.111.236349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tabas I., García-Cardeña G., Owens G. K. Recent insights into the cellular biology of atherosclerosis. The Journal of Cell Biology. 2015;209(1):13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kolodgie F. D., Burke A. P., Nakazawa G., Virmani R. Is pathologic intimal thickening the key to understanding early plaque progression in human atherosclerotic disease? Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27(5):986–989. doi: 10.1161/ATVBAHA.0000258865.44774.41. [DOI] [PubMed] [Google Scholar]
- 113.Bolton E., Rajkumar C. The ageing cardiovascular system. Reviews in Clinical Gerontology. 2011;21(2):99–109. doi: 10.1017/S0959259810000389. [DOI] [Google Scholar]
- 114.Virmani R., Avolio A. P., Mergner W. J., et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis: Comparison between occidental and Chinese communities. The American Journal of Pathology. 1991;139(5):1119–1129. [PMC free article] [PubMed] [Google Scholar]
- 115.O'Rourke M. F., Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. Journal of the American College of Cardiology. 2007;50(1):1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 116.Ragnauth C. D., Warren D. T., Liu Y., et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation. 2010;121(20):2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 117.Wang J. C., Bennett M. Aging and atherosclerosis: mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circulation Research. 2012;111(2):245–259. doi: 10.1161/CIRCRESAHA.111.261388. [DOI] [PubMed] [Google Scholar]
- 118.Childs B. G., Baker D. J., Wijshake T., Conover C. A., Campisi J., Van Deursen J. M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dimri G. P., Lee X., Basile G., et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proceedings of the National Acadamy of Sciences of the United States of America. 1995;92(20):9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Childs B. G., Durik M., Baker D. J., van Deursen J. M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nature Medicine. 2015;21(12):1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gisterå A., Hansson G. K. The immunology of atherosclerosis. Nature Reviews Nephrology. 2017;13(6):368–380. doi: 10.1038/nrneph.2017.51. [DOI] [PubMed] [Google Scholar]
- 122.Bouhlel M. A., Derudas B., Rigamonti E., et al. PPARγ activation primes human monocytes into alternative m2 macrophages with anti-inflammatory properties. Cell Metabolism. 2007;6(2):137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 123.Boyle J. J., Harrington H. A., Piper E., et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. The American Journal of Pathology. 2009;174(3):1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fontana L., Vinciguerra M., Longo V. D. Growth factors, nutrient signaling, and cardiovascular aging. Circulation Research. 2012;110(8):1139–1150. doi: 10.1161/CIRCRESAHA.111.246470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zanetti M., Gortan Cappellari G., Burekovic I., Barazzoni R., Stebel M., Guarnieri G. Caloric restriction improves endothelial dysfunction during vascular aging: Effects on nitric oxide synthase isoforms and oxidative stress in rat aorta. Experimental Gerontology. 2010;45(11):848–855. doi: 10.1016/j.exger.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 126.Kitada M., Ogura Y., Koya D. The protective role of Sirt1 in vascular tissue: its relationship to vascular aging and atherosclerosis. Aging. 2016;8(10):2290–2307. doi: 10.18632/aging.101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin X., Zhan J.-K., Wang Y.-J., et al. Function, role, and clinical application of MicroRNAs in vascular aging. BioMed Research International. 2016;2016:15. doi: 10.1155/2016/6021394.6021394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Freitas M., Rodrigues A. R., Tomada N., et al. Effects of aging and cardiovascular disease risk factors on the expression of sirtuins in the human corpus cavernosum. The Journal of Sexual Medicine. 2015;12(11):2141–2152. doi: 10.1111/jsm.13035. [DOI] [PubMed] [Google Scholar]
- 129.Rutanen J., Yaluri N., Modi S., et al. SIRT1 mRNA expression may be associated with energy expenditure and insulin sensitivity. Diabetes. 2010;59(4):829–835. doi: 10.2337/db09-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Furuya T. K., Da Silva P. N. O., Payão S. L. M., et al. SORL1 and SIRT1 mRNA expression and promoter methylation levels in aging and Alzheimer's Disease. Neurochemistry International. 2012;61(7):973–975. doi: 10.1016/j.neuint.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 131.van Rooij E., Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Molecular Medicine. 2014;6(7):851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ouimet M., Ediriweera H., Afonso M. S., et al. MicroRNA-33 Regulates Macrophage Autophagy in Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2017;37(6):1058–1067. doi: 10.1161/ATVBAHA.116.308916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Griffith E. C., Zhuang S. U., Niwayama S., Ramsay C. A., Chang Y.-H., Liu J. O. Molecular recognition of angiogenesis inhibitors fumagillin and ovalicin by methionine aminopeptidase 2. Proceedings of the National Acadamy of Sciences of the United States of America. 1998;95(26):15183–15188. doi: 10.1073/pnas.95.26.15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nilsson J., Hansson G. K., Shah P. K. Immunomodulation of atherosclerosis: implications for vaccine development. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;25(1):18–28. doi: 10.1161/01.ATV.0000149142.42590.a2. [DOI] [PubMed] [Google Scholar]
- 135.Bresalier R. S., Sandler R. S., Quan H., et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. The New England Journal of Medicine. 2005;352(11):1092–1102. doi: 10.1056/nejmoa050493. [DOI] [PubMed] [Google Scholar]
- 136.Hansson G. K. Mechanisms of disease: inflammation, atherosclerosis, and coronary artery disease. The New England Journal of Medicine. 2005;352(16):1626–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 137.Bäck M., Hansson G. K. Anti-inflammatory therapies for atherosclerosis. Nature Reviews Cardiology. 2015;12(4):199–211. doi: 10.1038/nrcardio.2015.5. [DOI] [PubMed] [Google Scholar]
- 138.Di Carlo A. Human and economic burden of stroke. Age and Ageing. 2009;38(1):4–5. doi: 10.1093/ageing/afn282. [DOI] [PubMed] [Google Scholar]
- 139.Feigin V. L., Lawes C. M. M., Bennett D. A., Anderson C. S. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. The Lancet Neurology. 2003;2(1):43–53. doi: 10.1016/S1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- 140.Rosamond W., Flegal K., Furie K., et al. Heart disease and stroke statistics-2008 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117(4):e25–e46. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 141.Lee J.-M., Zipfel G. J., Choi D. W. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399(6738):A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- 142.Rothwell P. M., Coull A. J., Silver L. E., et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) The Lancet. 2005;366(9499):1773–1783. doi: 10.1016/s0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 143.Koton S., Schwammenthal Y., Merzeliak O., et al. Cerebral leukoaraiosis in patients with stroke or TIA: Clinical correlates and 1-year outcome. European Journal of Neurology. 2009;16(2):218–225. doi: 10.1111/j.1468-1331.2008.02389.x. [DOI] [PubMed] [Google Scholar]
- 144.Scavone C., Munhoz C. D., Kawamoto E. M., et al. Age-related changes in cyclic GMP and PKG-stimulated cerebellar Na,K-ATPase activity. Neurobiology of Aging. 2005;26(6):907–916. doi: 10.1016/j.neurobiolaging.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 145.Brustovetsky T., Li V., Brustovetsky N. Stimulation of glutamate receptors in cultured hippocampal neurons causes Ca2+-dependent mitochondrial contraction. Cell Calcium. 2009;46(1):18–29. doi: 10.1016/j.ceca.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mitchell G. F. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. Journal of Applied Physiology. 2008;105(5):1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qin C. C., Hui R. T., Liu Z. H. Aging-related cerebral microvascular degeneration is an important cause of essential hypertension. Medical Hypotheses. 2008;70(3):643–645. doi: 10.1016/j.mehy.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 148.De Jong G. I., Farkas E., Stienstra C. M., et al. Cerebral hypoperfusion yields capillary damage in the hippocampal CA1 area that correlates with spatial memory impairment. Neuroscience. 1999;91(1):203–210. doi: 10.1016/S0306-4522(98)00659-9. [DOI] [PubMed] [Google Scholar]
- 149.Stoquart-ElSankari S., Balédent O., Gondry-Jouet C., Makki M., Godefroy O., Meyer M.-E. Aging effects on cerebral blood and cerebrospinal fluid flows. Journal of Cerebral Blood Flow & Metabolism. 2007;27(9):1563–1572. doi: 10.1038/sj.jcbfm.9600462. [DOI] [PubMed] [Google Scholar]
- 150.Drake C. T., Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain and Language. 2007;102(2):141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 151.Ueno M., Tomimoto H., Akiguchi I., Wakita H., Sakamoto H. Blood-brain barrier disruption in white matter lesions in a rat model of chronic cerebral hypoperfusion. Journal of Cerebral Blood Flow & Metabolism. 2002;22(1):97–104. doi: 10.1097/00004647-200201000-00012. [DOI] [PubMed] [Google Scholar]
- 152.Marstrand J. R., Garde E., Rostrup E., et al. Cerebral perfusion and cerebrovascular reactivity are reduced in white matter hyperintensities. Stroke. 2002;33(4):972–976. doi: 10.1161/01.STR.0000012808.81667.4B. [DOI] [PubMed] [Google Scholar]
- 153.Farkas E., de Vos R. A. I., Donka G., Jansen Steur E. N., Mihály A., Luiten P. G. M. Age-related microvascular degeneration in the human cerebral periventricular white matter. Acta Neuropathologica. 2006;111(2):150–157. doi: 10.1007/s00401-005-0007-y. [DOI] [PubMed] [Google Scholar]
- 154.Bertsch K., Hagemann D., Hermes M., Walter C., Khan R., Naumann E. Resting cerebral blood flow, attention, and aging. Brain Research. 2009;1267:77–88. doi: 10.1016/j.brainres.2009.02.053. [DOI] [PubMed] [Google Scholar]
- 155.Shah G. N., Mooradian A. D. Age-related changes in the blood-brain barrier. Experimental Gerontology. 1997;32(4-5):501–519. doi: 10.1016/S0531-5565(96)00158-1. [DOI] [PubMed] [Google Scholar]
- 156.Farrall A. J., Wardlaw J. M. Blood-brain barrier: ageing and microvascular disease—systematic review and meta-analysis. Neurobiology of Aging. 2009;30(3):337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 157.Lakatta E. G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107(1):139–146. doi: 10.1161/01.CIR.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 158.Seals D. R., Jablonski K. L., Donato A. J. Aging and vascular endothelial function in humans. Clinical Science. 2011;120(9):357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li Z., Froehlich J., Galis Z. S., Lakatta E. G. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999;33(1):116–123. doi: 10.1161/01.hyp.33.1.116. [DOI] [PubMed] [Google Scholar]
- 160.Asai K., Kudej R. K., Shen Y.-T., et al. Peripheral vascular endothelial dysfunction and apoptosis in old monkeys. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(6):1493–1499. doi: 10.1161/01.ATV.20.6.1493. [DOI] [PubMed] [Google Scholar]
- 161.Pober J. S., Min W., Bradley J. R. Mechanisms of endothelial dysfunction, injury, and death. Annual Review of Pathology: Mechanisms of Disease. 2009;4:71–95. doi: 10.1146/annurev.pathol.4.110807.092155. [DOI] [PubMed] [Google Scholar]
- 162.Widlansky M. E., Gokce N., Keaney J. F., Jr., Vita J. A. The clinical implications of endothelial dysfunction. Journal of the American College of Cardiology. 2003;42(7):1149–1160. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 163.Mitchell G. F., Parise H., Vita J. A., et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart study. Hypertension. 2004;44(2):134–139. doi: 10.1161/01.hyp.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 164.Yamamoto K., Takeshita K., Shimokawa T., et al. Plasminogen activator inhibitor-1 is a major stress-regulated gene: Implications for stress-induced thrombosis in aged individuals. Proceedings of the National Acadamy of Sciences of the United States of America. 2002;99(2):890–895. doi: 10.1073/pnas.022608799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pelegrí C., Canudas A. M., del Valle J., et al. Increased permeability of blood-brain barrier on the hippocampus of a murine model of senescence. Mechanisms of Ageing and Development. 2007;128(9):522–528. doi: 10.1016/j.mad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 166.Lesniewski L. A., Connell M. L., Durrant J. R., et al. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2009;64(1):9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jablonski K. L., Pierce G. L., Seals D. R., Gates P. E. Low dietary sodium intake is associated with enhanced vascular endothelial function in middle-aged and older adults with elevated systolic blood pressure. Therapeutic Advances in Cardiovascular Disease. 2009;3(5):347–356. doi: 10.1177/1753944709345790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Walker A. E., Eskurza I., Pierce G. L., Gates P. E., Seals D. R. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: Influence of habitual exercise. American Journal of Hypertension. 2009;22(3):250–256. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Walker A. E., Seibert S. M., Donato A. J., Pierce G. L., Seals D. R. Vascular endothelial function is related to white blood cell count and myeloperoxidase among healthy middle-aged and older adults. Hypertension. 2010;55(2):363–369. doi: 10.1161/HYPERTENSIONAHA.109.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kaplon R. E., Walker A. E., Seals D. R. Plasma norepinephrine is an independent predictor of vascular endothelial function with aging in healthy women. Journal of Applied Physiology. 2011;111(5):1416–1421. doi: 10.1152/japplphysiol.00721.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Devan A. E., Eskurza I., Pierce G. L., et al. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clinical Science. 2013;124(5):325–331. doi: 10.1042/CS20120291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Basu R., Man C. D., Campioni M., et al. Effects of age and sex on postprandial glucose metabolism differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006;55(7):2001–2014. doi: 10.2337/db05-1692. [DOI] [PubMed] [Google Scholar]
- 173.Issa J. S., Diament J., Forti N. Postprandial lipemia: influence of aging. Arquivos Brasileiros de Cardiologia. 2005;85(1):15–19. doi: 10.1590/S0066-782X2005001400004. [DOI] [PubMed] [Google Scholar]
- 174.Chakrabarti S., Davidge S. T. High glucose-induced oxidative stress alters estrogen effects on ERα and ERβ in human endothelial cells: Reversal by AMPK activator. The Journal of Steroid Biochemistry and Molecular Biology. 2009;117(4-5):99–106. doi: 10.1016/j.jsbmb.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 175.Mortuza R., Chen S., Feng B., Sen S., Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0054514.e54514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Costa E., Santos-Silva A., Paúl C., González Gallego J. Aging and cardiovascular risk. BioMed Research International. 2015;2015:2. doi: 10.1155/2015/871656.871656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.North B. J., Sinclair D. A. The intersection between aging and cardiovascular disease. Circulation Research. 2012;110(8):1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Koopman J. J. E., Kuipers R. S. From arterial ageing to cardiovascular disease. The Lancet. 2017;389(10080):1676–1678. doi: 10.1016/S0140-6736(17)30763-8. [DOI] [PubMed] [Google Scholar]
- 179.Wang A. Y.-M., Lam C. W.-K., Chan I. H.-S., Wang M., Lui S.-F., Sanderson J. E. Sudden cardiac death in end-stage renal disease patients. Hypertension. 2010;56(2):210–216. doi: 10.1161/HYPERTENSIONAHA.110.151167. [DOI] [PubMed] [Google Scholar]
- 180.Gao C., Howard-Quijano K., Rau C., et al. Inflammatory and apoptotic remodeling in autonomic nervous system following myocardial infarction. PLoS ONE. 2017;12(5) doi: 10.1371/journal.pone.0177750.e0177750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Stirbys P. Neuro-atriomyodegenerative origin of atrial fibrillation and superimposed conventional risk factors: Continued search to configure the genuine etiology of “eternal arrhythmia”. Journal of Atrial Fibrillation. 2016;9(4) doi: 10.4022/jafib.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lymperopoulos A., Rengo G., Koch W. J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circulation Research. 2013;113(6):739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zipes D. P. Heart-brain interactions in cardiac arrhythmias: role of the autonomic nervous system. Cleveland Clinic Journal of Medicine. 2008;75(supplement 2):S94–S96. doi: 10.3949/ccjm.75.Suppl_2.S94. [DOI] [PubMed] [Google Scholar]
- 184.Triposkiadis F., Karayannis G., Giamouzis G., Skoularigis J., Louridas G., Butler J. The sympathetic nervous system in heart failure. physiology, pathophysiology, and clinical implications. Journal of the American College of Cardiology. 2009;54(19):1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 185.Jensen-Urstad K., Storck N., Bouvier F., Ericson M., Lindblad L. E., Jensen-Urstad M. Heart rate variability in healthy subjects is related to age and gender. Acta Physiologica Scandinavica. 1997;160(3):235–241. doi: 10.1046/j.1365-201X.1997.00142.x. [DOI] [PubMed] [Google Scholar]
- 186.Katholi R. E., Couri D. M. Left Ventricular Hypertrophy: Major Risk Factor in Patients with Hypertension: Update and Practical Clinical Applications. International Journal of Hypertension. 2011;2011:1–10. doi: 10.4061/2011/495349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Levy D. The progression from hypertension to congestive heart failure. Journal of the American Medical Association. 1996;275(20):1557–1562. doi: 10.1001/jama.1996.03530440037034. [DOI] [PubMed] [Google Scholar]
- 188.Zhang Y., Mi S.-L., Hu N., et al. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radical Biology & Medicine. 2014;71:208–220. doi: 10.1016/j.freeradbiomed.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang Y., Wang C., Zhou J., et al. Complex inhibition of autophagy by mitochondrial aldehyde dehydrogenase shortens lifespan and exacerbates cardiac aging. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2017;1863(8):1919–1932. doi: 10.1016/j.bbadis.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 190.Wu B., Yu L., Wang Y. Aldehyde dehydrogenase 2 activation in aged heart improves the autophagy by reducing the carbonyl modification on SIRT1. Oncotarget . 2016;7:2175–2188. doi: 10.18632/oncotarget.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]