Abstract
Aging is the predominant risk factor for both genetic and sporadic Parkinson’s disease (PD). The majority of PD cases are nonfamilial, and the connection between aging and PD-associated genes is not well understood. Haploinsufficiency of the GBA gene, leading to a reduction in glucocerebrosidase (GCase) activity, is one of the most common genetic risk factors for PD. Furthermore, GCase activity is also reduced in brain regions of sporadic PD patients, with a corresponding accumulation of its glycosphingolipid (GSL) substrates. Recent findings in PD patients and aging control cases, and in human PD patient induced pluripotent stem cell neurons, have shown an age-dependent reduction in GCase activity and an elevation of some GSLs. We therefore asked whether aging-induced changes to both lysosomal and nonlysosomal GCase activity and GSL homeostasis in the brain could also be reflected in other nonhuman mammalian systems. Increases in brain polyubiquitin and the lysosomal-associated membrane protein, LAMP2A, were found in 24-month-old wild-type mice compared to 1.5-month-old mice. A lipidomic analysis was performed on brains of wild-type mice of different strains between 1.5 and 24 months of age. Aging created GSL changes that are reminiscent of sporadic PD. Levels of glucosylceramide, glucosylsphingosine, lactosylceramide, and GM1a were elevated in the brain of aged mice, and levels of complex gangliosides, GD1a, GD1b, and GT1b, were reduced with age. Parallel biochemical analyses revealed a change in lipid metabolism probably mediated by lysosomal hydrolases, with reduced GCase and increased neuraminidase activity. Based on these data, we hypothesize that perturbation of GSL metabolism in the aging brain may precede or may be part of abnormal protein handling and may accelerate PD pathophysiological processes in vulnerable neurons in PD and other age-related neurodegenerative disorders.
Keywords: Aging, Lipid, Neurodegeneration, Lysosome, Glucosylceramide, Parkinson’s disease
1. Introduction
Aging is the most powerful influence and risk factor for several neurodegenerative diseases, including Parkinson’s disease (PD). Genetic links to PD are well described, giving rise to monogenic forms of the disease [such as involving SNCA, parkin, and LRRK2 genes (Kumaran and Cookson, 2015)], as well as identification of high-risk gene variants such as mutations in GBA (Sidransky et al., 2009), and risk factor genes for idiopathic PD from genome-wide association study meta-analyses (Kumaran and Cookson, 2015). The connection between aging and genetic links to PD is not well understood, but one interesting hypothesis to test is whether cell biological processes associated with aging phenocopy the pathophysiological changes that occur in some forms of the disease that have a genetic cause.
One of the most common genetic risk factors of PD identified to date is mutations causing haploinsufficiency of GBA, the gene encoding lysosomal glucocerebrosidase (GBA, also referred to as GBA1). GBA is the lysosomal hydrolase responsible for the metabolism of the lysosomal glycosphingolipids (GSLs), glucosylceramide (GlcCer) and glucosylsphingosine (GlcSph). Outside the lysosome, GlcCer and GlcSph are degraded by GBA2. Many studies combine lysosomal GBA and nonlysosomal GBA2 activities and therefore refer to a combined glucocerebrosidase (GCase) activity. Such combined GCase activity has been reported to be reduced in several brain regions of PD patients carrying a loss of function GBA mutation (Gegg et al., 2012). In addition, compared to healthy controls, brain GCase activity is also reduced in sporadic PD patients without a GBA mutation, together with an increase in GlcSph substrate levels (Rocha et al., 2015a), thereby showing an epigenetic and biological congruence with the GBA loss of function mutations. A loss of GCase enzymatic activity in PD is also reflected in the blood and cerebrospinal fluid of patients (Alcalay et al., 2015; Parnetti et al., 2017). An age-dependent reduction in brain GCase activity is measured in healthy subjects in the brain regions most affected by PD, and by the time an individual reaches the 7th to 8th decade of life, GCase activity in the substantia nigra and putamen is reduced to the same extent as subjects with sporadic PD (Rocha et al., 2015a). The potential implication of these findings is that haploinsufficiency of GBA is not only phenocopied in nonhereditary forms of PD but also by the process of aging. With respect to changes in GSLs in the human brain during aging, previous reports have indicated alterations to several complex gangliosides in multiple brain regions, including reduced GM1a and GD1a (Kracun et al., 1991; Svennerholm et al., 1994). In normal aging in mice, or in models of accelerated senescence, measurements of brain ganglioside content have indicated increased GM1a and reduced levels of GD1a, GD1b, and GT1b (Ohsawa, 1989; Ohsawa and Shumiya, 1991). The processes underlying normal brain aging involve cellular organelles, including mitochondria. Aging is associated with accumulation of mitochondrial DNA mutations in substantia nigra dopaminergic neurons (Kraytsberg et al., 2006). Several PD-associated gene mutations have been shown to impair mitochondrial function, including PINK1 and parkin that are involved in mitochondrial quality control and mitophagy (Nguyen et al., 2016). It is therefore conceivable that with age, changes to multiple cell biological pathways, such as those associated with lysosomal function, GSL homeostasis, and mitochondrial integrity, may lead to cell dysfunction in vulnerable neurons and lower the threshold for developing PD and may represent new therapeutic targets.
Following up on our findings of an age-dependent reduction in GCase activity and an elevation of some GSLs in PD patients and aging control cases, and in human PD patient induced pluripotent stem cell neurons (Mazzulli et al., 2016; Rocha et al., 2015c), we asked whether PD- and aging-induced changes to either, or both, lysosomal and non-lysosomal GCase activity and GSL homeostasis in the brain could be reflected in other nonhuman mammalian systems. In the present study, we demonstrate that aging in the mouse brain results in a similar lipid profile to that seen in the aging human brain and in PD, with elevated levels of GlcCer, GlcSph, lactosylceramide (LacCer), and GM1a and decreased levels of GD1a, GD1b, and GT1b. These data are important for understanding normal brain aging and potentially also challenge the popular view that proteinopathy is the only trigger for neuronal demise in PD because altered GSL homeostasis may parallel or even precede changes in protein aggregation. These findings may have relevance for understanding pathophysiological mechanisms in age-associated neurodegenerative diseases.
2. Results
2.1. Accumulation of GlcCer and GlcSph in the brain of mice during normal aging
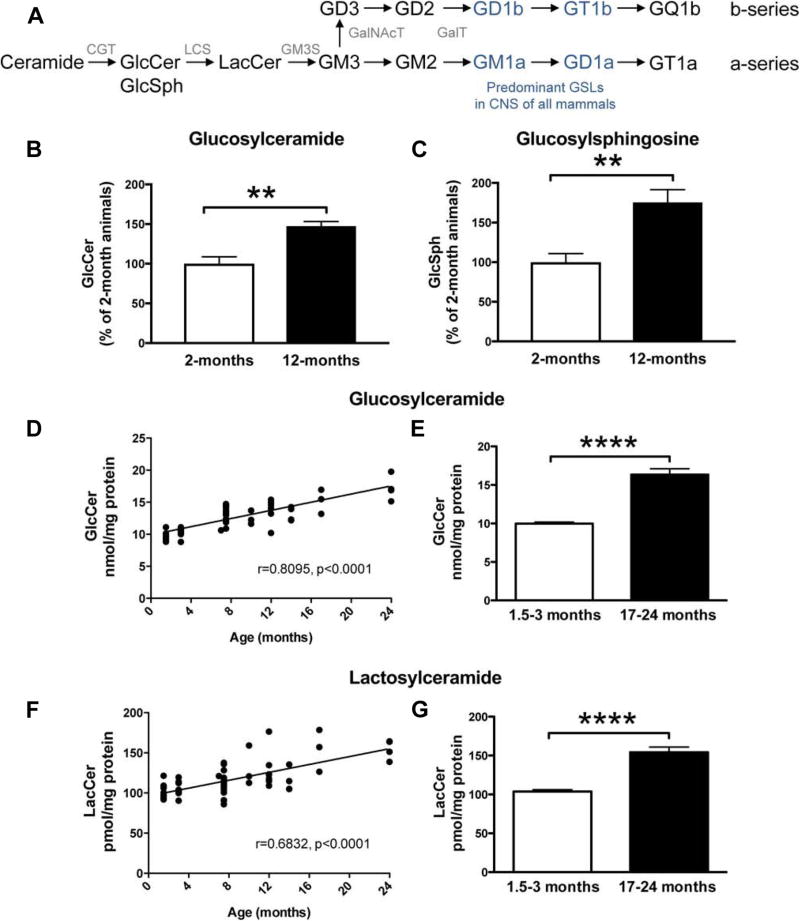
Whole-brain homogenates from wild-type (WT) BDF1 mice were collected at 2 and 12 months of age, and liquid chromatography/tandem mass spectrometry (LC-MS/MS) analysis was used to measure levels of the GSLs, GlcCer and GlcSph (Fig. 1A). A significant increase in both GlcCer (147.3 ± 5.8%, p < 0.01) and GlcSph (175.4 ± 16.1%, p < 0.01) was observed in the brains of 12-month-old animals compared to 2-month-old animals (Fig. 1B, C). Next, to establish whether the aging-induced increase in GlcCer was also observed in a different inbred mouse strain, whole-brain homogenates from FVB/N mice between 1.5 and 24 months of age were used for normal-phase high-performance liquid chromatography (NP-HPLC) analysis of GlcCer. Increasing age was significantly correlated with GlcCer levels (r = 0.8095, p < 0.0001) (Fig. 1D). In mice at 17–24 months, GlcCer levels were increased to 163.8% of young mice aged 1.5–3 months (Fig. 1E) (p < 0.0001, unpaired t-test).
Fig. 1.
GlcCer, GlcSph, and LacCer levels are increased with age in brains of WT FVB and BDF1 mice. (A) Biosynthetic pathway of GSLs: GlcCer, GlcSph, LacCer, CGT, LCS, GM3S, GalNAcT, and GalT. (B, C) Tissue homogenates from WT BDF1 mice (whole brain) at 2 (n = 4) and 12 (n = 6) months of age were used to determine GlcCer (B) and GlcSph (C) levels using mass spectroscopy chromatography (** = p < 0.01, unpaired t-test). (D) Tissue homogenates from the WT FVB mouse whole brain at 1.5–24 months of age were used to determine GlcCer levels. GlcCer was digested with Cerezyme, and released oligosaccharides were 2AA-labeled and analyzed using NP-HPLC. Data were analyzed using Pearson correlation analysis. (E) Comparison of GlcCer levels in young (1.5–3 months, n = 16) and aged (17–24 months, n = 7) WT FVB mice (**** = p < 0.0001, unpaired t-test). (F, G) Tissue homogenates from WT FVB mice at 1.5–24 months of age were used to determine LacCer levels. LacCer and other GSLs apart from GlcCer were digested with ceramide glycanase, and the released oligosaccharides were 2AA-labeled and analyzed using NP-HPLC. Data were analyzed using Pearson correlation analysis (F) and unpaired t-test (G) (n = 16, 1.5–3 months; n = 17, 17–24 months; **** = p < 0.0001). Abbreviations: 2AA, anthranilic acid; CGT, ceramide glucosyl transferase; GSL, glycosphingolipid; GlcCer, glucosylceramide; GlcSph, glucosylsphingosine; GM3S, GM3 synthase; GalNAcT; N-acetylgalactosamine transferase; GalT, galactosyl transferase; LacCer, lactosylceramide; LCS, lactosylceramide synthase; NP-HPLC, normal-phase high-performance liquid chromatography; WT, wild-type.
2.2. Levels of LacCer and complex gangliosides are associated with normal aging of the mouse brain
To assess whether aging induced a selective increase in brain GlcCer and GlcSph, levels of LacCer and complex gangliosides were measured. Brain LacCer was also significantly correlated with age (Fig. 1F, r = 0.6832, p < 0.0001) in FVB/N mice. At 17–24 months of age, levels of LacCer were 148.7% of levels detected at 1.5–3 months (p < 0.0001) (Fig. 1G).
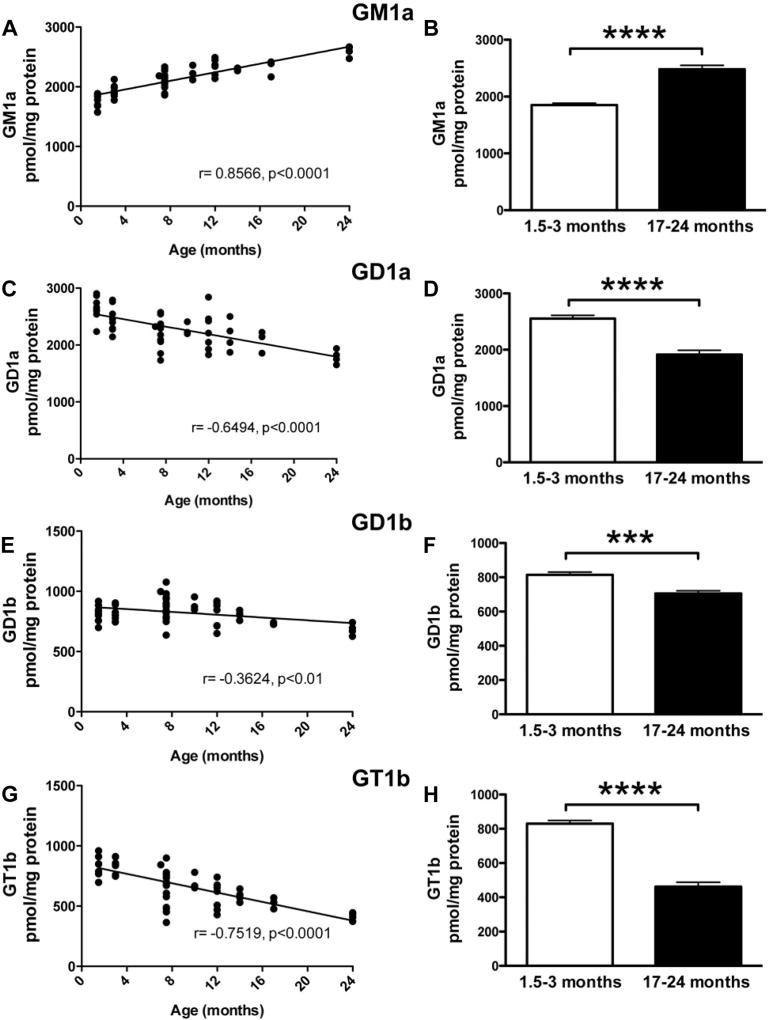
The major GSLs found in the mammalian nervous system are GM1a, GD1a, GD1b, and GT1b. Levels of GM1a were significantly increased with age in the mouse brain (Fig. 2A, r = 0.8566, p < 0.0001). At 17–24 months, GM1a levels were 134.0% of levels at 1.5–3 months of age (Fig. 2B, p < 0.0001, unpaired t-test). Conversely, GD1a, GD1b, and GT1b levels were all negatively correlated with age (Fig. 2C, E, G) (GD1a: r = −0.6494, p < 0.0001; GD1b: r = −0.3624, p < 0.01; and GT1b: r = −0.7519, p < 0.0001). In mice at 17–24 months of age, GD1a, GD1b, and GT1b were all significantly reduced compared to mice at 1.5–3 months of age (Fig. 2D, F, H) (GD1a: 74.9% cf. young mice, p < 0.0001, unpaired t-test; GD1b: 86.5% cf. young mice, p < 0.001, unpaired t-test; and GT1b: 55.6% cf. young mice, p < 0.0001, unpaired t-test).
Fig. 2.
Altered levels of gangliosides are associated with normal aging in WT FVB mice. Tissue homogenates from WT FVB mice at 1.5–24 months of age were used to determine levels of GM1a (A, B), GD1a (C, D), GD1b (E, F), and GT1b (G, H). Gangliosides were extracted and digested with ceramide glycanase. Released oligosaccharides were 2AA-labeled and analyzed using NP-HPLC. Data were analyzed using Pearson correlation analysis (A, C, E, G) and unpaired t-test (B, D, F, H) (n = 16, 1.5–3 months; n = 17, 17–24 months; *** = p < 0.001; **** = p < 0.0001). Abbreviations: 2AA, anthranilic acid; NP-HPLC, normal-phase high-performance liquid chromatography; WT, wild-type.
2.3. Total brain GSL load is increased with age in mice
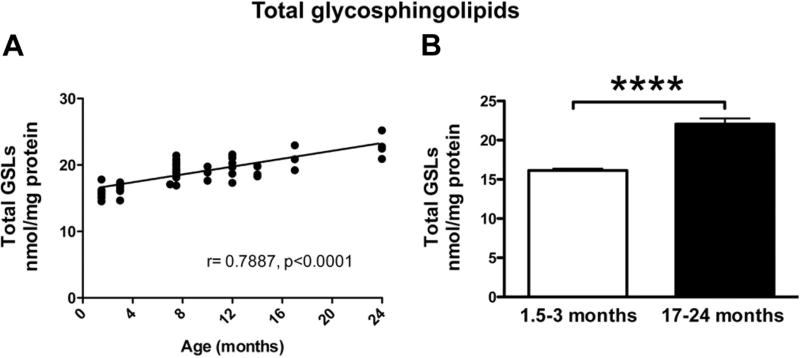
To evaluate whether total brain GSL load was changed with age, GlcCer, LacCer, and ganglioside levels were summed (Fig. 3). Total GSLs were significantly correlated with age (r = 0.7887, p < 0.0001) (Fig. 3A), and total GSLs in 17- to 24-month-old FVB/N mice were 136.8% of those measured in 1.5- to 3-month-old animals (p < 0.0001, unpaired t-test) (Fig. 3B).
Fig. 3.
Normal aging increases total levels of GSLs in brains of WT FVB mice. Pearson correlation analysis (A) of the sum of GlcCer + LacCer + GM1a + GD1a + GD1b + GT1b levels in whole-brain homogenates from WT FVB mice at 1.5–24 months of age shows that normal aging in mice is associated with increased load of GSLs with age. (B) Comparison of total GSL levels in young (1.5–3 months, n = 16) versus old (17–24 months, n = 7) (**** = p < 0.0001, unpaired t-test).
2.4. Age-dependent changes to GSLs are conserved across different strains of wild-type mice
To assess whether the increase in GlcCer and GM1a and the decrease in GD1b, GT1b, and GD1b in the brain during aging are a phenomenon specific for WT FVB/N mice, we performed lipidomic analysis of whole-brain homogenates of WT BALB/c mice at 1–2 months and 20–21 months of age and WT C57BL/6-DBA/2-BDF1 mice at 3–4 months and 17–18 months of age.
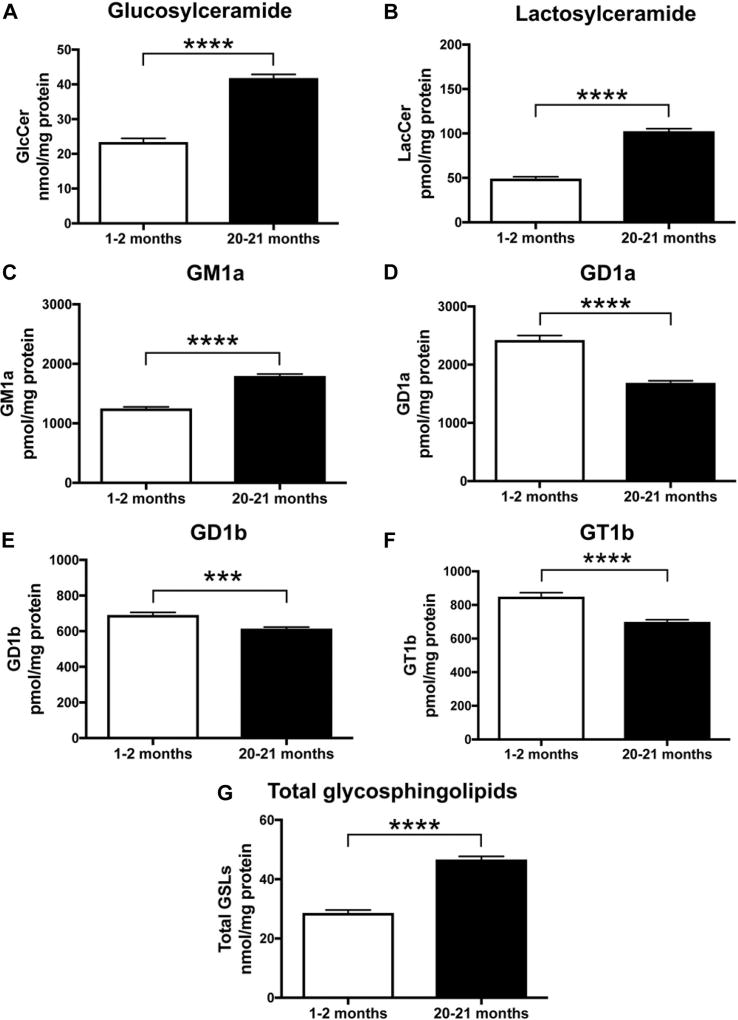
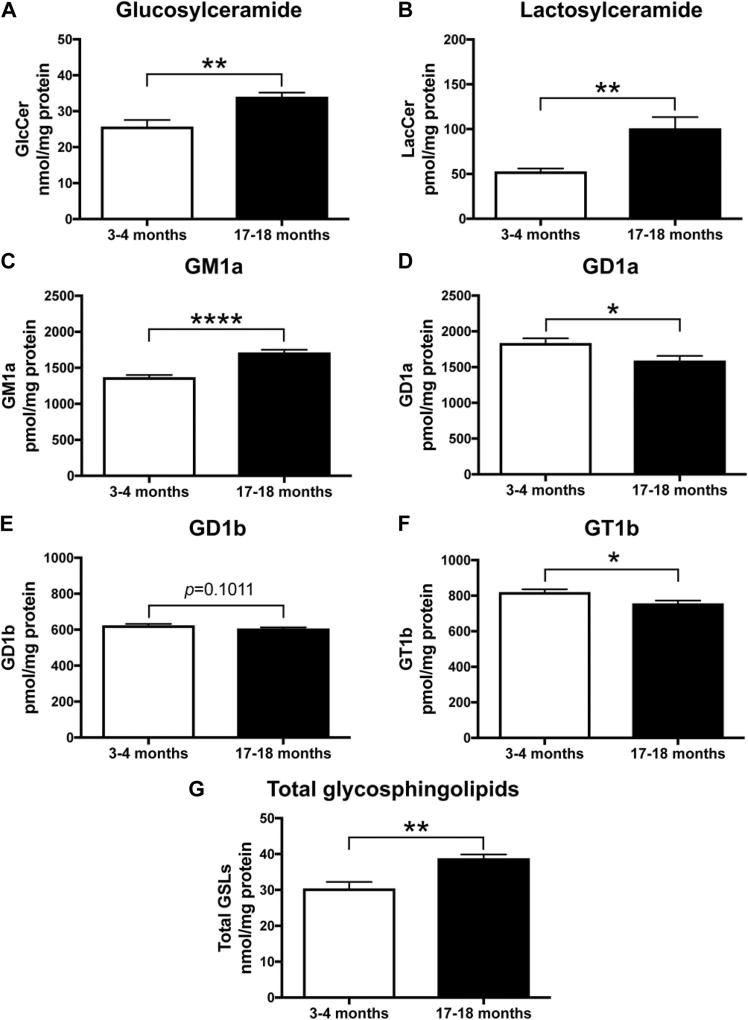
In accordance with previous results, a significant increase in GlcCer and LacCer in both strains was observed in the brains of old animals compared to young animals (Fig. 4A, B and 5A, B) (GlcCer: p < 0.0001 and p = 0.0021, LacCer: p < 0.0001 and p = 0.0059, unpaired t-test). Levels of GM1a were significantly increased with age in the mouse brain of both strains (Figure 4C and 5C, p < 0.0001 and p < 0.0001, respectively, unpaired t-test). Conversely, in old mice, GD1a, GD1b, and GT1b were all significantly reduced compared to young mice (Fig. 4D, E, F and 5D, E, F) (GD1a: p < 0.0001 and p = 0.0237; GD1b: p = 0.0005 and p = 0.1011; and GT1b: p < 0.0001 and p = 0.0176). To evaluate whether total brain GSL load was changed with age, GlcCer, LacCer, and ganglioside levels were summed. The level of total GSLs in 20- to 21-month-old BALB/c mice was increased to 163% of those measured in 1- to 2-month-old animals (Fig. 4G, p < 0.0001). Total GSLs in 17- to 18-month-old C57BL/6-DBA/2-BDF1 mice were increased to 127.6% of those measured in 3- to 4-month-old animals (Fig. 5G, p = 0.0017).
Fig. 4.
GlcCer, LacCer, and GM1a levels are increased in brains of old WT BALB/c mice, whereas levels of GD1a, GD1b, and GT1b are decreased with age. Whole-brain homogenates of WT BALB/c mice at 1–2 months and 20–21 months of age were used to determine levels of GlcCer (A), LacCer (B), GM1a (C), GD1a (D), GD1b (E), and GT1b (F) using NP-HPLC. Data were analyzed using unpaired t-test (n = 13, 1–2 months; n = 9, 20–21 months; *** = p < 0.001, **** = p < 0.0001). (G) Comparison of total GSL levels in young (1–2 months, n = 13) versus old (20–21 months, n = 9) mice (**** = p < 0.0001, unpaired t-test). Abbreviations: GlcCer, glucosylceramide; LacCer, lactosylceramide; NP-HPLC, normal-phase high-performance liquid chromatography; WT, wild-type.
Fig. 5.
GlcCer, LacCer, and GM1a levels are increased in brains of old WT BDF1 mice, whereas levels of GD1a, GD1b, and GT1b are decreased with age. Whole-brain homogenates of WT C57BL/6-DBA/2-BDF1 mice at 3–4 months and 17–18 months of age were used to determine levels of GlcCer (A), LacCer (B), GM1a (C), GD1a (D), GD1b (E), and GT1b (F) using NP-HPLC. Data were analyzed using unpaired t-test (n = 6, 3–4 months; n = 7, 17–18 months; * = p < 0.05, ** = p < 0.01, and **** = p < 0.0001). (G) Comparison of total GSL levels in young (3–4 months, n = 6) versus old (17–18 months, n = 7) mice (** = p < 0.01, unpaired t-test). Abbreviations: GlcCer, glucosylceramide; GSL, glycosphingolipid; LacCer, lactosylceramide; WT, wild-type.
In summary, age-dependent changes to GSLs are conserved across different strains of WT mice.
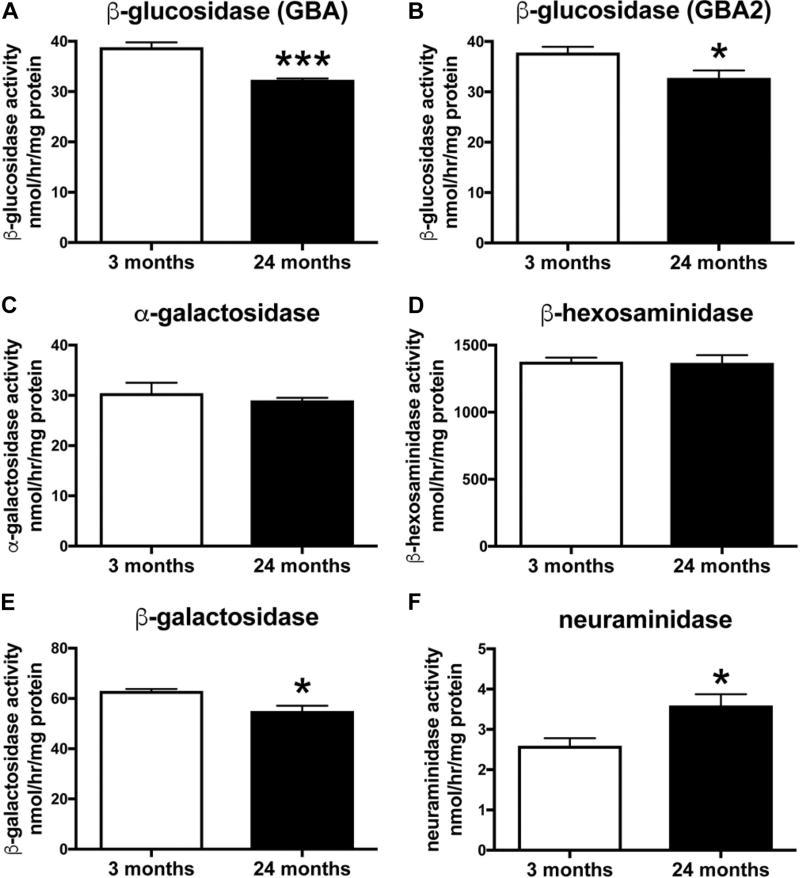
2.5. Reduced glucocerebrosidase and increased neuraminidase activity in the brain of aged mice
Not all GSLs are necessarily exclusively formed from de novo biosynthesis as they recycle and can be remodeled by neuraminidases. It is interesting to note that the increase in GM1a may likely be a result of sequential removal of sialic acid residues from GD1a, GD1b, and GT1b by neuraminidases, explaining their selective concomitant reduction with aging. Consequently, the activities of various lysosomal hydrolases were assayed in whole-brain homogenates of 3- and 24-month-old WT FVB mice (n = 4 per age). Activities of lysosomal β-glucosidase (GBA) and non-lysosomal β-glucosidase (GBA2) were measured independently. A significant decrease in GBA β-glucosidase activity was observed in brain homogenates of 24-month-old WT FVB/N mice compared to 3-month-old WT FVB/N mice (Fig. 6A, p = 0.0007, unpaired t-test, 17% reduction at 24 vs. 3 months). In addition, a significant decrease in GBA2 β-glucosidase activity was observed in brain homogenates of aged WT FVB/N mice compared to young WT FVB/N mice (Fig. 6B, p = 0.0292, unpaired t-test, 13% reduction at 24 vs. 3 months). There was no difference in α-galactosidase or β-hexosaminidase activity in brain homogenates of 3- and 24-month-old WT FVB/N mice (Fig. 6C, D). Also, a significant decrease in β-galactosidase activity was observed in brain homogenates of 24-month-old WT FVB/N mice compared to 3-month-old WT FVB/N mice (Fig. 6E, p = 0.0103, unpaired t-test, 12% reduction at 24 vs. 3 months). Furthermore, a significant increase in neuraminidase activity was observed in brain homogenates of old WT FVB/N mice compared to young WT FVB mice (Fig. 6F, p = 0.0247, unpaired t-test, 38% increase at 24 vs. 3 months). Importantly, we verify these changes in two independent mouse strains, BALB/c and BDF1 (data not shown).
Fig. 6.
Reduced GBA and GBA2 activities and increased neuraminidase activity in the brain of aged WT FVB mice. Lysosomal hydrolase activities were measured in whole-brain homogenates of 3- and 24-month-old WT FVB mice using artificial 4-MU-substrates. Lysosomal β-glucosidase acitivity is defined as GBA, and non-lysosomal β-glucosidase as GBA2. Data are presented as mean ± SEM (n = 4 per age, * = p < 0.05, *** = p < 0.001, unpaired t-test). Abbreviations: SEM, standard error of the mean; WT, wild-type.
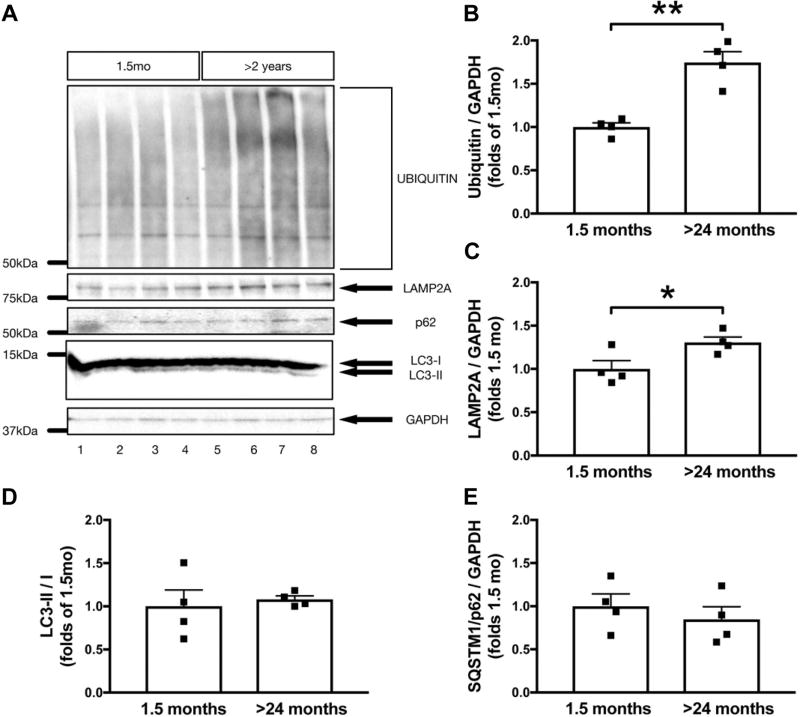
2.6. Aged mice display markers of impaired protein degradation and lysosomal dysfunction
To correlate age-dependent changes in lipid homeostasis to the level of protein, young and aged mouse whole-brain homogenates were lysed and probed for a variety of protein degradation and lysosomal markers (Fig. 7). Although the steady-state markers of autophagy, LC3 and SQSTM1/p62, were unchanged between and young and aged mice (Fig. 7D, E), we found significant increases in levels of LAMP2A (Figure 7C, 30 ± 12%, p < 0.05) and polyubiquitin (Figure 7B, 74 ± 13%, p < 0.01).
Fig. 7.
Proteolytic impairments in the aging WT FVB mouse brain. Levels of ubiquitin (A, B), LAMP2A (A, C), LC3-II/I (A, D), and SQSTM/p62 (A, E) were measured in whole-brain lysates of young (1.5 months) and aged (24 months) WT FVB mice. The optical density of ubiquitin, LAMP2A, LC3-II/I, and SQSTM/p62 bands was normalized by GAPDH and expressed as folds of 1.5-month-old animals. Data are presented as mean ± SEM (* = p < 0.05, ** = p < 0.01, unpaired t-test, n = 8 for all quantifications). Abbreviations: SEM, standard error of the mean; WT, wild-type.
3. Discussion
Aging is the most important risk factor for developing PD, and brain GCase activity is reduced over time in normal aging in humans (Rocha et al., 2015a). Aging mirrors changes to the GBA pathway similar to those that occur in genetic (GBA haploinsufficiency) and sporadic (non-GBA) forms of PD, and our previous data from the human substantia nigra demonstrated that GCase activity is reduced and levels of GlcSph are increased in sporadic PD (Rocha et al., 2015a). In the present study, we provide evidence that decreased lysosomal and non-lysosomal GCase activity and increased lipid load, as observed in brains of sporadic PD patients, also occur in normal aging in the mouse brain. We hypothesize that the increased GSL load associated with aging of the brain accelerates degenerative processes in vulnerable neurons, such as midbrain dopaminergic neurons, and lowers the threshold for developing PD (Rocha et al., 2015a). Critically, this hypothesis challenges the prevailing view that proteinopathy is the primary cause of PD because lipids and lysosomal changes could precede or exacerbate the protein load and aggregation.
Our data show that total GSL levels are progressively increased in the aging mouse brain, including elevations in levels of GlcCer, LacCer, and GM1a. These changes to GSLs are accompanied by a reduction in the activity of GBA and GBA2 glucocerebrosidases, as well as β-galactosidase, whereas lysosomal β-hexosaminidase and α-galactosidase activities were unaffected, and neuraminidases were increased. GCase activity is decreased in the brain during aging in humans, as well as in sporadic PD (Rocha et al., 2015a), and reduced activity of both GCase and β-galactosidase are reported in human neurons from PD patients carrying a GBA mutation (Schondorf et al., 2014). Future studies will determine the possible antecedent cellular mechanism(s) underlying changes in lysosomal hydrolases in the aging brain, as well as identify whether specific brain regions relevant to neurodegeneration are affected.
GlcCer is the biosynthetic precursor of all more-complex GSLs and the final product of GSL catabolism in the lysosome. It is markedly elevated in the brain in human neuronopathic Gaucher disease (GD) and related experimental models (Nilsson and Svennerholm, 1982; Rocha et al., 2015c). This increase in GlcCer is associated with neuropathological changes in neurons and degeneration (Farfel-Becker et al., 2014). GBA deficiency in neuronopathic GD also causes accumulation of GlcSph, which is the deacylated form of GlcCer. Our data showed that both GlcCer and GlcSph levels in brain were increased between 2 and 12 months of age in BDF1 WT mice. GlcSph levels have been shown to be directly neurotoxic (Schueler et al., 2003) and are correlated with central nervous system involvement in GD patients (Orvisky et al., 2002). Although genetic and pathological links between GD and PD are well documented (Sidransky et al., 2009), the case for a general lysosomal capacity loss associated with increased risk for PD is supported by links between parkinsonism and/or α-synucleinopathy and other lysosomal storage disorders including GM1 gangliosidosis (Roze et al., 2005), Niemann-Pick disease type C (Saito et al., 2004), Fabry disease (Buechner et al., 2006), and Krabbe disease (Smith et al., 2014). In adult-onset GM1 gangliosidosis, which is caused by β-galactosidase deficiency inducing central nervous system accumulation of ganglioside GM1a, patients present with generalized dystonia associated with akinetic-rigid parkinsonism (Roze et al., 2005). Our current data show that β-galactosidase activity in brains of aged mice is decreased and GM1a levels are increased in normal aging in mice. GM1a levels are increased in lipid rafts in synaptosomes in aged animals (Yamamoto et al., 2008), and interestingly in early PD, nigrostriatal degeneration is thought to occur in a retrograde manner with synaptic terminals affected first (Chung et al., 2009). Links between GM1a and the dopaminergic system also suggest that a loss of GM1a is associated with PD-like pathophysiological changes (Wu et al., 2012). GM1a is involved in signaling of the neurotrophic factor, GDNF (Hadaczek et al., 2015), and elevated levels of GM1a, as we observed with murine aging, could represent an age-related compensatory mechanism to enhance neurotrophic activity. Interestingly, pharmacological supplementation of PD patients with GM1a has been shown to have beneficial effects on clinical motor and neuropsychological functions in a 5-year study (Schneider et al., 2010). Curiously, in addition to having essential functions in membrane lipid rafts (Schnaar, 2016), GM1a is enriched in myelin (Heinecke et al., 2015) and the GM1a increase observed here may reflect age-related changes in myelin structures.
The association of lysosomal storage disorders and parkinsonism, and normal aging and GSL accumulation observed here in mice, is intriguing for understanding the pathophysiology of age-related diseases, and it suggests a complex interaction between multiple GSLs, aging, and neurodegeneration. Indeed, the principal risk factor for developing most adult-onset neurodegenerative diseases including PD is aging (Niccoli and Partridge, 2012). Specific mechanisms related to aging in PD have been obscured for many years in the literature by very limited clinical pathological human studies (Fearnley and Lees, 1991), and only recently, a broader and more accurate perspective has been provided showing the precise neuropathological resemblance between PD and aging in the human and primate brain—in fact, there is selective vulnerability of midbrain substantia nigra A9 dopamine neurons in both PD and normal aging (Cabello et al., 2002; Collier et al., 2011; Ma et al., 1999; McGeer et al., 1977; Rodriguez et al., 2015; Rudow et al., 2008). The age-dependent reduction of nigral dopamine neurons is conserved between species and in the substantia nigra pars compacta of WT mice, a reduction of ~10% dopaminergic neurons is observed between 7 and 25 months of age (Lee et al., 2016). It is not known what drives age-related decline, and original theories suggested that the cellular mechanisms associated with aging of midbrain dopamine (and other vulnerable) neurons and PD were unrelated. However, it has become apparent that normal aging in animals and humans, and degeneration of substantia nigra pars compacta dopamine neurons in PD, are linked by conserved pathophysiological mechanisms, including lysosomal dysfunction, protein aggregation, and accumulation of mitochondrial deletions (Collier et al., 2011). We have previously shown that accumulation of GlcSph occurs in several brain regions in sporadic PD (Rocha et al., 2015a); however, the selective vulnerability of the midbrain A9 dopamine neurons in PD exemplifies that this population of neurons may be particularly vulnerable to GSL accumulation. The elevations of GSLs found in normal aging are not associated with the same degree of storage and neuronal death observed in neuronopathic GD and other lysosomal storage disorders, but given that the current analyses represent whole-brain homogenates, individual cell types may show greater fold differences. Further studies will determine GSL and enzymatic alterations in young and aged mice in individual brain regions affected in PD and other age-related neurodegenerative disorders. It is reasonable to expect that moderate elevations of GSLs would be associated with multiple pathophysiological consequences and disrupted neuronal function. In their functional roles, GSLs are located in cellular membranes, cluster in lipid rafts, and are involved in cell-cell recognition, regulation of signal transduction, and protein cargo sorting (Lingwood, 2011). Indeed, many membrane-associated receptor and nonreceptor proteins are influenced by the lipid microenvironment. A moderate accumulation of GSLs and changes in the relative amounts of GSLs in aging could have marked physiological effects on these cellular functions. Whereas levels of GlcCer, LacCer, and GM1a are increased with age, our present data also show significant reductions in the levels of GD1a, GD1b and GT1b. Not all GSLs are necessarily exclusively formed from de novo biosynthesis as they recycle and can be remodeled by neuraminidases. Indeed, our data demonstrate that neuraminidase activity is increased in aged mice, and this may account for the observed age-related increase in GM1a and concomitant reduction in GD1a, GD1b, and GT1b through sequential removal of sialic acid residues. Conversely, we have previously shown that genetic deficiency of neuraminidases 3 and 4 in mice causes a reduction in levels of GM1a (Pan et al., 2017). Our findings on altered ganglioside levels with aging are consistent with earlier reports describing similar patterns of changes both in aging mouse brain (Ohsawa, 1989), and in a mouse model of accelerated senescence (Ohsawa and Shumiya, 1991). Reductions in GM1a, GD1a, GD1b, and GT1b have been reported in the frontal and temporal cortices, and in the basal telencephalon of patients with Alzheimer’s disease, another age-associated disease, compared to the same area of control patients (Kracun et al., 1991). Our current data indicating disrupted balance in GSL composition in aging are therefore potentially relevant for determining underlying disease mechanisms not only in PD but also in several age-related neurodegenerative disorders.
The cellular processes altered by pathologically increased GSLs can provide mechanistic clues for treatment and prevention of age-related neurodegenerative disease. There is evidence for lysosomal dysfunction, impairment of autophagy and ubiquitin proteasome system protein degradation pathways, disrupted calcium homeostasis, mitochondrial dysfunction, and oxidative stress, in several lysosomal storage disorders (Platt et al., 2012), and these perturbations are also common to age-related neurodegenerative disorders including PD (Reeve et al., 2014). Moreover, there is considerable evidence for an association between GSL metabolism and α-synuclein, a key pathogenic protein in PD (Rocha et al., 2015b,c; Sardi et al., 2017; Taguchi et al., 2017). A recent report demonstrates that Lewy bodies and Lewy neurites, which contain α-synuclein and are pathological hallmarks of PD, are also enriched in lipids, membrane fragments, and distorted organelles (Shahmoradian et al., 2017). We have previously shown that modulating the activity of lysosomal GBA in rodents causes inverse changes in α-synuclein accumulation, such that reducing lysosomal GBA activity (with a concomitant elevation of GlcCer and GlcSph) increases α-synuclein high molecular weight species and aggregation, whereas elevation of GBA through gene delivery reduces α-synucleinopathy and protects dopamine neurons from degeneration in the rodent substantia nigra (Rocha et al., 2015b,c).
This work has important implications for developing novel therapeutics for age-related neurodegenerative disorders. We hypothesize that aging-induced changes in GSLs will accelerate degenerative processes in vulnerable neurons in neurodegenerative disorders; such changes can potentially be targeted by effective interference in relevant enzymatic pathways, either using gene delivery to increase the hydrolysis of substrates, using small molecules to decrease GSL biosynthesis (using substrate reduction therapy), or pharmacological chaperones that facilitate transport of lysosomal hydrolases to the lysosomal compartment. Indeed, such strategies are already in use or are being developed clinically in several lysosomal storage disorders (Cox, 2015; Platt and Jeyakumar, 2008).
In conclusion, these new data show that normal aging alters several GSLs in the mammalian brain, including an elevation of GlcCer, LacCer, GM1a, and total GSL content, and selective reductions in GD1a, GD1b, and GT1b gangliosides. Importantly, changes in some GSLs in aging appear to be conserved between mice and humans and resemble the changes observed in sporadic PD. This may allow the identification of rational treatments to normalize these altered patterns of GSL expression.
3.1. Experimental procedures
3.1.1. Mice
All animal procedures were performed in accordance with the guidelines of the National Institute of Health and were approved by the Institutional Animal Care and Use Committee at McLean Hospital, Harvard Medical School. All procedures conducted in Oxford were performed according to the Animals (Scientific Procedures) Act 1986 under a project license (PPL No. P8088558D) from the UK Home Office. Animals were housed according to standard conditions, in a dark/light cycle of 12 hours, with ad libitum access to food and water. Male (n = 20) and female (n = 36) FVB mice at 1.5–24 months of age (The Jackson Laboratory, Bar Harbor, ME, USA) and male BDF1 (mixed C57/BL/6-DBA-2 background) mice at 2–12 months of age (Charles River, Wilmington, MA, USA) (n = 4, 2 months; n = 6, 12 months) were used. Furthermore, a separate cohort of BDF1 male mice at 3–4 months (n = 6) and 17–18 months (n = 7) of age and of male and female BALB/c mice at 1–2 months (n = 13) and 20–21 months (n = 9) of age (The Jackson Laboratory, Charles River, UK) were used. Mice were terminally anesthetized by intraperitoneal injection of sodium pentobarbital (130 mg/kg) and intracardially perfused with heparinized saline. Brains were rapidly removed, homogenized in water using a handheld Polytron homogenizer and aliquoted before being snap-frozen and stored at −80 °C.
3.1.2. NP-HPLC analysis
GlcCer and downstream GSLs were analyzed essentially as described by Neville et al. (2004). Lipids from brain homogenates were extracted with chloroform:methanol (1:2, v/v) overnight at 4 °C. The GSLs were then further purified using solid phase C18 columns (Telos, Kinesis, UK). After elution, the GSL fractions were split in half, dried down under a stream of nitrogen and treated with either Cerezyme (Genzyme, Cambridge, MA, USA) to obtain glucose from GlcCer or ceramide glycanase (prepared in house from the medicinal leech Hirudo medicinalis/verbana) to obtain oligosaccharides from other GSLs. Liberated glucose and free glycans were then fluorescently-labeled with anthranilic acid (2AA). Excess 2AA label was removed using DPA-6S SPE columns (Supelco, PA, USA). Purified 2AA-labeled glucose and 2AA-labeled oligosaccharides were separated and quantified by NP-HPLC as previously described (Neville et al., 2004). The NP-HPLC system consisted of a Waters Alliance 2695 separations module and an in-line Waters 2475 multi λ-fluorescence detector set at Ex λ360 nm and Em λ425 nm. The solid phase used was a 4.6 × 250 mm TSKgel Amide-80 column (Anachem, Luton, UK). A standard 2AA-labeled glucose homopolymer ladder (Ludger, UK) was included to determine the glucose units (GUs) of the HPLC peaks. Individual GSL species were identified by their GU values and quantified by comparison of integrated peak areas with a known amount of 2AA-labeled BioQuant chitotriose standard (Ludger). Results were normalized to protein content.
3.1.3. LC-MS/MS analysis
Quantification of GlcCer and GlcSph tissue levels using LC-MS/MS in aliquots of brain homogenates from BDF1 mice was performed as previously described (Rocha et al., 2015c). Briefly, brains were homogenized in water at a ratio of 14 mg wet weight tissue per 50 µL water, and GlcCer and GlcSph were extracted from the tissue homogenate in 800 µL of solvent. Samples were sonicated, vortexed, and centrifuged before the supernatant was transferred to an injection plate for LC-MS/MS analysis.
3.2. Lysosomal hydrolase activity assays
Lysosomal hydrolase activities were assayed fluorometrically using artificial sugar substrates containing the fluorophore 4-methylumbelliferone (4-MU). For measuring β-glucosidase (glucocerebrosidase) activities, samples were incubated in the presence or absence of 0.3 mM NB-DGJ for 30 minutes on ice before the assay. The substrate for GBA β-glucosidase activity was 4.5 mM 4-MU β-D-glucoside in 200 mM citrate/phosphate buffer, pH 5.2, 0.25% Triton X-100, 0.25% sodium taurocholate, 1.25 mM EDTA, and 4 mM 2-mercaptoethanol. GBA activity was defined as the NB-DGJ nonsensitive activity at pH 5.2. The substrate for GBA2 β-glucosidase activity was 4.5-mM 4-MU β-D-glucoside in 200mM citrate/phosphate buffer, pH 5.5, and 0.1% Triton X-100. GBA2 activity was defined as the NB-DGJ sensitive activity at pH 5.5. For α-galactosidase activity, 5 mM 4-MU α-D-galactoside in 100 mM sodium citrate buffer, pH 4.0, and 0.1% Triton X-100 was used as substrate. For β-hexosaminidase activity, 3 mM 4-MU N-acetyl-β-D-glucosaminide in 200 mM sodium citrate buffer, pH 4.5, and 0.1% Triton X-100 was used as substrate. For β-galactosidase activity, 1 mM 4-MU β-D-galactopyranoside in 200 mM sodium acetate buffer, pH 4.3, 100 mM NaCl, and 0.1% Triton X-100 was used as substrate. The substrate for neuraminidase activity was 0.8 mM 4-MU N-acetylneuraminic acid in 0.1 M acetate buffer, pH 4.6, and 0.1% Triton X-100. The digests (in triplicate) containing tissue homogenates in PBS with 0.1% Triton X-100 and artificial 4-MU substrate were incubated at 37 °C for 30 minutes (or 2 hours for neuraminidases). The reaction was stopped by adding cold 0.5 M Na2CO3, and the released fluorescent 4-MU was measured in a FLUOstar OPTIMA plate reader (BMG Labtech, Ortenberg, Germany) with an excitation at 360 nm and emission at 460 nm. A standard curve of free 4-MU was used to calculate the enzyme activity. Results were normalized to protein content.
3.3. Western blotting
For the biochemical analysis, whole-brain homogenates of mice were thawed on ice with protease and phosphatase inhibitors added (Halt Protease and Phosphatase Inhibitor Cocktail (100×); Thermo Fisher Scientific 1861284). Lysis was carried out for 30 minutes on ice in lysis buffer (150 mM NaCl, 50 mM Tris pH 7.6, 1% Triton X-100, and 2 mM EDTA), and the lysates sonicated for 30 seconds (5-second pulses, on ice). Membrane-enriched fractions were pelleted in 1% Triton-X by ultracentrifugation (100,000 × g, 1 hour, 4 °C) and reconstituted in lysis buffer supplemented 2% SDS. Further insoluble materials were pelleted at 100,000 × g (1 hour, room temperature), and the supernatant was utilized in subsequent experiments. Equal amount of protein from each fraction was separated using polyacrylamide gel electrophoresis. Primary antibodies included antibodies to LAMP2A (1:1000, ab18528; Abcam), LC3 (1:1000, PM036; MBL Life Science), SQSTM1/p62 (1:1000, PM045; MBL Life Science), ubiquitin (1:5000, Z0458; DAKO), and GAPDH (1:2000, AB2302; Millipore Sigma). The intensities of the immunoreactive bands were estimated using the gel analyzer suite in ImageJ (v1.7).
3.4. Statistical analysis
All statistical analyses were performed with GraphPad Prism, version 7.0 (GraphPad Software, San Diego, CA, USA).
Acknowledgments
This work was supported by the Consolidated Anti-Aging Foundation, the Poul Hansen family, the Harold and Ronna Cooper family, the Orchard Foundation, and NIH/NINDS 1R01NS092667-01. MH is funded by Parkinson’s UK (grant H-1501), FMP is a Royal Society Wolfson Research Merit Award holder and a Wellcome Trust Investigator in Science.
Footnotes
Disclosure statement
Authors have no conflict of interest to declare.
Authors’ contributions: PJH, MH, DAP, FMP, and OI designed research; PJH, MH, ORB, and EMR performed research. PJH, MH, EBM, ORB, EMR, DAP, FMP, and OI analyzed and interpreted data. PJH, MH, FMP, and OI wrote the article.
References
- Alcalay RN, Levy OA, Waters CC, Fahn S, Ford B, Kuo SH, Mazzoni P, Pauciulo MW, Nichols WC, Gan-Or Z, Rouleau GA, Chung WK, Wolf P, Oliva P, Keutzer J, Marder K, Zhang X. Glucocerebrosidase activity in Parkinson’s disease with and without GBA mutations. Brain. 2015;138:2648–2658. doi: 10.1093/brain/awv179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buechner S, De Cristofaro MT, Ramat S, Borsini W. Parkinsonism and Anderson Fabry’s disease: a case report. Mov Disord. 2006;21:103–107. doi: 10.1002/mds.20675. [DOI] [PubMed] [Google Scholar]
- Cabello CR, Thune JJ, Pakkenberg H, Pakkenberg B. Ageing of substantia nigra in humans: cell loss may be compensated by hypertrophy. Neuropathol. Appl. Neurobiol. 2002;28:283–291. doi: 10.1046/j.1365-2990.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J. Neurosci. 2009;29:3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat. Rev. Neurosci. 2011;12:359–366. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TM. Innovative treatments for lysosomal diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2015;29:275–311. doi: 10.1016/j.beem.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Farfel-Becker T, Vitner EB, Kelly SL, Bame JR, Duan J, Shinder V, Merrill AH, Jr, Dobrenis K, Futerman AH. Neuronal accumulation of glucosylceramide in a mouse model of neuronopathic Gaucher disease leads to neurodegeneration. Hum. Mol. Genet. 2014;23:843–854. doi: 10.1093/hmg/ddt468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, Schapira AH. Glucocerebrosidase deficiency in substantia nigra of Parkinson disease brains. Ann. Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P, Wu G, Sharma N, Ciesielska A, Bankiewicz K, Davidow AL, Lu ZH, Forsayeth J, Ledeen RW. GDNF signaling implemented by GM1 ganglioside; failure in Parkinson’s disease and GM1-deficient murine model. Exp. Neurol. 2015;263:177–189. doi: 10.1016/j.expneurol.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Heinecke KA, Luoma A, d’Azzo A, Kirschner DA, Seyfried TN. Myelin abnormalities in the optic and sciatic nerves in mice with GM1-gangliosidosis. ASN Neuro. 2015;7 doi: 10.1177/1759091415568913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracun I, Rosner H, Drnovsek V, Heffer-Lauc M, Cosovic C, Lauc G. Human brain gangliosides in development, aging and disease. Int. J. Dev. Biol. 1991;35:289–295. [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat. Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Kumaran R, Cookson MR. Pathways to Parkinsonism Redux: convergent pathobiological mechanisms in genetics of Parkinson’s disease. Hum. Mol. Genet. 2015;24:R32–R44. doi: 10.1093/hmg/ddv236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YI, Kang H, Ha YW, Chang KY, Cho SC, Song SO, Kim H, Jo A, Khang R, Choi JY, Lee Y, Park SC, Shin JH. Diaminodiphenyl sulfone-induced parkin ameliorates age-dependent dopaminergic neuronal loss. Neurobiol. Aging. 2016;41:1–10. doi: 10.1016/j.neurobiolaging.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Lingwood CA. Glycosphingolipid functions. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004788. https://doi.org/10.1101/cshperspect.a004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SY, Ciliax BJ, Stebbins G, Jaffar S, Joyce JN, Cochran EJ, Kordower JH, Mash DC, Levey AI, Mufson EJ. Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J. Comp. Neurol. 1999;409:25–37. doi: 10.1002/(sici)1096-9861(19990621)409:1<25::aid-cne3>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. Alpha-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc. Natl. Acad. Sci. U. S. A. 2016;113:1931–1936. doi: 10.1073/pnas.1520335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Suzuki JS. Aging and extrapyramidal function. Arch. Neurol. 1977;34:33–35. doi: 10.1001/archneur.1977.00500130053010. [DOI] [PubMed] [Google Scholar]
- Neville DC, Coquard V, Priestman DA, te Vruchte DJ, Sillence DJ, Dwek RA, Platt FM, Butters TD. Analysis of fluorescently labeled glycosphingolipid-derived oligosaccharides following ceramide glycanase digestion and anthranilic acid labeling. Anal. Biochem. 2004;331:275–282. doi: 10.1016/j.ab.2004.03.051. [DOI] [PubMed] [Google Scholar]
- Nguyen TN, Padman BS, Lazarou M. Deciphering the molecular signals of PINK1/parkin mitophagy. Trends Cell Biol. 2016;26:733–744. doi: 10.1016/j.tcb.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr. Biol. 2012;22:R741–R752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Nilsson O, Svennerholm L. Accumulation of glucosylceramide and glucosylsphingosine (psychosine) in cerebrum and cerebellum in infantile and juvenile Gaucher disease. J. Neurochem. 1982;39:709–718. doi: 10.1111/j.1471-4159.1982.tb07950.x. [DOI] [PubMed] [Google Scholar]
- Ohsawa T. Changes of mouse brain gangliosides during aging from young adult until senescence. Mech. Ageing Dev. 1989;50:169–177. doi: 10.1016/0047-6374(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Ohsawa T, Shumiya S. Age-related alteration of brain gangliosides in senescence-accelerated mouse (SAM)-P/8. Mech. Ageing Dev. 1991;59:263–274. doi: 10.1016/0047-6374(91)90137-o. [DOI] [PubMed] [Google Scholar]
- Orvisky E, Park JK, LaMarca ME, Ginns EI, Martin BM, Tayebi N, Sidransky E. Glucosylsphingosine accumulation in tissues from patients with Gaucher disease: correlation with phenotype and genotype. Mol. Genet. Metab. 2002;76:262–270. doi: 10.1016/s1096-7192(02)00117-8. [DOI] [PubMed] [Google Scholar]
- Pan X, De Aragao CBP, Velasco-Martin JP, Priestman DA, Wu HY, Takahashi K, Yamaguchi K, Sturiale L, Garozzo D, Platt FM, Lamarche-Vane N, Morales CR, Miyagi T, Pshezhetsky AV. Neuraminidases 3 and 4 regulate neuronal function by catabolizing brain gangliosides. FASEB J. 2017;31:3467–3483. doi: 10.1096/fj.201601299R. [DOI] [PubMed] [Google Scholar]
- Parnetti L, Paciotti S, Eusebi P, Dardis A, Zampieri S, Chiasserini D, Tasegian A, Tambasco N, Bembi B, Calabresi P, Beccari T. Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in Parkinson’s disease patients. Mov. Disord. 2017;32:1423–1431. doi: 10.1002/mds.27136. [DOI] [PubMed] [Google Scholar]
- Platt FM, Boland B, van der Spoel AC. The cell biology of disease: lysosomal storage disorders: the cellular impact of lysosomal dysfunction. J. Cell Biol. 2012;199:723–734. doi: 10.1083/jcb.201208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt FM, Jeyakumar M. Substrate reduction therapy. Acta Paediatr. 2008;97:88–93. doi: 10.1111/j.1651-2227.2008.00656.x. [DOI] [PubMed] [Google Scholar]
- Reeve A, Simcox E, Turnbull D. Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res. Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha EM, Smith GA, Park E, Cao H, Brown E, Hallett P, Isacson O. Progressive decline of glucocerebrosidase in aging and Parkinson’s disease. Ann. Clin. Transl. Neurol. 2015a;2:433–438. doi: 10.1002/acn3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha EM, Smith GA, Park E, Cao H, Brown E, Hayes MA, Beagan J, McLean JR, Izen SC, Perez-Torres E, Hallett PJ, Isacson O. Glucocerebrosidase gene therapy prevents alpha-synucleinopathy of midbrain dopamine neurons. Neurobiol. Dis. 2015b;82:495–503. doi: 10.1016/j.nbd.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Rocha EM, Smith GA, Park E, Cao H, Graham AR, Brown E, McLean JR, Hayes MA, Beagan J, Izen SC, Perez-Torres E, Hallett PJ, Isacson O. Sustained systemic glucocerebrosidase inhibition induces brain alpha-synuclein aggregation, Microglia and Complement C1q Activation in mice. Antioxid. Redox Signal. 2015c;23:550–564. doi: 10.1089/ars.2015.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Rodriguez-Sabate C, Morales I, Sanchez A, Sabate M. Parkinson’s disease as a result of aging. Aging Cell. 2015;14:293–308. doi: 10.1111/acel.12312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E, Paschke E, Lopez N, Eck T, Yoshida K, Maurel-Ollivier A, Doummar D, Caillaud C, Galanaud D, Billette de Villemeur T, Vidailhet M, Roubergue A. Dystonia and parkinsonism in GM1 type 3 gangliosidosis. Mov. Disord. 2005;20:1366–1369. doi: 10.1002/mds.20593. [DOI] [PubMed] [Google Scholar]
- Rudow G, O’Brien R, Savonenko AV, Resnick SM, Zonderman AB, Pletnikova O, Marsh L, Dawson TM, Crain BJ, West MJ, Troncoso JC. Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol. 2008;115:461–470. doi: 10.1007/s00401-008-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Suzuki K, Hulette CM, Murayama S. Aberrant phosphorylation of alpha-synuclein in human Niemann-Pick type C1 disease. J. Neuropathol. Exp. Neurol. 2004;63:323–328. doi: 10.1093/jnen/63.4.323. [DOI] [PubMed] [Google Scholar]
- Sardi SP, Viel C, Clarke J, Treleaven CM, Richards AM, Park H, Olszewski MA, Dodge JC, Marshall J, Makino E, Wang B, Sidman RL, Cheng SH, Shihabuddin LS. Glucosylceramide synthase inhibition alleviates aberrations in synucleinopathy models. Proc. Natl. Acad. Sci. U. S. A. 2017;114:2699–2704. doi: 10.1073/pnas.1616152114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar RL. Gangliosides of the vertebrate nervous system. J. Mol. Biol. 2016;428:3325–3336. doi: 10.1016/j.jmb.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider JS, Sendek S, Daskalakis C, Cambi F. GM1 ganglioside in Parkinson’s disease: results of a five year open study. J. Neurol. Sci. 2010;292:45–51. doi: 10.1016/j.jns.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Schondorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B, Sardi SP, Valsecchi M, Hoffmann S, Schwarz LK, Hedrich U, Berg D, Shihabuddin LS, Hu J, Pruszak J, Gygi SP, Sonnino S, Gasser T, Deleidi M. iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat. Commun. 2014;5:4028. doi: 10.1038/ncomms5028. [DOI] [PubMed] [Google Scholar]
- Schueler UH, Kolter T, Kaneski CR, Blusztajn JK, Herkenham M, Sandhoff K, Brady RO. Toxicity of glucosylsphingosine (glucopsychosine) to cultured neuronal cells: a model system for assessing neuronal damage in Gaucher disease type 2 and 3. Neurobiol. Dis. 2003;14:595–601. doi: 10.1016/j.nbd.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Shahmoradian SH, Genoud C, Graff-Meyer A, Hench J, Moors T, Schweighauser G, Wang J, Goldie KN, Sütterlin R, Castaño-Díez D, Pérez-Navarro P, Huisman E, Ipsen S, Ingrassia A, de Gier Y, Rozemuller AJ, De Paepe A, Erny J, Staempfli A, Hoernschemeyer J, Großerüschkamp F, Niedieker D, El-Mashtoly SF, Quadri M, van IJcken WF, Bonifati V, Gerwert K, Bohrmann B, Frank S, Britschgi M, Stahlberg H, van de Berg WD, Lauer ME. Lewy pathology in Parkinson’s disease consists of a crowded organellar membranous medley. bioRxiv. 2017 doi: 10.1038/s41593-019-0423-2. https://doi.org/10.1101/137976. [DOI] [PubMed]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BR, Santos MB, Marshall MS, Cantuti-Castelvetri L, Lopez-Rosas A, Li G, van Breemen R, Claycomb KI, Gallea JI, Celej MS, Crocker SJ, Givogri MI, Bongarzone ER. Neuronal inclusions of alpha-synuclein contribute to the pathogenesis of Krabbe disease. J. Pathol. 2014;232:509–521. doi: 10.1002/path.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm L, Bostrom K, Jungbjer B, Olsson L. Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J. Neurochem. 1994;63:1802–1811. doi: 10.1046/j.1471-4159.1994.63051802.x. [DOI] [PubMed] [Google Scholar]
- Taguchi YV, Liu J, Ruan J, Pacheco J, Zhang X, Abbasi J, Keutzer J, Mistry PK, Chandra SS. Glucosylsphingosine promotes alpha-synuclein pathology in mutant GBA-associated Parkinson’s disease. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.1525-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Lu ZH, Kulkarni N, Ledeen RW. Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans. J. Neurosci. Res. 2012;90:1997–2008. doi: 10.1002/jnr.23090. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Matsubara T, Sato T, Yanagisawa K. Age-dependent high-density clustering of GM1 ganglioside at presynaptic neuritic terminals promotes amyloid beta-protein fibrillogenesis. Biochim. Biophys. Acta. 2008;1778:2717–2726. doi: 10.1016/j.bbamem.2008.07.028. [DOI] [PubMed] [Google Scholar]