Abstract
Budesonide, a synthetic glucocorticoid used for treating asthma, and pioglitazone, a synthetic peroxisome proliferator-activated receptors γ ligand used for the treatment of diabetes, were evaluated for their combinational chemopreventive efficacy on mouse lung cancer using female A/J mice with benzo(a)pyrene used as the carcinogen. All chemopreventive treatments began 2-wk post-carcinogen treatment and continued daily for 20 wk. Budesonide was administered by the aerosol route using an improved aerosol delivery system. Pioglitazone was introduced by oral gavage. The characterization of drug distribution showed that budesonide introduced by aerosol delivery accumulated only in the lung. Budesonide alone reduced tumor load by 78% and pioglitazone alone reduced tumor load by 63%. By combining aerosolized budesonide with pioglitazone, the inhibition on tumor load was 90%. In vitro experiments using human cancer cells showed that budesonide and pioglitazone exhibited independent, additive inhibitory effects on cell growth. Our results provide evidence that aerosolized budesonide and oral pioglitazone could be a promising drug combination for lung cancer chemoprevention.
Keywords: budesonide, pioglitazone, mouse, lung tumorigenesis, chemoprevention
INTRODUCTION
Lung cancer is the most common form of cancer in the world, and the leading cancer-related cause of death in both men and women in the United States in 2009 [1]. Despite improvements in traditional therapies, such as surgery, radiation, and chemotherapy, over 85% of lung cancer patients die from their disease [1]. In addition to curative approaches to lung cancer, alternative approaches involving chemoprevention, defined as intervention with natural or synthetic compounds in the early precancerous stages of carcinogenesis, have been proposed [2]. Target populations for chemoprevention include those who are at high risk of developing lung cancer, for example, current and former smokers. Cigarette smoke is a complex mixture of carcinogens and toxicants. Thus, lung cancer caused by years of smoking is unlikely to be prevented through intervention in a single pathway. Combinations of drugs that act through different mechanisms may achieve greater chemopreventive efficacy.
Inhalation is widely accepted as the optimal route of administration in treating lung diseases. In comparison to systemic means of administration, drugs can be delivered directly to the target tissue resulting in better efficacy and at lower doses resulting in decreased toxicity [3]. Aerosol delivery of therapeutic drugs for lung cancer in humans has been reported to be an effective route of delivery with little systemic distribution [4,5]. Our previous study in mice using aerosolized polyphenon E showed similar inhibitory effects on benzo(a)pyrene (B(a)P)-induced lung tumorigenesis using a lower dose level when compared to oral administration [6]. No systemic toxicity or weight loss was observed during the experiments.
Budesonide is a synthetic glucocorticoid steroid used frequently for the treatment of asthma. Aerosol delivery of budesonide inhibited all stages of progression from hyperplasia formation to cancer in B(a)P-induced mouse lung carcinogenesis without systemic toxicity [7,8]. Gene expression analysis indicates that the chemopreventive effects of budesonide involve altered expression of genes in multiple signaling pathways [9]. By combining aerosolized budesonide with R115777 (ZarnestraMT, Tipifarnib) or myo-inositol, greater inhibition was achieved [10,11].
The peroxisome proliferator-activated receptors γ (PPARγ) is a member of the nuclear hormone receptor superfamily of ligand-activated transcription factors. It has a critical role in the regulation of multiple cellular processes including lipid metabolism and differentiation [12–14]. PPARγ expression was increased in non-small cell lung cancer (NSCLC) and PPARγ expression correlated with lung cancer histologic type and grade [15]. PPARγ is a molecular target for thiazolidinediones (TZDs), such as troglitazone, rosiglitazone, and pioglitazone. Pioglitazone and rosiglitazone are synthetic PPARγ ligands used clinically to treat type II diabetes. Recent epidemiological studies indicated a significant decrease in lung cancer risk in patients receiving TZDs to treat diabetes, suggesting that TZDs may have chemopreventive effects on lung cancer [12]. The mechanisms of the inhibition effect of TZDs on NSCLC were reviewed recently [16]. Troglitazone has been shown to inhibit the growth of NSCLC cells in vitro and in vivo [17,18]. Pioglitazone has a favorable safety profile compared to other TZDs in regards to cardiovascular side effects [19]. This is a strong rationale for testing pioglitazone versus rosiglitazone or troglitazone [20,21]. Pioglitazone-induced apoptosis and inhibited tumor growth in xenograft models of lung cancer [17]. In a lung adenocarcinoma model using mice, Wang et al. [22] found that oral dose of pioglitazone-inhibited tumor load by 50% or higher.
Both budesonide and pioglitazone have shown good inhibition effects in lung cancer using animal models without causing systemic toxicity or weight loss, which indicates they are good candidates for chemoprevention study. Here, the chemopreventive effects of the combination of pioglitazone and aerosolized budesonide for the inhibition of B(a)P-induced lung tumorigenesis in A/J mice was studied and compared with single agents. Our results showed that the combination of the two drugs inhibited tumor growth significantly.
MATERIALS AND METHODS
Reagents and Animals
Ethanol, dimethyl sulfoxide, polyethylene glycol 400, B(a)P (99% pure), tricaprylin, and budesonide were purchased from Sigma–Aldrich (St. Louis, MO). B(a)P was prepared just before use by dissolving in tricaprylin. Female A/J mice at 6 wk of age were obtained from Jackson Laboratories (Bar Harbor, ME). The use of animals was approved by the Washington University’s Institutional Animal Care and Use Committee.
Animal Experiments
Female A/J mice were given a single intraperitoneal dose of B(a)P (100 mg/kg body weight) in 0.2 ml of tricaprylin at 8 wk of age. The mice were housed at a constant temperature and humidity, and received a standard diet and water. Two weeks after B(a)P injection, the mice were randomly divided into six groups with 12 mice per group: (i) solvent control group (50% dimethyl sulfoxide in ethanol); (ii) gavage control group (polyethylene glycol: 0.5 g/L carboxy-methylcellulose in PBS = 1:1); (iii) solvent and gavage control group; (iv) budesonide group (2.25 mg/ml); (v) pioglitazone group (10 mg/kg body weight); (vi) budesonide and pioglitazone group. Budesonide was administered by aerosol delivery while pioglitazone was introduced by oral gavage. All treatments were started 2 wk after B(a)P, and then continued for 20 wk. Solutions were freshly prepared prior to use. The inhalation exposures were conducted 2 min/d and 5 d/wk using a custom-made nose-only exposure chamber. The design of the chamber ensures that the mice place their noses into the cone of each exposure port in the chamber. Mice were randomly placed into exposure ports to minimize any bias inside the chamber. Mice in gavage control groups and pioglitazone-treated groups received oral gavage 5 d/wk. The body weights of the mice were measured every week for the treatment duration. Mice were sacrificed by CO2 asphyxiation 22 wk after the exposure to the carcinogen. Lungs from each mouse were fixed in Tellyesniczky’s solution [23] overnight, followed by 70% ethanol. The fixed lungs were evaluated under a dissecting microscope to obtain fixed surface tumor count and individual tumor size. Tumor volume (V) was calculated using tumor radius (r) based on the formula: V (mm3) = 4πr3/3 [23]. The total tumor volume in each mouse was calculated by the sum of all tumor volumes. Tumor load was determined by averaging the total tumor volume of each mouse in each group. Tumor multiplicity and tumor load were analyzed by one-way analysis of variance followed by the Tukey’s multiple comparisons to determine differences in the number and in the size of lung tumors per mouse between groups. The level of statistical significance was set at P < 0.05.
Aerosol Procedure
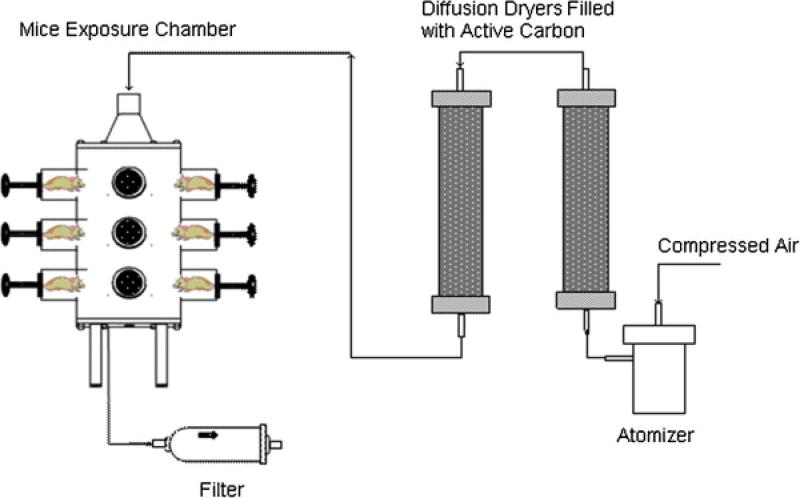
Budesonide was dissolved in a 50:50 ethanoldimethyl sulfoxide solution and atomized into droplets by the custom-made Collison Type atomizer. The rate of carry air flow outputs from the atomizer was 2.27 L/min. The aerosol stream was passed through two diffusion columns, having active carbon to remove organic solvents in droplets. Resulting dry aerosol stream was then introduced into the nose-only exposure chamber from the inlet located on the top of the chamber. Effluent aerosol was discharged from an opening at the bottom of the chamber. The schematic diagram of the aerosol delivery system is shown in Figure 1.
Figure 1.
Schematic diagram of aerosol delivery system. A custom-built atomizer was used to generate budesonide droplets. Aerosol flow was then passed through two diffusion dryers containing active carbon to remove dimethyl sulfoxide and ethanol. The resulting dry aerosol flow of budesonide particles was introduced into the nose-only exposure chamber from the top inlet. Effluent aerosol was discharged from an opening at the bottom of the chamber. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The size distribution of the aerosol generated by the above system was determined by Scanning Mobility Particle Sizer (SMPS) spectrometer, which includes an Electrostatic Classifier (TSI model 3080), a Differential Mobility Analyzer (DMA, TSI model 3081) and an Ultrafine Condensation Particle Counter (UCPC, TSI model 3025). The geometric median diameter (GMD), mass median diameter (MMD), geometric standard deviation (GSD), and particle concentration were obtained. Online samples were taken inside the chamber right at the locations where mice inhaled aerosols. No spatial difference in the particle concentration was found at different sample points in the aerosol delivery chamber.
Assuming that all the drug particles could completely deposit in the mouse’s lung once they were inhaled, the dose of aerosolized budesonide was estimated as follows [8]:
where Caerosol is the aerosol mass concentration (mg/L) which was measured by the SMPS system, RMV is the respiratory minute volume of the mouse (0.025 L/min, based on Guyton’s formula [24]), t is the exposure time (2 min), and Mbody is the body weight which was taken to be 0.020 kg. However, in reality, the deposition ratio is always <1, so the actual dose is less than the estimate value.
Tissue Assay Method
Lung, spleen, and liver tissues were weighed and homogenized in 200 µl dimethyl sulfoxide. The sample was vortexed for 2 min and centrifuged at 13,000 rpm for 15 min at 4°C. Supernatants were transferred and dried under nitrogen gas stream. Residues were reconstituted in 0.05 ml solvent and centrifuged. The supernatants were analyzed by high-performance liquid chromatography (HPLC). The serum was extracted as above for lung tissue homogenate.
Drug concentrations in lung and serum samples were determined by HPLC. The HP 1100 series HPLC system consisted of an autosampler, a binary pump, a column compartment, and a diode array detector (Agilent Tech, Santa Clara, CA). The HPLC column was 4.6 × 150 mm2 Zorbax SB-C18 3.5 µm column, with a 4.6 × 12.5 mm2 ZORBAX Eclipse XDB-C18 5-µm guard cartridge. The flow rate of the mobile phase was 1 ml/min. The mobile phase was phosphate buffer and acetonitrile, 45:55 for budesonide and 70:30 for pioglitazone. The column temperature was maintained at room temperature (~20°C).
Cell Proliferation Assays
Human NSCLC cell lines A549 and H1299 were purchased from American Type Culture Collection (Manassas, VA), and cultured in RPMI 1640 media with 10% fetal bovine serum (Gibco®, Invitrogen, Carlsbad, CA) and penicillin and streptomycin cocktail (Gibco). All cells were cultured in a humidified incubator at 37°C at 5%CO2. A549 and H1299 cells were seeded in 96-well plates (BD Falcon®, Franklin Lakes, NJ) at a density of 2 × 103 cells per well. Twenty-four hours after seeding, cells were exposed to different concentrations of budesonide, pioglitazone or the combination as indicated for 24, 48, or 72 h. Proliferation rate was measured by Alamar Blue, a cell viability indicator with resazurin as its active ingredient, which could be converted to the fluorescent molecule, resorufin, by active cells [25]. Alamar Blue (Invitrogen, Carlsbad, CA) was added to the culture media at 10% of the media volume during the last 10 h of the exposure period. Fluorescence was detected on Synergy HT microplate reader (Biotek, Winooski, VT) using an excitation wavelength of 544 nm and an emission wavelength of 590 nm.
Combination Effects Analysis
The linear model was used to test if there were combination effects of budesonide and pioglitazone and the type of combination effects (i.e., antagonism, additivity, and synergy). The model is:
where y is live cell numbers for the kth cell line and the lth replication, l = 1, 2, or 3; B is Budesonide effect, i = 1 or 0; P is Pioglitazone effect, t = 1 or 0; C represents the difference of two cell lines, k = 1 for A549 and 0 for H1299; e is an error term. The statistical analyses were performed in R (www.rproject. org).
RESULTS
Combination of Aerosolized Budesonide and Oral Pioglitazone in Chemoprevention of Lung Carcinogenesis in A/J Mice
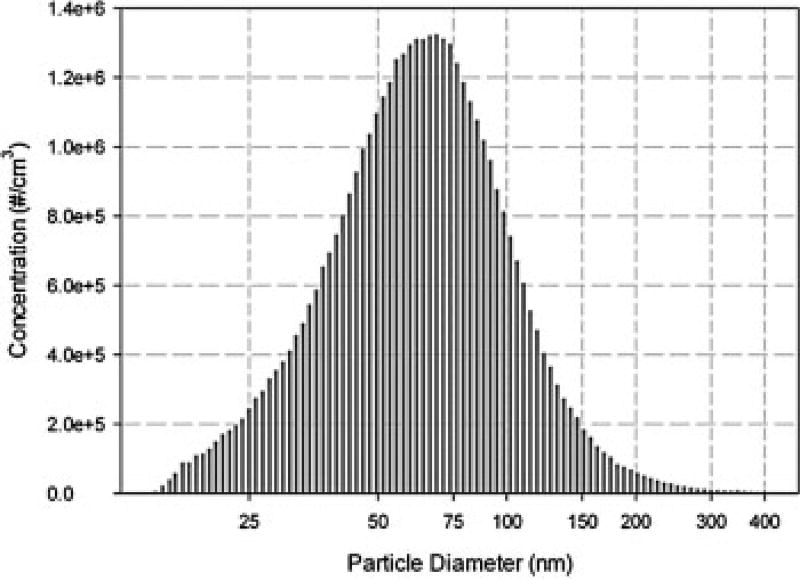
We first determined the aerosol characteristics of budesonide generated by our aerosol delivery system. The custom-made Collison type atomizer could provide stable aerosol size distribution, which ensured that the mice received the same dose everyday throughout the experiment. The size distribution of budesonide particles generated by the atomizer is shown in Figure 2. The GMD was 0.078 µm, and the GSD was 1.7. The MMD was 0.12 µm. The aerosol mass concentration Caerosol was 83.7 µg/L. No difference was found in GMD, GSD, and MMD among measurements at different sample points inside the exposure chamber, indicating that the drug particles were uniformly distributed in the chamber.
Figure 2.
Size distribution of budesonide particles in exposure chamber. The diameter of budesonide particles ranged from 0.01 to 0.4 µm, which was in the favorable ranges for mice inhalation study [29].
Mice were treated with aerosolized budesonide for 20 wk. The initial dose of budesonide was estimated to be about 209 µg/kg body weight. The body weight in all budesonide-treated groups decreased slowly during the first 7 wk of treatment. The average body weight in budesonide-treated groups was about 8% less than the corresponding control group by the end of 7th week. As a result, we decreased the dose of budesonide by 25% (about 157 µg/kg body weight) for the duration of treatment. After the dose adjustment, body weight increased in budesonide-treated mice, indicating that the budesonide was well-tolerated at this dose. There were no significant body weight differences between budesonide-treated groups and their corresponding control groups at the end of the experiment. Pioglitazone also had no obvious effects on body weight.
B(a)P induced an average of 5.0 ± 2.0 (n = 12) tumors per mouse in the solvent control group. Average tumor load in the control group was 0.30 ± 0.22 mm3. Aerosolized budesonide significantly decreased tumor multiplicity by 57% and tumor load by 78% when compared to the solvent control group (Table 1).
Table 1.
Effects of Combined Budesonide and Pioglitazone on Lung Tumorigenesis in Female A/J Mice
| Group | Final body weighta,b (g) |
Tumor multiplicitya |
Inhibition | Tumor loada (mm3) |
Inhibition | Percentage of tumors (<0.5 mm) |
|---|---|---|---|---|---|---|
| Solvent Control | 22.3 ± 1.2 | 5.00 ± 2.00 | — | 0.30 ± 0.22 | — | 28.3 |
| Budesonide | 20.0 ± 2.1 | 2.17 ± 1.64 | 56.7%* | 0.07 ± 0.06 | 78.4%* | 42.3 |
| Gavage control | 20.9 ± 1.9 | 4.33 ± 1.63 | — | 0.36 ± 0.33 | — | 23.1 |
| Pioglitazone | 23.0 ± 2.5 | 4.08 ± 2.11 | 5.8% | 0.13 ± 0.10 | 63.3%* | 50.8 |
| Solvent and Gavage control | 20.1 ± 2.5 | 4.70 ± 2.41 | — | 0.26 ± 0.24 | — | 39.2 |
| Budesonide and pioglitazone | 19.2 ± 1.7 | 2.09 ± 1.45 | 55.5%*,† | 0.03 ± 0.02 | 89.6%*,† | 87.0 |
Data shown as mean ± SD.
Average body weight after 20 wk treatment (22 wk after B(a)P injection).
P < 0.05 versus control.
P < 0.01 versus pioglitazone group.
Pioglitazone was given by oral gavage at a dose of 10 mg/kg body weight. When compared to the gavage control group, no inhibitory effects on tumor number were observed, while the tumor load was significantly decreased by 63%.
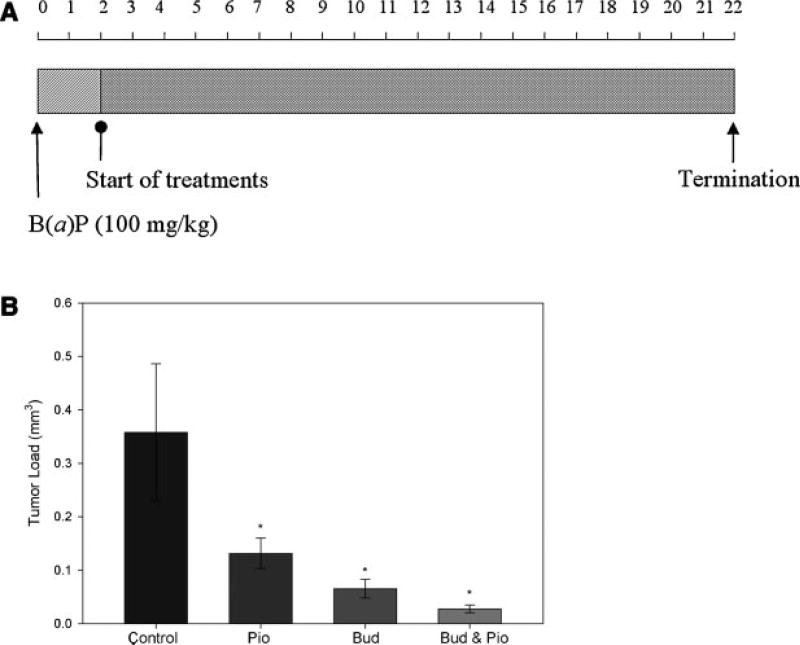
Pioglitazone was also studied for its chemoprevention effects when combined with aerosolized budesonide. We observed a 90% inhibition in tumor load compared to the corresponding control group. Eighty-seven percent of all the tumors observed in the combination group were smaller than 0.5 mm in diameter, compared to 42% in budesonide-treated group and 51% in pioglitazone-treated group. The combination of pioglitazone and budesonide did not significantly affect tumor multiplicity compared to budesonide alone. The results are summarized in Table 1 and Figure 3.
Figure 3.
A: Treatment protocol. treatments began 2 wk after i.p. injection of B(a)P. The solvent control groups and budesonide-treated groups received aerosol treatment for 2 min/d, 5 d/wk. The gavage control groups and both pioglitazone-treated groups received oral gavage 5 d/wk. Treatment duration was 20 wk. All mice were sacrificed 22 wk after the B(a)P injection. B: Effects of budesonide (bud) and pioglitazone (pio), individually introduced or in combination, on lung tumorigenesis in female A/J mice. *P < 0.05. Bar, SEM.
Tissue distributions of drugs were also examined. At the end of the study, four mice from each group were randomly selected and the left lobe of the lung was homogenized to analyze agent concentration. Serum, liver, and spleen tissue were obtained for the same purpose. Tissue concentrations of each agent are summarized in Table 2. The concentration of budesonide was 2.7 µg/g of lung. Budesonide was not detected in serum, liver, or spleen tissue via HPLC. Assuming that there were no budesonide accumulated in other unanalyzed organs, the total concentration of budesonide in mice was thus estimated to be 20.25 µg/kg body weight. In contrast to aerosolized budesonide, orally administered pioglitazone was detected in all tissues analyzed. Serum concentrations of pioglitazone were significantly higher than in lung tissue. The difference in drug distribution observed between aerosol and oral delivery indicates that aerosol delivery achieved an elevated lung tissue concentration while minimizing systemic drug levels.
Table 2.
Chemopreventive Agent Concentrations After 20 Wk of Treatment
| Concentrations ina | |||||
|---|---|---|---|---|---|
|
|
|||||
| Groups | Drug | Lung (µg/g) | Blood (µg/ml) | Liver (µg/g) | Spleen (µg/g) |
| Budesonide | Budesonide | 2.66 ± 0.17 | < 0.1 | < 0.1 | < 0.1 |
| Pioglitazone | Pioglitazone | 2.51 ± 0.41 | 5.24 ± 2.6 | 1.50 ± 0.18 | 3.25 ± 0.45 |
| Budesonide and pioglitazone | Budesonide | 2.73 ± 0.35 | < 0.1 | < 0.1 | < 0.1 |
| Pioglitazone | 1.44 ± 0.17 | 4.03 ± 2.01 | 1.62 ± 0.21 | 4.40 ± 0.53 | |
Data shown as mean ± SD.
An Additive Inhibitory Effect of Budesonide and Pioglitazone on Proliferation of Human Lung Cancer Cells
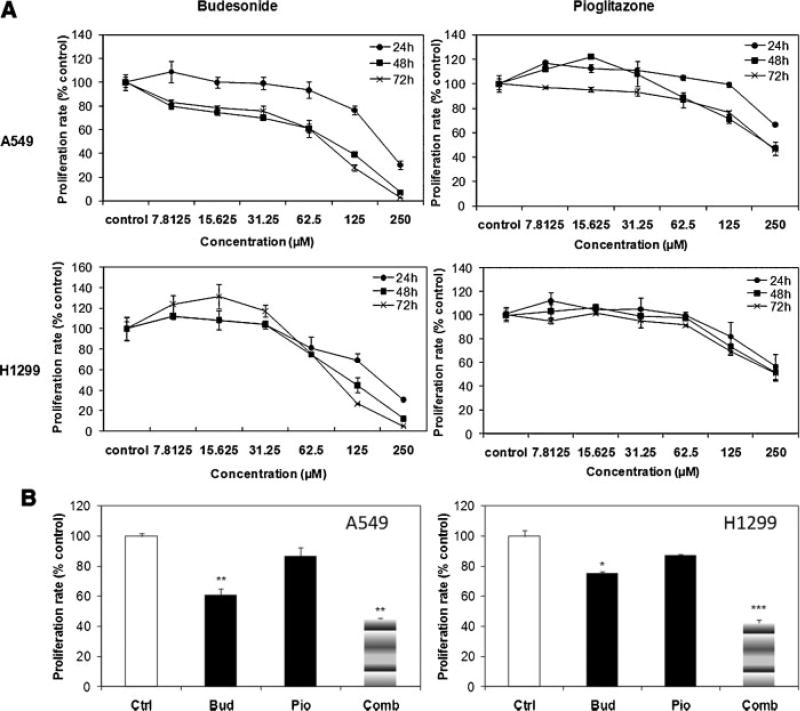
Human non-small lung cancer cells A549 and H1299 were exposed to different concentrations of budesonide or pioglitazone. Proliferation of A549 and H1299 cells decreased significantly following budesonide treatment in a dose-, and time-dependent manner. The proliferation was 3%, 28%, and 64% of control with 250, 125, and 62.5 µMof budesonide in A549 cells, and 5%, 27%, and 75% of control in H1299 cells. Pioglitazone at 250 µM inhibited cell proliferation by 50% but lower concentrations were ineffective, indicating low-efficacy of pioglitazone in inhibiting the proliferation of these cells (Figure 4A).
Figure 4.
An additive combined effect of budesonide and pioglitazone in inhibition of cell proliferation of human lung cancer cells. (A) Budesonide inhibits the proliferation of human non-small lung cancer cells in a dose- and time-dependent manner. (B) Combination (Comb) of budesonide (Bud) and pioglitazone (Pio) additively inhibits the proliferation of human non-small lung cancer cells.
When cells were treated with the combination of budesonide and pioglitazone, a strong growth-inhibition was observed (Figure 4B). 62.5 µM pioglitazone had no obvious effect on proliferation, while 62.5 µM budesonide inhibited the proliferation to around 60% in A549 cells, and 75% in H1299 cells. When 62.5 µM pioglitazone and budesonide were combined (at the same doses as in individual dosing), proliferation rates were decreased to approximately 40% in both lines. To further demonstrate the combination effects of these two agents, the linear model was used, as shown in Table 3, both budesonide and pioglitazone have significant effects in inhibiting cell growth (P < 0.005), but there is no agent interaction effect. Thus, the two agents exhibit independent, additive inhibitory effects on cell growth.
Table 3.
The Estimation of Combinational Effects of Budesonide and Pioglitazone
| Estimate | SE | t-value | Pr (>|t|) | |
|---|---|---|---|---|
| Budesonide | −3150.5 | 414.3 | −7.605 | 1.76e-8* |
| Pioglitazone | −1462.7 | 414.3 | −3.531 | 0.00136† |
| Cell | 4207.9 | 295.1 | 14.258 | 6.70e-15* |
| Budesonide:Pioglitazone | −496.2 | 625.1 | −0.761 | 0.45269 |
P < 0.001.
P < 0.005.
DISCUSSION
Chemoprevention using combinations of agents targeting different pathways may offer significant advantages compared to single agents by increasing efficacy. In addition, this approach may allow the use of lower doses with the potential to reduce adverse side effects. Budesonide inhibits all stages of lung tumorigenesis induced by B(a)P in the A/J mouse model [7,8] and is a good candidate for combinational studies [10,11]. Pioglitazone acts via distinct mechanisms [26–28] and was selected as a combination agent with aerosolized budesonide. Using a post-initiation protocol, we determined the effect of combining pioglitazone and budesonide on lung adenoma prevention in A/J mice. Significant inhibition of tumor load was observed compared to either agent alone.
In this study, budesonide was introduced through aerosol delivery using an aerosol generation and exposure system which could ensure that mice received constant dose of drug throughout the experiment [6]. Since mice have much smaller respiratory tract than human, the optimal particle size for mice inhalation studies is <0.3 µm [29]. In our study, the diameter of budesonide particles ranged from 0.01 to 0.4 µm, with the mass median diameter at 0.12 µm(Figure 2), which is more favorable for aerosolized drug delivery in mouse model compared with previous studies in which nebulizers was used for generating aerosolized agents [30,31].
After 20 wk treatment, aerosolized budesonide alone inhibited tumor multiplicity by 57% and tumor load by 78%, similar to what has been observed previously [8]. As expected, aerosol delivery achieved high budesonide concentrations in lung tissue but undetectable amounts in other tissues tested. Thus, by delivering agent particles directly to the mouse lung, the systemic dose could be significantly reduced without affecting efficacy. This in turn should reduce the risk of side effects and minimize systemic toxicity. Since chemoprevention targets healthy people with a high risk of developing lung cancer, efficacy and safety are two key criteria for the selection of chemopreventive agents. Compared to other means of administration, aerosol delivery has distinct advantages in satisfying the above two criteria.
Pioglitazone is a synthetic PPARγ ligand which has been approved by the Food and Drug Administration (FDA) for the treatment of type II diabetes. PPARγ agonists have anticancer effects on cell lines by binding with PPARγ receptor which then forms an active heterodimer with the retinoid X receptors [32]. After 20-wk administration of pioglitazone by oral gavage, the number of tumors in the pioglitazone treatment group was not affected. In contrast, tumor load was decreased by 63%.
When combined with budesonide, pioglitazone did not enhance the inhibition of tumor multiplicity compared to the budesonide-treated group. However, tumor load in the combination group was decreased by ~90% compared to its corresponding control group. Approximately 87% of total tumors were smaller than 0.5 mm in diameter in the combination group, compared to 51% in pioglitazone group and 42% in budesonide group. Large tumors are significantly inhibited in combination group compared to either single agent. The in vitro study using A549 and H1299 cells showed that the combination of budesonide and pioglitazone inhibited cell proliferation in both cell lines, while single agent had less or no obvious effects on proliferation. When the two agents were introduced at the same time, no antagonism effects were observed in both animal models and cell lines. Budesonide and pioglitazone have different mechanisms in inhibiting lung cancer [9,12,16]. The combination effects of budesonide and pioglitazone were shown to be independent and additive on cell growth by using a linear model, which indicated that the two agents functioned individually in inhibiting lung tumorigenesis and the overall inhibition was increased due to the additive effect.
The two drugs tested here for their efficacy on lung cancer chemoprevention, budesonide, and pioglitazone, are approved by the FDA to be used on human. In this study, both drugs were introduced in the same ways as they have been using to treat certain diseases—budesonide was introduced through inhalation, while pioglitazone was given orally. Our results showed that by combining those two drugs, the tumor number and tumor load was reduced by 56% and 90%, respectively. The combination of aerosolized budesonide and oral pioglitazone might be a good candidate for clinical trials.
In summary, aerosolized budesonide inhibited lung adenoma formation. Furthermore, aerosol delivery can achieve high concentration in target lung with low chemopreventive agent dose compared to other administration routes. Pioglitazone showed inhibition of tumor load while had no effect on tumor multiplicity. By combining aerosolized budesonide with oral pioglitazone, the tumor load of test mice could be further reduced. In vitro experiments using human cancer cells demonstrate that budesonide and pioglitazone acted dose-responsively and independently to exert a additive inhibitory effect on cell growth. Our result shows that the combination of agents targeting different pathways could be a promising approach in lung cancer chemoprevention.
Acknowledgments
We thank Drs. Haris Vikis, Michael James, and Jay Tichelaar for their critical comments and suggestions on the manuscript.
Abbreviations
- B(a)P
benzo(a)pyrene
- PPARγ
peroxisome proliferator-activated receptors γ
- NSCLC
non-small cell lung cancer
- TZDs
thiazolidinediones
- SMPS
scanning mobility particle sizer
- DMA
differential mobility analyzer
- UCPC
ultrafine condensation particle counter
- GMD
geometric median diameter
- MMD
mass median diameter
- GSD
geometric standard deviation
- HPLC
high-performance liquid chromatography
- FDA
the Food and Drug Administration
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Hecht SS, Kassie F, Hatsukami DK. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat Rev Cancer. 2009;9:476–488. doi: 10.1038/nrc2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippman SM, Lee JJ, Sabichi AL. Cancer chemoprevention: Progress and promise. J Nat Cancer Inst. 1998;90:1514–1528. doi: 10.1093/jnci/90.20.1514. [DOI] [PubMed] [Google Scholar]
- 4.Tatsumura T, Koyama S, Tsujimoto M, Kitagawa M, Kagamimori S. Further study of nebulisation chemotherapy, a new chemotherapeutic method in the treatment of lung carcinomas: Fundamental and clinical. Br J Cancer. 1993;68:1146–1149. doi: 10.1038/bjc.1993.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verschraegen CF, Gilbert BE, Loyer E, et al. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(s)-camptothecin in patients with advanced pulmonary malignancies. Clin Cancer Res. 2004;10:2319–2326. doi: 10.1158/1078-0432.ccr-0929-3. [DOI] [PubMed] [Google Scholar]
- 6.Fu HJ, He J, Mei F, et al. Lung cancer inhibitory effect of epigallocatechin-3-gallate is dependent on its presence in a complex mixture (polyphenon E) Cancer Prev Res. 2009;2:531–537. doi: 10.1158/1940-6207.CAPR-08-0185. [DOI] [PubMed] [Google Scholar]
- 7.Wattenberg LW, Estensen RD. Studies of chemopreventive effects of budenoside on benzo[a]pyrene-induced neoplasia of the lung of female A/J mice. Carcinogenesis. 1997;18:2015–2017. doi: 10.1093/carcin/18.10.2015. [DOI] [PubMed] [Google Scholar]
- 8.Wattenberg LW, Wiedmann TS, Estensen RD, Zimmerman CL, Steele VE, Kelloff GJ. Chemoprevention of pulmonary carcinogenesis by aerosolized budesonide in female A/J mice. Cancer Res. 1997;57:5489–5492. [PubMed] [Google Scholar]
- 9.Yao RS, Wang Y, Lemon WJ, Lubet RA, You M. Budesonide exerts its chemopreventive efficacy during mouse lung tumorigenesis by modulating gene expressions. Oncogene. 2004;23:7746–7752. doi: 10.1038/sj.onc.1207985. [DOI] [PubMed] [Google Scholar]
- 10.Wattenberg LW, Wiedmann TS, Estensen RD, et al. Chemoprevention of pulmonary carcinogenesis by brief exposures to aerosolized budesonide or beclomethasone dipropionate and by the combination of aerosolized budesonide and dietary myo-inositol. Carcinogenesis. 2000;21:179–182. doi: 10.1093/carcin/21.2.179. [DOI] [PubMed] [Google Scholar]
- 11.Alyaqoub FS, Tao LH, Kramer PM, et al. Prevention of mouse lung tumors and modulation of DNA methylation by combined treatment with budesonide and R115777 (Zarnestra(MT)) Carcinogenesis. 2007;28:124–129. doi: 10.1093/carcin/bgl136. [DOI] [PubMed] [Google Scholar]
- 12.Nemenoff RA. Peroxisome proliferator-activated receptor-gamma in lung cancer: Defining specific versus “off-target” effectors. J Thorac Oncol. 2007;2:989–992. doi: 10.1097/JTO.0b013e318158cf0a. [DOI] [PubMed] [Google Scholar]
- 13.Lehrke M, Lazar MA. The many faces of PPARγ. Cell Mol Immunol. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: Complex stories. Nat Rev Cancer. 2004;4:61–70. doi: 10.1038/nrc1254. [DOI] [PubMed] [Google Scholar]
- 15.Theocharis S, Kanelli H, Politi E, et al. Expression of peroxisome proliferator activated receptor-γ in non-small cell lung carcinoma: Correlation with histological type and grade. Lung Cancer. 2002;36:249–255. doi: 10.1016/s0169-5002(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 16.Nemenoff RA, Weiser-Evans M, Winn RA. Activation and molecular targets of peroxisome proliferator-activated receptor-gamma ligands in lung cancer. PPAR Res. 2008:8. doi: 10.1155/2008/156875. (Article ID 156875) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keshamouni VG, Reddy RC, Arenberg DA, et al. Peroxisome proliferator-activated receptor-gamma activation inhibits tumor progression in non-small-cell lung cancer. Oncogene. 2004;23:100–108. doi: 10.1038/sj.onc.1206885. [DOI] [PubMed] [Google Scholar]
- 18.Satoh T, Toyoda M, Hoshino H, et al. Activation of peroxisome proliferator-activated receptor-gamma stimulates the growth arrest and DNA-damage inducible 153 gene in non-small cell lung carcinoma cells. Oncogene. 2002;21:2171–2180. doi: 10.1038/sj.onc.1205279. [DOI] [PubMed] [Google Scholar]
- 19.Shah P, Mudaliar S. Pioglitazone: Side effect and safety profile. Expert Opin Drug Saf. 2010;9:347–354. doi: 10.1517/14740331003623218. [DOI] [PubMed] [Google Scholar]
- 20.Gegick CG, Altheimer MD. Comparison of effects of thiazolidinediones on cardiovascular risk factors: Observations from a clinical practice. Endocr Pract. 2001;7:162–169. doi: 10.4158/EP.7.3.162. [DOI] [PubMed] [Google Scholar]
- 21.Stafylas PC, Sarafidis PA, Lasaridis AN. The controversial effects of thiazolidinediones on cardiovascular morbidity and mortality. Int J Cardiol. 2009;131:298–304. doi: 10.1016/j.ijcard.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, James M, Wen W, et al. Chemopreventive effects of pioglitazone on chemically induced lung carcinogenesis in mice. Mol Cancer Ther. 2010;9:3074–3082. doi: 10.1158/1535-7163.MCT-10-0510. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Liu Q, Lantry LE, et al. A germ-line p53 mutation accelerates pulmonary tumorigenesis: p53-independent efficacy of chemopreventive agents green tea or dexamethasone/myo-inositol and chemotherapeutic agents taxol or adriamycin. Cancer Res. 2000;60:901–907. [PubMed] [Google Scholar]
- 24.Guyton AC. Measurement of the respiratory volumes of laboratory animals. Am J Physiol. 1947;150:70–77. doi: 10.1152/ajplegacy.1947.150.1.70. [DOI] [PubMed] [Google Scholar]
- 25.White MJ, DiCaprio MJ, Greenberg DA. Assessment of neuronal viability with Alamar blue in cortical and granule cell cultures. J Neurosci Methods. 1996;70:195–200. doi: 10.1016/s0165-0270(96)00118-5. [DOI] [PubMed] [Google Scholar]
- 26.Han S, Roman J. Rosiglitazone suppresses human lung carcinoma cell growth through PPARγ-dependent and PPARγ-independent signal pathways. Mol Cancer Ther. 2006;5:430–437. doi: 10.1158/1535-7163.MCT-05-0347. [DOI] [PubMed] [Google Scholar]
- 27.Keshamouni VG, Arenberg DA, Reddy RC, Newstead MJ, Anthwal S, Standiford TJ. PPAR-gamma activation inhibits angiogenesis by blocking ELR + CXC chemokine production in non-small cell lung cancer. Neoplasia. 2005;7:294–301. doi: 10.1593/neo.04601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hazra S, Batra RK, Tai HH, Sharma S, Cui XY, Dubinett SM. Pioglitazone and rosiglitazone decrease prostaglandin E-2 in non-small-cell lung cancer cells by up-regulating 15-hydroxyprostaglandin dehydrogenase. Mol Pharmacol. 2007;71:1715–1720. doi: 10.1124/mol.106.033357. [DOI] [PubMed] [Google Scholar]
- 29.Raabe OG, Al-Bayati MA, Teague SV, Rasolt A. Regional deposition of inhaled monodisperse coarse and fine aerosol particles in small laboratory animals. Ann Occup Hyg. 1988;32:53–63. [Google Scholar]
- 30.Dahl AR, Grossi IM, Houchens DP, et al. Inhaled isotretinoin (13-cis retinoic acid) is an effective lung cancer chemopreventive agent in A/J mice at low doses: A pilot study. Clin Cancer Res. 2000;6:3015–3024. [PubMed] [Google Scholar]
- 31.Liao XM, Liang W, Wiedmann T. Lung distribution of the chemopreventive agent difluoromethylornithine (DFMO) following oral and inhalation delivery. Exp Lung Res. 2004;30:755–769. doi: 10.1080/01902140490517836. [DOI] [PubMed] [Google Scholar]
- 32.Ondrey F. Peroxisome proliferator-activated receptor gamma pathway targeting in carcinogenesis: Implications for chemoprevention. Clin Cancer Res. 2009;15:2–8. doi: 10.1158/1078-0432.CCR-08-0326. [DOI] [PubMed] [Google Scholar]