Abstract
Heterocyclic amines (HCAs) are primarily produced during high temperature meat cooking. These compounds have been intensively investigated as mutagens and carcinogens. However, converging data suggest that HCAs may also be neurotoxic and potentially relevant to neurodegenerative diseases such as Parkinson’s disease (PD). The identification of new potential etiological factors is important because most PD cases are sporadic. Our group previously showed that 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) was selectively neurotoxic to dopaminergic neurons. However, PhIP is one of many HCAs, a class of compounds that exhibits wide structural variability. The goal of this study was to determine the neurotoxicity of the most prevalent and best studied HCAs from three subclasses: aminoimidazoaazarenes (AIA), α-carbolines, and β-carbolines. Using E17 rat primary midbrain cultures, we tested dopaminergic and non-dopaminergic neurotoxicity elicited by the following compounds: 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ), 2-amino-3,8-dimethylmidazo[4,5-f]quinoxaline (MeIQx), 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (4,8-DiMeIQx), PhIP, 1-methyl-9H-pyrido[3,4-b]indole (harmane), 9H-pyrido[3,4-b]indole (norharmane) and 2-amino-9H-pyrido[2,3-b]indole (AαC) at concentrations ranging from 100 nM – 5 µM. All tested HCAs were selectively neurotoxic, though the dose required to elicit selective loss of dopaminergic neurons or selective decreases in dopaminergic neurite length was compound specific. Non-dopaminergic neurons were unaffected at all tested doses. The sensitivity (determined by threshold dose required to elicit selective neurotoxicity) appears to be unrelated to published mutagenic potency. Both AIA and α/β-carbolines produced oxidative damage, which was magnified in dopaminergic neurons vs. non-dopaminergic neurons as further evidence of selective neurotoxicity. These studies are expected to prompt clinical and mechanistic studies on the potential role of HCA exposure in PD.
Keywords: Parkinson’s disease, heterocyclic amine, aminoimidazoaazarene, carboline, primary midbrain culture
1. Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that is a major public health concern because of the debilitating symptoms affecting ~10 million people worldwide (Dorsey et al., 2007). PD motor symptoms typically include postural instability, resting tremor, bradykinesia, and rigidity. While numerous non-motor and systemic symptoms may stem from a variety of pathologies, the PD motor symptoms are thought to primarily arise from loss of nigrostriatal dopaminergic (DA) neurons and resultant dopamine depletion (Dauer and Przedborski, 2003; Langston et al., 1983; Shulman et al., 2011). The etiology of PD has been intensely investigated. Currently, ~90% of PD cases are thought to arise from unknown causes (‘sporadic’) (Lesage and Brice, 2009). Numerous environmental factors have been investigated with respect to a role in PD pathogenesis (Cannon and Greenamyre, 2011). Given that exposures currently linked to PD likely account for few overall cases, the search for additional etiological factors and gene-environment interactions that may increase PD risk is an active area of research.
Dietary factors could potentially be encountered in higher doses and more frequently through one’s life span compared to other environmental contaminants. Diet has been investigated for a role in the etiology of neurodegenerative diseases. For example, it has been suggested that diets high in saturated fat such as animal fat increase the risk of PD (Logroscino et al., 1998). An emerging body of literature suggests that heterocyclic amines (HCAs) should be investigated as potential neurotoxicants, and specifically for relevance to PD. HCAs are primarily produced in meat cooked at high temperatures. Multiple subclasses have been identified, notably aminoimidazoaazarenes (AIA) and α/β-carbolines. AIA HCAs are formed through the Maillard reaction between amino acids and sugars, producing pyridine or pyrazine, which later reacts with creatine in a heat-dependent reaction (Skog et al., 1998), while the formation of α/β-carbolines is dependent on the pyrolysis of glutamic acid and tryptophan exposed to high temperatures over a period of time (Matsumoto et al., 1981).
To date, specific HCAs have been identified that may affect the dopamine system. 2-Amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1) and 2-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp-P-2) were found to increase DA and to decrease DA metabolites specifically in rat striatum, possibly due to inhibition of monoamine oxidase (MAO) (Ichinose et al., 1988). In examining the most prevalent AIA HCA, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), our group showed that exposure in primary cultures was selectively toxic to dopaminergic neurons. Here, we showed that PhIP and its metabolite N-OH-PhIP produced selective loss of dopamine neurons, while other neuronal cells were unaffected (Griggs et al., 2014). β-carbolines, which have been found to be produced endogenously as well as exogenously, have also been identified as possibly neurotoxic (Louis et al., 2011). Specifically, harmane blood levels have been associated with essential tremor (Louis et al., 2011). Further, PD patients have been found to have elevated blood harmane levels compared to healthy controls (Louis et al., 2014b). There is limited information on the α-carboline, AαC. However, there are some data on carcinogenic potential. AαC exposure in humans has been linked to hepatocellular carcinomas and hemangioendothelial sarcomas occur in exposed rodents (Ohgaki et al., 1984; Sugimura, 1985). Although AαC is implicated in many different carcinomas via the activation through CYP1A family, the neurological effects of this agent remain unknown (Niwa et al., 1982).
Although the examples above indicate that several HCAs are potentially neurotoxic, there is a significant gap in the literature with respect to tested neurotoxicity (Griggs et al., 2014; Ichinose et al., 1988). Importantly, to date, there have been roughly ~30 HCAs found to be present in the diet, with many more likely that are currently unknown (Roemer et al., 2016), and these compounds vary broadly in structural and physical properties, as well as mutagenicity (Felton et al., 1999; Kawamori et al., 2004; Layton et al., 1995; Totsuka et al., 2004). Our goal here was to test HCAs from three important subclasses that are found in the diet. Using primary midbrain cultures, we aimed to provide data on which HCAs were selectively toxic to dopaminergic neurons and the threshold dose for selective neurotoxicity.
2. Materials and Methods
2.1. HCAs to be tested
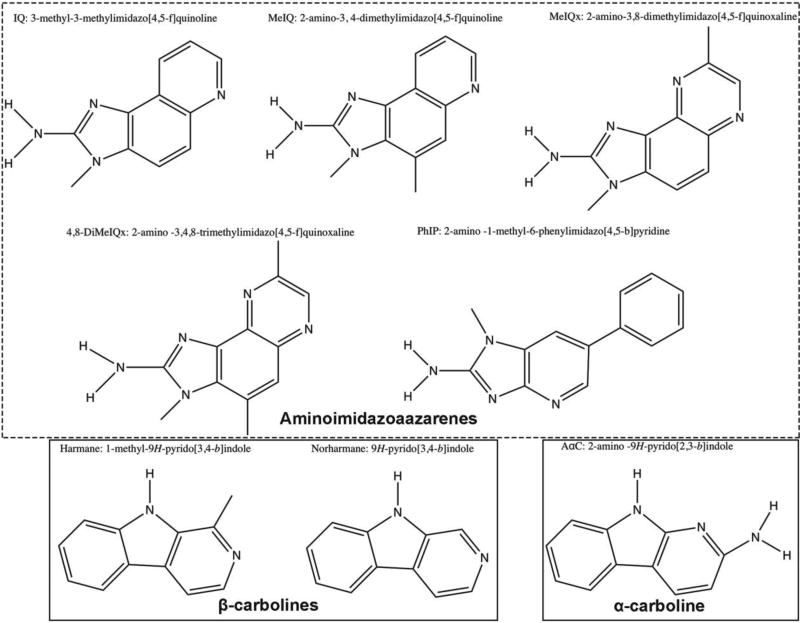
The structures of the HCAs to be used in these studies are shown in Figure 1. Structures were drawn using ChemDraw (Version 15). HCAs were chosen based on a body of literature in mutagenicity studies, dietary prevalence, and estimated consumption (Table 1) (Gibis, 2016).
Figure 1. Chemical structures of HCAs used in this study.
Aminoimidazoaazarenes used are IQ, MeIQ, MeIQx, 4,8-DiMeIQx and PhIP (dashed box). α/β-Carbolines used are harmane, norharmane, AαC (solid boxes). Structures were drawn using ChemDraw (Version 15).
Table 1.
Chemical properties of heterocyclic amines tested. Log P values are from PubChem Compound Database.
| Full chemical name | Abbreviation | Log P |
Exposed functional groups |
Estimated consumption (ng/kg/day) |
References |
|---|---|---|---|---|---|
| 2-amino-3-methylimidazo[4,5-f]quinoline | IQ | 1.5 | 1 Amine 1 Methyl | 0.23 – 0.28 | (Keating and Bogen, 2004; Layton et al., 1995; PubChem, 2017b) |
| 2-amino-3,4-dimethylimidazo[4,5-f]quinoline | MeIQ | 2.0 | 1 Amine 2 Methyl | ND | (PubChem, 2017c) |
| 2-amino-3,8-dimethylmidazo[4,5-f]quinoxaline | MeIQx | 1.0 | 1 Amine 2 Methyl | 1.10 – 2.61 | (Bogen and Keating, 2001; Keating and Bogen, 2004; Layton et al., 1995; PubChem, 2017d) |
| 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline | 4,8-DiMeIQx | 1.4 | 1 Amine 3 Methyl | 0.20 – 0.81 | (Bogen and Keating, 2001; Keating and Bogen, 2004; PubChem, 2017g) |
| 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine | PhIP | 2.2 | 1 Amine 1 Methyl | 6.00 – 16.64 | (Bogen and Keating, 2001; Keating and Bogen, 2004; Layton et al., 1995; PubChem, 2017a) |
| 1-methyl-9H-pyrido[3,4-b]indole | Harmane | 3.6 | 1 Methyl | NQ | (PubChem, 2017h) |
| 9H-Pyrido[3,4-B]indole | Norharmane | 3.2 | None | NQ | (PubChem, 2017f) |
| 2-amino-9H-pyrido[2,3-b]indole | AαC | 2.6 | 1 Hydrogen 1 Amine | 1.50 – 5.17 | (Bogen and Keating, 2001; Layton et al., 1995; PubChem, 2017e) |
ND: not detected, NQ: not quantified.
2.2. Chemicals
PhIP (A617000), IQ (A616500), MeIQ (A605200), MeIQx (A606600), 4,8-DiMeIQx (A631000) and AαC (A629000) were obtained from Toronto Research Chemicals (Ontario, Canada). Harmane (103276) and norharmane (N6252) were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.3 Animals
Embryonic day 14 (E14) timed-pregnant Sprague Dawley rats weighing 200 – 230 grams were purchased from Envigo (Indianapolis, IN, USA). Rats were given 3 days of acclimation and were housed in individual cages with unlimited access to food and water and exposure to 12-hour light and dark cycles. E17 pregnant rats were sacrificed with carbon dioxide delivered at a half flow rate until they reached unconsciousness and then at a full flow rate for rapid euthanasia (total exposure time: 5 min). All animal procedures were approved by the Purdue Animal Care and Use Committee (PACUC).
2.4. Preparation of Primary Midbrain Cultures and HCA Exposure
Primary midbrain cultures were prepared as previously described (Griggs et al., 2014; Strathearn et al., 2014). Briefly, midbrains obtained from E17 pregnant Sprague Dawley rat embryos were mechanically and enzymatically dissociated with trypsin (Life Technologies, 25200-072) at 26 µg/mL in 0.9% NaCl at 37°C for 20min. Then, trypsin inhibitor from Glycine max (soybean) (Sigma, T6522-25) (2.7 mg/ml) and DNase (4 mg/ml) were added to the cell suspension. After two rounds of centrifugation at room temperature (10 min at 180 × g and 5 min at 353 × g), the pellet was filtered (70 µm cell strainer) and resuspended in primary culture media (Low glucose DMEM, 35 mM sodium bicarbonate, 10% FBS premium, 10% horse serum, penicillin (10 U/mL), and streptomycin (10 µg/mL) and plated on 48-well plates previously coated with poly-L-lysine (10 µg/mL). Cells were plated at a density of 163,500 cells per well. After 5 d in culture the cells were treated with 17 µM cytosine arabinofuranoside (AraC) for an additional 5 d in order to inhibit the growth of glial cells. Ten days after the initial plating, the cultures were treated with HCAs at concentrations of 100 nM – 5 µM for 24 h. For select exposures, treatment times were extended up to 72 h to assess temporal development of cell loss and neurite retraction.
2.5. Immunocytochemistry
4% paraformaldehyde in PBS was used to fix the cells for 20 min. Cellular membranes were then permeabilized with PBS with 0.3% Triton X-100, 1% BSA and 10% FBS for 1 h at room temperature. The cells were incubated overnight at 4°C with primary antibodies: chicken anti-MAP2 (1:2000; EnCor Biotechnology Inc., CPCA-MAP2) to identify the general neuron population and rabbit anti-tyrosine hydroxylase (TH) (1:1000; Millipore, AB152) to identify dopaminergic neurons. The wells were then washed with PBS and incubated with secondary antibodies, Alexa Fluor 594 goat anti-chicken IgG (1:1000; Life Technologies, A11042) and Alexa Fluor 488 goat anti-rabbit IgG (1:1000; Life Technologies, A11008) at room temperature for 1 h. A Cytation 3 cell imaging reader (BioTek, USA) equipped with a 4× objective was used to obtain images that were further analyzed using the NIS-elements viewer (Nikon Instruments, USA).
2.6. Quantification of Dopaminergic and Non-Dopaminergic Neurons
Selective dopaminergic neurotoxicity was assessed by determining the percentage of total neurons that were dopaminergic (Griggs et al., 2014; Ysselstein et al., 2015). Neurons were counted in a blinded manner after obtaining images from the Cytation 3 by counting immunoreactive neurons stained with MAP2 and TH. One well consisted of a set of 16 images. Images taken near the edge of the plate were not counted because of variability of the cell density in this region. Specific morphological criteria were utilized in counting a cell as a neuron. A morphologically viable DA neuron was characterized as having a clearly visible nucleus and definitive TH+ staining (Cannon et al., 2009). Data obtained were expressed as a percent ratio of TH+ neurons to MAP2+ neurons to correct for variations in cell density. Both dopaminergic (TH+, MAP2+) and non-dopaminergic (TH−, MAP2+) neurons were assessed separately. Raw non-dopaminergic (TH−, MAP2+) cell counts were also reported to determine if non-dopaminergic neurons were affected. The majority of these TH− neurons are GABAergic (Cooper et al., 2006; Liu et al., 2008; Strathearn et al., 2014). Each experiment was repeated six times utilizing cultures prepared on different days.
2.6. Neurite Length Quantification
Neurite length measurements were performed on the same primary midbrain neuronal cultures that were imaged with the Cytation 3 and used for counting (staining as described above). The analysis was carried out using the NIS Elements BR 2.30 software as previously described (Griggs et al., 2014; Ysselstein et al., 2015). The software was calibrated prior to obtaining measurements to 1.6 microns per pixel. Neurite lengths for all dopaminergic (TH+) neurons were assessed at n = 100 – 200 neurites/treatment for 3 biological replicates. Neurites of MAP2+ cells were also measured in the same manner (n=300–400 neurites/treatment from 3 biological replicates). Neurites that were clearly attached to a viable cell body and also branched were included in the analysis. If multiple neurites branched off, then the longest was measured.
2.7. 3-Nitrotyrosine (NT) Quantification
Production of reactive nitrogen species (RNS) indicates that the cell is undergoing oxidative/nitrosative stress. To determine if these neurons experienced oxidative damage, one representative compound from each tested HCA subclass was examined. MeIQ and harmane at 1 µM were tested, representing the AIA and carboline groups, respectively. Primary cells were plated and treated on nitric acid treated coverslips and fixed as previously described. The following primary antibodies were used for immunocytochemistry: Chicken anti-MAP2 (1:2000; EnCor Biotechnology Inc, CPCA-MAP2), rabbit anti-NT (1:500; Millipore, 06-284) and mouse anti-TH (1:2000; Millipore, AB1542). Incubation of the primary antibodies occurred overnight at 4°C. Coverslips were then washed two times with PBS prior to secondary antibodies being added: Alexa Fluor 647 donkey anti-rabbit IgG (1:500; Jackson IR Laboratories, 711-606-152), Alexa Fluor 594 goat anti-chicken IgG (1:1000; Life Technologies, A11042) and Alexa Fluor 488 donkey anti-mouse IgG (1:500; Jackson IR Laboratories, 715-545-151). Incubations with secondary antibody took place for 1 h at room temperature. Cells were washed with PBS and mounted on microscope glass slides using ProLong Gold antifade mountant (Life Technologies, P36930)
Oxidative damage in DA neurons was quantified as described (Griggs et al., 2014). Oxidative damage was also assessed in non-dopaminergic neurons (TH−, MAP2+). Images from 3 separate experiments were taken in a blinded manner with a Nikon A1R confocal microscope using a 60× objective. To quantify oxidative damage, regions of interest (ROIs) were drawn around DA neurons with clear evidence of TH staining and a visible nucleus. The average NT intensity of ROIs for individual dopaminergic neurons were quantified using Nikon NIS-Elements AR software and normalized to the mean of the control in each experiment. Each dopaminergic ROI served as a distinct data point with a rationale for analysis similar to that previously described (Horowitz et al., 2011).
2.8. Statistical Analysis
Analysis was performed under the consultation of a biostatistician and with similar methodologies to our previous primary culture studies (Griggs et al., 2014). To conform with ANOVA assumptions for low-value percentage data, cytotoxicity data were transformed using a square root transformation. The results suggested that transformation did not alter the statistical outcome. One-way ANOVA with Dunnett’s post hoc test (repeated measures) was performed to test which HCA doses produced cytotoxicity relative to control (Prism 6, GraphPad, La Jolla, CA, USA). For neurite length measurements, each neurite was considered a biological replicate and was analyzed in that manner. Our previous studies suggest that neurite length data should be subjected to a more robust analysis to account for the potential of multiple neurites arising from a single cell and comparison across experiments conducted on different days (Griggs et al., 2014). Here, the term “experiment” is used to refer to a collection of measurements performed on a variety of treatment conditions with controls on the same day. Because the distribution of neurite lengths is strongly skewed to the right, the lengths were transformed using logs. The log least squares means data were analyzed using general linear models that included terms for treatments (5 HCA doses and controls), experiments, neurons (a random effect), and neurites within neurons followed by Dunnett’s test to compare each treatment with the corresponding controls. Proc glm in SAS Version 9.3, Cary, N.C., USA was used for the analyses. Immunofluorescence intensity for the oxidative stress marker (NT) was normalized to the mean control value for a given experiment and cell-type. These data failed the D'Agostino-Pearson normality test. Therefore, they were analyzed by nonparametric methods. The Mann-Whitney U-test was used when comparing 2 groups and the Kruskal-Wallis H-test followed by Dunn’s multiple comparisons post hoc test was used for 3 groups. For all results, p<0.05 deemed significant.
3. Results
3.1. HCAs in the AIA group are selectively toxic to DA neurons
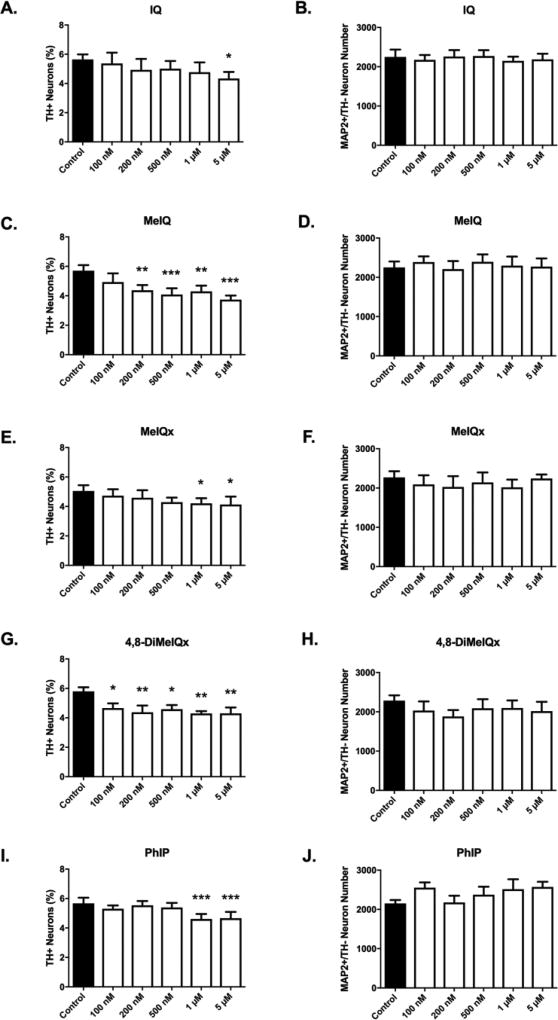
All HCAs in the AIA group (IQ, MeIQ, MeIQx, 4,8-DiMeIQx and PhIP) were toxic to dopamine neurons (Figures 2, 3). Selective toxicity to dopaminergic neurons was evident by quantifiable decreases in the percentage of neurons that were dopaminergic (Figure 3A,C,E,G,I) (n = 6/group). The required threshold dose to elicit a statistically significant decrease in the percentage of dopaminergic neurons was HCA-specific. At doses as low as 100 nM, 4,8-DiMeIQx exhibited a selective loss of 20% of dopaminergic neurons (4.663 ± 0.3194 vs. 5.798 ± 0.2806%; mean % dopaminergic neurons ± S.E.M; 4,8-DiMeIQx vs. control, p<0.05) (Figure 3G). Selective toxicity was also observed for all 4,8-DiMeIQx tested doses (100 nM – 5 µM). A dose of 200 nM was required for MeIQ to elicit selective dopaminergic toxicity (23% loss; 4.372 ± 0.3579 vs. 5.703 ± 0.3805%, p<0.01) (Figure 3C). For MeIQx and PhIP, a dose of 1 µM was required to elicit selective dopaminergic toxicity [MeIQx (17% loss; 4.207 ± 0.3535 vs. 5.052 ± 0.391%, p<0.05); PhIP (19% loss; 4.612 ± 0.3403 vs. 5.67 ± 0.3843%, p<0.001)]. This dose of PhIP was the same dose that was required to elicit selective dopaminergic neurotoxicity in our previous report (Griggs et al., 2014). IQ required the highest dose, at 5 µM to produce selective dopaminergic neurotoxicity (23% loss; 4.338 ± 0.4525 vs. 5.643 ± 0.346%, p<0.05).
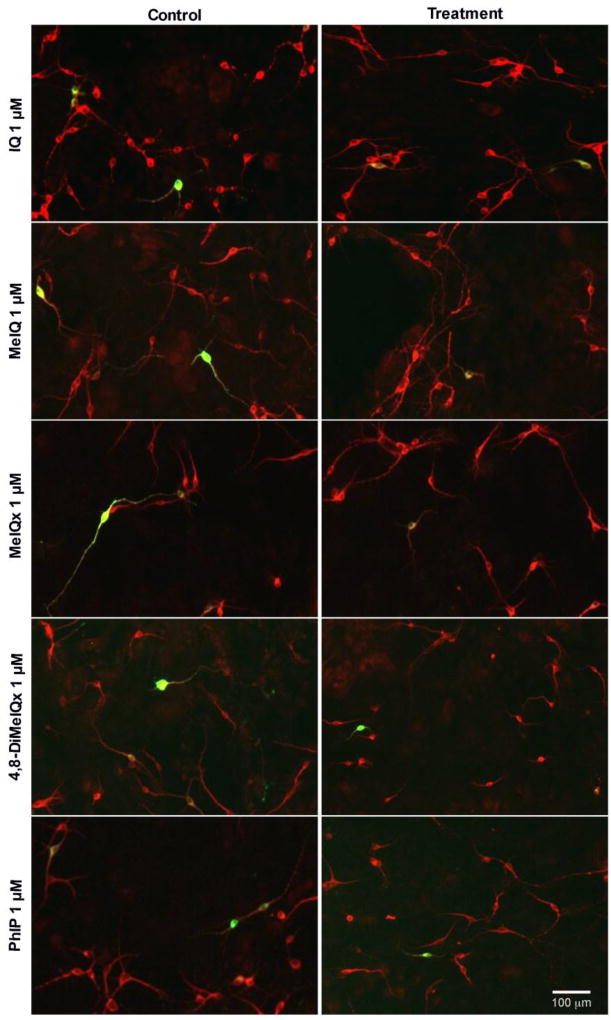
Figure 2. Representative images of primary midbrain neuronal cultures exposed to aminoimidazoaazarene HCAs (1 µM) show selective dopaminergic neurotoxicity.
Primary midbrain cultures from E17 rat embryos were treated with IQ, MeIQ, MeIQx, 4,8-DiMeIQx and PhIP for 24 h at concentrations of 100 nM – 5 µM. Cells were stained for tyrosine hydroxylase, TH (green) to identify dopamine neurons and microtubule-associated protein 2, MAP2 (red) to identify all neurons. Quantification was performed at all doses, while representative images are shown here from a 1 µM treatment, where the majority of tested HCAs exhibited selective dopamine toxicity. Images were obtained used an automated Cytation 3 cell imaging reader with a 4× objective. Scale bar represents 100 µm.
Figure 3. Aminoimidazoaazarene treatment produces selective dopaminergic neurotoxicity.
Selective DA neuronal loss was detected for all tested aminoimidazoaazarene HCAs (IQ, MeIQ, MeIQx, 4,8-DiMeIQx, PhIP). The threshold dose required to elicit selective dopamine neuron toxicity is compound-dependent (A,C,E,G,I). Non-dopaminergic neurons are not affected at tested doses (B,D,F,H,J) as evidenced by undetectable changes in MAP2+/TH− cell counts. Cells were stained for TH (green) to identify dopamine neurons and MAP2 (red) to identify all neurons. The percentage of TH+ neurons to MAP2+ neurons (TH+ Neurons %) was calculated to determine DA neuronal loss. Data presented as the mean ± SEM; one way ANOVA with Dunnett’s post-hoc test, *p<0.05, **p<0.01, ***p< 0.001 compared to control. n = 6/group.
Decreases in the percentage of dopaminergic neurons (dopamine neurons relative to total neurons) provide evidence of selective dopaminergic cell loss. To further determine whether nondopaminergic neurons were affected at any dose, but to a lesser extent than dopaminergic neurons, raw cell counts of non-dopaminergic (MAP2+/TH−) neurons were also determined. At all doses, for all AIA HCAs, there were no detectable effects on non-dopaminergic cell numbers, providing further evidence of selective dopaminergic neurotoxicity (n = 6/group) (Figure 3B,D,F,H,J).
3.2. AIA HCAs produce neurite length alterations in DA neurons
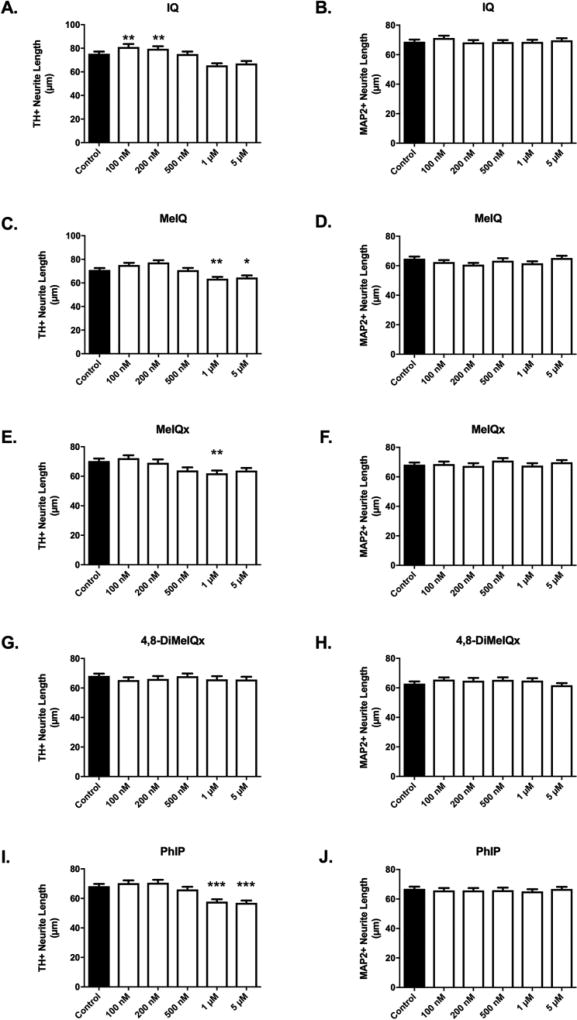
Neurite retraction is also evidence of ongoing toxicity in surviving neurons (Griggs et al., 2014). Quantifiable retraction was observed for most AIAs (Figure 4). Interestingly, IQ treatment produced significant increases at low doses (100 and 200 nM) and apparent though not significant decreases at higher doses (Figure 4A). MeIQ, MeIQx, and PhIP treatment produced significant retraction of dopaminergic (TH+) neurites at various concentrations (Fig. 4C,E,I). Similar to cytotoxicity data (Figure 3), the required threshold dose was HCA-dependent. MeIQ produced decreases that were detectable at 1 µM and above, where a 10% decrease was observed (63.58 ± 1.584 vs. 70.93 ± 1.668 µm, p<0.01, n = 563 – 645) (Figure 4C). Treatment with MeIQx at 1 µM produced a 12% decrease in neurite length (61.97 ± 1.94 vs. 70.28 ± 1.776 µm, p<0.01, n = 409 – 577) (Figure 4E). PhIP treatment produced a 15% retraction at 1 µM (57.76 ± 1.656 vs. 68.22 ± 1.595 µm, p<0.001, n = 587 – 648) (Figure 4I). There was no significant alteration in neurite lengths of 4,8-DiMeIQx-treated TH+ neurons (Figure 4G).
Figure 4. Aminoimidazoaazarene treatment produces selective decreases in dopaminergic neurite lengths.
Decreased dopaminergic neurite lengths were detected in cultures exposed to IQ (A) and MeIQ (C) at 1 µM – 5 µM and to MeIQx (E) at 1 µM, while 4,8-DiMeIQx (G) did not have an effect on neurite lengths in DA neurons. DA neurons treated with 1 µM – 5 µM PhIP (I) exhibited decreased neurite lengths (n = 326 – 648 TH+ neurites/group analyzed). No changes in neurite length were detected when pan-neuronal analysis was conducted (MAP2+), suggesting that changes were specific to dopaminergic neurons (B,D,F,H,J) (n = 428 – 818). Data presented as the mean ± SEM; general linear model with Dunnett’s post-hoc test, *p<0.05, **p<0.01, ***p<0.001 compared to control. n = total number of neurites measured from 3 different experiments.
Similar to cytotoxicity data, neurite retraction was selective to dopaminergic neurites. Neurite lengths were also analyzed in total neurons (MAP2+), where <10% are dopaminergic. In this population, no differences in neurite lengths were detected, regardless of compound or dose, further suggesting selectiveness of toxicity to dopaminergic neurons for AIA HCAs (Figure 4B,D,F,H,J).
3.3. HCA in the α/β-carboline group are selectively toxic to DA neurons
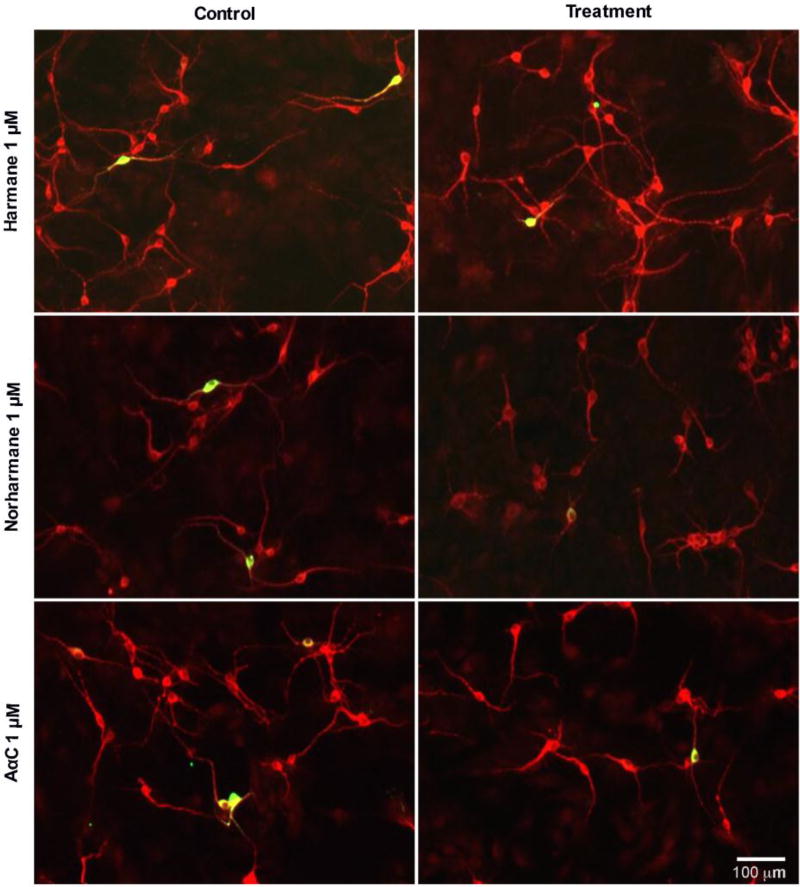
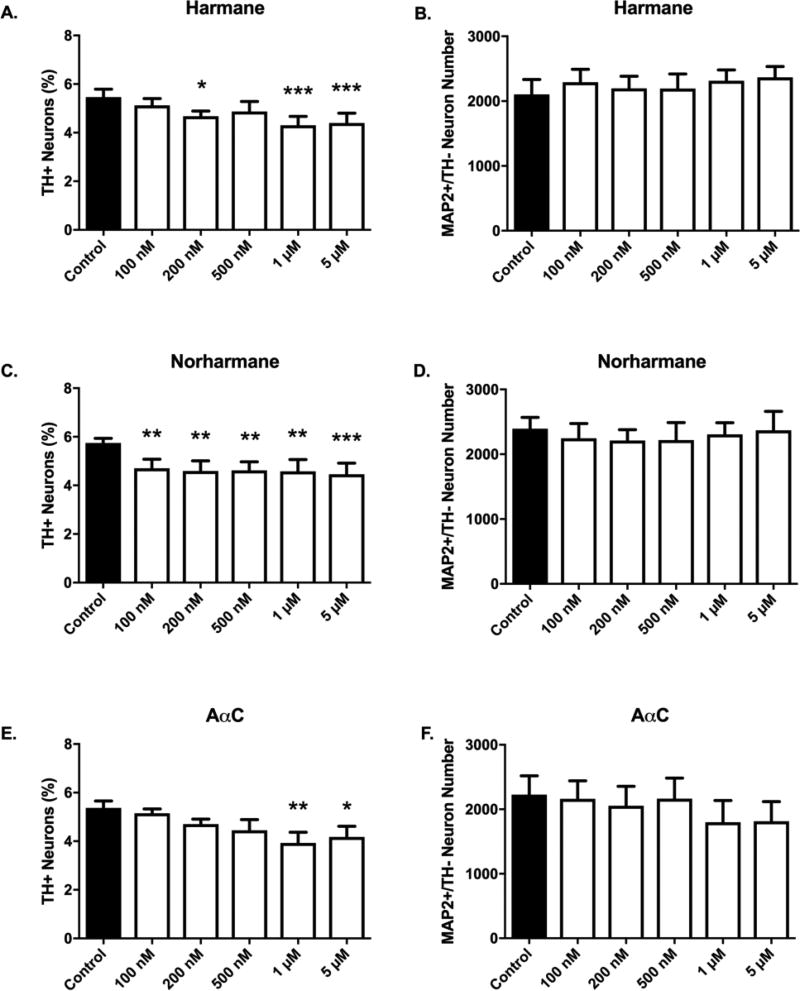
α/β-carboline treatment produced selective toxicity in dopaminergic neurons (Figures 5, 6). Selective toxicity to dopaminergic neurons was evident by quantifiable decreases in the percentage of neurons that were dopaminergic (Figure 6A,C,E) (n = 6/group). The required threshold dose to elicit a statistically significant decrease in the percentage of dopaminergic neurons was HCA-specific. In cultures treated with 100 nM norharmane, there was a detectable 18% decrease in the percentage of dopaminergic neurons (4.703 ± 0.3711 vs. 5.747 ± 0.190%, p<0.01; mean % dopaminergic neurons ± S.E.M) (Figure 6C). Harmane required a dose of 200 nM to elicit a significant decrease in dopaminergic neurons (15% loss; 4.677 ± 0.2123 vs. 5.467 ± 0.3244%, p<0.05) (Figure 6A). Finally, AαC required a dose of 1 µM to elicit a significant decrease in dopaminergic neurons (27% loss; 3.932 ± 0.4382 vs. 5.363 ± 0.2877%, p<0.01) (Figure 6E). Similar to AIAs, α/β-carboline treatments also showed no effect on raw counts of non-dopaminergic neurons (n = 6/group), providing further evidence that neurotoxicity is selective to dopaminergic neurons (Figure 6B,D,F).
Figure 5. Representative images of primary midbrain neuronal cultures exposed to α/β-carboline HCAs (1 µM) show selective dopaminergic neurotoxicity.
Primary midbrain cultures from E17 rat embryos were treated with β-carbolines (harmane or norharmane), or the α-carboline, AαC, for 24 h at concentrations of 100 nM – 5 µM. Cells were stained for tyrosine hydroxylase, TH (green) to identify dopamine neurons and microtubule-associated protein 2 MAP2 (red) to identify all neurons. Quantification was performed at all doses, while representative images are shown here from a 1 µM treatment, where the majority of tested HCAs exhibited selective dopamine toxicity. Images were obtained used an automated Cytation 3 cell imaging reader with a 4× objective. Scale bar represents 100 µm.
Figure 6. α/β-carboline treatment produces selective dopaminergic neurotoxicity.
Selective DA neuronal loss was detected for all tested aminoimidazoaazarene β-carboline (harmane or norharmane) and α-carboline (AαC) HCAs. The threshold dose required to elicit selective dopamine neuron toxicity is compound-dependent (A,C,E). Nondopaminergic neurons are not affected at tested doses (B,D,F) as evidenced by undetectable changes in MAP2+/TH− cell counts. Cells were stained for tyrosine hydroxylase, TH (green) to identify dopamine neurons and microtubule-associated protein 2 MAP2 (red) to identify all neurons. The percentage of TH+ neurons to MAP2+ neurons (TH+ Neurons %) was calculated to determine DA neuronal loss. Data presented as the mean ± SEM; one way ANOVA with Dunnett’s post-hoc test, *p<0.05, **p<0.01, ***p< 0.001 compared to control. n = 6/group.
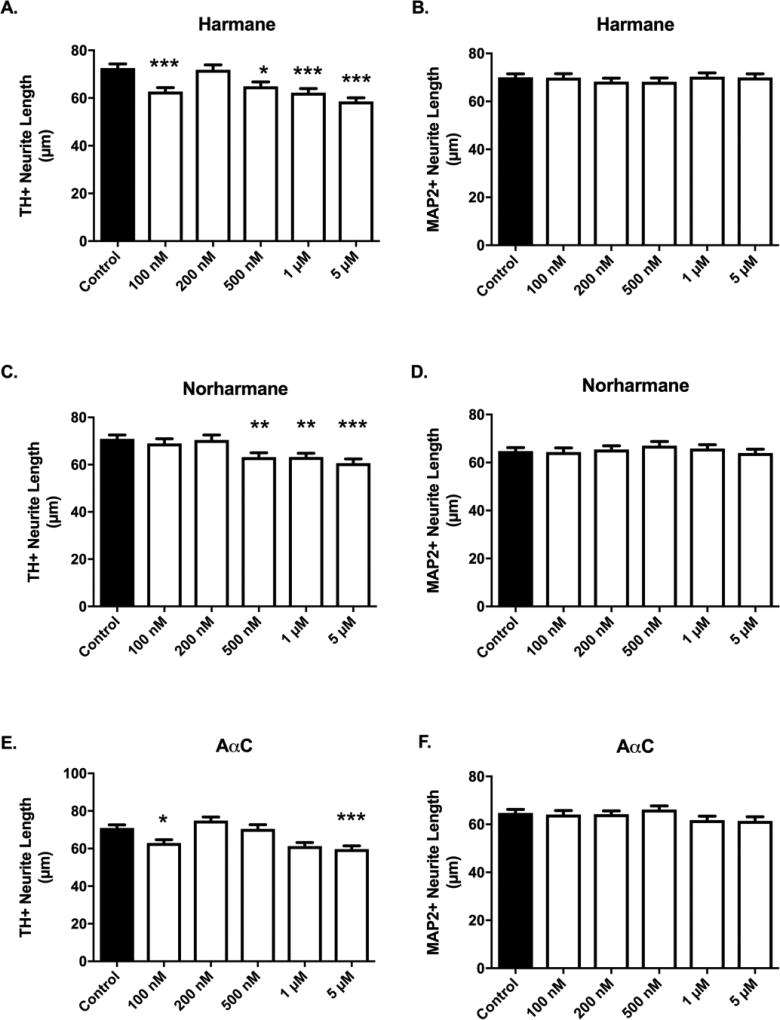
3.4. α/β-Carboline HCAs produce decreased neurite lengths in DA neurons
Harmane, norharmane and AαC treatments produced significant retraction in neurites of dopaminergic neurons at specific concentrations (Figure 7A,C,E). Similar to cytotoxicity data (Figure 6), the required threshold dose was HCA-dependent. Decreases in neurite length following harmane treatment were detectable as low as 100 nM, with 14% retraction (62.67 ± 1.713 vs. 72.57 ± 1.702 µm, p<0.001, n = 511 – 617) (Figure 7A). Norharmane required a higher dose to elicit neurite retraction, where 500 nM treatment produced significant retraction (11% decrease; 63.15 ± 1.864 vs. 70.93 ± 1.668 µm, p<0.01, n = 535 – 645) (Figure 7C). Data obtained from AαC treatments were similar to that of harmane, where there is a detectable 11% decrease at 100 nM (62.93 ± 1.743 vs. 70.93 ± 1.668 µm, p<0.05, n = 491 – 645) (Figure 7E). In harmane, there is a suggestion of a non-monotonic response in neurite length decreases, with detectable differences at low concentrations (100 nM) and high concentrations (1 and 5 µM), but a lack of detectable changes at an intermediate dose (200 nM). AαC exhibited a somewhat similar pattern, with less statistically identified differences (Figure 7A,C).
Figure 7. α/β-carboline treatment produces selective decreases in dopaminergic neurite lengths.
Decreased dopaminergic neurite lengths were detected in cultures exposed to harmane (A) at 100 nM, 500 nM, 1 µM, 5 µM, norharmane (C) at ≥ 500 nM, or AαC (E) at 100 nM, 1 µM, 5 µM (n = 347 – 645 TH+ neurites/group analyzed). No changes in neurite lengths were detected when pan-neuronal analysis was conducted (MAP2+), suggesting that changes were specific to dopaminergic neurons (B,D,F) (n = 479 – 793). Data presented as the mean ± SEM; general linear model with Dunnett’s post-hoc test, *p<0.05, **p<0.01, ***p< 0.001 compared to control. n = total number of neurites measured from 3 different experiments.
Similar to cytotoxicity data, neurite retraction was selective to dopaminergic neurites. Neurite lengths were also analyzed in total neurons (MAP2+), where <10% are dopaminergic. In this population, no differences in neurite lengths were detected, regardless of compound or dose, further suggesting selectiveness of toxicity to dopaminergic neurons for α/β-carboline HCAs (Figure 7B,D,F).
3.5. Threshold dose to elicit selective dopaminergic neurotoxicity
Data in sections 3.1 and 3.3 show that all HCAs tested are selectively neurotoxic to dopaminergic neurons regardless of HCA subclass, as measured by both loss of dopaminergic neurons (Figures 3,6) and decreases in neurite length (Figures 4,7). While the threshold dose required to elicit selective dopaminergic neurotoxicity did not appear to be subclass specific, the threshold dose varied by 50× between compounds (100 nm – 5 µM) (Table 2). A clear dose-response relationship was not observed in the tested endpoints, across a somewhat narrow dose range (Figures 3,4,6,7).
Table 2.
Comparison of quantified neurotoxicity
| Heterocyclic amine | Subclass | Threshold for detectable selective DA neurotoxicity |
DA neuron loss at 1 µM |
|---|---|---|---|
| IQ | Aminoimidazoaazarene | 5 µM | Not significant |
| MeIQ | Aminoimidazoaazarene | 200 nM | 25% |
| MeIQx | Aminoimidazoaazarene | 1 µM | 17% |
| 4,8-DiMeIQx | Aminoimidazoaazarene | 100 nM | 26% |
| PhIP | Aminoimidazoaazarene | 1 µM | 19% |
| Harmane | β-carboline | 200 nM | 21% |
| Norharmane | β-carboline | 100 nM | 20% |
| AαC | α-carboline | 1 µM | 27% |
3.6. Prolonged treatment times increases dopaminergic neuron loss in the absence of a temporal effect on neurite lengths
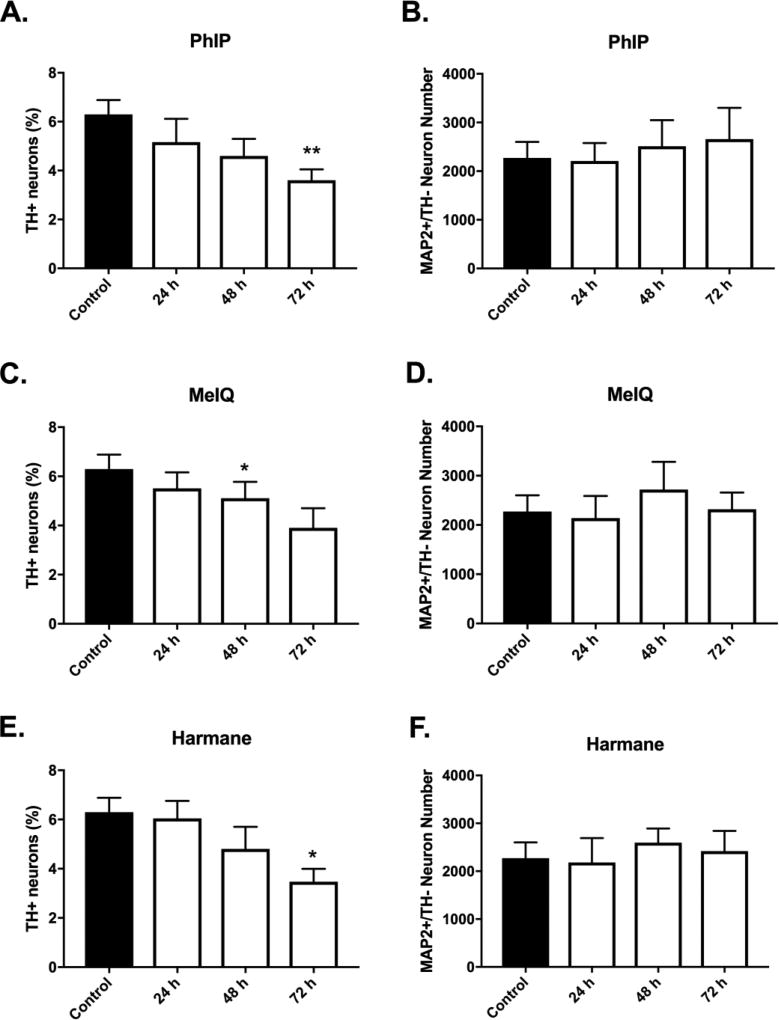
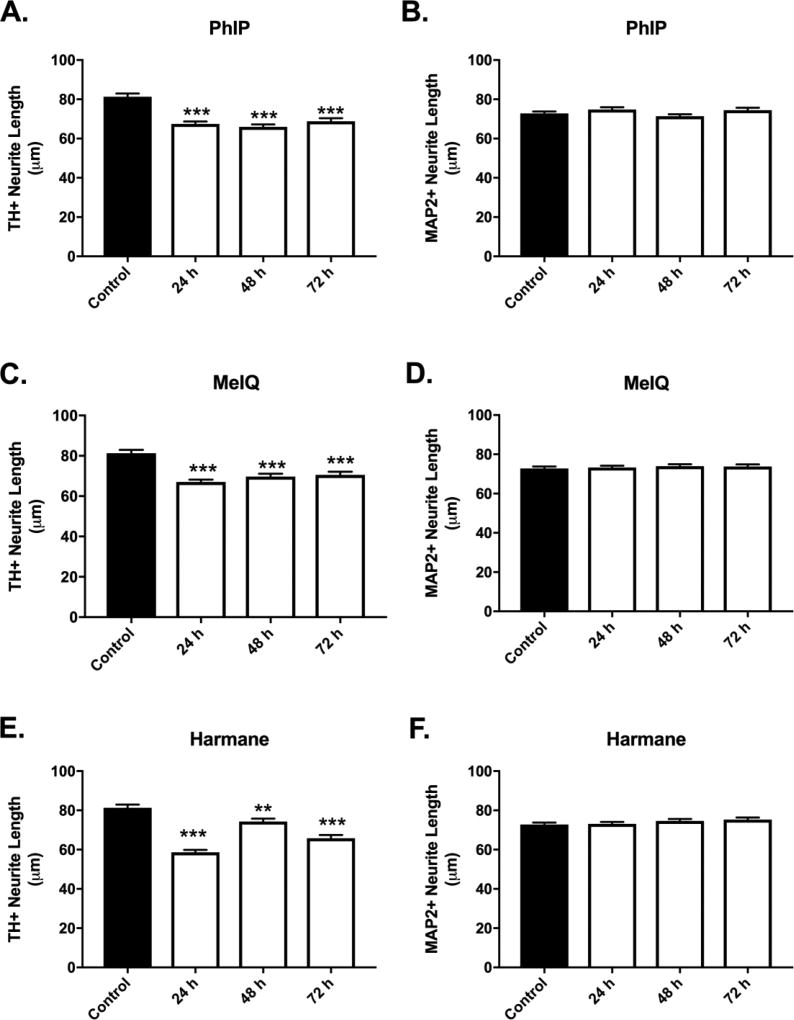
To determine if prolonging HCA treatment time increased the magnitude of dopaminergic cell loss, treatment times for select HCAs were extended up to 72 h. For these studies, a single dose of 1 µM was chosen based upon the dose response data from Figures 2–7. Temporal evaluation of dopaminergic cell loss provided evidence of an increase in the magnitude of cell loss over time (Figure 8A,C,E). PhIP treatment produced progressive loss of dopaminergic neurons, which was quantified as the percentage of dopaminergic neurons (Figure 8A) (6.30 ± 0.58 vs. 5.17 ± 0.95, 4.60 ± 0.70, 3.61 ± 0.45; control vs. PhIP at 24, 48, or 72 h, respectively; mean ± SEM; p<0.05 for control vs. 72 h). Treatment with MeIQ and harmane also provided evidence of progressive selective toxicity. MeIQ (Figure 8C): (6.30 ± 0.58 vs. 5.51 ± 0.66, 5.11 ± 0.66, 3.90 ± 0.81; control vs. MeIQ at 24, 48, or 72 h, respectively; mean ± SEM; p<0.05 for control vs. 48 h). Harmane (Figure 8E): (6.30 ± 0.58 vs. 6.04 ± 0.72, 4.80 ± 0.90, 3.47 ± 0.53; control vs. harmane at 24, 48, or 72 h, respectively; mean ± SEM; p<0.05 for control vs. 72 h. These experiments also confirmed our findings of specificity, where non-dopaminergic neurons were unaffected at all time-points (Figure 8B,D,F). Not all treatments achieved significance identical to that in Figures 3,6. Here (Figure 8), it is worth noting that the comparisons were different (across time vs. dose), with a lower number of repeats (n = 4 vs n = 6 in Figures 3,6). Importantly, the data in both sets of experiments show the effects of both dose and time on selective dopaminergic neurotoxicity.
Figure 8. Temporal development of HCA-induced selective dopaminergic neuron loss.
Progressive selective DA neuronal loss was detected for PhIP (A), MeIQ (C), and harmane (E) at 1 µM (24 – 72 h). Non-dopaminergic neurons are not affected at the tested dose at all time-points (B,D,F) as evidenced by undetectable changes in MAP2+/TH− cell counts. Cells were stained for TH (green) to identify dopamine neurons and MAP2 (red) to identify all neurons. The percentage of TH+ neurons to MAP2+ neurons (TH+ Neurons %) was calculated to determine DA neuronal loss. Data presented as the mean ± SEM; one way ANOVA with Dunnett’s post-hoc test, *p<0.05, **p<0.01 compared to control. n = 4/group.
Neurite length analysis in time-course studies (Figure 9) produced similar evidence of toxicity as in 24 hour studies. These studies further confirm specificity in earlier experiments. However, a clear time-response effect is not overtly apparent as with cell loss.
Figure 9. Temporal development of HCA-induced selective decreases in dopaminergic neurite lengths.
Decreased dopaminergic neurite lengths were detected in cultures exposed to PhIP (A), MeIQ (C), and harmane (E) at 1 µM (24 – 72 h) (n = 519 – 834 TH+ neurites/group analyzed). Non-dopaminergic neurites are not affected at the tested dose at all time-points (B,D,F) (n = 859 – 1204). Cells were stained for TH (green) to identify dopamine neurons and MAP2 (red) to identify all neurons. Data presented as the mean ± SEM; general linear model with Dunnett’s post-hoc test, **p<0.01, ***p< 0.001 compared to control. n = total number of neurites measured from 3 different experiments.
3.7. MeIQ and harmane produce oxidative damage in DA neurons
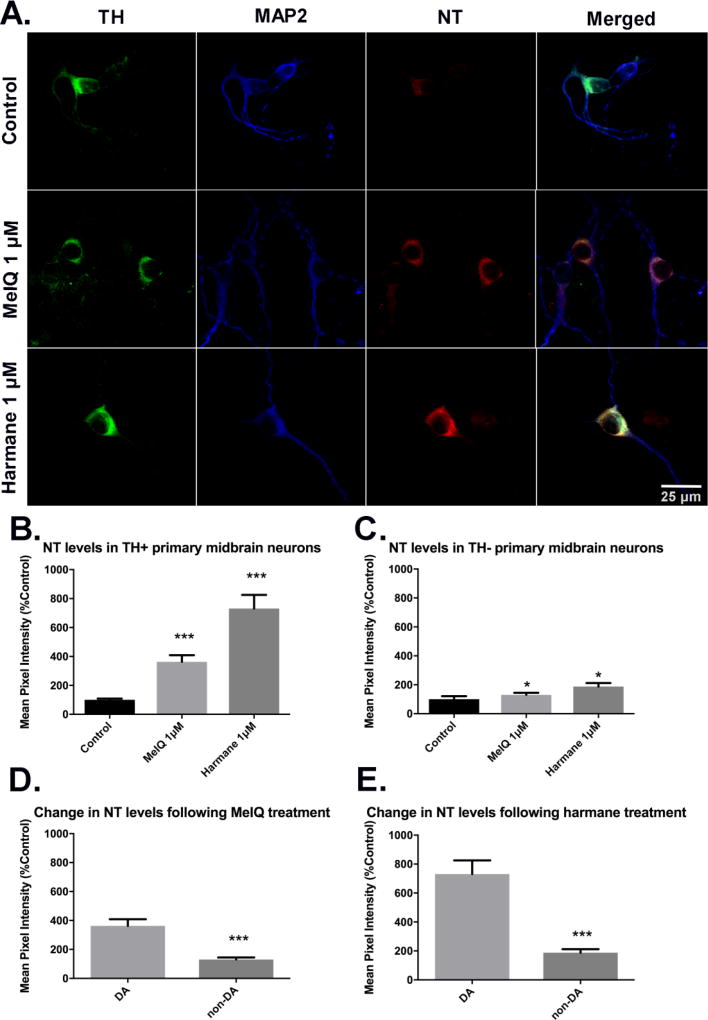
Oxidative damage was measured by quantifying nitrotyrosine intensity in cell bodies of dopaminergic (TH+, MAP2+) and non-dopaminergic (TH−, MAP2+) neurons. Oxidative damage was measured in cultures treated with MeIQ or harmane, as representative compounds for the AIA and carboline groups, respectively (Figure 10). In the AIA group, MeIQ was one of the few compounds that showed an observable downward trend in percentage of dopaminergic neurons with significance starting at 200 nM carried through 5 µM. MeIQ-treated primaries at 1 µM showed a > 5-fold increase in immunofluorescence signal for NT, indicating oxidative damage (362.9 ± 45.8 vs. 100 ± 9.1, p<0.001, n =137 – 138 dopaminergic neurons analyzed as ROIs, over 3 biological replicates; fluorescence intensity normalized to mean control value ± SEM and analyzed by Kruskal-Wallis ANOVA followed by Dunn’s post hoc test) (Figure 10B). Harmane was chosen to represent the α/β-carboline group with the same criteria that were used to choose MeIQ as a representative compound from the AIA group. Primary cultures treated with 1 µM harmane exhibited increased NT vs. control (731.3 ± 94.03 vs. 100 ± 9.1, p<0.001, n = 138 – 140 dopaminergic neurons analyzed as ROIs, over 3 biological replicates) (Figure 10B). Non-dopaminergic neurons also exhibited evidence of oxidative damage after HCA treatment, where cells treated with 1 µM MeIQ exhibited increased NT vs. control (130.1 ± 14.91 vs 100 ± 21.61, p<0.001), and those treated with 1 µM harmane also exhibited increased NT vs. control (188.1 ± 23.92 vs. 100 ± 21.6, p<0.001) n = 71 – 111 non-dopaminergic neurons analyzed as ROIs, over 3 biological replicates)]. The magnitude of NT level change after HCA treatment in nondopaminergic neurons was far less than in dopaminergic neurons (Figure 10C). Direct comparison of NT level change for a given HCA treatment showed that for both MeIQ and harmane, the increases were significantly greater in dopaminergic neurons vs. non-dopaminergic neurons (362.9 ± 45.8 vs. 130.1 ± 14.91 for MeIQ, p<0.001; 731.3 ± 94.03 vs. 188.1 ± 23.92 for harmane, p<0.001; Mann-Whitney U-test) (Figure 10D,E).
Figure 10. Harmane and MeIQ produce oxidative damage in DA neurons expressed through nitrotyrosine (NT) intensity.
A representative compound from the aminoimidazoaazarene subclass (MeIQ) and the α/β-carboline subclass (harmane) was chosen to test for the production of oxidative damage in dopaminergic neurons as evidenced by nitrotyrosine staining intensity. Representative images of untreated cultures or cultures treated with harmane (1 µM) or MeIQ (1 38 µM). Images captured via confocal microscopy after immunostaining using antibodies specific to TH (green), NT (red) and MAP2 (blue). Scale bars represent 25 µm (A). For quantification, NT fluorescence intensities in ROIs surrounding DA neuron cell bodies were quantified, then normalized to the mean value of the untreated culture. DA (B) and non-DA (C) neurons treated with harmane or MeIQ exhibited significantly increased NT levels. Data presented as the mean ± SEM; Kruskal-Wallis ANOVA with Dunn’s post-hoc test, *p<0.05, ***p<0.001 compared to control. Direct comparison between DA and non-DA neurons for each HCA shows (D, E) that DA neurons exhibited significantly increased oxidative damage vs. non-DA neurons after treatment. Data presented as the mean ± SEM; Mann-Whitney U-test, ***p<0.001 compared to DA. n = 71 – 141 ROIs analyzed/group over 3 biological repeats.
4. Discussion
Substantial data implicate a role for environmental exposures in the etiology of PD. Yet, specific exposures that are causative and likely to have a role in a significant number of cases have been very difficult to identify. Many classes of compounds have been implicated as potential PD risk factors, notably pesticides, metals, and other occupational and environmental exposures (Cannon and Greenamyre, 2011). Dietary exposures have been examined to a lesser extent; however, emerging data suggest a potential role in PD etiology (Agim and Cannon, 2015). HCAs are ubiquitous dietary components that have been suggested to be neurotoxic, with very limited neurotoxicity testing. Although we showed that one member of the HCA class of compounds, PhIP, was selectively toxic to dopaminergic neurons (Griggs et al., 2014), numerous other HCAs with significant differences in structural and physical properties had not been previously tested for neurotoxicity. Our goal here was to test a variety of HCAs, across multiple subclasses, for selective dopaminergic neurotoxicity and potential relevance to PD. We used a primary rat midbrain culture system that has been repeatedly utilized to model PD and test selective toxicity (Griggs et al., 2014; Strathearn et al., 2014). Here, we show that virtually all tested HCAs are selectively toxic to dopamine neurons, based on our observation that cell loss and neurite retraction occurred with the TH+ neuronal subpopulation of rat primary midbrain cultures, but not with non-dopaminergic neurons. While selective toxicity was broad across HCA subclass, the threshold dose required to elicit selective dopaminergic neurotoxicity was HCA-dependent. HCA neurotoxicity is important to investigate because both clinical and animal model studies show that multiple HCA subclasses enter the brain (Enokizono et al., 2008; Louis et al., 2013; Teunissen et al., 2010). Thus, our results are expected to prompt more detailed mechanistic and epidemiological studies to further examine the potential role of single and cumulative HCA exposures in PD etiology.
In examining HCA neurotoxicity, numerous compounds were considered for inclusion in this study. Here, we focused on HCAs that are prevalent in cooked meats, from three HCA subclasses, AIA and α and β-carbolines, which we characterized in terms of their effects on dopaminergic cell viability and oxidative stress. We chose to test the following AIAs: IQ, MeIQ, MeIQx, 4,8-DiMeIQx, and PhIP because extensive literature exists on the potential roles of these compounds in mutagenesis and carcinogenesis (Gibis, 2016). Harmane and norharmane were selected to represent β-carbolines in our study due to previous findings suggesting that these compounds are elevated in the plasma of patients with PD and ET (Louis et al., 2008; Louis et al., 2014b). α–Carboline, AαC, is known to possess mutagenic properties as well as being abundant like PhIP in certain foods and was chosen to represent this subclass (Raza et al., 1996).
The rat primary midbrain culture system utilized to evaluate selective dopaminergic neurotoxicity produced by HCA treatment is ideal due to the fact that the cultures consist of a mixed cell population that reproduces key features of the midbrain in vivo (Liu et al., 2009). Unlike immortalized cell lines such as SH-SY5Y, N27, and PC12, this model system contains different types of neurons including dopaminergic, serotonergic, and GABAergic neurons (Heusinkveld and Westerink, 2017), providing a system to observe selective neurotoxicity of compounds tested. In addition, the use of mixed cultures of glial cells and mature neurons enables one to mimic the environment in the mesencephalic region in humans (Gaven et al., 2014).
We found that all tested HCAs, both AIA and α/β-carbolines, produced selective loss of dopaminergic neurons as evidenced by decreases in the percentage of TH+ neurons. A lack of effect on non-dopaminergic neurons was further evident by direct analysis of MAP2+ cell counts. We used PhIP (Griggs et al., 2014), previously shown by our group to-induce dopaminergic neurotoxicity as a positive control in this study. Here, we found that the dose of PhIP required to induce selective dopaminergic neurotoxicity (1 µM) matched that determined in our previous report, strengthening our conclusions. While harmane and norharmane were previously examined in PD patients and in patients with other neurological diseases, the compounds were not thoroughly evaluated for selective dopaminergic neurotoxicity in a primary culture system (Kuhn et al., 1996; Kuhn et al., 1995; Louis et al., 2013; Louis et al., 2014a; Louis et al., 2014b). It is worth noting that one group did report evidence of dopaminergic neurotoxicity in mice after norharmane injections (~80 mg/kg, 2× day for 7 days) and also in a primary culture system exposed to the methylated cation of norharmane at 1 µM and above (Matsubara et al., 1998). There are very limited data on any potential relevance to neurotoxicity for the other HCAs tested in this report. Far more effort and interest have generally been directed to carbolines than AIAs, due to structural similarities to the known PD-relevant toxicant, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Matsubara et al., 1998). Interestingly, our data using an AIA (PhIP) and a β-carboline (harmane) have suggested that transport through the dopamine transporter (DAT) was unlikely, whereas studies in non-neuronal cell lines expressing DAT suggest that 2[N]-methylated β-carboline toxicity is mediated through DAT. Thus, the uptake and toxicity mechanisms may differ between subclasses (Griggs et al., 2014; Sammi et al., 2017; Storch et al., 2004).
For all compounds tested, neurite length alterations were observed only for neurites of dopaminergic neurons. Alterations in dopaminergic neurites were typically in the form of retraction as measured by decreased length. When total neurons were analyzed, no differences were observed among groups regardless of compound tested or dose, providing further evidence of specificity. In general, neurite length data strengthened the conclusions from the cytotoxicity data. However, there were some interesting differences. Most of these were in the doses that elicited significant effects on cell viability versus neurite length. Interestingly, IQ produced increases in length at low doses and apparent, though nonsignificant decreases at very high doses. Of all HCAs tested, IQ required the highest dose to produce cell loss. Thus, the observed effect may represent compensation in the absence of cell death. Also of note, dopaminergic neurons appeared to be most sensitive to 4,8-DiMeIQx, exhibiting selective cell loss as low as 100 nM and all tested doses, whereas no changes in neurite length were observed for TH+ neurons exposed to this compound at any tested dose. It is possible that all sensitive neurons were already lesioned, and surviving dopaminergic neurons were less sensitive in terms of neurite retraction. To this point, it is interesting that neurite length was typically a less sensitive endpoint than cell loss, whereas in vivo axonal retraction is thought to precede cell death. Such differences may result from neurites in culture being fundamentally different than in vivo. For example, in animals, axon lengths of dopaminergic neurons may reach nearly 1 meter due to a very convoluted path (Matsuda et al., 2009). Such morphological characteristics are thought to be one key aspect of selective sensitivity of the nigrostriatal dopamine system.
For both cell loss and neurite retraction, there was a general lack of a dose-response relationship. There are several possible explanations for a lack of dose-response. A classic dose-response curve is typically generated over a dose range of several logs, while here, the doses tested ranged over a factor of 50× (100 nM – 5 µM). Thus, the tested dose range may have been far too narrow to identify a dose response. The rationale for the included dose range was based on previous studies (Griggs et al., 2014). While our tested doses were likely higher than those humans are exposed to (Table 1), it is worth noting that HCAs may accumulate in human brain tissue, with reported levels (harmane) in the low nanomolar range (Louis et al., 2013). Thus, with respect to risk assessment practices, our lowest dose was within the typical 10× factor for translation from models to humans and 10× factor to account for individual variability (Faustman and Omenn, 2013). Doses above 5 µM were avoided due to solubility limits of lipophilic HCAs and decreased relevance to actual exposures. Another possibility for a lack of dose response is that the population of neurons in the culture system contains dopaminergic neurons from both the substantia nigra and ventral tegmental area because it is challenging to separate these adjacent nuclei during microdissection. Dopaminergic neurons in the substantia nigra are extensively lesioned in PD, while those from the ventral tegmental area are comparatively spared (Cannon and Greenamyre, 2011). Further, within the substantia nigra, there are dopaminergic neuronal subpopulations that exhibit differential sensitivity in PD and in animals exposed to PD toxicants (Fearnley and Lees, 1991; German et al., 1992; Lavoie and Parent, 1991; Liang et al., 1996). Thus, it is possible that at tested doses, specific subpopulations of dopamine neurons are affected and that comparatively insensitive dopamine neuronal populations would require higher doses to lesion. Finally, it is worth noting that longer exposure produced evidence that the magnitude of dopaminergic cell loss increases when treatment times are extended, further suggesting that temporal effects need to be considered when conducting in vivo studies.
HCAs have to date been primarily investigated as mutagens and for a role in carcinogenesis (Turner and Lloyd, 2017). Through the Ames assay, it has been determined that PhIP is the least mutagenic amongst most HCAs, although it is the most abundant in cooked meat, while MeIQ is the most mutagenic but the least abundant (Alaejos and Afonso, 2011). HCAs in the AIA group contain different degrees of known mutagenic properties that contribute to the development of colorectal cancer (Turner and Lloyd, 2017). Published mutagenicity of these HCAs are as follows MeIQ> IQ> 4,8-DiMeIQx> MeIQx> PhIP (Felton et al., 1999; Layton et al., 1995). In contrast, our findings suggest that AIA HCAs are ranked as follows in terms of cytotoxicity (lowest threshold dose for dopaminergic cell loss to highest), 4,8-DiMeIQx > MeIQ > MeIQx = PhIP > IQ. Thus, it appears that there is little relationship between mutagenicity potency and neurotoxicity, though further mechanistic studies will be needed. Data from the β-carboline group further support a disconnect between mutagenicity and neurotoxicity. Unlike the AIA group, β-carbolines are not known for mutagenic properties due to the lack of an active amine group. The α-carboline, AαC, does possess this amine group and is mutagenic. However, AαC is known to be less mutagenic than the other HCAs in the AIA group (Layton et al., 1995). When ranking our findings of carboline HCAs in terms of cytotoxicity (lowest threshold dose for dopaminergic cell loss to highest), the results are norharmane > harmane > AαC.
Ranking our findings in terms of cytotoxicity for all tested HCAs (lowest threshold dose for dopaminergic cell loss to highest), 4,8-DiMeIQx = norharmane > MeIQ = harmane > MeIQx = PhIP = AαC > IQ (Table 1). In examining the compounds lipophilicities and chemical structures (Tables 1 and 2), no obvious relationship emerges with these parameters and toxicity. However, in examining the AIA with the lowest threshold dose to elicit selective dopaminergic toxicity, 4,8-DiMeIQx, compared to that which required the highest dose, IQ, it is possible that both the presence and location of methyl groups may affect toxicity. Clearly, a far more detailed mechanistic analysis is needed in future studies, and our findings suggest that metabolites should also be examined for neurotoxicity.
Our previous findings suggested that PhIP exerts neurotoxicity through oxidative stress, where we showed evidence of oxidative damage in proteins and lipids, and that antioxidants were protective (Griggs et al., 2014). To determine if this mechanism is conserved across multiple HCAs, one HCA from each of the AIA and α/β-carboline groups was chosen to measure oxidative damage. MeIQ was chosen from the AIA group due to its potency in eliciting both TH+ neuron % decrease and neurite retraction. Harmane was chosen as a representative of the α/β-carboline group due to its response at almost all concentrations in both cytotoxicity and neurite length assays. Our findings indicate that MeIQ and harmane produce approximately a 4-fold and a 7-fold increase in NT staining vs. control, respectively. While oxidative protein modification also occurred in nondopaminergic neurons, the magnitude of the increase was far lower compared to dopaminergic neurons, suggesting selective sensitivity. In PD, oxidative stress has a critical role in the death of DA neurons (Hwang, 2013). Importantly, we have assessed oxidative modifications to protein, not direct production of oxygen free radicals. While upstream mechanisms still need to be elucidated, it appears that oxidative stress may also be a critical pathogenic event in HCA-induced neurotoxicity.
In summary, we have shown that multiple HCA subclasses produce selective dopaminergic neurotoxicity, an effect that should be investigated for relevance to PD. HCAs as an exposure are important because they are a common dietary component that may be consumed regularly and at higher doses than other neurotoxicants. The work presented here should prompt clinical and mechanistic studies to yield further insights into the role of HCAs in modulating PD risk.
Highlights.
HCAs produce selective loss of dopamine neurons in primary midbrain cultures.
The threshold dose for selective neurotoxicity is HCA-dependent.
HCAs generally elicit selective dopaminergic neurite retraction.
MeIQ and harmane increases oxidative damage in dopamine neurons.
Acknowledgments
Funding This work has been supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [R01ES025750 to J.R.C.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agim ZS, Cannon JR. Dietary factors in the etiology of Parkinson's disease. BioMed research international. 2015;2015:672838. doi: 10.1155/2015/672838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaejos MS, Afonso AM. Factors That Affect the Content of Heterocyclic Aromatic Amines in Foods. Comprehensive Reviews in Food Science and Food Safety. 2011;10(2):52–108. doi: 10.1111/j.1541-4337.2010.00141.x. [DOI] [Google Scholar]
- Bogen KT, Keating GA. U.S. dietary exposures to heterocyclic amines. J Expo Anal Environ Epidemiol. 2001;11(3):155–68. doi: 10.1038/sj.jea.7500158. [DOI] [PubMed] [Google Scholar]
- Cannon JR, Greenamyre JT. The role of environmental exposures in neurodegeneration and neurodegenerative diseases. Toxicological sciences : an official journal of the Society of Toxicology. 2011;124(2):225–50. doi: 10.1093/toxsci/kfr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson's disease. Neurobiology of Disease. 2009;34(2):279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science. 2006;313(5785):324–8. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68(5):384–6. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Enokizono J, Kusuhara H, Ose A, Schinkel AH, Sugiyama Y. Quantitative investigation of the role of breast cancer resistance protein (Bcrp/Abcg2) in limiting brain and testis penetration of xenobiotic compounds. Drug metabolism and disposition: the biological fate of chemicals. 2008;36(6):995–1002. doi: 10.1124/dmd.107.019257. [DOI] [PubMed] [Google Scholar]
- Faustman EL, Omenn GS. Risk Assessment. In: Klaassan CD, editor. Casarett & Doull's Toxicology: The Basic Science of Poisons. 8. McGraw Hill; New York: 2013. pp. 123–150. doi: [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain : a journal of neurology. 1991;114(Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Felton JS, Knize MG, Hatch FT, Tanga MJ, Colvin ME. Heterocyclic amine formation and the impact of structure on their mutagenicity. Cancer letters. 1999;143(2):127–34. doi: 10.1016/s0304-3835(99)00141-x. [DOI] [PubMed] [Google Scholar]
- Gaven F, Marin P, Claeysen S. Primary culture of mouse dopaminergic neurons. J Vis Exp. 2014:e51751. doi: 10.3791/51751(91). 10.3791/51751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German DC, Manaye KF, Sonsalla PK, Brooks BA. Midbrain dopaminergic cell loss in Parkinson's disease and MPTP-induced parkinsonism: sparing of calbindin-D28kcontaining cells. Annals of the New York Academy of Sciences. 1992;648:42–62. doi: 10.1111/j.1749-6632.1992.tb24523.x. [DOI] [PubMed] [Google Scholar]
- Gibis M. Heterocyclic Aromatic Amines in Cooked Meat Products Causes, Formation, Occurrence, and Risk Assessment, Comprehensive Reviews in Food Science and Food Safety Volume 15, Issue 2. Comprehensive Reviews in Food Science and Food Safety. 15:269–302. doi: 10.1111/1541-4337.12186. [serial online] Available at http://onlinelibrary.wiley.com/doi/10.1111/1541-4337.12186/abstract. Accessed 01. [DOI] [PubMed] [Google Scholar]
- Griggs AM, Agim ZS, Mishra VR, Tambe MA, Director-Myska AE, Turteltaub KW, McCabe GP, Rochet JC, Cannon JR. 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) is selectively toxic to primary dopaminergic neurons in vitro. Toxicological sciences : an official journal of the Society of Toxicology. 2014;140(1):179–89. doi: 10.1093/toxsci/kfu060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusinkveld HJ, Westerink RHS. Comparison of different in vitro cell models for the assessment of pesticide-induced dopaminergic neurotoxicity. Toxicol In Vitro. 2017 doi: 10.1016/j.tiv.2017.07.030. 10.1016/j.tiv.2017.07.030. [DOI] [PubMed] [Google Scholar]
- Horowitz MP, Milanese C, Di Maio R, Hu X, Montero LM, Sanders LH, Tapias V, Sepe S, van Cappellen WA, Burton EA, Greenamyre JT, Mastroberardino PG. Single-cell redox imaging demonstrates a distinctive response of dopaminergic neurons to oxidative insults. Antioxidants & redox signaling. 2011;15(4):855–71. doi: 10.1089/ars.2010.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang O. Role of oxidative stress in Parkinson's disease. Exp Neurobiol. 2013;22(1):11–7. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose H, Ozaki N, Nakahara D, Naoi M, Wakabayashi K, Sugimura T, Nagatsu T. Effects of heterocyclic amines in food on dopamine metabolism in nigro-striatal dopaminergic neurons. Biochemical pharmacology. 1988;37(17):3289–95. doi: 10.1016/0006-2952(88)90641-7. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Totsuka Y, Uchiya N, Kitamura T, Shibata H, Sugimura T, Wakabayashi K. Carcinogenicity of aminophenylnorharman, a possible novel endogenous mutagen, formed from norharman and aniline, in F344 rats. Carcinogenesis. 2004;25(10):1967–72. doi: 10.1093/carcin/bgh189. [DOI] [PubMed] [Google Scholar]
- Keating GA, Bogen KT. Estimates of heterocyclic amine intake in the US population. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2004;802(1):127–33. doi: 10.1016/j.jchromb.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Kuhn W, Muller T, Grosse H, Rommelspacher H. Plasma harman and norharman in Parkinson's disease. Journal of neural transmission. Supplementum. 1995;46:291–5. [PubMed] [Google Scholar]
- Kuhn W, Muller T, Grosse H, Rommelspacher H. Elevated levels of harman and norharman in cerebrospinal fluid of parkinsonian patients. J Neural Transm (Vienna) 1996;103(12):1435–40. doi: 10.1007/BF01271257. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–80. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Dopaminergic neurons expressing calbindin in normal and parkinsonian monkeys. Neuroreport. 1991;2(10):601–4. doi: 10.1097/00001756-199110000-00012. [DOI] [PubMed] [Google Scholar]
- Layton DW, Bogen KT, Knize MG, Hatch FT, Johnson VM, Felton JS. Cancer risk of heterocyclic amines in cooked foods: an analysis and implications for research. Carcinogenesis. 1995;16(1):39–52. doi: 10.1093/carcin/16.1.39. [DOI] [PubMed] [Google Scholar]
- Lesage S, Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Human molecular genetics. 2009;18(R1):R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- Liang CL, Sinton CM, Sonsalla PK, German DC. Midbrain dopaminergic neurons in the mouse that contain calbindin-D28k exhibit reduced vulnerability to MPTP-induced neurodegeneration. Neurodegeneration. 1996;5(4):313–8. doi: 10.1006/neur.1996.0042. [DOI] [PubMed] [Google Scholar]
- Liu F, Nguyen JL, Hulleman JD, Li L, Rochet JC. Mechanisms of DJ-1 neuroprotection in a cellular model of Parkinson's disease. Journal of neurochemistry. 2008;105(6):2435–53. doi: 10.1111/j.1471-4159.2008.05333.x. [DOI] [PubMed] [Google Scholar]
- Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, Yang H, Ueda K, Chan P, Yu S. alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci Lett. 2009;454(3):187–92. doi: 10.1016/j.neulet.2009.02.056. [DOI] [PubMed] [Google Scholar]
- Logroscino G, Marder K, Graziano J, Freyer G, Slavkovich V, Lojacono N, Cote L, Mayeux R. Dietary iron, animal fats, and risk of Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 1998;13(Suppl 1):13–6. [PubMed] [Google Scholar]
- Louis ED, Factor-Litvak P, Gerbin M, Slavkovich V, Graziano JH, Jiang W, Zheng W. Blood harmane, blood lead, and severity of hand tremor: evidence of additive effects. Neurotoxicology. 2011;32(2):227–32. doi: 10.1016/j.neuro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Factor-Litvak P, Liu X, Vonsattel JP, Galecki M, Jiang W, Zheng W. Elevated brain harmane (1-methyl-9H-pyrido[3,4-b]indole) in essential tremor cases vs. controls. Neurotoxicology. 2013;38:131–5. doi: 10.1016/j.neuro.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Factor-Litvak P, Michalec M, Jiang W, Zheng W. Blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentration in dystonia cases vs. controls. Neurotoxicology. 2014a;44C:110–113. doi: 10.1016/j.neuro.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Jiang W, Pellegrino KM, Rios E, Factor-Litvak P, Henchcliffe C, Zheng W. Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in essential tremor. Neurotoxicology. 2008;29(2):294–300. doi: 10.1016/j.neuro.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Michalec M, Jiang W, Factor-Litvak P, Zheng W. Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in Parkinson's disease. Neurotoxicology. 2014b;40:52–6. doi: 10.1016/j.neuro.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara K, Gonda T, Sawada H, Uezono T, Kobayashi Y, Kawamura T, Ohtaki K, Kimura K, Akaike A. Endogenously occurring beta-carboline induces parkinsonism in nonprimate animals: a possible causative protoxin in idiopathic Parkinson's disease. Journal of neurochemistry. 1998;70(2):727–35. doi: 10.1046/j.1471-4159.1998.70020727.x. [DOI] [PubMed] [Google Scholar]
- Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(2):444–53. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Yoshida D, Tomita H. Determination of mutagens, amino-alpha-carbolines in grilled foods and cigarette smoke condensate. Cancer letters. 1981;12(1–2):105–10. doi: 10.1016/0304-3835(81)90045-8. [DOI] [PubMed] [Google Scholar]
- Niwa T, Yamazoe Y, Kato R. Metabolic activation of 2-amino-9H-pyrido[2,3-b]indole by rat-liver microsomes. Mutation research. 1982;95(2–3):159–70. doi: 10.1016/0027-5107(82)90254-8. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Matsukura N, Morino K, Kawachi T, Sugimura T, Takayama S. Carcinogenicity in mice of mutagenic compounds from glutamic acid and soybean globulin pyrolysates. Carcinogenesis. 1984;5(6):815–9. doi: 10.1093/carcin/5.6.815. [DOI] [PubMed] [Google Scholar]
- PubChem. [Accessed August 8th, 2017];National Center for Biotechnology Information. PubChem Compound Database; CID=1530. 2017a Available at: https://pubchem.ncbi.nlm.nih.gov/compound/1530. doi.
- PubChem. [Accessed August 8, 2017];National Center for Biotechnology Information. PubChem Compound Database; CID=53462. 2017b Available at: https://pubchem.ncbi.nlm.nih.gov/compound/53462. doi.
- PubChem. National Center for Biotechnology Information. PubChem Compound Database; CID=62274. 2017c Available at: https://pubchem.ncbi.nlm.nih.gov/compound/62274 doi.
- PubChem. National Center for Biotechnology Information. PubChem Compound Database; CID=62275. 2017d Available at: https://pubchem.ncbi.nlm.nih.gov/compound/62275 doi.
- PubChem. [Accessed August 8th, 2017];National Center for Biotechnology Information. PubChem Compound Database; CID=62805. 2017e Available at: https://pubchem.ncbi.nlm.nih.gov/compound/62805. doi.
- PubChem. National Center for Biotechnology Information. PubChem Compound Database; CID=64961. 2017f Available at: https://pubchem.ncbi.nlm.nih.gov/compound/64961 doi.
- PubChem. National Center for Biotechnology Information. PubChem Compound Database; CID=104739. 2017g Available at: https://pubchem.ncbi.nlm.nih.gov/compound/104739 doi.
- PubChem. [Accessed July 20, 2017];National Center for Biotechnology Information. PubChem Compound Database; CID=5281404. 2017h Available at: https://pubchem.ncbi.nlm.nih.gov/compound/5281404. doi.
- Raza H, King RS, Squires RB, Guengerich FP, Miller DW, Freeman JP, Lang NP, Kadlubar FF. Metabolism of 2-amino-alpha-carboline. A food-borne heterocyclic amine mutagen and carcinogen by human and rodent liver microsomes and by human cytochrome P4501A2. Drug Metab Dispos. 1996;24(4):395–400. [PubMed] [Google Scholar]
- Roemer E, Meisgen T, Diekmann J, Conroy L, Stabbert R. Heterocyclic aromatic amines and their contribution to the bacterial mutagenicity of the particulate phase of cigarette smoke. Toxicology letters. 2016;243:40–7. doi: 10.1016/j.toxlet.2015.12.008. [DOI] [PubMed] [Google Scholar]
- Sammi SR, Agim ZS, Cannon JR. Harmane-induced selective dopaminergic neurotoxicity in C. elegans. Toxicological sciences : an official journal of the Society of Toxicology. 2017 doi: 10.1093/toxsci/kfx223. 10.1093/toxsci/kfx223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman JM, De Jager PL, Feany MB. Parkinson's disease: genetics and pathogenesis. Annual review of pathology. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- Skog KI, Johansson MA, Jagerstad MI. Carcinogenic heterocyclic amines in model systems and cooked foods: a review on formation, occurrence and intake. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 1998;36(9–10):879–96. doi: 10.1016/s0278-6915(98)00061-1. [DOI] [PubMed] [Google Scholar]
- Storch A, Hwang YI, Gearhart DA, Beach JW, Neafsey EJ, Collins MA, Schwarz J. Dopamine transporter-mediated cytotoxicity of beta-carbolinium derivatives related to Parkinson's disease: relationship to transporter-dependent uptake. Journal of neurochemistry. 2004;89(3):685–94. doi: 10.1111/j.1471-4159.2004.02397.x. [DOI] [PubMed] [Google Scholar]
- Strathearn KE, Yousef GG, Grace MH, Roy SL, Tambe MA, Ferruzzi MG, Wu QL, Simon JE, Lila MA, Rochet JC. Neuroprotective effects of anthocyanin-and proanthocyanidin-rich extracts in cellular models of Parkinsons disease. Brain Res. 2014;1555:60–77. doi: 10.1016/j.brainres.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura T. Carcinogenicity of mutagenic heterocyclic amines formed during the cooking process. Mutation research. 1985;150(1–2):33–41. doi: 10.1016/0027-5107(85)90098-3. [DOI] [PubMed] [Google Scholar]
- Teunissen SF, Vlaming ML, Rosing H, Schellens JH, Schinkel AH, Beijnen JH. Development and validation of a liquid chromatography-tandem mass spectrometry assay for the analysis of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and its metabolite 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine (N-OH-PhIP) in plasma, urine, bile, intestinal contents, faeces and eight selected tissues from mice. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2010;878(25):2353–62. doi: 10.1016/j.jchromb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Totsuka Y, Takamura-Enya T, Nishigaki R, Sugimura T, Wakabayashi K. Mutagens formed from beta-carbolines with aromatic amines. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences. 2004;802(1):135–41. doi: 10.1016/j.jchromb.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Turner ND, Lloyd SK. Association between red meat consumption and colon cancer: A systematic review of experimental results. Exp Biol Med (Maywood) 2017;242(8):813–839. doi: 10.1177/1535370217693117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ysselstein D, Joshi M, Mishra V, Griggs AM, Asiago JM, McCabe GP, Stanciu LA, Post CB, Rochet JC. Effects of impaired membrane interactions on alpha-synuclein aggregation and neurotoxicity. Neurobiol Dis. 2015;79:150–63. doi: 10.1016/j.nbd.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]