Abstract
Objective
Advanced age is an important risk factor for fracture. The ACTIVE (Abaloparatide Comparator Trial In Vertebral Endpoints) trial showed that abaloparatide-SC increased bone mineral density and reduced the risk of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis. This study describes the effects of abaloparatide-SC in the subgroup of patients aged 80 or more years in ACTIVE.
Methods
Post hoc analyses of bone mineral density and fracture incidence in this subgroup of patients who received abaloparatide-SC or placebo in the 18-month, phase 3, double-blind, randomized controlled ACTIVE trial.
Results
The mean ages of the patients ≥80 years were 81.9 and 81.7 years in the placebo (n=43) and abaloparatide-SC (n=51) groups, respectively. The increases in bone mineral density from baseline to 18 months with abaloparatide-SC treatment were 3.9% at the total hip (P <0.001), 3.6% at the femoral neck (P <0.01), and 12.1% at the lumbar spine (P <0.001), and were similar to those observed in the overall population. Abaloparatide-SC therapy was associated with numerical, but not statistically significant, reductions in the risk of vertebral and nonvertebral fractures in this subpopulation, compared to placebo. The proportion of patients reporting adverse events was similar between treatment groups and between the older subgroup and the overall population.
Conclusion
Abaloparatide-SC was effective in increasing bone mineral density in the very elderly subgroup of ACTIVE, with a safety profile similar to that of the overall study population.
Keywords: abaloparatide, bone mineral density, elderly, fracture risk, osteoporosis, postmenopausal women
Introduction
Osteoporosis is a disorder in which progressive bone loss damages skeletal architecture and impairs bone strength, resulting in increasing risk of fragility fracture.1 The prevalence of osteoporosis and the risk of fracture increase progressively with advancing age, and the clinical consequences of vertebral and hip fractures are greater among older compared to younger patients.2–4 A myriad of skeletal and non-skeletal risk factors for fracture occur among elderly patients. It is important to know whether drugs used to reduce fracture risk in patients with osteoporosis are effective in elderly patients, particularly given our aging society.
Abaloparatide is a 34-amino acid peptide that selectively binds to the parathyroid hormone receptor type 1 with higher selectivity for the RG versus R0 conformation resulting in transient receptor signaling with a net anabolic effect.5 In the multinational phase 3 Abaloparatide Comparator Trial In Vertebral Endpoints (ACTIVE) trial, treatment of women with postmenopausal osteoporosis with subcutaneous abaloparatide (abaloparatide-SC) for 18 months significantly increased bone mineral density (BMD) and decreased the risk of vertebral and nonvertebral fractures compared with placebo, and was well-tolerated.6 In a pre-planned subgroup analysis of ACTIVE, no interactions were observed between age at baseline as a function of 3 categories (<65, 65 to <75, and ≥75 years) and the treatment effect of abaloparatide-SC on new morphometric vertebral fractures, nonvertebral fractures, or changes in BMD.7 In this report, we describe a post hoc analysis of the effects and safety of abaloparatide-SC in the subgroup of patients aged 80 or more years in ACTIVE.
Methods
Patients and Procedures
The multicenter ACTIVE study enrolled postmenopausal women, ages 49 to 86 years, with osteoporosis as defined by prior radiographic vertebral fracture or recent (within 5 years of enrollment) nonvertebral fracture with a BMD T-score ≤ -2.5 and > -5.0 at the lumbar spine or femoral neck if aged ≤ 65 years or ≤ -2.0 and > -5.0 if aged > 65 years. For those aged > 65 years, no prior fracture was required if the lumbar spine or femoral neck BMD T-score was ≤ -3.0 and > -5.0. Other inclusion/exclusion criteria have been previously described.6 After informed written consent was obtained, patients were screened and then randomized 1:1:1 to receive either blinded daily injections of abaloparatide-SC 80 µg, matching placebo, or open-label daily subcutaneous injections of teriparatide 20 µg for 18 months. All patients received supplements of 500 to 1000 mg/day elemental calcium and 400 to 800 IU vitamin D based on regional standard of care. The endpoints were assessed as previously described,6 including the primary endpoint of the incidence of new vertebral fractures from baseline to 18 months in patients treated with abaloparatide-SC compared to placebo. Nonvertebral fractures, a secondary endpoint, were initially self-reported and then verified from source documents and excluded those of the spine, sternum, patella, toes, fingers, skull, and face and fractures associated with high trauma. Study oversight was performed and safety was assessed as previously described6 and all assessors of fracture were blinded to all treatments.
Statistical analyses
The primary and key secondary endpoints in ACTIVE were included in these post hoc analyses of a subset of patients ≥80 years of age in the abaloparatide-SC and placebo arms. Results for the overall population in the abaloparatide-SC and placebo arms are included for comparison. The intent-to-treat (ITT) population included all patients who were randomized to receive study medication and was used for all efficacy analyses except for those of vertebral fracture. The modified ITT population included all ITT patients who received both pretreatment and post baseline spine radiographs and the radiographs from this population were used for analysis of new morphometric vertebral fractures. The safety population included all patients who received one or more doses of study medication. The percent change in BMD from baseline to each study visit was compared using a mixed-effect repeated measures model. Relative risk ratios for new vertebral fractures were calculated using the Fisher’s exact test. Time to nonvertebral fracture was compared using the log-rank test in all participants through the entire observational period of 19 months (18 months of treatment plus 1 month of follow-up), as previously described.6 Hazard ratios for nonvertebral fractures were calculated using the Cox proportional hazards model. Since this was a hypothesis generating exploratory analysis, the P-values were not adjusted for multiple comparisons and were considered significant if <0.05.
Results
Among the 1645 women in the abaloparatide-SC and placebo blinded arms of the ACTIVE trial, 94 (5.7%) were age 80 years or older. Baseline characteristics for these elderly patients and the overall population are presented in Table 1. The mean ages of the patients in the elderly subgroup were 81.9 and 81.7 years in the placebo and abaloparatide-SC groups, respectively, and these patients were approximately 13 years older than those in the overall population at baseline. As expected, the BMD T-scores at the total hip and femoral neck were slightly lower in the older age cohort than in the overall population. Consistent with the inclusion criterion for women >65 years of age, fewer elderly patients reported having a nonvertebral fracture within 5 years prior to enrollment. The baseline characteristics were well-matched between treatment groups, with no statistically significant differences.
Table 1.
Demographics and Baseline Characteristics, ACTIVE Trial
| Characteristic | Placebo | Abaloparatide-SC | ||
|---|---|---|---|---|
| All, n=821 | ≥ 80 yr, n=43 | All, n=824 | ≥ 80 yr, n=51 | |
| Age, mean years (SD) | 68.7 (6.5) | 81.9 (1.5) | 68.9 (6.5) | 81.7 (1.4) |
| BMI, mean kg/m2 (SD) | 25.1 (3.6) | 25.2 (3.8) | 25.0 (3.5) | 24.7 (3.3) |
| Race, n (%) | ||||
| White | 655 (79.8) | 27 (62.8) | 663 (80.5) | 34 (66.7) |
| Asian | 131 (16.0) | 14 (32.6) | 128 (15.5) | 13 (25.5) |
| Black or African-American | 23 (2.8) | 2 (4.7) | 26 (3.2) | 4 (7.8) |
| Other | 12 (1.5) | 0 | 7 (0.8) | 0 |
| Hispanic or Latino, n (%) | 199 (24.2) | 18 (41.9) | 199 (24.2) | 21 (41.2) |
| BMD T-score, mean (SD) | ||||
| Total hip | −1.9 (0.8) | -2.4 (0.7) | −1.9 (0.7) | -2.2 (0.8) |
| Femoral neck | −2.2 (0.7) | -2.6 (0.6) | −2.2 (0.6) | -2.5 (0.6) |
| Lumbar spine | −2.9 (0.8) | -3.0 (0.9) | −2.9 (0.9) | -2.6 (1.3) |
| Prevalent vertebral fracture at baseline, n (%) | 188 (22.9) | 9 (20.9) | 177 (21.5) | 19 (37.3) |
| Prior nonvertebral fracture within 5 years of study day 1, n (%) | 266 (32.4) | 8 (18.6) | 248 (30.1) | 7 (13.7) |
BMD, bone mineral density; BMI, body mass index; yr, years. Values for overall population from reference 6.
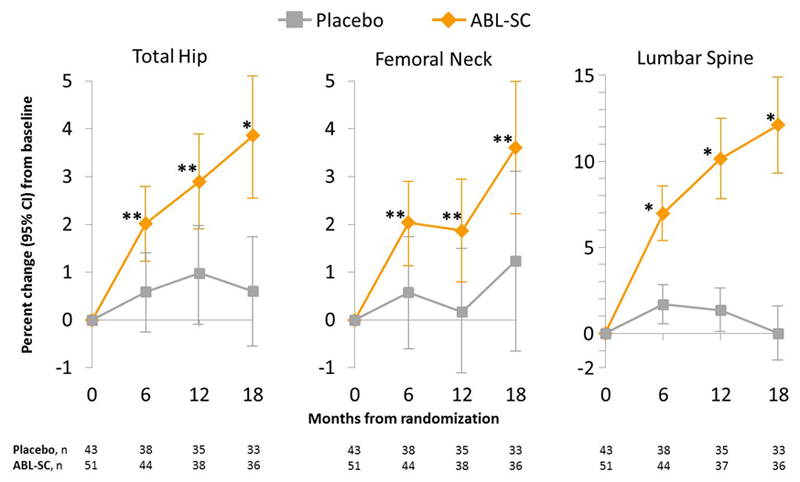
Changes in BMD over the 18-month treatment period are shown in Figure 1. In this older age group treated with abaloparatide-SC the increase in BMD at the total hip was significant at the first post-baseline measurement at 6 months (2.0% [95% CI, 1.2% to 2.8%]; P < 0.01 compared to placebo). After 18 months of treatment with abaloparatide-SC, the increases in BMD from baseline were 3.9% [95% CI, 2.5% to 5.2%], P < 0.001 compared to placebo at the total hip; 3.6% [95% CI, 2.2% to 5.0%], P < 0.01 compared to placebo at the femoral neck; and 12.1% [95% CI, 9.3% to 14.9%], P < 0.001 compared to placebo at the lumbar spine. These increases in BMD from baseline in the patients aged ≥80 years were similar to those reported for the overall population, with BMD increases from baseline at 6 months of 2.3% at the total hip, and at 18 months of 4.18% at the total hip, 3.6% at the femoral neck, 11.2% at the lumbar spine.6
Figure 1.
Mean percent change (95% confidence interval) in BMD at total hip, femoral neck, and lumbar spine among patients aged ≥80 years.
*P <0.001 vs placebo, **P <0.01 vs placebo.
ABL-SC, abaloparatide-SC; CI, confidence interval
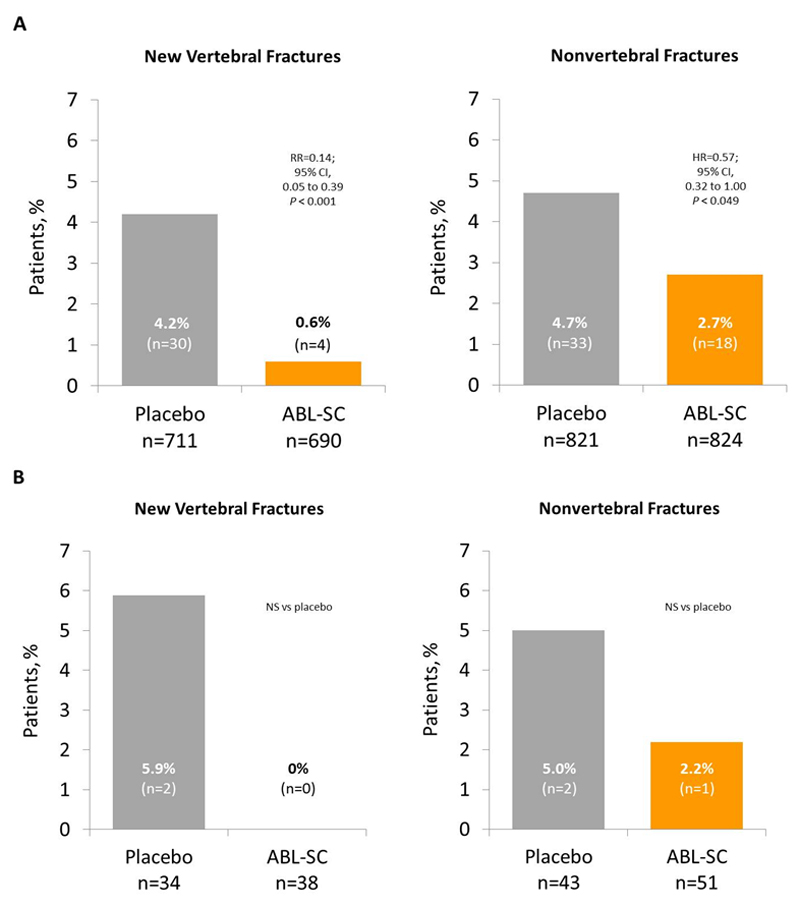
Vertebral and nonvertebral fracture risk reductions in the overall population are shown in Figure 2A. Although the number of fractures was small in the older age cohort, abaloparatide-SC therapy was associated with numerical, but not statistically significant, reductions in the risks of vertebral and nonvertebal fractures for this cohort, compared with placebo (Figure 2B). The proportions of patients reporting adverse events were similar between treatment groups and between the older age cohorts and the overall population (Table 2). As expected in an elderly population, the proportion of patients 80 years and older who reported serious adverse events was higher than in the entire study cohort but did not differ between the abaloparatide-SC and placebo groups. The overall discontinuation rate for the older age cohort (30.9%) was higher than for the entire study cohort (24.4%).
Figure 2.
Fracture risk reduction after 18 months of treatment among (A) the overall population and (B) patients aged ≥80 years. Percentages for new vertebral fractures were calculated using the modified intent-to-treat population. Percentages of nonvertebral fractures were Kaplan-Meier estimates using the intent-to-treat populations.
ABL-SC, abaloparatide-SC; CI, confidence interval; HR, hazard ratio; NS, not significant; RR, relative risk.
Table 2.
Safety and Adverse Events, ACTIVE Trial
| Event, n (%) | Placebo | Abaloparatide-SC | ||
|---|---|---|---|---|
| All, n=820 | ≥ 80 yr, n=43 | All, n=822 | ≥ 80 yr, n=51 | |
| ≥1 TEAE | 718 (87.6) | 34 (79.1) | 735 (89.4) | 44 (86.3) |
| ≥1 Serious TEAE | 90 (11.0) | 8 (18.6) | 80 (9.7) | 11 (21.6) |
| ≥1 TEAE Leading to Deatha | 5 (0.6) | 0 | 3 (0.4) | 3 (5.9) |
| ≥1 TEAE leading to discontinuation | 50 (6.1) | 1 (2.3) | 81 (9.9) | 6 (11.8) |
| Common TEAEsb | ||||
| Upper respiratory tract infection | 63 (7.7) | 6 (14.0) | 68 (8.3) | 10 (19.6) |
| Dizziness | 50 (6.1) | 5 (11.6) | 82 (10.0) | 7 (13.7) |
| Hypertension | 54 (6.6) | 3 (7.0) | 59 (7.2) | 7 (13.7) |
| Nasopharyngitis | 66 (8.0) | 2 (4.7) | 48 (5.8) | 4 (7.8) |
| Influenza | 39 (4.8) | 1 (2.3) | 52 (6.3) | 4 (7.8) |
| Muscle spasms | 16 (2.0) | 1 (2.3) | 22 (2.7) | 4 (7.8) |
| Osteoarthritis | 31 (3.8) | 1 (2.3) | 34 (4.1) | 4 (7.8) |
| Pain in extremity | 49 (6.0) | 3 (7.0) | 40 (4.9) | 3 (5.9) |
| Hypercalciuria | 74 (9.0) | 4 (9.3) | 93 (11.3) | 3 (5.9) |
| Urinary tract infection | 38 (4.6) | 4 (9.3) | 43 (5.2) | 2 (3.9) |
| Back pain | 82 (10.0) | 4 (9.3) | 70 (8.5) | 2 (3.9) |
| Anemia | 15 (1.8) | 3 (7.0) | 23 (2.8) | 2 (3.9) |
| Bronchitis | 20 (2.4) | 3 (7.0) | 19 (2.3) | 2 (3.9) |
| Constipation | 42 (5.1) | 3 (7.0) | 37 (4.5) | 1 (2.0) |
| Arthralgia | 80 (9.8) | 5 (11.6) | 71 (8.6) | 0 |
| Hypertriglyceridemia | 21 (2.6) | 3 (7.0) | 20 (2.4) | 0 |
Causes of death among the overall population in the placebo group: bowel cancer, intestinal obstruction, myocardial infarction, dissecting aneurysm of the aorta, sudden death; in the abaloparatide group: sepsis, bronchiectasis, ischemic heart disease.
Occurring in ≥5% in either of the ≥ 80 years treatment groups.
TEAE, treatment-emergent adverse event; yr, years. Values for overall population from reference 6 and data on file.
Discussion
In the subgroup of patients aged 80 years or older, abaloparatide-SC significantly increased BMD of the total hip, femoral neck, and lumbar spine, with increments of similar magnitude to those seen in the overall study. The numerical reductions in the risk of vertebral and nonvertebral fracture with abaloparatide-SC were also similar to the effects described in the entire ACTIVE trial, albeit with these analyses limited by the very small number of fracture events in the elderly subgroup. Tolerability and safety were also similar in the elderly cohort vs the entire ACTIVE cohort.
Older age is a consistent risk factor for both falls and for osteoporosis as diagnosed by low BMD, both of which are important and independent risk factors for fracture.8,9 In patients age 75 years and older, patients with hip T-score values consistent with osteoporosis are at higher risk of fracture than are patients of the same age with higher BMD values, although it appears that the contribution of low bone mineral density to fracture risk is less among older compared to younger postmenopausal women, perhaps related to the accumulation of fall-related risk factors with advancing age.10 Prevalence of vertebral fracture is also higher in the elderly, regardless of BMD.11
The effectiveness of osteoporosis drugs in elderly patients was initially addressed in the risedronate hip fracture trial in which risedronate significantly decreased the risk of hip fracture in postmenopausal women aged 70-79 years with low BMD.12 However, in a group of women at least 80 years old enrolled primarily on the basis of fall related risk factors, the 20% reduction in hip fracture risk was not statistically significant. Subsequently, in a post hoc analysis of the subgroup of patients in this study who were at least 80 years old with osteoporosis by BMD or prevalent vertebral fracture criteria, risedronate statistically significantly reduced the risk of hip fracture.13 Additionally, in a post hoc analysis of pooled data from the phase 3 risedronate fracture endpoint trials, risedronate reduced the risk of vertebral fracture in patients who were at least 80 years old with osteoporosis or at least one prevalent vertebral fracture.14 A retrospective subgroup analysis in men and women with osteoporosis ≥80 years treated with teriparatide demonstrated similar effects of therapy on BMD compared to treatment of women <80 years.15 In a post hoc analysis of the phase 3 trial with zoledronic acid, there were significant reductions in clinical vertebral and nonvertebral fractures in postmenopausal women with osteoporosis ≥75 years of age.16 In a post hoc analysis of a phase 3 study, denosumab significantly reduced the risk of hip fracture in a subset of postmenopausal women aged 75 years or older at high risk for fracture.17 Our results with abaloparatide-SC presented here are consistent with these subgroup analyses.
The strength of these analyses is the clinically important subgroup of very elderly women studied. The limitations include the post hoc nature of the analyses, as well as those limitations related to subgroup analyses including lack of adjustment for multiple comparisons, possible confounding as a result of the lack of stratification by age in the original randomization, and the small number of fracture events.
Conslusions
In the subgroup of patients aged 80 years or older, abaloparatide-SC therapy was associated with significantly increased BMD of the lumbar spine and proximal femur, numerically fewer vertebral and nonvertebral fractures, and no differences in the safety profile. These findings are consistent with efficacy of abaloparatide-SC in the very elderly comparable to that in the general older population. Since life expectancy is increasing and a growing proportion of individuals in the next 30 years will be in the at risk elderly category, it will be important to further develop such therapeutic options that clearly reduce fractures in the very old if we hope to make a substantial impact on disability, loss of independence, and mortality from complications of osteoporosis.
Acknowledgments
Medical writing support was provided by John L. Stock, MD, and funded by Radius Health, Inc.
Financial support: This work was supported by Radius Health, Inc.
Financial disclosures/conflicts of interest:
MM: Consultant/advisor for Amgen, Radius Health, Inc; NCH: consultancy, lecture fees and honoraria from Alliance for Better Bone Health, AMGEN, MSD, Eli Lilly, Servier, Shire, Consilient Healthcare and Internis Pharma; LAF, GH, YW: employees of Radius Health, Inc. and own company stock; PDM: member of scientific advisory boards for AgNovos, Amgen, Eli Lilly, Merck, Radius Health, Roche, Ultragenyx; research grants from Alexion, Amgen, Boehringer-Ingelheim, Daiichi-Sankyo, Eli Lilly, Immunodiagnostics, Merck, Merck Serono, National Bone Health Alliance, Novartis, Radius Health, Regeneron, Roche Diagnostics, Ultragenyx; data safety committees for Allergan, Grunenthal Group
FC: Consultant/advisor and Speaker for Amgen, Eli Lilly, Radius Health; Research Grant Recipient: Amgen, Eli Lilly; Consultant/Advisor: Tarsa
References
- 1.Consensus development conference: diagnosis, prophylaxis and treatment of osteoporosis. Am J Med. 1993;94:646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ., III Perspectives: How many women have osteoporosis now? J Bone Miner Res. 1995;10:175–177. doi: 10.1002/jbmr.5650100202. [DOI] [PubMed] [Google Scholar]
- 3.Looker AC, Wahner HW, Dunn WL, et al. Proximal femur bone mineral levels of US adults. Osteoporos Int. 1995;5:389–409. doi: 10.1007/BF01622262. [DOI] [PubMed] [Google Scholar]
- 4.Söderqvist A, Ekström W, Ponzer S, et al. Prediction of mortality in elderly patients with hip fractures: a two-year prospective study of 1,944 patients. Gerontology. 2009;55:496–504. doi: 10.1159/000230587. [DOI] [PubMed] [Google Scholar]
- 5.Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology. 2016;157:141–149. doi: 10.1210/en.2015-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis. JAMA. 2016;316:722–733. doi: 10.1001/jama.2016.11136. [DOI] [PubMed] [Google Scholar]
- 7.Cosman F, Hattersley G, Hu M, Williams GC, Fitzpatrick LA, Black DM. Effects of abaloparatide-SC on fractures and bone mineral density in subgroups of postmenopausal women with osteoporosis and varying baseline risk factors. J Bone Miner Res. 2017;32:17–23. doi: 10.1002/jbmr.2991. [DOI] [PubMed] [Google Scholar]
- 8.Dargent-Molina P, Favier F, Grandjean H, et al. Fall-related factors and risk of hip fracture: the EPIDOS prospective study. Lancet. 1996;348:145–149. doi: 10.1016/s0140-6736(96)01440-7. [DOI] [PubMed] [Google Scholar]
- 9.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 10.Schott AM, Cormier C, Hans D, et al. How hip and whole-body bone mineral density predict hip fracture in elderly women: the EPIDOS Prospective Study. Osteoporos Int. 1998;8:247–254. doi: 10.1007/s001980050061. [DOI] [PubMed] [Google Scholar]
- 11.Cosman F, Krege JH, Looker AC, et al. Spine fracture prevalence in a nationally representative sample of US women and men aged ≥40 years: results from the National Health and Nutrition Examination Survey (NHANES) 2013-2014. Osteoporos Int. 2017;28:1857–1866. doi: 10.1007/s00198-017-3948-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClung MR, Geusens P, Miller PD, et al. Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 13.Masud T, McClung M, Geusens P. Reducing hip fracture risk with risedronate in elderly women with established osteoporosis. Clin Interv Aging. 2009;4:445–449. doi: 10.2147/cia.s8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boonen S, McClung MR, Eastell R, El-Hajj Fuleihan G, Barton IP, Delmas P. Safety and efficacy of risedronate in reducing fracture risk in osteoporotic women aged 80 and older: implications for the use of antiresorptive agents in the old and oldest old. J Am Geriatr Soc. 2004;52:1832–1839. doi: 10.1111/j.1532-5415.2004.52506.x. [DOI] [PubMed] [Google Scholar]
- 15.Niimi R, Kono T, Nishihara A, et al. Usefulness of daily teriparatide treatment in elderly patients over 80 years of age. Osteoporos Int. 2016;27:1869–1874. doi: 10.1007/s00198-015-3479-1. [DOI] [PubMed] [Google Scholar]
- 16.Boonen S, Black DM, Colón-Emeric CS, et al. Efficacy and safety of a once-yearly intravenous zoledronic acid 5 mg for fracture prevention in elderly postmenopausal women with osteoporosis aged 75 and older. J Am Geriatr Soc. 2010;58:292–299. doi: 10.1111/j.1532-5415.2009.02673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boonen S, Adachi JD, Man Z, et al. Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab. 2011;96:1727–1736. doi: 10.1210/jc.2010-2784. [DOI] [PubMed] [Google Scholar]