Abstract
Functional connectivity using task-residual data capitalizes on remaining variance after mean task-related signal is removed from a time series. The degree of network specificity in language and attention domains featured by task-residual and resting-state data types were compared. Functional connectivity based on task-residual data evidenced stronger laterality of the language and attention connections and thus greater network specificity compared to resting-state functional connectivity of the same connections. Covariance between network nodes of task-residuals may thus reflect the degree to which two regions are coordinated in their specific activity, rather than a general shared co-activation. Task-residual functional connectivity provides complementary data to that of resting-state, emphasizing network relationships during task engagement.
Keywords: functional connectivity, language, attention, resting-state, task-residual
1. Introduction
The purpose of this study was to compare the sensitivity of task-residual and resting-state functional connectivity in addressing domain-specific and domain-general connectivity related to language production, using a simple, transparent method.
1.1. Task-Residual and Resting-State fMRI for Determining Functional Connectivity
Resting-state functional connectivity (rsFC) MRI is the dominant methodology used to capture functional connectivity networks (Greicius et al., 2009; Biswal et al., 2010). Task-residual functional connectivity (trFC) is an alternative approach that may offer additional information about coherence of brain systems. In trFC analysis, the effects of an active block or event-related task are regressed out of the fMRI time series and the resulting residual time series is used to define a covariance matrix (Fair et al., 2007; Andrews-Hanna et al., 2007; Zhang & Li, 2010; Fornito et al., 2012). Residuals are typically considered error variance when calculating the mean task-evoked signal. However, areas that are functionally related still show covariation and are related to behavioral differences (Al-Aidroos et al., 2012; Davies-Thompson & Andrews, 2012).
Task-residual functional connectivity may provide more specific information about networks in cognitive states than resting-state functional connectivity (Rogers & Gore, 2008; Fair et al., 2007; Norman-Haignere et al., 2011). When the mean effects of the task are regressed out, block-by-block (or trial-by-trial) variability relevant to the task remains in the residual signal (Fair et al., 2007). Block-by-block variability may encompass coordinated activity not consistently represented in modeling techniques of the hemodynamic response function that assume time invariance. As items differ in their neural demands (e.g., variation in task difficulty between individual items or blocks), functional network components that cooperate to meet those demands may show covarying fluctuations. Thus, in analysis techniques assuming time invariance, the activity associated with individual items and/or blocks is not captured in the task signal but may accumulate in the residuals (Fair et al., 2007). Thus, trFC may be more sensitive to functional interactions of specific, task-relevant network connections compared to rsFC.
1.2. Functional Connectivity Networks related to Language
In the present study, participants completed a resting-state scan and a covert verbal fluency task (semantic and phonemic word generation) as part of a larger project (Cognitive Connectome Project; Gess et al. 2016). To observe activity related to language function during the verbal fluency task, strength of laterality was used (left-hemisphere functional connectivity compared to functional connectivity of right-hemisphere homologues). Because language networks are known to be left-hemisphere lateralized and task-residual data may contain time-invariant effects of the language task, our central hypothesis was that language-based task-residual data would show stronger left-hemisphere lateralization than resting-state data. We used three network relationships to demonstrate our central hypothesis.
The three network relationships used to examine functional connectivity were the following: 1) nodes within the domain-specific language network, 2) nodes that intersect language and attention networks, and 3) nodes within the domain-general intention-attention network. We hypothesized that in each of the three network relationships, task-residual data would show stronger left-lateralized functional connectivity than resting-state data. The first network (language) nodes were comprised of cortex in Broca’s area of the inferior frontal gyrus (IFG) and the posterior perisylvian region (PPS) (Zlatar et al., 2013; Binder et al., 2009), based on their involvement in verbal fluency tasks. Given the positive correlation between these two regions (Tomasi & Volkow, 2012), we expected functional connectivity between the IFG and PPS to show stronger left-lateralized functional connectivity in task-residual data compared to resting-state data.
The second network relationship investigated was the intersection between anterior language and posterior attention nodes. Portions of PPS, such as the angular gyrus, are involved in both the task-positive language network and task-negative default mode network (DMN) (Wirth et al., 2011; Davey et al. 2015; Humphreys & Lambon Ralph, 2015). Posterolateral DMN regions included the angular gyrus are believed to be involved in self-referential attention and internal processes (Wirth et al., 2011) and show deactivation during effortful language tasks (Seghier et al., 2010; Meinzer et al., 2012). In resting-state functional connectivity studies, goal-directed regions, such as the IFG, and DMN attention regions typically show an inverse functional relationship, also referred to as an anticorrelation (Fox et al., 2006a). We thus expected the left IFG (task-positive) and areas of the left PPS converging with DMN functions (task-negative) to be anti-correlated. We expected this anti-correlation to have stronger left-lateralized functional connectivity in task-residual data compared to resting-state data.
The third network relationship of investigation comprised nodes of the domain-general executive attention, or intention-attention network. The pre-supplementary motor area (pre-SMA) was used for its involvement in task-positive activity and intentional response selection relevant to verbal fluency (Lau et al., 2004; Nachev et al., 2007). The posterior cingulate/precuneus (PC/Pc) region is associated with the DMN and involved in various forms of attention, (Cavanna & Trimble, 2005; Vanhaudenhuyse et al., 2011; Cato, et al. 2004; Nadeau et al., 1997). These two anterior and posterior regions are consistently found to be anti-correlated in resting-state functional connectivity literature, such that as dorsal anterior goal-directed regions (e.g., pre-SMA) are invoked (Fox et al., 2006a), activity in the posterior attentional regions is suppressed (Fox et al., 2005). Thus, we expected an anti-correlation between these two network nodes that would have stronger left-lateralized functional connectivity in task-residual data compared to resting-state data.
2. Method
2.1. Participants
A subset of 21 participants were selected from participants recruited for a parent study, the Cognitive Connectome Project at the University of Arkansas for Medical Sciences (UAMS) (Gess et al., 2014). The parent study consisted of healthy adults between the ages of 18–50. Study procedures were approved by the UAMS Institutional Review Board in accordance with the Declaration of Helsinki. Informed consent was obtained for all participants in the study. Inclusion criteria for this study were healthy right-handed, native English speakers with at least an eighth-grade reading and writing proficiency. We restricted the age range (18–30) of our adult sample because age-related functional alterations are evident at midlife (McGregor et al., 2013). Other exclusion criteria and recruitment procedures are described in a previous study (Gess et al., 2014). Demographic information for the sample is presented in Table 1.
Table 1.
Demographic characteristics
| Number of participants | 21 |
|---|---|
| Age (years) | |
| Mean (SD) | 23.24 (2.57) |
| Range | 18–30 |
| Sex, n (%) | |
| Female | 12 (57) |
| Male | 9 (43) |
| Ethnicity, n (%) | |
| African American | 8 (38) |
| Caucasian | 12 (57) |
| Hispanic Latino | 0 |
| Other | 1 (5) |
2.2. Verbal Fluency Task and Resting-state Scans
During the MRI session, participants were asked to silently generate as many words as possible that began with a specific category or letter prompt. Covert word generation has shown to reliably recruit language regions while minimizing motion artifact (La et al., 2016). The task consisted of one run containing 15-second blocks of alternating letter and semantic category prompts separated by 15 seconds of rest. The letter or cue word was presented for the entire 15 seconds of word generation. A total of five letters (i.e., R, P, W, S, J) and five categories (i.e., plants & flowers, clothing, foods, states, jobs) were presented. During rest (non-task) blocks, participants were shown a screen-centered fixation cross, and instructed to cease word generation until the next trial. For the resting-state scan, participants were instructed to relax, rest, and keep their eyes focused on the fixation cross in the center of the screen for the 7-minute acquisition.
2.3. Scanning Procedures
Imaging data were acquired using a Philips 3T Achieva X-series MRI scanner. Anatomic images were acquired with a MPRAGE sequence with the following parameters: matrix = 256 × 256; 22 sagittal slices; TR = shortest; TE = shortest; FA = 8°; resolution = 0.94 × 0.94 × 1 mm3. Functional images for the early participants (1–50) were acquired using an 8-channel head coil with an echo planar imaging (EPI) sequence and the following parameters: TR = 2000 msec; TE = 30 msec; FA = 90°; FOV = 240 × 240 mm2; matrix = 80 × 80, 37 oblique slices parallel to orbitofrontal cortex; “Philips interleaved” for participants 1–28 and interleaved for participants 29–49; resolution = 3.0 × 3.0 × 4.0 mm3. Functional images for the remaining participants (51–79) were acquired using a 32-channel head coil with the following parameters: TR = 2000 msec; TE = 30 msec; FA = 90°; FOV = 240 × 240 mm; matrix = 80 × 80, 37 oblique axial slices parallel to orbitofrontal cortex; sequential ascending acquisition; slice thickness = 2.5 mm with a 0.5 mm gap, resolution = 3.0 × 3.0 × 3.0 mm3. Three image volumes (6s) at the beginning of each functional run were discarded to allow the spin lattice magnetization to stabilize.
2.5. Imaging Data Analysis
2.5.1. Data Preprocessing
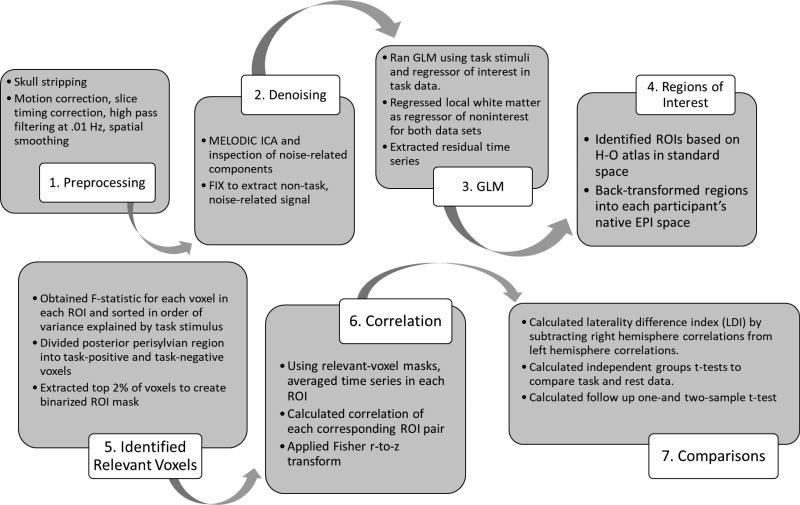
Functional images were pre-preprocessed using Analysis of Functional NeuroImages Software (AFNI; http://afni.nimh.nih.gov; Cox, 1996) and fMRI Software Library (FSL) (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL; Smith et al., 2004; Jenkinson et al., 2012). Skull stripping was performed on anatomic data using FSL’s Brain Extraction Tool (BET) (Smith, 2002). Functional data underwent motion correction, slice timing correction, and Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) (Smith et al., 2004) for denoising motion artifacts. Figure 1 outlines the processing steps.
Fig. 1.
Flow chart of processing steps.
2.5.2. Denoising
All the following processing steps were performed in native space. Within MELODIC, independent component analysis (ICA) was used to decompose each participant’s functional time series into different spatial and temporal components. FSL’s FMRIB’s ICA-based Xnoiseifier (FIX) was used to identify motion-related noise components using classifiers provided by the software authors (Salimi-Khorshidi et al., 2014; Griffianti et al., 2014). All components labeled as noise were visually inspected via their IC spatial maps, IC time courses, and power spectral density profiles to confirm that no probable signal was removed. Probable signal was defined as components in which suprathreshold focal activity was present in regions of interest, with an absence of patterns indicative of noise. Examples include heavily clustered activity at the frontal pole, ring pattern around edges of brain and “saw-tooth” pattern time courses. These criteria were based on Kelly and colleagues’ (2010) descriptions of common non-signal related patterns in MELODIC output.
The first and last two TRs were removed from each functional run to eliminate artifacts introduced by slice timing correction. For task data, the block basis function was used to model the mean time series using BLOCK5. Data were run through 3dREMLfit after 3dDeconvolve to correct for temporal correlations and to prepare for local white matter regression. Local white matter was regressed using ANATICOR to reduce bias in correlations contained within gray matter (Jo, et al., 2010). The resultant residual time series, after extraction of effects of non-interest and time-invariant task-related hemodynamic response estimates, were then promoted for further analysis.
2.5.3. Region of Interest Selection
Regions of interest (ROIs) were based on the Harvard-Oxford cortical brain atlas appropriate to the goals in this study. Each ROI was back-transformed from standard space into each participant’s native EPI space using FSL’s Non-linear Image Registration Tool (FNIRT).
2.5.4. Time Series Extraction
Within each ROI, the top two percent of voxels active in response to the language task were selected to extract the residual time series. This was performed to compensate for structural and functional anatomic heterogeneity, thus ensuring that each ROI was task-relevant and subject-specific (e.g., Amunts et al., 2004). This procedure comprised the following steps: The F-statistic was obtained for each voxel within each ROI and sorted by variance explained by the task. A threshold was applied such that the top two percent of voxels were retained to generate a binarized relevant-voxel ROI mask for each region. This absolute statistical threshold was used as an objective means to equate for differences in sensitivity across subjects. The PPS was divided into two separate masks of task-positive and task-negative voxels, to distinguish portions of the PPS associated with language and the DMN, respectively. The spatially distinct task-positive and task-negative masks underwent the same thresholding procedure as the other ROIs.
For each participant, the relevant-voxel ROI masks were then registered to rest EPI space. For both task-residual and resting-state analyses, the time series was extracted from the active-voxel ROI masks in each participant’s native task and rest EPI space. The time series of each voxel in each mask was averaged and correlated between ROI pairs. Fisher’s r-to-z transformation was used to yield normalized functional connectivity values for each ROI pair.
2.5.5. Comparison of Task-Residual and Resting-State Data
We hypothesized that left-hemisphere lateralization of language and attention connections derived from task-residual data would be stronger than those derived from resting-state data. We compared task-residual and resting-state functional connectivity by calculating a "laterality difference index" (LDI) for each subject in IBM SPSS Statistics for Windows version 21.0. The LDI was calculated by the difference between z-normalized functional connectivity of left-hemisphere ROIs and their right-hemisphere homologues. Thus, the index represents the magnitude of difference between FC of the two hemispheres. For ROI pairs with a positive correlation, higher values indicate stronger left-hemisphere connectivity and values closer to or less than zero indicate weaker left-hemisphere connectivity, or stronger right-hemisphere connectivity, respectively. Conversely, for ROI pairs with a negative correlation, more negative values indicated stronger left-hemisphere connectivity.
Paired-samples tests were conducted to compare the LDI of resting-state and task-residual data. Although the LDI comparison provides an omnibus indication of whether a laterality difference exists, the relative contribution of each hemisphere is unknown. Hence, to better understand the contribution of the two hemispheres to the laterality comparison, both paired- and one-sample follow up tests were performed. The follow up analyses were performed to explain the magnitude and direction of connectivity contributing to the LDI results. These follow up analyses included 1) paired samples t-tests to compare FC between left- and right-hemisphere ROI pairs in both data types and 2) one-sample tests to characterize the nature of the laterality findings, by determining whether each intra-hemispheric ROI pair was significantly correlated.
3. Results
3.1. Comparison of Task-Residual and Resting-State Data
3.1.1. Network 1: Laterality of Language Connectivity
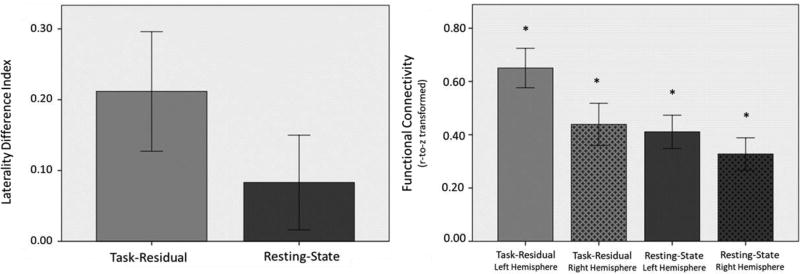
Based on normality test results from the Shapiro-Wilk test, a paired sample t-test was appropriate to compare resting-state and task-residual data. The task-residual time series between language ROIs (left IFG and left PPS task positive nodes) were positively correlated, consistent with their participation in the language network. Our laterality hypothesis was partially supported by the data. Results showed that in language ROIs, task-residual data demonstrated a trend toward stronger left-hemisphere laterality compared to resting-state data, as indicated by a higher positive LDI in task-residual data, p = 0.05 (Table 2; Fig. 3).
Table 2.
Comparison of task-residual and resting-state laterality difference indices
| Network Nodes | Task-residual | Resting state | |||
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | t | p | d | |
| Language LDI | 0.21 (0.39) | 0.08 (0.31) | −1.72 | 0.05† | 0.37 |
| Language-Attention LDI | −0.29 (0.39) | −0.07 (0.29) | −3.31 | 0.003* | 0.68 |
| Intention-Attention LDI | −0.09 (0.31) | 0.01 (0.24) | −1.85 | 0.04* | 0.36 |
p<0.05;
denotes a trend
Fig. 3.
(LEFT) Laterality of functional connectivity between language nodes, IFG and PPS, in task-residual and resting-state data. There was a trend that task-residual showed a greater LDI than resting-state data, p=0.05. (RIGHT) Functional connectivity of language nodes in each hemisphere based on task-residual and resting-state data. (*Significant at p < 0.05).
Follow up tests were conducted to clarify the contribution of the two hemispheres to the laterality findings. Paired samples t-tests revealed that in task-residual data, left-hemisphere language regions were significantly more correlated (M = 0.65, SD = 0.34) than their right-hemisphere homologues (M = 0.44, SD = 0.36), t(20) = 2.51, p = 0.02. Contrastingly, in resting-state data, no significant difference was found in functional connectivity between left-hemisphere language regions (M = 0.41, SD = 0.29) and their right-hemisphere homologues (M = 0.33, SD = 0.28), t(20) = 1.24 p = 0.23.
One sample t-tests, conducted to examine whether intra-hemispheric normalized correlations were significantly different than zero (Fig. 3), revealed that all intra-hemispheric correlations were significant: left-hemisphere task-residual data (M = 0.65, SD = 0.34), t(20) = 8.78, p = .000; right-hemisphere task-residual data (M = 0.44, SD = 0.36), t(20) = 5.55, p = .000; left-hemisphere resting-state data (M = 0.41, SD = 0.29), t(20) = 6.56, p = .000; right-hemisphere resting-state data (M = 0.33, SD = 0.28), t(20) = 5.39, p = .000. To summarize the follow up analyses, results show that although functional connectivity exists between language regions and their homologues in both task-residual and resting-state data, there is stronger connectivity in the left hemisphere in task-residual data but not resting-state data.
3.1.2. Network 2: Laterality of Language-Attention Connectivity
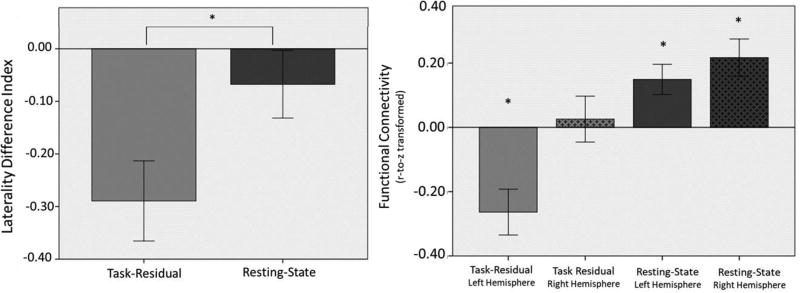
In addition to having language eloquent functions, areas of PPS are also associated with the DMN. This analysis examined the intersection between anterior language regions (IFG) and posterior language regions associated with the DMN (areas characterized by negative BOLD response of the PPS). Task-residual time series for these regions were anticorrelated, consistent with their involvement in task-positive vs. task-negative networks. Results supported our laterality hypothesis, in which task-residual data demonstrated stronger laterality than resting-state data as indicated by greater absolute value of the LDI in task-residual data, p = 0.003 (Table 2; Fig. 4).
Fig 4.
(LEFT) Laterality of functional connectivity between language-attention nodes, IFG and negative PPS, in task-residual and resting-state data. (RIGHT) Functional connectivity of language-attention nodes in each hemisphere based on task-residual and resting-state data. (*Significant at p < 0.05).
Follow up paired samples t-tests revealed that in task-residual data, left-hemisphere regions were more negatively correlated (M = −0.26, SD = 0.33) than their right-hemisphere homologues (M = 0.03, SD = 0.33), t(20) = −3.80, p = .001. In resting-state data, no significant difference was found in the functional connectivity between left- (M = 0.15, SD = 0.21) and right-hemisphere (M = 0.22, SD = 0.27) regions, t(20) = −1.06, p = 0.30. One sample t-tests conducted to examine whether intra-hemispheric normalized correlations were significantly different than zero (Fig. 4) revealed that the correlation between left-hemisphere task data was significant (M = −0.26, SD = 0.33), t(20) = −3.70, p = .001, but the correlation between right-hemisphere task data was not significant (M =0.03, SD = 0.33), t(20) = 0.36, p = .72. In resting-state data, both left and right intra-hemispheric correlations were positive and significant: left (M = 0.15, SD = 0.21), t(20) = 3.18, p = .005; right (M = 0.22, SD = 0.27), t(20) = 3.74, p = .001.
3.1.3. Network 3: Laterality of Intention-Attention Connectivity
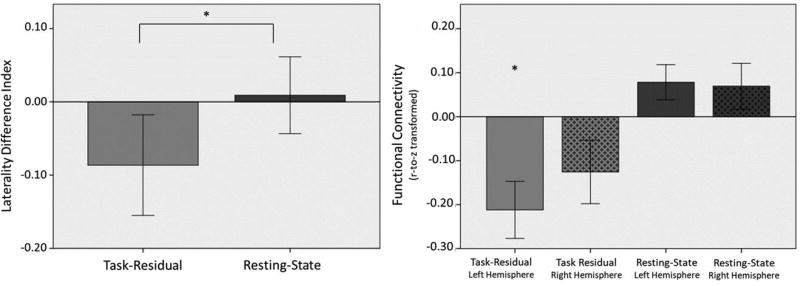
Task-residual time series for pre-SMA and PC/Pc were anti-correlated, consistent with their involvement in task-positive vs. task-negative networks. Consistent with our laterality hypothesis, a paired samples t-test showed that task-residual data demonstrated stronger left-hemisphere laterality than resting-state data, p = 0.04 (Table 2; Fig. 5). The intention-attention connections were more left-lateralized in task-residual data than in resting-state data, as indicated by greater absolute value of the LDI in task-residual data.
Fig. 5.
(LEFT) Laterality of functional connectivity between intention-attention nodes, preSMA and PC/Pc, in task-residual and resting-state data. (RIGHT) Functional connectivity of intention-attention nodes in each hemisphere based on task-residual and resting-state data. (*Significant at p < 0.05).
In spite of the significantly stronger LDIs for task-residual than resting-state data, follow up paired samples t-tests revealed that in task data, no significant difference was found in functional connectivity between left- (M = −0.21, SD = 0.30) and right-hemisphere (M = −0.13, SD = 0.33) intention-attention regions, t(20) = −1.26 p = 0.22. Similarly, in resting-state data, no significant difference was found in the functional connectivity between left (M = 0.08, SD = 0.18) and right (M = 0.07, SD = 0.23) hemisphere intention-attention regions, t(20) = 0.17 p = 0.87. One sample t-tests (Fig. 5) revealed that in task-residual data, left-hemisphere correlations were significant (M = −0.21, SD = 0.30), t(20) = −3.26, p = .004, but right-hemisphere correlations were not (M = −0.13, SD = 0.33), t(20) = −1.74, p = .097. In resting-state data, there were no significant differences from zero in the left-hemisphere correlations (M = 0.08, SD = 0.24), t(20) = 1.96, p = .064, or the right-hemisphere correlations (M = 0.07, SD = 0.24), t(20) = 1.33, p = .199. These results show that the greater laterality in task-residual data compared to resting-state data may be driven by the magnitude of the task-residual connectivity between the left pre-SMA and PC/Pc.
4. Discussion
Using transparent methods, we compared task-residual and resting-state approaches to characterize the FC of three network relationships. Task-residuals accentuated left-lateralized communication between intra-hemispheric nodes in both domain-specific and domain-general systems, suggesting that FC is altered during task engagement. The implications of these findings are discussed below.
4.1 Task-Residual Versus Resting-state Data
Our hypothesis that connectivity from task-residual data would be more lateralized (left-dominant) than resting-state data was partially supported. Nodes in the intention-attention network and language-attention network were more left-dominant in task-residual data compared to resting state data. However, language nodes showed a trend in the hypothesized direction.
Follow-up testing of language network laterality demonstrated that left-hemisphere regions were more strongly correlated than the right-hemisphere in task-residual data but not resting-state data. Despite significant right-hemisphere connectivity in both data types, left-hemisphere regions were still more tightly coupled than right in task-residual data. Although resting-state data showed significant FC in both hemispheres, it lacked the degree of network specificity that task-residual data afforded. Hence, laterality, one of the hallmarks of neural systems for language, is captured by task-residual comparisons but not by resting-state analyses. Overall, the data suggest that task-residuals highlight network specificity to a greater degree than in resting-state data, consistent with other context-dependent functional connectivity studies (Al-Aidroos et al., 2012).
The correlation between the anterior language region (IFG) and the posterior language region converging with DMN functions (characterized by negative activity in the posterior perisylvian region) had stronger laterality in task-residual data than resting-state data. In the follow-up comparisons, left-hemisphere correlations were significantly stronger than right in task-residuals but not resting-state data, implicating an upregulation of left-hemisphere functional connectivity and downregulation of irrelevant right-hemisphere connectivity. Again, task-residual data were more sensitive to lateralized relationship of frontal language cortices to lateral portions of the DMN than resting-state data. A possible explanation for this cross-network interaction is suppression of attentional mechanisms in the DMN so that subjects can focus on the demands of word generation in verbal fluency.
Task-based residual activity appears sensitive to functional interactions between task-relevant regions and may deemphasize functional interactions between task-irrelevant regions (Al-Aidroos et al., 2012; Davis-Thompson & Andrews, 2012; Norman-Haignere et al., 2012). One study involving face processing and visual attention (Al-Aidroos et al., 2012) reported that attending to faces strengthened the connectivity between occipital areas and the fusiform face area (FFA) but not between occipital areas and the parahippocampal place area (PPA), whereas attending to scenes strengthened the connectivity between occipital areas and the PPA but not between the occipital areas and the FFA. This pattern was observed despite robust responses from task-irrelevant areas. Task-residuals are sensitive to the functional interactions between task-relevant regions as opposed to regions that are evoked in response to a stimulus but do not interact.
Our study extends this line of research by exploring language and intention-attention networks. Although our study examined different networks, these investigations converge on the finding that task-based residuals can be used to feature interdependence between domain-specific regions. Additionally, the functional interdependence in task-residuals reflect synchronized activity in the context of a task even when time-invariant stimulus-evoked responses have been accounted for.
4.3 Study Limitations
It is possible that the trend detected in the language system did not reach significance due to a lack of statistical power. However, that the observed trend was in the hypothesized direction offers an empirical warrant for further investigation. Additionally, the use of both semantic and phonemic stimuli may have biased the selection of voxels in language cortices toward those more highly activated in both semantic and phonological processing than those activated more highly for one of these processes versus the other (Devlin et al., 2003). Thus, when averaging the response of active voxels in each ROI, the spatial distribution of activity reflected more heterogeneity than a single stimulus class would have, potentially reducing the coherence of correlations. Considering this possibility, however, the robustness of task-residual data in demonstrating the laterality expected in language processing is impressive.
4.4 Conclusion
The present findings indicate that task-residual data can be used to characterize specificity of network coherence. The degree to which domain-general and domain-specific regions share variance above and beyond the mean hemodynamic response during a task highlights context-relevant functional connectivity that is not present in resting-state data. Thus, task-residual data can offer complementary information to resting-state functional connectivity data. Future studies should continue to explore the sensitivity of this approach in detecting early age- or disease-related network alterations.
Highlights.
Task-residual functional connectivity captures synchrony between regions recruited for a task after removing task-evoked signal.
Task-residual data revealed greater network specificity than resting-state as measured by functional connectivity of left-hemisphere language regions and right-hemisphere homologs.
Reduced functional connectivity in domain-general attention regions is associated with reduced opposing coherence between task-positive and task-negative regions.
Acknowledgments
The authors would like to thank Stephen Towler and Dr. Gwen Frishkoff for training and helpful input.
Funding Sources
Behavioral and neuroimaging data for this project were collected by Dr. James at the University of Arkansas for Medical Sciences (UAMS) via funding from the UAMS Translational Research Institute (TRI) through grants UL1TR000039 and KL2TR000063 from the NIH National Center for Research Resources and the National Center for Advancing Translational Sciences. This work was also supported by the following grants from the US Department of Veterans Affairs Rehabilitation Research and Development Service: Grant # B6364L, Senior Research Career Scientist Ward to BC; Grant # E0596W, Effects of Exercise Intervention on Aging Related Motor Decline to KMM; a Georgia State Language and Literacy fellowship to SMT. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Archives of Neurology. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. https://doi.org/10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aidroos N, Said CP, Turk-Browne NB. Top-down attention switches coupling between low-level and high-level areas of human visual cortex. Proceedings of the National Academy of Sciences. 2012;109(36):14675–14680. doi: 10.1073/pnas.1202095109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Marshall JC, Shah NJ, Fink GR, Zilles K. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space—The roles of Brodmann areas 44 and 45. NeuroImage. 2004;22(1):42–56. doi: 10.1016/j.neuroimage.2003.12.031. https://doi.org/10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where Is the Semantic System? A Critical Review and Meta-Analysis of 120 Functional Neuroimaging Studies. Cerebral Cortex (New York, NY) 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Bucker RL, Colcombe S, Dogonowski A, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li S-J, Lin C-P, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng G, Veijola J, Villringer A, Walter M, Wang L, Weng X-C, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang Y-F, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato MA, Crosson B, Gökçay D, Soltysik D, Wierenga C, Gopinath K, Himes N, Belanger H, Bauer RM, Fischler IS, Gonzales-Rothi L, Briggs RW. Processing Words with Emotional Connotation: An fMRI Study of Time Course and Laterality in Rostral Frontal and Retrosplenial Cortices. Journal of Cognitive Neuroscience. 2004;16(2):167–177. doi: 10.1162/089892904322984481. https://doi.org/10.1162/089892904322984481. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chan MY, Park DC, Savalia NK, Petersen SE, Wig GS. Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences. 2014;111(46):E4997–E5006. doi: 10.1073/pnas.1415122111. http://doi.org/10.1073/pnas.1415122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Davey J, Cornelissen PL, Thompson HE, Sonkusare S, Hallam G, Smallwood J, Jefferies E. Automatic and Controlled Semantic Retrieval: TMS Reveals Distinct Contributions of Posterior Middle Temporal Gyrus and Angular Gyrus. The Journal of Neuroscience. 2015;35(46):15230–15239. doi: 10.1523/JNEUROSCI.4705-14.2015. http://doi.org/10.1523/JNEUROSCI.4705-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies-Thompson J, Andrews TJ. Intra- and interhemispheric connectivity between face-selective regions in the human brain. Journal of Neurophysiology. 2012;108(11):3087–3095. doi: 10.1152/jn.01171.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience. 2003;15(1):71–84. doi: 10.1162/089892903321107837. http://doi.org/10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NUF, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LK, Regina ACB, Kovacevic N, Martin M da GM, Santos PP, Carneiro C de G, Kerr DS, Amaro E, McIntock AR, Busatto GF. Aging Effects on Whole-Brain Functional Connectivity in Adults Free of Cognitive and Psychiatric Disorders. Cerebral Cortex. 2015 doi: 10.1093/cercor/bhv190. bhv190. http://doi.org/10.1093/cercor/bhv190. [DOI] [PubMed]
- Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proceedings of the National Academy of Sciences. 2012;109(31):12788–12793. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Coherent spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nature Neuroscience. 2006;9(1):23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic Fluctuations within Cortical Systems Account for Intertrial Variability in Human Behavior. Neuron. 2007;56(1):171– 184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Gess JL, Fausett JS, Kearney-Ramos TE, Kilts CD, James GA. Task-dependent recruitment of intrinsic brain networks reflects normative variance in cognition. Brain and Behavior. 2014;4(5):650–664. doi: 10.1002/brb3.243. https://doi.org/10.1002/brb3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex (New York, NY) 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, Moeller S, Xu Jungian, Yacoub E, Baselli G, Uqurbil K, Miller KL, Smith SM. ICA-based artefact and accelerated fMRI acquisition for improved Resting State Network imaging. NeuroImage. 2014;95:232–247. doi: 10.1016/j.neuroimage.2014.03.034. http://doi.org/10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E, Goldberg ME. Comprehensive Physiology. John Wiley & Sons, Inc; 2011. Attention: Behavior and Neural Mechanisms. https://doi.org/10.1002/cphy.cp010511. [Google Scholar]
- Humphreys GF, Lambon Ralph MA. Fusion and Fission of Cognitive Functions in the Human Parietal Cortex. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu198. bhu198. http://doi.org/10.1093/cercor/bhu198. [DOI] [PMC free article] [PubMed]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. NeuroImage. 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Saad W, Simmons K, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52(2):571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. Journal of Neuroscience Methods. 2010;189(2):233–245. doi: 10.1016/j.jneumeth.2010.03.028. http://doi.org/10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La C, Garcia-Ramos C, Nair VA, Meier TB, Farrar-Edwards D, Birn R, Meyerand ME, Prabhakaran V. Age-related changes in BOLD activation pattern in phonemic fluency paradigm: an investigation of activation, functional connectivity and psychophysiological interactions. Frontiers in Aging Neuroscience. 2016;8:110. doi: 10.3389/fnagi.2016.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to Intention. Science. 2004;303(5661):1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Lee MH, Smyser CD, Shimony JS. Resting-State fMRI: A Review of Methods and Clinical Applications. American Journal of Neuroradiology. 2013;34(10):1866–1872. doi: 10.3174/ajnr.A3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor KM, Patten C, Kleim JA, Crosson B, Butler AJ. Effects of aerobic fitness on aging-related changes of interhemispheric inhibition and motor performance. Frontiers in Aging Neuroscience. 2013;5:66. doi: 10.3389/fnagi.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Wydell H, O’Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. NeuroImage. 2007;36:T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau SE, Hammond E, Williamson DJ, Crosson B. Resting and stimulated states in functional imaging studies: Evidence of differences in attentional and intentional set. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1997;10:162–163. [PubMed] [Google Scholar]
- Norman-Haignere SV, McCarthy G, Chun MM, Turk-Browne NB. Category-Selective Background Connectivity in Ventral Visual Cortex. Cerebral Cortex (New York, NY) 2012;22(2):391–402. doi: 10.1093/cercor/bhr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers BP, Gore JC. Empirical Comparison of Sources of Variation for FMRI Connectivity Analysis. PLoS ONE. 2008;3(11):e3708. doi: 10.1371/journal.pone.0003708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiology of Aging. 2010;31(8):1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. https://doi.org/10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic Denoising of Functional MRI Data: Combining Independent Component Analysis and Hierarchical Fusion of Classifiers. NeuroImage. 2014;90:449–468. doi: 10.1016/j.neuroimage.2013.11.046. http://doi.org/10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Supplement 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M, Phillips C, Soddu A, Luxen A, Moonen G, Laureys S. Two Distinct Neuronal Networks Mediate the Awareness of Environment and of Self. Journal of Cognitive Neuroscience. 2010;23(3):570–578. doi: 10.1162/jocn.2010.21488. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li CR. A neural measure of behavioral engagement: task-residual low-frequency blood oxygenation level-dependent activity in the precuneus. NeuroImage. 2010;49(2):1911–1918. doi: 10.1016/j.neuroimage.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatar ZZ, Towler S, McGregor KM, Dzierzewski JM, Bauer A, Phan S, Crosson B. Functional language networks in sedentary and physically active older adults. Journal of the International Neuropsychological Society: JINS. 2013;19(6):625–634. doi: 10.1017/S1355617713000246. [DOI] [PMC free article] [PubMed] [Google Scholar]